Abstract
Dysregulation of intracellular Ca2+ is a major cause of neurologic dysfunction and likely plays an important role in the pathophysiology of numerous acute and chronic neurodegenerative conditions. The Ca2+-dependent protease, calpain, and the Ca2+/calmodulin (Ca2+/CaM)-dependent protein phosphatase, calcineurin, are primary effectors of multiple deleterious functions arising from altered Ca2+ handling. Increasing evidence suggests that the calpain-dependent, irreversible conversion of calcineurin to a constitutively active phosphatase occurs in intact cellular systems as a result of injury and disease. In this chapter, a brief overview of calpain and calcineurin functions in nervous tissue is given, followed by a more in-depth discussion of calpain/calcineurin interactions in vitro and in vivo. Particular emphasis is placed on recent studies that have identified calpain proteolysis of calcineurin as a key step in neurodegeneration associated with acute neurologic insults as well as chronic terminal diseases, like Alzheimer’s.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The calcium ion (Ca2+) is a ubiquitous messenger involved in countless, diverse cellular functions. In biological systems, Ca2+ leads a dual existence of sorts. On the one hand, Ca2+ is essential for life. In the nervous system, the release of neurotransmitters, remodeling of growth cones and dendritic spines in response to extracellular stimuli, activation and termination of transcriptional programs at the proper stages of development, and many, many other cellular functions depend critically on Ca2+. On the other hand, Ca2+ is also commonly the prelude to cellular degeneration and death. Studies in the early mid-1980s suggested that neuronal Ca2+ regulation is disrupted during normal aging, leading to deleterious changes in neuronal excitability and plasticity [1–4]. Around the same time, cytosolic Ca2+ overload was demonstrated to be one of the primary mechanisms of neuronal death following excitotoxic insults [5–7]. These findings led to the hypothesis that Ca2+ dysregulation is a general mechanism for neurologic dysfunction and/or neurodegeneration associated with aging, stroke, acute brain injury, and progressive neurodegenerative diseases [1–3, 8–13]. Today, the Ca2+ hypothesis remains viable but has evolved in important ways to emphasize selective changes in discrete Ca2+ signaling mechanisms in different cell types and/or in different disorders (e.g., see [14–23]).
Of the numerous Ca2+-sensitive proteins and enzymes, the protease calpain and the phosphatase calcineurin have emerged as two of the most common effectors of Ca2+-induced dysfunction and degeneration. Interestingly, calpains and calcineurin are present in many of the same subcellular domains and exhibit similarly high levels of activity following many of the same types of insults. Comparable changes in the expression/activity of calpains and calcineurin have also been observed in several distinct neurodegenerative diseases and/or conditions, while pharmacologic and genetic inhibitors of these enzymes ameliorate deleterious changes in common biomarkers. Taken together, the evidence suggests that calpain/calcineurin interactions may be a fundamental neurodegenerative mechanism and an opportune target for future therapeutic strategies. The purpose of this chapter is to provide a brief review of calpains and calcineurin and their roles in neurologic dysfunction, with particular emphasis placed on calcineurin signaling and the ramifications of calcineurin proteolysis in human neurodegenerative disease. Outstanding comprehensive reviews of the biochemistry and regulation of each of these Ca2+-dependent enzymes (as well as historical backgrounds) can be found here [24–27] (for calpain) and here [28–30] (for calcineurin).
2 Calpain
2.1 Calpain Structure
Calpains are a family of intracellular, nonlysosomal cysteine proteases that belong to the papain superfamily of proteases. Calpains are regulatory proteases found in most mammalian species and show varying degrees of sensitivity to fluctuating Ca2+ concentrations. Calpain proteins are heterodimers consisting of a large (~80 kDa) catalytic subunit and a smaller (28 kDa) regulatory subunit derived from different genes (Fig. 2.1). The catalytic subunit is made up of four distinct domains (I–IV), including a regulatory Ca2+ binding domain (IV) containing five EF-hand motifs; a “C2-like” domain (III) that includes Ca2+ and phospholipid binding sites; a catalytic domain (II) that consists of at least two Ca2+ binding sites along with two proteolytic core subdomains (IIa and IIb) that come together upon Ca2+ binding to form a functional cysteine protease core domain (CysPc); and an N-terminal domain (I) that may be autolyzed upon activation of the holoenzyme. Though relatively poorly understood, the regulatory subunit appears to remain associated with the catalytic subunit during activation [31] (contrary to earlier findings, e.g., see [32]) and is essential for maintaining the stability of the catalytic subunit in vivo. The regulatory subunit contains two domains (I and II): a glycine-rich domain (I) believed to regulate calpain interactions with membranes and/or membrane-related proteins and a Ca2+ binding domain (II) that includes five EF-hand motifs.
μ- and m-calpain subunits. Schematic illustration of the structure of the typical calpain (i.e., μ- and m-calpain) catalytic and regulatory subunit. NT N-terminus region, CysPC calpain-like cysteine protease core region, PEF pentameric EF-hand domain, GR glycine-rich domain. See text for a description of these subunits and their domains
In humans, there are more than a dozen calpain (or calpain-like) catalytic subunit genes (termed CAPN1, CAPN2, CAPN3, …), while there are at least two distinct regulatory subunit genes (CAPNS1 and CAPNS2). These genes are expressed in most cell types and/or tissues, though some genes can show tissue-type specific expression. The best characterized calpain holoenzymes consist of CAPN1 or CAPN2 and are commonly referred to as μ- and m-calpains, respectively (or calpains 1 and 2). CAPN1/μ-calpain is activated by micromolar concentrations of Ca2+in vitro, while CAPN2/m-calpain is activated when Ca2+ is in the millimolar range. In addition to Ca2+, the catalytic activity of calpain is also held in check by endogenous proteins called calpastatins. These proteins very specifically suppress the activity of μ- and m-calpains and are the only known endogenous proteins to serve this function. Unlike the calpain catalytic and regulatory subunits, there is only one human gene for calpastatin (CAST), though splicing variations can give rise to many distinct protein products [25, 27].
While Ca2+ is clearly the critical activating factor for calpains—binding to EF-hand motifs on both subunits as well as to multiple other binding sites within the catalytic and C2-like domains—the precise biochemical mechanisms/interactions that couple Ca2+ binding to increased protease activity have remained surprisingly elusive. One of the central issues is that concentrations of Ca2+ required for calpain activation in vitro seem too high to be physiologically relevant, since cytosolic Ca2+ concentrations are not likely to rise into the high micromolar range. This has led to much speculation that an additional biochemical event—such as autolysis, subunit dissociation, and/or the binding of some other cofactor—is necessary for calpain activation [24].Of these possibilities, Ca2+-dependent autolysis of the N-terminal region of the large calpain subunit has been widely accepted as an essential step in calpain activation. Early studies showed that autolysis reduces the Ca2+ concentration for activation of both μ and m-calpain in vitro [25]. However, many later studies have shown that autolysis is not necessary for activation in vivo, though it may still play an important regulatory function (for in-depth discussions, see [24, 25, 27]). Conversely, it has been suggested (i.e., [24]) that Ca2+ elevations in cellular microdomains (e.g., in postsynaptic spines and/or immediately adjacent to Ca2+ channels) may indeed be high enough to meet calpain activation requirements without the need of autolysis. In this case, calpain activation would only be brief due to the rapid drop in Ca2+ concentration in these microdomains and/or due to inhibition by calpastatins. Prolonged calpain activation would therefore only occur under pathologic conditions in which Ca2+ levels are chronically elevated and/or calpastatin function/expression is downregulated [24].
2.2 Calpain Functions in Nervous Tissue
Calpains are highly expressed in nervous tissue and have long been recognized for their important roles in modulating cellular structure and function. Numerous substrates for calpains have been identified and include cytoskeletal proteins, membrane receptors, ion channels, protein kinases, protein phosphatases (as discussed later), other proteases, and many other protein targets. Consequently, calpains are believed to take part in numerous and diverse signaling cascades. For a comprehensive list of calpain substrates and description of calpain functions, see [25, 27]. One of the earliest proposed functions of calpain in nervous system was the rapid, activity-dependent degradation of cytoskeletal proteins, such as spectrin, leading to the structural reorganization of dendritic spines and other neuronal processes [33–37]. Subsequently, calpain was also shown to target key glutamatergic receptors [38–42], as well as the proteins that modulate glutamate receptor expression/function including membrane-anchoring proteins [43–45] and protein kinases and phosphatases [46–48]. Calpain-mediated cleavage of protein kinases, such as protein kinase C, and phosphatases, such as CN, results in high levels of kinase/phosphatase activity that can persist long after the restoration of basal Ca2+ levels [46, 48]. The reorganization of the dendritic cytoskeleton, along with the generation of so-called memory molecules by calpain, may be critical to the expression and maintenance of long-term synaptic potentiation (LTP) and other forms of synaptic plasticity involved in neurodevelopment and cognition.
In addition to these beneficial functions, calpains also mediate numerous deleterious functions and are commonly implicated in neurodegenerative processes associated with severe Ca2+ dysregulation [26]. High levels of calpain expression/activity (or downregulation of calpastatins) are consistently found in primary neural cultures exposed to ischemia/hypoxia, glutamate/kainate, amyloid-β peptides (Aβ), and numerous other neurotoxic insults (e.g., [36, 48–51]). In intact animal models, elevated forms of activated calpain (both μ and m) have been reported in the brain within hours following injury due to carotid artery occlusion [52, 53], glutamate/kainate insult [48, 54], controlled cortical impact [55], or fluid percussion [56, 57]. In many of these same studies, calpain inhibitors exhibited strong neuroprotective and/or nootropic properties. Aberrant calpain activation also appears to be an excellent biomarker for chronic, progressive neurodegenerative disorders including Alzheimer’s disease (AD) [50, 58, 59], Parkinson’s disease [60], multiple sclerosis [61], and glaucoma [62, 63], to name a few. Moreover, similar to acute injury models, inhibition of calpains using pharmacologic or genetic approaches generally ameliorates functional and pathologic changes in cell culture and/or animal models of these disorders [50, 64–69].
The cellular mechanisms for calpain-mediated neurotoxicity can be difficult to pin down and may vary considerably depending on the brain region/cell type investigated or on the nature of the injury or disease state. One of the difficulties is that calpain interacts with so many different target proteins linked to cell death and degeneration. Indeed, distinct proapoptotic factors including caspase-3, BAX, apoptosis-inducing factor, and several others are directly targeted by calpains and have been proposed to mediate the deleterious actions of calpains in nervous tissue [52, 70–74]. Another complicating issue is that calpains interact extensively with other Ca2+ signaling mechanisms, many of which play a critical role in regulating Ca2+ homeostasis. For instance, several kinds of Ca2+ channels and pumps responsible for shuttling Ca2+ from the cytosol to the extracellular space, or into intracellular stores, are degraded by calpains leading to elevated cytosolic Ca2+ levels [75–77]. Calpains also appear to be involved in the cleavage of the pore-forming subunit of the L-type voltage-sensitive Ca2+ channel to a smaller, higher-conductance channel [78]. Each, or all of these changes, would be expected to exacerbate Ca2+ dysregulation, promoting further calpain activation and/or hyperactivation of other Ca2+-dependent enzymes.
Herein lies an additional complication: Does hyperactivation of other Ca2+-dependent enzymes following injury arise simply from increased Ca2+ binding or from direct calpain-mediated proteolysis? High activity levels resulting from increased Ca2+ binding is perhaps less troublesome because an ebb in cytosolic Ca2+ levels would be expected to result in a corresponding decrease in enzyme activity. Calpain-dependent activation, on the other hand, would appear to be a far greater threat to the cell because the resulting proteolytic enzyme fragments are generally uncoupled from their normal regulatory mechanisms and prone to dangerously high and enduring activity levels. The Ca2+-/calmodulin-dependent protein phosphatase, calcineurin, is one potential target of calpain. The following sections will discuss the structure and function of calcineurin, with particular emphasis on recent studies that have uncovered important calpain/calcineurin interactions in neurodegeneration and disease.
3 Calcineurin
3.1 Calcineurin Structure
Calcineurin, or protein phosphatase 3 (PPP3, formerly protein phosphatase 2b), is a nearly ubiquitously expressed serine/threonine protein phosphatase and the only known phosphatase to exhibit direct regulation by Ca2+/CaM. Calcineurin is typically found in intact cells as a heterodimer (see Fig. 2.2a) consisting of a catalytic subunit (CN A or PPP3C, ~61 kDa) and a smaller regulatory subunit (CN B or PPP3R, ~19 kDa). The catalytic subunit contains the catalytic core region, CN B binding domain, a Ca2+/calmodulin binding domain, and an autoinhibitory domain (AID) near the C-terminus that lies over the cleft of the catalytic domain and precludes substrate binding when cytosolic Ca2+ levels are low [79]. The regulatory CN B subunit is a calmodulin-like Ca2+ binding protein with four EF-hand motifs, two of which show very high affinity for Ca2+ and are likely occupied at normal resting Ca2+ levels (<10 nM). Ca2+ binding to CN B is thought to increase the physical association between CN A and CN B and appears to promote low levels of catalytic activity [80]. Early in vitro experiments suggested that the physical association between CN A and B could only be disrupted under supraphysiologic conditions, such as protein denaturation [80]. However, recent investigations on intact primary neurons indicate an increased physical association between catalytic and regulatory subunits in response to neurotoxic stimuli [81], suggesting the possibility that these subunits are not always bound to one another in vivo. These observations are consistent with other work showing that the CN B subunit can associate with and regulate specific target proteins in a CN A-independent manner [82–85]. Additional distinct roles of the CN B subunit in neurologic function are largely unknown but will likely be forthcoming in the next few years.
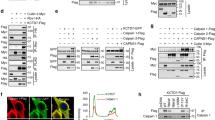
Calcineurin and its regulation by calpain proteolysis. (a) Schematic illustration of the structure of the CN A catalytic and CN B regulatory subunits. See text for a description of these subunits and their domains. (b) Cartoon illustration of the regulation of CN A activity by Ca2+/CaM, the CN A AID, and calpain proteolysis. Under normal conditions when intracellular Ca2+ levels are low, CN A activity is held in check by the CN A AID. When Ca2+ levels rise, Ca2+/CaM binds to the CN A subunit displacing the AID from the catalytic domain, resulting in high levels of phosphatase activity. Under abnormal conditions, like severe Ca2+ dysregulation, the protease calpain cleaves CN A at several locations near the C terminus, thus removing the AID. Without the AID, the CN A catalytic domain is no longer occluded resulting in high levels of phosphatase activity, even after the local Ca2+ concentration falls to basal levels. (c) Schematic illustration of the 57, 48, and 45 kDa CN A fragments generated by calpain-dependent cleavage, as demonstrated by Wu et al. [48]. (d) Western blot showing CN Aα proteolysis to 57 and 48 kDa fragments in primary hippocampal neural cultures 24 h after addition of neurotoxic amyloid-β peptides (Aβ). The lower 37 kDa band in the CN Aα blot was not sensitive to local Ca2+ levels nor to the addition of calpain, suggesting it represents a nonspecific band, a calpain-insensitive fragment, or an alternative splice variant. Proteolytic breakdown of the calpain substrate, a-spectrin, occurred in parallel with CN A proteolysis. Blockade of calpain activity with calpeptin prevented CN Aα proteolysis to 57 and 48 kDa fragments. However, a specific caspase 1 inhibitor (Z-YVAD-FMK) was without effect. Blot shown in panel d was from Mohmmad Abdul et al. [50] and used with permission
There are two major CN A isoforms expressed in brain (CN Aαor PPP3CA and CN Aβ or PPP3CB), of which, the CN Aα isoform is the most abundant [86]. A “testis”-specific CN A isoform (CN Aγ or PPP3CC) is also expressed in nervous tissue but at comparatively much lower levels. At least two regulatory CN B isoforms (CN Bα and CN Bβ or PPP3R1 and PPP3R2) have been characterized. CN Bα exhibits similar expression patterns as CN Aα and CN Aβ, while CN Bβ is testes specific [30]. Although isoform-specific differences in tissue distribution and cellular function have been well characterized outside of the brain, much less is known about the expressional/functional differences of CN Aα and CN Aβ inside the brain. However, as discussed in a later section, studies from our lab have shown that CN Aα is the isoform that exhibits the most striking changes and is most susceptible to calpain-mediated proteolysis during the progression of Alzheimer’s disease (AD) [14, 50, 87].
3.2 Calcineurin Function in Nervous Tissue
Calcineurin is perhaps best known and characterized in T and B lymphocytes where it coordinates transcriptional programs involved in lymphocyte activation, cytokine production, and lymphocyte anergy [88, 89]. However, calcineurin is most abundantly expressed in brain, especially in regions like the hippocampus [90], which is important for learning and memory and highly susceptible to age-related neurodegenerative disease [91, 92]. In fact, calcineurin was originally named for its high abundance in nervous tissue and its critical dependence on Ca2+ [93]. In healthy brain tissue, calcineurin is primarily enriched in neurons [86, 94] where it is highly expressed in dendrites and postsynaptic spines. Glial cells, in contrast, appear to express very low levels of calcineurin under normal conditions [94]. However, after injury, or during aging and age-related neurodegenerative disease, activated glial cells (especially astrocytes) can label very intensely for the presence of calcineurin [87, 95–97]. As discussed below, the major functions of calcineurin are likely very different in neurons and glia.
While not nearly as promiscuous as other related serine/threonine phosphatases (e.g., protein phosphatase 1 and 2a), calcineurin nevertheless acts on a broad range of substrates; many of which are directly involved in the structural and functional regulation of synapses. In neurons, calcineurin has long been known to dephosphorylate a host of cytoskeletal proteins involved in the dynamic modulation of dendritic spines, including MAP2b and cofilin [98–100]. Calcineurin has also been shown to modulate (i.e., reduce) glutamate receptor activity and/or surface expression via direct dephosphorylation of glutamate receptor subunits [101, 102] and/or through indirect activation of protein phosphatase 1 or other accessory proteins [103, 104]. Through these interactions, neuronal calcineurin is widely believed to play an essential role in mediating long-term synaptic depression (LTD) [105].
In addition to its close functional association with the cytoskeleton, calcineurin is also one of the primary mechanisms for coupling fluctuations in cytosolic Ca2+ to changes in gene expression. In neurons, calcineurin-dependent dephosphorylation of transcription factors, such as the cyclic AMP response element binding protein, is widely believed to underlie long-term reductions in key synaptic proteins involved in activity-dependent plasticity and cognitive function [106, 107]. Among the numerous transcription factors that exhibit sensitivity to calcineurin, perhaps none are as closely associated with calcineurin or are more important to overall calcineurin signaling than nuclear factor of activated T cells (NFATs). These transcriptions typically reside in the cytosol in a heavily phosphorylated state when the cell is at rest and Ca2+ levels are low. However, with cellular activation and elevated Ca2+, NFATs are bound tightly by calcineurin and dephosphorylated. This event leads to the transport of NFATs into the nucleus, where they remain until they are re-phosphorylated by a variety of “NFAT kinases” and transported back to the cytosol.
NFATs are clearly best known for their role in coupling calcineurin activation in lymphocytes to the transcriptional induction of numerous cytokines and immune/inflammatory mediators [108]. While much less is known about NFAT functions in neural cells, the existing data suggest these calcineurin-dependent factors play unique roles in different cell types and are likely key players in neurologic dysfunction and disease [14]. In neurons, activation of NFATs leads to the upregulation of proteins involved in Ca2+ signaling and homeostasis including inositol type 3 receptors [109, 110]. In glial cells, NFATs play a critical role in the induction of immune/inflammatory signaling factors, including a number of cytokines [111–113]. These functions are similar to that observed in T and B lymphocytes, as well as other peripheral immune/inflammatory cells [108]. Interestingly, glial-specific excitatory amino acid transporters (EAATs), particularly Glt-1/EAAT2 (i.e., the major glutamate transporter in the brain), also show high sensitivity to calcineurin/NFAT activity [87, 113]. However, unlike many cytokine factors, Glt-1/EAAT2 appears to be downregulated by calcineurin/NFAT in response to inflammatory and/or neurotoxic insults. Thus, in addition to its immune/inflammatory functions, the glial calcineurin/NFAT pathway also appears to be critical for regulating glutamate homeostasis.
Similar to calpain, calcineurin is often a “usual suspect” when it comes to neurodegeneration associated with Ca2+ dysregulation (e.g., see [114]). Through its actions on cytoskeletal proteins and glutamate receptors, neuronal calcineurin has been shown to mediate dendritic spine retraction and/or impaired synaptic function in response to a variety of injurious stimuli (e.g., see [115–118]). Aberrant calcineurin activity has also been linked to cell death cascades through the direct dephosphorylation of proapoptotic factors, such as BAD [119, 120], or through the transcriptional induction of other proapoptotic proteins such as the Fas ligand (FasL) [121]. In glial cells, calcineurin activity induces the expression of numerous proinflammatory mediators [97, 111–113, 122] and promotes glutamate dysregulation and excitotoxicity through activation of NFAT transcription factors [87, 113]. Finally, as alluded to above, the transcriptional and posttranslational modulation of Ca2+ channels and pumps by calcineurin may be a key mechanism for promoting and maintaining neuronal Ca2+ dysregulation in aging and age-related neurodegenerative diseases [109, 123–125].
Consistent with these observations, elevated calcineurin activity/signaling is often observed following acute injury to nervous tissue [126, 127] or during CNS aging [128] and/or disease [58, 87, 118, 129, 130]. In aged animals and transgenic animal models of AD, increased calcineurin activity/expression is linked to synaptic dysfunction [131, 132], dendritic spine irregularities [133, 134], elevated neuroinflammation [97, 135, 136], and cognitive decline [128, 129]. In human brain tissue, calcineurin/NFAT signaling is elevated during the emergence of clinical symptoms associated with AD [50, 87] and continues to increase with the progression of amyloid pathology and dementia [87]. Suppression of calcineurin activity using commercially available immunosuppressants, or through genetic manipulations, provides strong neuroprotection in many experimental models of acute injury [137, 138]. Glial activation and neuroinflammation in animal models of AD or stroke are also blunted by calcineurin inhibitors (or NFAT inhibitors) [132, 135, 136], as are numerous other biomarkers including synaptic dysfunction/degeneration [132–134, 139], amyloid pathology [132, 140], and cognitive impairment[129, 132, 141, 142]. However, despite all this evidence implicating a causative role of calcineurin in neurodegeneration, it deserves noting that other studies have shown that calcineurin can also activate cell survival pathways in neurons [143] and help resolve harmful neuroinflammatory signaling in glial cells under certain conditions [112, 144]. The precise conditions and cellular mechanisms that transform calcineurin from cellular protector to killer remain unclear and will require further investigation.
3.3 Mechanisms for Calcineurin Regulation
Clearly, changes in the activation state of calcineurin can spell the difference between optimal physiologic function and neurodegeneration. Consequently, multiple mechanisms are available for keeping calcineurin activity in check and/or for directing calcineurin to its proper substrates [29]. Anchoring proteins, such as A-kinase anchoring proteins (AKAPs), FK-506 binding protein 12 (FKBP12), and postsynaptic density 95 (PSD-95), can help sequester calcineurin to the membrane, thus limiting the access of calcineurin to cytosolic substrates. Usually, calcineurin is anchored along with other protein kinases to provide rapid and dynamic regulation over nearby membrane channels and pumps [145]. Moreover, membrane anchoring of calcineurin close juxtaposition to ligand and/or voltage-gated Ca2+ ionophores (e.g., NMDA receptors and L-type Ca2+ channels) allows calcineurin to respond rapidly to, and/or provide feedback regulation over, Ca2+ influx. In addition to sequestration mechanisms, calcineurin activity is also directly modulated by a handful of endogenous proteins, the most widely studied of which are cabins (calcineurin binding) and RCANs ((Regulator of Calcineurin) Down Syndrome Critical Region) [146]. These modulating proteins have gone by several different names (i.e., cabins–cains; RCANs–MCIPs and DSCRs) depending on the species investigated. Cabins and RCANs are highly expressed in brain and exhibit distribution patterns similar to calcineurin. While both proteins can bind to and inhibit calcineurin activity in vitro and in vivo (especially when inhibitors are overexpressed), RCANs may also facilitate calcineurin activity at physiologic levels, depending on the presence of other accessory proteins, as well as the phosphorylation state of RCAN [147, 148]. Finally, similar to calpains, calcineurin shows high redox sensitivity. Oxidation of the Fe2+–Zn2+ binuclear center in the calcineurin A catalytic domain, due to elevated superoxide and peroxide levels, is typically associated with reduced calcineurin activity, and a number of antioxidants have been shown to preserve calcineurin function [149].
3.4 The Importance of the Calcineurin AID
Among the numerous mechanisms for calcineurin regulation, none are more important than Ca2+/calmodulin and the calcineurin AID (Fig. 2.2a). Indeed, the interaction between these mechanisms is what permits discrete and high-fidelity coupling of calcineurin activity to local Ca2+ gradients [150]. Calcineurin is exquisitely sensitive to Ca2+ and has a Kd to Ca2+-saturated CaM in the picomolar range (28–100 pM) [151]. This value is far lower than that for other CaM-regulated enzymes, including the CaM kinases [152]. When Ca2+ is very low, calcineurin phosphatase activity is allosterically blocked by the AID (Fig. 2.2) [150]. Binding of Ca2+ to the four EF-hand motifs of the CN B subunit during elevations in cellular Ca2+ triggers a conformational change in the CN A subunit, exposing the Ca2+/CaM-binding site. Though Ca2+/CN B can, by itself, stimulate low levels of phosphatase activity and modulate the affinity of CN A for substrate phosphoproteins [80], it is the exposure of the calmodulin binding domain and subsequent binding of Ca2+/CaM that fully unleashes catalytic activity. Indeed, this binding event physically displaces the AID from the CN A catalytic core region [153], where it remains fully accessible to phosphosubstrates for as long as Ca2+/CaM is bound. Subsequently, when Ca2+ levels in the cell fall, allosteric inhibition of the catalytic domain by the AID is rapidly restored as Ca2+/calmodulin dissociates from calcineurin. Without the AID, calcineurin loses much of its sensitivity to Ca2+ and, as discussed below, becomes a highly disruptive constitutively active phosphatase (Fig. 2.1) [48].
3.5 Early In Vitro Evidence for Proteolysis of the Calcineurin AID
It has been known since the early to mid-1980s that calcineurin is susceptible to proteolysis in vitro. Early studies showed that exposure of the CN A/CN B holoenzyme to trypsin or chymotrypsin produced an enzyme complex containing the CN B subunit and a truncated ~40–46 kDa CN A subunit [154–156]. This proteolized CN A fragment retained physical interactions with the CN B subunit, but was incapable of binding Ca2+/CaM, and did not require CaM for high enzymatic activity. Application of trypsin/chymotrypsin to Ca2+/CaM-bound CN A resulted in slower rates of proteolysis with the additional appearance of 57, 55, and 54 kDa CN A fragments, suggesting that Ca2+/CaM binding offers some degree of protection from proteolysis. Interestingly, these proteolytic fragments also retained the capacity to bind to Ca2+/CaM. In subsequent studies, CN A was shown to undergo proteolysis in vitro by exposure to Ca2+ and calpain [157, 158]. Similar to earlier work, calpain protein was applied in vitro at different Ca2+ concentrations, in the presence or absence of CaM. Again, proteolysis of CN A did not affect interactions with the regulatory CN B subunit but did produce high levels of phosphatase activity independent of Ca2+/CaM. However, unlike earlier studies with trypsin, the presence of Ca2+/calmodulin did not protect calcineurin from calpain and instead hastened the rate of proteolytic cleavage. Under these conditions, calpain exposure produced CN A fragments of 55 and 48 kDa, which retained some capacity to bind to and/or respond to Ca2+/CaM [157]. These results suggest that calpain-mediated proteolysis greatly reduces but does not fully eliminate the responsiveness of CN A to Ca2+. In contrast to CN A, the regulatory CN B subunit does not appear to be vulnerable to proteolysis.
3.6 Effects of Overexpressing Truncated CN A in Intact Cell Systems
The proteolysis studies discussed above provided the first evidence that calcineurin activity is held in check by an AID located near the CaM binding domain in the C-terminus of the CN A subunit. Later work confirmed the existence of a C-terminus AID; demonstrated that the AID physically obscures the CN A catalytic core when Ca2+/CaM is absent; and showed that the binding of Ca2+/CaM to CN A displaces the AID from the catalytic region. Studies on calcineurin proteolysis also led to the development of cDNA clones that encode an ~48 kDa C-terminus truncated CN A fragment (ΔCN) that retains CN B binding properties but exhibits high levels of activity in the absence of Ca2+/CaM. The use of ΔCN, combined with newly developing gene delivery techniques, provided a convenient way to produce elevated calcineurin signaling without stimulating key cellular receptors and/or indiscriminately raising intracellular Ca2+ levels, which, in turn, greatly increased our understanding of calcineurin’s role(s) in cellular physiology. A consistent theme to emerge from ΔCN overexpression studies is that unchecked calcineurin activity, whether in peripheral tissues or in brain, leads to severe cellular dysfunction and/or death. In primary neuron cultures, ΔCN has been shown to induce numerous detrimental outcomes including postsynaptic spine retraction, dendritic atrophy, and/or apoptosis [118, 119, 159]. In astrocytes, ΔCN was found to trigger cellular hypertrophy and induce numerous genes involved in immune/inflammatory signaling [97]. In intact rodents, forebrain expression of ΔCN caused deficits in LTP and spatial memory [160–162]. Interestingly, these alterations are very similar to those observed in animal models of aging, injury, and or neurodegenerative disease in which endogenous calcineurin activity is aberrantly high.
4 Calpain Proteolysis of Calcineurin in Intact Nervous Tissue
Given the early evidence showing that calcineurin is highly susceptible to calpain-mediated proteolysis in vitro, it is somewhat surprising that the first demonstrations of calcineurin proteolysis in intact cellular systems were not provided until relatively recently [48]. It’s possible that the presence of proteolyzed calcineurin in neurologic disease and other disorders escaped detection because the majority of commercially available calcineurin antibodies target the CN A carboxy-terminus which is, of course, missing in smaller proteolyzed calcineurin fragments. Regardless, in the early to mid-2000s, proteolysis of calcineurin was shown to occur in both heart and neural tissue under pathologic conditions [48, 163, 164]. Western blots of CN A (using a primary antibody targeting amino acid residues 264–283) performed on primary neuronal cultures exposed to an excitotoxic glutamate insult revealed at least three truncated CN A products in conjunction with an elevation in Ca2+/CaM-independent calcineurin activity [48]. In the same study, a similar banding pattern for CN A was observed in Western blots of whole hippocampal lysates from mice treated with a kainic acid insult. However, when nervous tissue was treated with distinct calpain inhibitors prior to the administration of glutamate/kainite, CN A appeared as a single 60 kDa band. Using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ProTOF/MS), it was shown that calpains induce cleavage of the CN A subunit in vitro at amino acid residues 392, 424, and 501 resulting in cleavage products of 45, 48, and 57 kDa, respectively (see Fig. 2.2c). Note that these bands corresponded very closely to the CN A truncation products observed in cell cultures following excitotoxic injury. These results demonstrated that the majority of the AID remains intact in the 57 kDa CN A fragment but is excluded from the 45 and 48 kDa fragments. In addition to lacking the AID, the 45 kDa fragment (but not the 48 kDa fragment) is also devoid of the Ca2+/CaM binding domain. The work by Wu et al. [48] not only showed that calcineurin undergoes calpain-mediated proteolysis in intact cellular systems but that it also likely plays a significant role in driving pathologic outcomes. Consistent with this study, investigations across multiple laboratories have discovered calpain-dependent proteolysis in several distinct neurologic injuries and disease states including Alzheimer’s disease, ischemia, and glaucoma. The major findings of these studies are highlighted in Table 2.1 and discussed further below.
4.1 Calcineurin Proteolysis in Alzheimer’s Disease
Alzheimer’s disease (AD) is a devastating and terminal neurodegenerative disorder leading to profound cognitive deficits, personality alterations, and the eventual loss of most all daily life skills. The pathologic hallmarks of AD are extracellular Aβ plaques, intracellular neurofibrillary tangles, and extensive neuronal degeneration and neuronal death [165]. There is also ample evidence implicating neuroinflammation [166, 167], Ca2+ dysregulation [16, 20], and excitotoxic mechanisms [168, 169] in the pathophysiology of the disorder and, by corollary, calpain, and calcineurin signaling pathways, as well.
In 2005, it was demonstrated that changes in calpain and calcineurin during AD are extensively and directly intertwined, providing a novel mechanism for AD-related neurologic dysfunction and degeneration [58]. In this study by Liu et al., levels of the 57 kDa CN A truncation product were detected at significantly higher levels in medial temporal cortex of human subjects with severe AD pathology, compared to age-matched, non-demented control subjects. Higher levels of the 57 kDa fragment were directly correlated with levels of the 76 kDa active calpain fragment and, importantly, corresponded to greater calcineurin phosphatase activity. Furthermore, levels of proteolyzed calcineurin showed a direct positive correlation with neurofibrillary tangle load, suggesting that calpain/calcineurin interactions play an important role in disease pathology. Consistent with this observation, a later study from this group showed that calcineurin proteolysis in human neocortical regions coincided with elevated phospho-tau levels [170]. These results are particularly intriguing because tau hyperphosphorylation in AD was previously suggested to result from a decrease, rather than an increase, in calcineurin activity. It therefore appears that the relationship between calcineurin phosphatase activity and tau pathology may be different, or perhaps more complicated, than originally proposed.
Subsequent studies have provided further evidence that calcineurin is proteolyzed and activated to a greater degree during the progression of AD, though there are some discrepancies among the reports. In 2010, Wu et al. [118] observed increased expression of calcineurin proteolytic fragments in human AD cortical tissue, but unlike the Liu et al. study [58], the calcineurin truncation product associated with AD had a molecular weight of 48 kDa and was detected primarily in nuclear fractions. Increased nuclear localization of the 48 kDa fragment corresponded to increased nuclear levels of the NFAT3 isoform. Similar observations were observed in primary neuronal cultures from transgenic amyloidogenic mice. Moreover, forced overexpression of the 48 kDa calcineurin fragment in wild-type neuron cultures recapitulated dendritic dystrophy and spine loss typically observed with elevated amyloid levels. Whether the 48 kDa calcineurin fragment found in human AD tissue resulted from increased calpain-mediated proteolysis was not investigated in this study.
A year later, Mohmmad Abdul et al. [50] reported an increase in the expression of the 48 kDa CN A product in the hippocampus of human subjects diagnosed with mild cognitive impairment (MCI): a putative transition state between normal age-related cognitive decline and AD-related dementia [171]. Generation of the 48 kDa fragment showed a direct positive correlation with levels of the activated calpain 1 fragment, suggesting an increased interaction between calpain and calcineurin during the early clinical stages of AD. While both the CN Aα and CN Aβ isoforms each exhibited signs of proteolysis in human brain tissue, significant differences between subject categories were only observed for the CN Aα isoform. Consistent with an earlier report [172], proteolytic conversion of full-length CN Aα to the 48 kDa fragment as assessed by Mohmmad Abdul et al. [50] was also observed in primary rat hippocampal cultures 24 h after treatment with cytotoxic amyloid peptides (also see Fig. 2.2d). This proteolysis was associated with increased NFAT transcriptional activity, elevated proteolysis of the NR2B isoform of the NMDA receptor, and increased neuronal degeneration. Blockade of calpain activity significantly attenuated each of these effects, while inhibition of caspase 1 was largely ineffective, suggesting selective involvement of calpains in calcineurin proteolysis.
Interestingly, unlike the Wu et al. [118] report, the 48 kDa CN A fragment reported by Mohmmad Abdul et al. [50] was localized to cytosolic, rather than nuclear, fractions. The reason for this discrepancy is unclear but may be due in large measure to disease severity. Work on cardiomyocytes suggests that maintenance of truncated calcineurin in the nucleus may be more disruptive to cellular structure and function than cytosolic calcineurin [164]. It’s possible that the nuclear localization of calcineurin AD brain results from a more toxic stage of Ca2+ dysregulation. If true, nuclear localization of proteolyzed calcineurin may reflect a critical transition state between MCI and AD-related dementia.
4.2 Brain Ischemia/Hypoxia
Brain ischemia resulting from stroke or other vascular accidents can cause irreversible neuronal damage and/or death due to excitotoxicity and/or other deleterious processes. Recent studies on several rodent species subjected to ischemic insults (i.e., carotid artery occlusion) discovered the appearance of truncated CN A products in damaged brain tissue, in conjunction with elevations in activated calpain and/or with the breakdown of spectrin, a major calpain substrate [121, 173, 174]. In one report by Shioda et al. [174], proteolysis of calcineurin to a 48 kDa fragment occurred within hours of the ischemic insult and was associated with an increase in Ca2+/CaM-independent phosphatase activity along with an increase in the nuclear localization of NFAT4 in hippocampal CA1 pyramidal neurons. A follow-up study from this group suggested that proteolytic activation of calcineurin after ischemia underlies delayed neuronal death in the hippocampus [121]. Neuronal loss was hypothesized to occur via the nuclear translocation of NFAT4 and forkhead, followed by the transcriptional induction of the proapoptotic factor, FasL. Indeed, each of the events in this pathway was prevented in ischemic animals treated with the calcineurin inhibitor, FK-506. In the Rosenkranz et al. study, a proteomics approach was used to identify modified proteins following perinatal hypoxic−ischemic brain damage in rats [173]. CN A was among the proteins significantly upregulated after ischemia. In addition to elevated levels of full-length calcineurin, several CN A truncation products were also observed including 54, 48, and 46 kDa fragments. The appearance of these smaller calcineurin products were associated with reduced phosphorylation of the calcineurin substrate, DARP32, suggestive of elevated calcineurin activity.
4.3 Glaucoma
Glaucoma is one of the leading causes of blindness and involves the progressive death of retinal ganglion cells (RGC), followed by the degeneration of optic nerve fibers. Increased intraocular pressure (IOP), which leads to RGC apoptosis and optic nerve degeneration in experimental models (e.g., see [175, 176]), is widely believed to be a primary cause of glaucoma [177]. In 2005, a study by Huang et al. [178] reported on the progressive accumulation of a 45 kDa CN A fragment in rat retina during treatments that increase IOP. A similar calcineurin proteolytic fragment was also observed in retinal cell lysates harvested from transgenic mice that spontaneously develop increased IOP and other glaucoma-like symptoms. The appearance of the 45 kDa CN A fragment coincided with a reduction in the phosphorylation state of the proapoptotic factor BAD and an increase in the mitochondrial release of cytochrome C. Consistent with previous reports linking calcineurin to mitochondrial dysfunction and apoptosis [119, 159], pretreatment of rats with the calcineurin inhibitor FK-506 suppressed the dephosphorylation of BAD, reduced cytochrome C release, and ameliorated RGC death and degeneration. Using MALDI-ProTOF/MS to identify cleavage sites in CN A, a follow-up study from the same research group suggested that IOP-related calcineurin proteolysis is most likely attributable to the activation of calpains, rather than other proteases, such as caspases [62].
5 Unresolved Issues and Future Avenues of Research
5.1 Calpain/Calcineurin Interactions in Brain: Role of Different Isoforms and the Contribution of Different Cell Types
There are multiple isoforms of calpain and at least two major CN A isoforms expressed in brain. As alluded to above, we know relatively little about isoform-specific differences in calcineurin, in terms of function and distribution in the brain. However, our previous work on human AD tissue indicates that disease-related changes in both the subcellular localization and proteolysis of calcineurin are far more prominent for the CN Aα isoform [50, 87]. It’s possible that the different CN A isoforms show different patterns of co-localization with calpains or interact with different affinities to the calpains, among other possibilities. One complicating factor in resolving this issue is that we don’t really know which cell types exhibit calcineurin proteolysis. Most investigations of calpain/calcineurin interactions have dealt with neurodegenerative processes in neurons (discussed above and see Fig. 2.3). However, in recent years it has become increasingly clear that calcineurin also appears at high levels in activated glial cells, especially astrocytes [97], where it likely contributes to increased neuroinflammation during aging, injury, and disease. Calpains, too, are found in activated astrocytes and microglia with certain types of injury [179–182], and there is evidence that several astrocyte-enriched proteins, including the glial fibrillary acidic protein (GFAP) and vimentin, are targets of calpain-mediated proteolysis [183, 184].
Calpain/calcineurin interactions in neurons and astrocytes. Cartoons showing putative functions/outcomes of calpain/calcineurin interactions in neurons (top panel) and astrocytes (bottom panel). Based on the literature discussed, calpain-mediated proteolysis of calcineurin in neurons is strongly linked to neurodegenerative processes. Dephosphorylation of BAD leads to its translocation to the mitochondrial membrane and the subsequent release of cytochrome C, followed by caspase activation and apoptosis (e.g., see [119, 178]), while activation of NFAT3 and 4 isoforms triggers the transcriptional induction of the FasL proapoptotic factor and/or hastens the degeneration of dendrites and synapses (e.g., see [118, 121]). Though astrocytic calpain/calcineurin interactions (bottom panel) have yet to be investigated extensively, both appear at high levels in activated astrocytes as a result of injury. In astrocytes, calpain-dependent proteolysis of calcineurin could lead to extensive activation of the NFAT1 isoform followed by the upregulation of numerous cytokines involved in neuroinflammation, as well as the downregulation of EAATs resulting in excitotoxicity (e.g., see [87, 97, 111–113]). Note that calpain/calcineurin interactions in either cell type could lead to deleterious processes common to many neurologic disorders. CP calpain, CN CN, Cyt. C cytochrome C, Mito. mitochondria, CaM calmodulin, AID autoinhibitory domain
Early work in spinal cord tissue further showed that calpain inhibitors attenuated several markers of gliosis following acute injury [185], suggesting that calpains may help drive astrocyte activation. If so, neuroinflammatory signaling in glial cells may be yet another disease-related process in which calpains and calcineurin are common mechanisms (Fig. 2.3). This possibility raises an interesting dilemma: i.e., are calpain and calcineurin inhibitors neuroprotective because they suppress harmful neuroinflammatory cascades? Or, do calpain and calcineurin inhibitors reduce neuroinflammation by stemming neurodegeneration and/or apoptosis? The extent to which calpain-mediated proteolysis contributes to calcineurin signaling in glial cells is not presently known and difficult to assess in intact tissue with available research tools. Although N-terminus antibodies to the CN A subunit detect the appearance of full-length and truncated forms of CN A in Western blots, these antibodies do not make a distinction between full-length and truncated forms in immunohistochemistry applications. Thus, until a primary antibody is generated that selectively identifies CN A proteolytic products (i.e., recognized proteolyzed but not full-length CN A), it will be very difficult to determine where (i.e., which cell type) calcineurin is actually proteolyzed in heterogeneous tissues, such as brain.
5.2 Are There More Effective Ways to Selectively Target Calpain/Calcineurin Interactions for Therapeutic Purposes?
The evidence to date suggests that calpain/calcineurin interactions are possibly an important upstream mechanism of numerous deleterious changes associated with a variety of neurodegenerative diseases. A critical question is this: can the physical interaction between calpains and calcineurin be exploited for the development of new treatment strategies? Separately, calpain and calcineurin inhibitors have shown neuroprotective properties in numerous disease models. However, the use of these inhibitors in the clinic is fraught with many difficulties. For instance, commercially available calcineurin inhibitors are notorious for their numerous adverse effects, many of which are potentiated in elderly populations, who also show greatest susceptibility to neurodegenerative disease [186]. The relatively high level of toxicity associated with these drugs is very likely due to their poor specificity. Indeed, the most commonly used calcineurin inhibitors (cyclosporine and tacrolimus) are well known to bind to an inhibit immunophilins [30], which participate in many calcineurin-independent signaling cascades [187]. In addition, calcineurin is ubiquitously expressed and has pleiotropic functions all of which are suppressed by calcineurin inhibitors. Based on the toxic responses to these drugs, it seems clear that many calcineurin and immunophilin-dependent signaling pathways are critical for cell function and viability and should be left unperturbed.
The ideal drug or treatment would prevent calpain from proteolyzing CN A into a constitutively active fragment but would not interfere with the normal activation of calpains and calcineurin or interfere with the interactions of these enzymes with other substrates. This would require extensive investigation into the molecular mechanisms through which calpains recognize, bind to, and proteolyze the CN A subunit. Is there something unique about the primary sequence of CN A that makes it a good substrate for calpain? Is there a way that we could pharmacologically modify the CN A subunit to permit normal Ca2+/CaM binding but exclude binding of calpain? In this regard it may be instructive to consider strategies that have been used successfully to disrupt calcineurin interactions with NFATs for the purpose of developing safer and more effective immunosuppressive agents. Calcineurin interacts with NFATs, in part, by binding to a specific amino acid substrate, PxIxIT, located upstream from the NFAT DNA binding domain. In the late 1990s, Rao and colleagues developed a peptide (i.e., MAGPHPVIVITGPHEE or VIVIT) based on the PxIxIT sequence in an attempt to disrupt calcineurin/NFAT interactions [188]. VIVIT was shown to prevent NFAT activation as effectively as commercial calcineurin inhibitors but did not inhibit calcineurin catalytic activity, per se, in vitro. Since this report, VIVIT has been used by many labs as an alternative to calcineurin inhibitors for the study of diverse processes in numerous and distinct cell types. Proof of principle studies on intact animal models has also shown the potential of VIVIT as a prophylactic in allogenic tissue transplants [189] and as a neuroprotectant in AD-like amyloid pathology [132, 133]. Development of similar peptide or chemical-based reagents to selectively prevent calpain/calcineurin interactions could have a similar impact on therapeutic strategies for treating neurodegenerative disease. At the least, reagents of this type would rapidly advance our understanding of the specific functional consequences of calpain/calcineurin interactions and would therefore have great value to basic research.
6 Conclusions
Calpain and calcineurin are fascinating enzymes and important effectors of Ca2+-mediated neurotoxicity. Recent work has shown us that calpain and calcineurin are not merely regulators of their own discrete signaling pathways but interact extensively. This interaction could prove to be a key step in the transition from normal cellular function to pathological function. Extensive work will be necessary to determine not only when, but where, calpain/calcineurin interactions occur. Moreover, a greater understanding of the molecular basis of this interaction could lead to more specific and effective therapies for a variety of neurodegenerative disorders and diseases.
References
Gibson GE, C Peterson (1987) Calcium and the aging nervous system. Neurobiol Aging 8:329-343.
Khachaturian ZS (1987) Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol Aging 8:345-346.
Landfield PW, TA Pitler (1984) Prolonged Ca2+-dependent after hyperpolarizations in hippocampal neurons of aged rats. Science 226:1089-1092.
Landfield PW, TA Pitler, MD Applegate (1986) The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol 56:797-811.
Choi DW (1985) Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett 58:293-297.
Choi DW (1987) Ionic dependence of glutamate neurotoxicity. J Neurosci 7:369-379.
Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1:623-634.
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11:465-469.
Choi DW (1994) Calcium and excitotoxic neuronal injury. Ann N Y Acad Sci 747:162-171.
Gibson G, P Perrino, GA Dienel (1986) In vivo brain calcium homeostasis during aging. Mech Ageing Dev 37:1-12.
Khachaturian ZS (1989) The role of calcium regulation in brain aging: reexamination of a hypothesis. Aging (Milano) 1:17-34.
Landfield PW (1987) ‘Increased calcium-current’ hypothesis of brain aging. Neurobiol Aging 8:346-347.
Landfield PW, LW Campbell, SY Hao, et al (1989) Aging-related increases in voltage-sensitive, inactivating calcium currents in rat hippocampus. Implications for mechanisms of brain aging and Alzheimer’s disease. Ann N Y Acad Sci 568:95-105.
Abdul HM, JL Furman, MA Sama, et al (2010) NFATs and Alzheimer’s Disease. Mol Cell Pharmacol 2:7-14.
Berridge MJ (2012) Calcium signalling remodelling and disease. Biochem Soc Trans 40:297-309.
Bezprozvanny I, MP Mattson (2008) Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci 31:454-463.
Crish SD, DJ Calkins (2011) Neurodegeneration in glaucoma: progression and calcium-dependent intracellular mechanisms. Neuroscience 176:1-11.
Gasperini RJ, DH Small (2012) Neurodegeneration in familial amyloidotic polyneuropathy. Clin Exp Pharmacol Physiol 39:680-683.
Goodison WV, V Frisardi, PG Kehoe (2012) Calcium channel blockers and Alzheimer’s disease: potential relevance in treatment strategies of metabolic syndrome. J Alzheimers Dis 30 Suppl 2:S269-282.
Green KN, FM LaFerla (2008) Linking calcium to Abeta and Alzheimer’s disease. Neuron 59:190-194.
Stutzmann GE, MP Mattson (2011) Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev 63:700-727.
Thibault O, JC Gant, PW Landfield (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6:307-317.
Toescu EC, A Verkhratsky, PW Landfield (2004) Ca(2+) regulation and gene expression in normal brain aging. Trends Neurosci 27:614-620.
Campbell RL, PL Davies (2012) Structure-function relationships in calpains. Biochem J 447:335-351.
Goll DE, VF Thompson, H Li, et al (2003) The calpain system. Physiol Rev 83:731-801.
Nixon RA (2003) The calpains in aging and aging-related diseases. Ageing Res Rev 2:407-418.
Ono Y, H Sorimachi (2012) Calpains: an elaborate proteolytic system. Biochim Biophys Acta 1824:224-236.
Mansuy IM (2003) Calcineurin in memory and bidirectional plasticity. Biochem Biophys Res Commun 311:1195-1208.
Musson RE, NP Smit (2011) Regulatory mechanisms of calcineurin phosphatase activity. Curr Med Chem 18:301-315.
Rusnak F, P Mertz (2000) Calcineurin: form and function. Physiol Rev 80:1483-1521.
Gil-Parrado S, O Popp, TA Knoch, et al (2003) Subcellular localization and in vivo subunit interactions of ubiquitous mu-calpain. J Biol Chem 278:16336-16346.
Yoshizawa T, H Sorimachi, S Tomioka, et al (1995) Calpain dissociates into subunits in the presence of calcium ions. Biochem Biophys Res Commun 208:376-383.
Nixon RA (1986) Fodrin degradation by calcium-activated neutral proteinase (CANP) in retinal ganglion cell neurons and optic glia: preferential localization of CANP activities in neurons. J Neurosci 6:1264-1271.
Siman R (1992) Proteolytic mechanism for the neurodegeneration of Alzheimer’s disease. Ann N Y Acad Sci 674:193-202.
Siman R, M Baudry, G Lynch (1984) Brain fodrin: substrate for calpain I, an endogenous calcium-activated protease. Proc Natl Acad Sci U S A 81:3572-3576.
D’Orsi B, H Bonner, LP Tuffy, et al (2012) Calpains are downstream effectors of bax-dependent excitotoxic apoptosis. J Neurosci 32:1847-1858.
Siman R, M Baudry, G Lynch (1985) Regulation of glutamate receptor binding by the cytoskeletal protein fodrin. Nature 313:225-228.
Bi X, V Chang, E Molnar, et al (1996) The C-terminal domain of glutamate receptor subunit 1 is a target for calpain-mediated proteolysis. Neuroscience 73:903-906.
Bi X, J Chen, S Dang, et al (1997) Characterization of calpain-mediated proteolysis of GluR1 subunits of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors in rat brain. J Neurochem 68:1484-1494.
Bi X, Y Rong, J Chen, et al (1998) Calpain-mediated regulation of NMDA receptor structure and function. Brain Res 790:245-253.
Bi X, G Tocco, M Baudry (1994) Calpain-mediated regulation of AMPA receptors in adult rat brain. Neuroreport 6:61-64.
Dong YN, EA Waxman, DR Lynch (2004) Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J Neurosci 24:11035-11045.
Gascon S, M Sobrado, JM Roda, et al (2008) Excitotoxicity and focal cerebral ischemia induce truncation of the NR2A and NR2B subunits of the NMDA receptor and cleavage of the scaffolding protein PSD-95. Mol Psychiatry 13:99-114.
Jourdi H, X Lu, T Yanagihara, et al (2005) Prolonged positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors induces calpain-mediated PSD-95/Dlg/ZO-1 protein degradation and AMPA receptor down-regulation in cultured hippocampal slices. J Pharmacol Exp Ther 314:16-26.
Lu X, Y Rong, M Baudry (2000) Calpain-mediated degradation of PSD-95 in developing and adult rat brain. Neurosci Lett 286:149-153.
Sessoms JS, SJ Chen, DM Chetkovich, et al (1992) Ca(2+)-induced persistent protein kinase C activation in rat hippocampal homogenates. Second Messengers Phosphoproteins 14:109-126.
Suzuki T, K Okumura-Noji, A Ogura, et al (1992) Calpain may produce a Ca(2+)-independent form of kinase C in long-term potentiation. Biochem Biophys Res Commun 189:1515-1520.
Wu HY, K Tomizawa, Y Oda, et al (2004) Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem 279:4929-4940.
Kuwako K, I Nishimura, T Uetsuki, et al (2002) Activation of calpain in cultured neurons overexpressing Alzheimer amyloid precursor protein. Brain Res Mol Brain Res 107:166-175.
Mohmmad Abdul H, I Baig, H Levine, 3rd, et al (2011) Proteolysis of calcineurin is increased in human hippocampus during mild cognitive impairment and is stimulated by oligomeric Abeta in primary cell culture. Aging Cell 10:103-113.
Tamada Y, C Fukiage, S Daibo, et al (2002) Involvement of calpain in hypoxia-induced damage in rat retina in vitro. Comp Biochem Physiol B Biochem Mol Biol 131:221-225.
Blomgren K, C Zhu, X Wang, et al (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem 276:10191-10198.
Ostwald K, H Hagberg, P Andine, et al (1993) Upregulation of calpain activity in neonatal rat brain after hypoxic-ischemia. Brain Res 630:289-294.
Bi X, V Chang, R Siman, et al (1996) Regional distribution and time-course of calpain activation following kainate-induced seizure activity in adult rat brain. Brain Res 726:98-108.
Schoch KM, HN Evans, JM Brelsfoard, et al (2012) Calpastatin overexpression limits calpain-mediated proteolysis and behavioral deficits following traumatic brain injury. Exp Neurol 236:371-382.
McGinn MJ, BJ Kelley, L Akinyi, et al (2009) Biochemical, structural, and biomarker evidence for calpain-mediated cytoskeletal change after diffuse brain injury uncomplicated by contusion. J Neuropathol Exp Neurol 68:241-249.
Saatman KE, D Bozyczko-Coyne, V Marcy, et al (1996) Prolonged calpain-mediated spectrin breakdown occurs regionally following experimental brain injury in the rat. J Neuropathol Exp Neurol 55:850-860.
Liu F, I Grundke-Iqbal, K Iqbal, et al (2005) Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem 280:37755-37762.
Saito K, JS Elce, JE Hamos, et al (1993) Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A 90:2628-2632.
Mouatt-Prigent A, JO Karlsson, Y Agid, et al (1996) Increased M-calpain expression in the mesencephalon of patients with Parkinson’s disease but not in other neurodegenerative disorders involving the mesencephalon: a role in nerve cell death? Neuroscience 73:979-987.
Shields DC, KE Schaecher, TC Saido, et al (1999) A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A 96:11486-11491.
Huang W, J Fileta, I Rawe, et al (2010) Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci 51:3049-3054.
Oka T, Y Tamada, E Nakajima, et al (2006) Presence of calpain-induced proteolysis in retinal degeneration and dysfunction in a rat model of acute ocular hypertension. J Neurosci Res 83:1342-1351.
Crocker SJ, PD Smith, V Jackson-Lewis, et al (2003) Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson’s disease. J Neurosci 23:4081-4091.
Das A, DP Garner, AM Del Re, et al (2006) Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res 1084:146-157.
Grant RJ, LH Sellings, SJ Crocker, et al (2009) Effects of calpain inhibition on dopaminergic markers and motor function following intrastriatal 6-hydroxydopamine administration in rats. Neuroscience 158:558-569.
Hassen GW, J Feliberti, L Kesner, et al (2008) Prevention of axonal injury using calpain inhibitor in chronic progressive experimental autoimmune encephalomyelitis. Brain Res 1236:206-215.
Trinchese F, M Fa, S Liu, et al (2008) Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest 118:2796-2807.
Vaisid T, NS Kosower, A Katzav, et al (2007) Calpastatin levels affect calpain activation and calpain proteolytic activity in APP transgenic mouse model of Alzheimer’s disease. Neurochem Int 51:391-397.
Chen Q, M Paillard, L Gomez, et al (2011) Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun 415:533-538.
Choi WS, EH Lee, CW Chung, et al (2001) Cleavage of Bax is mediated by caspase-dependent or -independent calpain activation in dopaminergic neuronal cells: protective role of Bcl-2. J Neurochem 77:1531-1541.
McGinnis KM, ME Gnegy, YH Park, et al (1999) Procaspase-3 and poly(ADP)ribose polymerase (PARP) are calpain substrates. Biochem Biophys Res Commun 263:94-99.
Ozaki T, T Yamashita, S Ishiguro (2009) Mitochondrial m-calpain plays a role in the release of truncated apoptosis-inducing factor from the mitochondria. Biochim Biophys Acta 1793:1848-1859.
Valero JG, A Cornut-Thibaut, R Juge, et al (2012) micro-Calpain conversion of antiapoptotic Bfl-1 (BCL2A1) into a prodeath factor reveals two distinct alpha-helices inducing mitochondria-mediated apoptosis. PLoS One 7:e38620.
Kopil CM, AP Siebert, JK Foskett, et al (2012) Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor impairs ER Ca(2+) buffering and causes neurodegeneration in primary cortical neurons. J Neurochem 123:147-158.
Kopil CM, H Vais, KH Cheung, et al (2011) Calpain-cleaved type 1 inositol 1,4,5-trisphosphate receptor (InsP(3)R1) has InsP(3)-independent gating and disrupts intracellular Ca(2+) homeostasis. J Biol Chem 286:35998-36010.
Pottorf WJ, 2nd, TM Johanns, SM Derrington, et al (2006) Glutamate-induced protease-mediated loss of plasma membrane Ca2+ pump activity in rat hippocampal neurons. J Neurochem 98:1646-1656.
Hell JW, RE Westenbroek, LJ Breeze, et al (1996) N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci U S A 93:3362-3367.
Kissinger CR, HE Parge, DR Knighton, et al (1995) Crystal structures of human calcineurin and the human FKBP12-FK506-calcineurin complex. Nature 378:641-644.
Stemmer PM, CB Klee (1994) Dual calcium ion regulation of calcineurin by calmodulin and calcineurin B published erratum appears in Biochemistry 1995 Dec 5;34(48):15880.. Biochemistry 33:6859-6866.
Wu HY, E Hudry, T Hashimoto, et al (2012) Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-beta revealed by a novel fluorescence resonance energy transfer assay. J Neurosci 32:5298-5309.
Li N, Z Zhang, W Zhang, et al (2011) Calcineurin B subunit interacts with proteasome subunit alpha type 7 and represses hypoxia-inducible factor-1alpha activity via the proteasome pathway. Biochem Biophys Res Commun 405:468-472.
Li W, RE Handschumacher (2002) Identification of two calcineurin B-binding proteins: tubulin and heat shock protein 60. Biochim Biophys Acta 1599:72-81.
Liu L, Z Su, S Xin, et al (2012) The calcineurin B subunit (CnB) is a new ligand of integrin alphaM that mediates CnB-induced Apo2L/TRAIL expression in macrophages. J Immunol 188:238-247.
Saeki M, Y Irie, L Ni, et al (2007) Calcineurin potentiates the activation of procaspase-3 by accelerating its proteolytic maturation. J Biol Chem 282:11786-11794.
Kuno T, H Mukai, A Ito, et al (1992) Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem 58:1643-1651.
Abdul HM, MA Sama, JL Furman, et al (2009) Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci 29:12957-12969.
Aramburu J, J Heitman, GR Crabtree (2004) Calcineurin: a central controller of signalling in eukaryotes. EMBO Rep 5:343-348.
Baine I, BT Abe, F Macian (2009) Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol Rev 231:225-240.
Takaishi T, N Saito, T Kuno, et al (1991) Differential distribution of the mRNA encoding two isoforms of the catalytic subunit of calcineurin in the rat brain. Biochem Biophys Res Commun 174:393-398.
de Leon MJ, A Convit, S DeSanti, et al (1995) The hippocampus in aging and Alzheimer’s disease. Neuroimaging Clin N Am 5:1-17.
Scheff SW, DA Price (2006) Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimers Dis 9:101-115.
Klee CB, TH Crouch, MH Krinks (1979) Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A 76:6270-6273.
Goto S, Y Matsukado, Y Mihara, et al (1986) The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res 397:161-172.
Celsi F, M Svedberg, C Unger, et al (2007) Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol Dis 26:342-352.
Hashimoto T, T Kawamata, N Saito, et al (1998) Isoform-specific redistribution of calcineurin A alpha and A beta in the hippocampal CA1 region of gerbils after transient ischemia. J Neurochem 70:1289-1298.
Norris CM, I Kadish, EM Blalock, et al (2005) Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer’s models. J Neurosci 25:4649-4658.
Halpain S, P Greengard (1990) Activation of NMDA receptors induces rapid dephosphorylation of the cytoskeletal protein MAP2. Neuron 5:237-246.
Meberg PJ, S Ono, LS Minamide, et al (1998) Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil Cytoskeleton 39:172-190.
Wang Y, F Shibasaki, K Mizuno (2005) Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem 280:12683-12689.
Snyder GL, S Galdi, AA Fienberg, et al (2003) Regulation of AMPA receptor dephosphorylation by glutamate receptor agonists. Neuropharmacology 45:703-713.
Kam AY, D Liao, HH Loh, et al (2010) Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci 30:15304-15316.
Kim SM, SM Ahn, BS Go, et al (2009) Alterations in AMPA receptor phosphorylation in the rat striatum following acute and repeated cocaine administration. Neuroscience 163:618-626.
Unoki T, S Matsuda, W Kakegawa, et al (2012) NMDA receptor-mediated PIP5K activation to produce PI(4,5)P(2) is essential for AMPA receptor endocytosis during LTD. Neuron 73:135-148.
Malinow R, RC Malenka (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103-126.
Bito H, K Deisseroth, RW Tsien (1996) CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87:1203-1214.
Lin CH, SH Yeh, HY Lu, et al (2003) The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci 23:8310-8317.
Macian F (2005) NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol 5:472-484.
Graef IA, PG Mermelstein, K Stankunas, et al (1999) L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature 401:703-708.
Groth RD, PG Mermelstein (2003) Brain-derived neurotrophic factor activation of NFAT (nuclear factor of activated T-cells)-dependent transcription: a role for the transcription factor NFATc4 in neurotrophin-mediated gene expression. J Neurosci 23:8125-8134.
Canellada A, BG Ramirez, T Minami, et al (2008) Calcium/calcineurin signaling in primary cortical astrocyte cultures: Rcan1-4 and cyclooxygenase-2 as NFAT target genes. Glia 56:709-722.
Fernandez AM, S Fernandez, P Carrero, et al (2007) Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci 27:8745-8756.
Sama MA, DM Mathis, JL Furman, et al (2008) Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem 283:21953-21964.
Reese LC, G Taglialatela (2011) A role for calcineurin in Alzheimer’s disease. Curr Neuropharmacol 9:685-692.
Halpain S, A Hipolito, L Saffer (1998) Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci 18:9835-9844.
Shankar GM, BL Bloodgood, M Townsend, et al (2007) Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci 27:2866-2875.
Tackenberg C, R Brandt (2009) Divergent pathways mediate spine alterations and cell death induced by amyloid-beta, wild-type tau, and R406W tau. J Neurosci 29:14439-14450.
Wu HY, E Hudry, T Hashimoto, et al (2010) Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci 30:2636-2649.
Wang HG, N Pathan, IM Ethell, et al (1999) Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284:339-343.
Springer JE, RD Azbill, SA Nottingham, et al (2000) Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci 20:7246-7251.
Shioda N, F Han, S Moriguchi, et al (2007) Constitutively active calcineurin mediates delayed neuronal death through Fas-ligand expression via activation of NFAT and FKHR transcriptional activities in mouse brain ischemia. J Neurochem 102:1506-1517.
Nagamoto-Combs K, CK Combs (2010) Microglial phenotype is regulated by activity of the transcription factor, NFAT (nuclear factor of activated T cells). J Neurosci 30:9641-9646.
Carafoli E, A Genazzani, D Geurini (1999) Calcium controls the transcription of its own transporters and channels in developing neurons. Biochem Biophys Res Comm 266:624-632.
Groth RD, RL Dunbar, PG Mermelstein (2003) Calcineurin regulation of neuronal plasticity. Biochem Biophys Res Commun 311:1159-1171.
Norris CM, EM Blalock, KC Chen, et al (2010) Hippocampal ‘zipper’ slice studies reveal a necessary role for calcineurin in the increased activity of L-type Ca(2+) channels with aging. Neurobiol Aging 31:328-338.
Kurz JE, JT Parsons, A Rana, et al (2005) A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J Neurotrauma 22:476-490.
Miletic G, KM Sullivan, AM Dodson, et al (2011) Changes in calcineurin message, enzyme activity and protein content in the spinal dorsal horn are associated with chronic constriction injury of the rat sciatic nerve. Neuroscience 188:142-147.
Foster TC, KM Sharrow, JR Masse, et al (2001) Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci 21:4066-4073.
Dineley KT, D Hogan, WR Zhang, et al (2007) Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem 88:217-224.
Cho HJ, SM Jin, HD Youn, et al (2008) Disrupted intracellular calcium regulates BACE1 gene expression via nuclear factor of activated T cells 1 (NFAT 1) signaling. Aging Cell 7:137-147.
Norris CM, S Halpain, TC Foster (1998) Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. J Neurophysiol 80:1567-1570.
Furman JL, DM Sama, JC Gant, et al (2012) Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci 32:16129-16140.
Hudry E, HY Wu, M Arbel-Ornath, et al (2012) Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer’s disease. J Neurosci 32:3176-3192.
Kuchibhotla KV, ST Goldman, CR Lattarulo, et al (2008) Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron 59:214-225.
Yoshiyama Y, M Higuchi, B Zhang, et al (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53:337-351.
Zawadzka M, B Kaminska (2005) A novel mechanism of FK506-mediated neuroprotection: downregulation of cytokine expression in glial cells. Glia 49:36-51.
Kaminska B, K Gaweda-Walerych, M Zawadzka (2004) Molecular mechanisms of neuroprotective action of immunosuppressants – facts and hypotheses. J Cell Mol Med 8:45-58.
Saganova K, J Galik, J Blasko, et al (2012) Immunosuppressant FK506: focusing on neuroprotective effects following brain and spinal cord injury. Life Sci 91:77-82.
Rozkalne A, BT Hyman, TL Spires-Jones (2011) Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis 41:650-654.
Hong HS, JY Hwang, SM Son, et al (2010) FK506 reduces amyloid plaque burden and induces MMP-9 in AbetaPP/PS1 double transgenic mice. J Alzheimers Dis 22:97-105.
Taglialatela G, D Hogan, WR Zhang, et al (2009) Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav Brain Res 200:95-99.
Dineley KT, R Kayed, V Neugebauer, et al (2010) Amyloid-beta oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J Neurosci Res 88:2923-2932.
Vashishta A, A Habas, P Pruunsild, et al (2009) Nuclear factor of activated T-cells isoform c4 (NFATc4/NFAT3) as a mediator of antiapoptotic transcription in NMDA receptor-stimulated cortical neurons. J Neurosci 29:15331-15340.
Fernandez AM, S Jimenez, M Mecha, et al (2012) Regulation of the phosphatase calcineurin by insulin-like growth factor I unveils a key role of astrocytes in Alzheimer’s pathology. Mol Psychiatry 17:705-718.
Sanderson JL, ML Dell’Acqua (2011) AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist 17:321-336.
Liu JO (2003) Endogenous protein inhibitors of calcineurin. Biochem Biophys Res Commun 311:1103-1109.
Hilioti Z, DA Gallagher, ST Low-Nam, et al (2004) GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev 18:35-47.
Liu Q, JC Busby, JD Molkentin (2009) Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol 11:154-161.
Ullrich V, D Namgaladze, D Frein (2003) Superoxide as inhibitor of calcineurin and mediator of redox regulation. Toxicol Lett 139:107-110.
Klee CB, H Ren, X Wang (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273:13367-13370.
Quintana AR, D Wang, JE Forbes, et al (2005) Kinetics of calmodulin binding to calcineurin. Biochem Biophys Res Commun 334:674-680.
Klee CB (1991) Concerted regulation of protein phosphorylation and dephosphorylation by calmodulin. Neurochem Res 16:1059-1065.
Rumi-Masante J, FI Rusinga, TE Lester, et al (2012) Structural basis for activation of calcineurin by calmodulin. J Mol Biol 415:307-317.
Manalan AS, CB Klee (1983) Activation of calcineurin by limited proteolysis. Proc Natl Acad Sci U S A 80:4291-4295.
Tallant EA, WY Cheung (1984) Activation of bovine brain calmodulin-dependent protein phosphatase by limited trypsinization. Biochemistry 23:973-979.
Kincaid RL, PR Giri, S Higuchi, et al (1990) Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem 265:11312-11319.
Tallant EA, LM Brumley, RW Wallace (1988) Activation of a calmodulin-dependent phosphatase by a Ca2+-dependent protease. Biochemistry 27:2205-2211.
Wang KK, BD Roufogalis, A Villalobo (1989) Characterization of the fragmented forms of calcineurin produced by calpain I. Biochem Cell Biol 67:703-711.
Asai A, J Qiu, Y Narita, et al (1999) High level calcineurin activity predisposes neuronal cells to apoptosis. J Biol Chem 274:34450-34458.
Winder DG, IM Mansuy, M Osman, et al (1998) Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell 92:25-37.
Mansuy IM, M Mayford, B Jacob, et al (1998) Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell 92:39-49.
Mansuy IM, DG Winder, TM Moallem, et al (1998) Inducible and reversible gene expression with the rtTA system for the study of memory. Neuron 21:257-265.
Lakshmikuttyamma A, P Selvakumar, R Kakkar, et al (2003) Activation of calcineurin expression in ischemia-reperfused rat heart and in human ischemic myocardium. J Cell Biochem 90:987-997.
Burkard N, J Becher, C Heindl, et al (2005) Targeted proteolysis sustains calcineurin activation. Circulation 111:1045-1053.
Braak H, E Braak (1996) Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol Scand Suppl 165:3-12.
McGeer EG, PL McGeer (2010) Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis 19:355-361.
Wyss-Coray T (2006) Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med 12:1005-1015.
Molinuevo JL, A Llado, L Rami (2005) Memantine: targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. Am J Alzheimers Dis Other Demen 20:77-85.
Hynd MR, HL Scott, PR Dodd (2004) Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int 45:583-595.
Qian W, X Yin, W Hu, et al (2011) Activation of protein phosphatase 2B and hyperphosphorylation of Tau in Alzheimer’s disease. J Alzheimers Dis 23:617-627.
Mufson EJ, L Binder, SE Counts, et al (2012) Mild cognitive impairment: pathology and mechanisms. Acta Neuropathol 123:13-30.
Li Q, J Fang, M Yang, et al (2010) Galantamine inhibits calpain-calcineurin signaling activated by beta-amyloid in human neuroblastoma SH-SY5Y cells. Neurosci Lett 480:173-177.
Rosenkranz K, C May, C Meier, et al (2012) Proteomic analysis of alterations induced by perinatal hypoxic-ischemic brain injury. J Proteome Res 11:5794-5803.
Shioda N, S Moriguchi, Y Shirasaki, et al (2006) Generation of constitutively active calcineurin by calpain contributes to delayed neuronal death following mouse brain ischemia. J Neurochem 98:310-320.
Quigley HA, RW Nickells, LA Kerrigan, et al (1995) Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci 36:774-786.
Guo L, SE Moss, RA Alexander, et al (2005) Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci 46:175-182.
Nickells RW, GR Howell, I Soto, et al (2012) Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 35:153-179.
Huang W, JB Fileta, A Dobberfuhl, et al (2005) Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci U S A 102:12242-12247.
Shields DC, KE Schaecher, EL Hogan, et al (2000) Calpain activity and expression increased in activated glial and inflammatory cells in penumbra of spinal cord injury lesion. J Neurosci Res 61:146-150.
Shields DC, WR Tyor, GE Deibler, et al (1998) Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A 95:5768-5772.
Shields DC, WR Tyor, GE Deibler, et al (1998) Increased calpain expression in experimental demyelinating optic neuritis: an immunocytochemical study. Brain Res 784:299-304.
Feng ZH, J Hao, L Ye, et al (2011) Overexpression of mu-calpain in the anterior temporal neocortex of patients with intractable epilepsy correlates with clinicopathological characteristics. Seizure 20:395-401.
Gray BC, P Skipp, VM O’Connor, et al (2006) Increased expression of glial fibrillary acidic protein fragments and mu-calpain activation within the hippocampus of prion-infected mice. Biochem Soc Trans 34:51-54.
Cao X, Y Zhang, L Zou, et al (2010) Persistent oxygen-glucose deprivation induces astrocytic death through two different pathways and calpain-mediated proteolysis of cytoskeletal proteins during astrocytic oncosis. Neurosci Lett 479:118-122.
Du S, A Rubin, S Klepper, et al (1999) Calcium influx and activation of calpain I mediate acute reactive gliosis in injured spinal cord. Exp Neurol 157:96-105.
Aliabadi AZ, AO Zuckermann, M Grimm (2007) Immunosuppressive therapy in older cardiac transplant patients. Drugs Aging 24:913-932.
Marks AR (1996) Cellular functions of immunophilins. Physiol Rev 76:631-649.
Aramburu J, MB Yaffe, C Lopez-Rodriguez, et al (1999) Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133.
Noguchi H, M Matsushita, T Okitsu, et al (2004) A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med 10:305-309.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Norris, C.M. (2014). Calpain Interactions with the Protein Phosphatase Calcineurin in Neurodegeneration. In: Dhalla, N., Chakraborti, S. (eds) Role of Proteases in Cellular Dysfunction. Advances in Biochemistry in Health and Disease, vol 8. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9099-9_2
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9099-9_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9098-2
Online ISBN: 978-1-4614-9099-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)