Abstract
Tumor cells employ multiple elaborate, evolutionarily conserved mechanisms that enable them to respond to stress conditions in the tumor microenvironment, including hypoxia. Although the most studied cellular signaling pathway induced by hypoxia is mediated by the transcriptional activity of hypoxia-inducible factors (HIFs), several HIF-independent mechanisms have been implicated in hypoxic adaptation, especially in the regulation of macromolecular synthesis. One such mechanism, known as the unfolded protein response (UPR), encompasses a trio of cellular signaling cascades. The UPR is activated by the accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (ER). Numerous in vitro and in vivo studies using genetic and pharmacological modifications of UPR signaling components have demonstrated an important role for the UPR in determining tumor cell survival following transient and chronic hypoxia. This review summarizes the important aspects of UPR signaling and the role of the UPR in determining tumor cell survival or death under hypoxic stress. We also discuss novel pharmacological approaches for targeting critical UPR components as potential anti-tumor strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Unfolded protein response
- Hypoxia
- PERK
- ATF4
- IRE1
- ATF6
- Translation initiation factor
- Autophagy
- GCN2
- Hypoxia tolerance
1 Introduction
A common characteristic of most solid tumors is the presence of hypoxic regions, where the oxygen concentration can be as low as 0.01 %, while oxygen levels in normal tissue range from 3.1 to 8.7 % (Kizaka-Kondoh et al. 2003). Chronic hypoxia occurs when the tumor outgrows the existing vasculature, increasing the distance oxygen must diffuse to reach portions of the tumor. Tumors can also experience acute, or intermittent, hypoxia, which is caused by abnormal blood flow dynamics in the newly forming vessels (Kizaka-Kondoh et al. 2003). Tumor cells can survive adverse hypoxic conditions for extended periods of time depending on factors such as expression of anti-apoptotic genes, mutations in proapoptotic genes, and activation of prosurvival programs. Clinically, hypoxic tumors are more resistant to both chemotherapy, due to limited drug diffusion and cell-cycle dysregulation, and radiation therapy, due to chemical repair of DNA damage produced by free radicals (Brown and Wilson 2004). Moreover, the metastatic potential of cancer cells has been shown to be increased by hypoxia (Brown 1990; Cairns and Hill 2004; Chang et al. 2011). It is not surprising then that hypoxia has been associated with poor local tumor control and reduced overall survival (Höckel and Vaupel 2001; Vaupel et al. 2001; Koumenis 2006).
Cells adapt to hypoxic stress using hypoxia-inducible factor (HIF)-dependent and HIF-independent pathways (Koumenis 2006; Bertout et al. 2008; Semenza 2009). The HIF-dependent pathway regulates the expression of proteins important for anaerobic glycolysis, angiogenesis, and cell survival and is discussed at greater length in Chap. 2. HIF-independent effects are designed to curtail oxygen consumption by energy-expensive processes, such as DNA replication and protein synthesis. In particular, the unfolded protein response (UPR) is upregulated by hypoxia to decrease global messenger RNA (mRNA) translation (Koumenis 2006) .
2 Overview of the UPR
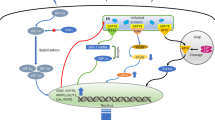
The UPR is a coordinated cellular program induced by cells to adapt to transient and chronic endoplasmic reticulum (ER) stress caused by factors such as unfolded proteins, hypoxia, nutrient deprivation, and oncogenic transformation (Kaufman 2002; Schönthal 2012). Induction of the UPR relies on three ER transmembrane proteins: PKR-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1), and activating transcription factor 6 (ATF6) (Fig. 10.1) . During unstressed conditions, these signaling transducers are maintained in an inactive state by an ER lumenal chaperone protein called GRP78 (glucose-regulated protein, 78 kDa; also known as BiP). When misfolded proteins begin to accrue, GRP78 dissociates from PERK, IRE1, and ATF6 to aid in protein folding and degradation, which allows the activation of these three proteins (Bertolotti et al. 2000; Shen et al. 2002). Activation of PERK involves homodimerization and transautophosphorylation. One major target of PERK is eukaryotic translation initiation factor 2 α-subunit (eIF2α) (Harding et al. 1999; Harding et al. 2000b). Normally, eIF2α binds guanosine triphosphate (GTP) as part of the initiation of cap-dependent translation . To begin the elongation phase of translation, eIF2α-GTP is hydrolyzed to eIF2α-guanosine diphosphate (GDP), which then disassociates from the ribosome. When PERK phosphorylates eIF2α at Ser51, the exchange of GDP for GTP is inhibited. This leads to a global reduction in translation, preventing further accumulation of unfolded proteins in the ER. Paradoxically, the translation of a few mRNAs is substantially elevated under these conditions. The most extensively studied of these mRNAs is that encoding activating transcription factor 4 (ATF4) (Shi et al. 1998; Harding et al. 2000a; Vattem and Wek 2004) . ATF4 is involved in the transcription of genes encoding proteins that assist the cell in neutralizing the stress, such as chaperones (GRP78) and proteins important for amino acid metabolism (asparagine synthetase (ASNS), glycine transporter 1, and several transfer RNA (tRNA) synthetases), redox homeostasis (cystathionine γ-lyase and heme oxygenase 1), angiogenesis (vascular endothelial growth factor A, angiogenin, and fibroblast growth factor 2), and autophagy (unc-51-like kinase 1 (ULK1), beclin 1, and microtubule-associated protein 1 light chain 3 beta (LC3B)) (He et al. 2001; Harding et al. 2003; Pereira et al. 2010; Rouschop et al. 2010; Rzymski et al. 2010; Avivar-Valderas et al. 2011; Dickhout et al. 2012; Pike et al. 2013) . ATF4 also upregulates the transcription of C/EBP homologous protein (CHOP), a proapoptotic protein that will be discussed in more detail later (Friedman 1996; Harding et al. 2000a) .
Consequences of the activation of the unfolded protein response (UPR) following hypoxia. Hypoxia-induced ROS disrupt proper protein folding, resulting in an accumulation of unfolded proteins in the endoplasmic reticulum (ER). ER stress is detected by three transmembrane UPR sensors—PERK, IRE1, and ATF6—by disassociation of the chaperone GRP78/BiP. Activation of the UPR leads to the upregulation of protein chaperones and other proteins important for neutralizing the cellular stress. Hypoxia-induced UPR signaling also mediates a cytoprotective autophagy response. However, chronic activation of PERK and IRE1 following hypoxia can lead to cellular apoptosis by various mechanisms
The second branch of the UPR is mediated by IRE1. Like PERK, dissociation of GRP78 allows IRE1 homodimerization and autophosphorylation (Bertolotti et al. 2000) . Activated IRE1 also possesses endonuclease activity, which processes unspliced X-box binding protein 1 (XBP1) mRNA by removing an inhibitory intron (Tirasophon et al. 1998; Yoshida et al. 2001). The spliced mRNA can then be translated to the functional XBP1-s protein, a transcription factor important for the upregulation of genes involved in protein folding and disulfide bond formation (protein disulfide isomerase-P5 and protein kinase inhibitor p58) and ER-associated protein degradation (ER degradation enhancer mannosidase alpha-like 1 (EDEM) and homocysteine-induced ER protein) (Yoshida et al. 2001; Calfon et al. 2002; Lee et al. 2003a; Yamamoto et al. 2004).
ATF6 regulates the third branch of the UPR. Unlike PERK and IRE1, when ATF6 is released from GRP78 binding, it translocates to the Golgi apparatus (Chen et al. 2002) . Once in the Golgi, two resident proteases, S1P and S2P, cleave ATF6 to release the cytosolic transcription factor domain (Ye et al. 2000). This activated ATF6 domain then translocates to the nucleus, where it stimulates the transcription of XBP1 and genes encoding protein chaperones (GRP78, GRP94, and calreticulin) (Haze et al. 1999; Yoshida et al. 2001; Okada et al. 2002) .
Cells also cope with ER stress by activating ER-associated degradation (ERAD). ERAD is a quality control pathway which allows cells to eliminate, as well as degrade, any terminally misfolded proteins from the ER and avoid proteotoxicity. The process of ERAD is regulated via two regulatory proteins in the lumen of the ER, namely EDEM and Yos9p. These proteins can recognize the irreversibly misfolded protein and target it for degradation (Smith et al. 2011; Walter and Ron 2011) the 26S proteasome in the cytosol with the help of critical ER membrane proteins Hrd1p and Hrd3p (hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase degradation proteins), which possess really interesting new gene (RING) domains as present in various ubiquitin ligases (Bays et al. 2001).
UPR activation leads to an increase in oxidative stress due to an increase in protein folding—a process further accentuated by ER oxidoreductase 1α (ERO1α) (Walter and Ron 2011). ERO1α oxidizes protein disulfide isomerase (PDI), which introduces new disulfide bonds in nascent or misfolded proteins to reduce ER load. Along with oxidizing PDI, ERO1α reduces oxygen to form H2O2, a primary causative agent for oxidative stress (Frand and Kaiser 1999; Ron and Walter 2007). However, PERK can neutralize the increasing oxidative nature of the ER lumen to maintain ER homeostasis by directly phosphorylating and activating Nrf2 (nuclear factor, erythroid 2-like 2), a transcription factor which induces antioxidant genes (Cullinan et al. 2003) .
3 Activation of the UPR and the Integrated Stress Response by Hypoxia and Other Stresses
Although the mechanism is not well understood, hypoxia is thought to activate the UPR by disrupting the redox balance of cells. The ER contains a variety of chaperones that assist in the folding of nascent polypeptides. In budding yeast, the enzyme Ero1p initiates polypeptide folding by oxidizing PDI, which then directly interacts with client proteins to promote proper disulfide bond formation (Tu and Weissman 2004). Importantly, this cascade of folding events requires molecular oxygen as the final electron acceptor (Tu and Weissman 2004) . Thus, chaperone activity is compromised under hypoxia, leading to an accumulation of unfolded proteins. Although a similar pathway has not been formally identified in mammals, it is hypothesized that chaperone activity in mammalian cells is also oxygen dependent.
Hypoxia has also been shown to activate the UPR through the generation of reactive oxygen species (ROS) . Liu and colleagues demonstrated that exposure of cells to the reactive oxygen species H2O2 results in phosphorylation of PERK and eIF2α (Liu et al. 2008) . Treatment with catalase diminishes hypoxia-induced phosphorylation of eIF2α, indicating that H2O2 generated under hypoxia contributes toward activation of the UPR (Liu et al. 2008). Future studies are required to determine the mechanism through which ROS disrupt protein folding .
In addition to hypoxia, the tumor microenvironment is often characterized by shortages of other nutrients such as glucose and amino acids. One pathway tumor cells utilize to cope with these stressors is the integrated stress response (ISR). The ISR consists of four kinases, including PERK. The three remaining kinases are general control nonderepressible 2 (GCN2), RNA-dependent protein kinase (PKR), and heme-regulated inhibitor (HRI) (Wek et al. 2006) . The GCN2 arm of the ISR is particularly important for tumor cells to respond to nutrient stress (Ye et al. 2010). Under conditions of amino acid deprivation, uncharged tRNAs accumulate in cells. GCN2 binds to uncharged tRNAs, causing it to undergo a conformational change and become catalytically active (Wek et al. 1989; Padyana et al. 2005). Activated GCN2 phosphorylates eIF2α to attenuate translation to conserve amino acids (Harding et al. 2000a). Additionally, upregulation of ATF4 by GCN2-mediated eIF2α phosphorylation initiates a transcriptional program to induce genes such as amino acid biosynthetic enzymes and amino acid transporters to promote recovery from nutrient stress (Harding et al. 2003) . GCN2 is also activated under conditions of glucose deprivation because cells consume amino acids as an energy source when glucose is unavailable (Ye et al. 2010) . Interestingly, PERK also contributes to ISR activation under glucose deprivation. Glycosylation is one important protein modification that occurs in the ER. If glucose is unavailable, glycosylation is reduced and unmodified proteins accumulate in the ER and activate the UPR (Lee 1992). Thus, the glycosylation inhibitor tunicamycin is frequently used as an experimental inducer of the UPR in the laboratory .
It should be noted that although hypoxia and nutrient deprivation are major sources of noncell autonomous activation of the UPR, cell intrinsic stresses can also induce this pathway. Our lab has recently identified the c-Myc oncogene as a cell autonomous activator of the UPR in cancer cells (Hart et al. 2012). As one of its many functions as a transcription factor, c-Myc upregulates genes involved in ribosome biogenesis (Meyer and Penn 2008). Oncogenic activation of c-Myc places increased demands on the protein synthetic machinery of a cell, resulting in dependence on the UPR to manage the folding of a large number of client proteins in the ER. Activation of the UPR is required for c-Myc-dependent tumorigenesis, as inhibition of this pathway through genetic or pharmacological means greatly reduces tumor cell viability in vitro and compromises the ability of cells to form tumors in vivo (Hart et al. 2012) .
4 Evidence of In Vivo Activation of the UPR and ISR in Human Tumors
A wealth of clinical data has established that all three branches of the UPR are activated in human tumors. Evidence for PERK pathway activation under hypoxia comes from cervical carcinoma samples. Here, high levels of ATF4 and CHOP colocalize with staining for pimonidazole, a hypoxia-sensitive dye (Bi et al. 2005) . It has also been demonstrated in breast carcinoma that ATF4 expression is increased in areas of necrosis known to be anoxic (Ameri et al. 2004). Additionally, ATF4 overexpression has been found in brain, breast, cervical, and skin cancers as compared to normal tissue controls (Bi et al. 2005). Activation of the IRE1 arm of the UPR has been found in lymphoma, liver, and breast cancer patient samples . In B-cell lymphoma and liver cancer, increased levels of spliced XBP1 are found in malignant tissue as compared to adjacent normal tissue, while levels of total XBP1 are elevated in breast cancer (Fujimoto et al. 2003; Shuda et al. 2003; Hart et al. 2012). Hepatocellular carcinoma samples also express higher levels of ATF6 mRNA than normal tissue (Shuda et al. 2003). Additionally, the increased expression of the ER chaperone GRP78 is found in a variety of tumor types, including stomach, breast, and lung cancer, relative to normal tissue controls (Fernandez et al. 2000; Uramoto et al. 2005; Wang et al. 2005; Zheng et al. 2008) .
4.1 GRP78, Hypoxia, and Tumor Progression
Several models have now been developed to better understand the consequences of UPR inhibition in tumor cells. One of the first components of the UPR studied in vivo was GRP78. Previous work in cell culture demonstrated that knocking down GRP78 sensitized tumor cells to hypoxia (Koong et al. 1994). Jamora and colleagues extended these studies to an animal model by demonstrating that B/C10ME fibrosarcoma cells expressing an anti-sense construct against GRP78 failed to form tumors as efficiently as their wild-type counterparts. When injected subcutaneously into mice, these cells either completely failed to form tumors or formed tumors that regressed after a period of time (Jamora et al. 1996).
4.2 The PERK Arm of the UPR in Malignancy
Perhaps one of the most extensively studied arms of the UPR in vivo is the PERK pathway. In cell culture, PERK-/- mouse embryonic fibroblasts (MEFs) fail to phosphorylate eIF2α in response to hypoxia and exhibit reduced clonogenic survival after hypoxic stress (Bi et al. 2005) . When Ras-transformed PERK-/- MEFs are subcutaneously injected into mice, they grow more slowly and exhibit lower levels of p-eIF2α than PERK +/+ cells (Bi et al. 2005). Additionally, PERK-/- tumors have smaller areas of hypoxia that overlap with areas of apoptosis, indicating that the PERK arm of the UPR supports tumor cell survival under hypoxia in vivo (Bi et al. 2005). Similar results were found with HT29 human colorectal carcinoma cell lines stably expressing a dominant-negative PERK construct (Bi et al. 2005).
PERK has also been studied in genetic mouse models of cancer. Mammary carcinoma-prone MMTV-Neu transgenic mice crossed to PERKfl/fl/MMTV-Cre mice demonstrated that deletion of PERK in the mammary gland slows tumor growth, resulting in improved overall survival (Bobrovnikova-Marjon et al. 2010) . Mice with deletion of PERK in the mammary gland also have fewer lung metastases than their wild-type counterparts. Interestingly, MMTV-Neu/PERKΔ / Δ tumor cells have significantly higher levels of ROS and DNA damage, indicating that PERK can also promote tumor growth by regulating the redox balance of cells. However, this is a double-edged sword as MMTV-Neu/PERKΔ / Δ mice develop spontaneous mammary carcinomas as they age, due to an increased level of genomic instability (Bobrovnikova-Marjon et al. 2010).
Gupta and colleagues also characterized the role of PERK in a genetically engineered mouse model of insulinoma (Gupta et al. 2009). In this model, PERK knockout mice develop fewer and smaller insulinomas due to slower rates of proliferation rather than increased rates of apoptosis. Interestingly, PERK knockout mice also have more poorly vascularized tumors than wild-type mice. This observation corroborates research performed in vitro demonstrating that PERK regulates the angiogenic switch and controls the expression of VEGF and other proangiogenic factors (Ghosh et al. 2010; Wang et al. 2012). Thus, PERK may not only control tumor cells’ response to hypoxia through cell autonomous activation of the UPR but also through noncell autonomous mechanisms by controlling oxygen delivery to the tumor by promoting angiogenesis .
4.3 UPR and Tumor Immunity
The PERK arm of the UPR has also been shown to influence tumor growth by promoting an anti-tumor immune response. Particular kinds of stressors, such as ultraviolet C (UV-C) irradiation and anthracycline treatment, induce exposure of the ER chaperone calreticulin on the plasma membrane of cells (Obeid et al. 2007). Exposed calreticulin serves as an engulfment signal for dendritic cells, thus eliciting an immune response. Knocking down calreticulin blocks immunogenic cell death (Obeid et al. 2007). Furthermore, cells that lack ERp57, a disulfide isomerase required for calreticulin exposure, become resistant to chemotherapy because they are unable to elicit an immune response in the host (Panaretakis et al. 2008).
Stressors that induce calreticulin exposure have been shown to induce both PERK and eIF2α phosphorylation . Cells with PERK knockdown or a nonphosphorylatable knock-in mutation of eIF2α, in which serine 51 is converted to alanine, fail to expose calreticulin in response to stress (Panaretakis et al. 2009). However, knockdown of ATF6 or knockdown of IRE1 does not affect calreticulin exposure, indicating that this stress response is specific to the PERK arm of the UPR . Vaccination with CT26 murine colon cancer cells treated with chemotherapeutics that induce calreticulin exposure was able to prevent tumor growth in mice after a later challenge of live, untreated CT26 cells. This effect was attenuated with vaccination of chemotherapy-treated CT26 cells with PERK knockdown, demonstrating that PERK can also exert noncell autonomous effects on tumor growth through inducing an immune response to cancer cells (Panaretakis et al. 2009) .
5 Role of PERK Pathway Effectors in Hypoxic and Nutrient Stress Adaptation and Tumor Growth
The consequences of PERK pathway inhibition have also been studied at the level of eIF2α phosphorylation and ATF4 induction . Similar to PERK-/- MEFs, MEFs containing the eIF2α S51A knock-in mutation experience reduced clonogenic survival after exposure to extreme hypoxia (Bi et al. 2005). These results were recapitulated in vivo: Ras-transformed eIF2α S51A MEFs form much smaller tumors than their wild-type counterparts (Bi et al. 2005). Additionally, a large majority of apoptotic cells in eIF2α S51A tumors are found within hypoxic areas, while in wild-type tumors, apoptotic cells are mostly located within areas of necrosis. As expected, ATF4-/- MEFs experience increased levels of apoptosis under both moderate and severe hypoxia (Bi et al. 2005). In vivo, HT1080 human fibrosarcoma cells stably transfected with a short hairpin RNA (shRNA) against ATF4 form much smaller tumors than cells transfected with a nontargeting shRNA when injected subcutaneously into nude mice (Ye et al. 2010) .
However, not all effects of ATF4 on tumor growth can be attributed to PERK . As previously mentioned, GCN2, another kinase member of the ISR, phosphorylates eIF2α and induces ATF4 in response to amino acid deprivation . When grown in culture, HT1080 shATF4 cells exhibit increased apoptosis that is rescued by addition of the nonessential amino acid asparagine (Ye et al. 2010). This effect can be attributed to the ATF4 target gene ASNS, which transfers an amino group from glutamine to aspartate, forming asparagine. Indeed, overexpression of ASNS in HT1080 shATF4 cells partially rescues the ability of these cells to form tumors in mice (Ye et al. 2010). These results highlight the variety of stressors that exist in the tumor microenvironment and the necessity to study the interplay between various survival pathways in tumor cells.
6 The IRE1 and ATF6 Arms of the UPR in Hypoxic Adaptation and Tumor Progression
The IRE1 arm of the UPR has also been studied extensively in vivo. Work from the Koong lab was the first to establish that this pathway is required for tumor growth (Romero-Ramirez et al. 2004). MEFs lacking XBP1 have greatly reduced clonogenic survival after exposure to severe hypoxia. These cells also fail to form tumors when injected subcutaneously into mice. Tumors formed from XBP1 wild-type MEFs showed excellent colocalization of XBP1 with the hypoxia-sensitive dye EF5, indicating that XBP1 is expressed in hypoxic areas of tumors. Similar results were obtained with HT1080 cells with knockdown of XBP1 (Chen et al. 2005). Since XBP1 is required for plasma cell differentiation, it has been frequently studied in multiple myeloma (Iwakoshi et al. 2003). In fact, sustained expression of spliced XBP1 in the B-cell compartment of mice recapitulates the human disease (Carrasco et al. 2007). Lee and colleagues demonstrated that inhibition of XBP1 through RNA interference or expression of a dominant negative mutant rendered multiple myeloma cells sensitive to ER stress (Lee et al. 2003b) .
Finally, ATF6 also shows promise as an anti-tumor target, but it has not been as extensively characterized as the other members of the UPR. Work in HEp3 human epidermoid carcinoma cells shows that ATF6 is constitutively activated in dormant, but not tumorigenic cells (Schewe and Aguirre-Ghiso 2008). This effect is attributed to ATF6-dependent induction of Rheb (Ras homolog enriched in brain), which increases mechanistic target of rapamycin (mTOR) activity, thus promoting tumor survival during periods of dormancy. Mice injected with dormant HEp3 cells with shATF6 experienced increased tumor-free survival as compared to mice injected with dormant HEp3 cells with an empty vector control. Further studies are required to characterize the role that ATF6 plays in survival under hypoxia and the consequences of inhibition of ATF6 signaling .
7 The ISR: Players and Consequences of its Activation
PERK-mediated phosphorylation of eIF2α following hypoxia leads to preferential translation of ATF4, which ultimately leads to remediation of the stress condition or to apoptosis (Harding et al. 2003; Blais et al. 2004; Ron and Walter 2007) . The expression of the protein from the ATF4 mRNA following cellular stress is regulated by a translation control mechanism involving the 5′ untranslated region (UTR) . The 5′-UTR of ATF4 contains two upstream open reading frames (uORFs) that function differentially to regulate stress-specific enhancement of ATF4 expression (Fig. 10.1). The proximal uORF1, which is three amino acid residues in length, acts as a ‘positive element’ by promoting ribosome reinitiation at downstream start codons, while the distal uORF2, which overlaps the start codon, acts as a negative element for mRNA translation. During an abundance of oxygen supply, high eIF2-GTP levels cause ribosome reinitiation at uORF2, resulting in inhibition of ATF4 translation. However, hypoxia-mediated eIF2α phosphorylation reduces eIF2α-GTP levels, causing delayed ribosome reinitiation and bypass of the negative uORF2. As a result, the 40S and the 60S ribosomal subunits can reinitiate at the ATF4 start codon, leading to increased expression (Blais et al. 2004; Vattem and Wek 2004). Expression of ATF4 is also shown to be modulated by hypoxia through increased mRNA stability (Ameri and Harris 2008). It has been previously shown that several mRNAs that encode critical members of the ISR, including ATF4, are highly labile and undergo rapid degradation by a mechanism involving nonsense-mediated mRNA decay (NMD) (Gardner 2010). Hypoxia-induced eIF2α phosphorylation leads to inhibition of NMD—a mechanism which involves localization of important factors of the NMD machinery to cytoplasmic dense aggregates known as stress granules, which act as storehouses for several mRNAs as well as components of the translation preinitiation complex. This sequestration of the NMD machinery allows stabilization of ATF4 mRNA following hypoxic stress (Gardner 2008).
Hypoxic induction of ATF4 has been shown to be solely dependent on PERK-induced eIF2α phosphorylation, as PERK-/- and the eIF2α S51A knock-in mutant MEFs fail to induce ATF4 expression (Bi et al. 2005; Koumenis et al. 2007) . Induction of ATF4 following hypoxia and anoxia has been shown to be independent of HIF1 expression and involves a mechanism of protein stabilization by the oxygen sensor prolyl hydroxylase 3 (PHD3) (Ameri et al. 2004; Koditz et al. 2007). Once expressed, ATF4 homodimerizes or heterodimerizes with other basic leucine zipper domain (bZIP) transcription factors such as CCAAT-enhancer-binding protein β (C/EBPβ) to upregulate genes involved in remediation of oxidative stress, amino acid metabolism, differentiation, and hypoxic tolerance (Harding et al. 2003).
One of the genes that is transcriptionally upregulated by ATF4, is CHOP which has major implications in determining cellular fate (Fawcett et al. 1999; Harding et al. 2000a). CHOP transcriptionally activates several key genes that lead to cell death following chronic hypoxic ER stress. Analysis of human, as well as mouse, tumor tissues has shown colocalization of ATF4 and CHOP expression with hypoxic regions (Bi et al. 2005). Acute hypoxia has been shown to be a major inducer of CHOP (Bi et al. 2005). This induction of CHOP was ATF4-dependent, as silencing of ATF4 significantly reduces its induction. Expression of CHOP following induction of the UPR has also been shown to be under the transcriptional control of ATF6 (Ma et al. 2002) .
ATF3 is another member of the ATF/CREB family of transcription factors that has been shown to be overexpressed in various human tumors and mouse cancer models (Janz et al. 2006; Pelzer et al. 2006). Although the expression of ATF3 is thought to be primarily controlled by the binding of ATF4 to its promoter to upregulate its transcription, several studies have shown that non-ISR pathways including nuclear factor-kappa B (NF-κB), p53, and c-Jun N-terminal kinase (JNK) play important roles in the regulation of its expression (Thompson et al. 2009). Along with the above mentioned transcriptional control, ATF3 was also shown to be regulated by mRNA stabilization via a mechanism involving recruitment of RNA binding proteins to the 3′ untranslated region of the mRNA (Pan et al. 2005). However, the regulation of ATF3 following hypoxia has been debated. Several studies have shown that ATF3 is expressed only following anoxia but not during hypoxic conditions. Such expression of ATF3 following anoxia is independent of HIF1α and p53 and involves stabilization of the mRNA (Ameri et al. 2007). In contrast, circulating tumor cells (CTCs), which are associated with highly aggressive metastatic tumors, were highly hypoxic but had increased expression of ATF3 (Ameri et al. 2010).
Another transcriptional target of ATF4 that has been shown to be upregulated following nutrient stress (low amino acids and glucose) is ASNS . Intriguingly, increased ASNS expression has also been observed in hypoxic CTCs (Ameri et al. 2010). L-asparaginase is currently used as treatment in patients with childhood acute lymphoblastic leukemia and acute myeloblastic leukemia (Richards and Kilberg 2006). ATF4 transcriptionally regulates ASNS by binding to the C/EBP-ATF response elements (CAREs) in its promoter, which are comprised of two nutrient-sensing response elements (NSRE-I and NSRE-II) (Zhong et al. 2003). In response to ER stress, ATF4 binds to the NSREs, resulting in increased rate of ASNS transcription. However, with sustained ER stress, ATF3 and C/EBPβ replace ATF4 from the CARE elements, resulting in decreased promoter activity. This type of feedback inhibition following chronic ER stress is regarded as self-limiting regulation of ATF4 (Su and Kilberg 2008).
8 Hypoxia-Induced UPR and the Decision between Cell Survival and Apoptosis
Stimulation of prolonged ER stress causes apoptosis in cells via hyperactivation of CHOP and IRE1, in addition to other various mechanisms (Fig. 10.1) . CHOP interacts with liver inhibitory protein (LIP), an inhibitory isoform of C/EBPβ, following chronic ER stress. Interaction with LIP allows CHOP to translocate to the nucleus and repress transcription of BCL2 (Chiribau et al. 2010). BCL2 interacts with several proteins from the BH3 family, such as BAD, NOXA, and PUMA (BCL2-associated agonist of cell death, phorbol-12-myristate-13-acetate-induced protein 1, BCL2 binding component 3, respectively), and sequesters these factors from BAX (BCL2-associated X protein)/BAD-mediated permeabilization of the mitochondrial membrane, leading to increased mitochondrial apoptosis (Tabas and Ron 2011). CHOP can also induce apoptosis in a more direct mechanism by heterodimerizing with C/EBPα and binding to the BIM (BCL2-like 11 (apoptosis facilitator)) promoter to increase its expression (Puthalakath et al. 2007). Thus, decreased BCL2 and increased BIM expression following chronic ER stress contribute to increased cellular apoptosis. In contrast, hypoxia-mediated chronic activation of ATF4 is beneficial for cellular survival, and ablation of ATF4 results in increased tumor cell sensitivity in vitro and in vivo (Bi et al. 2005) . It is possible that the proapoptotic properties of CHOP may be modest compared to the much stronger anti-apoptotic activity of ATF4, which has multiple transcriptional targets including autophagy genes (see section on autophagy below) .
Chronic ER stress is often associated with increased oxidative stress due to an increase in aberrant misfolding of proteins in the lumen of the ER. An important factor that contributes to the hyperoxidation of the ER is ERO1α, which is transcriptionally upregulated by CHOP (Marciniak et al. 2004). The mechanism through which CHOP-activated ERO1α increases cell death is via activation of inositol triphosphate receptor 1(IP3R1), an ER calcium release channel (Li et al. 2009). Hypoxia causes rapid efflux of ER Ca2+ into the cytoplasm by a similar activation of IP3R1 as observed in PC12 rat adrenal pheochromocytoma cells and cerebellar Purkinje cells (Patterson et al. 2004). Increased cytoplasmic Ca2+ levels activate Ca2+/calmodulin-dependent protein kinase II (CaMKII), a calcium-activated kinase, which in turn upregulates NADPH oxidase subunit (NOX2). This ultimately leads to generation of ROS, leading to cell death (Tabas and Ron 2011). Chronic ER stress also contributes to upregulation (via ATF4 and CHOP) of death receptor 5 (DR5) and tribbles 3 (TRB3)—two factors with proapoptotic functions (Yamaguchi and Wang 2004; Ohoka et al. 2005). Finally, overexpression of the CHOP target GADD34 (growth arrest and DNA damage-inducible protein 34), a phosphatase cofactor responsible for dephosphorylating eIF2α, can result in premature resumption of protein synthesis, which, during extended periods of ER stress, has been suggested to cause cellular apoptosis due to overloading of the ER (Marciniak et al. 2004) .
Even though CHOP is often regarded as the primary factor responsible for inducing cellular apoptosis, other arms of the UPR, especially IRE1, have also been linked to cell death . The apoptotic properties of IRE1 have been mostly attributed to the fact that IRE1 interacts with tumor necrosis factor receptor-associated factor 2 (TRAF2), an adapter protein that can stimulate cell death (Urano et al. 2000). There has also been evidence from coimmunoprecipitation experiments showing that activated IRE1 can directly interact with BAK and BAX to stimulate mitochondria-induced apoptosis (Hetz et al. 2006). Finally, chronic ER stress has been shown to induce ER-associated mRNA degradation via the endoribonuclease activity of IRE1 (Hollien et al. 2009). Hyperactivation of the endonuclease function of IRE1 has been shown to induce apoptosis by a mechanism which has not been clearly explored (Han et al. 2009).
High levels of ATF6 have been detected in regions of ischemic tissue which have an activated UPR (Doroudgar et al. 2009) . Unlike the other two arms of the UPR, the role of ATF6 following chronic ER stress is not well understood. This is partly because ATF6 has been shown to regulate the induction of several cytoprotective chaperones, such as BiP, as well as apoptotic factors such as CHOP (Yoshida et al. 2000) .
9 Hypoxia-Induced UPR and Autophagy
Eukaryotic cells have evolved several adaptive pathways to cope with various environmental and intracellular stress conditions. One of these key pathways is autophagy—a self-eating catabolic process that involves the formation of double membrane vesicles known as autophagosomes, which engulf cellular organelles and mediate their lysosomal breakdown. Thus, autophagy acts as a major regulatory mechanism to sequester harmful components of the cells and to replenish essential components, maintaining cellular homeostasis. Autophagy has been shown to be upregulated in highly aggressive and metastatic tumors (Kenific et al. 2010; Ravikumar et al. 2010; Rubinsztein et al. 2012). It has been suggested that autophagy represents an important mechanism in tumor progression by enabling cancer cells to survive when they encounter prolonged metabolic and hypoxic stress (Rubinsztein et al. 2012). In fact, several clinical trials have been initiated to test if inhibitors of autophagy sensitize cancer cells to chemotherapeutic agents (Yang et al. 2011; Rubinsztein et al. 2012).
Even though autophagy was discovered in lower eukaryotes as a mechanism to cope with nutritional stress conditions primarily, the UPR has now also been linked to the autophagic process. Of the three branches of the UPR, the PERK pathway has been shown to be the primary inducer of autophagy (Fig. 10.1) (He and Klionsky 2009; Rouschop and Wouters 2009; Hart et al. 2012) . Cells which lack PERK or express mutant eIF2α (S51A) fail to induce a proper autophagic response. This inability to induce autophagy following chronic ER stress leads to increased cellular apoptosis (Ogata et al. 2006). Several studies suggest direct regulation of autophagy by UPR components that transcriptionally regulate several key autophagy-related genes (Atg). Hypoxia-mediated activation of the UPR leads to upregulation of LC3 and Atg5—a process under direct transcriptional regulation of ATF4 and CHOP (Rouschop et al. 2010) . Recently, ATF4 was also shown to transcriptionally upregulate ULK1, a serine/threonine kinase which acts upstream of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and induces formation of autophagosomes (Pike et al. 2012). Therefore, if global protein synthesis is downregulated following chronic hypoxic stress, the ISR allows the cells to preferentially upregulate genes required for autophagy, presumably to replenish cellular nutrient pools .
Enhanced tumorigenesis is often characterized by hyperactivation of oncogenes that upregulate cell cycle progression, proliferation, and protein synthesis. One such oncogene that is hyperactivated in cancers such as Burkitt’s lymphoma is c-Myc. Even though chronic hypoxia has been shown to block c-Myc-mediated gene transcription by a mechanism that involves HIF1α-mediated disruption of the active c-Myc/Max complex, transient hypoxia, followed by reoxygenation, leads to its activation (Huang 2008). As c-Myc enhances protein synthesis by increasing ribosome biogenesis, overexpression of c-Myc results in activation of the UPR and an accompanying PERK-mediated cytoprotective autophagy response (Hart et al. 2012). UPR-mediated autophagy by overexpression of c-Myc was also observed in vivo as isolated B-cell lymphocytes from transgenic Eμ-Myc mice, which are characterized by hyperactive c-Myc, showed increased UPR activation and a concomitant induction of autophagy (Hart et al. 2012). Even though PERK enhances the levels of autophagy in cells, the IRE1-dependent arm of the UPR has been shown to prevent autophagy induction . Inhibition of IRE1 and its downstream target XBP1 were shown to induce basal autophagy in vitro and in vivo (Hetz et al. 2009; Kroemer et al. 2010). However, the mechanism by which IRE1 inhibits autophagy has yet to be elucidated.
Cellular autophagy is negatively regulated by activation of mTOR. As a consequence, cells have developed several negative feedback mechanisms to prevent mTOR activation in response to cellular stress conditions such as hypoxia (Rubinsztein et al. 2012). As mentioned before, chronic hypoxia leads to activation of JNK, which is mediated by IRE1/TRAF2 . Activated JNK phosphorylates and inactivates insulin receptor substrate 1 (IRS1), an inducer of mTOR activity (Wouters and Koritzinsky 2008). Alternatively, mTOR can also be negatively regulated by hypoxia-mediated increased efflux of calcium ions from the ER to the cytoplasm. Increased calcium concentration in the cytosol activates CAMKK2 (calcium/calmodulin-dependent protein kinase kinase 2, beta), which in turn activates AMPK (protein kinase, AMP-activated), leading to inhibition of mTOR activity (Wouters and Koritzinsky 2008).
Hypoxia-induced autophagy may also play a role in breast tumor development. Hypoxia has been detected in early breast cancer lesions. Activation of the UPR, and specifically PERK , was shown to be important for effective acini formation from early breast cell carcinomas (Avivar-Valderas et al. 2011). PERK was shown to initiate a cytoprotective autophagic response in these cells that provides resistance to anoikis-mediated cell death—a phenomenon which causes cell death following detachment from the extracellular matrix. The absence of an effective UPR in these cells prevents acinar formation and sensitizes cells to anoikis-mediated cell death (Avivar-Valderas et al. 2011) .
10 Therapeutic Targeting of the UPR
Therapeutic targeting of the UPR is an attractive approach for cancer treatment, since this pathway is frequently activated in animal and human tumors but less so in normal tissues (with exceptions being cells with high secretory capacity such as pancreatic β cells and B lymphocytes) (Todd et al. 2008; Fonseca et al. 2010; Li et al. 2011). Strategies for doing so fall into two main categories: inhibition of the UPR components or overactivation of the UPR pathway. It is well established that tumor cells rely on the UPR to cope with the nutrient-deprived, hypoxic microenvironment, so that inhibiting one or more branches of this pathway would impair the ability of the tumor cells to counteract these stresses, leading to cell death. On the other hand, overactivating the UPR by pharmacological means would also be a feasible method for specifically targeting tumor cells because, as explained earlier, prolonged or overwhelming stresses shift the balance in favor of the proapoptotic functions of this signaling cascade (Fels and Koumenis 2006) .
10.1 Small-Molecule Inhibitors of UPR Components
One approach for inhibiting the UPR is to develop small-molecule drugs that would target the initial activators of the pathway, preventing the tumor cell from coping with hypoxic stress. Indeed, a group from GlaxoSmithKline recently reported having identified an orally available PERK inhibitor, GSK2606414A, that exhibited potent antitumor activity against human pancreatic xenograft tumors (Axten et al. 2012) . Another group has characterized a compound that inhibited the endonuclease activity of IRE1 without affecting its kinase activity and reduced the tumor burden in mice subcutaneously implanted with human multiple myeloma cells (Papandreou et al. 2011) . Other inhibitors of IRE1 endonuclease activity, such as trierixin, could also prove to be clinically useful (Tashiro et al. 2007).
A different method for blocking the prosurvival function of the UPR would be to target GRP78 activity. Epigallocatechin gallate (EGCG), which targets the adenosine triphosphate (ATP)-binding domain of GRP78, and versipelostatin, which downregulates transcription of GRP78, both induce cytotoxicity in tumor cells in vitro (Park et al. 2004; Ermakova et al. 2006; Matsuo et al. 2009). The catalytic A subunit of bacterial AB5 subtilase cytotoxin selectively cleaves and inactivates GRP78 leading to tumor cell death in vivo and in vitro; however, subtilase causes unresolved ER stress and is lethal in mice, indicating it would not be a useful cancer therapy (Paton et al. 2006). Versipelostatin has the most promise as a therapeutic strategy though, as it appears to be specific for GRP78, whereas EGCG is not (Healy et al. 2009) .
10.2 Overactivation of the UPR
The other approach for preferential killing of hypoxic tumor cells would be to apply additional ER stress to further activate the UPR and tip the balance toward the proapoptotic signaling function of the UPR. One extensively studied class of compounds shown to activate the UPR is proteasome inhibitors (Fribley et al. 2004; Schönthal 2012) . Inhibiting the proteasome prevents protein degradation and results in an accumulation of unfolded and misfolded proteins, thereby activating the UPR. Our lab has previously demonstrated that the reversible proteasome inhibitor bortezomib preferentially kills hypoxic over normoxic tumor cells (Fels et al. 2008). In addition, treating multiple myeloma cells, which have a constitutively high level of UPR activation due to their role as secretory cells, with bortezomib induced apoptosis (Obeng et al. 2006). In fact, several clinical trials are ongoing to test the efficacy of various proteasome inhibitors for treatment of multiple myeloma (Li et al. 2011). One example is carfilzomib (CFZ), a peptide epoxyketone that irreversibly inhibits the proteasome, which was recently approved by the Food and Drug Administration (FDA) for patients with relapsed and refractory multiple myeloma. In addition, CFZ is currently being examined in clinical trials for the treatment of acute myeloid leukemia, acute lymphocytic leukemia, and chronic lymphocytic lymphoma (Crawford et al. 2011; Goldberg 2012). Other proteasome inhibitors, such as ONX0912, MLN9708, and NPI-0052, are in various phases of clinical trials for treatment of solid tumors, multiple myeloma, and lymphoma, respectively (Crawford et al. 2011; Goldberg 2012), and could also demonstrate synergistic effects when combined with UPR inhibitors.
As discussed earlier, activation of the UPR induces autophagy to facilitate degradation of the misfolded proteins and inhibition of autophagy aggravates ER stress, so another tactic for exploiting the UPR in hypoxic tumor cells would be to inhibit autophagy . Chloroquine, which blocks autophagy by inhibiting lysosome acidification, has been shown to sensitize tumor cells to hypoxia and to decrease the hypoxic fraction of xenograft tumors while increasing tumor response to radiation (Bertolotti et al. 2000; Rouschop et al. 2010). Other agents that inhibit autophagy, such as bafilomycin A or 3-methyladenine, could also be useful agents for inducing overactivation of the UPR in hypoxic cells (Aronson and Davies 2012) .
Several other strategies could also be utilized to hyperactivate the UPR in conjunction with hypoxia. For instance, the cyclooxygenase 2 inhibitor celecoxib was shown to cause ER stress and CHOP induction by inhibiting ER calcium pumps responsible for maintaining the calcium concentration within the ER (Tsutsumi et al. 2004; Pyrko et al. 2007). Both celecoxib and its noncoxib analog dimethyl-celecoxib act synergistically with bortezomib to induce tumor cell killing (Kardosh et al. 2008). Another approach that has shown some promise is inhibition of ER-associated protein degradation. Eeyarestatin I, a compound identified in a screen for inhibitors of proteasome-independent misfolded protein degradation, potentiates the cytotoxic effects of bortezomib in vitro and reduces tumor burden in vivo (Fiebiger et al. 2004; Wang et al. 2009; Valle et al. 2011). Additionally, our group also recently published a study describing the identification and characterization of a small-molecule compound, E235. While the mechanism by which it exerts its effects remains unclear, E235 upregulates ATF4 expression only in tumor cells, suggesting that it could have specific cytotoxic effects on hypoxic tumor cells (Sayers et al. 2013) .
In summary, there have been a multitude of studies showing promising antitumor effects via inhibition or hyperactivation of the UPR. In addition, combining two agents that overactivate the UPR has also yielded encouraging results. Therefore, ER stress activators or inhibitors could have selective cytotoxic effects in hypoxic cells, which rely on UPR signaling for survival .
References
Ameri K, Harris AL (2008) Activating transcription factor 4. Int J Biochem Cell Biol 40:14–21
Ameri K, Lewis CE, Raida M, Sowter H, Hai T, Harris AL (2004) Anoxic induction of ATF-4 through HIF-1–independent pathways of protein stabilization in human cancer cells. Blood 103:1876–1882
Ameri K, Hammond EM, Culmsee C, Raida M, Katschinski DM, Wenger RH, Wagner E, Davis RJ, Hai T, Denko N, Harris AL (2007) Induction of activating transcription factor 3 by anoxia is independent of p53 and the hypoxic HIF signalling pathway. Oncogene 26:284–289
Ameri K, Luong R, Zhang H, Powell AA, Montgomery KD, Espinosa I, Bouley DM, Harris AL, Jeffrey SS (2010) Circulating tumour cells demonstrate an altered response to hypoxia and an aggressive phenotype. Br J Cancer 102:561–569
Aronson LI, Davies FE (2012) DangER: protein ovERload. Targeting protein degradation to treat myeloma. Haematologica 97:1119–1130
Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA (2011) PERK Integrates Autophagy and Oxidative Stress Responses To Promote Survival during Extracellular Matrix Detachment. Mol Cell Biol 31:3616–3629
Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, Li WHH, Heerding DA, Minthorn E, Mencken T, Atkins C, Liu Q, Rabindran S, Kumar R, Hong X, Goetz A, Stanley T, Taylor JD, Sigethy SD, Tomberlin GH, Hassell AM, Kahler KM, Shewchuk LM, Gampe RT (2012) Discovery of 7-Methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a Potent and Selective First-in-Class Inhibitor of Protein Kinase R (PKR)-like Endoplasmic Reticulum Kinase (PERK). J Med Chem 55:7193–7207
Bays NW, Gardner RG, Seelig LP, Joazeiro CA, Hampton RY (2001) Hrd1p/Der3p is a membrane-anchored ubiquitin ligase required for ER-associated degradation. Nat Cell Biol 3:24–29
Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D (2000) Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2:326–332
Bertout JA, Patel SA, Simon MC (2008) The impact of O2 availability on human cancer. Nat Rev Cancer 8:967–975
Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 24:3470–3481
Blais JD, Filipenko V, Bi MX, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC (2004) Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24:7469–7482
Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C, Cavener D, Diehl JA (2010) PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene 29:3881–3895
Brown JM (1990) Tumor Hypoxia, Drug Resistance, and Metastases. J Natl Cancer Inst 82:338–339
Brown JM, Wilson WR (2004) Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
Cairns RA, Hill RP (2004) Acute Hypoxia Enhances Spontaneous Lymph Node Metastasis in an Orthotopic Murine Model of Human Cervical Carcinoma. Cancer Res 64:2054–2061
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS, Munshi N, Horner J, Ivanova EV, Protopopov A, Anderson KC, Tonon G, DePinho RA (2007) The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11:349–360
Chang Q, Jurisica I, Do T, Hedley DW (2011) Hypoxia Predicts Aggressive Growth and Spontaneous Metastasis Formation from Orthotopically Grown Primary Xenografts of Human Pancreatic Cancer. Cancer Res 71:3110–3120
Chen X, Shen J, Prywes R (2002) The Luminal Domain of ATF6 Senses Endoplasmic Reticulum (ER) Stress and Causes Translocation of ATF6 from the ER to the Golgi. J Biol Chem 277:13045–13052
Chen Y, Feldman DE, Deng C, Brown JA, De Giacomo AF, Gaw AF, Shi G, Le QT, Brown JM, Koong AC (2005) Identification of Mitogen-Activated Protein Kinase Signaling Pathways That Confer Resistance to Endoplasmic Reticulum Stress in Saccharomyces cerevisiae. Mol Cancer Res 3:669–677
Chiribau CB, Gaccioli F, Huang CC, Yuan CL, Hatzoglou M (2010) Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol 30:3722–3731
Crawford LJ, Walker B, Irvine AE (2011) Proteasome inhibitors in cancer therapy. J Cell Commun Signal 5:101–110
Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23:7198–7209
Dickhout JG, Carlisle RE, Jerome DE, Mohammed-Ali Z, Jiang H, Yang G, Mani S, Garg SK, Banerjee R, Kaufman RJ, Maclean KN, Wang R, Austin RC (2012) Integrated Stress Response Modulates Cellular Redox State via Induction of Cystathionine γ-Lyase: Cross-Talk Between Integrated Stress Response and Thiol Metabolism. J Biol Chem 287:7603–7614
Doroudgar S, Thuerauf DJ, Marcinko MC, Belmont PJ, Glembotski CC (2009) Ischemia Activates the ATF6 Branch of the Endoplasmic Reticulum Stress Response. J Biol Chem 284:29735–29745
Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma W-Y, Bode AM, Dong Z (2006) (–)–Epigallocatechin Gallate Overcomes Resistance to Etoposide-Induced Cell Death by Targeting the Molecular Chaperone Glucose-Regulated Protein 78. Cancer Res 66:9260–9269
Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ (1999) Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339:135–141
Fels D, Koumenis C (2006) The PERK/eIF2α/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther 5:723–728
Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, Koong AC, Koumenis C (2008) Preferential Cytotoxicity of Bortezomib toward Hypoxic Tumor Cells via Overactivation of Endoplasmic Reticulum Stress Pathways. Cancer Res 68:9323–9330
Fernandez PM, Tabbara SO, Jacobs LK, Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA, Patierno SR (2000) Overexpression of the glucose-regulated stress gene GRP78 in malignant but not benign human breast lesions. Breast Cancer Res Treat 59:15–26
Fiebiger E, Hirsch C, Vyas JM, Gordon E, Ploegh HL, Tortorella D (2004) Dissection of the Dislocation Pathway for Type I Membrane Proteins with a New Small Molecule Inhibitor, Eeyarestatin. Mol Biol Cell 15:1635–1646
Fonseca SG, Urano F, Burcin M, Gromada J (2010) Stress hypERactivation in the β-cell. Islets 2:1–9
Frand AR, Kaiser CA (1999) Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol Cell 4:469–477
Fribley A, Zeng Q, Wang C-Y (2004) Proteasome Inhibitor PS-341 Induces Apoptosis through Induction of Endoplasmic Reticulum Stress-Reactive Oxygen Species in Head and Neck Squamous Cell Carcinoma Cells. Mol Cell Biol 24:9695–9704
Friedman AD (1996) GADD153/CHOP, a DNA Damage-inducible Protein, Reduced CAAT/Enhancer Binding Protein Activities and Increased Apoptosis in 32D cl3 Myeloid Cells. Cancer Res 56:3250–3256
Fujimoto T, Onda M, Nagai H, Nagahata T, Ogawa K, Emi M (2003) Upregulation and overexpression of human X-box binding protein 1 (hXBP-1) gene in primary breast cancers. Breast Cancer 10:301–306
Gardner LB (2008) Hypoxic inhibition of nonsense-mediated RNA decay regulates gene expression and the integrated stress response. Mol Cell Biol 28:3729–3741
Gardner LB (2010) Nonsense-mediated RNA decay regulation by cellular stress: implications for tumorigenesis. Mol Cancer Res 8:295–308
Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F (2010) Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One 5:e9575
Goldberg AL (2012) Development of proteasome inhibitors as research tools and cancer drugs. J Cell Biol 199:583–588
Gupta S, McGrath B, Cavener DR (2009) PERK regulates the proliferation and development of insulin-secreting beta-cell tumors in the endocrine pancreas of mice. PLoS One 4:e8008
Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138:562–575
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000a) Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol Cell 6:1099–1108
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000b) Perk Is Essential for Translational Regulation and Cell Survival during the Unfolded Protein Response. Mol Cell 5:897–904
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633
Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M, Li Y, Gao Y, Liu H, Li C, Maity A, Thomas-Tikhonenko A, Perl AE, Koong A, Fuchs SY, Diehl JA, Mills IG, Ruggero D, Koumenis C (2012) ER stress-mediated autophagy promotes Myc-dependent transformation and tumor growth. J Clin Invest 122:4621–4634
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian Transcription Factor ATF6 Is Synthesized as a Transmembrane Protein and Activated by Proteolysis in Response to Endoplasmic Reticulum Stress. Mol Biol Cell 10:3787–3799
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93
He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AMK, Alam J (2001) Identification of Activating Transcription Factor 4 (ATF4) as an Nrf2-interacting Protein: Implication for Heme Oxygenase-1 Gene Regulation. J Biol Chem 276:20858–20865
Healy SJM, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A (2009) Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol 625:234–246
Hetz C, Bernasconi P, Fisher J, Lee A-H, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ (2006) Proapoptotic BAX and BAK Modulate the Unfolded Protein Response by a Direct Interaction with IRE1α. Science 312:572–576
Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH (2009) XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 23:2294–2306
Höckel M, Vaupel P (2001) Biological consequences of tumor hypoxia. Semin Oncol 28. Supplement 8:36–41
Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186:323–331
Huang LE (2008) Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation. Cell Death Differ 15:672–677
Iwakoshi NN, Lee A-H, Glimcher LH (2003) The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev 194:29–38
Jamora C, Dennert G, Lee AS (1996) Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA 93:7690–7694
Janz M, Hummel M, Truss M, Wollert-Wulf B, Mathas S, Johrens K, Hagemeier C, Bommert K, Stein H, Dorken B, Bargou RC (2006) Classical Hodgkin lymphoma is characterized by high constitutive expression of activating transcription factor 3 (ATF3), which promotes viability of Hodgkin/Reed-Sternberg cells. Blood 107:2536–2539
Kardosh A, Golden EB, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schönthal AH (2008) Aggravated Endoplasmic Reticulum Stress as a Basis for Enhanced Glioblastoma Cell Killing by Bortezomib in Combination with Celecoxib or Its Non-Coxib Analogue, 2,5-Dimethyl-Celecoxib. Cancer Res 68:843–851
Kaufman RJ (2002) Orchestrating the unfolded protein response in health and disease. J Clin Invest 110:1389–1398
Kenific CM, Thorburn A, Debnath J (2010) Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol 22:241–245
Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M (2003) Tumor hypoxia: A target for selective cancer therapy. Cancer Sci 94:1021–1028
Koditz J, Nesper J, Wottawa M, Stiehl DP, Camenisch G, Franke C, Myllyharju J, Wenger RH, Katschinski DM (2007) Oxygen-dependent ATF-4 stability is mediated by the PHD3 oxygen sensor. Blood 110:3610–3617
Koong AC, Auger EA, Chen EY, Giaccia AJ (1994) The regulation of GRP78 and messenger RNA levels by hypoxia is modulated by protein kinase C activators and inhibitors. Radiat Res 138:S60–S63
Koumenis C (2006) ER stress, hypoxia tolerance and tumor progression. Curr Mol Med 6:55–69
Koumenis C, Bi M, Ye J, Feldman D, Koong AC (2007) Hypoxia and the unfolded protein response. Methods Enzymol 435:275–293
Kroemer G, Marino G, Levine B (2010) Autophagy and the integrated stress response. Mol Cell 40:280–293
Lee AH, Iwakoshi NN, Glimcher LH (2003a) XBP-1 Regulates a Subset of Endoplasmic Reticulum Resident Chaperone Genes in the Unfolded Protein Response. Mol Cell Biol 23:7448–7459
Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH (2003b) Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci USA 100:9946–9951
Lee AS (1992) Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol 4:267–273
Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I (2009) Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186:783–792
Li X, Zhang K, Li Z (2011) Unfolded protein response in cancer: the Physician’s perspective. J Hematol Oncol 4:8
Liu L, Wise DR, Diehl JA, Simon MC (2008) Hypoxic Reactive Oxygen Species Regulate the Integrated Stress Response and Cell Survival. J Biol Chem 283:31153–31162
Ma Y, Brewer JW, Diehl JA, Hendershot LM (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318:1351–1365
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077
Matsuo J, Tsukumo Y, Sakurai J, Tsukahara S, Park H-R, Shin-ya K, Watanabe T, Tsuruo T, Tomida A (2009) Preventing the unfolded protein response via aberrant activation of 4E-binding protein 1 by versipelostatin. Cancer Sci 100:327–333
Meyer N, Penn LZ (2008) Reflecting on 25 years with MYC. Nat Rev Cancer 8:976–990
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, Endert P van, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61
Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Lee KP, Boise LH (2006) Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 107:4907–4916
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 24:1243–1255
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366:585–594
Padyana AK, Qiu H, Roll-Mecak A, Hinnebusch AG, Burley SK (2005) Structural basis for autoinhibition and mutational activation of eukaryotic initiation factor 2alpha protein kinase GCN2. J Biol Chem 280:29289–29299
Pan YX, Chen H, Kilberg MS (2005) Interaction of RNA-binding proteins HuR and AUF1 with the human ATF3 mRNA 3’-untranslated region regulates its amino acid limitation-induced stabilization. J Biol Chem 280:34609–34616
Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, Endert P van, Zitvogel L, Madeo F, Kroemer G (2008) The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 15:1499–1509
Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, Endert P van, Yuan J, Zitvogel L, Madeo F, Williams DB, Kroemer G (2009) Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 28:578–590
Papandreou I, Denko NC, Olson M, Van Melckebeke H, Lust S, Tam A, Solow-Cordero DE, Bouley DM, Offner F, Niwa M, Koong AC (2011) Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood 117:1311–1314
Park H-R, Tomida A, Sato S, Tsukumo Y, Yun J, Yamori T, Hayakawa Y, Tsuruo T, Shin-ya K (2004) Effect on Tumor Cells of Blocking Survival Response to Glucose Deprivation. J Natl Cancer Inst 96:1300–1310
Paton AW, Beddoe T, Thorpe CM, Whisstock JC, Wilce MCJ, Rossjohn J, Talbot UM, Paton JC (2006) AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443:548–552
Patterson RL, Boehning D, Snyder SH (2004) Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 73:437–465
Pelzer AE, Bektic J, Haag P, Berger AP, Pycha A, Schafer G, Rogatsch H, Horninger W, Bartsch G, Klocker H (2006) The expression of transcription factor activating transcription factor 3 in the human prostate and its regulation by androgen in prostate cancer. J Urol 175:1517–1522
Pereira ER, Liao N, Neale GA, Hendershot LM (2010) Transcriptional and Post-Transcriptional Regulation of Proangiogenic Factors by the Unfolded Protein Response. PLoS One 5:e12521
Pike LR, Phadwal K, Simon AK, Harris AL (2012) ATF4 orchestrates a program of BH3-only protein expression in severe hypoxia. Mol Biol Rep 39:10811–10822
Pike LRG, Singleton DC, Buffa F, Abramczyk O, Phadwal K, Li JL, Simon AK, Murray JT, Harris AL (2013) Transcriptional up-regulation of ULK1 by ATF4 contributes to cancer cell survival. Biochem J 449:389–400
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349
Pyrko P, Kardosh A, Liu Y-T, Soriano N, Xiong W, Chow RH, Uddin J, Petasis NA, Mircheff AK, Farley RA, Louie SG, Chen TC, Schönthal AH (2007) Calcium-activated endoplasmic reticulum stress as a major component of tumor cell death induced by 2,5-dimethyl-celecoxib, a non-coxib analogue of celecoxib. Mol Cancer Ther 6:1262–1275
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC (2010) Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev 90:1383–1435
Richards NG, Kilberg MS (2006) Asparagine synthetase chemotherapy. Annu Rev Biochem 75:629–654
Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee A-H, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le Q-T, Koong AC (2004) XBP1 Is Essential for Survival under Hypoxic Conditions and Is Required for Tumor Growth. Cancer Res 64:5943–5947
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Rouschop KM, Wouters BG (2009) Regulation of autophagy through multiple independent hypoxic signaling pathways. Curr Mol Med 9:417–424
Rouschop KMA, Beucken T van den, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, Kogel AJ van der, Koritzinsky M, Wouters BG (2010) The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest 120:127–141
Rubinsztein DC, Codogno P, Levine B (2012) Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 11:709–730
Rzymski T, Milani M, Pike L, Buffa F, Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I, Harris AL (2010) Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 29:4424–4435
Sayers CM, Papandreou I, Guttmann DM, Maas NL, Diehl JA, Witze ES, Koong AC, Koumenis C (2013) Identification and Characterization of a Potent Activator of p53-Independent Cellular Senescence via a Small-Molecule Screen for Modifiers of the Integrated Stress Response. Mol Pharmacol 83:594–604
Schewe DM, Aguirre-Ghiso JA (2008) ATF6α-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proc Natl Acad Sci USA 105:10519–10524
Schönthal AH (2012) Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol
Semenza GL (2009) Regulation of Oxygen Homeostasis by Hypoxia-Inducible Factor 1. Physiology 24:97–106
Shen J, Chen X, Hendershot L, Prywes R (2002) ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev Cell 3:99–111
Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC (1998) Identification and Characterization of Pancreatic Eukaryotic Initiation Factor 2 α-Subunit Kinase, PEK, Involved in Translational Control. Mol Cell Biol 18:7499–7509
Shuda M, Kondoh N, Imazeki N, Tanaka K, Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, Yamamoto N, Yamamoto M (2003) Activation of the ATF6, XBP1 and grp78 genes in human hepatocellular carcinoma: a possible involvement of the ER stress pathway in hepatocarcinogenesis. J Hepatol 38:605–614
Smith MH, Ploegh HL, Weissman JS (2011) Road to Ruin: Targeting Proteins for Degradation in the Endoplasmic Reticulum. Science 334:1086–1090
Su N, Kilberg MS (2008) C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 283:35106–35117
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190
Tashiro E, Hironiwa N, Kitagawa M, Futamura Y, Suzuki S, Nishio M, Imoto M (2007) Trierixin, a Novel Inhibitor of ER Stress-induced XBP1 Activation from Streptomyces sp. J Antibiot 60:547–553
Thompson MR, Xu D, Williams BR (2009) ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med (Berl) 87:1053–1060
Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12:1812–1824
Todd DJ, Lee A-H, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8:663–674
Tsutsumi S, Gotoh T, Tomisato W, Mima S, Hoshino T, Hwang HJ, Takenaka H, Tsuchiya T, Mori M, Mizushima T (2004) Endoplasmic reticulum stress response is involved in nonsteroidal anti-inflammatory drug-induced apoptosis. Cell Death Differ 11:1009–1016
Tu BP, Weissman JS (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346
Uramoto H, Sugio K, Oyama T, Nakata S, Ono K, Yoshimastu T, Morita M, Yasumoto K (2005) Expression of endoplasmic reticulum molecular chaperone Grp78 in human lung cancer and its clinical significance. Lung Cancer 49:55–62
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of Stress in the ER to Activation of JNK Protein Kinases by Transmembrane Protein Kinase IRE1. Science 287:664–666
Valle CW, Min T, Bodas M, Mazur S, Begum S, Tang D, Vij N (2011) Critical Role of VCP/p97 in the Pathogenesis and Progression of Non-Small Cell Lung Carcinoma. PLoS One 6:e29073
Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101:11269–11274
Vaupel P, Kelleher DK, Höckel M (2001) Oxygenation status of malignant tumors: Pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol 28. Supplement 8:29–35
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Wang Q, He Z, Zhang J, Wang Y, Wang T, Tong S, Wang L, Wang S, Chen Y (2005) Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev 29:544–551
Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, Ron D, Chen W, Trenkle W, Wiestner A, Ye Y (2009) ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA 106:2200–2205
Wang Y, Alam GN, Ning Y, Visioli F, Dong Z, Nör JE, Polverini PJ (2012) The Unfolded Protein Response Induces the Angiogenic Switch in Human Tumor Cells through the PERK/ATF4 Pathway. Cancer Res 72:5396–5406
Wek RC, Jackson BM, Hinnebusch AG (1989) Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci USA 86:4579–4583
Wek RC, Jiang HY, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34:7–11
Wouters BG, Koritzinsky M (2008) Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer 8:851–864
Yamaguchi H, Wang HG (2004) CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 279:45495–45502
Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K (2004) Differential Contributions of ATF6 and XBP1 to the Activation of Endoplasmic Reticulum Stress-Responsive cis-Acting Elements ERSE, UPRE and ERSE-II. J Biochem 136:343–350
Yang ZJ, Chee CE, Huang S, Sinicrope FA (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10:1533–1541
Ye J, Rawson RB, Komuro R, Chen X, Davé UP, Prywes R, Brown MS, Goldstein JL (2000) ER Stress Induces Cleavage of Membrane-Bound ATF6 by the Same Proteases that Process SREBPs. Mol Cell 6:1355–1364
Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN, Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C (2010) The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J 29:2082–2096
Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20:6755–6767
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA Is Induced by ATF6 and Spliced by IRE1 in Response to ER Stress to Produce a Highly Active Transcription Factor. Cell 107:881–891
Zheng HC, Takahashi H, Li XH, Hara T, Masuda S, Guan YF, Takano Y (2008) Overexpression of GRP78 and GRP94 are markers for aggressive behavior and poor prognosis in gastric carcinomas. Hum Pathol 39:1042–1049
Zhong C, Chen C, Kilberg MS (2003) Characterization of the nutrient-sensing response unit in the human asparagine synthetase promoter. Biochem J 372:603–609
Acknowledgments
The authors are supported by NIH R01 CA094214 and NIH R01 CA139362. C.M.S. and S.L.L. are supported by the National Institutes of Health National Cancer Institute Fellowship Training Grant [5-T32-CA-009677-20].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sayers, C., Dey, S., Lehman, S., Koumenis, C. (2014). The Unfolded Protein Response and Therapeutic Opportunities. In: Melillo, G. (eds) Hypoxia and Cancer. Cancer Drug Discovery and Development. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9167-5_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9167-5_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9166-8
Online ISBN: 978-1-4614-9167-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)