Abstract
For the heart to produce mechanical force, two cellular components are essential: myofibrils, which are responsible for generating contractile activity, and mitochondria, which provide most of the required energy. Diabetic cardiomyopathy is associated with defects in both mitochondria and myofibrils, indicating that changes in energy production and energy utilization are of foremost relevance in the etiology of cardiac dysfunction in chronic diabetes. Several elements including hyperglycemia, hyperlipidemia, and changes in the level of different hormones contribute directly or indirectly to contractile impairment in this multifactorial diabetic disorder. Metabolic imbalance, characterized by excessive fatty acid oxidation, Ca2+ overload, and oxidative stress are considered to reduce mitochondrial phosphorylation activity and impair the mitochondrial electron-transport chain in the diabetic heart. These subcellular alterations result in reduced level of adenosine triphosphate (ATP) in the diabetic heart, limiting cardiomyocyte contractile ability. Altered gene expression and excessive proteolytic activity caused by intracellular Ca2+ overload and oxidative stress in chronic diabetes promote changes in both composition and structure of myofibrils; this myofibril remodeling, characterized by diminished energy consumption and insensitivity to Ca2+, further impairs heart function in diabetic cardiomyopathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diabetic cardiomyopathy
- Mitochondria
- Myofibrils
- ATP
- Metabolic abnormalities
- Ca2+ overload
- Oxidative stress
- Cardiac dysfunction
1 Introduction
Diabetes mellitus is becoming a more relevant disease, as the global prevalence of diabetes was estimated to be 2.8 % in 2000 and is expected to increase more than 50 % until reaching 4.4 % in 2030 [1]. Diabetes is associated to a wide range of clinical manifestations related to either insulin deficiency in type 1 diabetes or insulin insensitivity in type 2 diabetes [2, 3]. This abnormal metabolic state is mainly characterized by, but not limited to, hyperglycemia (elevated glucose levels). Other hormonal and metabolic abnormalities, especially hyperlipidemia, are known to contribute to diabetes-related cardiovascular complications [4–9]. Although the name diabetes mellitus, in opposition to insipidus, is a remnant of a time when tasting urine was an acceptable medical procedure, the idea of a defective cardiac phenotype as a result of diabetes is a relatively new concept. Although diabetes mortality is primarily attributed to cardiovascular complications [10], the concept of diabetic cardiomyopathy was neglected for a long time, mainly because of the confounding effect of chronic diabetes on heart function by atherosclerosis and hypertension [9, 11]. It was in 1972, with the description of four diabetic patients showing congestive heart failure in the absence of coronary artery atherosclerosis by Rubler et al. [12], that the term diabetic cardiomyopathy was first used. Diabetic cardiomyopathy was defined as a cardiac dysfunction that occurs because of chronic diabetes, independently of coronary artery disease [13]. Patients with diabetes, in the absence of atherosclerosis, were found to suffer from ventricular dysfunction including shortened left ventricular ejection time, longer pre-ejection period, and also elevated end-diastolic pressure [14]. Some epidemiological studies also revealed that diabetes shows increased risk of heart failure even when atherosclerosis and hypertension risks are taken into account [15]. Animal models for diabetes also attest to the harmful effects of diabetes on heart function. Streptozotocin-induced diabetes in rats is associated with reduced heart rate, lower peak ventricular pressure- and also impaired left ventricular contractions and relaxations [16–21]. Further animal studies have also shown that diabetes, in conjunction with hypertension, leads to congestive heart failure [22, 23].
Although the molecular and cellular mechanisms of the cardiac dysfunction in diabetic cardiomyopathy are not completely understood [24], it is clear that an imbalance between energy production, in the form of adenosine triphosphate (ATP), and energy consumption is a key factor that contributes to the development of this pathological disorder [13, 24, 25]. The major players of the high-energy phosphate production and utilization cycle, in the cardiomyocytes, are the mitochondria (MT) and myofibrils (MF), the subcellular components responsible for the phosphorylation of adenosine diphosphate (ADP) into ATP and the hydrolysis of ATP by ATPase activity, respectively. Herein, this review focuses on the mechanisms by which chronic diabetes affects MT and MF functions, and also the consequences of the resulting energetic imbalance on cardiomyocytes and heart function.
2 Defects in Energy Production
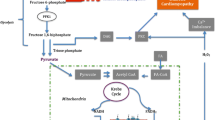
In cardiac tissue, as in most tissues, MT are accountable for most of the ATP production, and thus any deterioration of these organelles may lead to a state of energy restriction and consequently of cellular deficiency. In fact, MT are responsible, under normal conditions, for more than 95 % of all myocardial ATP synthesis [26]; cardiomyocytes have a rather limited ATP pool that would be consumed in approximately 10 s without continuous mitochondrial activity [26]. To maintain a stable ATP content, and proper myocardial function, energy consumption and production have to be tightly coupled in the cardiac muscle. Proper MT function is, therefore, essential for cardiac function as MT phosphorylation is commonly compromised in several different types of cardiac disorders [26–28], including diabetic cardiomyopathy [29–31]. MT dysfunction is mainly credited to metabolic alterations in diabetes, resulting in increased free fatty acid (FFA) utilization. Oxidative stress and Ca2+ overload are also relevant to the process that leads to MT damage and energy deficiency in the diabetic heart. These events are depicted in Fig. 1.
3 Defects in Energy Utilization
Cardiomyocytes require energy, in the form of ATP, to produce MF contractions and the mechanical force that ultimately allows the heart to pump blood. Because cardiomyocyte contractions are controlled, in both intensity and rhythm, by changes in intracellular Ca2+ concentration, a precise MF response to Ca2+ is crucial to overall heart performance. MF is the functional unit of the cardiac muscle, being composed mainly of actin and myosin; myosin is the protein that is actively responsible for muscular contractions, as it possesses ATPase activity. MF contractions are regulated by the troponin–tropomyosin complex (TnTm): Ca2+ binds to the TnTm complex, which exposes the myosin ATP-binding site, and this allows actin–myosin interactions to occur, resulting in the shortening of the muscle fiber. This contractile process requires energy in the form of ATP and represents about 60–70 % of all ATP consumption of the myocardium under normal conditions [26]. As a matter of fact, diabetic cardiomyopathy is associated with MF abnormalities that result in defects in the energy utilization process [13, 32–34]. Two main factors can be seen promoting cardiac contractile dysfunction: defects in energy utilization and abnormal MF regulation. Several cellular abnormalities involved in defects in energy utilization and regulation of MF in diabetic heart are depicted in Fig. 2.
Abnormal energy utilization is associated with MF remodeling, a process that is related to changes in gene expression, oxidative stress, intracellular Ca2+, and activation of proteolytic enzymes [13, 21, 32, 34, 35]. Several animal studies have reported decreased MF Ca2+-dependent ATPase activity in chronic diabetes [17, 19, 21, 36, 37], and MF insensibility to Ca2+ is most likely involved in diabetic cardiomyopathy [13]. Several factors interfere with myofibril function, involving both functional and regulatory enzymes [36–39]. Chronic diabetes, in animals, is associated with the prevalence of myosin heavy-chain (MHC) isoenzyme β over isoenzyme α [34, 40–48]. This shift in expression of MHC isoforms could explain the depressed ATPase activity in other animals; however, the relevance of this mechanism is uncertain in human hearts, in which the β-isoform is normally predominant over the α isoform [49, 50]. On the other hand, the phosphorylation of the myosin light chain (MLC) by the myosin light-chain kinase (MLCK) is a factor that could explain cardiac dysfunction in human diabetic cardiomyopathy, because MLC phosphorylation is related to increased MF sensitivity to Ca2+ [51]. However, MLC, MLCK, and MLC phosphorylation were shown to be significantly reduced in diabetic rat heart homogenate, and these changes were partially reversed by insulin treatment [38]. The activation of proteolytic enzymes, seen in cardiac dysfunction [35, 52–60], can also participate in the development of MF dysfunction. Intracellular Ca2+ overload and oxidative stress are related to the activation of proteases [61–63] that would lead to degradation of cardiomyocyte MF proteins in diabetic cardiomyopathy.
Chronic diabetes is also known to affect cardiac function through impaired MF regulation mainly caused by TnTm abnormalities [13]. The TnTm complex is formed by tropomyosin and three subunits of troponin, TnC, TnI, and TnT, that are responsible for Ca2+ binding, ATPase inhibition, and myosin binding, respectively. There is evidence that the phosphorylation of TnI and TnT by protein kinase A (PKA) and C (PKC) contribute to myofibrillar insensitivity to Ca2+, leading, in the given order, to reduced ATPase response to Ca2+ and reduced myosin–actin interactions [13]. These findings are supported by the fact that impaired actomyosin ATPase activity of diabetic animals could be partially normalized in the presence of TnTm extracted from healthy animals [64].
4 Mechanisms of Alterations in Mitochondria and Myofibrils
The primary energy source of cardiomyocytes are fatty acids, accounting for approximately 60–70 % of the myocardial substrate [65]. The participation of FFA in cardiac muscle energy metabolism is known to be even more expressive in a chronic diabetic state [66, 67]. Although lipids are essential for heart function, from both an energetic and structural point of view, excessive FFA uptake by myocytes is known to have deleterious effects on cardiac function, supporting the concept of ‘lipid paradox’ [65]. Studies conducted with both animal and human tissues have concluded that sarcolemmal (SL) glucose transporter (GLUT) 1, GLUT4, and sodium–glucose-linked transporter (SLGT) 1 expression is reduced in diabetic hearts [68–76]. In fact, glucose uptake is impaired in diabetic hearts by insulin deficiency or insensitivity [77–79]. The opposite is true for FFA uptake: high plasma levels of FFA not only increase cardiomyocyte FFA uptake but also depress glucose utilization by the diabetic heart [26, 28, 80]. Increased MT fatty acid oxidation, in chronic diabetes, is credited to the upregulation of the perioxisome proliferator-activated receptor α [71, 81–84]. Such an elevation in the rate of fatty acid oxidation, if maintained for a long period of time, is believed to impair MT oxidative phosphorylation, causing damage to the electron-transport chain and depressing MT Mg2+ ATPase [29–31]. In addition to the reduction of ATP reserves and depressed cardiac function [30, 31], MT dysfunction is associated with the formation of MT pores, leaking of MT proteins, and cellular dysfunction [9, 13, 24, 30, 85, 86]. Triglyceride synthesis is also remarkably upregulated in diabetic cardiomyopathy, leading to a phenomenon called lipotoxicity that is intimately related to cellular damage [9, 87]. Enhanced FFA uptake is associated with the accumulation of lipid droplets in the myocardium during the development of diabetic cardiomyopathy [9, 86].
Diabetes is intimately associated with defects in several metabolic pathways that are responsible for a marked increase in intracellular Ca2+ concentration [13, 18, 24, 73, 86, 88–95]. Ca2+-handling defects in chronic diabetes are known to be related to SL and sarcoplasmic reticulum (SR) alterations that favor the occurrence of intracellular Ca2+ overload [96–99]. In this regard, MT are known to function as Ca2+ sinks, in the event of Ca2+ overload, in an attempt to maintain the normal cytoplasmic level of free Ca2+ [24, 73, 100, 101]. Although this mechanism is intended to be protective in nature, excessive MT Ca2+ uptake depresses MT phosphorylation activity [13]. Different drugs that are capable of attenuating Ca2+ overload, such as Ca2+ channel blockers and angiotensin-receptor antagonists, have been shown to ameliorate cardiac dysfunction in diabetic cardiomyopathy [40, 95, 102, 103].
It is becoming clear that oxidative stress contributes to the development of several diabetic complications [104–108], including diabetic cardiomyopathy [13, 85, 109–113]. Hormonal imbalance in diabetes, marked by elevated levels of angiotensin II, catecholamines, and endothelins, plays a significant role in promoting oxidative stress and cardiac dysfunction [13, 114]. Damaged MT [13, 115, 116] and advanced protein glycation, caused by hyperglycemia, are also known to result in the development of oxidative stress in chronic diabetes [117, 118]. Initially, cardiomyocytes cope with increased oxidative stress by boosting their natural antioxidant defenses. Elevated activities of superoxide dismutase, glutathione peroxidase, and other antioxidant enzymes have been reported in the diabetic rat heart [119–121]. Most likely, these initial compensatory mechanisms are eventually exhausted, resulting in oxidative stress and leading to subcellular remodeling and myocardial cell damage [13]. Some reports have indicated that antioxidants are effective in preventing diabetic cardiomyopathy and cardiac dysfunction [13, 122, 123] and thus provide evidence regarding the relevance of oxidative stress in the pathophysiology of diabetic cardiomyopathy (Fig. 2).
5 Conclusions
From the foregoing discussion, it is apparent that a defect occurs in the process of energy production that leads to depression of ATP stores in the diabetic heart, primarily because of a shift in the balance between the utilization of glucose and the utilization of FFA as substrates by the myocardium. Excessive utilization of FFA for a prolonged period is considered to impair MT function with respect to oxidative phosphorylation and the electron-transport system. MT defects also includes opening of MT pores for leakage of cytoplasmic proteins that lead to the development of myocardial cell damage in the form of diabetic cardiomyopathy. Abnormalities in myocardial metabolism also promotes the occurrence of oxidative stress and intracellular Ca2+ overload, which results in the activation of different proteases and defects in gene expression in the diabetic myocardium. During this process of subcellular remodeling caused by diabetes, MF become defective in respect to their ability to utilize ATP as well as sensitivity to Ca2+ for the generation of contractile force. Thus, not only is cardiac dysfunction the result of defects in energy production by MT, but also defects in energy utilization by MF play a critical role during the development of diabetic cardiomyopathy.
References
Wild S, Roglic G, Green A et al (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Regan TJ, Ahmed S, Haider B et al (1994) Diabetic cardiomyopathy: experimental and clinical observations. N Engl J Med 91:776–778
Notkins AL (1979) The causes of diabetes. Sci Am 241(5):62–73
Christlieb AR (1973) Diabetes and hypertensive vascular disease. Am J Cardiol 32:592–606
Regan TJ (1983) Congestive heart failure in the diabetic. Annu Rev Med 34:161–168
Kannel WB, McGee DL (1979) Diabetes and cardiovascular disease. JAMA 241:2035–2038
Shah S (1980) Cardiomyopathy in diabetes mellitus. Angiology 31:502–504
Factor SM, Okun EM, Minase T (1980) Capillary microaneurysms in the human diabetic heart. N Engl J Med 302:384–388
Dhalla NS, Pierce GN, Innes IR, Beamish RE (1985) Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1:263–281
Garcia MJ, McNamara PM, Gordon T, Kannel WB (1974) Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes 23:105–111
Fein FS, Sonnenblick EH (1985) Diabetic cardiomyopathy. Prog Cardiovasc Dis 25:255–270
Rubler S, Dlugash J, Yuceoglu YZ et al (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol 30:595–602
Dhalla NS, Liu X, Panagia V et al (1998) Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res 40:239–247
Ahmed SS, Jaferi G, Narang RM, Regan TJ (1975) Preclinical abnormality of left ventricular function in diabetes mellitus. Am Heart J 89:153–158
Boudina S, Albel ED (2007) Diabetic cardiomyopathy revisited. Circulation 115:3213–3223
Fein FS, Malhotra A, Miller GB et al (1984) Diabetic cardiomyopathy in rats: mechanical and biochemical response to different insulin doses. Am J Physiol 247:H817–H823
Fein FS, Strobeck JE, Malhotra A, Scheuer J (1981) Reversibility of diabetic cardiomyopathy with insulin in rats. Circ Res 49:1251–1261
Ganguly PK, Pierce GN, Dhalla KS, Dhalla NS (1983) Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am J Physiol 244:E528–E535
Pierce GN, Dhalla NS (1981) Cardiac myofibrillar ATPase activity in diabetic rats. J Mol Cell Cardiol 13:1063–1069
Vadlamudi RVSV, Rodgers RL, McNeill JH (1982) The effect of chronic alloxan- and streptozotocin-induced diabetes on isolated rat heart performance. Can J Physiol Pharmacol 60:902–911
Takeda N, Dixon IMC, Hata T et al (1996) Sequence of alterations in subcellular organelles during the development of heart dysfunction in diabetes. Diabetes Res Clin Pract 30(Suppl):S113–S122
Factor SM, Minase T, Sonnenblick EH (1980) Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am Heart J 99:446–458
Factor SM, Minase T, Bhan R et al (1983) Hypertensive diabetic cardiomyopathy in the rat: ultrastructural features. Virchows Arch 398:305–317
Dhalla NS, Rangi S, Zieroth S, Xu YJ (2012) Alterations in sarcoplasmic reticulum and mitochondrial functions in diabetic cardiomyopathy. Exp Clin Cardiol 17:115–120
Dhalla NS, Takeda N, Rodriguez-Leyva D, Elimban V (2013) Mechanisms of subcellular remodeling in heart failure due to diabetes. Heart Fail Rev. doi:10.1007/s10741-013-9385-8
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Phys Rev 85:1093–1129
Dhalla NS, Das PK, Sharma GP (1978) Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 10:363–385
Rodrigues B, Cam MC, McNeill JH (1995) Myocardial substrate metabolism: implications in diabetic cardiomyopathy. J Mol Cell Cardiol 27:169–179
Kuo TH, Moore KH, Giacomelli F et al (1983) Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes 32:781–787
Pierce GN, Dhalla NS (1985) Heart mitochondrial function in chronic experimental diabetes in rats. Can J Cardiol 1:48–54
Flarsheim CE, Grupp IL, Matlib MA (1996) Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Am J Physiol Heart Circ Physiol 271:H192–H202
Dillman WH (1989) Diabetes and thyroid-hormone-induced changes in cardiac function and their molecular basis. Annu Rev Med 40:373–394
Malhotra A, Sanghi V (1997) Regulation of contractile proteins in diabetic heart. Cardiovasc Res 34:34–40
Machackova J, Barta J, Dhalla NS (2005) Molecular defects in cardiac myofibrillar proteins due to thyroid hormone imbalance and diabetes. Can J Physiol Pharmacol 83:1071–1091
Müller AL, Dhalla NS (2012) Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 17:395–409
Dillmann WH (1980) Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes 29:579–582
Pierce GN, Dhalla NS (1985) Mechanisms of the defect in cardiac myofibrillar function during diabetes. Am J Physiol 248:E170–E175
Liu X, Takeda N, Dhalla NS (1997) Myosin light-chain phosphorylation in diabetic cardiomyopathy in rats. Metabolism 46:71–75
Liu X, Takeda N, Dhalla NS (1996) Troponin I phosphorylation in heart homogenate from diabetic rat. Biochim Biophys Acta 1316:78–84
Afzal N, Pierce GN, Elimban V et al (1989) Influence of verapamil on some subcellular defects in diabetic cardiomyopathy. Am J Physiol 256:E453–E458
Rupp H, Elimban V, Dhalla NS (1989) Diabetes-like action of intermittent fasting on sarcoplasmic reticulum Ca2+-pump ATPase and myosin isoenzymes can be prevented by sucrose. Biochem Biophys Res Commun 164:319–325
Golfman L, Dixon IMC, Takeda N, Chapman D, Dhalla NS (1999) Differential changes in cardiac myofibrillar and sarcoplasmic reticular gene expression in alloxan-induced diabetes. Mol Cell Biochem 200:15–25
Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N et al (2000) Streptozotocin-induced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol 32:985–996
Dillmann WH (1982) Influence of thyroid hormone administration on myosin ATPase activity and myosin isoenzyme distribution in the heart of diabetic rats. Metabolism 31:199–204
Malhotra A, Penpargkul S, Fein FS et al (1981) The effect of streptozotocin-induced diabetes in rats on cardiac contractile proteins. Circ Res 49:1243–1250
Malhotra A, Mordes JP, McDermott L, Schaible TF (1985) Abnormal cardiac biochemistry in spontaneously diabetic Bio-Breeding/Worcester rat. Am J Physiol 249:H1051–H1055
Schaffer SW, Mozaffari MS, Artman M, Wilson GL (1989) Basis for myocardial mechanical defects associated with noninsulin-dependent diabetes. Am J Physiol 256:E25–E30
Takeda N, Nakamura I, Hatanaka T, Ohkubo T, Nagano M (1988) Myocardial mechanical and myosin isoenzyme alterations in streptozotocin-diabetic rats. Jpn Heart J 29:455–463
Mercadier JJ, Bouveret P, Gorza L et al (1983) Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res 53:52–62
Caforio ALP, Grazzini M, Mann JM et al (1992) Identification of α- and β-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation 85:1734–1742
Sweeney HL, Stull JT (1986) Phosphorylation of myosin in permeabilized mammalian cardiac and skeletal muscle cells. Am J Physiol 250:C657–C660
Ali MAM, Schulz R (2009) Activation of MMP-2 as a key event in oxidative stress injury to the heart. Front Biosci 14:699–716
Huang Y, Wang KK (2001) The calpain family and human disease. Trends Mol Med 7:355–362
Goll DE, Thompson VF, Li H et al (2003) The calpain system. Physiol Rev 83:731–801
Spinale FG (2007) Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 87:1285–1342
Schulz R (2007) Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 47:211–242
Müller AL, Hryshko LV, Dhalla NS (2013) Extracellular and intracellular proteases in cardiac dysfunction due to ischemia–reperfusion injury. Int J Cardiol 164:39–47
Müller AL, Freed D, Hryshko L, Dhalla NS (2012) Implications of protease activation in cardiac dysfunction and development of genetic cardiomyopathy in hamsters. Can J Physiol Pharmacol 90:995–1004
Kandasamy AD, Chow AK, Ali MAM, Schulz R (2010) Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85:413–423
León H, Baczkó I, Sawicki G et al (2008) Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte. Br J Pharmacol 153:676–683
Singh RB, Dhalla NS (2010) Ischemia-reperfusion-induced changes in sarcolemmal Na+/K+-ATPase are due to the activation of calpain in the heart. Can J Physiol Pharmacol 88:388–397
Singh RB, Hryshko L, Freed D, Dhalla NS (2012) Activation of proteolytic enzymes and depression of the sarcolemmal Na+/K+-ATPase in ischemia-reperfused heart may be mediated through oxidative stress. Can J Physiol Pharmacol 90:249–260
Müller AL, Freed D, Dhalla NS (2013) Activation of proteases and changes in Na+-K+-ATPase subunits in hearts subjected to ischemia-reperfusion. J Appl Physiol 114:351–360
Malhotra A, Lopez MC, Nakouzi A (1995) Troponin subunits contribute to altered myosin ATPase activity in diabetic cardiomyopathy. Mol Cell Biochem 151:165–172
Dhalla NS, Elimban V, Rupp H (1992) Paradoxical role of lipid metabolism in heart function and dysfunction. Mol Cell Biochem 116(1–2):3–9
Mazumder PK, O’Neill BT, Roberts MW et al (2004) Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 53:2366–2374
Herrero P, Peterson LR, McGill JB et al (2006) Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol 47:598–604
Berger J, Biswa C, Vicario PP et al (1989) Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature 340:70–72
Camps M, Castello A, Munoz P et al (1992) Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J 282:765–772
Dahl J, Herman WH, Hicks RJ et al (1993) Myocardial glucose uptake in patients with insulin-dependent diabetes mellitus assessed quantitatively by dynamic positron emission tomography. Circulation 88:395–404
Sivitz WI, DeSautel SL, Kayano T et al (1989) Regulation of glucose transporter messenger RNA in insulin-deficient states. Nature 340:72–74
Stanley WC, Lopaschuk GD, McCormack JG (1997) Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res 34:25–33
Dhalla NS, Elimban V (2013) Mechanisms of sarcolemmal defects in cardiac dysfunction due to chronic diabetes. In: Kimchi A (ed) Proceedings of the 17th world congress on heart disease, Medimond, Bologna (in press)
Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L et al (2010) Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J 31:100–111
Osborn BA, Daar JT, Laddaga RA, Romano FD, Paulson DJ (1997) Exercise training increases sarcolemmal GLUT-4 protein and mRNA content in diabetic heart. J Appl Physiol 82:828–834
Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F (2009) SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res 84:111–118
Kaestner KH, Flores-Riveros JR, McLenithan JC et al (1991) Transcriptional repression of the mouse insulin-responsive glucose transporter (GLUT4) gene by cAMP. Proc Natl Acad Sci U S A 88:1933–1937
Kainulainen H, Breiner M, Schurmann A et al (1994) In vivo glucose uptake and glucose transporter proteins GLUT1 and GLUT4 in heart and various types of skeletal muscle from streptozotocin-diabetic rats. Biochim Biophys Acta 1225:275–282
Stroedter D, Schmidt T, Bretzel RG, Federlin K (1995) Glucose metabolism and left ventricular dysfunction are normalized by insulin and islet transplantation in mild diabetes in the rat. Acta Diabetol 32:235–243
Randle PJ, Garland PB, Hales CN et al (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Finck BN, Kelly DP (2002) Peroxisome proliferator-activated receptor alpha (PPARalpha) signalling in the gene regulatory control of energy metabolism in the normal and diseased heart. J Mol Cell Cardiol 34:1249–1257
Banke NH, Wende AR, Leone TC et al (2010) Preferential oxidation of triacylglyceride-derived fatty acids in heart is augmented by the nuclear receptor PPAR alpha. Circ Res 107:233–241
Duncan JG, Fong JL, Medeiros DM et al (2007) Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115:909–917
Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A et al (2002) The cardiac phenotype induced by PPAR-alpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109:121–130
Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J Hypertens 18:655–673
Schaffer SW (1991) Cardiomyopathy associated with non-insulin dependent diabetes. Mol Cell Biochem 107:1–20
Yagyu H, Chen G, Yokoyama M et al (2003) Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 111:419–426
Dhalla NS, Golfman LS, Elimban V, Takeda N (1996) Remodelling of subcellular organelles during the development of diabetic cardiomyopathy. In: Chatham JC, Fodder JR, McNeill JH (eds) The heart in diabetes. Kluwer, Boston, pp 100–142
Penpargkul S, Fein F, Sonnenblick EH, Scheuer J (1981) Depressed cardiac sarcoplasmic reticular function from diabetic rats. J Mol Cell Cardiol 13:303–309
Lopaschuk GD, Tahiliani AG, Vadlamudi RV, Katz S, McNeill JH (1984) Cardiac sarcoplasmic reticulum function in insulin- or carnitine-treated diabetic rats. Am J Physiol 245:H969–H976
Lopaschuk GD, Katz S, McNeill JH (1984) The effect of alloxan- and streptozotocin-induced diabetes on calcium transport in rat cardiac sarcoplasmic reticulum. The possible involvement of long chain acylcarnitines. Can J Physiol Pharmacol 61:439–448
Lopaschuk GD, Eibschutz B, Katz S, McNeill JH (1984) Depression of calcium transport in sarcoplasmic reticulum from diabetic rats: lack of involvement by specific regulatory mediators. Gen Pharmacol 15:1–5
Yu Z, Tibbits GF, McNeill JH (1994) Cellular functions of diabetic cardiomyocytes: contractility, rapid-cooling contracture, and ryanodine binding. Am J Physiol 266:H2082–H2089
Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW et al (2002) Defective intracellular Ca2+ signaling contributes to cardiomyopathy in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 283:H1398–H1408
Yaras N, Bilginoglu A, Vassort G, Turan B (2007) Restoration of diabetes-induced abnormal local Ca2+ release in cardiomyocytes by angiotensin II receptor blockade. Am J Physiol Heart Circ Physiol 292:H912–H920
Lagadic-Gossmann D, Buckler KJ, Le Prigent K, Feuvray D (1996) Altered Ca2+ handling in ventricular myocytes isolated from diabetic rats. Am J Physiol 270:H1529–H1537
Ishikawa T, Kajiwara H, Kurihara S (1999) Alterations in contractile properties and Ca2+ handling in streptozotocin-induced diabetic rat myocardium. Am J Physiol 277:H2185–H2194
Allo SN, Lincoln TM, Wilson GL, Green FJ, Watanabe AM, Schaffer SW (1991) Non-insulin-dependent diabetes-induced defects in cardiac cellular calcium regulation. Am J Physiol 260:C1165–C1171
Ligeti L, Szenczi O, Prestia CM, Szabo C, Horvath K, Marcsek ZL et al (2006) Altered calcium handling is an early sign of streptozotocin-induced diabetic cardiomyopathy. Int J Mol Med 17:1035–1043
Dhalla NS, Pierce GN, Panagia V et al (1982) Calcium movements in relation to heart function. Basic Res Cardiol 77:117–139
Dhalla NS, Wang X, Beamish RE (1996) Intracellular calcium handling in normal and failing hearts. Exp Clin Cardiol 1:7–20
Afzal N, Ganguly PK, Dhalla KS et al (1988) Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes 37:936–942
Liu X, Suzuki H, Sethi R et al (2006) Blockade of the renin-angiotensin system attenuates sarcolemma and sarcoplasmic reticulum remodeling in chronic diabetes. Ann N Y Acad Sci 1084:1141–1154
Frank RN (1991) On the pathogenesis of diabetic retinopathy. A 1990 update. Ophthalmology 98:586–593
Wohaieb SA, Godin DV (1987) Alterations in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rat. Effects of insulin treatment. Diabetes 36:1014–1018
Thompon KH, Godin DV, Lee M (1992) Tissue antioxidant status in streptozotocin-induced diabetes in rats. Effects of dietary manganese deficiency. Biol Trace Elem Res 35:213–224
Giugliano D, Ceriello A, Paolisso G (1995) Diabetes mellitus, hypertension and cardiovascular disease: which role for oxidative stress? Metabolism 44:363–368
Fonseca VA, Stone A, Munshi M et al (1997) Oxidative stress in diabetic macrovascular disease: does homocysteine play a role? South Med J 90:903–906
Baynes JW, Thorpe SR (1999) Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9
Giardino I, Fard AK, Hatchell DL et al (1998) Aminoguanidine inhibits reactive oxygen species formation, lipid peroxidation, and oxidant-induced apoptosis. Diabetes 47:1114–1120
Ohuwa T, Sato Y, Naoi M (1995) Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci 56:1789–1798
Boudina S, Abel ED (2010) Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 11:31–39
Kowluru RA, Engerman RL, Kern TS (2000) Diabetes-induced metabolic abnormalities in myocardium: effect of antioxidant therapy. Free Radic Res 32:67–74
Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 10:339–359
Taniguchi N, Kaneto H, Asahi M et al (1996) Involvement of glycation and oxidative stress in diabetic macroangiopathy. Diabetes 45:S81–S83
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Brownlee M (1995) Advanced protein glycosylation in diabetes and aging. Annu Rev Med 46:223–234
Peiro C, Lafuente N, Matesanz N et al (2001) High glucose induces cell death of cultured human aortic smooth muscle cells through the formation of hydrogen peroxide. Br J Pharmacol 133:967–974
Kakkar R, Kalra J, Mantha V, Prasad K (1995) Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151:113–119
Kakkar R, Mantha SV, Kalra J, Prasad K (1996) Time course study of oxidative stress in aorta and heart of diabetic rat. Clin Sci 91:441–448
Mak DHF, Ip SP, Li PC, Poon MKT, Ko KM (1996) Alterations in tissue glutathione antioxidant system in streptozotocin-induced diabetic rats. Mol Cell Biochem 162:153–158
Ye G, Metreveli NS, Donthi RV et al (2004) Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes 53:1336–1343
Cai L, Wang Y, Zhou G et al (2006) Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol 48:1688–1697
Acknowledgments
The work referred in this article was supported by the infrastructure provided by the St. Boniface Hospital Research Foundation. A.H. Cunha-Volpato was supported by the National Council for Scientific and Technological Development (CNPq) of Brazil.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Dhalla, N.S., Cunha-Volpato, A.H., Xu, YJ. (2014). Abnormalities in ATP Production and Utilization in Diabetic Cardiomyopathy. In: Turan, B., Dhalla, N. (eds) Diabetic Cardiomyopathy. Advances in Biochemistry in Health and Disease, vol 9. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9317-4_18
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9317-4_18
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9316-7
Online ISBN: 978-1-4614-9317-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)