Abstract
Obesity has reached epidemic proportions, and excess body weight and adiposity have been linked to many adverse health conditions including various cancers. Rising obesity rates over the last few decades have been paralleled by concomitant reductions in nocturnal sleep duration, and epidemiological evidence has demonstrated a relationship between short sleep and increased weight gain and obesity. Causality cannot be inferred from these studies however, so laboratory-based interventions are essential to determine the nature of the short sleep-obesity link. The aim of this chapter is to summarize and evaluate the clinical intervention studies which altered sleep either by partially restricting sleep episode length or by completely eliminating the sleep episode to investigate the resulting effects on energy balance. Specific energy balance parameters considered include energy expenditure, subjective hunger/appetite ratings, appetite-regulating hormones, and food intake. Most studies support a role of short sleep in increasing food intake, but the results on energy expenditure, hunger, and hormonal regulation of food intake are less consistent. This chapter critically evaluates how methodological differences may contribute to discrepancies and inconsistencies between study results, with an emphasis on the roles of sex, the state of energy balance of study participants, and the timing of manipulated sleep schedules within the intervention studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sleep
- Sleep deprivation
- Sleep duration
- Energy balance
- Energy expenditure
- Hunger
- Appetite hormones
- Food intake
- Obesity
Introduction
Obesity has reached epidemic proportions worldwide, and a recent estimate indicates that almost a third of the adult population in the United States is obese (body mass index [BMI] ≥30 kg/m2) [1]. Obesity is a problem that has been linked to adverse health outcomes, including cardiovascular disease, diabetes, and overall reductions in life expectancy. Moreover, epidemiological evidence has demonstrated an association between obesity and increased risk of developing a variety of cancer types, including cancers of the esophagus, colon, breast, endometrium, kidney, liver, and pancreas [2]. Clearly understanding the various factors which contribute to the increased prevalence of obesity will therefore have widespread ramifications for many aspects of public health, including cancer.
One such potential contributor is sleep, which researchers increasingly point to as having a functional role in maintaining proper metabolism in addition to its more well-established roles in cognition and brain function. Over the past few decades, the drastic increase in the prevalence of obesity has been reflected by substantial decreases in the amount of sleep being obtained. For example, whereas in 1960 modal sleep duration was observed to be 8–8.9 h/night, by 2004 more than 30 % of adults aged 30–64 years reported sleeping <6 h/night [3]. More recently, the results of a large, cross-sectional population-based study of adults in the United States showed that 7.8 % report sleeping <5 h/night, 28.3 % report sleeping ≤6 h/night, and 59.1 % of those surveyed report sleeping ≤7 h/night [4].
These decreases in nocturnal sleep duration are likely due to modern technological advances, including widespread use of television and computers at night, and other light-emitting and alerting electronic appliances. Indeed, striking data from the 2011 Sleep in America Poll conducted by the National Sleep Foundation indicate that 95 % of those surveyed use some type of light-emitting electronic device, such as television, computer, cell phone, or tablet in the hour before going to sleep [5]. Exposure to bright, artificial light during the hours preceding bedtime can significantly suppress the release of the sleep-promoting hormone melatonin, which can delay sleep initiation and shorten sleep duration [6]. Related to this is the case of shift workers, who are exposed to high levels of ambient lighting during nighttime hours and frequently experience curtailment of sleep length by 1–4 h/night [7]. These workers show increased BMI and obesity prevalence compared to day workers [8, 9]. Additionally, a disproportionately high incidence of breast cancer was found in shift-working women [10]. One hypothesized mechanism underlying this association was exposure to light at night and subsequent melatonin suppression which can promote breast cancer development [11]. However, the contributions of chronic sleep restriction and obesity in individuals with atypical work schedules have not been established. Interestingly, it was recently observed that exposure to light at night is associated with higher odds of obesity and dyslipidemia [12], which further suggests an interaction between short sleep, obesity, and cancer.
Mounting epidemiological evidence supports the association between short sleep duration and the development of obesity, with increased odds of obesity observed in individuals habitually sleeping <7 h/night [13, 14]. Despite the associations, observational studies alone cannot establish a direct link between reduced sleep duration and increased obesity. Indeed, some authors have questioned the clinical relevance of the epidemiological studies and remain skeptical of the proposed causal links between short sleep and obesity [15]. It becomes apparent, therefore, that sleep-focused intervention studies are necessary to clearly determine the role of short sleep as a contributor to the development of obesity. An understanding of the mechanisms underlying this relationship will help determine if short sleep is a modifiable risk factor that affects obesity risk [16] and could potentially lead to targeted lifestyle treatment options for body weight management efforts.
This chapter will focus on laboratory-based clinical intervention studies which manipulated the duration of sleep to determine the resulting effects on energy balance-related parameters. We will consider studies that altered sleep either by partially restricting sleep episode length or by completely eliminating the sleep episode. The specific energy balance parameters included are energy expenditure (EE), hunger/appetite, appetite-regulating hormones, and food intake. A particular focus of this chapter will be on the specific methodological differences that characterize the various intervention studies and how these methodological differences may contribute to discrepancies and inconsistencies in the literature. Our aim, therefore, is to critically review the literature of laboratory-based sleep-focused intervention studies to more fully examine the functional implications of sleep restriction on energy balance while considering the confounding effects of differences in methods used in the various trials.
Energy Balance and Obesity
In practical terms, body weight gain and obesity are thought to develop as a consequence of excessive food intake and/or reduced physical activity [17]. Body weight stability is achieved when energy intake is equal to the energy output. Thus, energy balance is the quantifiable relationship between the intake and output of energy from the body. A major goal of the laboratory-based clinical intervention studies described in this chapter is to mechanistically support or disprove the epidemiological evidence in determining if sleep restriction is a causal factor in the pathway to obesity. If so, sleep restriction is expected to result in an energy imbalance such that energy intake is increased relative to EE (i.e., energy intake > energy output).
Total EE (TEE) is the summation of several components, including resting metabolic rate (RMR; the amount of energy fueling the body at rest), the thermic effect of food (TEF; energy associated with absorption and metabolism of food), and physical activity (PA; voluntary activity like exercise and non-exercise activity) [17]. The amount of food and composition of meals consumed under ad libitum conditions, either via totally free access or during a test meal, is a method of quantifying energy intake. Related to food intake is the hormonal and cognitive control of hunger and appetite. Thus, while measures of circulating appetite-regulating hormones, as well as current levels of subjective hunger and appetite, do not assess energy intake, per se, they represent an important aspect of the controls of food intake. It should be pointed out that while some have determined how sleep restriction conditions affect a calculated value of energy balance [18, 19], most researchers have rather investigated how sleep can influence the various components of energy balance (e.g., RMR, TEF, food intake) or energy balance-regulating factors (e.g., hunger, hormones).
Methodological Issues: Factors Which Can Influence Energy Balance Parameters or Their Assessment
A variety of issues arise when attempting to compare the results across various intervention studies which have used different methodological approaches to address the question of how sleep restriction affects energy balance. The following sections will systematically address the various methods used for experimental manipulation and data collection and their potential effects on the expression of the outcome variables, including EE, hunger, appetite-regulating hormones, and food intake.
Sleep Duration and Timing Effects
One of the most important aspects to consider when comparing across sleep-focused interventions is the nature of the manipulation, i.e., the duration of the sleep episode that is allowed. The most extreme case of sleep restriction is total sleep deprivation wherein sleep is completely eliminated for ≥24 h [20–24]. Partial sleep restriction, a model of sleep curtailment that is a closer approximation of what is experienced in daily life, allows for sleep episodes that are less than the “typical” sleep episode length (7–8 h/night) and ranges from an allowance of 3 h/night to 5.5 h/night in the studies included in this chapter [18, 19, 25–33]. Though not discussed here, it should be noted that some intervention studies have utilized manipulations which were designed to disturb sleep quality or the relative expression of specific sleep stages without affecting total sleep duration [34–36].
Hormone secretion within the hypothalamic-pituitary axis (HPA), which can affect metabolism and energy balance, is affected by the presence or absence of sleep, per se. Growth hormone and prolactin are observed to increase during sleep, whereas secretion of thyroid-stimulating hormone is inhibited by sleep [3]. Cortisol secretion is increased following total sleep deprivation [3], although the results are less consistent for partial sleep restriction [37]. Distinct sleep stages, as illustrated by cortical electroencephalographic activity, also play a role in the peripheral and central regulation of hormones and physiology, as slow-wave sleep (SWS) increases GH release and decreases sympathetic nerve activity [3], whereas rapid eye movement (REM) sleep appears to be associated with orexin (hypocretin) release [38]. A sleep stage-specific alteration in EE has also been reported [39]. The effects of total sleep deprivation may therefore be quite distinct from more moderate, partial sleep deprivation in which some sleep is allowed.
Related to the duration of the sleep episode is the length of the exposure to the sleep manipulation. Most studies of acute total sleep deprivation are 1 day [20–23], which may be similar to real-life circumstances in which it is rare to have total sleep elimination for longer than 24 h. Within the clinical laboratory setting, an even more realistic approximation of common real-life circumstances may be a chronic exposure to a milder partial sleep curtailment. Nonetheless, experimental designs have not been uniform, and sleep curtailment manipulations have lasted for as little 1 day to as long as 14 days [18, 19, 25–33]. Short-term sleep restriction may have different metabolic effects than longer-term periods where the body has time to habituate to a new sleep regimen and achieve a new equilibrium. This has not been studied thus far.
Although it may be less apparent than sleep episode length, the timing or scheduling of in-lab sleep opportunities may influence the expression of energy balance-related parameters. One common practice is to center the timing of the restricted sleep episode at the same or a similar clock time as the habitual/baseline sleep episode. For example, researchers may compare a short sleep episode scheduled from 0200 to 0600 h with a normal length episode scheduled from 0000 to 0800 h [25] or compare sleep episodes occurring at 0100–0500 h with those occurring at 2200–0800 h [29]. Alternatively, sleep duration may be restricted by eliminating the early portion of the sleep episode and anchoring sleep time to the wake time of the habitual sleep condition. An example of this would be comparing a short sleep episode scheduled from 0400 to 0800 h to a habitual sleep episode occurring from 2200 to 0800 h [30, 32]. A third option, used by at least one study, is to anchor the sleep episode to bedtime, such as from 2230 to 0400 h for short sleep and from 2230 to 0600 h for habitual sleep [22].
Sleep episode timing is important to consider since sleep is not a uniform process, and the amount and presence of specific sleep stages throughout the night are not constant. A sleep-regulatory interaction between circadian and homeostatic mechanisms [40] dictates that SWS, under a homeostatic regulation, is highest at the start of the sleep episode (regardless of clock time), whereas REM sleep expression, under a circadian regulation, is highest during the early morning hours [41]. Depending on the specifics of the experimental sleep manipulation, the amount of REM sleep may be disproportionately reduced compared to SWS which is expected to be conserved, as may be the case when short sleep episodes are anchored at the start or middle of habitual sleep. These nuances in the timing of the sleep episode are important since a growing body of literature is revealing that sleep architecture plays a role in energy balance regulation [42, 43]. Specifically, REM sleep expression has been demonstrated to be inversely related to hunger ratings and intake of fat and carbohydrate [42]. Moreover, recent epidemiological studies have described an effect of sleep episode timing on food intake and BMI [44, 45].
Methodology of Measurements
Measures of Energy Expenditure
In considering the effects of sleep restriction on energy output, researchers have typically focused on TEE and two of its main components, RMR and postprandial EE, or the TEF. TEE can be measured with the use of doubly labeled water (DLW) [18, 19] and also via whole-room indirect calorimetry (metabolic chamber) [21]. Whereas both can be used to estimate EE over a 24-h period, important differences between the two methods exist. DLW is best suited for measures of long-term free-living EE, whereas indirect calorimetry may be more suited for use within the controlled laboratory environment. However, most researchers may not have access to metabolic chambers at their facilities, and metabolic carts are used to measure RMR by indirect calorimetry over short periods (up to several hours). Caution should be used when making comparisons of TEE measured by each method, since it was observed that free-living EE estimated with DLW was 15 % greater than TEE measured in a metabolic chamber [46]. This is likely due to restricted movements within the confines of a small room. Participants’ PA levels are greatly reduced when they are restricted to the small room for 24-h metabolic recordings, compared to free-living conditions. To assess free-living PA, researchers have typically employed either wrist- [27] or waist-worn [18, 25] actigraphy. Of course, the site of attachment of actigraphic recording devices may influence recorded activity levels. Calmly sitting and reading or using the computer may manifest as high activity for wrist-placed recordings but minimal for waist-based recordings, though one study found that wrist actigraphy is a slightly more accurate estimate of calorimetry-based EE than waist placement [47]. Moreover, translation of activity counts to actual caloric expenditure relies on algorithms that have their own inherent errors. RMR [18–20, 26] and TEF [19, 20] are almost uniformly measured via indirect calorimetry using a ventilated hood metabolic cart.
Measures of Hunger/Appetite
In the laboratory setting, subjective ratings of hunger and appetite in response to sleep restriction have been made using either visual analogue scales (VAS) or Likert scales. A VAS typically consists of a bipolar horizontal 10-cm line, with one side expressing minimal extreme and the other side maximal extreme, on which participants rate their current level of hunger (or appetite) along the continuum. A Likert scale is a numeric rating scale on which participants select their current level of hunger (or appetite) by selecting a number between two extreme values. Both the VAS and Likert scales have been established as valid and reliable measurement tools and are thought to yield comparable results [48].
The timing of hunger/appetite assessments may influence the reported results. Indeed, a circadian rhythm of leptin secretion has been described [49] which is likely to drive a variation of hunger/satiety across the 24-h day. Moreover, food intake has an effect on subjective hunger. It may therefore be difficult to compare the results from ratings taken at one time point, e.g., in the morning in a fasted state [20, 22, 31], with those taken in the evening before dinner [28], 15–30 min before each meal [23], or throughout the day [18, 25, 27, 30].
Measures of Appetite-Regulating Hormones
As was described for appetite ratings, the timing and frequency of the sampling of appetite-regulating hormones is an important experimental detail to consider. This is particularly true for leptin, a satiety hormone, which, as described, circulates with an endogenous circadian rhythmicity [49]. Accordingly, a number of researchers have sampled plasma leptin in response to sleep restriction continuously over a ≥24-h span [18–20, 23, 24, 33] or repeatedly across the daytime and evening but not throughout the night [27, 29, 30]. Some studies discussed here included several leptin measurements selectively taken during the morning [22, 31, 32] or single morning and evening measurements to approximate the diurnal variation [28].
Similar considerations should be made for ghrelin, the other appetite-regulating hormone whose secretion has been most extensively studied in response to sleep restriction. Plasma ghrelin has been sampled in the context of sleep restriction across the 24-h day [18–20] or repeatedly across the daytime and evening but not throughout the night [27, 29]. Importantly, ghrelin, an appetite-stimulating hormone, is affected by food intake, showing a preprandial rise and a postprandial fall in its levels [50]. This should be considered when comparing the results of the study which sampled ghrelin selectively in the morning under fasting conditions [22] with the aforementioned studies with continuous sampling. It should also be noted that whereas most studies measured total ghrelin, only two have reported on active ghrelin levels in response to sleep restriction and these show different results [51, 52].
Availability of food and presentation of meals during the intervention period also makes the comparison of appetite-regulating hormones between studies difficult, since many of these hormones respond to energy intake. As stated, ghrelin levels decrease after a meal [50], whereas leptin levels are stimulated by food intake [53]. Most of the studies investigating the leptin or ghrelin response to sleep restriction presented food in fixed, standardized meals [18, 20, 28, 30, 31, 33], although others allowed for ad libitum food intake [19, 27, 32] and one sampled hormones under conditions of constant intravenous glucose infusion [29]. Unrestricted access to energy intake will often lead to a relative positive energy balance between restricted and habitual sleep, since sleep restriction leads to overeating relative to habitual sleep duration (see below) [18, 19, 25]. This can explain discordant hormonal responses to sleep restriction between studies and would also be expected to explain differences in hunger and appetite ratings, as discussed above. On the other hand, at least one sleep restriction study was conducted under a state of mild negative energy balance [51], which can also affect hormone levels and appetite regulation. In that case, however, food intake was matched during both sleep phases, and the degree of energy imbalance was equivalent under restricted and habitual sleep. In general, however, most studies did not determine and report energy balance state. Additionally, utilizing controlled feeding in sleep restriction experiments does not necessarily guarantee stable energy balance between sleep phase conditions. Specifically, participants can be over- or underfed, even on a controlled diet, due to slight inaccuracies in energy requirement estimation equations [54].
Measures of Food Intake
The effects of sleep restriction on food intake are assessed by allowing participants to eat freely while measuring total energy consumed and the macronutrient composition of what is eaten. Typically, food is weighed by the investigator before and after meals to determine consumption, and the nutritional content is determined with computer software [18–20, 25, 27]. Differences in the method of food presentation, however, may account for slight discrepancies in the literature. In the studies to date, food intake was measured with buffet/constant availability of food [27], food served in excess at fixed meal times [19, 20, 25], or under conditions of complete participant control over food selection and eating time [18].
Sex Effects
Sex-based differences can contribute to interindividual variability when exploring the interaction between sleep and energy balance. Important changes in physiology, hormone secretion, and behavior across the menstrual cycle in premenopausal women can also affect this relationship.
Whereas sleep macrostructure appears not to be affected by sex [55], sleep complaints are more prevalent in women who are 1.5–2 times more likely to report insomnia symptoms than men [56]. Alterations in sleep across the menstrual cycle have been reported, with reduced REM sleep observed during the postovulatory luteal phase compared to the preovulatory follicular phase [57]. Body temperature and thermoregulation are also significantly modulated by both sex [55] and menstrual phase [55, 58].
Sex-based differences in EE are not widespread [59], although decreased RMR has been observed in women compared to men [60]. In terms of menstrual cycle, some have demonstrated increases in 24-h EE [61, 62] and TEF [62] during the luteal phase compared to the follicular phase. Women have higher fasting serum leptin levels compared to men at similar total body fat mass [63], and they have a higher 24-h leptin profile [64]. Leptin levels appear to have a menstrual phase variation, with increased levels observed during the luteal phase compared to the follicular phase [65, 66]. Sex-based differences in ghrelin levels, however, have been inconsistently observed [67, 68], and ghrelin appears to be stable across the menstrual cycle [69]. Daily energy requirements are higher for men than women, and this is reflected in the significantly increased daily energy intake observed in men compared to women after controlling for age, height, and weight [70]. Food intake is often observed to have a menstrual cycle variation, with increased intake during the luteal phase compared to the follicular phase, though inconsistencies also exist [71]. Taken together, it is therefore important to be aware of the sex distribution within each study as well as phase of the menstrual cycle when measurements are taken when ovulating women were included. Although it is acceptable for studies to make assessments within subjects at the same phase of the menstrual cycle, this does not discriminate whether there are menstrual cycle phase effects on the impact of sleep duration on energy balance parameters. For example, it is possible that sleep restriction could have a greater impact on food intake in women studied in the luteal phase compared to those studied in the follicular since the luteal phase is a phase of relative hyperphagia. Such menstrual phase effects have not been studied to date. Furthermore, enrolling and testing women regardless of the phase of the menstrual cycle could attenuate the effects observed.
Sleep and Energy Balance
Sleep Restriction and Energy Expenditure
Methodological details and findings of studies which have focused on the effects of sleep restriction on EE are summarized in Table 11.1.
Four investigations included measures of RMR [18–20, 26]. St-Onge and colleagues exposed male and female participants to a time in bed (TIB) of either 9 h (2200–0700 h) or 4 h (0100–0500 h) for 4 days before measuring RMR in the morning via indirect calorimetry [18]. No significant differences were observed between conditions. A study by Buxton and colleagues compared RMR measured via indirect calorimetry at ~0820 h after either 10-h or 5-h TIB (sleep episodes centered at 0300 h) for 7 days and also observed no between-condition differences [26]. Similarly, Nedeltcheva and colleagues also observed no difference in RMR after awakening when comparing between 8.5-h and 5.5-h TIB (sleep episodes centered at habitual sleep midpoint) for 14 days [19]. The only instance of an effect on RMR was reported by Benedict and colleagues [20]. In that study, RMR recorded in the morning from 0745 to 0815 h was significantly reduced after one night of total sleep elimination compared to an 8-h (2300–0700 h) sleep opportunity. This indicates that RMR is not likely to be a factor which influences energy balance under conditions of chronic partial sleep restriction, although a complete elimination of sleep does seem to affect next-morning metabolism.
The results of the latter two studies by Nedeltcheva et al. [19] and Benedict et al. [20] demonstrate that, similar to the effects observed in RMR, an effect of sleep on TEF was only observed after a night of total sleep deprivation as opposed to a milder partial sleep curtailment. Specifically, TEF was unchanged after sleeping 5.5 h/night or 8.5 h/night for 14 days [19] but was significantly decreased after a night of sleep elimination compared to after an 8-h sleep opportunity [20].
Two of the studies described above [18, 19] utilized DLW to assess daily TEE. No significant changes in TEE were observed either after 4 days of restricting sleep to 4 h/night [18] or after 14 days of restricting sleep to 5.5 h/night [19]. A study conducted by Jung and colleagues in a metabolic chamber [21] compared TEE throughout 24 h when an 8-h sleep episode was allowed (sleep timing based on participants’ habitual sleep schedule) and when sleep was eliminated. TEE was significantly increased during sleep elimination compared to sleep allowance conditions. Increases were mainly observed during the habitual night, which supports a role of sleep in energy conservation [21]. Critical differences between these studies include the use of DLW or metabolic chamber for EE measures and the use of partial or total sleep deprivation.
Free-living PA under sleep-restricted conditions has been measured with the use of actigraphy. Two of these studies with somewhat similar experimental designs report contradictory results [25, 27]. Both studies included men exclusively. Schmid et al. found lower daytime PA after 2 days of 4.25-h TIB compared to 8.25-h TIB [27]. This study utilized wrist-worn actigraphy, and short sleep timing was anchored to habitual wake-up time (habitual, 2245–0700 h; short, 0245–0700 h) [27]. Conversely, Brondel et al. found increased afternoon-to-evening (1215–2015 h) PA after 2 days of 4-h TIB compared to 8-h TIB [25]. The Brondel et al. [25] study, on the other hand, utilized waist-worn actigraphy, and short sleep timing was anchored to the midpoint of habitual sleep (habitual, 0000–0800 h; short, 0200–0600 h). St-Onge et al. observed that less percentage of time was spent in heavy and very heavy activity after short (4-h TIB, 0100–0500 h) vs. habitual (9-h TIB, 2200–0700 h) sleep, when PA was assessed in men and women with waist-worn actigraphy [18].
Taken together, the findings of the studies described above suggest that a few nights of moderate partial sleep restriction do not have a large effect on energy metabolism. In contrast, a single night of total sleep elimination results in a wakefulness-associated increase in nocturnal EE. A speculative extension of this finding is that it may lead to compensatory decreases in next-day RMR [16]. Though contradictory results are present, partial sleep restriction may reduce the intensity and amount of PA, which could be a contributor in the pathway to obesity.
Sleep Restriction and Hunger/Appetite
Methodological details and findings of studies which have focused on the effects of sleep restriction on ratings of hunger/appetite are summarized in Table 11.2.
As described above, subjective hunger is commonly assessed in response to sleep curtailment, as a preliminary means of determining the effects of sleep restriction on energy intake. Omisade and colleagues exposed 15 women to 3-h TIB (0500–0800 h) or 10-h TIB (2200–0800 h) for 1 day and observed that hunger was not altered when assessed once at 1830 h [28]. Spiegel and colleagues reported significantly increased hunger as assessed continuously across the day when a group of men were exposed to 4-h TIB (0100–0500 h) compared to 10-h TIB (2200–0800 h) for 2 days [29]. Similarly, Brondel and colleagues reported significantly increased preprandial hunger values before breakfast and dinner after 2 days of short (4-h TIB, 0100–0500 h) vs. habitual (8-h TIB, 0000–0800 h) sleep [25]. No differences in hunger were observed in studies by Schmid et al. [27] and St-Onge et al. [18] comparing sleep durations of 4.25 h/night (0245–0700 h) vs. 8.25 h/night (2245–0700 h) and 4 h/night (0100–0500 h) vs. 9 h/night (2200–0700 h), respectively. Reynolds and colleagues exposed a group of men to 4-h TIB (0400–0800 h) for 5 days and found no change in hunger assessed throughout the day when compared to the baseline sleep condition of 10-h TIB (2200–0800 h) [30]. A similar study by van Leeuwen and colleagues exposed men to 4-h TIB (0300–0700 h) and 8-h TIB (2300–0700 h) for 5 days and found no difference in hunger when assessed at 0730 h in a fasted state [31].
The effects of total sleep deprivation on subjective hunger have also been investigated. Schmid et al. assessed hunger in a group of men at 0730 h in the fasted state after a night of 7.5-h TIB (2230–0600 h), a night of 5-h TIB (2230–0330 h), and after total sleep elimination. The authors noted an incremental impact of sleep restriction on subjective hunger: compared to the full sleep episode, participants reported double the hunger rating after total sleep elimination and ~50 % increased hunger (though not statistically significant) after short sleep compared to habitual sleep [22]. The study by Benedict et al., exposing men to 1 night of total sleep deprivation, also found increased hunger when compared to measures taken after habitual sleep length [20]. On the other hand, a study by Pejovic and colleagues including both men and women found no effect of sleep elimination on hunger when compared to an 8-h sleep opportunity [23].
Differences in experimental design may account for some of the discrepancies observed in the results on the impact of sleep restriction on subjective hunger. In the studies which compared short and habitual sleep conditions, those which anchored the short sleep episode to the time of habitual awakening reported no effect on hunger [27, 28, 30, 31]. Conversely, the studies which did observe a significant increase in hunger during restriction anchored short sleep timing to the midpoint of the habitual sleep episode, thereby cutting off the last 2–3 h of the sleep episode in the short sleep condition [25, 29]. Recalling the aforementioned circadian variation of REM sleep [41], it is assumed that the elimination of the latter portion of the sleep episode to achieve partial restriction will result in a significant decrease in the expression of REM sleep. This is important, since a recent study from our laboratory observed that REM sleep duration is inversely related to hunger [42]. Increased hunger under sleep curtailment in the Spiegel et al. [29] and Brondel et al. [25] studies may therefore be explained by the reduction of REM sleep, which is not expected to be observed in the other sleep restriction studies which maintained a similar wake-up time between short and habitual duration conditions. A caveat to this hypothesis is that the restricted sleep condition in the St-Onge et al. study was also anchored to the midpoint of habitual sleep and no effect of sleep duration was noted on hunger and appetite ratings [18]. Nevertheless, the inclusion of women in that study complicates a direct comparison with the Spiegel and Brondel studies, which were done exclusively with male participants. Interestingly, out of the three studies which compared hunger between total sleep elimination and habitual sleep conditions, those which included men exclusively observed significant effects of sleep deprivation [20, 22], whereas no differences were observed between elimination and habitual conditions when women were also included [23].
Subjective appetite after partial sleep restriction was assessed in four studies to date [18, 27, 29, 30]. No effect of 2- and 5-day partial sleep restriction was observed on appetite ratings in the studies by Schmid et al. [27] and Reynolds et al. [30], which included only men and anchored the short sleep episode to the time of habitual awakening (wake times in both conditions maintained at 0700 h and 0800 h, respectively). Similar to what was observed for hunger ratings, Spiegel et al. reported increased appetite for sweet, salty, and starchy food in men exposed to 2 days of short sleep (episodes anchored to the midpoint of sleep) [29]. No changes in appetite were observed by St-Onge et al. who exposed men and women to 3 days of short sleep (episodes anchored to the midpoint of sleep) [18].
As was seen for EE, the effects of a night of total sleep deprivation appear to induce substantial increases in hunger and appetite ratings, whereas the effects of partial sleep restriction remain less defined. In the study by Spiegel and colleagues, which was the first to report increased hunger and appetite after sleep restriction, participants were fed via constant intravenous glucose infusion throughout the measurement period [29]. The findings of that study are the most robust of all reported, and their particular method of administering calories could have amplified the effects of sleep restriction above what was induced by others. The effects of sleep restriction on subjective perceptions of hunger and appetite may also be modulated by sex, since differences between short and habitual sleep are more commonly observed in studies which included only male participants.
Sleep Restriction and Appetite-Regulating Hormones
Methodological details and findings of studies which have focused on the effects of sleep restriction on appetite-regulating hormones are summarized in Table 11.3.
Various circulating peptides and hormones have been demonstrated to play a role in the regulation of hunger, appetite, satiety, and food intake. These include hypothalamic factors (e.g., neuropeptide Y and agouti-related peptide), gut hormones (e.g., ghrelin, glucagon-like peptide-1 [GLP-1], peptide YY [PYY], and cholecystokinin), and adiposity signals (e.g., leptin and adiponectin) [72]. Leptin and ghrelin have been the most widely studied appetite-regulating hormones within the context of experimental sleep restriction studies. In fact, of all the energy balance-related parameters discussed in this chapter, the effects of sleep restriction on leptin have probably been studied the most. Though they will not be discussed here, two studies with inconsistent findings have investigated adiponectin levels in response to sleep restriction [23, 51], and PYY and GLP-1 have been sampled after sleep curtailment in one study [51].
In their innovative study, Spiegel and colleagues [29] were among the first to report altered leptin in response to partial sleep restriction. They exposed men to 2 days of 4-h TIB (0100–0500 h) or 10-h TIB (2200–0800 h) and noted a significant decrease in leptin, as measured continuously throughout the day, after short sleep. In a similar study by the same group [33], leptin levels were again compared between 4-h TIB and 10-h TIB, but the exposure was extended to 6 days. Samples were obtained regularly over 24 h. The authors noted a decrease in leptin levels and a 2-h advance in the time of leptin peak in response to short sleep. Schmid et al. [27] observed no difference in leptin levels across the day after short (4.25 h, 0245–0700 h) or habitual (8.25 h, 2245–0700 h) sleep for 2 days. Similarly, studies by St-Onge et al. [51] and Nedeltcheva et al. [19] found no effect on leptin levels sampled across 24 h in response to short sleep for 3 [51] or 14 days [19].
Some studies, however, noted increases in leptin after partial sleep restriction. A study by Omisade and colleagues [28] exposed women to 3-h (0500–0800 h) or 10-h TIB (2200–0800 h) for 1 day and sampled salivary leptin at 0830 h and 2000 h to approximate a diurnal variation of the hormone. They observed an increase in morning leptin levels compared to baseline sleep, but no change in evening levels. Simpson and colleagues [32] studied men and women under 4-h TIB (0400–0800 h) and 10-h TIB (2200–0800 h) for 5 days. They obtained a single blood draw between 1030 and 1200 h and observed an increase in response to short sleep. Reynolds and colleagues [30] utilized a similar design (sleep durations, schedule, and length of exposure) as Simpson et al. [32] and also noted an increase in leptin after short sleep when sampling across the day in men. Another similarly designed study by van Leeuwen et al. [31], comparing 4-h TIB (0300–0700 h) to 8-h TIB (2300–0700 h) for 5 days, sampled leptin at 0730 h and observed an increase in short vs. habitual sleep episodes.
Comparing between 1 day of 7.5-h TIB (2230–0600 h), 5-h TIB (2230–0400), and sleep elimination, Schmid et al. [22] found no difference in leptin when sampled in the morning. Similarly, Benedict et al. [20] compared between one night each of sleep elimination and habitual sleep in men and noted no change in leptin levels sampled across 24 h. On the contrary, Pejovic et al. [23] compared leptin levels sampled across 24 h in men and women after one night of sleep elimination or habitual sleep and observed an increase in levels after total sleep deprivation. In a slightly different design, Mullington and colleagues [24] sampled leptin levels continuously over a baseline day and 3 days of total sleep deprivation and noted that the amplitude of the circadian variation was reduced during sleep elimination compared to habitual sleep. The authors concluded that sleeping may have a role in controlling the nocturnal rise in leptin levels and that increased nocturnal eating may be a consequence of this attenuated nocturnal increase [24].
Thus, while widely studied, the effects of sleep curtailment on leptin secretion are inconsistent, and the reasons for this are not clear. Feeding protocols utilized in these studies varied and included either controlled feeding [20, 28, 33, 51], participant self-selection [19, 22, 23, 27], or constant intravenous glucose infusion [29]. Unfortunately, a pattern is not apparent between feeding protocols. Likewise, differences in the timing and frequency of sampling (either morning fasted, throughout the day, or across 24 h) are also likely to systematically contribute to disparate results. Nevertheless, increased awakening during sleep restriction intervention is associated with prolonged light exposure, which can delay the central circadian pacemaker [6]. It would therefore be important to sample continuously throughout the 24-h cycle to observe the dynamics of the secretory profile. Interestingly, each of the partial restriction studies that observed increases in leptin anchored the timing of short sleep episode to habitual wake-up time [28, 30–32], whereas the two studies by Spiegel et al. (exclusively studying men), which found a decrease in leptin, anchored short sleep to the center of the night [29, 33]. Similar decreases might have been expected in the St-Onge et al. [51] and Nedeltcheva et al. [19] studies which anchored the short sleep episode to the center of the night. However, these latter studies included both men and women. Together, the results may suggest that the effects of sleep restriction on leptin secretion are affected by the timing of sleep or subtle alterations in sleep architecture. A modulatory effect of sex is also possible.
In a finding mechanistically consistent with their initial observation of decreased leptin, Spiegel and colleagues [29] reported an increase in ghrelin in a group of men in response to 2-day exposure to 4-h TIB (0100–0500 h) compared to 10-h TIB (2200–0800 h). The ghrelin findings reported by St-Onge et al. [51] are somewhat consistent with that report: studying men and women, after 3-day exposure to 4-h TIB (0100–0500 h) and 9-h TIB (2200–0700 h), increases in fasting and morning levels were observed after short sleep, selectively in men but not women. Neither Schmid et al. [27] nor Nedeltcheva et al. [19], on the other hand, reported any changes in ghrelin after restricting sleep to 4.25 h/night for 2 days or 5.5 h/night for 14 days, respectively. Sampling in the morning between 0700 and 0730 h, another study by Schmid et al. [22] observed a trend for increased ghrelin in response to 5-h TIB (2230–0330) compared to 7.5-h TIB (2230–0600 h) and a significant increase in ghrelin in response to total sleep deprivation compared to habitual sleep. Sampling across 24 h during a day of habitual sleep and a day of total sleep elimination, Benedict et al. [20] observed reduced ghrelin levels at 1300 h but increased ghrelin levels at 0430–0730 h in sleep elimination compared to habitual sleep duration.
While the findings are mixed, increased ghrelin is often reported in response to sleep length curtailment, particularly in the morning [20, 22, 51]. Sex appears to have a modulatory role in regulating the effects of sleep restriction on ghrelin levels. St-Onge et al. [51] found a strong sex effect for ghrelin, with increased levels in response to short sleep in men but not women, which is in agreement with others who studied men exclusively [20, 22, 29]. The Nedeltcheva et al. study [19], which also included both men and women, reported no effect of sleep duration on ghrelin levels. Importantly, the study by St-Onge et al. was large enough to allow separate analyses by sex (males, n = 14; females, n = 13), whereas the study by Nedeltcheva et al. may have been underpowered to allow separate sex comparisons (males, n = 6; females, n = 5). The studies which observed increased ghrelin in men anchored either the short sleep episode to the middle of the night [29, 51] or the time of habitual lights-out [22], thereby eliminating sleep from the final 2–3 h of the night, whereas the study not observing a change in ghrelin in men anchored short sleep to the time of habitual awakening [27]. Thus, again, a role of sleep timing or the expression of sleep architecture influencing the effects of sleep length curtailment on appetite-hormone levels is possible.
Sleep Restriction and Food Intake
Methodological details and findings of studies which have focused on the effects of sleep restriction on food intake are summarized in Table 11.4.
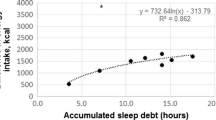
A critical aspect that should be accurately measured when researchers are concerned with energy balance is food intake. Accordingly, in addition to measuring subjective ratings of hunger/appetite and the circulation of appetite-regulating hormones, five laboratory-based sleep restriction intervention studies have measured food intake [18–20, 25, 27]. In the study by Brondel and colleagues [25], food intake was measured after 1-day exposure to short (4-h TIB, 0200–0600 h) or habitual sleep length (8-h TIB, 0000–0800 h). Afternoon and evening food intake was self-recorded, and ad libitum intake of in-lab breakfast and lunch was assessed. Energy intake was significantly increased in restricted compared to habitual sleep. In the study by Schmid and colleagues [27], food intake was measured after 2-day exposure to short (4.25-h TIB, 0245–0700 h) or habitual sleep length (8.25-h TIB, 2245–0700 h). Food was presented as a buffet breakfast until 1100 h, a snack buffet from 1100 h onward, and free access to meals on request. No differences in energy intake were observed. St-Onge et al. [18] included both men and women and measured food intake after 4-day exposure to short (4-h TIB, 0100–0500 h) or habitual (9-h TIB, 2200–0700 h) sleep duration. Participants were given free access to a variety of foods available in the lab and were given $25 to purchase food from local markets. The amount and timing of eating was decided by the participant. Energy intake was significantly increased after sleep restriction. Nedeltcheva and colleagues [19] also measured food intake in both men and women after exposure to 14 days of short (5.5-h TIB) or habitual (8.5-h TIB) sleep duration (timing centered at the midpoint of sleep). Participants were served meals at fixed times in excess and also had unlimited access to a snack bar with palatable snacks and soft drinks. Energy intake from snacks was increased after short sleep, compared to habitual sleep duration. Benedict and colleagues [20] compared food consumed by men from a buffet offered at 1730 h after one night of total sleep deprivation or a night of habitual sleep (2300–0700 h). No difference in energy consumed from this dinner buffet was seen.
Increased energy consumed under ad libitum conditions after sleep curtailment compared to habitual sleep duration was observed in three out of four studies which utilized partial sleep restriction. The Schmid et al. study [27] was the only partial curtailment study which did not report increased energy consumed. Interestingly, it is also the only study which anchored short sleep timing to habitual wake-up time, whereas the remaining three studies [18, 19, 25] centered restricted sleep to the midpoint of habitual sleep. This may imply that sleep timing or subtle changes in the expression of sleep architecture may influence food intake, or an interaction between sleep timing and sleep duration is important. The specific food presentation of the Benedict et al. study [20], namely, a single eating opportunity at 1730 h instead of access throughout the day, may account for the unexpected lack of a significant effect of total sleep deprivation on food intake.
Macronutrient composition of the food eaten was also measured in each of the five aforementioned studies that assessed ad libitum energy consumption [18–20, 25, 27]. Increased fat intake was observed in response to partial sleep restriction in the Brondel et al. [25], Schmid et al. [27], and St-Onge et al. [18] studies, and increased saturated fat intake was also observed in the latter [18]. The Nedeltcheva et al. study [19] reported increased intake of carbohydrate, but not fat, after short vs. habitual sleep duration and selective increase in snack food intake but not meals. The Benedict et al. study [20] which failed to detect differences in energy intake after a night of total sleep deprivation compared to a night of habitual sleep also did not detect any between-condition differences in macronutrient intake. This is likely due to its distinct methodological approach, described above.
Increased consumption of energy and fat seems to be the most consistently observed changes in an energy balance parameter in response to partial sleep restriction. Increases in energy intake beyond the energy which is expended by either metabolic processes or PA are therefore a viable link in establishing a causal pathway from sleep restriction to the development of obesity. Data obtained from the laboratory-based intervention studies described above support much of the epidemiological data, showing that short sleep is associated with a high-fat diet [73] and excess snacking [74].
Conclusions: Considerations and Future Steps
The results from the laboratory-based clinical intervention studies described in this chapter lend support to the epidemiological evidence showing an association between short sleep duration and increased prevalence of obesity. Although some results remain equivocal, experimental reduction of sleep duration was demonstrated to causally relate to parameters which would support a positive energy balance. As described in the introduction, a state of energy imbalance would exist when energy intake is not equal to energy output. Positive energy balance arises when food intake is excessive enough to surpass EE or, conversely, when EE is reduced to a level below normal energy intake. Based on the evidence described here, it appears that sleep restriction may affect energy balance mainly via energy input rather than EE. Specifically, the intervention studies have been convincing in demonstrating a causal link between reduced sleep duration and increased food intake. Reduced PA (which would be a logical consequence of increased daytime sleepiness) has been reported by some, although partial sleep restriction does not appear to affect RMR. To date, only one study has utilized a metabolic chamber to investigate 24-h EE in response to total sleep restriction. This tool should be used in the context of partial sleep curtailment to look more closely at TEE but also sleeping metabolic rate and non-exercise activity thermogenesis, which have not yet been described under short sleep conditions.
As far as the mechanism by which reduced sleep duration leads to increased food intake, the authors have mainly considered an alteration in the hormonal control of appetite and hunger. Conflicting results have been presented for leptin, a satiety-signaling hormone, although increases in ghrelin, an appetite-stimulating hormone, may be more uniformly observed. A potential modulation by sex on the interaction between short sleep and appetite hormones may exist, however, indicating a need for more intervention studies which are powered to detect differences between men and women. Indeed, within young, ovulating females, the menstrual cycle and its associated variations in hormones and physiology may further influence food intake or possibly EE in response to sleep restriction. Energy balance state itself is known to influence leptin and ghrelin. Under unrestricted feeding conditions, sleep-deprived participants are expected to consume excess food, leading to a positive energy balance state. Going forward, then, it will be important to conduct studies under highly controlled energy intake conditions which help determine the interaction between short sleep and energy balance on the hormonal control of food intake.
In real-life conditions, individuals who are habitually exposed to short sleep may have increased daily food intake because their prolonged wake episodes may afford them increased opportunities to eat. While this has not been extensively studied in the laboratory, one study considering this observed that nocturnal eating episodes (past the time of habitual bedtime) were present in ~27 % of participants [18]. It has recently been reported in observational studies that the timing of food intake is related to increased BMI [44] and reduced weight loss effectiveness [75]. This observation may be related to a study in mice showing that animals fed a high-fat diet during their inactive phase gained more weight than mice fed during their habitual active phase [76]. The effects of sleep restriction on the temporal distribution of food intake, under ad libitum and unrestricted conditions, should therefore be further pursued. Such studies may also have practical ramifications for the health of night shift workers, since these individuals often experience shortened sleep episodes, unusual feeding-fasting behavior characterized by nocturnal eating, and increased risk of weight gain and cancer.
As discussed in this chapter, small differences in the scheduling of the sleep within the intervention studies may influence specific energy balance outcomes. This implies that not just sleep duration alone but also the timing of sleep episodes should be considered as playing a role in the regulation of metabolism and body weight. Indeed, researchers have been paying increasing attention to this: an observational study found that individuals with later sleep schedules tended to have higher energy intakes throughout the day than those whose midpoint of sleep was earlier [44], and a laboratory-based manipulation study illustrated that sleep restriction combined with a chronic circadian misalignment resulted in reduced RMR and altered glucose homeostasis [52]. The problem of circadian misalignment, short sleep, and delayed sleep and meal timing is not limited to shift workers and presents an important health risk for a large portion of the population. More work should be done to determine if sleep timing affects energy balance and how it may be involved in the causal pathway to obesity.
Chronic partial sleep restriction likely leads to a state of positive energy balance, which can ultimately result in excess weight gain and obesity. Furthermore, obesity is associated with increased risk of a variety of cancers [2], which may suggest a role of sleep restriction in cancer as well. This role may be directly causal or indirectly, via increased risk of obesity. As described, shift workers, who experience reduced sleep duration combined with a circadian disruption, are at an increased risk of obesity and cancer development. Related to this, recent epidemiological work has shown an association between “social jet lag” (i.e., the discrepancy between sleep episode timing between work and free days that often results in sleep loss) and obesity [77], and chronic jet lag conditions increase tumor progression in mice [78]. An important angle of research may be to further assess the markers of cancer risk in the context of experimental sleep restriction studies. The effects of sleep episode schedule, in addition to duration, on the development of obesity, adverse metabolic outcomes, and cancer should be further studied with controlled laboratory interventions.
In conclusion, accumulating evidence from intervention studies has been delineating the ways in which restricted sleep duration may lead to obesity, corroborating much of the epidemiological studies on this sleep-obesity link. Nonetheless, many inconsistencies and questions within the laboratory-based data still remain, owing mainly to important methodological differences between studies. It is necessary that researchers continue to explore the mechanisms underlying the relationship between sleep and energy balance, as this line of research will continue to have major public health implications for various medical conditions, including, but not limited to, obesity and cancer.
References
Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41.
Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91.
Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, et al. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67 Suppl 1:2–9.
Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63.
National Sleep Foundation. Sleep in America Poll: communications technology in the bedroom 2011. http://www.sleepfoundation.org/sites/default/files/sleepinamericapoll/SIAP_2011_Summary_of_Findings.pdf
Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96:E463–72.
American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester: American Academy of Sleep Medicine; 2005.
Biggi N, Consonni D, Galluzzo V, Sogliani M, Costa G. Metabolic syndrome in permanent night workers. Chronobiol Int. 2008;25:443–54.
Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–52.
Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–11.
Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–62.
Obayashi K, Saeki K, Iwamoto J, Okamoto N, Tomioka K, Nezu S, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: a cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98:337–44.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26.
Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643–53.
Horne J. Obesity and short sleep: unlikely bedfellows? Obes Rev. 2011;12:e84–94.
Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–801.
Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126:126–32.
St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6.
Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33.
Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schioth HB, et al. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr. 2011;93(6):1229–36.
Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol. 2011;589(Pt 1):235–44.
Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res. 2008;17(3):331–4.
Pejovic S, Vgontzas AN, Basta M, Tsaoussoglou M, Zoumakis E, Vgontzas A, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res. 2010;19:552–8.
Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15:851–4.
Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9.
Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33.
Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–82.
Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6.
Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–50.
Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, et al. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7:e41218.
van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641.
Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53.
Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–71.
Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9.
Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. British Journal of Nutrition 2013;109(4); 748–756
Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr. 2011;94:804–8.
Shlisky JD, Hartman TJ, Kris-Etherton PM, Rogers CJ, Sharkey NA, Nickols-Richardson SM. Partial sleep deprivation and energy balance in adults: an emerging issue for consideration by dietetics practitioners. J Acad Nutr Diet. 2012;112:1785–97.
Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6.
Fontvieille AM, Rising R, Spraul M, Larson DE, Ravussin E. Relationship between sleep stages and metabolic rate in humans. Am J Physiol. 1994;267:E732–7.
Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythm. 1999;14:557–68.
Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38.
Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–9.
Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond). 2012;36:1346–52.
Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19:1374–81.
Golley RK, Maher CA, Matricciani L, Olds TS. Sleep duration or bedtime? Exploring the association between sleep timing behaviour, diet and BMI in children and adolescents. Int J Obes (Lond). 2013;37:546–51.
Seale JL, Rumpler WV, Conway JM, Miles CW. Comparison of doubly labeled water, intake-balance, and direct- and indirect-calorimetry methods for measuring energy expenditure in adult men. Am J Clin Nutr. 1990;52:66–71.
Swartz AM, Strath SJ, Bassett Jr DR, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32 Suppl 9:S450–6.
Hasson D, Arnetz BB. Validation and findings comparing VAS vs. Likert scales for psychosocial measurements. Int Electron J Health Educ. 2005;8:178–92.
Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–9.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10.
Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43.
Dallongeville J, Hecquet B, Lebel P, Edme JL, Le Fur C, Fruchart JC, et al. Short term response of circulating leptin to feeding and fasting in man: influence of circadian cycle. Int J Obes Relat Metab Disord. 1998;22:728–33.
St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9:73–80.
Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565–74.
Soares CN. Insomnia in women: an overlooked epidemic? Arch Womens Ment Health. 2005;8:205–13.
Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–56.
Shechter A, Boudreau P, Varin F, Boivin DB. Predominance of distal skin temperature changes at sleep onset across menstrual and circadian phases. J Biol Rhythms. 2011;26:260–70.
Klausen B, Toubro S, Astrup A. Age and sex effects on energy expenditure. Am J Clin Nutr. 1997;65:895–907.
Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol. 1993;75:2514–20.
Webb P. 24-hour energy expenditure and the menstrual cycle. Am J Clin Nutr. 1986;44:614–9.
Piers LS, Diggavi SN, Rijskamp J, van Raaij JM, Shetty PS, Hautvast JG. Resting metabolic rate and thermic effect of a meal in the follicular and luteal phases of the menstrual cycle in well-nourished Indian women. Am J Clin Nutr. 1995;61:296–302.
Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochem Mol Med. 1996;59:1–6.
Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB, et al. Sex differences in circulating human leptin pulse amplitude: clinical implications. J Clin Endocrinol Metab. 1998;83:4140–7.
Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf). 1997;47:101–6.
Ludwig M, Klein HH, Diedrich K, Ortmann O. Serum leptin concentrations throughout the menstrual cycle. Arch Gynecol Obstet. 2000;263:99–101.
Makovey J, Naganathan V, Seibel M, Sambrook P. Gender differences in plasma ghrelin and its relations to body composition and bone – an opposite-sex twin study. Clin Endocrinol (Oxf). 2007;66:530–7.
Bellone S, Rapa A, Vivenza D, Castellino N, Petri A, Bellone J, et al. Circulating ghrelin levels as function of gender, pubertal status and adiposity in childhood. J Endocrinol Invest. 2002;25:RC13–5.
Dafopoulos K, Sourlas D, Kallitsaris A, Pournaras S, Messinis IE. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil Steril. 2009;92:1389–94.
Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10:133–42.
Lovejoy JC. The influence of sex hormones on obesity across the female life span. J Womens Health. 1998;7:1247–56.
Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–72.
Shi Z, McEvoy M, Luu J, Attia J. Dietary fat and sleep duration in Chinese men and women. Int J Obes (Lond). 2008;32:1835–40.
Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr. 2011;14:889–95.
Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37(4):604–11.
Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring). 2009;17:2100–2.
Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol: CB. 2012;22:939–43.
Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, et al. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–85.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
St-Onge, MP., Shechter, A. (2014). Sleep-Focused Interventions: Investigating the Effects of Sleep Restriction on Energy Balance. In: Redline, S., Berger, N. (eds) Impact of Sleep and Sleep Disturbances on Obesity and Cancer. Energy Balance and Cancer, vol 8. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9527-7_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9527-7_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9526-0
Online ISBN: 978-1-4614-9527-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)