Abstract
In recent years, legume proteins have gained importance as food ingredients because of their nutraceutical properties. However, legumes are also considered to be a prime factor in the development of food allergies. Peanuts and soybeans are major food allergens in Western countries; while lentil, chickpea, and lupine allergens are more relevant in the Mediterranean area. Moreover, lupine seed flour is increasingly used in bakery, snack, and pastry products due its multifunctional properties. Information about the effects of thermal processing indicates that treatments combining heat and steam pressure, like autoclaving and instant controlled pressure drop (DIC), could decrease allergenicity. This work reviews the effect of DIC treatment at several pressure and time conditions on lupine, peanut, lentil, chickpea, and soybean by SDS-PAGE and immunoblotting. The data demonstrate that DIC treatment decreases the overall in vitro IgE-binding capacity of peanut, lentil, and chickpea and markedly reduces lupine and soybean immunoreactivity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In the past few decades, the prevalence of allergic diseases has increased dramatically and food allergies play a major role in this increase. Food allergy affects approximately 6–8 % children and 3–4 % adults in Europe (Mills et al. 2007). Legumes are increasingly regarded as beneficial food ingredients. In fact, they are recommended as a staple food by health organizations, and dieticians now tend to encourage their consumption in counseling (Leterme 2002). The allergenic potential of many legumes such as lupines (Lupinus albus), peanuts (Arachis hypogaea), lentils (Lens culinaris), chickpeas (Cicer arietinum), and soybeans (Glycine max) is well established (Verma et al. 2012). As legume allergy affects a significant proportion of the population, some effective methods should be adopted to minimize its risk. Consumption of legumes may provoke mild to severe anaphylactic symptoms in sensitized individuals. The usual symptoms are angioedema, vomiting, urticaria, allergic rhinitis, diarrhea, skin rashes, swelling of the tongue or throat, and asthma. Moreover, there is a significant degree of immunological cross-reactivity within the group of legume allergens and between this group and other plant allergens. Consequently, there is a growing interest in the development of newer methods to overcome this type of allergic problem and several strategies are being tested to minimize the allergenicity potential of legume crops (Chung and Reed 2011).
Heating promotes protein denaturation, aggregation, and structure disruption and it can therefore modify the allergenic properties of proteins. The molecular basis of changes in allergenic activity is the inactivation or destruction of epitope structures, the formation of new epitopes, or an enhanced access to cryptic epitopes by denaturation of the native allergen (Besler et al. 2001). Plant protein allergenicity may be variably affected by thermal processing due to an increase or decrease in IgE immunoreactivity. Thus, the overall effect of such procedures on a complex food matrix cannot be predicted (Beyer et al. 2001; Mondoulet et al. 2005). A better understanding of how thermal processing induces biochemical and immunological changes in food allergens may contribute to the development of new diagnostic tools and to alleviate the problem of allergies.
Our previous studies have shown that boiling in an autoclave under harsh conditions markedly reduced lupine, lentil, chickpea, soybean, and peanut allergenicity (Álvarez-Álvarez et al. 2005; Cabanillas et al. 2012; Cuadrado et al. 2007, 2009). However, several extremely resistant immunoreactive proteins still remained in some legumes, such as lentil and chickpea, even after these extreme treatments. Similar results have been found using instant controlled pressure drop (DIC®), a procedure that combines heat and steam pressure as in autoclaving (Cuadrado et al. 2011; Guillamón et al. 2008). In the present chapter, we have summarized the impact of DIC technology on the IgE-binding capacity of proteins from legumes such as lupine, peanut (raw and roasted), lentil, chickpea, and soybean. Its effect is compared with that produced by other thermal treatments, with and without pressure.
2 Major Legumes and Their Predominant Allergens
Legumes are a rich source of protein and other nutritious elements that have functional properties, which is why they are an important ingredient in manufactured foods today. Indeed legumes have become an important ingredient of the human diet throughout the world (Duranti 2006). Unfortunately, they are one of the common types of food that have the potential to elicit an allergenic response. Some common allergenic legumes are peanut, soybean, lentil, lupine, pea, chickpea, red gram, and black gram. Several legume allergens have been identified and characterized as belongings to the cupin superfamily of storage proteins (Mills et al. 2002). The major allergens of soybean and peanut have been extensively studied and Ara h 1, a 65 kDa glycoprotein belonging to the vicilin family, is one of the best characterized (Burks et al. 1998). Several legume allergens are strongly resistant to digestion and food processing, thus strengthening their allergenic potential (Mills et al. 2004). Fewer investigations have been carried out on lentil and chickpea allergens. No chickpea allergens have been identified and only three lentil allergens have been characterized until now. Research is being performed by various groups to identify more legume allergens (Verma et al. 2012).
The pattern of legume sensitization varies in different parts of the world, probably due to the genetic status of individuals, consumption habits, or maybe the involvement of other factors. A higher prevalence of peanut allergy is found in the UK, France, and North America, whereas a major incidence of soybean allergy has been reported in south-east Asia. Lentil, chickpea, and lupine allergic reactions are more widespread in the Mediterranean area (Crespo and Rodríguez 2003). According to UE regulations it is mandatory to label a list of 14 groups of potential allergenic foods. These include peanut and soybean, which were among the first to be included, and they have been followed more recently by lupine and any ingredients derived from it (Commission Directive 2006/142/EC).
3 Effect of Thermal Processing on Legume Allergenic Proteins
Foods are subjected to thermal and nonthermal processing methods to improve their quality, preservation, safety, and suitability for specific product applications. The degree of processing affects digestibility, solubility, and other related parameters. During processing, proteins can form oligomers, become denatured, aggregate, fragment, and reassemble, and these changes most often reduce solubility (Maleki 2004). Processing can alter the overall IgE-binding profiles of a particular extract, which can become more or less antigenic or result in new allergens (neoallergens) (Schmitt et al. 2010). Therefore, the study of processing is necessary to assess the allergenicity of existing and newly introduced foods (Wal 2003). The effect of thermal processing mainly depends on temperature and duration. Moreover, alteration in the structure of a protein also depends on interactions with other food matrix constituents. In general, when the temperature is around 70–80 °C a loss of secondary structure occurs, whereas at 80–90 °C the formation of new bonds and rearrangements of disulfide bonds occur. At higher temperatures (90–100 °C) there is aggregate formation (Davis and Williams 1998).
Studies demonstrated that extracts from roasted peanut bind IgE at approximately 90-fold higher levels than those from raw peanuts (Maleki et al. 2000; Chung et al. 2003). These studies showed that the major allergens from roasted peanuts, Ara h 1 and Ara h 2, undergo structural alterations that enhance their allergenic properties. Heat treatment probably increases the digestibility of proteins, so their absorption through the gastrointestinal tract may also increase, decreasing the possibility for an allergenic protein to elicit an allergenic response. However, in some cases thermal processing may reduce the digestibility of a particular allergen or neoantigens may be formed that were not originally present. This general phenomenon may enhance the allergenic problem in sensitized patients and the neoantigens may also present an additional problem. One major factor responsible for the formation of neoantigens is the Maillard reaction, i.e., the interaction of protein components with sugar residues upon heating, generating sugar conjugated protein derivatives which enhance the allergenicity of proteins (Maleki et al. 2000).
IgE antibodies recognize and interact with epitopes present on allergenic proteins. IgE-binding epitopes can be either linear or conformational. In linear epitopes, the amino acids are arranged in linear order along the polypeptide chain, while in the conformational epitopes amino acids that are far apart in the primary sequence may come together during the folding of the polypeptide chain. Linear epitopes may be more problematic compared to the conformational ones, as the former are mostly resistant to heat treatment. Thermal processing mainly affects conformational epitopes as heat can break the bonds. Refolding allows the formation of native conformational epitopes but a few new allergens may be formed, requiring further efforts to minimize the risk associated with these neoantigens (Sathe and Sharma 2009). Thus, thermal processing as well as other processing events can dramatically alter the structure, function, and allergenicity of foods. Incomplete knowledge of the allergens in processed foods increases the complexity of food allergy diagnosis.
4 Effect of DIC on Immunoreactivity of Legume Proteins
In accordance with the aim of this chapter, the changes in the IgE-binding capacity of lupine, raw and roasted peanut, lentil, chickpea, and soybean proteins produced by DIC technology will be summarized.
All DIC treatments were carried out according to the experimental design developed by Haddad et al. (2001). Briefly, the moistened product is placed in a processing chamber and exposed to steam pressure (up to 8 bar) at high temperature (up to 170 °C) for a relatively short time (a few seconds to some minutes). An instant pressure drop towards a vacuum at about 50 mbar follows this high temperature–short time stage. This abrupt pressure drop simultaneously provokes an autovaporization of part of the water in the product and an instantaneous cooling, which stops thermal degradation. Whole seeds of lupine, raw and roasted peanut, lentil, chickpea, and soybean were treated at different pressures for different time periods, using a 12 or 22 central point composite design (4 or 10 repetitions, respectively). Some DIC-treated samples were selected for SDS-PAGE and immunoblotting studies: 3 and 6 bar for 1 and 3 min, with a constant initial water content of 50 g of water per 100 g of dry matter.
4.1 Effect of DIC on Lupine (L. albus) Allergens
White lupine is considered to be a rich source of protein with a high lysine content and is increasingly used both for its multifunctional properties and its potential hypocholesterolemic and hypoglycemic effects (Duranti 2006). However, lupine seed flour has been reported to be a causative agent of allergic reactions, especially in patients allergic to peanut (Moneret-Vautrin et al. 1999). Moreover, lupine seed proteins have proved to be an interesting model for the study of the thermal conformational stability of proteins under different pH conditions from both biochemical and technofunctional points of view (Duranti et al. 2000).
In a previous study, microwave cooking, boiling, and extrusion cooking produced minimal changes on IgE binding to lupine proteins; however, boiling in an autoclave at 2.6 bar (138 °C) for 20 min produced a significant decrease in allergenicity (Álvarez-Álvarez et al. 2005). Only two proteins of 23 and 29 kDa had IgE-binding capacity after autoclaving for 20 min, whereas autoclaving for 30 min completely abolished the IgE binding of these components, suggesting that lupine allergens are relatively heat stable. Two main allergens were subsequently identified as Lup 1 (conglutin β, 34.5 kDa, 7S protein) and Lup 2 (a basic subunit of conglutin α, 20 kDa, 11S protein) (Guillamón et al. 2010). Both were partially sequenced and their high degree of homology with major allergens from peanut, lentil, pea, and soybean could explain the IgE cross-reactivity of lupine with these legumes.
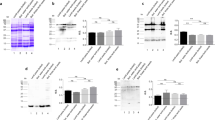
Guillamón et al. studied the effect of DIC treatment on lupine allergenicity (Guillamón et al. 2008). The SDS-PAGE and IgE-immunoblot analysis of raw and DIC-processed lupine proteins are shown in Fig. 1a, b. Raw lupine and lupine processed with DIC at 3 bar for 1 and 3 min had similar SDS and IgE-immunoblot band patterns; Lup 1 (34.5 kDa) and Lup 2 (20 kDa) were still present in these samples. A similar protein pattern was found by Álvarez-Álvarez et al. in autoclaved lupine seeds processed at 1.2 bar for 20 min (Álvarez-Álvarez et al. 2005). A major decrease in the number and intensity of the bands was observed when DIC was applied at 6 bar for 1 min, but Lup 1 (34.5 kDa) was still present. However, after 3 min at 6 bar neither Lup 1 nor Lup 2 were detected (Fig. 1b). This study confirmed the results of previous work that demonstrated the thermal resistance to autoclave treatment of the major lupine allergens (Álvarez-Álvarez et al. 2005). Both results suggest that lupine seeds treated with processing technologies combining heat and steam pressure, such as DIC and autoclaving, could almost completely eliminate the in vitro immunoreactivity of lupine.
4.2 Effect of DIC on Peanut (A. hypogaea) Allergens
Peanut allergy is one of the most common IgE-mediated reactions to food because of its severity and lifelong persistence (Sicherer and Sampson 2007). Considerable effort has been spent in characterizing peanut allergens and 11 allergenic proteins have been identified until now (Ara h 1–Ara h 11). The major peanut allergens, Ara h 1 (65 kDa, vicilin) and Ara h 2 (17 kDa, conglutin), are recognized by 70–90 % of sensitized subjects (Burks et al. 1998) while Ara h 3 (11S legumin) is considered to play a lesser allergenic role (Rabjohn et al. 1999). Thermal treatment has a significant effect on peanut immunoreactivity. As previously mentioned, roasting peanut enhances its IgE-binding capacity (Maleki et al. 2000), while boiling decreases its allergenicity (Beyer et al. 2001).
According to Cabanillas et al., the IgE immunoreactivity of roasted peanut decreases significantly at extreme conditions of autoclaving (2.6 bar, 30 min) (Cabanillas et al. 2012). Results obtained by circular dichroism spectroscopy indicated that most of the α-helical structure was lost after autoclave treatment. The fact that many of the IgE-binding epitopes of major peanut allergens (Ara h 1, Ara h 2 and Ara h 3) are located on the α-helical regions may explain this decrease (Barre et al. 2007; Mueller et al. 2011).
When DIC treatment was used at 3 bar for 1 and 3 min and at 6 bar for 1 min with raw and roasted peanut proteins, it did not produce any relevant change in the immunoblot profile compared to untreated samples (Cuadrado et al. 2011). However, DIC treatment at 6 bar for 3 min resulted in a marked decrease in the protein bands of 65 kDa (putative Ara h 1) (Fig. 2) and no immunoreactive bands of less than 20 kDa were recognized. However, some bands (e.g., 37 kDa, Ara h 3 acid subunit) behave differently in raw and roasted peanuts (Fig. 2). Taking into account the reduction of in vitro immunoreactivity observed at the highest pressure and longest time, DIC seems to be more effective on the immunoreactivity of roasted peanut proteins than on that of raw peanuts. From the literature, it is apparent that changes in the immunoreactivity of peanut proteins following thermal treatment may be in large part due to a modification in the structure and reactivity of each individual peanut allergen and also to their interaction with the food matrix, although more studies are required to provide a fuller understanding of this question.
4.3 Effect of DIC on Lentil (L. culinaris) Allergens
Lentil is commonly consumed in Mediterranean areas and it has been reported as a cause of IgE-mediated hypersensitivity reactions, particularly in pediatric patients. Multiple IgE-binding allergens have been detected in both raw and boiled lentil but studies investigating the allergenicity of lentils with well-documented clinical sera are scarce. Two major lentil allergens, Len c 1 (48 kDa vicilin) (López-Torrejón et al. 2003) and Len c 2 (66 kDa) were isolated from boiled lentils and characterized (Sánchez-Monge et al. 2000). Recently, a third allergen, Len c 3, has also been characterized (Akkerdaas et al. 2012).
Boiling lentil proteins does not seem to be an effective way of reducing their allergenic potential. However, autoclave treatment of lentil (1.2 and 2.6 bar, up to 30 min) significantly decreased the activity of IgE-binding proteins. Autoclaving at the highest pressure (2.6 bar) for 30 min significantly reduced the overall IgE-binding capacity, although extremely resistant immunoreactive proteins still remained even after this harsh treatment (Cuadrado et al. 2009).
When we studied the effect of DIC treatment on lentil, we observed that the control sample was composed of numerous immunoreactive bands with molecular weights of between 101 and 140 kDa, including a protein of 48 kDa (putative major allergen Len c1) (Fig. 3a) (Cuadrado et al. 2011). DIC processing at 3 and 6 bar for 1 and 3 min produced a marked decrease in the overall immunoreactivity (data not shown) but extreme DIC conditions (6 bar, 3 min) reduced the number of IgE-binding proteins in a manner similar to that observed with autoclave treatment under harsh conditions. After this kind of DIC treatment only four heat-stable allergenic proteins of MW 29, 48, 57, and 68 kDa were still present (Fig. 3a). These results confirmed the thermostability of some lentil allergens, which are also detected after autoclaving.
Relative band density (a.u., arbitrary units) of immunoreactive proteins vs. band molecular weight (MW, kDa) obtained from IgE immunoblots from control and DIC-treated (6 bar, 3 min) samples of lentils (a), chickpeas (b), and soybeans (c). The pool serum used was from subjects sensitized to each legume. Where no dark bands are present the amount of material is below the limit of detection (control: white; DIC: black)
4.4 Effect of DIC on Chickpea (C. arietinum) Allergens
Chickpea is an important source of proteins in several parts of the world. In Asian countries it is widely consumed in many traditional dishes. The high consumption rate of this crop in the Mediterranean area has also resulted in allergic problems in sensitive individuals (Crespo and Rodríguez 2003). Chickpea and lentil are the most common cause of allergic reactions to legumes in Spanish children and there is a cross-reactivity between them (Crespo et al. 1995). Some subjects allergic to this legume on ingestion also report symptoms when they inhale vapors from cooking chickpeas (Niphadkar et al. 1997). Previous studies have detected multiple IgE-binding bands in chickpea extracts in the molecular weight range of 10–106 kDa, of which the majority were found to be heat stable (Patil et al. 2001). So far, chickpea allergens have not been immunologically characterized; only two allergenic polypeptides from chickpea (2S albumin and 11S globulin) have been identified (Vioque et al. 1999).
Cuadrado et al. found multiple IgE-binding proteins in chickpea boiled for 30 min (Cuadrado et al. 2009). A decrease in the number and intensity of the bands was observed after autoclaving (1.2 bar, 12 min) and the immunoreactivity decreased as pressure and time increased. At 2.6 bar (30 min) only two bands (19 and 16 kDa) were still detected.
The effect of DIC treatment on allergenic proteins from chickpea and lentil was similar. Untreated chickpea had numerous IgE-binding proteins with molecular weights of between 12 and 82 kDa (Fig. 3b). The immunoreactive band pattern after DIC treatment at 6 bar for 3 min also showed a marked decrease in the number and intensity of IgE-binding proteins. However, in the chickpea experiment, there were no apparent distinctions among the different pressure and time conditions used (data not shown), and more heat-stable immunoreactive proteins were still present at the same extreme DIC conditions (6 bar, 3 min) (Fig. 3b).
4.5 Effect of DIC on Soybean (G. max) Allergens
Soybean and peanut are the two main legumes involved in hypersensitive responses in numerous countries. Soybean, with around 21 known allergenic proteins, is widely consumed throughout the world. It is mainly used as an ingredient in formulated foods, meat/poultry products, together with bakery, pastry, and dairy products, and has many pharmaceutical and industrial uses (Endres 2001). The large amount of soybean consumed has also been associated with a high risk of allergy for consumers and the prevalence rate in the general population is around 0.3–0.4 %. Several major allergens have been identified in soybean, namely, P34 (Gly m Bd 30 K), Gly m 1, Gly m 2, Gly m 3, Gly m 4, and Gly m Bd 28 K (Verma et al. 2012). Given the commercial use of soybean protein in food products, which frequently include thermally processed proteins, it is of great importance to investigate the effect of heat treatment on the main allergens of this legume. Burks et al. did not find any relevant decrease in IgE binding after heating soy proteins at various temperatures and for various times (Burks et al. 1991). Wilson et al. (2005) concluded that several procedures are needed to eliminate soybean allergenicity, particularly that of P 34, the major allergenic protein.
To evaluate the effect of DIC, untreated soybean was used as a control (Cuadrado et al. 2011). All the major allergens and other minor immunoreactive proteins (13–119 kDa) were detected in the control soybean (Fig. 3c). Although DIC treatment at 3 bar for 1 and 3 min resulted in a slight reduction in the soybean immunoreactive bands (data not shown), when the pressure was increased to 6 bar and applied for 3 min, no immunoreactive proteins could be detected on the immunoblot pattern (Cuadrado et al. 2011) (Fig. 3c). Similar results were found when this legume was autoclaved at 2.6 bar for 30 min (Cuadrado et al. 2007). The DIC technique employed here had the strongest effect on the immunoreactive proteins of soybean compared to the other legumes studied. The short processing time (3 min) represents an advantage for future potential applications in the food industry. Elimination of allergenic proteins via processing could eventually enhance the safety of soybean products, making them available for soy-sensitized individuals.
5 Conclusions
According to Thomas et al. (2007), food processing may impact the potential allergenicity of proteins, although there are no general rules regarding how allergenic foods respond to physical, chemical, or biochemical processing methods. The modifications may result in a loss of organized structure and protein denaturation. Moreover, it has been demonstrated that the degree of processing can dramatically affect digestibility, solubility, and other parameters related to IgE reactivity. In some allergenic proteins, the epitopes are destroyed but they are unaltered in others. Thus, processing can alter the overall IgE-binding profiles of legume proteins.
This chapter summarized the effect of DIC treatment (steam pressure, high temperature, and short time) on different legumes and compared it with other thermal procedures. DIC treatment significantly decreased the overall immunoreactivity of lentil, chickpea, and peanut, mainly roasted, and almost completely eliminated IgE recognition of lupine and soybean proteins. These DIC-treated legumes could constitute an alternative to intact proteins in the development of different food products. However, as in vitro IgE reactivity is an indicator of potential in vivo allergenicity, further in vitro assays and in vivo clinical data are required to confirm that DIC treatment can reduce the in vivo allergenicity of these legumes. Only after such studies could these putative hypoallergenic foods be safely consumed and even utilized as a desensitizing food.
References
Akkerdaas J, Finkina E, Balandin S, Santos Magadan S, Knulst A, Fernández-Rivas M, Asero R, van Ree R, Ovchinnikova T (2012) Lentil (Lens culinaris) lipid transfer protein Len c 3: a novel legume allergen. Int Arch Allergy Immunol 157(1):51–57. doi:10.1159/000324946
Álvarez-Álvarez J, Guillamón E, Crespo JF, Cuadrado C, Burbano C, Rodríguez J, Fernández C, Muzquiz M (2005) Effects of extrusion, boiling, autoclaving, and microwave heating on lupine allergenicity. J Agric Food Chem 53(4):1294–1298. doi:10.1021/jf0490145
Barre A, Jacquet G, Sordet C, Culerrier R, Rouge P (2007) Homology modelling and conformational analysis of IgE-binding epitopes of Ara h 3 and other legumin allergens with a cupin fold from tree nuts. Mol Immunol 44(12):3243–3255. doi:10.1016/j.molimm.2007.01.023
Besler M, Steinhart H, Paschke A (2001) Stability of food allergens and allergenicity of processed foods. J Chromatogr B Biomed Sci Appl 756:207–228
Beyer KB, Morrow E, X-M L, Bardina L (2001) Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol 107:1077–1081
Burks A, Williams L, Helm RM, Thresher W, Brooks J, Sampson H (1991) Identification of soy protein allergens in patients with atopic dermatitis and positive soy challenges: determination of change in allergenicity after heating or enzyme digestion. Adv Exp Med Biol 289:295–307
Burks A, Sampson H, Bannon G (1998) Peanut allergens. Allergy 53:725–730
Cabanillas B, Maleki SJ, Rodríguez J, Burbano C, Muzquiz M, Jimenez M, Pedrosa MM, Cuadrado C, Crespo JF (2012) Heat and pressure treatments effects on peanut allergenicity. Food Chem 132(1):360–366. doi:10.1016/j.foodchem.2011.10.093
Chung S-Y, Reed S (2011) Removing peanut allergens by tannic acid. Food Chem 134:1468–1473
Chung S, Butts C, Maleki SJ, Champagne E (2003) Linking peanut allergenicity to the processes of maturation, curing, and roasting. J Agric Food Chem 51:4273–4277
Commision Directive 2006/142/EC of the European Parlament and of the Council (2006) Official Journal of the European Union L 368/110.
Crespo JF, Rodriguez J (2003) Food allergy in adulthood. Allergy 58:98–113
Crespo JF, Pascual C, Burks A, Helm R, Martín-Esteban M (1995) Frequency of food allergens in a paediatric population from Spain. Pediatr Allergy Immunol 6:39–43
Cuadrado C, Cabanillas B, Pedrosa MM, Varela A, Guillamón E, Muzquiz M, Crespo JF, Rodríguez J (2007) Effect of heat treatments on legume allergenicity. . Paper presented at the Integrating Legume Biology for Sustainable Agriculture. Proceedings of the 6th European Conference on Grain Legumes, Lisbon, Portugal, 12–16 November
Cuadrado C, Cabanillas B, Pedrosa MM, Varela A, Guillamón E, Muzquiz M, Crespo JF, Rodríguez J, Burbano C (2009) Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol Nutr Food Res 53(11):1462–1468. doi:10.1002/mnfr.200800485
Cuadrado C, Cabanillas B, Pedrosa MM, Muzquiz M, Haddad J, Allaf K, Rodríguez J, Crespo JF, Burbano C (2011) Effect of instant controlled pressure drop on IgE antibody reactivity to peanut, lentil, chickpea and soybean proteins. Int Arch Allergy Immunol 156(4):397–404. doi:10.1159/000324443
Davis P, Williams S (1998) Protein modification by thermal processing. Allergy 53:102–105
Duranti M (2006) Grain legume proteins and nutraceutical properties. Fitoterapia 77:67–82
Duranti M, Sessa F, Scarafoni A, Bellini T, Dallocchio F (2000) Thermal stabilities of lupine seed conglutin ç protomers and tetramers. J Agric Food Chem 48:1118–1123
Endres J (ed) (2001) Soy protein products-characteristics, nutritional aspects, and utilization. AOCS, Champaign
Guillamón E, Burbano C, Cuadrado C, Muzquiz M, Pedrosa MM, Sánchez M, Cabanillas B, Crespo JF, Rodríguez J, Haddad J, Allaf K (2008) Effect of an instantaneous controlled pressure drop on in vitro allergenicity to lupines (Lupinus albus var Multolupa). Int Arch Allergy Immunol 145(1):9–14. doi:10.1159/000107461
Guillamón E, Rodríguez J, Burbano C, Muzquiz M, Pedrosa MM, Cabanillas B, Crespo JF, Sancho A, Mills C, Cuadrado C (2010) Characterization of lupine major allergens (Lupinus albus L). Mol Nutr Food Res 54:1168–1676
Haddad J, Louka N, Gadouleau M, Juhel F, Allaf K (2001) Application du nouveau procédé de séchage/texturation par Détente Instantanée Contrôlée DIC aux poissons: impact sur les caractéristiques physico-chemiques du produit fini. Sci Aliment 21:481–498
Leterme P (2002) Recommendations by health organizations for pulse consumption. Br J Nutr 88:239–242
López-Torrejón G, Salcedo G, Martín-Esteban M, Diaz-Perales A, Pascual CY, Sánchez-Monge R (2003) Len c 1, a major allergen and vicilin from lentil seeds: protein isolation and cDNA cloning. J Allergy Clin Immunol 112(6):1208–1215. doi:10.1016/j.jaci.2003.08.035
Maleki SJ (2004) Food processing: effects on allergenicity. Curr Opin Allergy Clin Immunol 4(3):241–245. doi:10.1097/00130832-200406000-00018
Maleki SJ, Chung SY, Champagne ET, Raufman JP (2000) The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol 106(4):763–768. doi:10.1067/mai.2000.109620
Mills C, Jenkins J, Marigheto N, Belton P, Gunning A, Morris V (2002) Allergens of the cupin superfamily. Biochem Soc Trans 30(6):925–929
Mills C, Jenkins JA, Alcocer M, Shewry P (2004) Structural, biological, and evolutionary relationships of plant food allergens sensitizing via the gastrointestinal tract. Crit Rev Food Nutr 44:379–407. doi:10.1080/104086904990489224
Mills C, Mackie A, Burney P, Beyer K, Frewer L, Madsen C, Botjes E, Crevel R, van Ree R (2007) The prevalence, cost and basis of food allergy across Europe. Allergy 62(7):717–722
Mondoulet L, Paty E, Drumare M-F, Sea A-L (2005) Influence of thermal processing on the allergenicity of peanut proteins. J Agric Food Chem 53:4547–4553
Moneret-Vautrin D, Guérin L, Kanny G, Flabbee J, Frémont S, Morisset M (1999) Cross-allergenicity of peanut and lupine: the risk of lupine allergy in patients allergic to peanuts. J Allergy Clin Immunol 04(4 Pt 1):883–888
Mueller GA, Gosavi RA, Pomes A, Wuenschmann S, Moon AF, London RE, Pedersen LC (2011) Ara h 2: crystal structure and IgE binding distinguish two subpopulations of peanut allergic patients by epitope diversity. Allergy 66(7):878–885. doi:10.1111/j.1398-9995.2010.02532.x
Niphadkar PV, Patil SP, Bapat MM (1997) Chickpea induced anaphylaxis. Allergy 52:480–482
Patil SP, Niphadkar PV, Bapat MM (2001) Chickpea: a major food allergen in the Indian subcontinent and its clinical and immunochemical correlation. Ann Allergy Asthma Immunol 87(2):140–145
Rabjohn P, Helm E, Stanley J, West C, Sampson H, Burks A, Bannon G (1999) Molecular cloning and epitope analysis of the peanut allergen Ara h 3. J Clin Invest 103:535–542
Sánchez-Monge R, Pascual CY, Díaz-Perales A, Fernández-Crespo J, Martín-Esteban M, Salcedo G (2000) Isolation and characterization of relevant allergens from boiled lentils. J Allergy Clin Immunol 106(5):955–961. doi:10.1067/mai.2000.109912
Sathe SK, Sharma GM (2009) Effects of food processing on food allergens. Mol Nutr Food Res 53(8):970–978. doi:10.1002/mnfr.200800194
Schmitt D, Nesbit J, Hurlburt B, Cheng H, Maleki SJ (2010) Processing can alter the properties of peanut extract preparations. J Agric Food Chem 58(2):1138–1143. doi:10.1021/jf902694j
Sicherer S, Sampson H (2007) Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol 120(3):491–503
Thomas K, Herouet-Guicheney C, Ladics G, Bannon G, Cockburn A, Crevel R, Fitzpatrick J, Mills C, Privalle L, Vieths S (2007) Evaluating the effect of food processing on the potential human allergenicity of novel proteins: international workshop report. Food Chem Toxicol 45:1116–1122
Verma A, Kumar S, Das M, Dwivedi P (2012) A comprehensive review of legume. Clin Rev Allerg Immunol. doi:10.1007/s12016-012-8310-6
Vioque J, Sánchez-Vioque R, Clemente A, Pedroche J, Bautista J, Millán F (1999) Purification and partial characterization of chickpea 2S albumin. J Agric Food Chem 47(4):1405–1409. doi:10.1021/jf980819k
Wal J (2003) Thermal processing and allergenicity of foods. Allergy 58:727–729
Wilson S, Blaschek K, Gonzalez de Mejia E (2005) Allergenic proteins in soybean: processing and reduction of P34 allergenicity. Nutr Rev 63:47–58
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Burbano, C., Cuadrado, C. (2014). Effect of DIC on the Allergenicity of Legume Proteins. In: Allaf, T., Allaf, K. (eds) Instant Controlled Pressure Drop (D.I.C.) in Food Processing. Food Engineering Series. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8669-5_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8669-5_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8668-8
Online ISBN: 978-1-4614-8669-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)