Abstract
The south-western Beni forest–grassland mosaic in central western Bolivia, a natural fragmented forest area, is home to two Bolivian primate endemics: the Beni titi monkey (Callicebus modestus) and the Olalla brothers titi monkey (Callicebus olallae). Despite extremely low sample sizes (n = 1 and n = 2, respectively), these species have been consistently recognized in taxonomic reviews of the genus since their discovery and description in the late 1930s; however, no new field data was available until 2002. In this chapter, we present a summary of our efforts to: describe the distributional limits of both species, determine their phenotypical characteristics in the field, conduct preliminary analyses of genetic differences between the two species, estimate population densities, describe the composition and structure of the naturally fragmented forest habitats where they occur, and document the behavioral ecology of both species. We also report the threats facing these two range restricted endemics through informal questionnaires with local actors and deforestation studies. Additionally, we detail efforts to conserve these species to date, such as local outreach campaigns, coordination with national government offices, and assistance to local municipalities for inclusion of these species requirements in municipal development plans and their prominence in the justification for the creation of a Municipal Protected Area in the Santa Rosa municipality. Finally, we identify priority conservation actions for the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
In 2002, the Wildlife Conservation Society “rediscovered” Bolivia’s two endemic primate species, the Beni titi monkey (Callicebus modestus) and Olalla’s titi monkey (C. olallae) (Felton et al. 2006), for whom no field data existed following their original collections in 1937 and 1938 (Lönnberg 1939). Subsequently, we have concentrated research efforts on determining the distribution of these range restricted primate endemics (Felton et al. 2006; Martinez and Wallace 2007), determining their genetic diversity (Barreta 2007), and taxonomic status (Martinez and Wallace 2007), assessing their conservation status (Mercado and Wallace 2010; Veiga et al. 2008a, b), estimating their natural abundance (López-Strauss 2008; López-Strauss and Wallace, submitted), describing their basic ecology (Arnez 2012; López 2011; Martinez and Carvajal, unpublished data; Martinez and Lopez, unpublished data), and assessing the threat of forest destruction, fire, and increased forest fragmentation in the southwestern Beni Department (Reinaga et al. unpublished data). In this paper we summarize results to date and assess the overall conservation challenges facing these threatened and endemic titi monkeys in a naturally fragmented Bolivian rainforest.
Synopsis of Biological Information
Field observations of phenotypic traits (Felton et al. 2006; López 2011; López-Strauss 2008; Martinez and Carvajal, unpublished data; Martinez and Lopez, unpublished data; Martinez and Wallace 2007) support historical taxonomic reviews (Hershkovitz 1990; Kobayashi 1995; van Roosmalen et al. 2002) that have consistently maintained a species status for both endemics (Figs. 33.1 and 33.2).
Genetic information on six loci derived from collected scats of wild C. modestus and C. olallae, and blood samples from one captive specimen of C. donacophilus clearly demonstrate that both C. modestus and C. olallae differ significantly from the allopatric C. donacophilus. Parameters of genetic differentiation between C. modestus and C. olallae are sufficiently large (F ST = 0.12) to warrant distinct subspecies status (Barreta 2007) although falling just short of the rule of thumb (F ST = 0.15) for automatic genetic qualification for species status. However, when considered in combination with distributional and phenotypic data, as well as incorporating a conservation perspective that tends to consider the phylogenetic concept of species definition, we continue to recommend full species status for C. modestus and C. olallae.
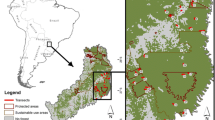
Callicebus olallae and C. modestus were originally described from one and two specimens respectively, and each known from one location separated by just 65 km (Lönnberg 1939). Subsequent field efforts have broadened the distributional knowledge for both species (Felton et al. 2006; Martinez and Wallace 2007, 2013; WCS, unpublished data) with C. modestus distribution defined by the Beni River in the west, the Maniqui River in the east, the beginning of the Andean piedmont forest in the south, and the beginning of true Amazonian forest near the Candado community in the north (Martinez and Wallace 2007, 2013) covering a total area of approximately 8,966.4 km2 (Fig. 33.3).
Callicebus olallae has an even smaller overall distribution of 267.4 km2 occupying riverine forests and associated forest along the Yacuma River between the headwaters of the Yacuma river and the area near Santa Rosa del Yacuma itself (Martinez and Wallace 2007; Fig. 33.3). One additional and isolated record exists for C. olallae along the Maniqui River to the east although this requires further confirmation, especially because of observed heavy hunting pressure in this area.
For both species the total areas reported above are significant overestimates of the potential distributions for two reasons. Firstly, the natural savanna-forest complex in which they occur means that forest habitat is only a percentage of the overall polygons detailed above: approximately 41.7 % in the case of C. modestus and 36.6 % in the case of C. olallae (WCS, unpublished data). Secondly, distributional surveys have revealed that titi monkey distribution is far from ubiquitous within the forested portion of the landscape (Felton et al. 2006; Martinez and Wallace 2007, 2013; WCS, unpublished data). Current studies are focusing on a finer-scale analysis of forest cover and vegetation type as well as predictive models of distribution using field information on presence, confirmed absence and probable absence of both species (WCS, unpublished data).
In the dry season of 2006, the abundance of both species was estimated using an adapted point count methodology for primate vocalizations (López-Strauss 2008; López-Strauss and Wallace, submitted). Seven selected locations were sampled for each endemic species, and distances to detected vocalizations were measured over four sample days at each location. Results revealed that minimum density estimates on the basis of observed and verified groups were 2.91 groups/km2 (SD ± 2.2) for C. olallae and 2.65 groups/km2 (SD ± 2.06) for C. modestus. Estimates on the basis of all groups detected through vocalizations were approximately double the minimum densities: 5.94 groups/km2 (SD ± 3.50) for C. olallae and 4.93 groups/km2 (SD ± 4.58) for C. modestus (López-Strauss 2008). Average observed group size is 2 individuals (SD ± 1.06) for C. olallae and 2.64 (SD ± 1.19) for C. modestus (López-Strauss 2008). Groups for both species generally consist of monogamous pairs, pairs and their offspring from 1 or 2 years, or solitary individuals (López-Strauss 2008; Martinez and Wallace 2007).
Finally, between 2007 and 2008, we conducted a behavioral ecology study on two groups of C. olallae (López 2011; Martinez et al. unpublished data), and between 2010 and 2011, we conducted a similar study on two groups of C. modestus (Arnez 2012; Martinez et al. unpublished data). Preliminary analyses have provided important management information for both species. For C. olallae observations of terrestrial movements of 5–40 m between patches of forest in the natural fragmented forest (Martinez and Wallace 2011) as well abandoning territories in direct response to fire (Martinez and Wallace, unpublished data) have important implications in terms of interpreting forest fragmentation and associated threats from the perspective of these monkeys. There are also observations of C. olallae fleeing in the presence of other primate species including Aotus azarae, Sapajus apella and Saimiri boliviensis, suggesting that interspecific competition may also be an important limiting factor for this species.
Preliminary analyses of dietary information demonstrate that C. olallae is frugivorous–folivorous, only incidentally insectivorous, with the diet shifting from fruit to leaves as a function of fruit availability in the forest (Martinez et al. unpublished data). Mampuesto (Tabebuia nodosa) was the most consumed species including leaves, flowers, and fruits. Other species such as a liana (Machaerium sp.) and the bibosillo (Coccoloba sp.) provided significant annual percentages of observed fruit consumption.
Observations of terrestrial movements were also registered for C. modestus during movements in their territory, as was fleeing behavior in the presence of Sapajus apella, though not Aotus azarae. Observed diet to date suggests that C. modestus is slightly more frugivorous than C. olallae, possibly due to a greater overall fruit availability at the study site including motacù (Attalea phalerata) and tutumilla (Coussarea hydrangeaefolia). Folivory is also important for this species, and insectivory of grasshoppers, caterpillars, stick insects, ants, and termites has also been observed markedly more frequently than for C. olallae (Arnez 2012; Martinez et al. unpublished data).
Summary of Threats and Human Activities
Bolivia is the 28th largest country in the world, but has a small population (80th in the world), and as a result has an extremely low national population density (216th in the world). As such, Bolivia has large tracts of intact wilderness in good conservation condition as demonstrated in a national conservation status evaluation (WCS, unpublished data). The conservation status of the southwestern Beni Department in this national evaluation is generally considered between good and moderate. Specific information regarding human activities and threats in the distribution area identify cattle ranching and associated fires and deforestation as the major threats (WCS, unpublished data), and observations during research in the area have verified this assessment (Felton et al. 2006; Martinez and Wallace 2007, 2011, 2013).
More recently, a specific analysis of deforestation within the distribution of both Callicebus endemics (Reinaga et al. unpublished data) revealed that for C. modestus deforestation rates between 2001 and 2005 were 0.71 %; whereas, between 2005 and 2009 there was actually a process of forest growth (0.27 %) resulting in an overall annual forest loss of 0.17 % between 2001 and 2009 representing 28.6 km2 or 1.49 % of the maximum distribution (forest cover within known distribution = 1,914.7 km2) for this endemic primate. This is well below the national average for the period 2000–2010 (0.5 % annual loss); however, it is important that forest regeneration between 2005 and 2009 does not necessarily signify an increase in habitat for C. modestus, at least not in the short term.
Due to the extremely restricted distribution of C. olallae the situation is even more dramatic with annual deforestation rates between 2001 and 2005 at 0.11 % increasing markedly to 0.67 % between 2005 and 2009 for an overall annual rate of 0.39 % between 2001 and 2009 representing 3.15 km2 or 3.1 % of the maximum theoretical distribution in 2001 (forest cover within known distribution = 101.7 km2) for this endemic primate. This is also below the national average for the period 2000–2010.
Conservation Opportunities, Priority Actions, and Future Challenges
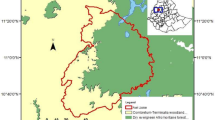
In 2007, the Santa Rosa del Yacuma municipal government declared the creation of the Pampas del Yacuma Municipal Reserve, the largest municipal reserve in Bolivia covering 616,453 ha. Similarly in 2008, the neighboring Reyes municipality also created the Los Santos Reyes Municipal Reserve covering 505,591 ha. These reserves do not have the same protection status as national protected areas; for example, land tenure is not largely in favor of the protected area. In addition, the large size of the Municipal Reserves has resulted in major management and administration challenges for the municipalities. Nevertheless, the two municipal reserves do cover the vast majority of the known distribution areas of both Callicebus endemics and as such represent a huge opportunity for their conservation.
Only Santa Rosa del Yacuma municipality has any kind of presence in the reserve, largely because the Yacuma River is a major ecotourism location with more than 15,000 tourists a year. Neither municipal reserve has a management plan to date. A priority action for the next five years will be to develop management plans in a participative manner with local stakeholders that include specific considerations for the endemic primates and their habitat. Given that much of the area within the reserves overlaps with existing cattle ranches, a key local stakeholder group will be the cattle ranching community. Encouraging them to retain forest patches within their ranch management schemes and manage pasture fires accordingly and/or switch to alternative pasture improvement techniques represents a major conservation action priority.
An imminent and game-changing infrastructure development project—el Corredor del Norte or Northern Corridor road improvement—is another huge challenge for the forests of southwestern Beni. In 2011, the road paving process was already underway for the adjacent Yucumo–Rurrenabaque section and the consultation process has begun for the Rurrenabaque–Santa Rosa del Yacuma section that bisects the distribution of both species (Fig. 33.3). The paving of this road is expected to increase pressures on remaining adjacent forests including forest loss due to colonization, agroforestry, and cattle ranching, all well-known phenomena observed at sites across the Amazon (Fleck et al. 2006, 2007).
Only elegant mitigation actions specifically designed for the Corredor del Norte will avoid significant habitat loss for both endemic species, a potentially catastrophic outcome for the extremely range-restricted Callicebus olallae. As such researchers and conservationists must work with the Bolivian national road authority (Autoridad Boliviana de Carreteras) to develop specific recommendations to reduce the risk of deforestation related to potential expansions in colonization, slash and burn agriculture, and intensified livestock production.
In the meantime, conservationists have prioritized local and national outreach campaigns regarding the existence of the virtually unknown Callicebus modestus and C. olallae, their endangered and endemic status, and ecology and habitat requirements. To date, at a national level this has consisted in a series of newspaper articles and the production of posters for each species as well as informing the scientific and environmental community in professional settings. At a local level, preliminary outreach activities included distribution of the aforementioned posters, as well as meetings with local communities, ranchers and schoolchildren to provide basic information on the habitat needs of these range-restricted endemics (Fig. 33.4). These local outreach activities have been intensified by a group of outstanding young Bolivian conservationists who are focused on raising local awareness about the endemic primates (Carvajal et al. unpublished).
Conclusion
In 2002, the very existence of Bolivia’s two endemic primates was in question. A decade later and both phenotypically distinct and apparently valid species are now exponentially better off in terms of available information, and even more encouragingly, local people in the Santa Rosa and Reyes municipalities are now aware and proud of their existence. This change has been achieved with a total investment of less than $150,000. The challenge of how to manage this naturally fragmented tropical forest–savanna landscape in the face of forthcoming threats will require more significant investment in the future, but the data summarized here will hopefully ensure that the conservation requirements of these endangered titi monkeys are appropriately considered therein.
References
Arnez A (2012) Dieta y comportamiento alimenticio de dos grupos de monos titi (Callicebus modestus) endémicos del Beni, Bolivia. Disertacion, Universidad Mayor San Simon, Cochabamba, Bolivia
Barreta J (2007) Caracterización genética de dos especies de monos tití Callicebus olallae y Callicebus modestus del Departamento del Beni. Informe Técnico. Wildlife Conservation Society & Instituto de Biología Molecular y Biotecnología, La Paz, Bolivia
Felton A, Felton AM, Wallace RB, Gómez H (2006) Identification, distribution and behavioral observations of the titi monkeys Callicebus modestus Lönnberg, 1939 and Callicebus olallae Lönnberg, 1939. Primate Conserv 20:41–46
Fleck LC, Painter L, Reid J, Amend M (2006) A road through Madidi: an environmental-economic analysis. Conservation strategy fund. Serie Técnica 6:1–95
Fleck LC, Painter L, Amend M (2007) Carreteras y áreas protegidas: un análisiseconómico integrado deproyectos en el norte de la Amazonía Boliviana. Conservation strategy fund. Serie Técnica 12:1–75
Hershkovitz P (1990) Titis, new world monkeys of the genus Callicebus (Cebidae, Platyrrhini): a preliminary taxonomic review. Fieldiana Zool New Ser 55:1–109
Kobayashi S (1995) A phylogenetic study of titi monkeys, genus Callicebus, based on cranial measurements: I. Phyletic groups of Callicebus. Primates 36:101–120
Lönnberg E (1939) Notes on some members of the genus Callicebus. Arkiv för Zoologi 31:1–18
López LA (2011) Dieta y comportamiento alimenticio de dos grupos de Callicebus olallae en la estancia ganadera “La Asunta”; Municipio de Santa Rosa del Yacuma, Beni-Bolivia. Disertación, Universidad Mayor de San Andrés, La Paz, Bolivia
López-Strauss H (2008) Estimación de densidad y composición de grupos de dos especies de primates, Callicebus olallae y Callicebus modestus, endémicos del sudoeste del Departamento del Beni, Bolivia. Disertación, Universidad Mayor de San Andrés, La Paz, Bolivia
López-Strauss H, Wallace RB (Submitted) Density estimates of two Bolivian primate endemics, Callicebus olallae and C. modestus. Mastozoologia Neotropical
Martinez J, Wallace RB (2007) Further notes on the distribution of endemic Bolivian titi monkeys, Callicebus modestus and Callicebus olallae. Neotrop Primates 14:47–54
Mercado N, Wallace RB (2010) Distribución de primates en Bolivia y áreas prioritarias para su conservación. Trop Conserv Sci 3:200–217
Martinez J, Wallace RB (2011) First reports of terrestrial travel of Callicebus olallae. Neotrop Primates 18:49–52
Martinez J, Wallace RB (2013) New information about the distribution of Callicebus in Bolivia. Ecología en Bolivia 48:57–62
Van Roosmalen MGM, van Roosmalen T, Mittermeier RA (2002) A taxonomic review of the titi monkeys, genus Callicebus Thomas, 1903, with the description of two new species, Callicebus bernhardi and Callicebus stephennashi from Brazilian Amazonia. Neotrop Primates 10:1–52
Veiga LM, Wallace RB, Martinez J (2008a) Callicebus modestus. In: IUCN 2010. IUCN Red List of Threatened Species. Version 2010.4. www.iucnredlist.org. Downloaded on 29 Dec 2010
Veiga LM, Wallace RB, Martinez J (2008b) Callicebus olallae. In: IUCN 2010. IUCN Red List of Threatened Species. Version 2010.4. www.iucnredlist.org. Downloaded on 29 Dec 2010
Acknowledgments
We would like to thank the Wildlife Conservation Society, Gordon and Betty Moore Foundation, Primate Conservation Inc., Margot Marsh Biodiversity Foundation, the BP Conservation Leadership Program, and the Conservation International Primate Action Fund for their continued financial support. We would like to thank the National Directorate for the Protection of Biodiversity for help in acquiring necessary research permits, as well as the collaboration of the Municipalities of Reyes, San Borja, and Santa Rosa del Yacuma, and especially the Nogales cattle ranches for access to the study sites.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Wallace, R.B., Martinez, J., Lopez-Strauss, H., Barreta, J., Reinaga, A., Lopez, L. (2013). Conservation Challenges Facing Two Threatened Endemic Titi Monkeys in a Naturally Fragmented Bolivian Forest. In: Marsh, L., Chapman, C. (eds) Primates in Fragments. Developments in Primatology: Progress and Prospects. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8839-2_33
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8839-2_33
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8838-5
Online ISBN: 978-1-4614-8839-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)