Abstract
Decisions regarding treatment of sarcoidosis rely on several factors. These include symptoms, organ involvement, signs of functional impairment, and current and prior therapy. Over the years, the treatment options for sarcoidosis have increased. While this has allowed the clinician to tailor therapy for the individual patient, it also has led to the need to consider risk and benefit each individual patient. Not all therapy is equally effective in sarcoidosis. The benefits from an individual therapy may be more apparent within a few weeks, such as with glucocorticoids and anti-TNF biologic agents, whereas cytotoxic drugs such as methotrexate (MTX) may take up to 6 months or longer to demonstrate their effectiveness. Some drugs such as pentoxifylline seem only to be useful as steroid-sparing agents for pulmonary disease. In addition drugs such as chloroquine and thalidomide may be effective for cutaneous disease but not pulmonary disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Decisions regarding treatment of sarcoidosis rely on several factors. These include symptoms, organ involvement, signs of functional impairment, and current and prior therapy. Over the years, the treatment options for sarcoidosis have increased. While this has allowed the clinician to tailor therapy for the individual patient, it also has led to the need to consider risk and benefit each individual patient.
Not all therapy is equally effective in sarcoidosis. The benefits from an individual therapy may be more apparent within a few weeks, such as with glucocorticoids and anti-TNF biologic agents [1, 2], whereas cytotoxic drugs such as methotrexate (MTX) may take up to 6 months or longer to demonstrate their effectiveness [3]. Some drugs such as pentoxifylline seem only to be useful as steroid-sparing agents for pulmonary disease [4, 5]. In addition, drugs such as chloroquine and thalidomide may be effective for cutaneous disease but not pulmonary disease [6, 7].
Despite our increasing number of agents for treating sarcoidosis, there is evidence to suggest that the rate of hospitalization and death from sarcoidosis is rising [8, 9]. There are several possible reasons for this, including increased recognition of the disease as a cause of mortality, complications of the disease such as pulmonary hypertension [10, 11], and complications of treatment such as infection [12]. As we add more potent treatments, we may be increasing the overall risk for the patient. Another limitation to treatment decisions is the relatively few well controlled double blind placebo-controlled trials [13, 14]. This is in part because of the diverse presentation of sarcoidosis. Nevertheless, evidence recommendations can often be given for individual patient situations [15]. In this chapter, we will review the indications for therapy, current treatment options, and propose guidelines for treatment and monitoring with various agents.
Indication for Therapy
Table 3.1 lists those indications proposed as relative and absolute indications for therapy [16]. For many patients, the decision to treat will be based upon the level of symptoms and presence of functional impairment [17]. This is especially true for pulmonary and cutaneous disease. For example, a small skin lesion on the arm or back may not warrant any treatment. However, lupus pernio and hypercalcemia will often be treated with systemic therapy [18].
Pulmonary disease is the most common manifestation of sarcoidosis. However, not all patients with pulmonary disease require systemic therapy. Dyspnea and cough are the major indications for therapy. In the USA, about half of patients with pulmonary disease require systemic therapy [19, 20]. A smaller percentage of patients seem to require systemic therapy for pulmonary disease in Europe and Asia [21, 22]. However, advanced pulmonary disease is encountered throughout the world. This includes pulmonary fibrosis, which can lead to significant morbidity and some mortality [23, 24].
For pulmonary disease, several parameters have been proposed to initially assess and monitor disease [25] and are summarized in Table 3.2. These include those obtained with pulmonary function testing, especially the forced vital capacity (FVC). Changes in FVC are the most widely used measure of response to therapy [26]. However, other static pulmonary function tests, including the DLCO may change with therapy [27]. Exercise capacity can be assessed by the 6-min walk test, which allows one to determine the 6-min walk distance (6MWD). While changes in 6MWD may be a reflection of changes in lung function, there are several other factors which can affect 6MWD [28]. These include other conditions such as pulmonary hypertension as well as other factors, such as fatigue [29] and impaired muscle strength [30]. Full cardiopulmonary exercise testing does provide information not obtained from routine spirometry [31, 32]. However, the variability of performing the test across centers has led to limited use in clinical trials of treatments.
Chest imaging has also been used to assess response to therapy. The Scadding staging system has proved a useful way of characterizing the majority of patients with pulmonary disease [33]. There are problems with reproducibility of the staging system [34]. In addition, most studies fail to show significant changes in stage with therapy. Muers et al. devised a detailed radiographic score similar to what has been used in pneumoconiosis [35]. Significant improvement in the Muers’ score were seen with prednisone therapy [27] and infliximab [34] compared to placebo. The Muers’ score is time consuming and others have reported that a simple comparison of chest X-ray before and after therapy has been useful [36, 37]. However, this method was not able to differentiate patients treated with infliximab versus those treated with placebo [34].
CT scanning, including high resolution CT (HRCT) imaging, has proved useful in identifying manifestations of sarcoidosis as well as complications of the disease such as bronchiectasis, fibrosis, and aspergillomas. Scoring systems for HRCT have been proposed [38, 39]. To date, no study assessing response to treatment in a systematic way of the utility of HRCT is published.
Positron emission tomography using fluor-18 fluorodeoxyglucose (PET) scanning of sarcoidosis has demonstrated that increased activity may be seen in the lungs with parenchymal lung disease. A negative correlation between 18F-FDG PET activity and FVC has been found [40]. Mostard et al. demonstrated that the severity of the pulmonary involvement, assessed by HRCT features and lung function parameters, appeared to be associated with PET activity in sarcoidosis [40]. The majority of patients with fibrotic changes demonstrated inflammatory activity at pulmonary and extra-thoracic sites. In addition, improvement in lung function and PET activity in the lung has been shown after several treatments [41, 42].
Finally several measures of quality of life (QOL) have been reported in the treatment of sarcoidosis. For the most part, the studies have failed to show convincing evidence of change, but this may be due to study design. Also, many of the questionnaires are not sarcoidosis specific. The fatigue assessment scale (FAS) was developed for sarcoidosis patients [43]. Minimal clinically important differences have been determined for this scale [44]. The FAS scale has been shown to significantly improve with neurostimulants [45] and neurostimulant-like [46] drugs.
Figures 3.1 and 3.2 summarize an approach to treating sarcoidosis. In Fig. 3.1, the level of disease is determined based on pulmonary function studies and level of symptoms. In some cases, patients with normal pulmonary function may still have an advanced level of disease because of extra pulmonary disease, such as ocular or neurologic disease. Also, patients with symptoms and normal lung function should lead to investigation of alternative causes of dyspnea. These would include cardiac or muscle disease or complications such as pulmonary hypertension. Figure 3.2 is a guide to the usual therapy for each of these levels of disease.
The level of disease is determined based on pulmonary function studies and level of symptoms. In some cases, patients with normal pulmonary function may still have an advanced level of disease because of extra pulmonary disease, such as ocular or neurologic disease. Also, patients with symptoms and normal lung function should lead to investigation of alternative causes of dyspnea. *Alternative causes would include cardiac or muscle disease or complications such as pulmonary hypertension
A guide to the usual therapy for each of these levels of disease determined in Fig. 3.1. *Anti-TNF agents should be used in caution in patients with moderate to severe cardiomyopathy
Individual Treatments
Glucocorticoids:
The most widely used treatment for sarcoidosis remains glucocorticoids [47]. This is based on the observation that these drugs can be quite effective for the disease. In addition, several trials have demonstrated a significant improvement in FVC, DLCO, or chest roentgenogram [27, 48]. The drug has also been used effectively in all other manifestations of sarcoidosis, including neurologic [49], cutaneous [18], cardiac [50], and ocular disease [51].
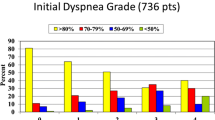
Several case series have demonstrated that corticosteroids are the preferred first drug for sarcoidosis patients. The overall frequency of initial corticosteroid therapy use is about 50 %, but varies from 30 to 80 % (Fig. 3.3) [20, 52–54]. In addition, some underlying conditions may be more likely to treat with corticosteroids. For example, neurological and cardiac involvement is almost always treated initially with corticosteroids. In one report of a large sarcoidosis population in the USA, the most common reason to use corticosteroid therapy was cardiac disease, while lung disease was the third most common, even though the overall prevalence of pulmonary disease was 18 times higher than cardiac disease [19]. For patients with cardiac or neurologic disease, a combination of corticosteroids and cytotoxic agents (such as MTX or azathioprine) are often used for initial therapy, although there is no specific trial demonstrating superiority of this approach over corticosteroids alone.
A bigger question is what dose to begin therapy. The usual dose is 20–40 mg a day. Tapering of corticosteroids is often over a prolonged period. One group did demonstrate a more rapid response to lower doses of prednisone and usually tapered over 2–6 weeks. However, this was more for an acute decompensation of the disease, not for standard management of the underlying condition. Figure 3.4 demonstrates the average daily prednisone dosage from one study comparing MTX to placebo for acute pulmonary sarcoidosis. The dose of prednisone was reviewed every 1–2 months, but significant reduction was not seen until 6 months of therapy. After that point, half of the placebo patients required an increase in their prednisone dosage.
Average prednisone dose at various time intervals for patients with acute pulmonary sarcoidosis receiving either placebo or methotrexate. There was a significant difference in the average prednisone dose after 6 months of therapy [59]
The approach to reduction of prednisone is almost as variable as the initial dose. In one multicenter trial, a steroid-tapering regimen was proposed and was able to be applied to over 80 % of visits. A modified version of this schedule evaluates the patient after the first 6–8 weeks of therapy. If the patient has improved, the dose of prednisone is halved. If the patient is stable, the dose is not changed for another 6–8 weeks. At the next and all subsequent visits, the dose is halved if the patient is stable or improved. If the patient relapses, the dose is doubled. For patients in whom the dose cannot be kept below 10 mg within 4–6 months, a steroid sparing alternative is added.
Unfortunately, once a patient begins corticosteroids, they may require long-term treatment. Figure 3.3 also demonstrates the percentage of patients who require long-term therapy in four large studies [20, 52–54]. These studies highlight that there is a subset of patients with chronic disease who are often considered for steroid sparing agent. These would be for patients with level B, C, or D symptoms. At most sarcoidosis centers, these patients represent the majority of patients who are still being followed 2–5 years after their initial diagnosis [55].
The toxicity of prednisone includes weight gain, mood swings, diabetes, cataracts, glaucoma, acne, and increased bruising. Osteopenia and osteoporosis are particular problems with long-term glucocorticosteroid use. Guidelines for preventing osteoporosis have been proposed by the American College of Rheumatology. These include supplemental calcium and vitamin D for sarcoidosis patients; there is an increased rate of hypercalcemia and hypercalcuria based on autonomous 1-alpha hydroxylase activity in the granulomas and increased levels of 1,25-diOH-vitamin D3, especially in patients with chronic sarcoidosis [56]. Therefore, modifications of the ACR recommendations have been made and are summarized in Table 3.3 [57].
Methotrexate:
This drug is the most commonly prescribed steroid-sparing cytotoxic agent for chronic sarcoidosis [47]. Initial reports suggested approximately two-thirds of patients will respond to the drug after 6 months of therapy [58]. It has been shown to be steroid sparing as compared to placebo in a double blind placebo-controlled trial [59]. The drug has been reported as effective in pulmonary [58], cutaneous [60], ocular [51], and neurologic disease [49]. Guidelines for MTX dosage and monitoring have mostly derived from those developed for rheumatoid arthritis and psoriasis. However, since bone marrow involvement is common in sarcoidosis [61], the dose used in sarcoidosis is often only 10–15 mg a week. In some cases, we have used as little as 2.5 mg a week in patients with significant leukopenia due to their sarcoidosis. In addition, liver involvement is common in sarcoidosis. Surveillance liver biopsies have been performed in sarcoidosis patients on prolonged courses of MTX [62]. However, it appears that routine liver function testing can be useful to detect potential MTX hepatotoxicity. This is especially true with changes in the transaminase levels over time. Folic and folinic acid supplementation may be useful to reduce MTX toxicity [63, 64]. Dosage and evidence-based recommendations for monitoring while prescribing MTX is provided in Table 3.4. These recommendations have been adapted from those made by an expert panel [65].
Azathioprine:
The cytotoxic agent azathioprine was most frequently used as a steroid-sparing agent for solid organ transplantation. It had been reported as effective for pulmonary sarcoidosis [66]. It has been used as an alternative to MTX in chronic sarcoidosis. Some groups use the agent because of familiarity with the drug [47]. Since azathioprine has less hepatotoxicity than MTX, it is often used in treating symptomatic hepatic disease [67, 68]. It also may be an alternative agent for patients who develop pulmonary toxicity from MTX. It is associated with a higher frequency of nausea and leukopenia than MTX [69] and therefore is not a likely candidate when those complications rise from MTX treatment. Azathioprine is associated with an increased rate of skin cancers and infections [70]. In a recent trial of idiopathic pulmonary fibrosis, azathioprine therapy was associated with an increased mortality compared to placebo treated patients [71]. Dosage and evidence-based recommendations for monitoring while prescribing azathioprine is provided in Table 3.4. These recommendations have been adapted from those made by an expert panel [65].
Leflunomide:
Developed as an alternative to MTX for rheumatoid arthritis [72], leflunomide has been reported as effective in treating chronic sarcoidosis [73, 74]. In one series, treatment was associated with a significant improvement in forced vital capacity compared to pretreatment values [73]. While the drug has similar toxicity as MTX, the frequency of nausea is less. In addition, pulmonary toxicity is much less frequent with leflunomide, although it can still occur [75]. Leflunomide is associated with systemic hypertension and peripheral neuropathy [75, 76], toxicities not associated with MTX. Dosage and evidence-based recommendations for monitoring while prescribing leflunomide is provided in Table 3.4. These recommendations have been adapted from those made by an expert panel [65].
Mycophenolate:
While not as commonly used as other cytotoxic agents, mycophenolate appears to be effective at least in some patients with chronic sarcoidosis. It has been reported in small case reports as effective in treating cutaneous [77], ocular [78], and neurologic disease [79]. While the drug has not been used that frequently in sarcoidosis, it appears to have some advantages over other cytotoxic agents. This includes a lower rate of leukopenia, an important issue in sarcoidosis patients who may have bone marrow involvement from the underlying disease [61, 80]. There is an increased rate of infection and cutaneous malignancies similar to that seen with azathioprine [81, 82]. Dosage and evidence-based recommendations for monitoring while prescribing mycophenolate is provided in Table 3.4. These recommendations have been adapted from those made by an expert panel [65].
Antimalarial agents:
Chloroquine and hydroxychloroquine represent the two antimalarial drugs that have been used in treating sarcoidosis [6, 83]. These agents have been reported as effective in both cutaneous and pulmonary disease. These drugs appear to be more effective for cutaneous than pulmonary disease [6, 84]. However one study did find chloroquine was superior to placebo in treating patients with chronic pulmonary disease [85]. The major toxicity of these drugs is ocular and routine eye examinations are recommended for both agents [86]. Hydroxychloroquine is associated with low risk of ocular toxicity, especially when low doses that are adjusted for patient’s weight are employed [87]. However, eye examinations on a regular basis should still be considered [88]. Dosage and evidence based recommendations for monitoring while prescribing the antimalarial agents is provided in Table 3.4. These recommendations have been adapted from those made by an expert panel [65].
Tumor Necrosis Factor Inhibitors
Tumor necrosis factor (TNF) was found to be secreted in high levels from alveolar macrophages of some patients with sarcoidosis [89]. Alveolar macrophages from patients with chronic sarcoidosis with worsening disease despite corticosteroid therapy still released high levels of TNF [90]. These observations suggested that inhibition of TNF may be a target of therapy in sarcoidosis patients [91]. When the chimeric monoclonal anti-TNF antibody infliximab became available for clinical use, there were a large number of case series demonstrating the effectiveness of some of these drugs in refractory sarcoidosis [92–94]. These drugs proved effective for cutaneous lesions, such as lupus pernio [95], neurologic [96], and other forms of refractory disease [97, 98]. Subsequently, two double-blind placebo were performed. Both of these found infliximab was superior to placebo in treating refractory pulmonary disease [2, 99]. In the larger of these studies, infliximab treatment was also superior to placebo in treating extra pulmonary disease [100]. Dosage and evidence-based recommendations for monitoring for infliximab and adalimumab are provided in Table 3.5. These recommendations have been adapted from those made by an expert panel [65].
Examining the various reports regarding the use of TNF-alpha inhibitors for sarcoidosis, guidelines have been proposed (Table 3.6) [101]. Analysis of the two randomized trials using infliximab for pulmonary sarcoidosis provides some insight regarding who is more likely to respond to treatment. However, these two studies have some major differences. The study led by Rossman included only 19 patients. Analysis was performed after only 6 weeks of therapy. The study led by Baughman consisted of 138 patients. Analysis was performed after 24 weeks of therapy. In the larger randomized trial comparing infliximab to placebo, the median FVC was 69 %. Subgroup analysis of response to infliximab found that those patients with a FVC > 69 % had no significant response to infliximab therapy compared to placebo, while there was significantly larger response for those whose FVC was less than 69 % [2]. In the other randomized trial, there was an even larger response to infliximab compared to placebo (Fig. 3.5). The median FVC for that study was 60 % [99]. These studies support the concept that more severe patients are more likely to respond to therapy.
Not all patients with chronic pulmonary sarcoidosis will respond to infliximab therapy [102]. There is little evidence to support that these drugs would be effective for patients with severe fibrosis [23]. There have been two markers which were identified retrospectively from a randomized trial of patients with treated with infliximab. An elevated C reactive protein (CRP) was associated with a significantly higher rate of response to infliximab compared to control [103]. Patients with reticulonodular infiltrates on chest roentgenogram were also more likely to respond to therapy [34]. That study also identified a inflammatory profile which was associated with an enhanced response to therapy [104]. These markers need to be studied prospectively before they can be applied in standard practice.
Anti-TNF therapy has been employed in refractory extra pulmonary disease as well. In one study, the overall response of extra-pulmonary disease responded better to infliximab compared to placebo [100]. That study employed a physician assessment of each organ and comparisons were made before and after therapy. Specific organ involvement has also been reported. In a study of over a hundred treatment regimens for patients with lupus pernio, Stagaki et al. found that infliximab was associated with significantly higher rate of resolution or near completed rate of resolution compared to any other regimen employed [18]. In patients with refractory neurosarcoidosis failing other regimens, two case series reported responses in all patients treated with infliximab [79, 105]. For ocular sarcoidosis, both infliximab [51, 106] and adalimumab [51, 107] have been reported as successful in treating patients who have failed other regimens.
Other Agents
Rituximab has been reported as effective in treating refractory cases of sarcoidosis. These include joint and eye disease [108, 109]. It is a monoclonal antibody which leads to B cell depletion and has proved useful in treating refractory rheumatoid arthritis [110]. It has a significantly different toxicity profile than the TNF-alpha inhibitors. Dosage and evidence-based recommendations for monitoring while administering rituximab is provided in Table 3.5. These recommendations have been adapted from those made by an expert panel [65].
The phosphodiesterase 4 (PDE-4) inhibitors have been reported as effective in some cases of sarcoidosis. These drugs inhibit TNF release by alveolar macrophages [111, 112]. Pentoxifylline was the first drug in this class reported as effective in treating sarcoidosis [113]. In a randomized, placebo-controlled trial, pentoxifylline was steroid sparing compared to placebo, but was not associated with significant improvement in lung function [5]. Aprelimast is a more selective PDE-4 inhibitor. In an open label trial of chronic cutaneous sarcoidosis, it was shown to be effective [114]. The major toxicity of this class of drugs has been nausea and tachycardia.
The antioxidants may have a role in treating some patients with sarcoidosis. Quercetin has been shown to reduce oxidant stress in sarcoidosis patients [115]. Its role in improving outcome in sarcoidosis is currently under study. Another antioxidant, N-acetyl cysteine (NAC), has been suggested as a potential treatment for pulmonary fibrosis. This was based on its effectiveness as a supplemental agent to azathioprine in patients with idiopathic pulmonary fibrosis [116]. The effectiveness of NAC in the treatment of sarcoidosis is also under study.
Special Considerations in Sarcoidosis
There are several clinical problems associated with sarcoidosis which do not always respond to just anti-inflammatory therapy. These conditions may respond to specific therapy for these complications. However, that specific therapy may not be effective for other aspects of the disease. Examples of this include sarcoidosis associated pulmonary hypertension, fatigue, and small fiber neuropathy. For all three of these conditions, alternative therapies have been reported as effective.
Sarcoidosis-associated pulmonary hypertension (SAPH) can occur in 5–15 % of unselected sarcoidosis patients an up to 50 % of persistently dyspneic sarcoidosis patients [10, 117, 118]. As noted in Fig. 3.1, SAPH can lead to hypoxia and/or significant dyspnea in patients with normal pulmonary function studies and no evidence of parenchymal lung disease. Diagnosis and treatment of this condition will be discussed elsewhere in this book.
Significant fatigue associated with sarcoidosis has been reported in over half of patients [119–121]. It may occur for years after all other evidence of disease activity has resolved [122]. In some cases, the fatigue may be due to sleep disturbances, which are common in sarcoidosis [123, 124]. In some cases, treatment of the underlying disease with TNF inhibitors has improved fatigue [125]. Neurostimulants have been reported as useful in treating sarcoidosis associated fatigue (SAF) [126]. Specific pharmacologic treatment for SAF has been studied using double blind, crossover design studies. The neurostimulant d-methylphenidate was found to be superior to placebo in treating SAF [45]. In that study, patients were receiving one or more systemic therapy for their sarcoidosis but still had clinically significant fatigue. In another report, r-modafinil was found to be superior to placebo in treating SAF [46]. That study found that there was no difference in improvement for fatigue in those patients with daytime hypersomnulence versus those without, as assessed by multiple sleep latency time. This would suggest these neurostimulants work for fatigue in patients with or without sleep disturbance.
Small fiber neuropathy is a clinical problem encountered in sarcoidosis [127]. Intravenous immunoglobulin therapy was reported as effective in a small case series [128]. A recent report suggests that ARA 290 may provide a novel solution to this problem [129].
Recently, Heij et al. demonstrated in a pilot study that ARA 290 reduced small fiber neuropathy-related symptoms including fatigue, autonomic dysfunction, and pain. ARA 290 (a peptide designed to activate the innate repair receptor that arrests injury and initiates cytoprotection, anti-inflammation, and healing) reduces allodynia in preclinical neuropathy models. Moreover, they found a significant improvement from baseline in the pain and physical functioning dimensions of the SF-36 QOL questionnaire [129].
Conclusion
While not all patients with sarcoidosis require treatment, a significant percent of patients do require systemic therapy. An approach to treatment based on lung function and other relevant clinical parameters including and the level of symptoms (see also Fig. 3.1) can lead to a step wise approach to therapy (Fig. 3.2). In patients placed on systemic therapy, the treating physician must monitor for toxicity.
References
McKinzie BP, Bullington WM, Mazur JE, Judson MA. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci. 2010;339(1):1–4.
Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, Du BR, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802.
Lower EE, Baughman RP. The use of low dose methotrexate in refractory sarcoidosis. Am J Med Sci. 1990;299:153–7.
Judson MA, Silvestri J, Hartung C, Byars T, Cox CE. The effect of thalidomide on corticosteroid-dependent pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23(1):51–7.
Park MK, Fontana JR, Babaali H, Gilbert-McClain LI, Joo J, Moss J, et al. Steroid sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:121–31.
Siltzbach LE, Teirstein AS. Chloroquine therapy in 43 patients with intrathoracic and cutaneous sarcoidosis. Acta Med Scand. 1964;425:302S–8.
Baughman RP, Judson MA, Teirstein AS, Moller DR, Lower EE. Thalidomide for chronic sarcoidosis. Chest. 2002;122:227–32.
Gerke AK, Yang M, Tang F, Cavanaugh JE, Polgreen PM. Increased hospitalizations among sarcoidosis patients from 1998 to 2008: a population-based cohort study. BMC Pulm Med. 2012;12:19. doi:10.1186/1471-2466-12-19.:19-12.
Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183(11):1524–30.
Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138:1078–85.
Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. 2003;124(3):922–8.
Baughman RP, Lower EE. Fungal infections as a complication of therapy for sarcoidosis. QJM. 2005;98:451–6.
Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev. 2005;(2):CD001114.
Paramothayan S, Lasserson T, Walters EH. Immunosuppressive and cytotoxic therapy for pulmonary sarcoidosis. Cochrane Database Syst Rev. 2003;(3):CD003536.
Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8(1):95–103.
Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, Du BR, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(Sep):149–73.
Baughman RP, Costabel U, du Bois RM. Treatment of sarcoidosis. Clin Chest Med. 2008;29(3):533–48.
Stagaki E, Mountford WK, Lackland DT, Judson MA. The treatment of lupus pernio: results of 116 treatment courses in 54 patients. Chest. 2009;135(2):468–76.
Judson MA, Boan AD, Lackland DT. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:119–27.
Baughman RP, Judson MA, Teirstein A, Yeager H, Rossman M, Knatterud GL, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006; 99(5):307–15.
Pietinalho A, Ohmichi M, Hiraga Y, Lofroos AB, Selroos O. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis. 1996;13:159–66.
Loddenkemper R, Kloppenborg A, Schoenfeld N, Grosser H, Costabel U. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis – results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft fur die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15(2):178–82.
Nardi A, Brillet PY, Letoumelin P, Girard F, Brauner M, Uzunhan Y, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38(6):1368–73.
Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis. 1997;14:154–8.
Baughman RP, Drent M, Culver DA, Grutters JC, Handa T, Humbert M, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:90–8.
Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Semin Respir Crit Care Med. 2010;31(4):409–18.
Gibson GJ, Prescott RJ, Muers MF, Middleton WG, Mitchell DN, Connolly CK, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51(3):238–47.
Baughman RP, Lower EE. Six-minute walk test in managing and monitoring sarcoidosis patients. Curr Opin Pulm Med. 2007;13(5):439–44.
Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132(1):207–13.
Marcellis RG, Lenssen AF, Elfferich MD, De VJ, Kassim S, Foerster K, et al. Exercise capacity, muscle strength and fatigue in sarcoidosis. Eur Respir J. 2011;38(3):628–34.
Marcellis RG, Lenssen AF, de Vries GJ, Baughman RP, van der Grinten CP, Verschakelen JA, et al. Is there an added value of cardiopulmonary exercise testing in sarcoidosis patients? Lung. 2013;191(1):43–52.
Wallaert B, Talleu C, Wemeau-Stervinou L, Duhamel A, Robin S, Aguilaniu B. Reduction of maximal oxygen uptake in sarcoidosis: relationship with disease severity. Respiration. 2011;82(6):501–8.
Scadding JG. Prognosis of intrathoracic sarcoidosis in England. Br Med J. 1961;4:1165–72.
Baughman RP, Shipley R, Desai S, Drent M, Judson MA, Costabel U, et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest. 2009;136:526–35.
Muers MF, Middleton WG, Gibson GJ, Prescott RJ, Mitchell DN, Connolly CK, et al. A simple radiographic scoring method for monitoring pulmonary sarcoidosis: relations between radiographic scores, dyspnoea grade and respiratory function in the British Thoracic Society Study of Long-Term Corticosteroid Treatment. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(1):46–56.
Zappala CJ, Desai SR, Copley SJ, Spagnolo R, Cramer D, Sen D, et al. Optimal scoring of serial change on chest radiography in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):130–8.
Judson MA, Gilbert GE, Rodgers JK, Greer CF, Schabel SI. The utility of the chest radiograph in diagnosing exacerbations of pulmonary sarcoidosis. Respirology. 2008;13(1): 97–102.
Drent M, de Vries J, Lenters M, Lamers RJ, Rothkranz-Kos S, Wouters EF, et al. Sarcoidosis: assessment of disease severity using HRCT. Eur Radiol. 2003;13(11):2462–71.
Oberstein A, von Zitzewitz H, Schweden F, Muller-Quernheim J. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14(1):65–72.
Mostard RL, Verschakelen JA, van Kroonenburgh MJ, Nelemans PJ, Wijnen PA, Voo S, et al. Severity of pulmonary involvement and (18)F-FDG PET activity in sarcoidosis. Respir Med. 2012;12:10.
Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, Vucinic-Mihailovic V, Artiko V, Saranovic D, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med. 2012;53(10):1543–9.
Keijsers RG, Verzijlbergen EJ, van den Bosch JM, Zanen P, van de Garde EM, Oyen WJ, et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(2):123–9.
de Vries J, Michielsen H, van Heck GL, Drent M. Measuring fatigue in sarcoidosis: the Fatigue Assessment Scale (FAS). Br J Health Psychol. 2004;9(Pt 3):279–91.
de Kleijn WP, De VJ, Wijnen PA, Drent M. Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis. Respir Med. 2011;105(9):1388–95.
Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest. 2008;133(5):1189–95.
Lower EE, Malhotra A, Surdulescu V, Baughman RP. Armodafinil for sarcoidosis-associated fatigue: a double-blind, placebo-controlled, crossover trial. J Pain Symptom Manage. 2013;45(2):159–69.
Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010;104(5):717–23.
Pietinalho A, Tukiainen P, Haahtela T, Persson T, Selroos O, the Finnish Pulmonary Sarcoidosis Study Group. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24–31.
Lower EE, Broderick JP, Brott TG, Baughman RP. Diagnosis and management of neurologic sarcoidosis. Arch Intern Med. 1997;157:1864–8.
Chapelon-Abric C, de Zuttere D, Duhaut P, Veyssier P, Wechsler B, Huong DL, et al. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore). 2004;83(6):315–34.
Baughman RP, Lower EE, Ingledue R, Kaufman AH. Management of ocular sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:26–33.
Gottlieb JE, Israel HL, Steiner RM, Triolo J, Patrick H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest. 1997;111(3):623–31.
Hunninghake GW, Gilbert S, Pueringer R, Dayton C, Floerchinger C, Helmers R, et al. Outcome of the treatment for sarcoidosis. Am J Respir Crit Care Med. 1994;149(4 Pt 1): 893–8.
Rizzato G, Montemurro L, Colombo P. The late follow-up of chronic sarcoid patients previously treated with corticosteroids. Sarcoidosis. 1998;15:52–8.
Baughman RP, Nagai S, Balter M, Costabel U, Drent M, Du BR, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28(1):56–64.
Kavathia D, Buckley JD, Rao D, Rybicki B, Burke R. Elevated 1, 25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med. 2010;104(4): 564–70.
Sweiss NJ, Lower EE, Korsten P, Niewold TB, Favus MJ, Baughman RP. Bone health issues in sarcoidosis. Curr Rheumatol Rep. 2011;13(3):265–72.
Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. 1995;155:846–51.
Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17: 60–6.
Webster GF, Razsi LK, Sanchez M, Shupack JL. Weekly low-dose methotrexate therapy for cutaneous sarcoidosis. J Am Acad Dermatol. 1991;24:451–4.
Lower EE, Smith JT, Martelo OJ, Baughman RP. The anemia of sarcoidosis. Sarcoidosis. 1988;5:51–5.
Baughman RP, Koehler A, Bejarano PA, Lower EE, Weber Jr FL. Role of liver function tests in detecting methotrexate-induced liver damage in sarcoidosis. Arch Intern Med. 2003;163(5):615–20.
Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, et al. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. Ann Intern Med. 1994;121:833–41.
van Ede AE, Laan RF, Rood MJ, Huizinga TW, van de Laar MA, van Denderen CJ, et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2001;44(7):1515–24.
Baughman RP, Meyer KC, Nathanson I, Angel L, Bhorade SM, Chan KM, et al. Monitoring of nonsteroidal immunosuppressive drugs in patients with lung disease and lung transplant recipients: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;142(5):e1S–111.
Muller-Quernheim J, Kienast K, Held M, Pfeifer S, Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J. 1999;14:1117–22.
Kennedy PT, Zakaria N, Modawi SB, Papadopoulou AM, Murray-Lyon I, du Bois RM, et al. Natural history of hepatic sarcoidosis and its response to treatment. Eur J Gastroenterol Hepatol. 2006;18(7):721–6.
Cremers JP, Drent M, Baughman RP, Wijnen PA, Koek GH. Therapeutic approach of hepatic sarcoidosis. Curr Opin Pulm Med. 2012;18(5):472–82.
McKendry RJR, Cyr M. Toxicity of methotrexate compared with azathioprine in the treatment of rheumatoid arthritis: a case-control study of 131 patients. Arch Intern Med. 1989;149:685–9.
Wang K, Zhang H, Li Y, Wei Q, Li H, Yang Y, et al. Safety of mycophenolate mofetil versus azathioprine in renal transplantation: a systematic review. Transplant Proc. 2004;36(7): 2068–70.
Raghu G, Anstrom KJ, King Jr TE, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–77.
Emery P, Breedveld FC, Lemmel EM, Kaltwasser JP, Dawes PT, Gomor B, et al. A comparison of the efficacy and safety of leflunomide and methotrexate for the treatment of rheumatoid arthritis. Rheumatology (Oxford). 2000;39(6):655–65.
Sahoo DH, Bandyopadhyay D, Xu M, Pearson K, Parambil JG, Lazar CA, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145–50.
Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:43–8.
Alcorn N, Saunders S, Madhok R. Benefit-risk assessment of leflunomide: an appraisal of leflunomide in rheumatoid arthritis 10 years after licensing. Drug Saf. 2009;32(12): 1123–34.
Osiri M, Shea B, Robinson V, Suarez-Almazor M, Strand V, Tugwell P, et al. Leflunomide for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2003;30(6):1182–90.
Kouba DJ, Mimouni D, Rencic A, Nousari HC. Mycophenolate mofetil may serve as a steroid-sparing agent for sarcoidosis. Br J Dermatol. 2003;148(1):147–8.
Bhat P, Cervantes-Castaneda RA, Doctor PP, Anzaar F, Foster CS. Mycophenolate mofetil therapy for sarcoidosis-associated uveitis. Ocul Immunol Inflamm. 2009;17(3):185–90.
Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology. 2009;72(4):337–40.
Browne PM, Sharma OP, Salkin D. Bone marrow sarcoidosis. JAMA. 1978;240:43–50.
Bichari W, Bartiromo M, Mohey H, Afiani A, Burnot A, Maillard N, et al. Significant risk factors for occurrence of cancer after renal transplantation: a single center cohort study of 1265 cases. Transplant Proc. 2009;41(2):672–3.
Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24(5):517–25.
Chloroquine in the treatment of sarcoidosis. A report from the Research Committee of the British Tuberculosis Association. Tubercle. 1967;48(4):257–72.
Baughman RP, Lower EE. Evidence-based therapy for cutaneous sarcoidosis. Clin Dermatol. 2007;25(3):334–40.
Baltzan M, Mehta S, Kirkham TH, Cosio MG. Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med. 1999;160(1):192–7.
Leecharoen S, Wangkaew S, Louthrenoo W. Ocular side effects of chloroquine in patients with rheumatoid arthritis, systemic lupus erythematosus and scleroderma. J Med Assoc Thai. 2007;90(1):52–8.
Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12(4):294–304.
Elder M, Rahman AM, McLay J. Early paracentral visual field loss in patients taking hydroxychloroquine. Arch Ophthalmol. 2006;124(12):1729–33.
Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med. 1990;115:36–42.
Ziegenhagen MW, Rothe E, Zissel G, Muller-Quernheim J. Exaggerated TNFalpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:185–90.
Baughman RP, Iannuzzi M. Tumour necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs. 2003;17(6):425–31.
Baughman RP, Lower EE. Infliximab for refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:70–4.
Pettersen JA, Zochodne DW, Bell RB, Martin L, Hill MD. Refractory neurosarcoidosis responding to infliximab. Neurology. 2002;59(10):1660–1.
Meyerle JH, Shorr A. The use of infliximab in cutaneous sarcoidosis. J Drugs Dermatol. 2003;2(4):413–4.
Doty JD, Mazur JE, Judson MA. Treatment of sarcoidosis with infliximab. Chest. 2005;127(3):1064–71.
Sollberger M, Fluri F, Baumann T, Sonnet S, Tamm M, Steck AJ, et al. Successful treatment of steroid-refractory neurosarcoidosis with infliximab. J Neurol. 2004;251(6):760–1.
Sweiss NJ, Welsch MJ, Curran JJ, Ellman MH. Tumor necrosis factor inhibition as a novel treatment for refractory sarcoidosis. Arthritis Rheum. 2005;53(5):788–91.
Saleh S, Ghodsian S, Yakimova V, Henderson J, Sharma OP. Effectiveness of infliximab in treating selected patients with sarcoidosis. Respir Med. 2006;100(11):2053–9.
Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller WJ, et al. A double-blind, randomized, placebo-controlled trial of infliximab in patients with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:201–8.
Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J. 2008;31(6):1189–96.
Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25: 76–89.
Maneiro JR, Salgado E, Gomez-Reino JJ, Carmona L. Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review. Semin Arthritis Rheum. 2012;42(1):89–103.
Sweiss NJ, Barnathan ES, Lo K, Judson MA, Baughman R. C-reactive protein predicts response to infliximab in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:49–56.
Loza MJ, Brodmerkel C, du Bois RM, Judson MA, Costabel U, Drent M, et al. Inflammatory profile and response to anti-TNF therapy in patients with chronic pulmonary sarcoidosis. Clin Vaccine Immunol. 2011;18:931–9.
Sodhi M, Pearson K, White ES, Culver DA. Infliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosis. Respir Med. 2009;103(2):268–73.
Baughman RP, Bradley DA, Lower EE. Infliximab for chronic ocular inflammation. Int J Clin Pharmacol Ther. 2005;43:7–11.
Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol. 2012;250(5):713–20.
Belkhou A, Younsi R, El Bouchti I, El Hassani S. Rituximab as a treatment alternative in sarcoidosis. Joint Bone Spine. 2008;75(4):511–2.
Lower EE, Baughman RP, Kaufman AH. Rituximab for refractory granulomatous eye disease. Clin Ophthalmol. 2012;6:1613–8. doi:10.2147/OPTH.S35521.
Higashida J, Wun T, Schmidt S, Naguwa SM, Tuscano JM. Safety and efficacy of rituximab in patients with rheumatoid arthritis refractory to disease modifying antirheumatic drugs and anti-tumor necrosis factor-alpha treatment. J Rheumatol. 2005;32(11):2109–15.
Spatafora M, Chiappara G, Merendino AM, D’Amico D, Bellia V, Bonsignore G. Theophylline suppresses the release of tumour necrosis factor-alpha by blood monocytes and alveolar macrophages. Eur Respir J. 1994;7(2):223–8.
Tong Z, Dai H, Chen B, Abdoh Z, Guzman J, Costabel U. Inhibition of cytokine release from alveolar macrophages in pulmonary sarcoidosis by pentoxifylline: comparison with dexamethasone. Chest. 2003;124(4):1526–32.
Zabel P, Entzian P, Dalhoff K, Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med. 1997;155:1665–9.
Baughman RP, Judson MA, Ingledue R, Craft NL, Lower EE. The safety and efficacy of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148(2):262–4.
Boots AW, Drent M, de Boer VC, Bast A, Haenen GR. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 2011;30(4):506–12.
Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–42.
Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J. 2008;32(2):296–302.
Palmero V, Sulica R. Sarcoidosis-associated pulmonary hypertension: assessment and management. Semin Respir Crit Care Med. 2010;31(4):494–500.
de Kleijn WP, Elfferich MD, de Vries J, Jonker GJ, Lower EE, Baughman RP, et al. Fatigue in sarcoidosis: American versus Dutch patients. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26(2):92–7.
de Kleijn WP, de Vries J, Lower EE, Elfferich MD, Baughman RP, Drent M. Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med. 2009;15(5):499–506.
Gvozdenovic BS, Mihailovic-Vucinic V, Ilic-Dudvarski A, Zugic V, Judson MA. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Respir Med. 2008;102(11):1636–42.
Korenromp IH, Heijnen CJ, Vogels OJ, van den Bosch JM, Grutters JC. Characterization of chronic fatigue in sarcoidosis in clinical remission. Chest. 2011;140(2):441–7.
Verbraecken J, Hoitsma E, van der Grinten CP, Cobben NA, Wouters EF, Drent M. Sleep disturbances associated with periodic leg movements in chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21(2):137–46.
Turner GA, Lower EE, Corser BC, Gunther KL, Baughman RP. Sleep apnea in sarcoidosis. Sarcoidosis. 1997;14:61–4.
Elfferich MD, Nelemans PJ, Ponds RW, de Vries J, Wijnen PA, Drent M. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration. 2010;80:212–9.
Wagner MT, Marion SD, Judson MA. The effects of fatigue and treatment with methylphenidate on sustained attention in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22(3):235.
Hoitsma E, Marziniak M, Faber CG, Reulen JP, Sommer C, De Baets M, et al. Small fibre neuropathy in sarcoidosis. Lancet. 2002;359(9323):2085–6.
Parambil JG, Tavee JO, Zhou L, Pearson KS, Culver DA. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir Med. 2011; 105(1):101–5.
Heij L, Niesters M, Swartjes M, Hoitsma E, Drent M, Dunne A, et al. Safety and efficacy of ARA290 in sarcoidosis patients with symptoms of small fiber neuropathy: a randomized, double blind, pilot study. Mol Med. 2013;18:1430–6.
Keijsers RG, Verzijlbergen JF, van Diepen DM, van den Bosch JM, Grutters JC. 18F-FDG PET in sarcoidosis: an observational study in 12 patients treated with infliximab. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25(2):143–9.
Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. The sarcoidosis health questionnaire. A new measure of health-related quality of life. Am J Respir Crit Care Med. 2003;168: 323–9.
Patel AS, Siegert RJ, Creamer D, Larkin G, Maher TM, Renzoni EA, et al. The development and validation of the King’s Sarcoidosis Questionnaire for the assessment of health status. Thorax. 2013;68(1):57–65.
Hagaman JT, Kinder BW, Eckman MH. Thiopurine S-methyltranferase testing in idiopathic pulmonary fibrosis: a pharmacogenetic cost-effectiveness analysis. Lung. 2010;188(2):125–32.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Baughman, R.P., Drent, M. (2014). The Treatment of Pulmonary Sarcoidosis. In: Judson, M. (eds) Pulmonary Sarcoidosis. Respiratory Medicine, vol 17. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-8927-6_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8927-6_3
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-8926-9
Online ISBN: 978-1-4614-8927-6
eBook Packages: MedicineMedicine (R0)