Abstract
Our increasing knowledge about health and disease suggests that vegetarian diets rich in fruits and vegetables have a significant impact in prevention and therapy of multiple types of cancer. Phytochemicals from both dietary and nondietary origins inhibit cancer growth and progression of human cancers by interacting at the cellular and molecular levels. The mitochondrion is the key organelle that provides energy to cells for their growth and survival. In addition, mitochondria play an important role in cancer cell apoptosis and may also regulate autophagy. Cancer cells have high proliferative and less apoptotic activities; therefore, agents that induce apoptosis selectively in cancer cells are desired for the treatment of cancer. Some dietary phytochemicals target mitochondria causing destruction of mitochondrial membranes leading to the discharge of proapoptotic mitochondrial protein, which initiates apoptotic cell death in cancer. Although the role of autophagy is debated as a mechanism of cell death or cell survival, many phytochemicals modulate autophagy in cancer cells. Therefore, selective targeting of cell-death pathways by phytochemicals in cancer cells could be an attractive strategy for the prevention and treatment of cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Living organisms are broadly classified as either unicellular or multicellular organisms. The complexity of many different cell types coming together in multicellular organisms mandates stringently regulated processes for cell division, proliferation, and differentiation, which are controlled by many signaling pathways. The rate of cell division is controlled by the process of apoptosis and differentiation so that the organism is not overburdened with unwanted cells. In humans, around 107 cells die in the body every second (Reed 2010) and a variety of pathways are used to coordinate cell death during development and morphogenesis to control cell numbers thus eliminating damaged cells. By this process, the body ensures that only healthy cells survive in the body and cells having any mutation or change that might be harmful, are eliminated. But some cells escape from the usual barricade mechanisms within the body and they further divide, accumulate, and may turn cancerous. These cells acquire the property of indefinite cell division and resist programmed cell death or apoptosis. Defects or resistance to normal programmed cell-death mechanisms are prerequisites in the pathogenesis of tumors.
Cell death as a process becomes very important in maintaining homeostasis and helps to evade pathogenesis. There are three mechanisms of cell death, namely apoptosis , autophagy, and necrosis (Ouyang et al. 2012) . Other than these mechanisms, anoikis is a special case of apoptosis in response to inappropriate cell/extracellular matrix (ECM) interactions , leading to complete detachment and eventually death. The importance of anoikis in vivo can readily be seen when alterations that perturb its normal control enhance tumor metastasis, a process that requires cells to survive in totally inappropriate ECM environments. Cancer cells are known to develop resistance towards these cell-death mechanisms and thereby continue to divide and proliferate in spite of accumulating mutations and other defects. Agents triggering cell death in cancer cells or sensitizing them to normal cell-death mechanisms are used as cancer therapeutics (Reed 2006) . Natural plants and vegetation have been well studied and documented by ancient literature for their medicinal values. With further development of technology, there have been studies on identifying active principles from these plants or various constituents of our daily diet and identifying their medicinal activities including anticancer efficacies. These studies have resulted in identification of numerous compounds or molecules from plant origins with anticancer activity against various human cancers including skin cancer, prostate cancer, oral cancer, and breast cancer (Surh 2003) . Although there are several factors that affect tumor cells, among them the most important is the daily lifestyle or diet. In this chapter, we review the three major death mechanisms and try to understand how the natural or chemopreventive agents modulate these processes and hence function as anticancer agents in cancer cells.
Phytochemicals in Chemoprevention of Cancer
Plants have been the main source of a significant percentage of potent anticancer agents that are discovered, developed, and being used in clinical practice. Prevention cancer by natural compounds needs better understanding of the mechanistic actions including their effect on cancer cell proliferation , angiogenesis , invasion, migration, and metastasis. After almost three and a half decades of research following the coining of the term “chemoprevention” by Michael Sporn in 1976, it is now getting recognition as a potent strategy against cancer prevention and control (Bode and Dong 2009) . Chemoprevention aims to arrest the cancer in its early stages so that disease eventually does not lead to the invasive and metastatic stage and seeks to reverse the preneoplastic condition. Phytochemicals have been isolated and characterized from the different sources of plants including fruits, vegetables, spices, beverages, cereals, and many others. Figure 1 shows various dietary agents from fruits, vegetables, dietary supplements, and medicinal spices that inhibit different processes of cancer including cell invasion, migration, metastasis, and angiogenesis, along with attenuating the exponential growth of cancer. Although a small portion of natural compounds have been explored, a large chunk of marine life and other potent sources are open for development and discovery of new drugs or chemopreventive agents. Thus phytochemicals serve as excellent molecules to inhibit the promotion and progression of carcinogenesis, to remove genetically damaged, preinitiated, or neoplastic cells from the body by inducing apoptosis or cell cycle arrest.
Diagram depicts various death-inducing pathways and potential modulatory targets for chemopreventive agents in cancer cells. Phytochemicals act as modulators of apoptosis pathways and also trigger the expression of proteins that play a central role in apoptosis. For example, apigenin, capsaicin, gallic acid, resveratrol, silibinin, and flavopiridol activate caspase-3 and -9 leading to apoptosis. However, ACA, butein, berberine, curcumin, EGCG, quercetin, sanguiarine, zerumbone, and capsaicin downregulate Bcl-2 and upregulate Bax which plays a major role in the intrinsic pathway of apoptosis induction
The incidence of cancer is increasing worldwide, however, fruit and vegetable intake has been shown to reduce the risk of cancer in numerous epidemiological studies (Block et al. 1992) . Recently, biochemical and molecular approaches have been applied to select naturally occurring dietary substances that showed more control over the key molecules regulating cancer pathways. These biomolecules are potentially able to modulate intracellular signaling pathways and may provide a molecular basis of chemoprevention . Table 1, shows various phytochemicals including silibinin , resveratrol , epigallocatechin-3-gallate (EGCG) , fisetin, capsaicin, curcumin , and genistein which have been reported to cause cell death via both extrinsic and intrinsic pathways of apoptosis in cancer cells. These phytochemicals have been explored in different types of cancer such as prostate, lung, breast, cervical, oral, and colon cancer at different doses, and it has been estimated that dietary modification may prevent more than two-thirds of human cancer (Surh 2003) . These agents have also been explored for their role as chemosensitizing and radiosensitizing agents, wherein they function by countering the resistance mechanisms in cancer cells (Garg et al. 2005; Nambiar et al. 2011) . The defective apoptotic pathways are well-known causes of carcinogenesis and, in addition, are one of the resistance mechanisms acquired by cancer cells in response to various therapeutic agents. However, cancer cells acquire resistance to apoptosis by overexpression of antiapoptotic proteins and/or by the downregulation or mutation of proapoptotic proteins . Therefore, identification and exploration of pathways that are involved in carcinogenesis could facilitate the use of dietary constituents as a key strategy to prevent cancer development. As shown in Fig. 1, phytochemicals modulate extrinsic and intrinsic pathways at the molecular level and also target the significant proteins that constitute the apoptosis core machinery including Bcl-2, Bax, cytochrome-c, and caspases which mediate apoptosis.
Death of Cancer Cells: Role of Mitochondria
All cell organelles in the mammalian cells serve a unique and well-demarcated purpose. Among these organelles, one that draws rigorous attention is the aptly called “powerhouse of the cell,” the mitochondrion. Cellular energy is almost solely controlled by the mitochondrion, which ultimately produces the energy for cell. As much as it governs life, it also governs one of the most illustrious mechanisms of cell death, apoptosis . Mitochondrial involvement in various diseases is also very well documented. The role of mitochondria in pathogenesis gained slow and steady importance in the previous two decades of research and is now a well-recognized target even for cancer therapeutics (Tatarkova et al. 2012) . Mitochondrial membrane permeabilization is one of the central steps to apoptosis. The aim of major chemotherapeutic agents has been the induction of apoptosis in cancer cells. Hence, targeting molecules controlling the mitochondrial membrane permeability is one of the most common mechanisms of cell death induction in cancer cells. Second, targeting the cellular energetic pathways could also be a triggering factor for cell death induction. Another recent study suggests that manipulating and targeting the number of mitochondria could also be one of the ways of targeting cancer cells. It is now known that the mitochondrial number per cell is regulated by two processes of fusion and fission, fission leading to doubling the number and fusion leading to reducing the number to half. In the study by Rehman et al. (2012) it was shown that by tipping the balance more towards fusion rather than fission, they could dramatically reduce cell proliferation and growth of lung cancer cells in mice. Even though novel ways of mitochondrial targeting are being explored, the most recognized involvement of mitochondria remains on cell death induction via apoptosis.
Although several years of research and observations have explained the process and morphology of apoptotic cells, such as chromatin condensation, nuclear fragmentation, plasma membrane blebbing, and cell shrinkage (Ferreira et al. 2002), with the recent advancement in technology we are capable of understanding the process of cell death at the cellular as well as molecular level. It is now not only possible to identify and decipher the extent of involvement of cell organelles, but also of the smallest of molecules coupled with the process. Hence, each and every molecule regulating mitochondrial function could be explored as a target for cancer prevention and control.
Mitochondrial Pathways of Apoptosis: Potential Targets of Phytochemicals
Mitochondrial involvement in the cell death program proceeds through two main pathways namely, intrinsic and extrinsic pathways. The mitochondrial pathway of apoptosis functions in response to various types of intracellular stress including growth factor withdrawal, DNA damage, unfolding stresses in the endoplasmic reticulum, and death receptor stimulation (Khosravi-Far and Esposti 2004) . The extrinsic apoptotic pathway is activated through the death receptors such as tumor necrosis factor (TNF) receptor, TNF-related apoptosis-inducing ligand (TRAIL) receptor, and APO1 receptor (also called Fas or CD95). Death receptors are cell surface receptors which are integral cell membrane proteins that transmit apoptotic signals initiated by specific ligands such as Fas ligand, TNF-α, and TRAIL. Although there are differences in the signaling pathways stimulated by the different death receptors, they perform a central role in inducing apoptosis and can activate the downstream cascade after the binding of its specific ligand. The ligand binding causes a conformational alteration in the intracellular domains of the receptors, which exposes the death domain and allows the recruitment of several apoptotic proteins to the receptor forming protein complexes. These protein complexes are known as death inducing signaling complexes (DISC) . The establishment of DISC initiates the caspase cascades and therefore the stimulation of apoptosis via this mechanism is very quick (Khosravi-Far and Esposti 2004) . Binding of TNF-α to TNFR1 results in receptor trimerization and clustering of intracellular death domain, that is, TNFR-associated death domain (TRADD) which associates with FADD and ultimately leads to the initiation of apoptosis via the recruitment and cleavage of procaspase-8. Similarly, in Fas receptor activated apoptosis, the process is triggered by the Fas and CD95 ligands, which leads to binding of TRAIL to the DR4 or DR5 receptor and activates the TRAIL death-receptor apoptosis pathway (Guicciardi and Gores 2009) . Following the reception of stress signals, proapoptotic Bcl-2 family proteins are activated and subsequently interact with and inactivate antiapoptotic Bcl-2 proteins.
The Bcl-2 is a family of proteins of pro- and antiapoptotic function. On the basis of structural homology and respective functions, Bcl-2 proteins had been classified into three groups. The first group shows the antiapoptotic function with structural homology in BH domains (BH1–BH4), for example, Bcl-2, Bcl-xL, and Mcl-1; the second group includes the pro-apoptotic members with three BH domains, for example, Bax and Bak; and the third group of proapoptotic proteins shows homology within the BH3 domain known as Bad, Bid, or Bim (Garrido et al. 2006) . These proteins potentially participate in signaling, which changes the mitochondrial membrane dynamics. On receiving an apoptotic signal, the proapoptotic members enhance the mitochondrial membrane permeability and hence lead to release of cytochrome c, followed by apoptosome complex formation. The level of Bax and Bcl-2 ratio is a pivotal factor and plays a significant role in determining future survival or apoptotic death of the cell. After the apoptosome formation, the program cell death is triggered and is performed by a family of highly conserved cysteinyl aspartate-specific protease known as caspases. Caspases cleave a large number of cellular substrates and thus dismantle the cell. Caspases-8 and -10 are activated by the death receptor of cell-like Fas/CD95, TNFR, DR-3, and DR4/5. caspase-9 is activated through the cytochrome c release of mitochondria , and caspases-8, -10, -9 are considered as most upstream caspases in apoptotic signaling (Fan et al. 2005) . Caspases-3, -6, and -7 are the downstream caspases activated by upstream caspases or the signal cascade of the death receptor of the cell. Most of the cancer cells acquire resistance to apoptotic stimuli via overexpression of antiapoptotic proteins such as Bcl-2 or under expression of proapoptotic factors. Many phytochemicals have the potential to modulate the levels of these regulatory proteins, and thereby, lead to caspase activation and apoptosis . Survivin is an inhibitor of apoptosis protein (IAP), which is highly expressed in cancer cells and is a target for chemopreventive agents. It has been observed that its expression interferes with caspase-9 and prevents caspase activation, and as a result inhibits apoptosis (Altieri 2006) . It is reported that mitochondrial expression of survivin is not found in normal cells, whereas mitochondrial survivin is absolutely related with tumor transformation of cells (Altieri 2003, Dohi et al. 2004) . Survivin increases apoptotic resistance in cancer cells mainly by blocking mitochondrial intrinsic pathways.
Autophagy as a Target of Phytochemicals for Cancer Prevention
Autophagy , as the term itself suggests is a cellular degradation mechanism in response to cellular stress. Over the past decade, many studies have established the exact mechanism of autophagic process, ranging from how it is executed, to the protein machinery involved, but until now, the context in which autophagy is triggered is still unclear. Questions remain about its utility in the cell. Is it just a homeostasis-maintaining cytoprotective mechanism or could it be a cytotoxic mechanism in a defined set of conditions or in a disease state? Most studies advocate that autophagy is a cytoprotective mechanism providing the cells support to sustain survival, but then it becomes tricky to explain why so many cytotoxic agents induce autophagy. If it is indeed a prosurvival mechanism, then it remains ambiguous why genes involved in autophagy are frequently mutated in human cancers (Mathew et al. 2007) . Different explanations have been provided for this process; one plausible explanation for this paradoxical process could be that autophagy in limited condition is prosurvival but if it exceeds the rate of cellular synthesis, then it may promote cell death. In the case of apoptosis -resistant tumors, autophagy helps to survive metabolic stress, hence helping those tumor cells to flourish and therefore autophagic inhibition could be seen as a mechanism to sensitize apoptosis-resistance cells to metabolic stress-induced death.
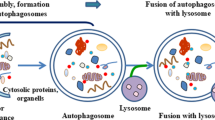
As a target in cancer prevention , autophagy play a vital role as it can inhibit tumor initiation by removing the damaged cells and limiting genomic instability. Furthermore, autophagy may also be able to limit chronic inflammation, which is considered to be a known precursor to carcinogenesis . This notion is well supported by the fact that autophagy-deficient mice are comparatively more tumor prone (Takamura et al. 2011) . Also, use of autophagic inducers such as rapamycin and metformin reduce the tumor initiation process. Autophagy is regulated through the PI3K/AKT/mTOR pathway, Beclin 1 complex, Bcr-Abl, and FOXO signaling (Sandri 2012; Wang et al. 2011) . The PI3K signaling network plays critical role in the regulation of cell growth, survival, proliferation, differentiation, and motility, but when dysregulated can lead to oncogenic transformation. and eventually mTORC1, which is one of its direct targets. mTORC1 is needed for protein translation and supports net cell-mass accumulation by inhibiting autophagy (Jung et al. 2010) . The inhibitory mechanism of mTORC1 is brought about by inhibitory phosphorylation of ULK1 and ATG13 proteins that are involved in phagophore initiation complex formation, especially when there is an abundance of nutrients and growth factors. When nutrients are limited, it dissociates from the ULK1 complex, initiating the autophagy process. The vesicle nucleation and assembly of the phagophore requires a Class III phosphatidylinositol 3-kinase (PI3K-III) complex containing the proteins VPS34, p150, ATG14, and Beclin 1. It is followed by the phagophore elongation which requires Atg16 and LC-3. The phagophore then elongates to form the autophagosome, which encapsulates cytoplasmic material and fusion with lysosome that involves proteins including Rab7, SKD1, LAMP1 and LAMP2, eventually delivering its cargo to the lysosome. These proteins that are involved in the formation of autophagosome and upstream signaling pathways governing the process could be potential targets of cancer chemopreventive agents. Autophagy as a process helps in the removal of unwanted elements from the cytoplasm and suppresses the accumulation of toxic protein aggregates and the production of damage-inducing reactive oxygen species (ROS) . The sustained state of oxidative stress is also a prelude to the genome and tissue damage, inflammation, and cell death.
Autophagy is also linked to mitochondria-mediated apoptosis . For instance, the role of autophagy in controlling the number of damaged mitochondria (mitophagy), which are a major source of ROS production in mammalian cells, is not yet well understood. Hence, both autophagy inducers and inhibitors are used in cancer therapeutics. Several of the natural compounds of plant origin, such as curcumin , silibinin , resveratrol , paclitaxel, quercetin, genistein are shown to induce these autophagic pathways as potential therapeutic agents to deal with carcinogenesis (Fig. 2). The context dependent role of autophagy and how it could be used in cancer therapeutics is very well summarised in the review by Cheong et. al. (Cheong et al. 2012) .The successful targeting of autophagy in cancer therapy by natural compounds would require molecular analysis of the components of distinct forms of autophagy , identifying the context of operation with respect to the tumor stage as well correlating how this process is related to apoptotic mechanisms in cancer cell.
The effect of phytochemicals on autophagic death in cancer cells. Autophagy involves (1) vesicle induction (2) vesicle elongation and maturation (3) autophagosome formation and docking (4) fusion and formation of autolysome which is regulated by PI3K/Akt/mTOR, RTK signaling, ROS and nutrient availability. (P) depicts the modulation of autophagy by various phytochemicals; PE, phosphoethaolamine
Phytochemicals Inducing Cell Death in Cancer Cells
Curcumin
Curcumin , is isolated from Curcuma longa and has been extensively evaluated for its anti-carcinogenic and chemopreventive efficacies in a wide range of cancers. Its potential anti-carcinogenic effects are exerted via mechanisms such as induction of cell cycle arrest, activation of tumor suppressor genes including p53 and various transcription factors such as Nrf2 and NF-kB modulation of inflammatory signaling cascades and by inducing apoptosis (Zhou et al. 2011) . These effects in turn are all known to be via its ability to modify or alter the response of various signaling pathways including mitogenic and inflammatory responses, cell cycle regulation, and cell death regulating pathways.
Curcumin has been shown to induce both mitochondria-dependent as well as -independent cell death mechanisms. Guo et al. showed that curcumin induced apoptosis in human colon carcinoma LoVo cells was mediated via mitochondrial death pathway involving activation of caspase 3 and 9. Curcumin also down-regulated survivin levels in these cells (Guo et al. 2012) . It has also been reported that curcumin induced cell death in nasopharyngeal carcinoma cells by inducing cytochrome c release and activation of caspase 9 and 3 (Kuo et al. 2011) . Similar results were reported with dimethoxycurcumin on MCF-7 breast cancer cells, wherein curcumin could induce loss of mitochondrial potential in these cells leading to apoptosis . In another study, curcumin could alter the cellular energy status of the cells by decreasing the ATP/ADP ratio. This effect was in turn brought in by the suppression of α, β, γ, ε subunits of ATP synthase. The study further showed that curcumin could reduce the levels of Bcl-2 and increase Bax levels (Kunwar et al. 2012) . Curcumin was also shown to induce apoptosis via intrinsic pathway which was evident by increase in mitochondrial calcium accumulation in murine mammary gland adenocarcinoma. The mechanism was further confirmed by use of a mitochondrial uniporter inhibitor prior to the treatment of the cells by curcumin , which resulted in inhibition of curcumin mediated loss of mitochondrial membrane potential and eventually decreased cell death (Ibrahim et al. 2011) . Recently, it has been shown that Curcumin induced cell death required Apaf-1, as Apaf-1 deficiency in these cells resulted in inhibition of caspase 3 activation by Curcumin (Gogada et al. 2011) . Role of curcumin in inducing the intracellular calcium levels as a mechanism of inducing cell death was also confirmed by Wang et al., who demonstrated that curcumin induced cell death in hepatocellular carcinoma cells could be abrogated by the use of intracellular calcium chelators (Wang et al. 2012) .
Another form of cell death mechanism shown by curcumin is induction of autophagy . After treatment with curcumin, levels of LC3-II and Beclin 1 have been detected to be elevated in K562 cells, suggesting that curcumin may affect autophagosome formation (Jia et al. 2009) . In glioma cells, curcumin has been found to inhibit the AKT/p70S6 kinase pathway while activate ERK1/2, resulting in autophagic induction, whereas PI3K activity was not affected (Aoki et al. 2007) . It is also suggested that in oral squamous carcinoma cells, curcumin induces formation of autophagic vesicles, which was confirmed by the use of an autophagic inhibitor, which suppressed the effects of curcumin on the cells. This study also showed that curcumin induced production of ROS in these cells and use of antioxidant N- acetyl cysteine (NAC) led to suppression of not only ROS but also the formation of autophagic vesicles and vacuoles, suggesting that curcumin mediated autophagy in these cells could be via generation of ROS and oxidative stress induction (Kim et al. 2012) .
Silibinin
Silibinin is a polyphenolic flavonoid isolated mainly from the seeds of milk thistle (Silybum marianum (L.) Gaertn) . Over a decade, many studies have shown that silibinin targets signaling pathways that are constitutively activated in cancer cells and are involved in tumor cell survival, growth, invasion and metastasis. Silibinin mediates these pleiotropic effects by targeting various signaling pathways including EGFR, IGF-1R and NF-κB pathways in (Singh and Agarwal 2006) . Silibinin induced inhibition of tumor cell growth is brought about by induction of cell cycle arrest, mainly in the G1 phase, which leads to prolonged doubling time of these cells. The induction of cell cycle arrest via silibinin in various in vitro and in vivo models has been attributed to its ability to reduce the levels of CDKs and cyclins and also by augmenting the levels of cell cycle inhibitory proteins, the CDK inhibitors, including Cip1/p21 and Kip1/p27. Triggering the process of cell death by silibinin is also one of its well studied mechanisms of tumor inhibition. Silibinin has been shown to induce apoptosis in a wide range of cancer cells. Here, we summarize few of the studies depicting its role in cancer cell death.
One of the earliest reports showing the role of silibinin in cell death came in 1999 for cervical carcinoma cells (Bhatia et al. 1999) , and later it was shown that silibinin inhibits both constitutive and TNF- alpha induced NF-κB activation in human prostate carcinoma DU145 cells, and significantly sensitizes them to TNF-alpha induced apoptotic death (Dhanalakshmi et al. 2002) . Further, it was also shown that silibinin could synergize with doxorubicin to enhance apoptosis induction in prostate cancer cells (Tyagi et al. 2002) . The combination of silibinin was further tried along with other chemotherapeutic agents including cisplatin and carboplatin, wherein it sensitized the cells to drug induced apoptosis in human prostate cancer cells (Dhanalakshmi et al. 2003) . The results obtained in vitro was further validated by in vivo models of PCa using athymic nude mice xenograft showing that the inhibition of tumor growth in treated mice were due to, in part, by silibinin induced apoptosis as evidenced by increased levels of cleaved caspase-3 (Singh et al. 2003) . Silibinin was shown to down-regulate survivin which was associated with a very strong and prominent caspase-9 and -3 activation as well as PARP cleavage in bladder cancer cells (Tyagi et al. 2003) . The role of silibinin in inducing cell death in bladder carcinoma cells has been further evaluated. Silibinin enhanced p53 activation which was mediated via ATM-Chk2 pathway, which in turn induced caspase-2-mediated apoptosis. Further, silibinin caused a rapid translocation of p53 and Bid into mitochondria leading to increased permeabilization of mitochondrial membrane and cytochrome c release into the cytosol. The study deciphered a novel mechanism for apoptosis induction by silibinin involving p53-caspase-2 activation and caspase-mediated cleavage of Cip1/p21 (Tyagi et al. 2006) . Recently, it was shown that in hepatocellular carcinoma cells, silibinin activated extrinsic apoptotic pathway by up-regulating the TRAIL and DR5 and caspase-3 and -8 activation (Bousserouel et al. 2012) .
Another mechanism of silibinin mediated cell death was observed in glioma cells. Kim et al. showed that silibinin could inhibit glioma cell proliferation via Ca+2/ROS/MAPK dependent mechanism in vitro and in vivo. Silibinin was shown to induce intracellular calcium levels and augment levels of ROS thereby inducing death. EGTA, calpain inhibitor or NAC could abrogate silibinin induced cell death (Kim et al. 2009) . A follow-up study by Jeong et al. using calpain inhibitors showed that calpains which are cytosolic calcium activated cysteine proteases, play an important role in silibinin-induced cell death. Further analysis revealed that use of calpain inhibitor eradicates silibinin induced AIF nuclear translocation and eventually silibinin induced cell death (Jeong et al. 2011) .
Apart from induction of apoptosis , silibinin is known to induce autophagic cell death mechanisms. Silibinin-induced autophagy led to increase in the conversion of LC3 I to LC3 II and an up-regulation of Beclin 1 expression, which was concomitant with p53 suppression and NF-κB activation in human melanoma cells (Jiang et al. 2011) . Use of NF-κB inhibitor led to the inhibition of silibinin-induced autophagy, and autophagic specific inhibitor 3-methyladenine (3-MA) treatment reversed silibinin-induced p53 expression as well as NF-κB activation. This study suggests a positive feedback loop between p53 inhibition-mediated NF-κB activation and autophagy (Jiang et al. 2011) . Similar studies were also reported in fibroblast HT1080 cells where silibinin induced Beclin 1 expression and generated ROS, and use of NAC abrogated silibinin induced autophagy in these cells (Duan et al. 2010) . Later, the same group further elucidated the mechanism of silibinin-induced autophagic cell death suggesting that autophagy induction was mediated via activation of p53-ROS-p38 and JNK pathways (Duan et al. 2011) . In cervical cancer cells, silibinin induced autophagy as well as apoptosis via induction of ROS generation. The study suggested the involvement of p53-mediated ROS generation as well as ROS-mediated p53 activation . Silibinin was also found to activate JNK, which in turn induces ROS formation and therefore, silibinin might activate p53-ROS-JNK loop to induce cell death (Fan et al. 2011) .
Although the above mentioned studies suggested a positive role of silibinin as anticancer mechanism , few study have also suggested a cytoprotective role of autophagy. Kauntz et al. showed that in human colon cancer cells, silibinin induced apoptosis via activation of both intrinsic and extrinsic pathways of apoptosis , but coincidently it also induced autophagy in these cells. But when autophagic inhibitor was used, there is was a significant enhancement in cell death, suggesting a cytoprotective function of autophagy in these cells (Kauntz et al. 2011) . Looking into the mechanism of silibinin mediated autophagy induction in cancer cells, most of the studies have attributed it to be orchestrated via ROS, and generation of ROS was further linked to major stress activated pathways in the cell including p53 induction , JNK activation and p38 signaling pathways.
EGCG or Green Tea Polyphenols
Green tea polyphenols (GTP) have been demonstrated to suppress tumorigenesis in several in vitro and in vivo models, and is one of the promising chemopreventive agents for human cancers (Yang et al. 2009) . Green tea catechins (GTCs) including (-)-EGCG, (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG) and (-)-epicatechin (EC) were shown to suppress cell growth and induce apoptosis in various cell types in addition to their chemopreventive effect. Chung et al., demonstrated the role of GTCs in growth suppression, apoptosis induction, ROS formation and mitochondrial depolarization which was in order of ECG>EGCG>EGC>EC. Even though GTCs were able to induce apoptosis, there was no effect observed on members of Bcl-2 family as EGCG did not alter the expression of Bcl-2, Bcl-xL and BAD in prostate cancer cells (Chung et al. 2001) . In a study done to evaluate the role of EGCG treatment on human monocytic leukemia U937 cells, it demonstrated an elevation of caspase 8 activity and its cleavage. The DNA ladder formation, a mark of apoptosis induction caused by the EGCG treatment was inhibited by the use of a caspase 8 inhibitor. These findings advocate the involvement of the Fas-mediated cascade in the EGCG-induced apoptosis in U937 cells (Hayakawa et al. 2001) .
In hepatocellular carcinoma cells, EGCG inhibited the proliferation of cells by inducing apoptosis and blocking cell cycle progression in the G1 phase. Its effects were partly brought about by significant increase in the expression of p53 and p21/WAF1 protein. An enhancement in Fas/APO-1 and its two ligand forms, membrane-bound Fas ligand (mFasL) and soluble Fas ligand (sFasL), as well as Bax protein was attributed for the apoptotic effect induced by EGCG (Kuo et al. 2003) . EGCG-induced apoptosis in human prostate carcinoma LNCaP cells was mediated via modulation of p53 stability and p14 mediated down-regulation of murine double minute 2 (MDM2) proteins. EGCG was also shown to negatively regulate NF-κB activity, thereby decreasing the expression of the pro-survival Bcl-2 proteins. Stabilization of p53 led to sustained transcriptional activation of its downstream targets Cip1/p21 and Bax. Thus, EGCG can regulate the expression of two major transcription factors in a manner that it shifts the balance towards triggering apoptosis rather than survival in these cells. The enhanced Bax/Bcl-2 ratio triggered the activation of initiator capsases 9 and 8 followed by activation of effector caspase 3 (Hastak et al. 2003) . EGCG-induced apoptosis of pancreatic cancer cells was reported to be accompanied by growth arrest at an early phase of the cell cycle. In addition, EGCG upregulated Bax oligomerization and depolarization of mitochondrial membranes to facilitate cytochrome c release into cytosol. EGCG downregulated XIAP, an inhibitor of apoptosis protein which resulted in downstream caspase activation. Along with these effects, cells treated with EGCG elicited the production of intracellular reactive oxygen species (ROS) , as well as the c-Jun N-terminal kinase (JNK) activation in pancreatic carcinoma cells (Qanungo et al. 2005). In head and neck carcinoma cells, EGCG increased Fas/CD95 expression along with a drastic decrease in the Tyr705 phosphorylation of STAT3. It is well known that STAT3 targets gene products such as Bcl-2, vascular endothelial growth factor (VEGF), Mcl-1, and cyclin D1 which are known to positively regulate cancer growth and progression. These molecules were also eventually down-regulated by EGCG treatment. The effect of EGCG was mediated via inhibition of STAT-3 activation was further validated by over expression of STAT3 in these cells, which led to resistance to EGCG treatment, suggesting that STAT3 could be a critical target of EGCG induced cell death in HNSCC cells (Lin et al. 2012a) .
EGCG treatment is shown to cause the loss of mitochondrial membrane potential with the increase of ROS generation, p53 expression , Bax/Bcl-2 ratio, cytochrome c release, and cleavage of procaspase-3 and -9 and poly(ADP-ribose)-polymerase in cervical carcinoma cells (Singh et al. 2011) . EGCG induced apoptosis of gastric carcinoma NUGC-3 cells has been found to be associated with lowered survivin and increased Bax and TRAIL expression and p73 activation. In this study, inhibition of p73 via siRNA diminished EGCG effects on survivin expression and cell viability. This study suggested that EGCG induces cell death in gastric cancer cells by apoptosis via inhibition of survivin which lies down-stream of p73 (Onoda et al. 2011) . Analysis of the gene expression patterns of the androgen-independent prostate cancer cell line DU145, treated with EGCG revealed that EGCG modulated the expression levels by more than two-fold of 40 genes. These gene products were mainly involved in the functions of transcription, RNA processing, protein folding, phosphorylation, protein degradation, cell motility, and ion transport. Among them, inhibitor of DNA binding 2 (ID2), known as a dominant anti-retinoblastoma (Rb) helix-loop-helix protein, was found to be down-regulated 4-fold by EGCG treatment. Overexpression of ID2 in DU145 cells reduced apoptosis and increased cell survival in the presence of EGCG, and its knock-down mimicked the apoptosis effect generated by EGCG treatment, although it was milder (Luo et al. 2010).
When NCI-H295 cells were treated with EGCG, the mitochondrial membrane potential decreased and intracellular free Ca2+ increased in a time-dependent manner. EGCG decreased the protein levels of Bcl-2, Bcl-xl, xIAP, cIAP, Hsp70 and Hsp90, but increased the protein expression of Bad, Bax, Fas/CD95, cytochrome c, Apaf-1, AIF, GADD153, GRP78, and cleaved caspase-3, -7,-8 and -9 (Wu et al. 2009). Overall, these studies suggest that EGCG has multiple targets for inducing cell death in various types of cancer cells.
Fisetin
Fisetin (3,7,3,4-tetrahydroxyflavone) , a naturally occurring flavonoid commonly found in the smoke tree (Cotinus coggygria) , is also found in fruits and vegetables such as strawberry, raspberries, apple, persimmon, grape, onion and cucumber. It exerts a wide variety of activities, including neurotrophic, anti-oxidant, anti-inflammatory and anti-angiogenic effects (Maher 2006) . It has been reported to inhibit the proliferation of a wide variety of cancer cells, including prostate cancer (Haddad et al. 2010) , liver cancer (Chen et al. 2002) , colon cancer (Lu et al. 2005) , and leukemia cells (Lee et al. 2002) . In colon cancer cells HCT-116, fisetin induced apoptosis which involved enhanced ser15 phosphorylation of p53 and activation of caspase 3 and PARP. The study demonstrated the role of fisetin in modulating securin levels. Inhibition of securin resulted in potentiated response to fisetin induced apoptosis (Yu et al. 2011) . In bladder cancer cells, fisetin induced apoptosis through mitochondrial pathway which was resultant of Bax up-regulation and cytochrome c release, especially at the higher (100 µM) dose (Li J et al. 2011) .
TRAIL , which is an endogenous proapoptotic regulator of apoptosis , is mainly expressed by immune cells, and hence plays a very significant role in protecting the body against tumorigenesis . Studies done on evaluating the role of fisetin in cells which are resistant to TRAIL mediated apoptosis, showed that fisetin could sensitize these cells to TRAIL mediated apoptosis . Fisetin was also shown to increase the expression of TRAIL R1 receptor and down-regulate NF-κB activity (Szliszka et al. 2011) . When the role of Fisetin was explored in human breast cancer cells , it displayed differential effects showing better efficacy in MCF-7 cells than MDA-MB-231 cells without any cytotoxity to MCF-10A cells, a non-tumorigenic breast epithelial cells. MCF-7 cells lack caspase 3, but fisetin could induce apoptosis in these cells, which was characterized by plasma membrane blebbing, mitochondrial depolarization and cleavage of caspase-7, -8, -9 and PARP. Interestingly, these cells did not show DNA fragmentation or phosphatidyl serine externalization which is one of the most common features of apoptosis (Yang et al. 2011) .
In cervical carcinoma HeLa cells, fisetin triggered sustained activation of Erk1/2 together with cleavage of caspase 3 and PARP. Treatment with synthetic Erk1/2 inhibitor PD98059 also inhibited fisetin mediated activation of caspase-3 and -8 (Yang et al. 2012). Erk1/2 activation is typically associated with enhanced proliferation in cancer cells, but recent literature also show that sustained activation of Erk1/2 could lead to apoptotic cell death.
Fisetin is also shown to induce autophagic cell death in cancer cells. The autophagic effect of fisetin was shown to be exerted via inhibition of mTOR which is a component of the AKT signaling pathway and is known to play an important role in survival signaling. The study evaluated the effect of fisetin on human prostate carcinoma PC-3 cells which are null for PTEN and hence have an constitutively activated PI3K/AKT signaling. Fisetin down-regulated mTOR complexes and thereby induced autophagy in these cells. Many studies debate that induced autophagy could be a mechanism of cytoprotection, but this study showed that fisetin induced autophagy in PC-3 cells was not a protective mechanism (Suh et al. 2010).
Grape Seed Extract
Grape seed extract (GSE) is a complex mixture of polyphenols containing dimers, trimers, and other oligomers (procyanidins) of catechin and epicatechin and their gallate derivatives together known as the proanthocyanidins. GSE, a commonly used dietary supplement, is known to possess activities ranging from antiinflammatory, antioxidant, antibacterial and antiviral activities. Strong efficacies are recently reported against prostate, lung, breast, skin and other cancers. In prostate carcinoma LNCaP cells, as early as ~ 5 h treatment time with GSE, the cells get rounded in shape and got detached from culture dish suggesting an anoikis-like apoptotic cell death by reducing the cellular adhesion to extracellular matrix by reducing the level and activity of focal adhesion kinase (FAK) . The study also showed that GSE could induce both anoikis and apoptosis involving decrease in FAK level and cleavage of caspases and PARP, and release of cytochrome c from the mitochondria to the cytosol, suggesting an activation of intrinsic pathway of apoptosis . However, the pan-caspase inhibitor z-VAD.fmk failed to completely attenuate GSE-induced apoptosis, implying that caspase-independent pathways were also involved in GSE-induced apoptosis (Kaur et al. 2006) .
Of the non-cysteine proteases, AIF is thought to mediate apoptosis through caspase-independent pathway, where following a death stimulus, mitochondrial AIF is released into cytosol, which then translocates to nucleus and causes nuclear condensation followed by massive chromatin fragmentation and cell death. GSE phosphorylated ATM-Ser1981 in LNCaP cells, which might be responsible for p53 phosphorylation at Ser15. Following DNA damage, autophosphorylation of ATM at Ser1981 is required for the activation of ATM, which then phosphorylates downstream targets such as Chk2, p53 and H2A.X. Indeed, all these downstream targets of activated ATM were found to be phosphorylated following GSE treatment of LNCaP cells. GSE-induced apoptosis , cell growth inhibition, and cell death were found to be attenuated by the pretreatment with N-acetylcysteine, suggesting the involvement of ROS in its apoptotic activity that might be a consequence of oxidative stress–caused DNA damage. The induction of ROS by GSE is also supported by another recent study showing that depending on its concentration, GSE itself could lead to the production of hydrogen peroxide (Kaur et al. 2006) . In Caco-2 cells, GSE treatment inactivates the PI3K pathway leading to a concomitant decrease in Bad, cAMP response element-binding protein (CREB) and forkhead in rhabdomyosarcoma (FKHR) phosphorylation (Engelbrecht et al. 2007) .
Another study done to assess whether the sources of GSE had any difference in effects, it revealed that irrespective of the sources, higher doses of GSE could induce apoptotic cell death in colon cancer cells. The apoptotic mechanism involved both intrinsic and extrinsic pathway, and release of AIF (Dinicola et al. 2010). This can be further validated by another study showing that GSE induced both intrinsic and extrinsic cell death in colorectal carcinoma cells involving caspase- dependent mechanism and release of AIF (Derry et al. 2012) . One of the major reasons attributed to GSE-induced cell death was the generation of ROS which was independent of the p53 status of the colorectal carcinoma cells unlike what was observed in LNCaP cells (Derry et al. 2012) .
The differential effect of GSE was also evident in head and neck carcinoma cells wherein it induced apoptosis in D-562 and FaDu cells but not in normal epidermal keratinocytes. The apoptotic response was characterized by activation of DNA damage checkpoint signaling ATM, ATR, Cdc-25C, which further led to the activation of caspase 8, 9 and 3 and inhibition of DNA repair BRCA1 and Rad 51, and enhanced ROS production (Shrotriya et al. 2012) . In another study done with oral carcinoma cells with grape seed procyanidins (GSPs) showed that the effect on the cell was dependent on the p53 status of the cells. It was observed that in cells with wild type p53, GSP could induce mitochondrial dependent apoptosis whereas in cells which were mutant for p53, GSP induced cell cycle arrest but not apoptosis and no down-regulation of Bcl-2 protein was observed (Lin et al. 2012a) . Overall, these studies suggest that GSE can selectively induce death of cancer cells involving ROS generation, extrinsic, intrinsic, caspase-dependent and –independent apoptotic pathways.
Other Natural Agents Inducing Cancer Cell Death
Other than these well studied natural plant molecules discussed above, many other compounds isolated from different plant sources have been shown to induce apoptosis in cancer cells (Table 1). Xanthohumol, a plant phytochemical was shown to act via extrinsic pathway in human colon carcinoma cells and activates FADD in the DISC that modulate release of caspase-8 and starts the induction of apoptosis (Pan et al. 2005) . Zerumbone was also shown to enhance TRAIL-induced apoptosis through the up-regulation of DRs and the down-regulation of cFLIP (Yodkeeree et al. 2010) . Zerumbone inhibited cell proliferation in liver cancer cells and induced apoptosis by decreasing the levels of anti-apoptotic protein, Bcl-2 and up-regulation of pro-apoptotic Bax (Sakinah et al. 2007). Sanguinarine induced apoptosis in human leukemia cells by down-regulation of anti-apoptotic Bcl-2 and up-regulation of pro-apoptotic Bax expression (Han et al. 2008). Similarly, genistein decreases the ratio of Bcl-2/Bax and Bcl-xL/Bax in ovarian cancer cells which led these cells to programmed cell death and also enhances the phosphorylation and activation of p53 (Ouyang et al. 2009) . Delphinidin significantly increased the levels of active caspases-3 and -9 in prostate cancer cells that leads to apoptosis, which was mediated via induction of Bax and inhibition of Bcl-2 protein (Hafeez et al. 2008) . Garcinol changed the ratio of the anti-apoptotic Bcl-2 and pro-apoptotic Bax proteins at the dose of 20 μM in colon cancer cells that resulted in cell apoptosis (Liao et al. 2005) .
Resveratrol , a natural product from grapes and present in red wine, is well known to have anti-tumorigenic effect involving induction of apoptosis through activation of death receptor like Fas/CD95/APO-1(Athar et al. 2006) . Resveratrol induces alterations in mitochondria permeability pore transition, release of cytochrome c into the cytosol and conformational change in Apaf-1 which recruits the components of apoptosome complexe and activate caspase cascade. Simultaneously, resveratrol promotes release of SMAC/DIABLO in cytosol which modulate IAPs activity and allow caspase-dependent apoptosis (Athar et al. 2010) . Apigenin is known to induce ROS that promotes mitochondrial permeability and causes decrease in the mitochondrial Bcl-2 expression. Further, apigenin induced cytochrome c release and activated cleavage of caspase-3, 7, 8 and 9 that finally follow the apoptosis in prostate cancer cells; although in neuroblastoma, apigenin increased the intracellular free Ca+2 and allow these cells to start mitochondrial mediated apoptosis (Shukla and Gupta 2010) . Human myeloid cells undergo apoptosis via activation of TNF up-regulation after the exposure of flavopiridol (Takada et al. 2008) . Thus, many dietary and non-dietary phytochemicals have ability to induce death in cancer cells involving the known basic mechanisms of apoptosis induction and autophagy .
Summary and Conclusions
Induction of cell death remains one of the most desired effects of cancer therapy. Most of the therapeutically important agents work by induction of apoptotic, autophagic or necrotic death in cancer cells. These agents induce death via modulation of various signaling pathways activated in cancer cells. Enhancement of autophagic and apoptotic cell death induction by therapeutic agents especially selectively in cancer cells is important. One of the reasons for preferring natural agents with chemopreventive efficacy over the synthetic chemical agents, which induce cytotoxicity is that they are specifically more cytotoxic to cancer cells as compared to normal cells. One of the interesting phenomenon exhibited by these phytochemicals is that in normal cells most of these agents act as potent antioxidants which reduce generation of ROS, but majority of studies with these phytochemicals in cancer cells depict that these agents activate the mechanisms of cell death mainly by generation of ROS and hence creating a condition of oxidative stress. This duality in function which leads to preferential cytotoxic effect towards cancer cells is the unique property of natural plant phytochemicals , and warrants further investigation at molecular levels.
References
Achenbach TV, Müller R, Slater EP (2000) Bcl-2 independence of flavopiridol-induced apoptosis. Mitochondrial depolarization in the absence of cytochrome c release. J Biol Chem 275:32089–32097
Aggarwal BB, Ichikawa H (2005) Molecular Targets and Anticancer Potential of Indole-3-Carbinol and Its Derivatives. Cell Cycle 49:1201–1215
Ahmad A, Banerjee S, Wang Z et al (2008) Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J Cell Biochem 105:1461–1471
Ahmad A, Wang Z, Ali R et al (2010) Apoptosis-inducing effect of garcinol is mediated by NF-kappaB signaling in breast cancer cells. J Cell Biochem 109(6):1134–1141
Altieri DC (2003) Validating survivin as a cancer therapeutic target. Nat Rev Cancer 3(1):46–54
Altieri DC (2006) Targeted therapy by disabling crossroad signaling networks: the survivin paradigm. Mol Cancer Ther 5(3):478–482
Aoki H, Takada Y, Kondo S et al (2007) Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol 72:29–39
Athar M, Back JH, Kopelovich L et al (2010) Multiple molecular targets of resveratrol: anti-carcinogenic mechanisms. Arch BiochemBiophys 486(2):95–102
Bat-Chen W, Golan T, Peri I et al (2010) Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer 62(7):947–957
Bhatia N, Zhao J, Wolf DM, Agarwal R (1999) Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin. Cancer Lett 147:77–84
Bhutani M, Pathak AK, Nair AS et al (2007) Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res 13(10):3024–3032
Block G, Patterson B, Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 18(1):1–29
Bode AM, Dong Z (2009) Cancer prevention research—then and now. Nat Rev Cancer 9:508–516
Bousserouel S, Bour G, Kauntz H et al (2012) Silibinin inhibits tumor growth in a murine orthotopic hepatocarcinoma model and activates the TRAIL apoptotic signaling pathway. Anticancer Res 32:2455–2462
Chen YC, Shen SC, Lee WR et al (2002) Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol 76:351–359
Cheong H, Lu C, Lindsten T, Thompson CB (2012) Therapeutic targets in cancer cell metabolism and autophagy. Nat Biotechnol 30:671–678
Chung LY, Cheung TC, Kong SK et al (2001) Induction of apoptosis by green tea catechins in human prostate cancer DU145 cells. Life Sci 68:1207–1214
Derry M, Raina K, Agarwal R, Agarwal C (2012) Differential effects of grape seed extract against human colorectal cancer cell lines: The intricate role of death receptors and mitochondria. Cancer Lett. doi:10.1016/j.canlet.2012.12.015
Dhanalakshmi S, Agarwal P, Glode LM, Agarwal R (2003) Silibinin sensitizes human prostate carcinoma DU145 cells to cisplatin- and carboplatin-induced growth inhibition and apoptotic death. International journal of cancer J Int Cancer 106:699–705
Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R (2002) Silibinin inhibits constitutive and TNFα-induced activation of NF-kB and sensitizes human prostate carcinoma DU145 cells to TNFα-induced apoptosis. Oncogene 21:1759–1767
Dohi T, Beltrami E, Wall NR et al (2004) Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 114(8):1117–1127
Duan W, Jin X, Li Q et al (2010) Silibinin induced autophagic and apoptotic cell death in HT1080 cells through a reactive oxygen species pathway. J Pharmacol Sci 113:48–56
Duan W-J, Li Q-S, Xia M-Y et al (2011) Silibinin activated ROS-p38-NF-κB positive feedback and induced autophagic death in human fibrosarcoma HT1080 cells. J Asian Nat Prod Res 13:27–35
Engelbrecht A-M, Mattheyse M, Ellis B et al (2007) Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer letters 258:144–153
Fan S, Li L, Chen S et al (2011) Silibinin induced-autophagic and apoptotic death is associated with an increase in reactive oxygen and nitrogen species in HeLa cells. Free Radical Res 45:1307–1324
Fan TJ, Han LH, Cong RS et al (2005) Caspase Family Proteases and Apoptosis. Acta Biochimicaet Biophysica Sinica 37(11):719–727
Ferreira CG, Epping M, Kruyt FAE et al (2002) Apoptosis: target of cancer therapy. Clin Cancer Res 8:2024–2034
Garg AK, Buchholz TA, Aggarwal BB (2005) Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 7:1630–1647
Garrido C, Galluzzi L, Brunet M (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Differ 13:1423–1433
Gogada R, Amadori M, Zhang H et al (2011) Curcumin induces Apaf-1-dependent, p21-mediated caspase activation and apoptosis. Cell Cycle 10:4128–4137. doi:10.4161/cc.10.23.18292
Guicciardi ME, Gores GJ (2009) Life and death by death receptors. The FASEB Journal 23:1625–1637
Guo L, Chen X-J, Hu Y-H et al (2012) Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother Res PTR
Haddad AQ, Fleshner N, Nelson C et al (2010) Antiproliferative mechanisms of the flavonoids 2,2’-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr Cancer 62:668–681
Hafeez B, Asim M, Siddiqui IA et al (2008) Delphinidin, a dietary anthocyanidin in pigmented fruits and vegetables: a new weapon to blunt prostate cancer growth. Cell Cycle 7(21):3320–3326
Hastak K, Gupta S, Ahmad N et al (2003) Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene 22:4851–4859
Hayakawa S, Saeki K, Sazuka M et al (2001) Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem Biophys Res Commun 285:1102–1106
Hsu JD, Kao SH, Ou TT et al (2011) Gallic Acid Induces G2/M Phase Arrest of Breast Cancer Cell MCF-7 through Stabilization of p27Kip1 Attributed to Disruption of p27Kip1/Skp2 Complex. J Agric Food Chem 59(5):1996–2003
Ibrahim A, El-Meligy A, Lungu G et al (2011) Curcumin induces apoptosis in a murine mammary gland adenocarcinoma cell line through the mitochondrial pathway. Eur J Pharmacol 668:127–132
Ichikawa H, Takada Y, Murakami A et al (2005) Identification of a Novel Blocker of IB Kinase That Enhances Cellular Apoptosis and Inhibits Cellular Invasion through Suppression of NF-B-Regulated Gene Products. The Journal of Immunology 174:7383–7392
Jeong JC, Shin WY, Kim TH et al (2011) Silibinin induces apoptosis via calpain-dependent AIF nuclear translocation in U87MG human glioma cell death. J Exp Clin Cancer Res 30:44
Jia Y-L, Li J, Qin Z-H, Liang Z-Q (2009) Autophagic and apoptotic mechanisms of curcumin-induced death in K562 cells. J Asian Nat Prod Res 11:918–928
Jiang Y-Y, Yang R, Wang H-J et al (2011) Mechanism of autophagy induction and role of autophagy in antagonizing mitomycin C-induced cell apoptosis in silibinin treated human melanoma A375-S2 cells. Eur J Pharmacol
Jung C.H, Ro S.H, Cao J, Otto N.M, Kim D.H (2010) mTOR regulation of autophagy. FEBS letters 584:1287–1295
Katiyar SK, Meeran SM, Katiyar N et al (2009) p53 Cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol Carcinog 48(1):24–37
Kauntz H, Bousserouel S, Gossé F, Raul F (2011) Silibinin triggers apoptotic signaling pathways and autophagic survival response in human colon adenocarcinoma cells and their derived metastatic cells. Apoptosis Int J Program Cell Death 16:1042–1053
Kaur M, Agarwal R, Agarwal C (2006) Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther 5:1265–1274
Khosravi-Far R, Esposti MD (2004) Death receptor signals to mitochondria. Cancer Biol Ther 3(11):1051–1057
Kim JY, Cho TJ, Woo BH et al (2012) Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol 57:1018–1025
Kim KW, Choi CH, Kim TH et al (2009) Silibinin inhibits glioma cell proliferation via Ca2+/ROS/MAPK-dependent mechanism in vitro and glioma tumor growth in vivo. Neurochem Res 34:1479–1490
Kunwar A, Jayakumar S, Srivastava AK, Priyadarsini KI (2012) Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch Toxicol 86:603–614
Kuo CL, Wu SY, Ip SW et al (2011) Apoptotic death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma cells is mediated through the ROS, mitochondrial depolarization and caspase-3-dependent signaling responses. Int J Oncol 39:319–328
Kuo PL, Lin CC (2003) Green tea constituent (-)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci 10:219–227
Lee WR, Shen SC, Lin HY et al (2002) Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)-dependent endonuclease. Biochem Pharmacol 63:225–236
Li J, Cheng Y, Qu W et al (2011) Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol 108:84–93
Lian F, Li Y, Bhuiyan M et al (1999) p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer 33(2):125–131
Liao CH, Sang S, Ho CT et al (2005) Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and Akt survival signaling in human colon cancer cells. J Cell Biochem 96(1):155–169
Lin H-Y, Hou S-C, Chen S-C et al (2012a) (-)Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J Agric Food Chem 60:2480–2489
Lu X, Jung J, Cho HJ et al (2005) Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr 135:2884–2890
Maher P (2006) A comparison of the neurotrophic activities of the flavonoid fisetin and some of its derivatives. Free Radic Res 40:1105–1111
Malikova J, Zdarilova A, Hlobilkova A (2006) Effects of sanguinarine and chelerythrine on the cell cycle and apoptosis. Biomedical papers of the Medical Faculty of the University Palacky. Olomouc 150(1):5–12
Mathew R, Karantza-Wadsworth V, White E (2007) Role of autophagy in cancer. Nature Reviews. Cancer 7:961–967
Nambiar D, Rajamani P, Singh RP (2011) Effects of phytochemicals on ionization radiation-mediated carcinogenesis and cancer therapy. Mutat Res 728:139–157
Nigam N, Bhui K, Prasad S et al (2009) [6]-Gingerol induces reactive oxygen species regulated mitochondrial cell death pathway in human epidermoid carcinoma A431 cells. Chem Biol Interact 181(1):77–84
Onoda C, Kuribayashi K, Nirasawa S et al (2011) (-)Epigallocatechin-3-gallate induces apoptosis in gastric cancer cell lines by down-regulating survivin expression. Int J Oncol 38:1403–1408
Ouyang L, Shi Z, Zhao S et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Proliferation 45:487–498
Pan L, Becker H, Gerhauser C (2005) Xanthohumol induces apoptosis in cultured 40–16 human colon cancer cells by activation of the death receptor- and mitochondrial pathway. Mol Nutr Food Res 49:837–843
Pandey MK, Sandur SK, Sung B et al (2007) Butein, a Tetrahydroxychalcone, Inhibits Nuclear Factor (NF)-B and NF-B-regulated Gene Expression through Direct Inhibition of IB Kinase on Cysteine 179 Residue. J Biol Chem 282(24): 17340–17350
Pavese JM, Farmer RL, Bergan RC (2010) Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev 29(3):465–482
Qanungo S, Das M, Haldar S, Basu A (2005) Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 26:958–967
Ramasamy K, Agarwal R (2008) Multitargeted therapy of cancer by silymarin. Cancer Lett 269(2):352–362
Reed JC (2006) Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nature clinical practice Oncology 3:388–398
Reed JC (2010) Apoptosis and cancer. In Hong WK (ed) Holland-Frei cancer medicine, 8th edn. PMPH–USA
Rehman J, Zhang HJ, Toth PT, Zhang Y, Marsboom G, Hong Z, Salgia R et al (2012) Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB Journal 26(5):2175–2186
Rong JJ, Hu R, Qi Q et al (2009) Gambogic acid down-regulates MDM2 oncogene and induces p21(Waf1/CIP1) expression independent of p53. Cancer Letters 284:102–112
Sandri M (2012) FOXOphagy path to inducing stress resistance and cell survival. Nat Cell Biol 14:786–788
Shrotriya S, Deep G, Gu M et al (2012) Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis 33:848–858
Shukla S, Gupta S (2010) Apigenin: A Promising Molecule for Cancer Prevention. Pharm Res 27(6):962–978
Singh M, Singh R, Bhui K et al (2011) Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol Res 19:245–257
Singh RP, Agarwal R (2006) Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer 13:751–778
Singh RP, Sharma G, Dhanalakshmi S, Agarwal C (2003) Suppression of Advanced Human Prostate Tumor Growth in Athymic Mice by Silibinin Feeding Is Associated with Reduced Cell Proliferation, Increased Apoptosis, and Inhibition of Angiogenesis 1. Prevention 12:933–939
Sung B, Pandey MK, Ahn KS et al (2008) Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood 111(10):4880–4891
Surh Y.J (2003) Cancer chemoprevention with dietary phytochemicals. Nature reviews. Cancer 3:768–780
Szliszka E, Helewski KJ, Mizgala E, Krol W (2011) The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int J Oncol 39:771–779
Takada Y, Sethi G, Sung B et al (2008) Flavopiridol Suppresses Tumor Necrosis Factor-Induced Activation of Activator Protein-1, c-Jun N-Terminal Kinase, p38 Mitogen-Activated Protein Kinase (MAPK), p44/p42 MAPK, and Akt, Inhibits Expression of Antiapoptotic Gene Products, and Enhances apoptosis through cytochrome c release and caspase activation in human myeloid cells. Mol Pharmacol 73(5):1549–1557
Takamura A, Komatsu M, Hara T, Sakamoto A, Kishi C, Waguri S, Eishi Y, Hino O, Tanaka K, Mizushima N (2011) Autophagy-deficient mice develop multiple liver tumors. Genes Dev 25:795–800
Tatarkova Z, Kuka S, Petráš M, Račay P, Lehotský J, Dobrota D, Kaplan P (2012). Why mitochondria are excellent targets for cancer therapy. Klinická onkologie: casopis Ceské a Slovenské onkologické spolecnosti 25(6):421–426
Tyagi A, Singh RP, Agarwal C, Agarwal R (2006) Silibinin activates p53-caspase 2 pathway and causes caspase-mediated cleavage of Cip1/p21 in apoptosis induction in bladder transitional-cell papilloma RT4 cells: evidence for a regulatory loop between p53 and caspase 2. Carcinogenesis 27:2269–2280
Tyagi AK, Agarwal C, Singh RP et al (2003) Silibinin down-regulates survivin protein and mRNA expression and causes caspases activation and apoptosis in human bladder transitional-cell papilloma RT4 cells. Biochem Biophys Res Commun 312:1178–1184
Tyagi AK, Singh RP, Agarwal C et al (2002) Silibinin Strongly Synergizes Human Prostate Carcinoma DU145 Cells to Doxorubicin-induced Growth Inhibition, G 2 -M. Clin Cancer Res 8:3512–3519
Wang W.H, Chiang I.T, Ding K et al (2012) Curcumin-induced apoptosis in human hepatocellular carcinoma j5 cells: critical role of ca(+2)-dependent pathway. Evid Based Complement Alternat Med eCAM 2012:512907
Wang S, Yu Q, Zhang R, Liu B (2011) Core signaling pathways of survival/death in autophagy-related cancer networks. Int J Biochem Cell Biol 43:1263–1266
Yang CS, Wang X, Lu G, Picinich SC (2009) Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Reviews Cancer 9:429–439
Yang P.M, Tseng H.H, Peng C.W et al (2012) Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol 40:469–478
Yodkeeree S, Sung B, Limtrakul P et al (2010) Zerumbone Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors in Human Colon Cancer Cells: Evidence for an Essential Role of Reactive Oxygen Species. Cancer Res 7(21):3320–3326
Yu SH, Yang PM, Peng CW et al (2011) Securin depletion sensitizes human colon cancer cells to fisetin-induced apoptosis. Cancer Lett 300:96–104
Zhang W, Ha M, Gong Y et al (2010) Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol Rep 24:1585–1592
Zhou H, Beevers CS, Huang S (2011) The targets of curcumin. Current Drug Targets 12:332–347
Acknowledgments
The original published work in this communication is supported by funds from DST, CSIR, and ICMR, India. V. Mohan and D. Nambiar acknowledge the fellowship support from UGC and CSIR, India, respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mohan, V., Nambiar, D., Kale, R., Singh, R. (2013). Cell-Death—Inducing Mechanisms of Cancer Chemopreventive Agents. In: Chandra, D. (eds) Mitochondria as Targets for Phytochemicals in Cancer Prevention and Therapy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9326-6_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9326-6_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9325-9
Online ISBN: 978-1-4614-9326-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)