Abstract
The importance of understanding and treating anorectal fistulae is evidenced not only by the prevalence of this condition but also by the variety of approaches involved. There is currently neither a consensus nor a set of guidelines on the management of anorectal fistulae. Whether the fistula is simple or complex, it is paramount to maintain fecal continence and decrease recurrence. Historical approaches have included fistulotomy with or without marsupialization, seton placement, and endorectal advancement flaps, each with variable success rates. More recently, synthetic and biologic plugs have become available as adjuncts in fistula management. In this chapter, we discuss the available plugs, their chemical composition, and contraindications to and recommended techniques of application, including optimal disk placement. We also review several studies with a focus on success and recurrence rates when using the Surgisis or Gore Bio-A plug. Overall the Gore Bio-A appears to be an improvement over the Surgisis plug. However, further studies are required to clearly define its indications and application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The importance of understanding and treating anorectal fistulae is evidenced not only by the prevalence of this condition but also by the variety of approaches involved. There is currently neither a consensus nor a set of guidelines on the management of anorectal fistulae. Whether the fistula is simple or complex, it is paramount to maintain fecal continence and decrease recurrence. Historical approaches have included fistulotomy with or without marsupialization, seton placement, and endorectal advancement flaps, each with variable success rates. More recently, synthetic and biologic plugs have become available as adjuncts in fistula management. In this chapter, we discuss the available plugs, their chemical composition, and contraindications to and recommended techniques of application, including optimal disk placement. We also review several studies with a focus on success and recurrence rates when using the Surgisis or Gore Bio-A plug. Overall the Gore Bio-A appears to be an improvement over the Surgisis plug. However, further studies are required to clearly define its indications and application.
Anal fistula is one of the most frequently treated anorectal diseases, with an estimated 10–30% of all colorectal interventions performed to treat anal fistula [1]. Although being a common condition, there is not a single surgical technique to repair this disease due to the complex anatomic and physiologic aspects of this disease. Prior to focusing on synthetic anal fistula plugs, it is first necessary to focus on the classification of fistula and the history leading up to the development and incorporation of synthetic anal fistula plugs.
Anal fistulas can be classified into two main groups: simple and complex fistulas. Simple fistulas are generally low fistulas with single tracts and carry a low risk of incontinence when treated. In contrast, a complex fistula involves greater than 30–50 % of the sphincter muscle; anterior in woman; or the patient has a history of preexisting incontinence, Crohn’s disease, or local irradiation [2].
When treating either type of fistula, maintaining fecal continence is essentially tantamount to healing the fistula itself. Garcia-Aguilar et al. published a report in 1996 of 75 patients who underwent surgery for anal fistulas between 1988 and 1992. Treatment included fistulotomy and marsupialization (n = 300), seton placement (n = 63), endorectal advancement flap (n = 3), and other (n = 9). Fistulas recurred in 31 patients (8 %) and 45 % reported some degree of postoperative incontinence [3].
The traditional treatment of complex anal fistulas often times involved advancement flaps. There is a wide variance of success rates. Jun and Choi reported an initial success rate of 95 % (38 of 40) patients who underwent anocutaneous flaps with zero reports of incontinence to either flatus or stool [4]. Conversely, Sonoda et al. reported the Cleveland Clinic experience. The study was a retrospective review of 105 patients. Sixty-two patients had anorectal fistulas while 37 patients had rectovaginal fistulas. The rate of primary healing was found to be 63.6 %. Greater body surface area, history of incision and drainage of a perianal abscess preceding advancement flap, previous placement of a seton drain, and short duration of fistula were associated with higher success rates. Conversely, Crohn’s disease and rectovaginal fistulas were associated with lower success rates [5].
Since the 1940s, fibrin glue has been used as surgical sealant [6]. Fibrin glue began to come into favor as a treatment for anal fistulas in the 1990s. Venkatesh et al. published a series of 30 patients treated with autologous fibrin glue treated over a 4-year period. Success rate was 60 % [6]. Urinary tract-related fistulas, Crohn’s disease-related fistulas, and fistulas related to AIDS failed to heal completely with fibrin glue application. Haim et al. looked at their long-term results [7]. The initial success rate was 53 % (32/60). Of the 32 patients who were initially treated successfully, 23 were located and contacted at a mean follow-up of 6.5 years. Seventeen of 23 patients (74 %) remained disease-free. Despite the low rate of success, the application of fibrin glue has several advantages. These include easy application, no risk of damage to the sphincter complex, minimal patient discomfort, and the capacity to repeat application if the initial treatment fails [7].
Moving forward to the 2000s, anal fistula plugs began to come into practice in the mid 2000s. In 2005, the FDA approved Cook Medical’s Surgisis Fistula Plug. It comprises porcine intestinal submucosa. It can be used in infected fields, host tissues colonize the graft, and it does not injure the sphincter complex [8]. Despite the scientific belief that this product would have excellent success rate, the results varied substantially. Success rates ranged from 13.9 to 87 % [9, 10]. This wide range of healing ultimately led to a consensus conference that was held in 2007. The results of the conference are presented here [8].
A consensus conference was held in Chicago on 27 May 2007 at the Illinois Airport Hilton Hotel to develop uniformity of opinion from surgeons with considerable experience in the use of the anal fistula plug. Of the 15 surgeons in attendance, 5 had performed 50 or more anal fistula plug procedures. Success rates with this approach have been reported to be as high as 85 %. Anecdotal communications have however suggested lower rates of success. Concerns have been expressed over plug extrusion and inadequacy of long-term follow-up. It was thought prudent to hold this conference because, despite a number of publications attesting to the safety and efficacy of the procedure, to date there has not been uniformity of opinion regarding indications and technique, nor has there been level I evidence of any actual benefit.
Plug Material, Mechanism, and Applications
Small intestinal submucosa (SIS) is a natural biomaterial harvested from porcine small intestine and fabricated into a biomedical product of various shapes and thicknesses [2]. As such it has been applied to a host of potential indications. These include reinforcement of soft tissue for incisional and inguinal herniorraphy; urethral sling placement in urogynaecology; staple line reinforcement; paraesophageal hernia repair and in the treatment of anal fistula. The fact that it has been demonstrably useful as a bioprosthetic material in infected fields makes its application in fistula surgery quite reasonable. The Surgisis AFP Anal Fistula Plug (the plug) has a biological configuration suitable for fistula disease. When SIS is implanted, host tissue cells and blood vessels colonize the “graft.” In essence, SIS provides a scaffold or matrix to allow infiltration of the patient’s connective tissue. The material is supplied in a sterile, peel-open package and is intended for one-time use.
Recommendations
All of the following recommendations and opinions of the Consensus Panel were unanimously agreed by those present unless otherwise indicated.
Inclusion⁄exclusion criteria indications for the use of the plug include:
-
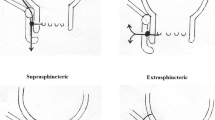
Transsphincteric Fistula
-
This was considered to be the ideal indication for the use of the plug.
-
-
Anovaginal Fistula
-
While it was recognized that the shorter the tract the less likely the procedure would be successful, the plug was felt to be a reasonable alternative to other operations. Besides the financial cost of failure there appeared to be no disadvantage in attempting its use in this circumstance.
-
-
Intersphincteric Fistula
-
The Consensus Panel felt that the use of the plug for this indication was valid, if conventional fistulotomy posed a significant risk of incontinence. This would include those patients with inflammatory bowel disease and those who had previously undergone radiation therapy.
-
-
Extrasphincteric Fistula
-
While this was recognized to be an uncommon indication for fistula surgery, it was regarded as an indication for the fistula plug. Suturing the plug to the site of the internal opening was considered to be potentially technically difficult.
-
Contraindications for the Use of the Plug
Conventional, Uncomplicated Intersphincteric Fistula
Success approaches 100 % with minimal morbidity with standard fistulotomy. Thus, the cost⁄failure rate with the use of the plug cannot be justified. In addition, the following conditions were felt to be inappropriate because of the extremely low probability of success:
-
1.
Pouch-vaginal fistula.
-
2.
Rectovaginal fistula (because of the short length of the track).
-
3.
Fistula with a persistent abscess cavity.
-
4.
Fistula with any suggestion of infection. Examples included those with associated anorectal abscess formation, persistent cavity (as above), and a fistula with induration or purulent drainage.
-
5.
Allergy to porcine products.
-
6.
Inability of the surgeon to identify both the external and internal openings. This is an absolute contraindication for undertaking this procedure.
Preoperative Preparation
Some surgeons experienced in using the plug have prepared the bowel as for a major colon resection, with laxatives and antibiotics. The Consensus Panel questioned the value of attempts to delay defecation. They queried whether liquid stool was preferable to solid for prevention of extrusion of the plug. When the use of a small-volume preoperative enema was considered, there was no consensus: half of the Panel felt it would be useful. It was accepted that there was no evidence base for this consideration. Therefore, in the absence of data, the Panel concluded that bowel preparation and⁄or the use of a small-volume enema should be left to the individual surgeon’s personal preference. The Panel did recommend a single preoperative dose of systemic antibiotics but felt that this should not be continued for longer.
Intraoperative Management
Anaesthetic
This was deemed to be a matter of the patient’s or surgeon’s preference.
Positioning the Patient
This was regarded as a matter of the surgeon’s preference. The critical element, however, was to ensure adequate visualization of the internal opening to place the suture correctly.
Surgical Technique
Identifying the Internal and External Openings
The plug cannot be inserted unless there is clear delineation of the primary and secondary openings. Irrigation of the track with saline or peroxide was recommended.
Passing a Probe
Gentle passage of a probe was essential to confirm the position of the track and to facilitate insertion of the plug. The Panel unanimously affirmed that debridement, curettage, or brushing of the tract should not be performed. Such maneuvers would enlarge the fistula track.
Using a Seton
There was uniformity of opinion that a seton should always be employed temporarily until there was no evidence of acute inflammation, purulence, or excessive drainage. This would often take 6–12 weeks. However, the use of a seton prior to implantation was unnecessary if there was no acute inflammatory process.
Preparing the Plug
The AFP plug should be completely immersed in sterile saline for 2 min. Allowing immersion for >5 min risks fragmentation of the plug. Conversely, implantation of a nonhydrated plug is extremely painful.
Managing the Recessed Internal Opening
If there is epithelialization of the internal opening (dimpled or recessed), limited mobilization of the mucosal edges with debridement prior to suture placement should be considered.
Passing the Plug
The use of a suture or ligature was recommended to pull the narrow end of the plug from the internal opening through the track to the external opening until the plug is snug.
Trimming the Plug
Any excess plug should be trimmed at the level of the internal opening (the wide end) and sutured with 2-0 long-term, braided, absorbable material (e.g., Vicryl, Ethicon, Inc., Somerville, NJ, USA), incorporating the underlying internal anal sphincter. Monofilament material should not be used. There was no consensus, however, as to whether the plug should be buried under the mucosa. The excess external plug should be trimmed flush with the skin without fixation. The external opening may be enlarged if necessary to facilitate drainage.
Postoperative Care
The Panel had a stimulating discussion on various postoperative management alternatives. However, in the absence of evidence-based data, opinions revolved around what seemed reasonable and appropriate with more emphasis on the “art” rather than the “science” of surgery. There were nevertheless several unanimous conclusions.
-
1.
Diet: No dietary restrictions.
-
2.
Activity: No strenuous activity, exercise, or heavy lifting for 2 weeks. Abstinence from sexual intercourse for 2 weeks.
-
3.
External dressing: For patient comfort only.
-
4.
Topical antibiotics: Not indicated.
-
5.
Cleansing: Showers with gentle cleaning.
-
6.
Bowel management: Medications as necessary to prevent constipation or diarrhea.
-
7.
Follow-up visits. Surgeon’s preference: The tract should not be probed during these visits.
Outcome
Defining Failure
Early extrusion of the plug is either a technical error [the track being too large, the plug pulled too tightly, faulty fixation (i.e., to the mucosa rather than to the internal sphincter)] or infection. The Panel unanimously agreed that the overwhelming majority of fistulas which heal do so within 3 months, although some will take longer. The decision whether the operation should be considered a failure rests with the individual surgeon, but should not be taken for a minimum of 3 months.
Conclusions
The anal fistula plug was felt to be a reasonable alternative for the treatment of anal fistula. Members of the Panel were asked to state what they felt to be a reasonable rate of success and concluded that 50–60 % should be considered acceptable. To achieve the highest possibility of success, the Panel concluded that patient selection, avoidance of local infection, and meticulous technique were required. Besides the consideration of cost it was felt that the patient would not be adversely affected by insertion of the fistula plug because all other management options were still available. It was recognized, however, that even in patients with apparent healing the rate of subsequent recurrence was unknown. Prospective randomized trials comparing the anal fistula plug with other treatments such as seton fistulotomy were recommended. Finally it was unanimously agreed that the procedure should be undertaken only by trained surgeons familiar with anorectal anatomy and experienced in conventional anal fistula surgery and in the management of its complications.
Gore Bio-A Plug
In 2009, the synthetic Gore Bio-A plug was FDA approved. The Gore Bio-A is a synthetic plug as compared to Surgisis, which is a biologic plug. The Gore Bio-A fistula plug is a porous fibrous structure composed solely of a synthetic bioabsorbable polyglycolide–trimethylene carbonate copolymer (67 % polyglycolide, 33 % trimethylene carbonate). The copolymer has been found to be both biocompatible and nonantigenic because it is degraded via a combination of hydrolytic and enzymatic pathways. In vivo studies with this copolymer indicate that the bioabsorption process should be complete within 6 months [11]. The device consists of a disk 16 mm in diameter, attached to six tubes, each 9 cm in length. The size of the plug can be tailored by changing the number and length of the tubes so that it occupies the fistula tract until the bioabsorbable nature of the material allows the body to fill the defect with native tissue [12]. In comparison to the Surgisis plug, the disk was devised to decrease the incidence of dislodgement. The plug is depicted in Fig. 13.1.
Fistula plug placement. The fistula tract is identified and curetted. A silk tie is then placed through the tract. The tie is then affixed to the fistula plug so that it will be pulled through the internal orifice out the external. The plug is trimmed for optimal passage. The plug is gently brought through the fistulous tract. The plug is sutured into place and the internal orifice is closed with figure-of-8 absorbable suture. The external end of the plug is trimmed to skin level for patient comfort. Images courtesy of Dr. Alex Ky
The following technique is provided by Gore Medical (Flagstaff, AZ) and comprises the corporate recommendations for procedure [13].
Preparation
-
Prepare the patient and surgical site using standard techniques appropriate for anal fistula repair.
-
Remove the device from its sterile packaging using aseptic technique.
-
Using sharp sterile scissors, trim the disk diameter to a size appropriate for the defect allowing for adequate fixation of the disk to the rectal mucosa. Care should be taken to avoid the creation of sharp edges or corners when trimming the disk.
-
Individual tubes can be removed from the device to accommodate the diameter of the fistula tract. When removing tubes, begin with the center-most tubes, carefully cutting the tube as close to the disk as possible (proximally adjacent) without compromising tube attachment.
-
To facilitate introduction and deployment of the device in the fistula tract, it is recommended that a suture be used to gather the tubes and pull the device through the fistula tract. To do so, run a suture through the distal ends of the tubes. A bite depth of approximately 3 mm is recommended to ensure adequate suture retention strength.
Note: The use of a resorbable suture is recommended to minimize the potential that any permanent material is implanted.
-
The GORE® BIO-A® Fistula Plug does not need to be hydrated prior to use. However, to facilitate passage of the tubes through the fistula tract, briefly immerse the entire device in sterile saline.
Device Placement
-
Use standard techniques to define, clean, and prepare the fistula tract. If necessary, the tract may be defined with a curette to remove any granulation tissue.
-
Insert a fistula probe or other suitable instrument through the fistula tract, entering through the external (secondary) opening and exiting via the internal (primary) opening.
-
Grasp the suture attached to the distal end of the GORE® BIO-A® Fistula Plug.
-
Gently draw the suture into the internal (primary) opening of the fistula tract. Continue to draw the suture through the fistula tract.
-
Once the suture is visible at the external (secondary) opening, slowly draw the GORE® BIO-A® Fistula Plug into the defect until slight resistance is felt and the device disk is securely seated at the internal (primary) opening.
Note: Take care to ensure that disk lies flat and is well apposed to the rectal mucosa at the internal (primary) opening of the fistula tract.
-
After the device is properly positioned in the fistula tract, one of the following fixation methods should be used to secure the disk at the internal (primary) opening.
Fixation Method I
-
Using a suitable resorbable suture, secure the disk of the GORE® BIO-A® Fistula Plug to the adjacent tissue, obtaining adequate bites of rectal wall to prevent device migration and minimize the potential for leakage of bowel contents into the fistula tract.
Fixation Method II
-
Using a suitable resorbable suture, close the rectal mucosa over the disk portion of the device to prevent device migration and minimize the potential for leakage of bowel contents into the fistula tract. Figures 13.2, 13.3, 13.4, 13.5, 13.6, 13.7, and 13.8 depict the set-up and installation of the fistula plug.
Fig. 13.2 Fig. 13.3 Fig. 13.4 Fig. 13.5 Fig. 13.6 Fig. 13.7
Results
Buchberg et al. published a retrospective review of a prospectively maintained database between 2007 and 2009 comparing the Surgisis anal fistula plug to the Gore Bio-A fistula plug [14]. There were a total of 27 plug insertions over a 28-month period in 16 patients. Twelve patients underwent 16 Cook plug insertions and 10 patients underwent 11 Gore plug insertions. Several patients who initially failed the Cook plug were subsequently receiving Gore plugs.
Successful closure (healing) was clinically defined as the absence of any discharge or swelling, with the internal opening closed by the time the anoscopy was performed and all external openings closed at the perineal examination at the last follow-up session. In regard to technique, the button was secured flush with the anal mucosa and secured with two to three 2-0 Vicryl sutures.
The overall procedural success rate in the Gore group was 54.5 % (6 of 11) vs. 12.5 % (2 of 16) in the Cook group. Of note, patients whose fistula plug was inserted after pretreatment of the fistula with a draining (loose) seton appeared to heal more often (55.6 %, 5/9) compared to those treated without a seton (28.6 %, 2/7).
De la Portilla et al. reported a series a total of 19 patients (18 men, 1 woman) with transsphincteric anal fistulas [12]. The median age was 49 (range, 33–65) years. Patients with known hypersensitivity to materials in the plug, those who had more than three external openings or Crohn’s disease, and those who were under 18 years of age or were pregnant were excluded form the study. The follow-up duration was 12 months and successful closure was clinically defined as the absence of any discharge or swelling, with the internal opening closed by the time the anoscopy was performed and all external openings closed at the perineal examination at the last follow-up session. In regard to technique, the disk was secured flush with the mucosa with interrupted absorbable sutures.
Concerning results, relapse occurred in 16 patients (with a perianal abscess in one patient). Successful closure was observed in only 3 out of 19 patients (15.8 %). The poor results from this study were attributed to the learning curve of the surgeons, the small number of patients, and the varied nature of the fistulas.
Ratto et al.’s study included 11 patients with a median age of 42 [15]. The fistulas were cryptoglandular in origin in all of the patients. There were five high anterior transsphincteric fistulas and six high posterior transsphincteric. The median duration of the procedure was 40 min. There were between 2 and 4 tubes inserted into the fistula tact. In regard to surgical technique, the submucosal pocket was closed with 3-0 Vicryl stitches. The disk was included in the suture to prevent plug migration and the protruding tubes were trimmed 2–3 mm beyond the surface of the perianal skin. The median follow-up was 5 months. All patients were evaluated by physical examination and endo-anal ultrasound. Success was defined as the absence of drainage, closure of the external opening, and the absence of perianal swelling or abscess formation. In regard to results, there was no early dislodgement, and closure occurred in 8 of 11 patients (72.7 %).
Ommer and colleagues recently published the initial results from Germany [16]. This series comprised 40 patients (30 male, 10 female, age 51 ± 12 years) who underwent closure of a high transsphincteric (n = 28) or supra-sphincteric (n = 12) fistula with Gore Bio-A Fistula Plug® in three surgical departments by five colorectal surgeons. Healing of the fistula was defined as complete closure of the internal opening and the external wound and no symptoms of inflammation. In describing the surgical technique, all arms of the plug were pulled tight and the head was fixated to the internal sphincter muscle using 2–3 sutures (PDS 2-0). The head was then covered with a mucosa-submucosa-flap (Vicryl 2-0).
The overall healing rate was 57.5 % (23/40). Six months after surgery the fistula had healed in 20 patients (50.0 %). Three additional fistulas healed after 7, 9, and 12 months. One patient developed an abscess which required surgical drainage and the plug had become dislodged in two patients during the first 2 weeks postoperatively. The healing rate varied significantly amongst the five surgeons with a range of 0–75%. In patients having only prior drainage of the abscess healing occurred in 63.6% (14/22) whereas in patients after one or more flap fistula reconstruction the healing rate decreased to 50% (9/18). No patient complained about any impairment of his or her preoperative continence status. Additionally, four Crohn’s patients were treated with a success rate of 25 %. The following Table 13.1 is a summary of the recent studies.
Conclusion
The Gore Bio-A fistula plug is a new and evolving treatment modality for anal fistulas. There is a wide range of results in the data that has been published up to this point. This is due to the learning curve of the surgery, patient selection, and the small number of patients. One technical aspect that needs to be clarified is in regard to the fixation method of the disk. It is not clear whether it is optimal to secure the disk on top of the rectal mucosa or secure it beneath a flap. Intuitively, it seems that covering the disk with a mucosal flap would produce better results. This plug has also not been extensively studied in Crohn’s patients. There are only four patients in the literature who have been treated with the Gore Bio-A plug. Overall, the Gore Bio-A plug is a new technique that appears to be an improvement over the Surgisis plug and updated research will more clearly define the proper fixation method and surgical indications.
References
Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12.
Ellis CN. Bioprosthetic plugs for complex anal fistulas: an early experience. J Surg Educ. 2007;64:36–40.
Garcia-Aguilar J, Belmonte C, Wong WD, Goldberg SM, Madoff RD. Anal fistula surgery: factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39:723–9.
Jun SH, Choi GS. Anocutaneous advancement flap closure of high anal fistulas. Br J Surg. 1999;86(4):490–2.
Sonoda T, Hull T, Piedmonte MR, Fazio VW. Outcomes of primary repair of anorectal and rectovaginal fistulas using the endorectal advancement flap. Dis Colon Rectum. 2002;45:1622–8.
Venkatesh KS, Ramanujam P. Fibrin glue application in the treatment of recurrent anorectal fistulas. Dis Colon Rectum. 1999;42:1136–9.
Haim N, Neufeld D, Ziv Y, Tulchinsky H, et al. Long-term results of fibrin glue treatment for cryptogenic perianal fistulas: a multicenter study. Dis Colon Rectum. 2011;54(10):1279–83.
The Surgisis AFP. anal fistula plug: report of a consensus conference. Colorectal Dis. 2008;10:17–20.
Champagne BJ, O’Connor LM, Ferguson M, et al. Efficacy of anal fistula plug in closure of cryptoglandular fistulas: long-term follow-up. Dis Colon Rectum. 2006;49:1817–21.
Safar B, Jobanputra S, Cera S, et al. Anal fistula plug: Initial experience and outcomes meeting abstract. Dis Colon Rectum. 2007;50:725.
Katz AR, Mukherjee DP, Kaganov AL, Gordon S. A new synthetic monofilament absorbable suture made from poly-trimethylene carbonate. Surg Gynecol Obstet. 1985;161:213–22.
De la Portilla F, Rada R, Jiménez-Rodríguez R, et al. Evaluation of a new synthetic plug in the treatment of anal fistulas: results of a pilot study. Dis Colon Rectum. 2011;54(11):1419–22.
GORE® BIO-A® Fistula Plug instructions for use http://www.goremedical.com/resources/dam/assets/AM0175-ML3_English.pdf. Accessed Nov 18, 2012.
Buchberg B, Masoomi H, Choi J, Bergman H, Mills S, Stamos MJ. A tale of two (anal fistula) plugs: is there a difference in short-term outcomes? Am Surg. 2010;76:1150–3.
Ratto C, Litta F. Parello et al. Gore Bio-A® Fistula Plug: a new sphincter-sparing procedure for complex anal fistula. Colorectal Dis. 2012;14(5):e264–9.
Ommer A, Herold A, Joos A, et al. Gore BioA Fistula Plug in the treatment of high anal fistulas—initial results from a German multicenter-study. Ger Med Sci. 2012;10:13. doi:10.3205/000164. Epub 2012 Sep 11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ky, A.J., Polcino, M., Nanna, A.T. (2014). Synthetic Fistula Plug. In: Abcarian, H. (eds) Anal Fistula. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-9014-2_13
Download citation
DOI: https://doi.org/10.1007/978-1-4614-9014-2_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-9013-5
Online ISBN: 978-1-4614-9014-2
eBook Packages: MedicineMedicine (R0)