Abstract
Background: Traditional cytotoxic drugs are characterized by a narrow therapeutic window and significant interpatient variability in therapeutic and toxic effects. The new targeted therapies have a larger therapeutic window and some have different drug clearance mechanisms. Objective: To provide an insight into history, rationales, and limitations of current dosing methods in traditional cytotoxic drugs and new targeted therapies and to suggest a practical framework for dose calculation and a basis for future research and clinical studies. Methods: Review of relevant literature related to dose calculation of anticancer drugs. Results: Body surface area (BSA) or weight-based dosing and fixed dosing fail to standardize systemic anticancer drug exposure between individuals. Strategies using clinical parameters, genotype and phenotype markers, and therapeutic drug monitoring all have potential and each has a role for specific drugs. However, no one method is a practical dose calculation strategy for many or all drugs. Neither body size nor fixed dosing alone can be used for currently available drugs. Conclusion: Dosing strategies for anticancer drugs should be individualized according to elimination mechanisms and individual patient characteristics. Ways to determine these factors require further investigation and should be a component of early phase studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Body surface area
- Interindividual variability
- Pharmacokinetics
- Drug disposition
- Flat dose
- Toxicity-adjusted dosing
- Therapeutic drug monitoring
- Dose individualization
1 Introduction
There are three issues that set the scene for defining the starting dose of novel anticancer drugs:
-
1.
There is a revolution in the understanding and identification of drug elimination mechanisms at the molecular level.
-
2.
Some of the new targeted therapies have a larger therapeutic window than traditional cytotoxic agents.
-
3.
The traditional monopoly held by body surface area for dose calculation of cytotoxic agents is inaccurate for many drugs.
Cytotoxic drug disposition is minimally affected by body size. At best, body size accounts for less than 30 % of the interindividual variation in drug exposure. Most of the variation is due to genetic and phenotypic differences in elimination and absorption processes. Drug elimination is largely determined by mechanisms that are unrelated to body size and other methods that account for these variations are needed for dose calculation. Even with targeted therapies that may have a larger safety margin, reduction in interpatient variability in drug exposure is critical to minimizing underdosing.
2 History of BSA in Dose Calculation
In 1916, when Delafield and Eugene DuBois developed a formula to approximate body surface area (BSA), they would not have realized the implications that this would later have on the millions of cancer patients treated with cytotoxic chemotherapy [27]. BSA is the two-dimensional surface area of an individual’s skin, using height and weight. They developed the nomogram to normalize measurement of basal metabolic rate among individuals, but in the late 1950s, it was suggested after minimal investigation that BSA should be used to normalize cytotoxic drug calculation.
One of the first uses of BSA in drug dose calculation was in 1950, when Crawford et al. [16] showed that plasma drug levels for sulfadiazine (an acetylated and renally excreted antibiotic) and acetylsalicylic acid (a renally excreted analgesic) linearly correlated with administered dosage per unit of BSA in patients varying widely in size. BSA has also been used to extrapolate preclinical animal toxicology data to allow an estimation of a safe starting dose for phase I studies of cytotoxic agents in humans [31, 40]. In 1958, an attempt was made to define a more accurate method of dose calculation for cytotoxic drugs in children [97]. Pinkel examined the literature and found that the “conventional” dose of five cytotoxic drugs (mercaptopurine, methotrexate, mechlorethamine, tri-ethylenethiophosphoramide, and actinomycin) for pediatric and adult humans and for experimental animals was similar if corrected for “representative” BSAs for humans and animals. Pharmacokinetic analyses were not performed and actual patients were not included in the study so comparison of other variables such as antitumor effect or toxicity could not be undertaken. Three of the drugs are renally excreted (mercaptopurine, methotrexate, and actinomycin), and the apparent relationship may have been due to the known correlation of BSA and renal function. Pinkel recommended that the potential use of BSA for dose calculation should be further investigated, but this was not undertaken until the last decade. In the meantime, the use of BSA for dose calculation in oncology became dogma, without further investigation into the relationship between dose and BSA or other parameters of body size.
3 Does Body Size Correlate with Drug Disposition?
3.1 Drug Disposition
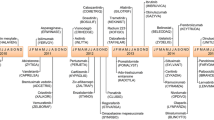
Drug disposition or blood concentration is determined by absorption, distribution, and clearance (Fig. 1). Absorption and clearance are largely determined by activity of transmembrane transporters and metabolizing enzymes in the gut, kidney, and liver. For some drugs, hepatic and renal blood flows are also important. Drug distribution is dictated by the degree of plasma protein binding and whether the drug freely distributes into extravascular tissue. For instance, drugs that are highly plasma protein bound such as warfarin, tolbutamide, and ibuprofen have a low volume of distribution (approximately 0.1 L/kg) which roughly equates to blood volume [105]. Since blood volume and the amount of total body water is related to body size, volume of distribution may relate to body size in some circumstances [1]. Aminoglycosides, phenobarbitone, ibuprofen, carboplatin, vinorelbine, irinotecan, and tacrolimus are some drugs where measures of body size have correlated with the apparent volume of distribution [74, 80, 86, 91, 109, 127, 138].
Schematic representation of drug disposition. ABC: ATP-binding cassette; SLC: Solute carrier. Reproduced with permission from [36]
However, it must be remembered that volume of distribution (V d) is not a physiological measurement but a pharmacokinetic ratio. It is the theoretical volume into which a drug is distributed and is described by the formula; V d = Dose/Concentration. Therefore, the volume of distribution for an intravenous dose is determined by peak blood concentration. The possible relationship between body size and volume of distribution may be important in circumstances where peak plasma concentration determines toxicity or drug efficacy. Intuitively, one would expect a relationship between peak plasma concentration and toxicity for cytotoxic agents. However, limited information is available. No correlation was found between toxicity and peak concentration of irinotecan SN-38 or epirubicin [107, 124]. A correlation has been shown for oral etoposide [129]. However, for this drug and also for paclitaxel, the time above critical plasma concentration, rather than peak concentration, appears to be more important [37, 56, 79]. Where a relationship has been shown between a pharmacokinetic parameter and drug efficacy or toxicity for anticancer treatment, it is usually the area under the time–concentration curve (AUC) or steady-state plasma concentration rather than V d or peak plasma concentration that correlates (Table 1).
The AUC is determined by dose and clearance and defined by the formula, AUC = Clearance/Dose. As mentioned before, metabolism and elimination by the kidneys and liver determines the drug clearance of most drugs. Very few of these processes would be expected to be determined by body size. A few drugs such as aminoglycosides are almost solely eliminated by glomerular filtration. It has been suggested that GFR correlates with body size [113], and dose of gentamicin and tobramycin is now determined by adjusting for body weight. However, even for carboplatin, a cytotoxic drug that is mostly eliminated by glomerular filtration, dose calculated using GFR is more accurate than using BSA [57].
3.2 Body Size and Cytotoxic Drug Clearance
Giving a larger dose to a larger person makes intuitive sense, and to some extent this is true, but overall body size is a minor determinant of drug exposure. BSA is known to be proportional to blood volume [1]. It has been claimed that BSA is also proportional to glomerular filtration rate (GFR) [113], but a more recent assessment has questioned this relationship [24]. Liver function decreases with advancing age in parallel to the loss of liver volume [110]. Liver volume as determined by helical CT scanning has been shown to correlate with BSA (r 2 = 0.54) and total body weight (r 2 = 0.61) in 21 patients with a history of cancer but without liver metastases [87].
Over the last decade, the relationship between BSA and drug disposition of cytotoxic drugs has been revisited [43, 44, 99, 101]. Table 2 is a list of drugs that have been reported to show a correlation between drug clearance and BSA. Even for some of these drugs the correlation coefficients are low, indicating that BSA accounts for less than 30 % of the variability in clearance between individuals. For most cytotoxic drugs, no correlation can be seen with BSA and drug clearance (Table 3). The most compelling evidence against the use of BSA alone for dose calculation is the fact that a large interpatient variability in drug exposure remains despite “normalization” of dose by BSA.
In adult populations the extremes of BSA vary from approximately 1.4 to 2.3 m2—a little over a 1.5-fold range, but the majority of individuals fall into a range much less than this. Even for drugs where the use of BSA may reduce variability, there is minimal contribution in reduction in variability from the use of body size for a person 1.7 m2 compared to 1.8 m2. Assuming a maximum contribution of BSA to drug disposition of 30 %, it is only in the situations of extreme BSA (e.g., an individual of 150 cm and 40 kg compared to one of 185 cm and 120 kg) where this parameter may become a significant factor in dose calculation.
The issue of substantial variation in body size is amplified in pediatric oncology where body weight ranges from a few kilograms to adult size. In these situations of extreme difference, body size must come into play. This is more akin to the interspecies scaling of chemotherapy dose, such as in estimating the dose for humans based on toxicology studies in rodents. BSA has proved useful in this situation of interspecies scaling of dose [31, 40]. It would therefore be reasonable to use BSA to scale an approximate starting dose of a drug for clinical trials in children based on adult data. However, even here it is unreasonable to use BSA as the sole determinant of dose for individual infants. The same inaccuracies of using BSA alone would hold when differentiating dose between children within a small range of body size.
3.3 Body Size and Targeted Therapies
3.3.1 Small Molecules
A few studies have looked at the relationship between body size and dose or drug exposure for small molecules. The pharmacokinetics of tyrosine kinase inhibitors (TKI) has recently been reviewed by van Erp et al. [130]. The effect of body size on small molecule inhibitors is summarized below.
3.3.1.1 Sunitinib
In a predominantly pharmacogenetic study, van Erp et al. found no correlation between BSA and toxicity in 183 patients who received at least one cycle of 50 mg single-agent sunitinib [131]. In a population pharmacokinetic meta-analysis of sunitinib, body size was found to affect the volume of distribution (V d/F) but not clearance of sunitinib [54]. However, simulated exposure of sunitinib varied considerably and it was predicted that body size effect on AUC was minimal. Clearance and AUC rather than V d/F is more likely to affect the steady-state level of sunitinib.
3.3.1.2 Imatinib
Two studies in Japanese populations have suggested an effect of body size and dose of imatinib [63, 108]. Sakai et al. found that trough imatinib concentration did not correlate with body weight or BSA but did correlate with imatinib dose and dose adjusted for BSA or weight. Kawaguchi et al., in 31 patients in complete cytogenetic response (CCR), found that BSA was significantly smaller in patients receiving a reduced dose due to toxicity compared with those receiving a standard dose, pointing to a weak effect of body size on “optimal dose” (defined as the dose of imatinib that could achieve and maintain a CCR with acceptable adverse effects). Again there was no relationship between BSA and imatinib trough level. Both these studies indicated that reduced dose of imatinib may be sufficient in smaller patients to achieve adequate drug exposure and clinical benefit. However, both studies showed a wide interpatient variation in imatinib exposure regardless of the dose taken. In the Sakai et al. study, even among those taking the same dose of 400 mg/day, the imatinib concentration was widely distributed (582–2,420 ng/mL) regardless of body size. In other words, an individual was just as likely to get an effective (or ineffective) drug concentration regardless of whether they ingested 300, 400, or 500 mg of imatinib daily. Just like cytotoxic chemotherapy, factors other than dose of imatinib are more important in determining drug exposure and body size has a weak effect.
3.3.1.3 Erlotinib
Lu et al., in 1,047 patients with non-small cell lung cancer, showed that erlotinib clearance did not correlate with body weight but was affected by total bilirubin, α1-acid glycoprotein, and smoking status [73]. Interestingly, occurrence of skin rash has been associated with survival in erlotinib-treated patients [133] and erlotinib AUC correlated with occurrence of skin rash in the study of Lu et al., indicating a correlation between drug exposure and efficacy. More recently, Thomas et al., in a study of 42 patients with head and neck cancer, showed that erlotinib clearance was partly explained by patients’ age, hepatic function, ABCG2 genetic polymorphism, and smoking status but not by body weight [123].
3.3.2 Monoclonal Antibodies
Ten mAbs are currently approved by FDA for the treatment of cancer and all of them are of the IgG class. It is important to understand different clearance pathway between mAbs and traditional cytotoxic drugs. mAbs are given intravenously and once in the systemic circulation, entry to the extravascular compartment (intestinal fluid and tissue) is primarily driven by hydrostatic pressure, osmotic pressure, endothelia pore size, and vessel tortuosities [88]. The distribution is limited to 1–2 times plasma volume, indicating a poor penetration into tissue spaces, including tumors [139].
Unlike small molecules, IgG antibodies are large (150 kDa) and are therefore not filtered by the kidney or excreted in urine [3]. The dominant route for elimination of antibodies is via uptake and catabolism by the reticuloendothelial system (RES). The neonate receptor FcRn, expressed on macrophages and natural killer cells, binds to the Fc portion of IgG antibody and plays a major role in antibody clearance. Since blood volume correlates with body size, there is some logic to using weight or BSA to estimate dose for these agents.
3.3.2.1 Bevacizumab
For antibodies like bevacizumab, which targets soluble antigens, the pharmacokinetic profile is characterized by a linear two-compartment model with a rapid elimination phase from a short distribution and more prolonged elimination half-life, as a result of the nonspecific clearance by the RES and interaction with FcRn. In a population pharmacokinetic study of bevacizumab, Lu et al. demonstrated that body weight and gender were the covariates with the greatest influence on bevacizumab central compartment volume of distribution (V c) and clearance (CL), which support the body weight-based dosing [72]. Despite that, covariate effects of all factors only explained about 40 % of interpatient variance for V c and 60 % of interpatient variance for CL.
3.3.2.2 Trastuzumab, Rituximab, and Cetuximab
For antibodies targeting membrane-associated internalizing antigen, the total clearance is a combination of two different clearance pathway: (1) the nonspecific, linear pathway attributed to the RES and (2) the specific, nonlinear, and saturable antigen-mediated clearance pathway, which is mediated by the binding of the antibody to the antigen and subsequent internalization of the antibody–antigen complex, followed by degradation of the internalized antibody and antigen complex [121]. The contribution of antigen to mAb clearance depends on various antigen-related factors, such as antigen concentration, distribution, and turnover rate. These effects were demonstrated in a pharmacokinetic modeling of 476 patient with metastatic breast cancer treated with trastuzumab, where Bruno et al. found that body weight as well as burden of disease and serum level of extracellular domain of the Her 2 receptor affected trastuzumab clearance. However, these covariate effects on trastuzumab exposure were only modest in comparison with the large interpatient variability of CL which was 43 % [9]. Similarly Ng et al. showed that BSA accounted for about 19.7 % of interindividual CL variability of rituximab and that adjusting the dose as a function of body surface area does not seem to improve the predictability of rituximab exposure [89].
In a review of data from two studies of 143 patients with head and neck cancer treated with cetuximab, Dirks et al. found a fourfold variation in trough cetuximab level while receiving BSA-based dosing [22]. Together ideal body weight (not actual weight or BSA) and WBC accounted for almost 35 % of the total variability in maximum elimination rate, a parameter that determines trough level. A comparison of the trough concentrations of underweight patients (dosed according to body size) showed that these were lower than other patients (median 48.2 vs. 62.4 μg/mL, P = 0.014) and the authors questioned the use of the current practice of use of BSA for cetuximab dose.
4 Alternative Body Size Measures and Obesity
Lean body mass (LBM) consists of body cell mass, extracellular fluid, and nonfat connective tissue and is essentially fat-free mass [87]. LBM is commonly measured by dual-energy X-ray absorptiometry which distinguishes fat, fat-free mass, and bone.
It has been suggested that LBM correlates with systemic drug clearance, but so far this has not yet been substantiated [83]. Nawaratne et al. showed that LBM correlates with liver volume and antipyrine clearance, a nonspecific quantitative test of hepatic drug oxidation [87]. However, in this study there was no correlation between liver volume and antipyrine clearance indicating that other unknown factors account for the relationship. Further studies are required to determine the importance of LBM in dose calculation of hepatically eliminated drugs.
Ideal body weight (IBW) is the weight that insurance companies consider appropriate for height and is determined by a formula. The use of IBW for dose calculation (sometimes as a function for BSA) attempts to account for excess adipose tissue. Body constitution in the obese is characterized by a higher percentage of fat and a lower percentage of lean tissue and water. The effect of obesity on some cytotoxic drugs has been examined. In obese patients methotrexate clearance is increased, busulfan and ifosfamide clearances are unchanged, and doxorubicin and cyclophosphamide clearances are reduced [29, 39, 69, 98, 104]. Hepatic oxidative metabolism is unaffected by obesity as measured by antipyrine clearance or erythromycin breath test [13, 57]. It would be expected that the volume of distribution be affected by obesity especially for drugs that are lipid soluble. However, this expected relationship is variable with some lipid-soluble drugs increasing the volume of distribution (e.g., benzodiazepines, verapamil), while others have no effect (e.g., cyclosporine, propranolol) [14].
Obesity is an extreme of body size and in this special situation size may become an important determinant of drug disposition and so higher doses are required. Curiously, it is often in the obese patient where the strict practice of BSA dose calculation is abandoned and other arbitrary rules are applied such as capping of BSA or dose. However, retrospective studies of breast cancer patients have shown that obese patients seem to be less likely to develop neutropenic sepsis even if actual body weight (ABW) was used and that obese patients who receive arbitrary dose reductions had a worse outcome [15, 42, 61, 106].
Sparreboom et al. assessed actual plasma pharmacokinetics of eight anticancer agents in 1,206 lean and obese adult patients and found the disposition of some, but not all, drugs was significantly altered by obesity [118]. For example, absolute clearance of cisplatin, palitaxel and troxacabine (P < 0.023) were increased but decreased for doxorubicin (P = 0.013) and unchanged for carboplatin, docetaxel, doxorubicin, irinotecan, or topotecan. The selection of a better prediction of pharmacokinetics among alternate weight descriptors for dose calculation in obese, including actual body weight, predicted normal weight, lean body mass, (adjusted) ideal body weight, and the mean of ideal and actual body weight, is drug specific and sex dependent and seemed unrelated to the intrinsic physicochemical properties or route of elimination.
In conclusion, obesity may affect drug clearance and treatment outcome in a drug-specific manner, and empiric decrease in drug dose in obese patients (e.g., dose capping or by using IBW) should be discouraged because they may compromise efficacy in this group of patients. However, it is important to remember that variation in drug exposure still occurs even in the obese patient [48]. Obesity may not be the dominating factor in dose calculation for a patient who has reduced drug elimination for other reasons such as intrinsic variations in metabolism and/or drug transporter function. In other words, it is important to realize that drug elimination for all drugs varies widely between individuals, obese or otherwise, and often this variation eclipses any contribution body size has to drug disposition. In many individuals, accounting for obesity may be of minor importance compared to the normal overriding factors of interpatient variation in drug effect. Obesity is probably of most significance in the situation where the patient has “average” drug metabolism and elimination.
5 Significance of Getting the Wrong Dose
A common argument in support of the continued use of BSA for dose calculation of chemotherapy is that the degree of inaccuracy is not clinically significant. The obvious consequence of incorrect dose calculation is overdose and excessive toxicity, a situation most oncologists have learned to accept. But perhaps a more common but less appreciated consequence of using BSA alone is underdosing and reduced drug effect.
Individuals vary in their capability to eliminate xenobiotics by four- to tenfolds [44]. For drugs with a wide therapeutic window such as some antibiotics, this problem is not crucial since the recommended dose can be pitched towards the high end of the dose range without fear of significant dose-related toxicity. On the other hand, most cytotoxic drugs have a narrow therapeutic window. The dose that causes unacceptable or even fatal toxicity is not much higher than the optimal dose needed for anticancer effect for many drugs. For this reason, the main endpoint of dose-finding studies has traditionally been prevention of unacceptable toxicity. Coupling this with the wide interpatient variability in drug disposition, conservatism becomes intrinsic to the dose recommendation process for anticancer drugs. Mean dose is pitched towards the low range to minimize the number of patients with severe toxicity, and consequently a substantial proportion of cancer patients may be inadvertently underdosed (Fig. 2) [48].
Scheme of a phase I study for a drug with linear pharmacokinetics. The horizontal lines represent the variation in systemic exposure at various dose levels. At dose level 3, those patients with lower drug elimination capability develop dose-limiting toxicity and subsequently that dose level is defined as the maximum tolerated dose. Dose level 2 is recommended for phase II studies since it causes tolerable toxicity in all patients. However, due to the variation in drug handling, a proportion of patients will be relatively underdosed since they are more capable of eliminating the drug. This means the wide distribution of systemic exposure is skewed towards the ineffective range when dose is calculated using BSA
A number of studies have shown a significantly worse antitumor effect for those patients who failed to develop myelosuppression after treatment compared to those who did in patients receiving chemotherapy for breast cancer, ovarian cancer, advanced testicular cancer, and lung cancer [21, 47]. Similarly, lack of skin rash has been associated with worse outcome for lung cancer patients treated with erlotinib and for lack of hypertension with sunitinib therapy for renal cell cancer [103, 128].
Pharmacokinetic evidence for inadvertent underdosing and its consequence in cytotoxic drugs have been demonstrated clearly in a series of studies by Gamelin et al. [33]. This group first established an AUC0-8 of 20–25 mg h/L as the optimal level with a regimen using 5 FU in a dose of 1,300 mg/m2 infused over 8 h every week [32, 35]. In a group of 81 patients treated with dose calculated using BSA, 80 % of patients were found to have an ineffective 5 FU plasma concentration after the first dose [33]. In a subsequent study in 2008, they showed that pharmacokinetically guided 5 FU dose adjustment (targeted AUC 20–25 mg h/L) led to significantly improved objective response rate, a trend to higher survival rate, and fewer grade 3/4 toxicities, comparing to fixed BSA-based dosing [34].
Small molecule inhibitors and monoclonal antibodies (mAbs) that target key components of the signal transduction pathways that are commonly activated in cancer are transforming the care of patients with cancer. Some of these therapies, particularly the antibodies, have a larger therapeutic window than conventional chemotherapy and therefore have minimal acute toxicity at levels of drug exposure that causes an anticancer effect. Examples include the CD20 antibody rituximab, the EGFR antibody cetuximab, and the Her2 antibody trastuzumab. On the other hand, some of these new agents have significant toxicity at doses not far above those required for an effect on the tumor. Examples in this category include the kinase inhibitors sunitinib, sorafenib, and imatinib. But clear exposure–effects relationships have been reported consistently both in animal model and clinical studies (Table 4). During the early pharmacokinetic studies of imatinib, La Coutre et al. treated Bcr–Abl tumor-bearing nude mice with a regimen that assured a continuous block of Bcr–Abl kinase activity [66]. Administration of imatinib three times per day, over an 11-day period, cured 87–100 % of treated mice, whereas administration once or twice a day did not. This suggested that continuous adequate exposure is critical to the success of this inhibitor as a therapeutic agent [25]. Several studies showed that the mean plasma imatinib concentrations in nonresponder were significantly lower than those in responders for CML patients receiving a fixed standard dose [96, 108, 112]. In mouse xenograft models, sunitinib inhibited target receptors when plasma concentrations reached between 50 and 100 ng/mL, and similar results were obtained in a functional assay of VEGF-induced vascular permeability in vivo [77]. Subsequently, this concentration was selected to be the target for clinical applications. In a phase 1 study, sunitinib trough levels above 50 ng/mL were associated with tumor response [28]. In a study of sunitinib in patients with gastrointestinal stromal tumors (GIST), at the currently recommended dose of 50 mg/day, 21 % of patients (10 of 48) achieved trough concentrations of sunitinib and its major active metabolites SU12662 below 50 ng/mL [20].
6 Can Fixed Dose Be Used?
6.1 Cytotoxic Drugs
Since body size is not useful for the majority of anticancer drugs, is it reasonable to use a fixed dose for all patients [18, 76]? The advantages for using fixed doses of cytotoxic drugs are many including financial and safety issues. For example, what is the additional cost of prescribing 305 mg of paclitaxel instead of 300 mg? Can 215 mg of DTIC (instead of 200 or 220 mg), 85 mg of docetaxel (instead of 80 or 90 mg), or 63 mg of methotrexate be accurately compounded? The decimal point can easily be missed by an inexperienced technician when 2.2 mg of vincristine is prescribed.
Flat-fixed dosing has been studied for several cytotoxic drugs, including irinotecan [17], capecitabine [111], cisplatin [18], and paclitaxel [78, 85, 114] with or without comparison with BSA-based dosing. As predicted, fixed doses may result in comparable pharmacokinetic variability in some drugs, but it is no more accurate than BSA-derived dose. A large interpatient variation in drug exposure will remain with all the implications of overdosing and underdosing as discussed above. Our group has examined the use of epirubicin (150 mg continuous infusion) and vinorelbine (60 mg every 21 days) in separate studies and found that this approach was safe for both drugs [45, 137]. However, interpatient variability in clearance was still eightfold and fourfold, respectively, for each drug which is similar to the variability if BSA was used for dose calculation [45].
Loos et al. compared flat-fixed dosing of cisplatin with BSA-based dosing in 25 Dutch patients with extreme BSA values (exceeding the average ± 1 standard deviation). The results suggested that a fixed dose calculated on the average BSA of all patients might lead to exacerbated toxicities in small patients and underdosing in large patients. This resulted in the recommendation of fixed dose per BSA cluster (e.g., 100 mg for patients with BSA ≤ 1.65 m2; 130 mg for those with BSA between 1.66 and 2.04 m2; 150 mg for those with BSA ≥ 2.05 m2) [70].
6.2 Targeted Agents
6.2.1 Flat Dose in Targeted Agents
Small molecular inhibitors are mainly metabolized by the phase I liver enzyme CYP3A and are substrates for the ABC transporters [130]. Given the potential substantial interindividual variation in the activity of these mechanisms [52], it is not surprising to see the wide interindividual variation in systemic exposure of most small molecular inhibitors (Table 5). For example, one study showed a fivefold variation in estimated imatinib clearance (CL/F) on day 1 with fixed dose (600 mg/day) in patients with CML and GIST [49]. This implies that a fixed dose should not be used for these agents unless they have a very wide therapeutic window. In general terms, the small molecules are less specific in their action compared to antibody therapies and are associated with more off-target toxicity. Strangely, the more toxic small molecules are given as a fixed-flat dose, while the antibody therapies, which have less acute toxicity, are dosed according to body weight or BSA. For example, in phase III studies of 4 weeks on 2 weeks off schedule of sunitinib, 38 % of patients with metastatic renal cell carcinoma and 28 % of patients with GIST required dose interruption, whereas a dose reduction was necessary in 32 % and 11 %, respectively [20, 84]. However, some small molecules are less toxic. A maximum tolerated dose for imatinib was never reached in the phase 1 trials of imatinib [26] and some studies have used a high fixed dose (800 mg/day) so that almost all patients reach a pharmacokinetic threshold where the drug might be active if the target is willing.
For some of these targeted agents, the toxicity is so low that a “lack of toxicity trigger” for dose increase for these drugs cannot be depended upon. However, these same drugs may be suitable for a high fixed dose, ensuring an active drug concentration is achieved provided that drug exposure-dependent cumulative toxicity (such as cardiotoxicity) is not present.
Small molecule inhibitors are largely given orally and continuously which introduces additional factors that can increase variation in drug exposure apart from body size. Oral bioavailability of some small molecular inhibitors is highly dependent on gastrointestinal absorption and first-pass drug metabolism by the liver, two processes that both vary considerably among individuals. For example, high-fat meals can lead to more than threefold increase of AUC of lapatinib [100] and 82 % increase in bioavailability of nilotinib [122]. On the other hand, no obvious influence of food is found with sorafenib [120], imatinib [38], or sunitinib [4]. Additionally, patient adherence to oral antineoplastic agents is quite variable with reported adherence rates ranging from 20 to 100 % [93].
Chronic administration may cause induction of drug elimination pathways [10]. For example, imatinib clearance increased by 33 % after chronic exposure over 12 months in one study [62]. This may be a contributing factor in the amelioration of imatinib toxicity that occurs with time or partial overcome of tumor resistance by imatinib dose escalation in CML patients [115].
6.2.2 Therapeutic Drug Monitoring in Small Molecular Targeted Therapy
Therapeutic drug monitoring (TDM) entails the measurement and interpretation of drug concentration in biological fluids and the individualization of drug dosages or schedules to maximize the therapeutic effect and to minimize toxicities [82]. Trough drug level, an indicator for drug level at steady state, has been used as a useful and practical TDM method and provided valuable guidance for dose adjustment in several selected drugs, including antibiotics, immunosuppressives, antiepileptic, and anti-HIV treatment. The use of TDM in traditional cytotoxic drugs, however, has been limited to few drugs only [41, 94, 136], due to several factors including lack of established “therapeutic ranges” and concentration/effect relationship, frequent use of combined drugs with overlapping therapeutic and toxic effects, and intermittent drug schedules [36].
Clear concentration and effect relationship has been shown in several small molecules (Table 4). For example, a number of studies have demonstrated that trough imatinib levels were strongly associated with efficacy in patients with chronic myeloid leukemia [96, 112]. Trough sorafenib concentrations were evaluated in 67 patients in early phase studies and were found to be moderately predictive of prolonged progression-free survival (sorafenib investigator brochure). Dose-limiting toxicities of sunitinib were associated with combined trough levels of sunitinib and SU 12662, an equipotent metabolite (Sunitinib investigator brochure).
Similarly, correlation of trough drug level and clinical outcomes were also found in monoclonal antibodies. In a Japanese study, serum trough levels of rituximab of responders were higher than nonresponder [125]. Fracasso et al. recently reported a correlation between cetuximab trough levels and antitumor response on cetuximab monotherapy [30].
Based on these findings and the fact that small molecules are given chronically and usually as a single agent, trough level monitoring may be a useful tool to ensure an effective target concentration is maintained.
7 A Compromise
Since BSA-based dosing is inaccurate in most anticancer drugs and it is unlikely that using a single fixed dose for all patients is the answer, consideration should be given to using a range of “fixed doses” for a particular drug that could be used as the starting dose and for dose adjustments. However, the original question remains. How should we determine the starting dose for anticancer drugs? The answer must be in defining ways to predict drug handling in each individual. We do this currently when carboplatin is dosed using GFR. However, as previously stated, the use of simple formulae for other drugs will not be possible because of complex elimination mechanisms. Complex formulae using obscure parameters also should not be favored. Dose calculation must be kept relatively simple to allow the busy clinician to adopt any new system.
Studies are underway to define the drug handling genotype and phenotype before drug administration so an individualized dose can be given on the first cycle [58, 102, 119]. Assessment of both hepatic metabolism and active biliary excretion is essential since these are the important elimination processes for the majority of cytotoxic drugs. Such in vivo tests of drug handling would have the advantage of being applicable to a range of cytotoxic and non-cytotoxic drugs, cleared by similar mechanisms.
Tamoxifen is activated to endoxifen by CYP2D6 and breast cancer patients with certain polymorphisms of this gene have lower endoxifen levels and may have worse anticancer outcome [53, 64]. We are undertaking a trial to determine whether dose escalation in such patients will overcome the detrimental effect of possessing particular CYP2D6 polymorphisms (ClinicalTrials.gov Identifier: NCT01075802). As we learn more about the pharmacogenetics of other drugs, similar fixed dose-range system for dose calculation could be applied for other anticancer drugs based on genotype. A number of polymorphisms of the UGT1A1 and other genes are associated with a variation in irinotecan exposure and toxicity, but so far a dose cluster recommendation based on different genotypes has not been made [59, 126].
One scenario worth investigating is whether pretreatment in vivo tests of genotype or phenotype can identify the estimated 20–30 % of patients who fall into the extremes of drug elimination capability. The starting dose can then be selected from a range of fixed doses according to low, normal, or high drug elimination/disposition type. If body size is found in phase 1/2 studies to be important in determining variability of drug exposure, then this can also be accounted for also. Fine-tuning of doses can be based on the presence or absence of toxicity or some other parameter that measures biological effect or by therapeutic drug monitoring. An example of development of such a method for dose calculation of a theoretical new drug is summarized in Table 6.
In summary, body size should be only one of a number of key parameters that are considered when determining chemotherapy dose for a new drug. For some drugs the effect of body size on drug disposition will be insignificant. For others, body size may contribute up to 30 % of interpatient variability. Body size may theoretically affect peak plasma concentrations for drugs with a low volume of distribution and care should be exercised when examining these drugs in phase 1 studies.
It should not be assumed that body size affects drug disposition of a new drug. This parameter should be examined in phase 1 studies along with other parameters after a fixed dose is given. For this reason, individuals with extremes of body size should be excluded from initial phase 1 studies. Drug disposition in individuals with extremes of body size should be examined in separate studies if appropriate, as occurs with other factors such as renal and hepatic function. Special attention should be applied to factors that are probably more important in determining variability such as measures of drug elimination phenotype and genotype. These should not be confined to drug metabolism alone but also include transmembrane influx and efflux pumps and key regulatory nuclear receptors.
References
Baker RJ, Kozoll DD, Meyer KA (1957) The use of surface area as a basis for establishing normal blood volume. Surg Gynecol Obstet 104:183–189
Baselga J, Carbonell X, Castaneda-Soto NJ et al (2005) Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol 23:2162–2171
Beckman RA, Weiner LM, Davis HM (2007) Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer 109:170–179
Bello CL, Sherman L, Zhou J et al (2006) Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs 17:353–358
Bence AK, Anderson EB, Halepota MA et al (2005) Phase I pharmacokinetic studies evaluating single and multiple doses of oral GW572016, a dual EGFR-ErbB2 inhibitor, in healthy subjects. Invest New Drugs 23:39–49
Britten CD, Kabbinavar F, Hecht JR et al (2008) A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol 61:515–524
Bruno R, Hille D, Riva A et al (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Bruno R, Vivier N, Vergniol JC et al (1996) A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J Pharmacokinet Biopharm 24:153–172
Bruno R, Washington CB, Lu JF et al (2005) Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 56:361–369
Burger H, Nooter K (2004) Pharmacokinetic resistance to imatinib mesylate: role of the ABC drug pumps ABCG2 (BCRP) and ABCB1 (MDR1) in the oral bioavailability of imatinib. Cell Cycle 3:1502–1505
Burris HA 3rd, Hurwitz HI, Dees EC et al (2005) Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol 23:5305–5313
Calvert AH, Newell DR, Gumbrell LA et al (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Caraco Y, Zylber-Katz E, Berry EM et al (1995) Antipyrine disposition in obesity: evidence for negligible effect of obesity on hepatic oxidative metabolism. Eur J Clin Pharmacol 47:525–530
Cheymol G (1993) Clinical pharmacokinetics of drugs in obesity. An update. Clin Pharmacokinet 25:103–114
Colleoni M, Li S, Gelber RD et al (2005) Relation between chemotherapy dose, oestrogen receptor expression, and body-mass index. Lancet 366:1108–1110
Crawford JD, Terry ME, Rourke GM (1950) Simplification of drug dosage calculation by application of the surface area principle. Pediatrics 5:783–790
de Jong FA, Mathijssen RH, Xie R et al (2004) Flat-fixed dosing of irinotecan: influence on pharmacokinetic and pharmacodynamic variability. Clin Cancer Res 10:4068–4071
de Jongh FE, Verweij J, Loos WJ et al (2001) Body-surface area-based dosing does not increase accuracy of predicting cisplatin exposure. J Clin Oncol 19:3733–3739
Delbaldo C, Chatelut E, Re M et al (2006) Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res 12:6073–6078
Demetri GD, van Oosterom AT, Garrett CR et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Di Maio M, Gridelli C, Gallo C et al (2005) Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol 6:669–677
Dirks NL, Nolting A, Kovar A et al (2008) Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol 48:267–278
Dobbs NA, Twelves CJ (1998) What is the effect of adjusting epirubicin doses for body surface area? Br J Cancer 78:662–666
Dooley MJ, Poole SG (2000) Poor correlation between body surface area and glomerular filtration rate. Cancer Chemother Pharmacol 46:523–526
Druker BJ, Lydon NB (2000) Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J Clin Invest 105:3–7
Druker BJ, Talpaz M, Resta DJ et al (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031–1037
DuBois D (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17:863–871
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24:25–35
Fleming RA, Eldridge RM, Johnson CE et al (1991) Disposition of high-dose methotrexate in an obese cancer patient. Cancer 68:1247–1250
Fracasso PM, Burris H 3rd, Arquette MA et al (2007) A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res 13:986–993
Freireich EJ, Gehan EA, Rall DP et al (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 50:219–244
Gamelin E, Boisdron-Celle M, Delva R et al (1998) Long-term weekly treatment of colorectal metastatic cancer with fluorouracil and leucovorin: results of a multicentric prospective trial of fluorouracil dosage optimization by pharmacokinetic monitoring in 152 patients. J Clin Oncol 16:1470–1478
Gamelin E, Boisdron-Celle M, Guerin-Meyer V et al (1999) Correlation between uracil and dihydrouracil plasma ratio, fluorouracil (5-FU) pharmacokinetic parameters, and tolerance in patients with advanced colorectal cancer: a potential interest for predicting 5-FU toxicity and determining optimal 5-FU dosage. J Clin Oncol 17:1105
Gamelin E, Delva R, Jacob J et al (2008) Individual fluorouracil dose adjustment based on pharmacokinetic follow-up compared with conventional dosage: results of a multicenter randomized trial of patients with metastatic colorectal cancer. J Clin Oncol 26:2099–2105
Gamelin EC, Danquechin-Dorval EM, Dumesnil YF et al (1996) Relationship between 5-fluorouracil (5-FU) dose intensity and therapeutic response in patients with advanced colorectal cancer receiving infusional therapy containing 5-FU. Cancer 77:441–451
Gao B, Klumpen HJ, Gurney H (2008) Dose calculation of anticancer drugs. Expert Opin Drug Metab Toxicol 4:1307–1319
Gianni L, Kearns CM, Giani A et al (1995) Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13:180–190
Gibbons J, Egorin MJ, Ramanathan RK et al (2008) Phase I and pharmacokinetic study of imatinib mesylate in patients with advanced malignancies and varying degrees of renal dysfunction: a study by the National Cancer Institute Organ Dysfunction Working Group. J Clin Oncol 26:570–576
Gibbs JP, Gooley T, Corneau B et al (1999) The impact of obesity and disease on busulfan oral clearance in adults. Blood 93:4436–4440
Goldsmith MA, Slavik M, Carter SK (1975) Quantitative prediction of drug toxicity in humans from toxicology in small and large animals. Cancer Res 35:1354–1364
Graf N, Winkler K, Betlemovic M et al (1994) Methotrexate pharmacokinetics and prognosis in osteosarcoma. J Clin Oncol 12:1443–1451
Griggs JJ, Sorbero ME, Lyman GH (2005) Undertreatment of obese women receiving breast cancer chemotherapy. Arch Intern Med 165:1267–1273
Grochow LB, Baraldi C, Noe D (1990) Is dose normalization to weight or body surface area useful in adults? J Natl Cancer Inst 82:323–325
Gurney H (1996) Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol 14:2590–2611
Gurney H, Ackland S, Gebski V et al (1998) Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol 16(7):2299–2304
Gurney H (2001) Determining the drug elimination phenotype: hepatic sestamibi scan and midazolam clearance as in vivo tests for drug metabolism and biliary elimination. Proc Am Soc Clin Oncol 20:abs 305
Gurney H (2005) I don’t underdose my patients…do I? Lancet Oncol 6:637–638
Gurney H, Shaw R (2007) Obesity in dose calculation: a mouse or an elephant? J Clin Oncol 25:4703–4704
Gurney H, Wong M, Balleine RL et al (2007) Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther 82:33–40
Hammond LA, Eckardt JR, Baker SD et al (1999) Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol 17:2604–2613
Hidalgo M, Bloedow D (2003) Pharmacokinetics and pharmacodynamics: maximizing the clinical potential of Erlotinib (Tarceva). Semin Oncol 30:25–33
Ho RH, Kim RB (2005) Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther 78:260–277
Hoskins JM, Carey LA, McLeod HL (2009) CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer 9:576–586
Houk BE, Bello CL, Kang D et al (2009) A population pharmacokinetic meta-analysis of sunitinib malate (SU11248) and its primary metabolite (SU12662) in healthy volunteers and oncology patients. Clin Cancer Res 15:2497–2506
Houk BE, Bello CL, Poland B et al (2009) Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic meta-analysis. Cancer Chemother Pharmacol 66:357–371
Huizing MT, Vermorken JB, Rosing H et al (1995) Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: a European Cancer Centre (ECC) trial. Ann Oncol 6:699–704
Hunt CM, Westerkam WR, Stave GM (1992) Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol 44:275–283
Innocenti F, Iyer L, Ratain MJ (2000) Pharmacogenetics: a tool for individualizing antineoplastic therapy. Clin Pharmacokinet 39:315–325
Innocenti F, Kroetz DL, Schuetz E et al (2009) Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J Clin Oncol 27:2604–2614
Jen JF, Cutler DL, Pai SM et al (2000) Population pharmacokinetics of temozolomide in cancer patients. Pharm Res 17:1284–1289
Jenkins P, Elyan S, Freeman S (2007) Obesity is not associated with increased myelosuppression in patients receiving chemotherapy for breast cancer. Eur J Cancer 43:544–548
Judson I, Ma P, Peng B et al (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55:379–386
Kawaguchi T, Hamada A, Hirayama C et al (2009) Relationship between an effective dose of imatinib, body surface area, and trough drug levels in patients with chronic myeloid leukemia. Int J Hematol 89:642–648
Kiyotani K, Mushiroda T, Imamura CK et al (2010) Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 28:1287–1293
le Coutre P, Kreuzer KA, Pursche S et al (2004) Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol 53:313–323
le Coutre P, Mologni L, Cleris L et al (1999) In vivo eradication of human BCR/ABL-positive leukemia cells with an ABL kinase inhibitor. J Natl Cancer Inst 91:163–168
Leyland-Jones B, Gelmon K, Ayoub JP et al (2003) Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 21:3965–3971
Li J, Brahmer J, Messersmith W et al (2006) Binding of gefitinib, an inhibitor of epidermal growth factor receptor-tyrosine kinase, to plasma proteins and blood cells: in vitro and in cancer patients. Invest New Drugs 24:291–297
Lind MJ, Margison JM, Cerny T et al (1989) Prolongation of ifosfamide elimination half-life in obese patients due to altered drug distribution. Cancer Chemother Pharmacol 25:139–142
Loos WJ, de Jongh FE, Sparreboom A et al (2006) Evaluation of an alternate dosing strategy for cisplatin in patients with extreme body surface area values. J Clin Oncol 24:1499–1506
Loos WJ, Gelderblom H, Sparreboom A et al (2000) Inter- and intrapatient variability in oral topotecan pharmacokinetics: implications for body-surface area dosage regimens. Clin Cancer Res 6:2685–2689
Lu JF, Bruno R, Eppler S et al (2008) Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 62:779–786
Lu JF, Eppler SM, Wolf J et al (2006) Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer. Clin Pharmacol Ther 80:136–145
Madden T, Sunderland M, Santana VM et al (1992) The pharmacokinetics of high-dose carboplatin in pediatric patients with cancer. Clin Pharmacol Ther 51:701–707
Maloney DG, Grillo-Lopez AJ, Bodkin DJ et al (1997) IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 15:3266–3274
Mathijssen RH, Verweij J, de Jonge MJ et al (2002) Impact of body-size measures on irinotecan clearance: alternative dosing recommendations. J Clin Oncol 20:81–87
Mendel DB, Laird AD, Xin X et al (2003) In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 9:327–337
Miller AA, Rosner GL, Egorin MJ et al (2004) Prospective evaluation of body surface area as a determinant of paclitaxel pharmacokinetics and pharmacodynamics in women with solid tumors: Cancer and Leukemia Group B Study 9763. Clin Cancer Res 10:8325–8331
Millward MJ, Newell DR, Yuen K et al (1995) Pharmacokinetics and pharmacodynamics of prolonged oral etoposide in women with metastatic breast cancer. Cancer Chemother Pharmacol 37:161–167
Miya T, Goya T, Fujii H et al (2001) Factors affecting the pharmacokinetics of CPT-11: the body mass index, age and sex are independent predictors of pharmacokinetic parameters of CPT-11. Invest New Drugs 19:61–67
Miya T, Goya T, Yanagida O et al (1998) The influence of relative body weight on toxicity of combination chemotherapy with cisplatin and etoposide. Cancer Chemother Pharmacol 42:386–390
Moore MJ, Erlichman C (1987) Therapeutic drug monitoring in oncology. Problems and potential in antineoplastic therapy. Clin Pharmacokinet 13:205–227
Morgan DJ, Bray KM (1994) Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet 26:292–307
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Mross K, Hollander N, Frost A et al (2006) PAC fixed dose: pharmacokinetics of a 1-hour paclitaxel infusion and comparison to BSA-normalized drug dosing. Onkologie 29:444–450
Murry DJ, Oermann CM, Ou CN et al (1999) Pharmacokinetics of ibuprofen in patients with cystic fibrosis. Pharmacotherapy 19:340–345
Nawaratne S, Brien JE, Seeman E et al (1998) Relationships among liver and kidney volumes, lean body mass and drug clearance. Br J Clin Pharmacol 46:447–452
Newsome BW, Ernstoff MS (2008) The clinical pharmacology of therapeutic monoclonal antibodies in the treatment of malignancy; have the magic bullets arrived? Br J Clin Pharmacol 66:6–19
Ng CM, Bruno R, Combs D et al (2005) Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol 45:792–801
Nguyen L, Chatelut E, Chevreau C et al (1998) Population pharmacokinetics of total and unbound etoposide. Cancer Chemother Pharmacol 41:125–132
Nguyen L, Tranchand B, Puozzo C et al (2002) Population pharmacokinetics model and limited sampling strategy for intravenous vinorelbine derived from phase I clinical trials. Br J Clin Pharmacol 53:459–468
Nikolova Z, Peng B, Hubert M et al (2004) Bioequivalence, safety, and tolerability of imatinib tablets compared with capsules. Cancer Chemother Pharmacol 53:433–438
Partridge AH, Avorn J, Wang PS et al (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94:652–661
Pearson AD, Amineddine HA, Yule M et al (1991) The influence of serum methotrexate concentrations and drug dosage on outcome in childhood acute lymphoblastic leukaemia. Br J Cancer 64:169–173
Peng B, Hayes M, Resta D et al (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22:935–942
Picard S, Titier K, Etienne G et al (2007) Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109:3496–3499
Pinkel D (1958) The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res 18:853–856
Powis G, Reece P, Ahmann DL et al (1987) Effect of body weight on the pharmacokinetics of cyclophosphamide in breast cancer patients. Cancer Chemother Pharmacol 20:219–222
Ratain MJ (1998) Body-surface area as a basis for dosing of anticancer agents: science, myth, or habit? J Clin Oncol 16:2297–2298
Ratain MJ, Cohen EE (2007) The value meal: how to save $1,700 per month or more on lapatinib. J Clin Oncol 25:3397–3398
Reilly JJ, Workman P (1993) Normalisation of anti-cancer drug dosage using body weight and surface area: is it worthwhile? A review of theoretical and practical considerations. Cancer Chemother Pharmacol 32:411–418
Rivory LP, Slaviero K, Seale JP et al (2000) Optimizing the erythromycin breath test for use in cancer patients. Clin Cancer Res 6:3480–3485
Rixe O, Billemont B, Izzedine H (2007) Hypertension as a predictive factor of Sunitinib activity. Ann Oncol 18:1117
Rodvold KA, Rushing DA, Tewksbury DA (1988) Doxorubicin clearance in the obese. J Clin Oncol 6:1321–1327
Roland M (1994) Clinical pharmacokinetics. Concepts and applications, 3rd edn. Williams & Wilkins, Baltimore
Rosner GL, Hargis JB, Hollis DR et al (1996) Relationship between toxicity and obesity in women receiving adjuvant chemotherapy for breast cancer: results from cancer and leukemia group B study 8541. J Clin Oncol 14:3000–3008
Rothenberg ML, Kuhn JG, Schaaf LJ et al (2001) Phase I dose-finding and pharmacokinetic trial of irinotecan (CPT-11) administered every two weeks. Ann Oncol 12:1631–1641
Sakai M, Miyazaki Y, Matsuo E et al (2009) Long-term efficacy of imatinib in a practical setting is correlated with imatinib trough concentration that is influenced by body size: a report by the Nagasaki CML Study Group. Int J Hematol 89:319–325
Sam WJ, Aw M, Quak SH et al (2000) Population pharmacokinetics of tacrolimus in Asian paediatric liver transplant patients. Br J Clin Pharmacol 50:531–541
Schnegg M, Lauterburg BH (1986) Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation and caffeine clearance. J Hepatol 3:164–171
Sharma R, Rivory L, Beale P et al (2006) A phase II study of fixed-dose capecitabine and assessment of predictors of toxicity in patients with advanced/metastatic colorectal cancer. Br J Cancer 94:964–968
Singh N, Kumar L, Meena R et al (2009) Drug monitoring of imatinib levels in patients undergoing therapy for chronic myeloid leukaemia: comparing plasma levels of responders and non-responders. Eur J Clin Pharmacol 65(6):545–549
Smith HW (1951) The kidney, structure and henobar in health and disease. Oxford University Press, New York
Smorenburg CH, Sparreboom A, Bontenbal M et al (2003) Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J Clin Oncol 21:197–202
Sohn SK, Moon JH, Cho YY et al (2007) Efficacy of dose escalation of imatinib mesylate in patients with cytogenetic or hematologic resistance. Leuk Lymphoma 48:1659–1661
Soulieres D (2003) Identifying predictive and surrogate markers of erlotinib antitumor activity other than rash. Oncology (Williston Park) 17:29–33
Soulieres D, Senzer NN, Vokes EE et al (2004) Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol 22:77–85
Sparreboom A, Wolff AC, Mathijssen RH et al (2007) Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol 25:4707–4713
Stoehlmacher J, Park DJ, Zhang W et al (2002) Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst 94:936–942
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23:965–972
Tabrizi MA, Tseng CM, Roskos LK (2006) Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 11:81–88
Tanaka C, Yin OQ, Sethuraman V et al (2010) Clinical pharmacokinetics of the BCR-ABL tyrosine kinase inhibitor nilotinib. Clin Pharmacol Ther 87:197–203
Thomas F, Rochaix P, White-Koning M et al (2009) Population pharmacokinetics of erlotinib and its pharmacokinetic/pharmacodynamic relationships in head and neck squamous cell carcinoma. Eur J Cancer 45:2316–2323
Tjuljandin SA, Doig RG, Sobol MM et al (1990) Pharmacokinetics and toxicity of two schedules of high dose epirubicin. Cancer Res 50:5095–5101
Tobinai K, Igarashi T, Itoh K et al (2004) Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol 15:821–830
Toffoli G, Cecchin E, Gasparini G et al (2010) Genotype-driven phase I study of irinotecan administered in combination with fluorouracil/leucovorin in patients with metastatic colorectal cancer. J Clin Oncol 28:866–871
Touw DJ, Graafland O, Cranendonk A et al (2000) Clinical pharmacokinetics of phenobarbital in neonates. Eur J Pharm Sci 12:111–116
Townsley CA, Major P, Siu LL et al (2006) Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer 94:1136–1143
van der Gaast A, Vlastuin M, Kok TC et al (1992) What is the optimal dose and duration of treatment with etoposide? II. Comparative pharmacokinetic study of three schedules: 1 x 100 mg, 2 x 50 mg, and 4 x 25 mg of oral etoposide daily for 21 days. Semin Oncol 19:8–12
van Erp NP, Gelderblom H, Guchelaar HJ (2009) Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat Rev 35:692–706
van Erp NP, Eechoute K, van der Veldt AA et al (2009) Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol 27:4406–4412
Verweij J, Casali PG, Zalcberg J et al (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364:1127–1134
Wacker B, Nagrani T, Weinberg J et al (2007) Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 13:3913–3921
Wen PY, Yung WK, Lamborn KR et al (2006) Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res 12:4899–4907
Widmer N, Decosterd LA, Csajka C et al (2006) Population pharmacokinetics of imatinib and the role of alpha-acid glycoprotein. Br J Clin Pharmacol 62:97–112
Wolfrom C, Hepp R, Hartmann R et al (1990) Pharmacokinetic study of methotrexate, folinic acid and their serum metabolites in children treated with high-dose methotrexate and leucovorin rescue. Eur J Clin Pharmacol 39:377–383
Wong M, Balleine RL, Blair EY et al (2006) Predictors of vinorelbine pharmacokinetics and pharmacodynamics in patients with cancer. J Clin Oncol 24(16):2448–2455
Xuan D, Lu JF, Nicolau DP et al (2000) Population pharmacokinetics of tobramycin in hospitalized patients receiving once-daily dosing regimen. Int J Antimicrob Agents 15:185–191
Yan L, Hsu K, Beckman RA (2008) Antibody-based therapy for solid tumors. Cancer J 14:178–183
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Gao, B., Klumpen, HJ., Gurney, H. (2014). Defining the Starting Dose: Should It Be mg/kg, mg/m2, or Fixed?. In: Rudek, M., Chau, C., Figg, W., McLeod, H. (eds) Handbook of Anticancer Pharmacokinetics and Pharmacodynamics. Cancer Drug Discovery and Development. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9135-4_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9135-4_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9134-7
Online ISBN: 978-1-4614-9135-4
eBook Packages: MedicineMedicine (R0)