Abstract
The packaging of chromosomal DNA by nucleosomes not only condenses and organizes the genome but also blocks access of DNA-binding proteins to regulatory DNA elements. However, this process also allows nucleosomes and other chromatin components to actively participate in the regulation of transcription, DNA replication, DNA repair, and other chromosomal processes. To enable dynamic regulation of nucleosomes, cells have evolved a set of specialized chromatin remodeling complexes (termed Remodelers) that use the energy of ATP hydrolysis to slide, destabilize, eject, or edit/restructure nucleosomes. Here, we address many aspects of Remodeler function, including their composition, specialization, mechanisms, and regulation. We also address their roles in embryonic stem cells, differentiation, development, cancer, and human disease syndromes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Introduction
Chromosomes must actively balance two opposing needs: the need to condense and organize (topologically) nearly 2 m of DNA—against the need for access to the genome by the factors that conduct transcription, replication, recombination, repair, and other chromosomal processes. The proteins involved in DNA packaging and condensation (as well as those that reverse these processes) are termed chromatin, and the most abundant constituents are histone proteins, which come together to form nucleosomes. The majority of chromatin assembly occurs during DNA replication, in coordination with the DNA replisome. This process involves histone delivery to nascent DNA, and also the action of chromatin Remodelers—which ensure proper density and spacing of nucleosomes after replication. Access to the DNA within chromatin involves the cooperative action of DNA sequence, site-specific transcription factors, histone modification enzymes, and a set of chromatin remodeling complexes.
Here, we review the roles of ATP-dependent chromatin remodeling complexes, termed hereafter “Remodelers,” a set of complexes with central and specialized roles in either the assembly of chromatin, the access of factors to chromatin, or the restructuring of nucleosomes (Fig. 3.1). Remodelers are distinguished from other chromatin factors by their use of the energy of ATP hydrolysis to promote these functions. Remodelers work with other chromatin factors to regulate both chromatin packaging and unpackaging, as the DNA elements that control chromosomal processes (enhancers, promoters, and replication origins) must be exposed in a regulated manner to properly regulate gene transcription, DNA replication, DNA repair, and recombination. Here, we examine dynamic chromatin from the perspective of Remodelers, discuss Remodeler specialization and mechanisms needed to accomplish their main processes [chromatin assembly, access, or restructuring/editing (Fig. 3.1)], and consider their biological roles and disease connections.
Chromatin processes and Remodeler involvement. Remodelers can be classified by their involvement in particular chromatin processes. (a) Chromatin Assembly: Most of ISWI- and CHD-family Remodelers help in the process of histone deposition, the full maturation/formation of nucleosomes, and their spacing—which can result in the blockage of the cognate site (red) of a DNA-binding protein (DBP) (Note: spacing can also result in consistent site exposure, Figs. 3.2 and 3.3). (b) Chromatin Editing: Remodelers of the INO80/SWR1 family (and others) modify the composition of resident nucleosomes by performing histone exchange, either removing or replacing noncanonical histone variants (blue disk). (c) Chromatin Access: SWI/SNF-family Remodelers (and others) alter nucleosomes by repositioning, ejecting the octamer, or evicting dimer(s), allowing exposure for a DNA-binding protein to its cognate site (green)
3.1.1 Composition and Biophysical Properties of Nucleosomes
To understand Remodelers, one must first understand their nucleosome substrate—addressed in detail in Chap. 1—with the salient features for Remodelers addressed here. The canonical nucleosome is a protein octamer consisting of two copies of each of the four core/canonical histone proteins (H3, H4, H2A, and H2B), around which 147 bp of DNA are wrapped. The octamer can be subdivided into four histone dimer pairs: two H3/H4 dimers form the central H3/H4 tetramer, which is capped on each end by an H2A/H2B dimer. These dimer pairs interact to form an interlocked right-handed helical staircase, forming a surface upon which the DNA climbs. Here, positively charged amino acids facing outward from the histone staircase contact the negatively charged phosphate backbone of the DNA. Each histone dimer pair contacts the DNA backbone along about three consecutive turns each involving 10–11 bp (totaling ~31 bp), with the four dimer pairs providing a total of 12 histone–DNA contact sites. Two additional histone–DNA contacts are provided by extensions from histone H3, which form the initial (weaker) contacts at the two entry/exit sites to the nucleosome—reaching 14 total histone–DNA contacts. Although each contact in isolation is relatively weak (~1 kcal/mol, requiring ~1 pN of force to disrupt), all 14 added together confer considerable positional stability (~12–14 kcal).
These histone–DNA contacts provide the energetic and biophysical obstacle that Remodelers must overcome, as Remodelers must disrupt these contacts to perform their roles. As ATP hydrolysis provides ~7.3 kcal/mol of free energy, Remodelers must either break only a few histone–DNA contacts at a time (providing a partially unwrapped intermediate), or alternatively utilize more than one ATP hydrolysis to yield a repositioned (or ejected) nucleosome product.
Beyond the four core/canonical histones, all eukaryotes also contain histone variant proteins that can be incorporated into nucleosomes to specialize chromatin regions. This chapter discusses the role of Remodelers in the loading or removal of the histone variants H2A.Z, macroH2A, CENPA, and H3.3. Variant nucleosomes can specialize a nucleosome by affecting its biophysical properties/stability, and by presenting unique epitopes that may affect protein associations, including Remodeler targeting or activity. Furthermore, higher eukaryotes also employ a “linker” histone (most commonly an H1 or H5 subtype), which joins the nucleosome (Kornberg 1974), to form the chromatosome, which may also provide a steric or thermodynamic barrier to Remodeler action. In general, linker histones help stabilize and assemble higher-order forms of chromatin and can affect Remodeler function.
3.1.2 Concepts of Nucleosome Phasing and Spacing
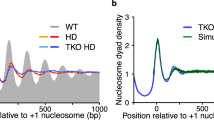
An important concept in chromatin biology is that nucleosomes compete with site-specific DNA-binding proteins for occupancy of sites in the genome. Here, most (but not all) DNA-binding factors are blocked from binding their cognate site if it is wrapped on the surface of a nucleosome (Fig. 3.2a, b), whereas sites placed between nucleosomes are exposed and available. Intuitively, random nucleosome deposition (which occurs initially following replication) results in a random likelihood of site exposure (Fig. 3.2a). As developed below, the process of chromatin assembly involves nucleosome “spacing”; the creation of arrays with nucleosomes placed a fixed distance apart. However, the spacing process—conducted on a population of genomes—does not necessarily result in uniform positioning of nucleosomes for all members of the population (Fig. 3.2b). Instead, site accessibility depends on (1) the extent of nucleosome “phasing” (the uniformity of nucleosome positioning in the population) and (2) the position of the site in relation to the phased nucleosomes: full site exposure involves a lack of overlap, partial exposure from partial overlap, and blockage from total overlap. Phasing can be observed even in instances where spacing is not enforced (Fig. 3.2c). Notably, arrays that are both phased and spaced create regions with uniform site exposure (Fig. 3.2d, green DNA) or blockage (Fig. 3.2d, red DNA), which is reflected in the extent to which histones/nucleosomes occupy those sites in the entire population (Fig. 3.2, bottom schematic). Below we explore the roles of Remodelers and other factors in creating these array architectures.
Concepts of nucleosome spacing and phasing, and their relationship to DNA site exposure. The positioning of nucleosomes in relation to important cis-acting sequences, and the consistency of their positioning in the population, determines the extent and homogeneity of access to particular sites. Four types of nucleosome arrays are shown, which differ in their use of spacing and phasing, and therefore impact access to defined sites. The extent of exposure of two defined DNA sites are depicted; red if blocked by a nucleosome, or green if exposed between (or at the edges of) a nucleosome. The bottom schematic depicts the density of histone (in arbitrary units) ranging from zero to full occupancy (in a population average)
3.2 Classification of Remodelers
3.2.1 Chromatin Processes and Remodeler Functions
Remodelers can be classified by their involvement in particular chromatin processes: chromatin assembly, genome access, and nucleosome editing/restructuring (Fig. 3.1). Although simplified, this representation provides a very useful framework for considering Remodeler functions (below) and their mechanisms (described later).
3.2.1.1 Chromatin Deposition and Assembly
Chromatin deposition and assembly during replication involves histone chaperone complexes delivering histone dimers (H3/H4 and H2A/H2B) to nascent DNA, and working in coordination with “assembly” Remodelers to facilitate the proper maturation, density and spacing of nucleosomes behind the replisome. Here, assembly Remodelers may initially help in the “maturation” of initial histone–DNA complexes into canonical octameric nucleosomes, and then conduct a spacing of those nucleosomes, typically placing them a fixed distance apart from one another (Figs. 3.1a and 3.3). Nucleosomes placed atop AT-rich DNA (Fig. 3.3, orange DNA segments) are unstable, as AT-rich sequences are rigid and disfavor nucleosomal DNA curvature, which can contribute to local nucleosome deficiency (Fig. 3.3, red nucleosomes).
Action and impact of remodelers and boundary factors on nucleosome spacing and phasing. Combinations of DNA sequence elements, DNA-binding factors, and Remodelers can arrive at particular chromatin architectures. Expanding on Fig. 3.2, the presence of particular sequence element (AT-rich DNA, orange) can deter nucleosome formation and/or stability (red nucleosome). Spacing of such arrays can place AT-rich elements either within, or between nucleosomes. However, spacing in the presence of a boundary factor by assembly Remodelers (ISWI or CHD) creates phased arrays in which the position of the AT-rich element is at a defined location within the population. Access Remodelers, such as SWI/SNF, may in the course of remodeling evict these nucleosomes more easily, due to their instability, creating a uniform exposed architecture (a nucleosome-depleted region) in the population. Notably, if a binding site for another transcription factor (not depicted) is located either within or adjacent to this nucleosome-depleted region, it will be constantly exposed
In the absence of a defined element that specifies phasing, spacing results in arrays with heterogeneous nucleosome density, positioning, and site exposure (Figs. 3.2b and 3.3). Notably, assembly Remodelers can create phased arrays through collaboration with a DNA-bound “boundary factor”; a chromatin or transcription factor (or complex) that helps define the position of the flanking nucleosome(s). Examples of boundary factors include the nucleosomes positionally stabilized by transcriptional repressors, or the first (+1) nucleosome adjacent to the transcription start site (TSS) in genes, stabilized by the preinitiation transcription complex. In the presence of such a boundary factor, the assembly Remodeler places the flanking nucleosome(s) a particular distance from the boundary factor, much in the same way that the Remodeler spaces nucleosomes a fixed distance apart. This process defines the position of an initial nucleosome, with the “phasing” of subsequent nucleosomes along the array determined via spacing from this initial nucleosome (Fig. 3.3). Notably, this mode of assembly can be used to create spaced and phased arrays that provide either full site exposure or blockage (Fig 3.2d). Furthermore, the precise nucleosome spacing provided by assembly Remodelers promotes the efficient loading of linker histones, and therefore the higher-order packing of nucleosome arrays. Taken together, the mode of assembly sets up the initial nucleosome/packaging landscape, which then defines the opportunities and barriers for site-specific DNA-binding proteins.
3.2.1.2 Chromatin Editing
Chromatin editing is a form of postreplicative chromatin assembly that involves a compositional change in a resident nucleosome, characterized by the incorporation or removal of a histone variant (Fig. 3.1b). Common examples of editing, developed below, include the replacement of H2A or H3 with related variants, assisted by editing Remodelers. Editing offers the ability to specialize a single nucleosome, or an array of nucleosomes, at a defined location—which can be important for factor recruitment, deterrence, or activity. Variants provide a new composition to a chromatin region, which may impact nucleosome stability and/or protein recognition. Certain editing Remodelers conduct both the removal and replacement process, whereas other Remodelers rely on other processes/factors (i.e., transcription and topoisomerase action) to remove the nucleosome and conduct only the replacement process. We note that this latter function constitutes postreplicative nucleosome assembly/replacement.
3.2.1.3 Chromatin Access
Chromatin access can be enabled by an “access” Remodeler, which can either slide or eject the histone octamer, or evict components such as an H2A/H2B dimer (Fig. 3.1c). In regard to transcription, “access” Remodelers can expose binding sites either for activators or repressors, with a corresponding impact on transcription, thus context is key. Chromatin access activities can be utilized in other processes including DNA repair and recombination. Although far from uniform, one common scenario involves the use of “assembly” Remodelers to promote gene silencing through site blockage at enhancers and promoters, and the use of “access” Remodelers to promote gene activation through site exposure (Fig. 3.1a, c). An important distinction between assembly and access Remodelers involves their ability to eject nucleosomes. Here, access Remodelers may conduct octamer ejection to expose larger regions of DNA—those that cannot be easily accessed by sliding. This ejection activity can be influenced by histone composition, transcription factors, and the underlying DNA sequence, which may make the octamer more prone to ejection (Fig 3.3, red nucleosome). Here, if a binding site for a transcription factor is embedded within this nucleosome-depleted region, it will be now exposed.
Taken together, Remodelers are needed for most aspects of nucleosome dynamics. Remodelers help ensure dense nucleosome packaging (at steady state) at the vast majority of locations in the genome, and at the same time allow factors the ability to rapidly access particular DNA sequences/loci in a regulated manner. As chromosomal processes (chromatin assembly, transcription, repair, etc.) are accompanied by particular histone modifications, a key question is how histone modifications might recruit or regulate these specialized Remodelers. First, we discuss the shared properties of all Remodelers and then focus on their specialization.
3.2.2 Compositional Attributes Shared by Remodelers
Although they have different functional properties, detailed below, all Remodelers share particular enzymatic and thematic properties including (1) an affinity for the nucleosome that is much greater than DNA itself, utilizing histone-binding domains that may also detect covalent histone modifications, (2) a single catalytic subunit, containing an ATPase domain that is split into two RecA-like lobes (termed DExx and HELICc), which functions as a DNA-translocating motor that breaks histone–DNA contacts (Fig. 3.4), (3) domains and/or proteins that regulate the ATPase domain, and (4) domains and/or proteins for interaction with other chromatin proteins, chaperones, or site-specific transcription factors. Together, these shared properties enable their selective engagement or action on particular nucleosomes, and in particular functional contexts. They also provide a framework for understanding their composition and assemblies described below.
Remodeler Families, defined by their ATPase, and domain properties. All Remodelers contain a SWI2/SNF2-related ATPase subunit characterized by an ATPase/Translocase domain (Tr) split into two RecA-like lobes termed DExx (red) and HELICc (orange). Remodelers can be separated into four families based on domain features, including the conserved domains flanking the ATPase domain, and the length and function of the insertion within the ATPase domain. While Remodelers of ISWI, CHD, and SWI/SNF families contain a short insertion (gray) within the ATPase domain, Remodelers of the INO80 family harbor a long insertion (yellow). Distinct signature domain (combinations and placement) define each family. ISWI: HSS module (HAND-SANT-SLIDE, cyan), AutoN region (pink) and NegC region (green). CHD: tandem chromodomains (purple), a DBD module (DNA-binding domain, cyan) and region with structural similarity to ISWI [asterisk; NegC (green)]. INO80: HSA module (dark green) and a long insertion (yellow). SWI/SNF: bromodomain (light green), an HSA module (dark green), a SnAC domain (blue) and AT-hooks (black). The domain sufficient for DNA Translocation (Tr), discussed in the text and utilized in Fig. 3.7, is depicted below
3.2.3 Remodeler Families and Compositional Specialization
Remodelers can be separated into families based on similarities/differences of domains residing within their catalytic ATPase subunits (Flaus et al. 2006), and their attendant subunit composition. These criteria define four separate Remodeler families: ISWI, CHD/Mi2–NuRD, INO80/SWR1, and SWI/SNF (Fig. 3.4). As developed in the section on Remodeler mechanisms, the domains flanking the ATPase domain either help regulate the ATPase domain and/or mediate Remodeler composition through assembly of additional proteins.
Remodeler family utilization is widespread, as almost all eukaryotes contain at least one Remodeler complex in each of the four families. Moreover, higher eukaryotes construct and employ a remarkable set of Remodeler subtypes within each of the four Remodeler families [Table 3.1, also compiled in Bao and Shen (2007)]. Within a Remodeler family, constructing subtypes with compositional diversity typically involves the use of (1) alternative ATPase paralogs, (2) alternative “signature/core” paralogs, selecting one paralog from a highly related set, and (3) alternative attendant subunits that vary between subtypes. A key concept for complex organisms is the use of these assembly principles to construct cell type- or developmentally specific Remodeler subtypes. Below is detailed how particular organisms blend both combinatorial and modular concepts for the construction of their Remodeler repertoire (an example involving human Remodelers subtypes is depicted in Fig. 3.5). For clarity, the species origin of Remodeling complexes (or subunits) will be preceded by a letter designating their origin: human (h), Saccharomyces cerevisiae/yeast (y), Schizosaccharomyces pombe (sp), Drosophila melanogaster (d), mouse (m), Xenopus laevis (x), and Arabidopsis thaliana (a). Below, we provide compositional information, but defer to later mechanistic sections details on how compositions specialize functions.
Examples of human Remodeler subtype compositions. Scheme depicting one human Remodeler subtype from each family: CHRAC for ISWI family, NuRD for CHD family, INO80 for INO80 family, and esBAF/BAF/PBAF for SWI/SNF family. All Remodelers contain an ATPase/translocase subunit (red), and additional “signature/core” and unique subunits (Table 3.1) that can be organized in modules. For the SWI/SNF family, examples of modular construction of subtypes (esBAF, BAF, and PBAF) are depicted
3.2.3.1 ISWI Family
All ISWI ATPases contain at their C-terminus a “HAND-SANT-SLIDE” (HSS) domain, which involves a combination of three domains: the HAND domain, the SANT domain (ySWI3, yADA2, hNCoR, hTFIIIB), and a SLIDE domain (SANT-like ISWI). The HSS binds to two different nucleosomal epitopes: the SANT domain interacts with the unmodified histone H3 tail (Boyer et al. 2004), while the adjacent and structurally related SLIDE domain contacts the DNA as it exits the nucleosome along with the DNA flanking the nucleosome, also known as “linker” DNA (Dang and Bartholomew 2007) (Fig. 3.6b) [notably, the functions of these two structurally related domains are swapped in the HSS domains of yeast ISWI members (Pinskaya et al. 2009)]. Interestingly, the HSS domain helps regulate dISWI remodeling activity in conjunction with two other regulatory domains that flank the ATPase lobes, AutoN and NegC (see below) (Grune et al. 2003; Clapier and Cairns 2012; Mueller-Planitz et al. 2013).
Structures of Remodelers. (a) Structure of yeast Chd1 (PDB access: 3MWY) highlighting the two adjacent RecA-like lobes DExx (red) and HELICc (orange), which are separated by the DNA-binding cleft (red dashed line), crossed one time by the short insertion (gray), and a second time by NegC* (green). Tandem chromodomains are also depicted (purple). (b) Structure of the HSS domain of ISW1 showing the successive distribution of the regions HAND (blue), SANT (green), Spacer/Helixα7 (purple) and SLIDE (yellow) away from the nucleosome core particle, and along the bent extranucleosomal DNA (orange) (partial from PDB access: 2Y9Z). (c) 3D reconstruction from electron microscopy of dimeric SNF2H Remodeler bound symmetrically to the nucleosome. The nucleosome structure was placed manually in the reconstruction, highlighting the histone H4 tails located into structural pockets [inspired from Racki et al. (2009)]. (d) 3D reconstruction from electron microscopy of RSC Remodeler with nucleosome modeled into the pocket (Leschziner et al. 2007)
The ISWI ATPase is a scaffold around which several different ISWI-family Remodeler subtypes are built (Table 3.1). At one extreme is Drosophila, which constructs all ISWI subtypes around a single ISWI ATPase. In contrast, most other organisms use at least two related ISWI paralogs for subtype construction (Table 3.1). For example, humans use two ISWI paralogs (SNF2H and SNF2L) to assemble multiple distinct ISWI Remodeler subtypes (with ACF, CHRAC, and NURF the most abundant), which can be distinguished by their core/signature subunits. ACF- and CHRAC-type Remodelers contain a common core protein, hACF1 (with both PHD and bromodomains in metazoans). CHRAC is distinguished further by the presence of two additional proteins, hCHRAC 15 and 17, which have DNA-binding histone fold motifs. This represents an example of modular subtype construction. In keeping, NURF-type Remodelers contain a signature protein, NURF301/BPTF, which is an analog of ACF1 (retaining PHD and bromodomains), which also contains DNA-binding HMGI(Y) motifs and interaction domains for the assembly of additional core NURF subunits. Functionally, most ISWI-family complexes function in an “assembly” mode to promote site blockage and gene repression; however, certain subtypes (i.e., NURF) have been adapted to function as “access” Remodelers to promote site exposure, chromatin opening, and gene activation (Fig. 3.1). In addition to the main ISWI subtypes, SNF2H is found in three additional specialized remodeling complexes: NoRC (bearing Tip5, for nucleolar regulation of RNAPI genes) (Strohner et al. 2001), RSF (bearing RSF1, for gene silencing) (Hanai et al. 2008), and WICH (bearing WSTF, contributing to DNA replication in heterochromatin and DNA repair) (Poot et al. 2004; Yoshimura et al. 2009). Within these, Drosophila contains a protein related to Tip5 (Toutatis), which associates with dISWI to form NoRC-related complexes (Emelyanov et al. 2012).
3.2.3.2 CHD Family
Members of this family contain two signature domains within the catalytic subunit: within the N-terminus are two tandemly arranged chromodomains (see below) and within the C-terminus resides a portion of the HSS domain—typically the DNA-binding SLIDE subdomain, and often also the SANT domain—described above for ISWI complexes (Fig. 3.6b) (Ryan et al. 2011). In keeping with their similarity to ISWI Remodelers, CHD ATPases are also flanked by sequences/structures similar to AutoN and NegC. Notably, CHD-family Remodelers display more diversity than any other Remodeler family. Certain yeast species (i.e., S. cerevisiae) employ a single CHD ATPase, which functions as a monomer. In contrast, humans encode nine separate CHD ATPases (and the related ALC1 ATPase, see “orphans” below), which have apparently evolved by duplication and subfunctionalization. Only a subset have been characterized compositionally (Table 3.1), and the characterized subset reveals a wide variety of assemblies. In certain cases, core/signature subunits can be used to define subtypes conserved in eukaryotes (such as Mi2–NuRD, below), but in many other instances classification is challenging due to compositional diversity or lack of information.
Functionally, CHD Remodelers are linked to all three general processes: assembly (spacing nucleosomes), composition/editing (histone H3.3 incorporation), and access (site exposure in promoters)—reflecting their compositional diversity—with functional contexts provided in later sections. The best characterized multisubunit CHD-family member is the Mi2/NURD (Nucleosome Remodeling and Deacetylase) subtype (Denslow and Wade 2007), which includes the ATPase Mi2, histone deacetylases (HDAC1/2) and methyl CpG-binding domain (MBD) proteins. As predicted by composition, this subtype is linked to gene repression in higher eukaryotes. Notably, invertebrates utilize a MEC subtype, which in Drosophila combines the Mi2 ATPase with dMEP1 (a protein harboring seven zinc fingers) to constitute the most abundant Mi2-containing complex. Interestingly, dMec confers proneural gene repression via an HDAC-independent mechanism (Kunert et al. 2009), showing that Mi2-containing complexes can repress through HDAC-dependent and independent modes. Presently, an analog of MEC in vertebrates is not known. However, as detailed later, certain CHD Remodelers subtypes slide or eject nucleosomes to promote transcription.
3.2.3.3 INO80 Family
INO80-family ATPases are characterized by a long insertion between the DExx and HELICc motifs (forming a “split” ATPase), to which are bound the enigmatic helicase-related (AAA-ATPase) Rvb1/2 proteins (Jha and Dutta 2009) and at least one actin-related protein (ARP5/6). Notably, a helicase-SANT (HSA) domain resides in the N-terminus, which is important for the assembly of two additional ARPs and β-actin itself. The family includes the highly related ATPases INO80 and SWR1 (or subtypes), around which the major Remodeler subtypes in this family are formed. Complexes in this family are most closely associated with editing functions. The SWR1/SRCAP/Tip60 subtypes remove canonical H2A–H2B dimers and replace them with histone variant H2A.Z–H2B dimers, whereas INO80 subtypes apparently have the reciprocal function. INO80 has diverse additional functions, including additional editing functions (H2A.X removal, likely underlying its DNA repair functions) and access functions to promote transcriptional activation.
SWR1 subtypes exhibit notable modularity, involving the association of the Remodeler with a set of AAA ATPases and also a histone acetyltransferase (HAT) module. In yeast, the ySWR1 complex associates with a separate HAT complex, yNuA4, whereas in flies and vertebrates the HAT module can be stably integrated within the Remodeler complex (i.e., dTip60 complexes). Notably, flies consolidate remodeling and HAT functions within a single SWR1-like complex (dTip60), whereas humans utilize both a hTip60 subtype and a separate dedicated Remodeler, hSRCAP.
3.2.3.4 SWI/SNF Family
Most eukaryotes utilize two to three related SWI/SNF-family subtypes, built around two related catalytic subunits (Table 3.1), though flies build their subtypes from a single ATPase. Domains present on most SWI/SNF-family catalytic ATPases include an N-terminal HSA domain (which binds actin and/or actin-related proteins), a C-terminal bromodomain, and often a pair of AT-hooks (which bind the minor groove of DNA). A pair of actin-related proteins (ARPs) is present in complexes from lower eukaryotes (Cairns et al. 1998), whereas complexes from higher orthologs contain a dimer consisting of actin and an actin-related protein (hBAF53a/b) (Lessard et al. 2007). Beyond ARPs, SWI/SNF complexes consistently contain a set of core/signature subunits that help define the family, and which in humans include BAF155/170, BAF60, and BAF47 (Table 3.1). A key concept for SWI/SNF Remodelers is “combinatorial” construction: “core” subunits are all derived from a set of paralogs, which are tissue and/or cell type specific and can help form specialized assemblies that drive ES cell self-renewal, cell differentiation, or developmental transitions—in concert with transcription factors (detailed later). SWI/SNF-family Remodelers are most closely associated with chromatin access (Fig. 3.1), as they slide and eject nucleosomes at many loci, but this access can be utilized for either activation or repression.
3.2.3.5 “Orphan” Remodelers
In addition to the four main families and their subtypes, there are a group of “Orphan” Remodelers, which have important specialized functions. Although phylogenetically most related to CHD Remodelers, the ALC1 (Amplified in Liver Cancer 1) subtype Remodeler ATPases lack a chromodomain and are therefore also named CHD1-like (CHD1L). Furthermore, the C-terminal DBD normally present in CHD Remodelers is replaced by a macrodomain, which interacts with PAR, allowing the rapid targeting of ALC1 to DNA breaks (Ahel et al. 2009; Gottschalk et al. 2009) (see later). Additional subunits are currently unknown.
The Fun30/Etl1 subfamily of Remodelers lack identifiable accessory domains or proteins. yFun30 performs chromatin editing by promoting histone turnover, both removal and replacement (Awad et al. 2010), and helps silence heterochromatic loci by direct interaction at chromatin boundaries and within silent loci (Neves-Costa et al. 2009). Similarly, spFft3 contributes to the maintenance of chromatin structure at centromeres and subtelomeres by preventing euchromatin invasion (Stralfors et al. 2011). Moreover, Fun30 promotes correct chromatin structure at the budding yeast point centromere, which is not embedded in heterochromatin (Durand-Dubief et al. 2012). In humans, SMARCAD1 interacts with PCNA and ensures that silent chromatin is correctly perpetuated throughout replication (Rowbotham et al. 2011). Finally, Fun30 and SMARCAD1 are decisive in DNA breaks repair by promoting DNA end resection (Chen et al. 2012; Costelloe et al. 2012; Eapen et al. 2012) (see later).
ATRX-containing Remodelers contain the large ATRX ATPase, which lacks known additional domains, binds to G-tetraplex DNA in vitro (Law et al. 2010) and associates with the histone H3.3 chaperone DAXX. Notably, ATRX–DAXX complexes perform replication-independent deposition of the H3.3 variant, especially at telomeres (Goldberg et al. 2010; Lewis et al. 2010; Drane et al. 2010; Elsasser et al. 2012). Surprisingly, ATRX also acts as a negative regulator of the incorporation of macroH2A, impacting gene expression (Ratnakumar et al. 2012) (see section on disease syndromes, below). Localization of ATRX changes during the cell cycle: while at centromeric heterochromatin during interphase and mitosis, ATRX is restricted to rDNA during metaphase (McDowell et al. 1999).
CSB is a SNF2-family DNA translocase involved in transcription-coupled nucleotide-excision repair (TC-NER) (Woudstra et al. 2002). CSB interacts directly with core histones and remodels nucleosomes in an ATP-dependent manner (Citterio et al. 2000). It also wraps DNA, suggesting that CSB may destabilize nucleosomes (Beerens et al. 2005). However, it is most intensively studied in its role in Cockayne’s syndrome, involving its association with RNAPII (see Disease Syndromes, below), and in control of rDNA transcription, involving RNAPI (below).
3.2.4 PTM-Interacting Motifs and Combinatorial Regulation
Chromatin-modifying enzymes work in concert with Remodelers to orchestrate nucleosome dynamics. Posttranslational modifications (PTMs) are recognized by Remodeler motifs and used to help guide Remodeler function; below, we discuss the most prevalent motifs and their functions in targeting/retention, and later their utilization in remodeling mechanisms.
3.2.4.1 Bromodomain
Acetylated lysines in histones and other proteins are bound by the bromodomain, a motif common in Remodelers of most families. For SWI/SNF-family Remodelers, a bromodomain always resides near the C-terminus of the ATPase (Fig. 3.4). Evidence for function includes work on the C-terminal bromodomain in the ATPase subunit (ySnf2/Swi2) of ySWI/SNF, where it is necessary for the retention of the Remodeler at the SUC2 gene (Hassan et al. 2002). Remodeler bromodomains can interact with specific acetylated histone residues. For example, yRsc4 interacts with H3K14ac in vitro and promotes gene activation in vivo (Kasten et al. 2004). Regarding SWR1 subtypes, the bromodomains of yBdf1 may recognize patterns of acetylation (including H3K14ac), which may influence the deposition of H2A.Z–H2B variant dimers into the appropriate nucleosome (Zhang et al. 2005). Thus, histone acetylation might help guide the location or efficiency of the replacement process. As yet, bromodomains present in ISWI Remodelers have not been connected to particular substrates.
Notably, in most organisms the two main SWI/SNF-family subtypes can often be distinguished by the presence or absence of subunit(s) bearing multiple bromodomains (Table 3.1). These multiple bromodomains can reside in a single protein (polybromo/BAF180 in higher eukaryotes) or be distributed among several (e.g., yRsc1/2/4/10), and functional work in multiple organisms supports functional roles for these bromodomains. The presence of multiple bromodomains raises the possibility for cooperative recognition of separate modifications, which is an active area of investigation. Mechanistic work in vitro has demonstrated that histone acetylation can increase the efficiency of SWI/SNF-family Remodelers, including their affinity for and activity on nucleosomes in different contexts (Ferreira et al. 2007a; Carey et al. 2006; Chatterjee et al. 2011), though much remains to be learned. Remodeler bromodomains may also be utilized for the intrinsic regulation (autoinhibition) of the Remodeler, as Remodeler bromodomains can bind specific acetylated residues within the Remodeler in competition with nucleosomal epitopes (VanDemark et al. 2007; Kim et al. 2010).
3.2.4.2 BAH Domain
The BAH (bromo-adjacent homology) domain is often found alongside bromodomains in multiple Remodeler proteins (Rsc1/2, polybromo, BAF180), primarily of the SWI/SNF family, and also resides alone in other chromatin regulators (e.g., Sir3 and Orc1). Recent structural and genetic evidence strongly support roles for the BAH domain in histone binding (Onishi et al. 2007), interacting either with the exposed top/bottom surface of the octamer or with histone tails and may be regulated by lysine methylation (Armache et al. 2011). Thus, the BAH has been adapted to become a versatile histone recognition module, and experiments in vivo involving both yeast and metazoan systems have verified its functional importance in multiple contexts.
3.2.4.3 CHD Domain
CHD-family Remodelers typically bear in their N-terminus two tandem chromodomains. Tandem CHD domains appear to function as a structural unit, and in certain circumstances bind one or two methylated lysines (Brehm et al. 2004). Chromodomains from human CHD1 clearly bind H3K4me2/3, marks correlated with active chromatin (Flanagan et al. 2005; Sims et al. 2005). However, methyl–lysine binding and H3K4me specificity is far from universal. For example, H3K4me2/3 specificity is not detectable with yCHD1 and dCHD1 (Morettini et al. 2011) or with dKismet (Srinivasan et al. 2008). Remarkably, Mi-2 may instead use its chromodomains for the recognition of DNA rather than methylated tails (Bouazoune et al. 2002). Thus far, a primary role for the chromodomains in targeting has not been clearly substantiated, and for dCHD1 localization has proven independent of the chromodomains (Morettini et al. 2011). It is therefore possible that other mechanisms drive the recruitment of CHD1, and that histone–chromodomain recognition contributes to a subsequent stabilization of that interaction, or instead helps regulate the Remodeler. Indeed, recent work on yChd1 strongly supports a role for the chromodomains in gating the access of the Remodeler to DNA (Hauk et al. 2010). Notably, Mi-2 lacking its chromodomains fails to bind or remodel nucleosomes at all, suggesting a more general enabling role for chromodomains.
3.2.4.4 PHD Domain
The PHD (plant homeodomain) finger is a methyl–lysine interaction motif found in subunits of multiple Remodeler family subtypes. In the ISWI-family Remodeler NURF, the PHD of the BPTF subunit interacts directly with H3K4me3, stabilizing BPTF/NURF on active chromatin (Wysocka et al. 2006). However, additional studies suggest alternative epitopes. For example, the PHD of dACF1 recognizes the globular domain of core histones (Eberharter et al. 2004). In regard to function, certain subtypes rely on their PHD domains (i.e., dACF) while other subtypes (i.e., dMi-2) do not. More than any other motif studied thus far, PHD domains have proven to cooperate functionally with other histone-recognition motifs for histone interaction. For example, the second PHD finger of hBPTF (the largest subunit of hNURF) binds H3K4me2/3, which then imparts to the adjacent bromodomain specificity toward H4K16ac, whereas in the absence of influence the bromodomain recognizes all H4 acetylations at low affinity. This remarkable bivalent recognition of a mononucleosomal histone modification pattern by hBPTF is decisive for proper localization of NURF (Ruthenburg et al. 2011). Analogously, the PHD finger and bromodomain residing on Tip5, the largest subunit of NoRC, cooperate to recruit NoRC to nucleosomes with H4K16ac, an interaction needed for rDNA silencing (Zhou and Grummt 2005). This PHD–bromo cooperativity also extends beyond Remodelers to key transcription factors like TRIM24 (Tsai et al. 2010). Finally, functional diversity and altered targeting specificities can arise from alternative splicing of a Remodeler subunit. For example, dNURF301 can be spliced in an isoform lacking the C-terminal PHD finger and bromodomain, usually recognizing H3K4Me3 and H4K16Ac. The presence of the C-terminal of NURF301 is required for a subset of NURF targets and proper spermatogenesis (Kwon et al. 2009).
Moreover, the tandem PHD fingers and chromodomains of hCHD4/Mi-2β (the catalytic core of the NuRD Remodeler) regulate nucleosome recognition, ATPase and remodeling activities of hCHD4 (Watson et al. 2012). While the tandem PHD fingers of hCHD4 possess individual histone-binding capacities, they associate with high affinity to two histone H3 tails in a single nucleosome, with H3K9 methylation or acetylation strengthening their binding (Musselman et al. 2012), promoting transcriptional repression.
3.2.5 Remodeler Motif Utilization
A key issue is whether the nucleosome-interacting motifs and domains residing on Remodelers are used for initial targeting or subsequent retention or are instead used to provide regulatory information to the ATPase subunit, possibly to tune either the activity or mode of remodeling. However, for many of the individual domains listed above, their affinities for nucleosomal epitopes are modest, typically in the 100 nM–10 μM range, questioning their sufficiency for targeting a Remodeler to a locus. However, as Remodelers can contain several histone-binding motifs, their use in combination could, in principle, provide an affinity sufficient for targeting or retention. Here, examples of combinatorial recognition are few (i.e., PHD–bromo and PHD–chromo), but growing in number. An additional possibility is that domain-modification interactions help regulate the Remodeler ATPase activity or other remodeling properties of the complex; the notion that histone modifications provide information to the Remodeler rather than targeting or retention.
In the examples above, Remodeler motifs are used to selectively engage nucleosomes bearing particular modifications, thus enhancing their affinity or activity. An equally important issue is how Remodelers avoid binding and/or acting on “improper” nucleosomes—as their action might impair the intended process. Here, avoidance can involve a covalent modification on the nucleosome that is sterically incompatible with Remodeler binding, or instead, one that renders the Remodeler inactive via an allosteric mechanism.
3.3 Remodeler Mechanisms and Regulation
3.3.1 DNA Translocation Underlies Remodeler Mechanisms and Outcomes
Remodeler families and subtypes exhibit different compositions and have specialized functions (assembly, editing, and access). However, all contain a single, similar catalytic domain, which functions as an ATP-dependent DNA translocase used to break histone–DNA contacts. A key emerging concept is that differences in the implementation and regulation of DNA translocation can define these different outcomes achieved by Remodeler subtypes.
Structural information can inform mechanisms but is limited for Remodelers; there is currently no high-resolution structure of a Remodeler bound to a nucleosome, nor a structure of a multisubunit Remodeler in the absence of a nucleosome. Presently, the only available structure of a chromatin remodeling ATPase is of yeast Chd1 (Fig. 3.6a) (Hauk et al. 2010). The structure is highly similar to known ATP-dependent DNA translocases/helicases (i.e., Rad54, PcrA), revealing two adjacent RecA-like lobes between which lies a DNA-binding cleft and a site for ATP-binding and hydrolysis—which constitutes the DNA translocation region/motor discussed later (Bowman 2010; Flaus and Owen-Hughes 2011). Thus, the yChd1 structure establishes a prototype DNA translocating motor for other Remodelers. An emerging concept in Remodeler regulation (developed below) involves the use of domains that flank the ATPase domain in regulating the functions of the ATPase domain. For Chd1, the domains flanking the translocase region include the C-terminal NegC domain and the N-terminal tandem chromodomains. Notably, the chromodomains are positioned to interfere with the path of DNA through the cleft, whereas the NegC domain bridges the two RecA-like lobes, features that may be important for regulation of DNA translocation (Fig. 3.6a). Biochemical experiments support a 1:1 Remodeler:nucleosome stoichiometry for most Remodelers with exceptions noted later.
DNA translocation has been intensively studied for SWI/SNF-, ISWI-, and CHD-family Remodelers, and these Remodelers share important mechanistic features. For example, for all three Remodelers their ATPase/Translocase domain (termed “Tr,” Fig. 3.7) binds to DNA within the nucleosome, about two turns from the central DNA dyad (Fig. 3.7, state 1) (Saha et al. 2005; Zofall et al. 2006). The position of the Tr domain remains fixed on the octamer, and for SWI/SNF Remodelers a domain (SnAC, Fig. 3.4) has been identified that helps “anchor” the Remodeler (“A,” Fig. 3.7) to the nucleosome (Sen et al. 2013). From this fixed position, the Tr domain performs directional DNA translocation by pulling in DNA from the proximal side of the nucleosome and pumping it toward the dyad (Fig 3.7b, state 2, note movement of red dot) (Saha et al. 2005; Zofall et al. 2006). This DNA “pumping” action is provided by the reciprocal action of two RecA-like subdomains, termed DExx and HELICc, which sequentially bind and release DNA—analogous to “inchworming”—apparently moving 1–2 bp of DNA per cycle of ATP binding/hydrolysis/release (Blosser et al. 2009; Sirinakis et al. 2011; Deindl et al. 2013). Here, it is important to reconsider how histone–DNA contacts are broken and reformed on both sides of the internal Tr domain. The act of translocating 1–2 bp by the Tr domain creates both DNA torsion and translational tension on both sides of the Tr domain, but of opposite polarity on each side: the proximal side is undertwisted and lacks sufficient DNA, whereas the distal side is overtwisted and contains excess DNA. On the distal side, this tension causes the breakage and reformation of histone–DNA contacts, propagating in a wave-like manner away from the location of the Tr domain toward the distal exit site of the nucleosome by diffusion—with histone–DNA contacts broken at the leading edge of the wave and reforming at the lagging edge of the wave—and the resolution of torsion and tension occurring through its arrival at the distal linker, resulting in the extension of the linker by 1–2 bp. A similar wave propagation mechanism occurs on the proximal side of the nucleosome, with resolution occurring through the pulling of 1–2 bp from the proximal linker into the nucleosome and toward the Tr domain. This model has been termed “wave-ratchet-wave” to denote the movement of DNA toward and then away from the internal Tr domain (Saha et al. 2005). Notably, the Tr domain also functions as an internal ratchet to ensure directional movement of the DNA. The overall reaction results in displacement of the octamer along the DNA (Fig. 3.7b, state 3), defining nucleosome sliding. By iteration, subsequent ATP hydrolysis cycles lead to additional directional displacement.
Models of DNA translocation during a Remodeling cycle by SWI/SNF and ISWI Remodelers. (a) On the left panel, side view of a nucleosome with the left-handed wrapping of DNA (light green-dark green) around the histone octamer (gray transparent cylinder). DNA color changes from light green to dark green when passing the nucleosomal dyad axis. On the right panel, two-dimensional depiction of the nucleosome after 90° rotation according to the axis. The perspective of DNA wrapping is strengthened by the depiction of the second gyre of the DNA in dark green dots, after passing the dyad axis. A red dot serves as a reference point on the DNA, convenient to trace (conceptually) DNA translocation at the surface of the octamer. (b) Depiction of the successive steps occurring during a remodeling cycle performed by SWI/SNF Remodelers (States 1–3). The SWI/SNF Remodeler interacts with the nucleosome via its ATPase/translocase domain (Tr, red) located near the dyad, and an anchoring domain (A, blue) that affixes the Remodeler to the surface of the octamer (State 1). The ATPase/translocase domain achieves a small DNA displacement (1–2 bp) at the surface of the nucleosome (State 1 to 2), generating DNA tension (orange DNA) on both the proximal and distal sides of the Tr domain, owing to the lack and excess of DNA, respectively (State 2). DNA tension is resolved (restoring green DNA color) on the proximal side DNA moving into the nucleosome from the proximal linker, and on the distal side by one-dimensional diffusion of the excess DNA, which moves around the distal side (second half) of the nucleosome in the form of a small wave, and then resolves in the distal linker (State 2 to 3). The SWI/SNF Remodeler performs iterations of this DNA translocation cycle, resulting in a progressive displacement of the histone octamer with respect to the DNA (State 4). (c) Depiction of the successive steps occurring during a remodeling cycle performed by ISWI Remodelers (States 1–4). ISWI interacts with the nucleosome via its ATPase/translocase domain (Tr, red) located near the dyad, via a hypothetical anchoring domain (A*, blue), and via a DNA-binding domain (DBD, cyan), which contacts extranucleosomal DNA (State 1). Remodeling by ISWI involves regulation of the Tr domain by the DBD, with binding of the DBD to the DNA linker activating the ATPase/translocase domain to perform DNA translocation (green arrow, State 1 to 2), generating DNA tension (orange DNA) on both sides, similar to SWI/SNF (State 2). On the distal side, DNA tensions are resolved as for SWI/SNF. On the proximal side, the release of the DBD from the DNA linker is required to ease DNA tension, which then allows DNA to be donated from the proximal linker into the nucleosome. However, DBD release from the proximal linker inhibits the translocase activity (red line, State 2 to 3). Once the DBD resumes binding extranucleosomal DNA in its new position, DNA translocation can again proceed (green arrow, State 4). As for SWI/SNF, ISWI performs iterations of DNA translocation described, resulting in a progressive displacement of the histone octamer with respect to the DNA (State 5)
An earlier section described the biophysical parameters needed to break histone–DNA contacts. Recently, the biophysical parameters of DNA translocation by ISWI- and SWI/SNF-family Remodelers have been determined using multiple single molecule formats, revealing their ability to implement sufficient force (7–12 pN) to break multiple histone–DNA contacts, and the ability to move DNA in a directional and processive manner, with considerable velocity (~8 bp/s) (Zhang et al. 2006b; Blosser et al. 2009; Sirinakis et al. 2011). All Remodelers that conduct sliding likely share this central DNA translocation mechanism, but likely apply and regulate this mechanism differently to achieve different outcomes, such as nucleosome spacing or ejection, developed below.
3.3.2 Mechanisms and Regulation of Chromatin Access
Chromatin access in different contexts involves Remodeler subtypes from all four families, though nucleosome disorganization and ejection is most strongly associated with SWI/SNF Remodelers. Intuitively, random sliding of nucleosomes on an array will, over time, provide access to virtually all sites on the array, regardless of their initial positions. Indeed, most SWI/SNF-family enzymes can conduct sliding to provide access of DNA-binding factors to nucleosomal templates (Logie and Peterson 1997). Although nucleosomes bind more strongly to certain types of DNA sequences—those with an intrinsic “left-handed” curvature compatible with nucleosome formation—the single molecule experiments described above demonstrate that SWI/SNF Remodelers can impart sufficient force to slide nucleosomes along any DNA sequence and can impose peak forces that can disassemble a nucleosome. The remodeling reaction likely involves the sequential interaction of one nucleosome with one remodeler, as biochemical and structural studies with SWI/SNF-family Remodelers strongly support a 1:1 Remodeler:nucleosome stoichiometry (Leschziner et al. 2005, 2007; Skiniotis et al. 2007; Chaban et al. 2008) along with a striking pocket of nearly perfect mononucleosome dimension, the access to which may involve more than one conformation, and be regulated by histone tail modifications (Dechassa et al. 2008; Asturias et al. 2002; Leschziner et al. 2007; Skiniotis et al. 2007) (Fig. 3.6d). A key issue is why and how ISWI- and CHD-family Remodelers achieve spaced nucleosome arrays, while SWI/SNF-family Remodelers act to randomize initially spaced arrays. As detailed below, a spacing function involves the use of a DBD to detect and measure extranucleosomal DNA, a domain and property lacking in SWI/SNF-family Remodelers.
In addition to sliding, Remodelers provide DNA access by nucleosome ejection, via one of two mechanisms (Lorch et al. 2006; Boeger et al. 2003; Reinke and Horz 2003). First, the disruption of histone–DNA contacts by DNA translocation (a shared attribute of Remodelers) can render the histones susceptible to loss, allowing either histone chaperones and/or specialized proteins on particular Remodelers access to remove the underlying histones. Here, INO80 Remodelers may employ specialized proteins to chaperone histones out of nucleosomes (Hogan et al. 2010). In the second mechanism, the nucleosome adjacent to the one undergoing remodeling is ejected—not the actual nucleosome bound to the Remodeler; here, the act of processive DNA translocation on the bound nucleosome initially draws the available linker DNA into the bound nucleosome and, when the linker DNA is exhausted, the Remodeler then “spools” the DNA off of the adjacent nucleosome, leading to octamer ejection (Cairns 2007; Boeger et al. 2008). Support for this mode comes from studies on ySWI/SNF (Dechassa et al. 2010). A key unanswered question is how ejecting Remodelers choose between a sliding versus an ejection mode. Contributing factors might include the stability of the nucleosome; here, particular histone variants and/or the underlying DNA sequence may facilitate ejection (Fig. 3.3, red nucleosome).
A related mechanistic and regulatory issue is how Remodeler ATPase activity is regulated by Remodeler subunits, histone variants, histone determinants, and modifications. Here, SWI/SNF-family Remodelers have both a higher affinity for and increased activity on nucleosomes with acetylation (Ferreira et al. 2007a; Chatterjee et al. 2011). Although initial links between particular bromodomains on Remodelers and particular acetylation marks have been made, there is still much to learn to fully understand their roles. Roles for the enigmatic actin-related proteins in regulating remodeling are provided into a later section.
3.3.3 Mechanisms and Regulation of Chromatin Assembly
Nucleosome assembly and spacing is conducted primarily by ISWI- and CHD-family Remodelers. Current models include an initial deposition phase, where the Remodeler may assist in the formation of fully mature, folded nucleosomes, followed by the use of regulated sliding activities to achieve ordered spacing (Fig. 3.1). As previewed above, the key to spacing involves use of a DNA-binding domain (DBD), residing in the C-terminus of ISWI (HSS domain) and certain CHD (SLIDE domain) ATPases (Fig. 3.4). This DBD measures the distance between nucleosomes by binding to linker/extranucleosomal DNA (Fig. 3.7c, state 1) (Fig. 3.6b) (McKnight et al. 2011). Interestingly, the DNA-binding status of the DBD actually regulates the activity of the ATPase/translocase domain. For ISWI, binding of the DBD/HSS to extranucleosomal DNA turns on the ATPase/translocase domain (Fig. 3.7c, state 1), by relieving autoinhibition by the flanking NegC domain (Fig. 3.4, not depicted in Fig. 3.7), which pulls in ~1 bp of DNA, causing tension in the DNA between these domains (Fig. 3.7c, state 2). Release of the DBD from the DNA then allows 1 bp to be drawn into the nucleosome, which relieves that tension (Fig. 3.7c, state 3)—with the entire process likely involving one cycle of ATP binding/hydrolysis/release. Recent measurements suggest that the DBD domain may, in certain circumstances, remain bound to linker DNA for more than one ATP hydrolysis cycle, leading to additional tension (involving several base pairs) between the ATPase/translocase domain and the DBD, before tension is released (Deindl et al. 2013). Regardless, the net translocation of DNA into the substrate nucleosome will draw the adjacent nucleosome ever nearer (and the linker DNA ever shorter). Notably, an additional attribute explains spacing. In its unbound state, the DBD does not stimulate the ATPase/translocase (Fig. 3.7c, state 3); thus, available extranucleosomal DNA is needed to reengage DNA translocation (Fig. 3.7c, state 5), and this cycle continues until linker DNA is exhausted or until the adjacent nucleosome interferes with binding of the DBD by steric hindrance (not depicted), leaving the adjacent nucleosome a fixed distance from the substrate nucleosome. Sequential application of the spacing process to all nucleosomes on the template will produce an array with all nucleosomes the same distance apart, and if combined with a boundary factor, a spaced array that is phased with respect to the boundary factor (Fig. 3.3). Notably, certain ISWI subtypes (i.e., ACF) contain a protein that extends the length of DNA bound by the HSS/DBD, yielding an array with a longer median internucleosomal distance, in keeping with the model described above.
Interestingly, nucleosomal epitopes (and their modification status) can regulate the activity and mechanism of assembly Remodelers. Here, the clearest example is the stimulation of ISWI ATPase activity by a small basic region on the histone H4 tail (residues 17–19) (Hamiche et al. 2001; Clapier et al. 2001, 2002), but not if the flanking lysine residue is acetylated (H4K16ac). Here, the H4 tail does not increase Remodeler affinity for the nucleosome, but rather affects ATPase activity through an allosteric mechanism that may involve relief of autoinhibition. Notably, the N-terminus of ISWI contains a “mimic” of the H4 tail basic patch, which inhibits ATPase activity (AutoN, Fig. 3.4) (Clapier and Cairns 2012), and is antagonized by the authentic (and unacetylated) H4 tail basic patch, helping to ensure that ISWI does not space/organize highly acetylated nucleosomes. This relationship makes biological sense, as nucleosomes bearing H4K16ac are found more often at active gene promoters and enhancers, where (intuitively) nucleosome dynamics are favored over order and assembly.
Interestingly, single molecule experiments have shown that the direction of DNA translocation on the nucleosome can abruptly change, though it is not presently clear how this is achieved. Possible solutions include a change in DNA translocation directionality, or instead a 180° flip of the nucleosome within the Remodeler. However, certain ISWI Remodelers appear to implement another option (Racki and Narlikar 2008). Here, certain ISWI complexes can operate via a 1:1 or 2:1 Remodeler:nucleosome stoichiometry (Strohner et al. 2005; Racki et al. 2009) (Fig. 3.6c). Notably, the 2:1 structure involves the second ISWI complex binding in a symmetrical position on the opposite side of the nucleosome, without a steric clash with the first complex; as the DNA translocation mechanism is directional (pumping toward the dyad), two ISWI complexes on opposite sides may alternate in activity, enabling octamer movement in alternative directions.
For ISWI-family Remodelers, additional noncatalytic subunits also impact the remodeling reaction. For example, the dNURF301 subunit of dNURF facilitates nucleosome sliding (Xiao et al. 2001). Similarly, the PHD fingers of dACF1 (in dACF and dCHRAC subtypes) enhance nucleosome sliding by stabilizing Remodeler–nucleosome interactions and affect the directionality of nucleosome movement (Eberharter et al. 2004; Fyodorov et al. 2004). Moreover, different noncatalytic subunits present in various hSNF2H-containing Remodelers (hACF, hCHRAC, hRSF, and hWICH) regulate hSNF2H activity through their interaction with extranucleosomal DNA (He et al. 2008). Remarkably, hACF1 improves nucleosome spacing by changing the length of extranucleosomal DNA required to perform sliding; a subunit fulfilling this function does not exist in hSWI/SNF (He et al. 2006). Furthermore, the Ioc3 subunit of yISW1a binds the yIsw1 HSS domain and facilitates an interaction with the linker DNA of the adjacent nucleosome (Yamada et al. 2011). Moreover, sliding activity of CHRAC relative to ACF is further enhanced by the additional histone-fold proteins, facilitating remodeling by binding and bending DNA at the edge of the nucleosome (Kukimoto et al. 2004; McConnell et al. 2004; Hartlepp et al. 2005; Dang et al. 2007).
Remodelers also contribute to the formation of higher-order chromatin structures (Varga-Weisz and Becker 2006). The first level of higher organization involves the association of the linker histone H1 with the nucleosome core particle, forming the chromatosome, which increases chromatin compaction. Interestingly, ISWI promotes H1 deposition in chromatin in vivo and likely conducts this activity within the ACF subtype (Fyodorov et al. 2004; Lusser et al. 2005; Corona et al. 2007; Siriaco et al. 2009), suggesting that a particular nucleosomal repeat length is optimal for H1 assembly. Remarkably, a chromatosome experimental context does not restrict sliding by dACF, but inhibits dCHD1 (Maier et al. 2008), suggesting a hierarchy of Remodeler action. Notably, remodeling activities of ySWI/SNF, hSWI/SNF, and xMi-2 can be inhibited by H1, but antagonized by H1 phosphorylation, which can rescue remodeling by ySWI/SNF (Hill and Imbalzano 2000; Horn et al. 2002). However, in other work, little or no inhibition was observed at stoichiometric levels of either canonical H1 isoforms (Clausell et al. 2009) or embryonic H1 variants (Saeki et al. 2005). Thus, much remains to be learned about how particular higher-order structures might prevent or permit particular Remodeler subtypes.
3.3.4 Mechanisms and Regulation of Chromatin Editing Involving H2A Variants
Nucleosome editing, which involves the incorporation or removal of histone variants, are largely performed by INO80-family Remodelers. Editing chromatin composition by histone variant incorporation allows the construction of specialized chromatin regions in a replication-independent manner. Among the key variants incorporated is the H2A variant H2A.Z. Here, elegant work has shown that the SWR1 subtype removes canonical H2A–H2B dimers and replaces them with H2A.Z–H2B dimers (Mizuguchi et al. 2004). Analogous to the mechanisms above for nucleosome ejection, SWR1 may utilize the tension and breakage of histone–DNA contacts created by ATP-dependent DNA translocation to facilitate H2A–H2B dimer removal—providing the first step in nucleosome editing. However, a professional “editing” Remodeler (unlike an “access” Remodeler, which simply ejects histones) must stabilize the hexasome and uniquely deliver the replacement variant dimer, and then release the finished product. In keeping, the SWR1 complex contains proteins specialized for H2A.Z/H2B dimer recognition (Wu et al. 2005), and conducts dimer replacement in a stepwise and unidirectional fashion—one dimer at a time—first generating heterotypic nucleosomes, then homotypic H2A.Z nucleosomes (Luk et al. 2010). As the ATPase activity of SWR1 is stimulated by canonical H2A-containing nucleosomes, yet further stimulated by the free H2AZ–H2B dimer, it is likely that the H2A.Z–H2B dimer acts as both an effector and substrate for this reaction. Notably, a nucleosome containing H2A.Z in combination with canonical histone H3 is stable, whereas combination with the H3 variant H3.3 creates an unstable nucleosome, prone to ejection and turnover (Jin et al. 2009), properties utilized for regulating genes and heterochromatin propagation, but not addressed further here (Zhang et al. 2005; Raisner et al. 2005).
Beyond gene regulation—during DNA double-stranded break repair, SWR1 is recruited by the modified histone variant γH2AX, which is phosphorylated during checkpoint activation (van Attikum et al. 2007; Xu et al. 2012), and performs H2A.Z incorporation. Recent work supports roles for INO80-family Remodelers (the INO80 subtype itself) in conducting a reciprocal process to SWR1—H2A.Z–H2B dimer removal and replacement with canonical H2A–H2B, with evidence clearest in vivo, a function needed to preserve genome integrity (Papamichos-Chronakis et al. 2011). Moreover, INO80 may conduct an analogous editing process during the DNA damage response in the removal of γH2AX.
3.3.5 Incorporation of H3 Variants H3.3 and CENPA via Editing–Assembly Hybrid Mechanisms
In contrast to H2A–H2B dimer replacement, which conserves the H3/H4 tetramer, incorporation H3–H4 variant histones into nucleosomes involves features both of assembly (replication-independent replacement of the entire octamer) and editing (focal conversion of canonical nucleosomes into variant nucleosomes). Replacement is most common within coding regions of highly transcribed genes, where the process of transcription causes limited nucleosome eviction, followed by the replication-independent placement of H3.3-containing histones by the HIRA–ASF1 assembly system. This process may be facilitated by assembly Remodelers using modes analogous to those described above for replication-dependent assembly. Notably, the incorporation of the H3.3 variant into chromatin in other contexts and locations can involve particular Remodelers. For example, dCHD1 is required for de novo assembly and deposition of H3.3 into decondensing sperm chromatin (Konev et al. 2007). Intriguingly, the CHD2 Remodeler is involved in the loading of H3.3 at myogenic gene promoters prior to their activation, contributing to myogenic cell fate (Harada et al. 2012). Moreover, the ATRX Remodeler, along with the histone chaperone DAXX, contributes to the replication-independent deposition of H3.3 variant, mainly at pericentric chromatin and telomeres (Goldberg et al. 2010; Lewis et al. 2010; Drane et al. 2010; Elsasser et al. 2012). Finally, PBAP, but not BAP, is recruited to chromatin boundaries along with the histone-interacting protein FACT, is required for the loading of H3.3 by HIRA–ASF1, and plays a crucial role in boundary functions (Nakayama et al. 2012).
Elucidating the mechanistic process defining the centromeric chromatin territory and identifying the Remodeler(s) involved in the incorporation of the centromeric-specific histone H3 variant CENP-A is of major interest. S. pombe yHrp1 CHD Remodeler contributes to the incorporation of CENP-A and is necessary for proper chromosome segregation (Walfridsson et al. 2005). Chicken CHD1 localizes to centromeres through interaction with SSRP1, a subunit of the histone chaperone FACT, and is required for centromeric localization of CENP-A (Okada et al. 2009). The Remodeler RSF, combining SNF2H and Rsf1, actively supports the assembly of CENP-A chromatin in HeLa cells, as Rsf1 depeletion induces loss of centromeric CENP-A (Perpelescu et al. 2009). Interestingly, the Drosophila homolog of RSF, combining ISWI and dRsf1, interacts with both Tip60 and H2Av, and may play a role in the early step of silent chromatin formation by assisting in H2Av replacement (Hanai et al. 2008).
While some Remodelers promote histone variant deposition, others prevent erratic incorporation. For example, SWI/SNF is involved in constraining the distribution of histone variant Cse4 by actively removing Cse4 from ectopic sites and maintaining point centromeres (Gkikopoulos et al. 2011b). Similarly, ATRX acts as a negative regulator of the incorporation of macroH2A, an H2A variant that imparts repression, thus impacting critical gene expression (Ratnakumar et al. 2012).
3.3.6 Impact of Histone Variants on Chromatin Remodeling Activities
Notably, noncanonical nucleosomes can either promote or preclude remodeling. For example, SWI/SNF and ACF are unable to remodel nucleosomes containing macroH2A variant, present on the inactive X chromosome (Doyen et al. 2006). In contrary, the incorporation of H2AZ variant in nucleosomes correlates with an increased association of various Remodelers involved in gene regulation (Goldman et al. 2010). Thus far, only ISWI Remodelers display a demonstrated stimulation of their remodeling activities by variants, first observed with H2AZ, with stimulation attributed to an extended basic patch on the nucleosome surface (Goldman et al. 2010). Finally, the remodeling outcomes might be altered by cooperating with histone chaperones: for example, while CHD Remodelers are usually involved in nucleosome assembly, S. pombe yHrp1 and yHrp3 Remodelers perform nucleosome disassembly at promoters and coding region in coordination with the yNap1 histone chaperone (Walfridsson et al. 2007).
3.3.7 Actin and Actin-Related Proteins in Remodeler Regulation
In the cytoplasm, actin is an abundant cytoskeletal protein that works with the ARP2/3 complex to branch actin filaments. Intriguingly, actin and/or actin-related proteins (ARPs) are consistent components of all SWI/SNF- and INO80-family Remodelers. Notably, most ARPs are nuclear, and all nuclear ARPs tested have proven to assemble into SWI/SNF- and INO80-family Remodelers, but not ISWI- or CHD-family Remodelers (Table 3.1) (Cairns et al. 1998; Shen et al. 2000; Mizuguchi et al. 2004; Zhao et al. 1998; Dion et al. 2010).
Actin and/or ARPs bind directly to one of two domains on the Remodeler ATPase: the HSA domain (located in the N-terminus) or the long insertion, located between the DExx and HELICc ATPase domains solely in INO80-family Remodelers (Fig. 3.4). HSA domains are necessary and sufficient for selective binding of particular ARPs and actin, typically an actin–ARP pair, which explains why SWI/SNF Remodelers have two ARP/actin proteins per Remodeler. INO80-family Remodelers utilize their HSA domain to assemble actin and two ARPs and utilize their long insertion domain to assemble one additional ARP (either ARP5 or ARP6), though the assembly of this additional ARP also requires other proteins that bind the long insertion, including RuvB homologs (Jonsson et al. 2004).
Much progress has been made recently in understanding the structures of individual ARPs and ARP modules. For yINO80, isolated structures of Arp4 and Arp8 have been solved, revealing strong similarity to actin. Notably, Arp8 forms a dimer, but utilizes a unique N-terminal extension not present in actin itself for dimerization rather than actin-related surfaces (Saravanan et al. 2012). For ySWI/SNF, a high-resolution structure has been solved of a four-protein module that includes two ARPs, the HSA domain, and an ARP-interacting factor (Schubert et al. 2013). Here, the two ARPs are likewise highly similar to actin in terms of their overall structure. However, the ARPs use a hydrophobic pocket to assemble atop the helical HSA domain and use their actin-like regions to dimerize in a manner that bears no relationship to the surfaces used by actin polymers or the ARP2/3 dimer complex. Thus, counter to expectation, interactions between ARPs (and likely actin) in Remodelers are very different from those utilized by ARP2/3 or actin polymers.
One function of ARPs is to regulate the function of the ATPase domain (Jonsson et al. 2004; Szerlong et al. 2008; Shen et al. 2003). Within yINO80, ARPs promote Remodeler ATPase activity, DNA binding, and nucleosome mobilization (Shen et al. 2003). Drugs that affect actin function also lower hSWI/SNF ATPase activity, arguing that actin likewise regulates the Remodeler ATPase (Zhao et al. 1998). ARPs in ySWR1 and yRSC positively regulate the remodeling activities (Mizuguchi et al. 2004; Wu et al. 2005; Szerlong et al. 2003, 2008), strongly suggesting this as a common property. For RSC and SWI/SNF, the catalytic Remodeler ATPase and the two ARPs form a stable module capable of DNA translocation and moderate nucleosome remodeling activity (Yang et al. 2007; Sirinakis et al. 2011). However, a key unanswered question is how ARPs regulate the ATPase domain, and whether ARPs have additional interactions with either histones or other chromatin proteins that are then communicated to the ATPase domain. Indeed, there are emerging links between ARPs and histones; certain ARPs have histone-binding activity in vitro (Downs et al. 2004), including selectivity for either H2A/B dimers or H3/H4 tetramers (Downs et al. 2004; Gerhold et al. 2012; Saravanan et al. 2012), and recent structural studies support interaction of the Arp8 dimer (from yINO80) with nucleosomes (Saravanan et al. 2012). Moreover, Arp6 within ySWR1 is part of a protein module that helps bind the H2A.Z variant (Wu et al. 2005). Beyond their possible roles in regulating ATPase function, targeting INO80 to a locus undergoing a DSB increases the mobility of this locus in an ARP8-dependent manner (Neumann et al. 2012). In contrast to actin, most Remodeler ARPs do not themselves bind or hydrolyze ATP, though low ATPase activity has been reported for particular nuclear ARPs (Dion et al. 2010), but the meaning of this activity is currently unclear.
Intriguingly, roles are emerging for particular nuclear ARPs that are independent of their functions in Remodelers, though not addressed here (Yoshida et al. 2010; Lee et al. 2007). Finally, it should be noted that actin/ARP dimers also reside in HAT complexes, where they might retain functions related to the binding of chromatin proteins, but not retain a function related to ATPase regulation.
3.3.8 Cooperation of Remodelers with High-Mobility Group Domains/Proteins
High-mobility group (HMG) proteins are abundant chromatin architectural proteins that bind to and alter DNA structure. Their binding energy might be used to facilitate Remodeler interaction with particular regions of the nucleosome and may further affect the activity or efficiency of remodeling. Here, separate HMG proteins can interact or cooperate with Remodelers, or instead an HMG domain can reside within particular Remodeler subunits. For example, for Drosophila ACF, nucleosomal DNA binding and sliding activities are enhanced by the interaction of HMGB1 with the extranucleosomal DNA (Bonaldi et al. 2002). Also, the HMGB1-related protein NHP6a associates with ARPs in SWI/SNF and RSC (Szerlong et al. 2003) and promotes remodeling. HMG domains present in hBAF57 and hBAF111, respectively subunits of hBAF and hBRM complexes, promote the in vivo function of those Remodelers (Chi et al. 2002; Papoulas et al. 2001). Notably, the recruitment of hSWI/SNF to the HIV-1 promoter by the transcription factor ATF3 is dependent on HMGA1 protein (Henderson et al. 2004). The vertebrate-specific HMGN family of HMG proteins contains a nucleosome-binding domain that can repress chromatin remodeling by antagonizing nucleosome binding and mobilization by ACF or BRG1 (Rattner et al. 2009). Finally, the intriguing yNhp10 protein residing in the INO80 Remodeler plays an important role in targeting the complex to sites of DNA damage, possibly through interaction with phosphorylated H2A.X (Morrison et al. 2004).
3.3.9 Posttranslational Modifications That Regulate Remodelers
An additional regulatory layer for Remodelers involves reversible covalent modifications, which also provides opportunities to coregulate them with their nucleosomal substrates.
3.3.9.1 Phosphorylation
One of the earliest examples of Remodeler modification involved the phosphorylation-induced inactivation of hSWI/SNF complex in mitosis, which involved phosphorylation of hSWI3 and hBRG1 by hERK1, and its reversal by hPP2A, which restores remodeling activity (Muchardt et al. 1996; Sif et al. 1998). Simultaneously, phosphorylation of one of the two alternative catalytic subunits of hSWI/SNF, hBRM, results in degradation of hBRM (Sif et al. 1998). Furthermore, phosphorylation of ySnf5 in ySWI/SNF occurs in G1, and snf5 mutants show cell cycle arrest; however, the link between ySnf5 phosphorylation and progression has not been established (Geng et al. 2001). Notably, phosphorylation of Baf60c orchestrates chromatin transitions for both lipogenesis and myogenesis genes, developed further in a later section (Forcales et al. 2012; Wang et al. 2013). For CHD-family Remodelers, relatively constitutive phosphorylation of dMi-2 by dCK2 has been demonstrated, which attenuates its ATPase activity and nucleosome sliding (Bouazoune and Brehm 2005). Interestingly, the DNA damage response is coordinated by phosphorylation of checkpoint proteins using Mec1/Tel1 kinases, which also phosphorylate the yIes4 subunit of yINO80 which does not impact repair itself, but does impact the repair checkpoint by an unknown mechanism (Morrison et al. 2007).
3.3.9.2 Acetylation
Early work suggested that hSWI/SNF function can be reduced by acetylation of hBRM using hPCAF, limiting transcription activation and cell growth (Bourachot et al. 2003). Notably, the yeast ortholog of hPCAF is Gcn5, which likewise is the catalytic subunit of several different yeast HAT complexes that promote gene activation. Remarkably, Gcn5 acetylates many Remodelers, affecting their function. For example, dGcn5 acetylates dISWI, which may contribute to dNURF function during metaphasic-chromosomal condensation (Ferreira et al. 2007b). Interestingly, yGcn5 may temporally assist and then deter interaction of the SWI/SNF-family member yRSC with nucleosomes; Gcn5 acetylation of H3K14 attracts a bromodomain residing in the yRsc4 subunit, whereas Gcn5 acetylation of Rsc4 itself causes that same bromodomain to bind the internal acetylation rather than H3K14ac, suggesting a model of autoinhibition (VanDemark et al. 2007). Similarly, yGcn5 acetylates the ATPase subunit of ySWI/SNF, ySnf2, at residues located between the AT-hook domains, facilitating an intramolecular interaction with the C-terminal bromodomain and regulating the dissociation of SWI/SNF from chromatin (Kim et al. 2010). Therefore, Gcn5 modulates ySWI/SNF occupancy on chromatin, facilitating retention via histone acetylation (see gene activation section below), and hindering it by ySnf2 subunit acetylation.
3.3.9.3 PARylation
Similar to acetylation, poly-ADP-ribosylation by PARP is a modification used to target particular Remodelers either to genes or to loci undergoing DNA repair (described later). The best characterized example involves dISWI, which can be PARylated, reducing its ATPase activity and its nucleosome binding affinity (Sala et al. 2008). dISWI and PARP therefore have antagonistic roles in regard to chromatin condensation. Finally, there are several instances of either ubiquitylation or sumoylation of Remodeler subunits, though their roles in remodeling have not been well defined (Wykoff and O’Shea 2005).
3.4 Remodeler Functions in Particular Chromosomal Processes
Many chromosomal processes are dynamic and complex and can involve several Remodelers acting sequentially or in concert. The logic of Remodeler choice relates to the needed task: an “assembly” Remodeler for chromatin organization, an “editing” Remodeler for specialization of specific chromatin regions, or an “access” Remodeler for DNA exposure. Below, the action of various Remodelers in selected chromosomal processes is described, including dosage compensation, chromatin domains, DNA replication, DNA repair and recombination, chromosome cohesion/segregation, and gene regulation.
3.4.1 Large Chromatin Domains, Insulators, and Boundaries
In addition to single nucleosomes and small regions, Remodelers can regulate large chromatin domains. A remarkable example of large-scale remodeling is provided by hSATB1 (Special AT-rich sequence Binding 1), a global nuclear organizer protein assembled in a cage-like network structure within the nucleus, that regulates gene expression by structuring higher-order chromatin organization into loop domains (Han et al. 2008). Hypothesized to affect gene expression by alteration of chromatin folding, aberrant expression of hSATB1 correlates with metastasis in various cancers, defining this regulator as a prognosis marker. Remarkably, hSATB1 targets hACF and hNuRD Remodelers to specific loci, mediating deacetylation of histones and nucleosome positioning over several kilobases (Yasui et al. 2002). Insulation describes the process that partitions chromosomes into distinct domains by forming chromatin loops bordered by boundary regions. Chromatin boundaries associate specific DNA sequences to insulator/boundary proteins, organizing the genome in limited functional units and facilitating independent gene regulation by insulation. The CTCF insulator is widely common in the human genome and its functional mechanism involves partner proteins cohesin and p68 (Parelho et al. 2008; Wendt et al. 2008; Yao et al. 2010). In addition, by interacting with hCTCF, the Remodeler hCHD8 localizes to many hCTCF-binding sites, and is necessary at the CTCF boundaries to perform enhancer-blocking function and to insulate the imprinted H19/Igf2 ICR locus (Ishihara et al. 2006). Remarkably, Drosophila Remodelers NURF and NuRD antagonistically regulate the homeotic genes by modulating the enhancer-blocking function of several locus-specific chromatin boundaries (Li et al. 2010). Insulation can be used in combination with Remodelers to drive cell differentiation or embryogenesis. For example, NURF complex collaborate with the histone methyltransferase hSET1 in regulating the USF-bound barrier insulator to prevent erythroid genes from being silenced by heterochromatin invasion during erythropoiesis (Li et al. 2011). Moreover, ISWI associates with the ArsI insulator protein, regulating aspects of sea urchin development by varying the extent of its interaction during embryogenesis (Yajima et al. 2012).
The CHD-family subtype dMi-2, but not dKismet (another CHD subtype), has the ability to alter chromosome structure by promoting local chromosome decondensation in vivo. In contrast to ISWI, dMi-2 does not regulate higher-order chromatin structure by altering histone H1 deposition, but by destabilizing the association of cohesin with interphase chromosomes (Fasulo et al. 2012). These results raise the interesting possibility that dMi-2 may regulate cellular differentiation by tuning cohesin activity and chromosome condensation.
3.4.2 Dosage Compensation
The clearest example of chromosome-wide regulation by a Remodeler involves the dosage compensation system in Drosophila, which equalizes the transcriptional output of X-linked genes in males and females. In Drosophila, dosage compensation is achieved by multiple factors that affect the male X compaction, resulting in approximately twofold upregulation of gene expression. The key characteristic of the X chromosome in males involves its hyperacetylation at H4K16 by dMOF, the HAT subunit of the Drosophila dosage compensation complex (DCC) [reviewed in Rea et al. (2007) and Gelbart et al. (2009)]. H4K16ac reduces chromatin compaction by inhibiting fiber formation and decreasing dISWI remodeling activity (Dorigo et al. 2003; Shogren-Knaak et al. 2006; Corona et al. 2002). Interestingly, when observing the polytene chromosomes of the salivary gland, mutations in iswi or nurf301 confer massive decondensation of the male X chromosome, moderate decompaction of mitotic chromosomes, and defects in the loading of histone H1 (Deuring et al. 2000; Badenhorst et al. 2002; Corona et al. 2007). Therefore, dISWI as a member of dNURF contributes globally to chromosome compaction, but is antagonized by acetylation by the DCC (as dISWI displays lower activity on acetylated nucleosomes), relieving compaction and promoting transcription (likely via enabling productive elongation through chromatin by RNAPII) (Larschan et al. 2011).
3.4.3 DNA Replication
The initiation of DNA replication at origins is regulated by chromatin, and its progression greatly challenges chromatin integrity, involving both DNA polymerase passage and the need to reassemble nucleosomes in the wake. The process of replication is controlled by the cell cycle, and defects or encountered obstacles activate checkpoints that can utilize chromatin in both in the processes of signaling and resolution. In yeast, access Remodelers, such as ySWI/SNF, are required to promote origin access and support DNA polymerase progression, as the firing of replication origins in vivo is inhibited by nucleosome positions (Simpson 1990; Flanagan and Peterson 1999).
Interestingly, assembly Remodelers are also involved in the timing of replication initiation and firing. In yeast, yIsw2 is accumulated at sites of active replication, helping the progression of the replication fork (Vincent et al. 2008). In higher cells, hSNF2H plays a major role in many replication contexts, tuned by its modular assembly with additional subunits, and its association with additional activities. For example, SNF2H alternatively associates with hACF1 to promote DNA replication through heterochromatin regions (Collins et al. 2002), or with hWSTF to target replication foci in heterochromatin (Poot et al. 2004), or with hTip5 to specifically promotes the late replication of inactive rRNA genes (Li et al. 2005). Notably, SNF2H in association with the histone deacetylases HDAC1/2 is needed for G1-specific chromatin remodeling and the initiation of DNA replication at the Epstein–Barr virus origin (Zhou et al. 2005). In a similar process, but with an opposite outcome, yChd1 cooperates with a histone methyltransferase to negatively regulate DNA replication (Biswas et al. 2008).
Regarding INO80-family Remodelers, work in yeast on the yINO80 have been particularly revealing: yIno80 associates with replication origins, actively contributes to normal S phase progression, improves fork stability and progression (via replisome stability), and both interacts with and migrates with PCNA during replication (Vincent et al. 2008; Shimada et al. 2008; Papamichos-Chronakis and Peterson 2008). Under stress conditions, yIno80 is essential for fork progression and associates with stalled replication forks (Shimada et al. 2008). Notably, INO80 has important though indirect functions in fork progression: while bound to autonomous replicating sequences (ARSs) during S phase, INO80 promotes PCNA ubiquitination and facilitates the recruitment of Rad18 and Rad51, proteins needed to process obstructed replication forks (Falbo et al. 2009). Moreover, RPA (Replication Protein A), which accumulates at stalled replication forks, interacts with both yIsw2 and yIno80, which may function to enable the stalled fork to back up and then reenter a productive phase.
3.4.4 Chromosome Cohesion and Segregation
Roles for Remodelers in various aspects of chromosome segregation are accumulating. Among the most studied aspect are roles in the loading of cohesin, which link sister chromatids together prior to anaphase. In humans, SNF2H mediates loading of cohesin on specifically modified chromatin in an ATPase-dependent manner (Hakimi et al. 2002). yRSC is also present at centromeres, participating in proper kinetochore function and chromosome segregation (Hsu et al. 2003; Huang and Laurent 2004). yRSC via its ATPase subunit Sth1p interacts directly with cohesin, promoting cohesin loading on chromosome arms and proper sister chromatid cohesion (Huang and Laurent 2004). Notably, the CHD-family Remodeler dMi-2 antagonizes cohesin association during interphase, primarily at genic regions (Fasulo et al. 2012).
Beyond cohesion, Remodelers have roles in centromere and spindle function. ISWI interacts with microtubule-associated proteins and is required to maintain spindle microtubules in anaphase and for proper chromosome segregation (Yokoyama et al. 2009). Curiously, ISWI performs this function in an ATPase-independent manner. Interestingly, hATRX (which deposits H3.3 at pericentric regions) requires global histone deacetylation at meiotic onset to bind to centromeric heterochromatin and promote a bipolar meiotic spindle and proper chromosome alignment (De La Fuente et al. 2004). Distinct from its role in replicative stress (see above) and DNA repair (see below), yINO80 and the Ies6 subunit prevent chromosome missegregation and increase in ploidy, by regulating the incorporation of H2AZ in pericentric chromatin (Chambers et al. 2012). In higher eukaryotes, loss of the YY1 protein, subunit of INO80, also leads to polyploidy and increase in aberrant chromosome structure (Wu et al. 2007b). In addition to INO80, yISW2 and yRSC are involved in the histone turnover regulation at pericentric chromatin, contributing to the maintenance of kinetochore organization (Verdaasdonk et al. 2012). Thus, the regulation of pericentric chromatin by Remodelers appears increasingly critical for proper centromere and kinetochore organization.
3.4.5 DNA Repair and Recombination
Breaks in DNA are relatively common and threaten genome integrity. Chromatin dynamics are involved in many aspects of their repair, including access to the break, cessation of local transcription, maintaining the association of DNA ends, recruitment of repair proteins, homolog pairing, coordinating repair pathways in a chromatin context, and restoring the initial chromatin landscape following the repair. Two alternative pathways can be followed to repair double-strand breaks (DSBs): homologous recombination (HR) or nonhomologous end-joining (NHEJ), and both involve Remodelers. However, repair by HR involves a homology search and pairing requiring the removal and/or modification of chromatin along long stretches of DNA, and therefore considerable chromatin remodeling.
One of the earliest chromatin responses to repair is the phosphorylation of H2AX by checkpoint kinases (at S139 in vertebrates, or H2A at S129 in yeast), referred as γH2AX. This phosphorylation occurs in a broad region around the site of damage and contributes to the recruitment of various repair factors and Remodelers. Damage recognition is most closely associated with INO80-family Remodelers. In yeast, the interaction of yINO80 Remodeler to γH2A involves the subunits Nhp10, Ies3, and Arp4 (Morrison et al. 2004; Downs et al. 2004). In contrast, the recruitment of INO80 to DSBs in mammals appears independent of γH2AX but requires ARP8 (Kashiwaba et al. 2010). Moreover, an additional ARP subunit, ARP5, seems to interact with γH2AX, facilitating its initial phosphorylation and spreading (Kitayama et al. 2009; Kandasamy et al. 2009). Interestingly, INO80 is retained at DSBs after H2A phosphorylation decreases, suggesting additional protein interactions contributing to its retention. INO80 activity may help exposing DNA for 5′–3′ resection, as its deletion fails to generate 3′ single-strand DNA overhang (van Attikum et al. 2004). In addition, INO80 facilitates H2AX phosphorylation and contributes to DNA damage checkpoints toward overcoming cell cycle arrest (Papamichos-Chronakis et al. 2006). Finally, in mouse, INO80 interacts with the transcription factor YY1 (Yin Yang-1), essential for development and DNA repair by HR (Wu et al. 2007b).
The recruitment of ySWR1 to DSBs also involves γH2AX and is necessary for yKu80 loading at DSBs and error-free NHEJ (van Attikum et al. 2007). While yINO80 removes both γH2A and H2AZ nucleosomes near the DSBs (Papamichos-Chronakis et al. 2006; van Attikum et al. 2007), SWR1 performs the opposite activity: depositing H2AZ at DSBs (Xu et al. 2012). H2AZ deposition, and therefore SWR1 activity, is critical to generate an open chromatin conformation, to restrict single-stranded DNA production, and to load RPA and Ku70/Ku80 proteins (Xu et al. 2012). Remarkably, the ortholog of SWR1 in Drosophila, dTip60, integrates within the complex a histone acetyltransferase dTip60 along with Domino ATPase, providing an additional layer of regulation (Kusch et al. 2004). Interestingly, dTip60 acetylates γH2Av prior to its removal by Domino. Although these HAT and ATPase activities are combined in Drosophila and human, they are found in separate complexes in yeast, though the regulatory principle is preserved: acetylation of H4 or H2A N-terminal lysine residues by the yeast HAT yNuA4 can independently promote the incorporation of H2AZ in chromatin by the ySWR1 Remodeler, in a manner dependent on the bromodomain subunit Brf1 (Altaf et al. 2010). Finally, yINO80 contributes to telomere length restriction by interacting with multiple telomerase components and helping regulate the recombination-dependent maintenance of telomere structures (Yu et al. 2007).
Both ySWI/SNF and yRSC Remodelers contribute to HR, with ySWI/SNF involved in the early steps before the strand invasion, and yRSC assisting later for completion (Chai et al. 2005). In keeping with its known roles in chromatin access, yRSC facilitates nucleosome eviction and yKy70 binding and assists error-free NHEJ (Shim et al. 2007). Interestingly, invasion of resected DNA strand during mating type switching requires SWI/SNF to evict the heterochromatin factor Sir3 from donor sequences (Sinha et al. 2009). Moreover, a cooperative chromatin activation loop has been proposed, involving self-reinforcing steps, to propagate chromatin access: phosphorylation of H2AX recruits GCN5, which acetylates the adjacent H3, and is then recognized by the bromodomain in BRG1 (Lee et al. 2010). Additional connections of SWI/SNF to repair include the association of ySWI/SNF with the proteins that recognize damage (Rad23–Rad4), and a clear deficiency of repair in swi/snf mutants (Gong et al. 2006), a result that has been extended to human cell lines.
Roles for ISWI-family Remodelers include hACF1, which interacts with hKu70, and accumulates at sites of DNA damage, with SNF2H ATPase activity required for efficient DSB repair (Lan et al. 2010). The accumulation of hACF1 at DNA damage sites in the early steps is related to the G2/M checkpoint, prior to γH2AX accumulation (Sanchez-Molina et al. 2011). Notably, the frequency of HR and NHEJ induced by DSBs is decreased in cells lacking hACF1, SNF2H, or CHRAC15/17, pointing toward a specific requirement of CHRAC complex—and not just ACF—in DSBs repair (Lan et al. 2010).
Certain Remodelers, especially those in the CHD family, appear to rely on PAR for recruitment to DNA damages sites, including ALC1 and NuRD. ALC1 is recruited to DNA damage sites and activated via its macrodomain, which binds a PARP1-nucleosome intermediate, and its remodeling activity is stimulated by PARylation (Ahel et al. 2009; Gottschalk et al. 2009, 2012). ALC1 is likely to prime DNA breaks for NHEJ by repositioning nucleosomes and interacting with NHEJ proteins. In addition, CHD4–NuRD is also recruited to DSB in a PARP-dependent manner, promoting transcription repression at DNA damage sites (Polo et al. 2010; Chou et al. 2010; Larsen et al. 2010). Notably, PAR can be also used to recruit Remodelers in the context of transcription activation (see below). PARylation might be therefore broadly used to rapidly attract factors required for quick and efficient transcriptional response to critical situations, such as DNA damage (above) or heat-shock stress (see later).
Notably, Fun30 and SMARCAD1 are essential for DNA break repair by promoting DNA end resection (Chen et al. 2012; Costelloe et al. 2012; Eapen et al. 2012). Fun30 promotes long-range DNA end resection and checkpoint adaptation through removal of Rad9 (Chen et al. 2012), and SMARCAD1 knockdowns results in HR and RPA foci formation defects (Costelloe et al. 2012). Remarkably, in order to facilitate repair in heterochromatin regions, DNA accessibility is increased by loss of CHD3–NuRD upon KAP-1 phosphorylation (Goodarzi et al. 2011). Roles for CHD2 are emerging, as mutant mice exhibit a defective clearance of γH2AX foci and aberrant DNA damage response after X-ray irradiation, suggesting that CHD2 plays a direct role in either the repair of DNA-strand breaks or the attenuation of the γH2AX signal after repair (Nagarajan et al. 2009). Following repair, the process restoring the chromatin landscape to its original status remains unknown. Finally, roles for the “orphan” Remodeler CSB in transcription-coupled repair are deferred to the section on disease syndromes (below).
3.4.6 Promoter Architectures and Transitions
Although roles for chromatin and Remodelers of chromatin at genes can be very complex, a significant portion conforms to a general logic—developed in the sections above—that Remodelers mediate the occupancy and positioning of nucleosomes that affects the exposure of important cis-controlling sites on the DNA. Here, an important consideration is the initial status of the promoter, whether it is generally “open” (lacking nucleosomes) or “closed,” bearing nucleosome which cover important sites (Cairns 2009).
3.4.6.1 Open Promoters
Open promoters are formed through a combination of DNA sequence features, histone variants, and the action of Remodelers (Fig. 3.3). DNA sequences such as AT-rich tracts disfavor nucleosomes (Segal et al. 2006), cis-sites embedded in and around those AT-tracts can (indirectly) attract SWR1-type Remodelers to create H2A.Z-containing nucleosomes at and adjacent to the AT-tracts, creating a situation of nucleosome instability, and lower occupancy. Furthermore, Remodelers can act to eject remaining nucleosomes, creating a clear nucleosome-depleted region (NDR) of ~100–200 bp located upstream of the TSS, flanked by H2A.Z variant nucleosomes that are well phased/positioned (Yuan et al. 2005). Many NDRs also contain binding sites for transcription factors, which are thus exposed, and a significant subset (in higher cells) may also have preloaded RNAPII. However, these genes (and the resident RNAPII) are not necessarily active, as proximal or distal enhancers can be wrapped in nucleosomes and require remodeling for exposure, or other types of chromatin modifications may exist that prevent RNAPII initiation or elongation. Thus, gene promoters can be “poised” for expression, but not currently active. The transition to the active state is accompanied by increased histone acetylation and other modifications, additional nucleosome loss around the TSS and enhancer regions (exposing additional cis-controlling elements), and significant nucleosome movements both in the promoter and coding regions.
3.4.6.2 Closed Promoters
Closed promoters are those that in the repressed state lack an NDR or associated RNAPII, due largely to the presence of competing nucleosomes. These promoters typically lack AT-rich sequences, bear lower levels of H2A.Z-containing nucleosomes and often utilize assembly Remodelers to help establish a repressed architecture characterized by relatively stable nucleosomes. To activate these promoters, nucleosomes must be either moved or evicted to expose promoter cis-controlling sites. However, due to the properties of their initial architecture, closed promoters require and experience more remodeling events and chromatin transitions before RNAPII association and activity are enabled. Therefore, closed promoters are more reliant on the action of access Remodelers and also chromatin-modifying complexes (especially HATs, which assist Remodeler function), than are open promoters. In one scenario, a “pioneer” activator (Act#1, Fig. 3.8b) may bind an open site and recruit both histone modifiers and an “access” Remodeler, which evicts flanking nucleosomes to enable binding of additional activators (Act#2, Fig. 3.8b).
Promoter architectures and nucleosome occupancy. Many genes in yeast conform to one of two promoter architectures, “open” or “closed,” which refer to the extent that nucleosomes occupy the proximal promoter in the repressed state. (a) Open promoters have a depleted proximal nucleosome adjacent to the transcription start site (TSS, black arrow), a feature common at constitutive genes. (b) Covered promoters have a nucleosome adjacent to the TSS in their repressed state, a feature common at highly regulated genes. The figure depicts the two contrasting types, but most yeast genes blend the features shown to provide appropriate regulation. Gray nucleosomes contain canonical H2A, whereas blue nucleosomes bear H2A.Z. Binding sites (green DNA) for transcriptional activators (ACT) are shown. These sites are largely exposed for open promoters and largely blocked by nucleosomes at covered promoters. Covered promoters typically have nucleosome positioning sequence elements of varying strength and locations and lack “phasing” in the population. In contrast, at open promoters the nucleosomes flanking the NDR (nucleosome-depleted region) termed “−1” and “+1”, are positionally defined in the population, especially the “+1” nucleosome
An interesting issue, recently addressed, is how Remodelers might be used to define the position of the “+1” and “−1” nucleosomes. Here, transcription factors (i.e., ACT, Fig. 3.8a) present in the NDR may serve as “boundary” elements for ISWI-family Remodelers, which will then place these promoter nucleosomes a fixed distance from the binding site (Yen et al. 2012). An important concept is the apparent antagonism between Remodelers that organize chromatin, and those that disorganize/eject nucleosomes (especially at closed promoters) setting up a dynamic flux of assembly/disassembly (Lorch et al. 2006). Below, we provide a set of selected results that illustrate this interplay during gene repression and activation.
3.4.7 Gene Repression
Globally, assembly Remodelers can help impose gene repression by building nucleosomal arrays that impair access of DNA-binding factors, promote the association of linker histones (see sections on chromatin assembly, above), and by attracting additional chromatin modifier complexes involved in repression. However, it is a misconception that silent chromatin is static; rather, it is occupied by dynamic Remodelers that interact with DNA-bound repressors and histone modifiers to consistently reinforce the repressed state. Early examples came from yeast, including the use of the assembly Remodeler yISW2. yISW2 can slide nucleosomes over important promoter elements, enforcing transcriptional repression (Whitehouse and Tsukiyama 2006), interfering with TBP binding (Alen et al. 2002; Moreau et al. 2003), prevent cryptic antisense transcription from intergenic regions (Whitehouse et al. 2007), in part by limiting the size of the NDRs (Yadon et al. 2010). Support in Drosophila includes work on polytene chromosomes, which reveal ISWI generally colocalized with repressed loci. In regard to targeting, the DNA-binding repressor yUme6, along with the corepressors and Ssn6-Tup1, recruit yISW2 Remodeler to various promoters, repressing transcription (Goldmark et al. 2000). Analagous examples of DNA-binding factors recruiting ISWI Remodelers abound. Beyond ISWI, there are analogous roles for CHD-family Remodelers in repression, and also instances where the action of SWI/SNF Remodelers is used to expose sites for DNA-binding repressors.
Earlier was detailed how a lack of histone H4 acetylation positively regulates ISWI Remodelers. Indeed, a consistent theme in gene repression the collaboration of histone deacetylases (HDACs) with assembly Remodelers to implement transcriptional repression (Burgio et al. 2008). In budding yeast, the association of HDACs with Ssn6-Tup1 and yISW2 Remodelers explains a portion of its repression activity. In S. pombe, the Remodeler SHREC also combines Remodeler ATPase and HDAC activities in one complex and is critical for the assembly of silent pericentromeric heterochromatin (Sugiyama et al. 2007). Moreover, SHREC cooperates with HP1 proteins Chp2 and Swi6 (which bind to H3K9me) as well as the histone chaperone Asf1, to promote histone deacetylation and nucleosome occupancy, preventing NDRs at heterochromatin regions (Yamane et al. 2011). In keeping, Mi-2/NuRD-type Remodelers in higher cells involve the embedding of HDACs within Remodeler complexes, and further include the embedding of DNA methyl-binding proteins (MBDs), supporting a coordinated role in silencing DNA-methylated regions. Thus, Remodeler evolution often involves the addition of coordinated modules to the Remodeler, enabling complex functions, such as gene repression.
Remodelers specialized for repression can be recruited by DNA-binding factors. For example, Mi-2 containing Remodelers are recruited by SUMOylated transcription factors, resulting in SUMO-dependent transcriptional repression (Ivanov et al. 2007; Stielow et al. 2008; Schultz et al. 2001; Siatecka et al. 2007; Reddy et al. 2010). hCHD8 and dKismet (its Drosophila ortholog) are recruited by β-catenin and negatively regulate β-catenin target gene expression, antagonizing the Wnt-β-catenin signaling pathway and recruiting linker histone H1 (Thompson et al. 2008; Nishiyama et al. 2012). Roles for ncRNAs in recruiting Remodelers for repression are emerging. For example, in A. thaliana, SWI/SNF helps confer RNA-mediated transcriptional silencing (Zhu et al. 2013). In this situation, SWI/SNF is recruited via its SWI3B subunit which interacts indirectly with RNAPV-produced lncRNAs. Here, SWI/SNF contributes to gene silencing by repositioning nucleosomes and facilitating DNA methylation.
3.4.8 Transcription Initiation
Sections above detailed, in a conceptual manner, how access Remodelers can help DNA-binding factors access their sites. From a chromatin perspective, the process of transcriptional initiation at RNAPII genes involves access and editing Remodelers (assisted by modifiers) enabling the ordered access of factors to enhancers and promoters, culminating in RNAPII activity. Notably, virtually all enhancers and promoters are occupied by multiple Remodelers, preventing a comprehensive description of remodeling in transcription initiation. Instead, we provide examples that support a key principle: the Remodeler tasks needed for RNAPII occupancy and initiation are logically those needed to reverse the initial blocked, repressed state.
3.4.8.1 SWI/SNF Remodelers
Early genetic work in yeast revealed that components of SWI/SNF complex were needed for the activation of many genes, and that SWI/SNF antagonized repression by histones (Winston and Carlson 1992). Furthermore, promoter chromatin transitions by Remodelers can occur independently of basal transcription factors (TBP) or RNAPII and can therefore precede transcription (Schmid et al. 1992; Hirschhorn et al. 1992; Yudkovsky et al. 1999). Influential work in Drosophila supported this work, showing SWI/SNF-family Remodelers present with RNAPII at many active genes, and providing evidence that active SWI/SNF is needed for RNAPII to occupy genes (Armstrong et al. 2002). However, the timing of SWI/SNF recruitment can vary and depends on the initial repression architecture: at the yeast HO promoter, SWI/SNF is recruited early by the initial DNA-binding protein (ySwi5) and is needed for early chromatin transitions, whereas SWI/SNF is one of the last factors to occupy the IFN-β promoter, which bears a large enhanceosome prior to SWI/SNF engagement (Cosma et al. 1999). Although SWI/SNF Remodelers contain bromodomains, targeting by an activator appears important for their recruitment. Among the dozens of examples in yeast and humans, some of the best studied are ySWI/SNF interaction with ySwi5 or yGcn4p (Cosma et al. 1999; Natarajan et al. 1999), and hSWI/SNF with HSF1 or the glucocorticoid receptor (GR) (Kwon et al. 1994; Hsiao et al. 2003). Interestingly, GR is displaced by hSWI/SNF in a periodic and cyclical manner (Nagaich et al. 2004). An emerging theme involves the use of specialized cell-type-specific Remodeler components for “tethering” the Remodeler to cell type-specific DNA-binding proteins and/or chromatin modifications. For example, the SWI/SNF subtype PBAF, but not BAF, interacts with and promotes transcriptional activation by particular nuclear receptors (Lemon et al. 2001). Furthermore, SWI/SNF complexes containing BAF200 (a specific targeting subunit containing an ARID domain) regulate the expression of particular interferon-responsive genes (Yan et al. 2005; Gao et al. 2008). Additional examples of specialized “tethering” proteins involving other Remodeler families are provided in later sections.
Notably, work in yeast has revealed the recruitment of SWI/SNF-family Remodelers to NDRs by specific DNA binding proteins, which helps reinforce the depth of nucleosome loss at the NDR (Raisner et al. 2005). Corepressors and coactivators can also be involved in SWI/SNF recruitment (Dimova et al. 1999). A large literature supports the recruitment of hSWI/SNF to cell-type-specific gene enhancers and promoters, typically by interaction with a specific master regulator (see later sections). Following targeting by a transcription factor, ySWI/SNF can be stabilized at locations by its bromodomain, which may help anchor the Remodeler to acetylated nucleosomes (Hassan et al. 2002). Combined with information in previous sections, SWI/SNF-family Remodelers use nucleosome sliding and eviction, likely in combination with histone acetylation and variants, to provide access to DNA-binding proteins.
3.4.8.2 ISWI Remodelers
The NURF-subtype of ISWI-family Remodelers utilizes their specific large subunit (dNURF301 in Drosophila, BPTF in humans) to effectively adapt an ISWI Remodeler into an access Remodeler by affecting promoter chromatin. Interestingly, dNURF301 interacts with many sequence-specific transcriptional regulators, including dGAGA, dHSF, the ecdysone receptor, and the dKen repressor (Xiao et al. 2001; Badenhorst et al. 2002; Kwon et al. 2008). In addition, dNURF interacts with a TATA-binding protein homolog, dTrf2, to activate gene expression (Hochheimer et al. 2002). Yeast utilizes particular ISWI subtypes, with yISW1b best linked to activation, contributing to promoter clearance and activation at particular promoters (Morillon et al. 2003). Therefore, although ISWI ATPases intrinsically space and organize nucleosomes, accessory proteins can subvert that function for other purposes.
3.4.8.3 CHD Remodelers
As emphasized throughout, CHD Remodelers are diverse in functions. Early studies in S. pombe had also shown that nucleosome eviction at promoters during activation involves CHD Remodelers Hrp1 and Hrp3 (Walfridsson et al. 2007). In keeping, yChd1 performs the selective removal of promoter nucleosome in an activator-dependent manner (Ehrensberger and Kornberg 2011). Notably, dKismet also contributes to activation just before the transition to elongation (Srinivasan et al. 2005). In humans, CHD7 cooperates with SWI/SNF Remodelers to activate genes in the neural crest lineage (Bajpai et al. 2010). Interestingly, CHD7 colocalizes strongly with H3K4me patterns indicative of enhancers (Schnetz et al. 2009). Furthermore, CHD8 appears to differentially regulate target genes: repressing β-catenin target genes (Thompson et al. 2008) and the HOXA2 gene (Yates et al. 2010), while activating androgen-responsive transcription (Menon et al. 2010). Finally, Remodelers can be recruited simultaneously and acts antagonistically to tune gene activation, as illustrated by SWI/SNF and Mi-2β at the lipopolysaccharide-stimulated genes in macrophages (Ramirez-Carrozzi et al. 2006). Other examples of simultaneous antagonistic actions of Remodelers are provided in sections below.
3.4.8.4 INO80 Remodelers
Early work in yeast revealed the INO80 factor as needed for full transcription of inositol-regulated genes, and cooperativity with ySWI/SNF complex (Ford et al. 2008). The transcription factor YY1, master regulator of development, has been described as a subunit of hINO80 Remodeler, gaining access to its binding sites in close proximity of the TSS, and activating transcription (Cai et al. 2007; Wu et al. 2007b). Extension of this work in the fission yeast, S. pombe, shows the presence of INO80 at genes involved in phosphate and adenine metabolism where it functions to evict promoter nucleosomes (Hogan et al. 2010).
3.4.9 Transcription Elongation
Chromatin within gene coding regions must accommodate RNAPII progression, which involves the chaperoning of nucleosomes around the advancing polymerase, and the replacement of evicted nucleosomes—which is needed to prevent promiscuous initiation from occurring within genes. Current evidence supports the widespread use of both assembly and access Remodelers within coding regions to perform these two functions. First, prior to transcription, coding regions display nucleosome spacing and phasing, with the +1 nucleosome acting as a type of boundary element to set the phase; an effect most evident in yeast, which requires the combined action of multiple Isw1 and/or Chd1 Remodelers (Gkikopoulos et al. 2011a; Pointner et al. 2012). Transcription through chromatin may be assisted by SWI/SNF-family Remodelers, with support in both yeast (Schwabish and Struhl 2004; Carey et al. 2006) and higher systems (Brown et al. 1996). This assistance may occur right at the transition from initiation to elongation, or at later steps along the coding region. Notably, hSWI/SNF has been shown to promote tat-dependent elongation of the HIV promoter (Treand et al. 2006).
Transcription elongation also involves the use of a factors (FACT, Spt6, others), which act as nucleosome chaperones to help pass histone octamers around RNAPII and promote their reassociation with the DNA to reform nucleosomes. Once deposited and matured, a clear concept is the use of assembly Remodelers to help reestablish an organized nucleosomal state in the wake of RNAPII action. Remarkably, the ISWI-family Remodeler yISW1b is targeted to nucleosomes harboring H3K36 methylation by the PWWP domain of the Ioc4 subunit (Maltby et al. 2012; Smolle et al. 2012), a mark added by a histone methyltransferase (Set2 in yeast), which travels with elongating RNAPII. In this context, yISW1b acts together with yCHD1 to maintain chromatin integrity during transcription elongation by preventing transhistone exchange (Smolle et al. 2012). Consistently, yISW1 has been shown to have a genome-wide function in transcription elongation by localizing at mid-coding sequence nucleosomes and its deletion result in initiation from cryptic intragenic promoters (Tirosh et al. 2010).
CHD-family Remodelers are involved in transcription elongation as yChd1 interacts physically and functionally with elongation factors (Simic et al. 2003), and all dCHD Remodelers colocalize with active sites of transcription (Marfella and Imbalzano 2007; Murawska et al. 2008), with dCHD1 localization mirroring the elongating RNAPII (Srinivasan et al. 2005). In higher eukaryotes, the nucleosomes of the coding region contain the H3.3 variant in a replication-independent manner (Ahmad and Henikoff 2002), possibly deposited by CHD1 in the wake of the RNAPII progression. Both in yeast and Drosophila, CHD1 regulates the replication-independent turnover of H3 and, while it promotes histone exchange at the 5′ of the genes, it prevents exchange at the 3′ ends of genes with length dependence (Radman-Livaja et al. 2012). Moreover, CHD1 modulates transcription termination (Alen et al. 2002; Walfridsson et al. 2007).
Surprisingly, and in contrast to its usual role in repression, the CHD-family Remodeler dMi-2 associates with active heat-shock (HS) genes, contributing to efficient gene transcription and RNA processing. Interestingly, the recruitment of dMi-2 involves a two-step process, an initial rapid recruitment by direct binding of dMi-2 to PAR, a cell response to stresses at specific chromatin regions, followed by an intriguing binding to nascent RNA transcripts (Murawska et al. 2011). Stress-induced PARylation might be broadly used to rapidly attract factors required for quick and efficient transcriptional response. Interestingly, ChIP-Seq reveals that dMi-2 binds with the body of the active HS genes, closely following nascent RNA synthesis, suggesting that transcription itself is a major determinant of the recruitment of Mi-2 Remodeler (Mathieu et al. 2012).
dKismet facilitates early elongation by RNAPII, by acting downstream of P-TEFb recruitment (Srinivasan et al. 2008). Surprisingly, the histone methyltransferases ASH1 and TRX involved in the elongation process require dKismet for their binding, while dKismet itself does not bind to the methylation marks deposited by these enzymes. Interestingly, it is proposed that dKismet counteracts Polycomb group repression and antagonizes H3K27 methylation indirectly by recruiting of ASH1 and TRX to chromatin (Srinivasan et al. 2008). Interestingly, CHD8 appears to interact with the elongating form of RNAPII, regulating the cyclin E2 gene (Rodriguez-Paredes et al. 2009).
3.4.10 Chromatin Remodeling in RNAPI and RNAPIII Regulation
RNA polymerase I (RNAPI) transcribes rDNA genes; and mammalian rDNA repeats utilize a particular Remodeler in a remarkable manner for rDNA repression. The transcription factor TTF-I has a binding site in the spacer region between rDNA repeats and recruits the “orphan” NoRC complex to RNAPI promoters. This imposes rDNA silencing by relocating the promoter-bound nucleosome to a position unfavorable for transcription and also attracts HDAC and DNA methyltransferase activity in an H4K16Ac-dependent manner (Strohner et al. 2004; Zhou and Grummt 2005; Li et al. 2006). Beyond its repressive role with NoRC, TTF-I plays also a key role in establishing chromatin features resulting in active rDNA genes by interacting with CSB (Cockayne Syndrome protein B) that recruits the histone methyltransferase G9a and promotes transcription elongation (Yuan et al. 2007). Moreover, B-WICH Remodeler attracts HAT at active rDNA promoters and is proposed to counteract NoRC to drive rDNA transcription (Percipalle et al. 2006; Vintermist et al. 2011). Finally, a fourth Remodeler also regulates rDNA genes: NuRD. NuRD establishes a specific chromatin landscape at rDNA genes to help poise them for transcription activation, involving unmethylated promoter DNA, association with components of the preinitiation complex, bivalent histone modifications, and collaboration with NoRC to establish the positioned promoter-bound nucleosome that is unfavorable for transcription (Xie et al. 2012). Ultimately, CSB is responsible for enabling transcription by resetting the promoter-bound nucleosome position.
RNA polymerase III (RNAPIII) transcribes small ncRNAs, related to protein synthesis (tRNAs) and other functions. Work in yeast has shown that the SWI/SNF-family Remodeler RSC plays a global role in the removal of nucleosomes from RNAPIII genes (such as tRNAs) (Parnell et al. 2008) and is recruited to RNAPIII loci by subunits of the basal transcription system (Soutourina et al. 2006). Moreover, B-WICH Remodeler is involved in the regulation of 5S rRNA/7SL transcription by RNAPIII (Cavellan et al. 2006). In humans, overlap between RNAPIII genes and Remodelers has been detected by genomics approaches, but the roles of Remodelers at those loci remain to be determined.
3.5 Remodelers in Pluripotency, Development, and Differentiation
Spatial and temporal regulation of gene expression is decisive for development, differentiation, and organogenesis. This requires cross-talk between signaling pathways, transcriptional machinery, and chromatin regulators. Here, knockout studies in mice support important roles for Remodelers in many developmental processes, and many reviews address their diverse roles in flies and mice (Simon and Tamkun 2002; Ho and Crabtree 2010). Briefly, vertebrates require at least one member of each Remodeler family for organism viability, and further rely on subtypes for proper differentiation of most, if not all tissues—consistent with the construction of specialized Remodelers to conduct cell-type-specific transcriptional programs. Here, we provide selected recent advances, with an emphasis on mouse and human systems, and reference earlier work in model organisms for conceptual precedent.
3.5.1 Remodeler Involvement in Stem Cell Circuitry and Pluripotency
Remodelers impact developmental capacity at multiple levels, including self-renewal and pluripotency of stem cells. Remarkably, pluripotent embryonic stem (ES) cells assemble and utilize a specific SWI/SNF-family Remodeler, termed esBAF, that is distinguishable from the typical BAF complex present in differentiated cells; it contains Brg1, BAF155, and BAF60a, and lacks the corresponding paralogs Brm, BAF170, and BAF60c subunits (Ho et al. 2009a, b). Regarding function, shRNA-mediated depletion of Brg in ES cells (and analogous experiments with the BAF250 component) triggers differentiation, indicating a role for maintaining pluripotency and self-renewal, rather than a role in differentiation (Gao et al. 2008; Yan et al. 2008). Interestingly, genome-wide binding sites of Brg1 in ES cells overlap with those of many critical regulators of pluripotency: Oct4, Sox2, Nanog, Smad1, and STAT3, supporting the notion that esBAF assists in regulating pluripotency and self-renewal circuitry (Ho et al. 2009a). A role for SWI/SNF/BAF complexes in pluripotency is not limited to ES cells, as the maintenance of hematopoietic stem cells requires BAF53a subunit of BAF, an actin-related protein (Krasteva et al. 2012). Notably, Brg1-containing Remodelers act very early to promote transcription, as maternal-effect mutations in the mouse greatly inhibit zygotic genome activation at the two-cell stage (Bultman et al. 2006).
Roles for CHD-family proteins in ES cells include CHD1, which helps maintain open chromatin and pluripotency, helps regulate ES cell self-renewal, and is required for somatic cell reprogramming to the pluripotent state (Gaspar-Maia et al. 2009). CHD7 targets active gene enhancer elements and has an antagonistic role to cobinders p300, Oct4, Sox2, and Nanog, fine-tuning the expression levels of ES cell-specific genes (Schnetz et al. 2010). Furthermore, CHD9 has roles in osteogenic cells and their differentiation (Shur et al. 2006). However, much attention has been focused recently on the Mi-2/NuRD Remodeler (CHD3/4), and its functional interactions with esBAF Remodeler, to address a key issue: how ES cells decide between self-renewal and differentiation (Yildirim et al. 2011). First, NuRD directly attenuates expression of the key genes involved in pluripotency and self-renewal, and creating transcriptional heterogeneity in the population, facilitating the ability for a subset of cells—those with the lowest levels of self-renewal factors—to enter into differentiation. Notably, Mi-2/NuRD works antagonistically to esBAF, which is targeted by Stat3 to the same key self-renewal genes to promote their activation. Furthermore, NuRD helps reinforce downregulation of self-renewal and pluripotency factors as cells commit to differentiation (Reynolds et al. 2012). Moreover, NuRD can associate with LSD1 at active ES cell enhancers and acts to decommission them via H3K4me1 removal, leading to additional downregulation and promotion of differentiation (Whyte et al. 2012). Part of Mi-2/NuRD targeting may involve the MBD3 component, which can bind hydroxymethylated cytosine (5hmC) (Yildirim et al. 2011). These molecular results support earlier genetic results showing that ES cells lacking MBD3–NuRD display more persistent self-renewal (Kaji et al. 2006). Overall, the antagonistic action of Mi-2/NuRD and esBAF can be influenced by signaling pathways, which then tilt the balance between self-renewal and differentiation. Finally, beyond ES cells, Mi-2β/NuRD regulates key self-renewal genes in hematopoietic stem cells and is needed for lineage priming (Yoshida et al. 2008).
For the INO80 family, Tip60-p400, which combines H2AZ or H2AX deposition activity and histone acetylation activity, is necessary to maintain ES cell identity and surprisingly represses transcription of genes induced during development by promoting H4 acetylation at both active and silent target promoters (Fazzio et al. 2008). Roles for ISWI-family Remodelers are clearest in Drosophila, where ACF1 is strongly decreased during embryonic development but persists at high levels specifically in undifferentiated cells, including the germ cell precursors and larval neuroblasts (Chioda et al. 2010). Moreover, dACF1 is required for the faithful establishment of diversified chromatin structures, such as heterochromatin. Also, studies of NURF301 in Drosophila testis revealed that NURF is specifically required for the maintenance of germline stem cells by preventing their premature entry into differentiation, via a positive regulation of the JAK-STAT pathway (Cherry and Matunis 2010). In contrast, ISWI is dispensable in follicle stem cells, while the INO80 ATPase Domino promotes follicle stem cell self-renewal (Xi and Xie 2005).
3.5.2 Building Lineage-Specific Remodelers for Differentiation and Commitment
Beyond regulating pluripotency and self-renewal, Remodelers actively determine cell fate, promote differentiation, and maintain lineage commitment. To mirror the diversity of cell lineages, a repertoire of specialized lineage-specific Remodelers is constructed by combinatorial and modular assembly, either by using paralog subunits and/or excluding subunits/modules. These new compositions create Remodeler subtypes that likely influence their interactions with master transcription factors and/or particular chromatin structures, which affect transcription programs that drive differentiation. Notable examples illustrating these concepts are presented below.
Specialized SWI/SNF/BAF-family Remodelers are intimately involved in neurogenesis. First, the transition from neural progenitors to postmitotic neurons is accompanied by a switch in BAF subunit composition: BAF45a and BAF53a are exchanged for BAF45b and BAF53b. Of particular importance, genetic experiments demonstrate their necessity and sufficiency for this developmental transition (Lessard et al. 2007) (Fig. 3.9). The mechanism underlying the switch in subunit composition is remarkable, involving the use of both microRNAs and neural corepressors: during the switch, the REST corepressor no longer occupies the microRNA loci, allowing miR-9/9* and miR-124 expression—which then attenuate BAF53a—leading to cell cycle exit, the activation of BAF53b and neural differentiation (Yoo et al. 2009) (Fig. 3.9). Interestingly, BAF53b is also essential for neuron dendritic pattern formation (Wu et al. 2007a). Changes in BAF subunit composition include neuron-specific isoforms of BAF57 to create alternative SWI/SNF subtypes that regulate neurogenesis (Kazantseva et al. 2009). The action of SWI/SNF in neuronal differentiation is likely antagonized by dMi2, which cooperates with the transcriptional repressor Tamtrack69 to suppress neuronal cell fate during early development (Murawsky et al. 2001; Yamasaki and Nishida 2006). Remarkably, CHD5, found in a NuRD-like complex and expressed exclusively in the brain, directly represses BAF45b and BAF53b (Potts et al. 2011), and is thus also involved in the regulation of the shift from neural progenitors to neurons. Furthermore, BAP55, a subunit shared by BAP, PBAP, and Tip60 Remodelers, and a homolog to human BAF53a,b, plays a specific role within Tip60 complex to regulate dendrite targeting in olfactory projection neurons (Tea and Luo 2011). Taken together, neurogenesis provides several examples of Remodeler compositions driving differentiation.
Neuronal differentiation orchestrated by a switch in hBAF Remodeler composition mediated by microRNA regulation (Adapted from Lessard et al., Annu. Rev. Cell Dev. Biol., 2010). During migration from the subventricular zone of the spinal cord to the postmitotic zone, neural progenitors differentiate into neurons. This process is orchestrated by a switch in BAF composition from BAF45a and BAF53a in neural progenitors, to BAF45b, BAF45c, and BAF53b in postmitotic neurons. In neural progenitors (left panel), the expression of the neuron-restrictive silencer factor (NRSF/REST) inhibits microRNA miR-9* and miR-124 expression, which in turn allow BAF53a expression and cellular proliferation. Moreover, the expression of BAF53a inhibits the one of BAF53b. In contrast, in differentiated neurons (right panel), NRSF/RSF is inactive, leading to the expression of the microRNA, which in turn repress BAF53a expression, allowing cell cycle exit. Moreover, BAF53a inhibition activates BAF53b expression, leading to dendritic morphogenesis. Text and arrows depicted in green and bold refer to factors that are active/activated/expressed, while depiction in red refers to factors that are inactive/inhibited/repressed
Roles for specialized SWI/SNF and CHD Remodelers in muscle differentiation abound. MyoD is a master regulator of muscle cell fate, which relies on SWI/SNF complex for activation at many target loci and for differentiation of nonmyogenic cells into skeletal muscle cells (de la Serna et al. 2001). MyoD binding with the myogenic locus occurs in two steps: first indirectly via tethering by Pbx1, and then directly to its cognate site following recruitment of, and remodeling by, hSWI/SNF (de la Serna et al. 2005). Remarkably, in undifferentiated proliferative myoblasts a specific BAF60 paralog, BAF60c, interacts at myogenic loci with the transcription factor MyoD, but does so by itself—independent of the SWI/SNF complex (Forcales et al. 2012). The signaling that accompanies skeletal muscle differentiation leads to BAF60c phosphorylation by p38 kinase, triggering the recruitment of SWI/SNF (BRG1 subtype) to myogenic loci, chromatin remodeling, and transcription initiation (Forcales et al. 2012) (Fig. 3.10). Notably, a similar process of SWI/SNF Remodeler recruitment by BAF60c occurs in lipogenesis; BAF60c residing at target gene promoters is phosphorylated by aPKC kinase upon insulin signaling, leading to subsequent formation of a lipoBAF complex, allowing remodeling and transcription activation at lipogenic genes (Wang et al. 2013). Furthermore, BAF60c is also a component of the Notch-dependent transcriptional activation required for the establishment of the left–right asymmetry (Takeuchi et al. 2007).
Myogenic differentiation by the recruitment of CHD2 and BAF Remodelers by MyoD. (a) Prior to differentiation, MyoD recruits the CHD2 Remodeler to myogenic loci and incorporates a H3.3 histone variant into promoter nucleosomes, prior to gene transcription. (b) In undifferentiated myoblasts, MyoD interacts with BAF60c, a BAF Remodeler subunit, at myogenic loci. Upon activation, BAF60c is phosphorylated by p38 kinase and recruits BAF Remodeler, which opens chromatin and allows RNAPII recruitment and expression of MyoD-target genes, leading to differentiation into muscle cells
During heart development, BAF60c is specifically required for generating beating cardiomyocytes from fibroblasts (Lickert et al. 2004; Takeuchi and Bruneau 2009; Ieda et al. 2010). Furthermore, PBAF utilizes its BAF180 subunit to promote cardiac chamber maturation and coronary development (Wang et al. 2004; Huang et al. 2008). Heart development in both mouse and zebrafish involves a dosage-sensitive interrelationship between transcription factors and the BAF Remodeler (Takeuchi et al. 2011). Interestingly, in adult mouse cardiomyocytes, Brg1 is not actively transcribed, but can be reactivated by cardiac stress, possibly through the reactivation of an embryonic program involving interactions with HDACs and PARP (Hang et al. 2010).
CHD2 is ubiquitiously expressed, but highly enriched in muscle tissues (Marfella et al. 2006). In muscle, exchange of histone variants appears to be involved in gene regulation. In mouse ES cells, H3.3 is found at many developmental regulatory genes that have “bivalent” chromatin, marked with coincident H3K27me (normally correlated with gene silencing) and also H3K4me3 (normally correlated with gene activation) (Goldberg et al. 2010). Remarkably, prior to their actual transcription, CHD2 deposits the H3.3 histone variant at genes for skeletal muscle differentiation and function, guided by CHD2 interaction with MyoD (Harada et al. 2012) (Fig. 3.10). Thus, MyoD can recruit successive Remodelers to perform different tasks along the path to gene activation.
Roles for multiple Remodelers in blood cell development are emerging. Here, roles for SWI/SNF subtypes have recently been revealed, including specific actin-related proteins (Krasteva et al. 2012). For ISWI, NURF recruitment to the Egr1 locus (important for thymocyte maturation) involves interaction with the transcription factor Srf by the NURF subunit BPTF, enabling its stable binding to promoters (Landry et al. 2011). In lymphocytes, the lineage-determining factor Ikaros tethers NuRD to active genes involved in lymphoid differentiation; remarkably, Ikaros inhibits both the remodeling and HDAC activities of NuRD at these locations, and also affects the presence of NuRD at locations that lack an Ikaros binding site (Zhang et al. 2012). Here, loss of Ikaros leads to the redistribution of NuRD and reactivation of transcriptionally poised genes involved in proliferation, mediating progression to a leukemic state. Thus, a DNA-binding protein (here, Ikaros) is capable of regulating Remodeler targeting and activity. The MTA (metastasis-associated) subunits specialize and help target NuRD subtypes via their interaction with transcription factors. For example, in B lymphocytes, MTA3 interacts with BCL6, the master regulator of B cell differentiation, targeting NuRD repression, and therefore preventing terminal differentiation into plasma cells (Fujita et al. 2004). Remarkably, expressing BCL6 in plasma cells, while MTA3 is functional, results in a reversion of the cell fate and reprogramming into B lymphocytes (Fujita et al. 2004). Moreover, MTA3 directs the repression of genes involved in converting mammary epithelial cells to breast cancer cells (Fujita et al. 2003). Finally, MTAs can enlist the histone H3K4/K9 demethylase LSD1 in association with NuRD, to abolishing the metastatic potential of breast cancer cell lines (Wang et al. 2009b) (developed below). Changes in subunit composition of the NuRD complexes are therefore decisive in establishing cell type-specific transcriptional programs.
3.5.3 Remodeler Regulation of Body Plan
Two ubiquitously expressed groups of proteins, the Polycomb group of repressors and the Trithorax group of activators, regulate the Hox genes, which code key factors for controlling body plan patterning (and hematopoiesis) during development. Chromatin Remodelers and “positive” modifiers belong to the Trithorax group proteins. The initial connection involved the observation that mutations in Drosophila BRM (the SWI/SNF-family ATPase in flies) suppressed Polycomb mutations (Tamkun et al. 1992). In addition, dNURF301 contributes to the activation of the homeotic selector genes (Badenhorst et al. 2002), and dNURF maintains Hox gene expression patterning during development, using H3K4me3 (Wysocka et al. 2006). In contrast, repression of the Hox genes involves dMi-2, likely transmitting the repression from the gap proteins to the Polycomb proteins (Kehle et al. 1998). Notably, Remodelers contribute to proper tissue organization during embryogenesis by regulating genes creating and responding to morphogen signals in spatial territories. For example, at gastrulation in X. laevis, xCHD4 abundance controls the balance and boundary along the animal–vegetal axis between mesoderm and neuroectoderm formation (Linder et al. 2007).
3.5.4 Plant Development
Many ATPases have been identified as possible Remodelers in A. thaliana [reviewed in Knizewski et al. (2008)]. Among them, four ATPases belong to the SWI/SNF family. aBRM is the only ATPase harboring a C-terminal bromodomain, the signature of SWI/SNF. The related SPLAYED (aSYD) ATPase is expressed in early development, while a truncated version is present in adult plants. Notably, aBRM and aSYD have different targets, as brm and syd mutations lead to nonoverlapping and pleiotropic developmental defects (Bezhani et al. 2007). Although aBRM is essential for accurate flowering and reproduction, neither aBRM nor aSYD are essential to embryonic development, in contrast to mammals. aBRM is involved in the regulation of the photoperiod pathway genes and is an essential repressor of Flowering Locus C (FLC) by creating repressive chromatin at the locus (Farrona et al. 2011). Interestingly, double mutants of noncanonical SWI/SNF ATPases CHR12 and CHR23 (MINUSCULE 1 and 2) result in embryonic lethality, with weak double mutants displaying dramatic defects in stem cell maintenance (Sang et al. 2012). The sole gene similar to SNF5 produces aBSH, a protein contributing to the control of auxin-responsive genes (Brzeski et al. 1999).
The aDDM1 ATPase performs chromatin remodeling in vitro (Brzeski and Jerzmanowski 2003), and its mutations result in inaccurate DNA methylation patterns, with hypomethylation of repeat regions and hypermethylation of low copy regions (Hirochika et al. 2000). Moreover, loss of aDDM1 results in loss of heterochromatin DNA methylation, along with a replacement of H3K9me by H3K4me (Gendrel et al. 2002). Interestingly, aDDM1 interacts with aMBDs and affects their subnuclear localization (Zemach et al. 2005). PICKLE, a CHD3-like Remodeler, regulates the transition from embryonic to vegetative development by repressing the expression of seed-associated genes during germination (Ogas et al. 1999). Moreover, PICKLE promotes the deposition of H3K27me3, a unique role for a Remodeler, and is independent of the plant growth regulator gibberellin (Zhang et al. 2008). Beyond its role as a transcriptional repressor, PICKLE can adopt a Trithorax-like function, acting as a transcriptional activator antagonizing Polycomb Group proteins (Aichinger et al. 2009). The antagonism between PICKLE and Polycomb Group proteins is important for the regulation of cell identity and meristem activity in Arabidopsis (Aichinger et al. 2009, 2011).
Although a SWR1 Remodeler has not been yet purified from plants, its existence is strongly supported by various experimental evidences [reviewed in March-Diaz and Reyes (2009)]. In Arabidopsis, the ATPase aPIE1 (PHOTOPERIOD INDEPENDENT EARLY FLOWERING1) possesses an ATPase domain with a long insertion and contains an HSA domain in its N-terminal region. Homologs of many SWR1 Remodeler subunits have been identified in Arabidopsis (Choi et al. 2007). Interestingly, aPIE1 directly interacts with H2AZ variants, suggesting a functional conservation (March-Diaz et al. 2008). Moreover, deposition of H2AZ at FLC requires aPIE1 and aARP6 (Deal et al. 2005, 2007). Remarkably, there is a strong inverse correlation between DNA methylation and the presence of H2AZ in Arabidopsis, suggesting that H2AZ protects genes from DNA methylation or DNA methylation prevents H2AZ deposition (Zilberman et al. 2008).
3.6 Remodelers and Cancer
Connections between Remodelers and cancer have been growing for over two decades, but have increased exponentially with the application of high-throughput sequencing to tumors. As described above, Remodelers are integral participants in self-renewal and differentiation decisions—issues central in cancer—which likely underlie their growing importance in cancer biology.
3.6.1 SWI/SNF Family
The advent of high-throughput sequencing has revealed that mutations in SWI/SNF complex members are present at a remarkable frequency: ~19 % across all tumor types, approaching the frequency observed for p53 (26 %) (Shain and Pollack 2013). More specifically, SWI/SNF mutations are found at exceptionally high frequency in human malignant rhabdoid tumors (MRTs, >95 %), ovarian clear cell carcinoma (75 %), clear cell renal carcinoma (57 %), hepatocellular carcinoma (40 %), gastric cancers (36 %), melanoma (34 %), and pancreatic cancer (26 %) (Shain and Pollack 2013). An early and clear connection of SWISNF to cancer involved the core hSWI/SNF subunit hSNF5/BAF47/INI1. Notably, biallelic loss of Snf5 occurs in nearly all human MRTs (Versteege et al. 1998; Jackson et al. 2009). Mouse models reveal that Snf5−/− mice develop cancers very similar to human MRTs with full penetrance by 11 weeks (Roberts and Orkin 2004). Although the precise role of SNF5 in regulating SWI/SNF function is not known, tumors display clear misregulation of genes related to stem cell self-renewal and targets of the PRC2 complex, which silences those genes by adding H3K27me. Remarkably, loss of the PRC2 component Ezh2 prevents the formation of tumors in Snf5−/− mice (Wilson et al. 2010). Thus, the work reveals an antagonism between SWI/SNF and PRC2, with Snf5 loss promoting an embryonic self-renewal transcriptional program. However, this relationship may not be universal, as certain tumor types can be found with both PRC2 and SWI/SNF mutations. Interestingly, tumor formation is also prevented following additional loss of BRG1, suggesting that oncogenic transformation might result partly from aberrant activity of a residual complex (Wang et al. 2009a).
Mutations in BRG1 (one of two alternative ATPase subunits) have been described in lung, pancreatic, breast and prostate tumors, and cell lines. For some, incidence is remarkably high; studies of nonsmall cell carcinomas show mutations in BRG1 in ~35 % of cell lines, and an even higher percentage of cell lines show a loss of BRG1 expression, raising the possibility of epigenetic silencing as well. Tumorigenesis can also be driven by reduced expression of Brg1 rather than full loss (Bultman et al. 2000). Moreover, BRM-containing and BRG1-containing SWI/SNF Remodelers have different transcriptional specificities and antagonistic roles in differentiation (Kadam and Emerson 2003; Flowers et al. 2009). Links to cancer are less clear with the alternative ATPase subunit BRM; however, Brm-deficient mice display androgen-independent growth and cellular proliferation (Shen et al. 2008). In addition, BAF57, a core subunit also common to all hSWI/SNF complexes, is involved in apoptosis by increasing expression of the tumor suppressor gene CYLD (Wang et al. 2005).
The presence of BAF180 helps define the PBAF subtype, and BAF180 harbors many distinctive domains including six bromodomains (in tandem), two BAH domains, and a HMG domain, which together contribute to functional specificity (Lemon et al. 2001; Moshkin et al. 2007). Notably, mutations in PBRM (which encodes BAF180) have been identified in >40 % of renal cell carcinomas (Varela et al. 2011). Along with BAF180, another PBAF-specific subunit, BRD7, is a tumor suppressor found only in the subset of breast cancers that lack p53 mutations (Drost et al. 2010; Burrows et al. 2010). Notably, mutations in the ARID1A and ARID1B paralogs (also termed BAF250a/b) are as prevalent as those in the two alternative ATPases. Mutations in the ARID1A/B paralogs are found at high frequency in gastric cancers, ovarian cancers, pancreatic cancers, and melanoma—though ARID1A mutations are much more common in certain cancers, such as melanoma. BAF250a/ARID1a is a DNA-binding protein (though not sequence specific) present in esBAF and BAF complexes, but excluded from PBAF complex. Like BAF180, the ARID1A/B paralogs are likely involved in targeting the Remodeler to particular genes. Undoubtedly, considerable future research will be focused on understanding their modes of targeting and gene targets.
Finally, SWI/SNF mutations appear to be largely mutually exclusive with p53, which might suggest that other mutagenic processes may operate to provide the spectrum of needed genetic mutations. However, tumors with SWI/SNF mutations typically contain very few additional genetic mutations, and most cancers with SWI/SNF mutations lack major structural changes. An alternative view is that impairment of SWI/SNF may impart epigenetic misregulation and transcriptional heterogeneity, followed by selection. As SWI/SNF Remodelers regulate genes at critical nodes (self-renewal, lineage-specific differentiation, cell cycle, cell migration, and signaling) their epigenetic misregulation may provide the needed combination (Wilson and Roberts 2011).
3.6.2 ISWI Family
Links between ISWI-family Remodeler to cancers have slowly emerged. For example, SNF2L (which resides in hNURF) suppresses cell proliferation and migration through an attenuation of the Wnt/β-catenin signaling (Eckey et al. 2012). Remarkably, SNF2L is strongly expressed in normal melanocytes but almost absent in melanoma. Therefore, depletion of SNF2L affects the migratory potential of melanoma cells. Furthermore, there is an inverse correlation between SNF2L expression and melanoma malignancy (Eckey et al. 2012). Notably, in Drosophila, larval blood cell development is regulated by dNURF, which represses STAT92E target genes (Badenhorst et al. 2002). Deficiency in dNURF leads to a neoplastic transformation of circulating hemocytes, resulting in blood cell overproliferation and melanotic tumors (Badenhorst et al. 2002).
3.6.3 CHD Family
Connections of NuRD to cancer abound, given its central role in gene regulation and ES cells differentiation [reviewed in Lai and Wade (2011)]. The metastasis-associated proteins MTA1-3 are components of NuRD, which regulate invasive behavior in multiple cancers. Rather than acting as similar and redundant factors, MTA1-3 shows unique and often antagonistic activities. In keeping, MTA1 and MTA3 display opposing expression patterns during breast cancer progression: MTA1 expression progressively increases, while MTA3 expression decreases during tumorigenesis (Zhang et al. 2006a). In breast cancers, estrogen receptor (ER) activity is repressed, in part by upregulation of MTA1 expression, resulting from heregulin–ERBB2 pathway activation (Mazumdar et al. 2001). Here, increased levels of MTA1 correlate with tumor progression in many cancer types. In contrast, MTA3 restrains breast tumor progression by repressing transcription of the master regulator Snail, inhibiting epithelial-to-mesenchymal transition (EMT), a critical step in metastasis (Fujita et al. 2003). Moreover, MTA3 expression correlates with ER expression, and MTA3 associates with NuRD in an ER-dependent manner (Fujita et al. 2003). Thus, MTA3 has a unique role as a tumor suppressor in breast cancer. In direct contrast to MTA3-dependent repression of Snail and EMT, MTA2-associated NuRD is recruited to key EMT genes by the master regulator Twist, thus promoting EMT (Fu et al. 2011). Finally, posttranslational modifications of MTAs appear to greatly regulate their function. For example, the lysine-specific demethylase 1 (LSD1) interacts with and apparently demethylates MTA1 to activate NuRD (Wang et al. 2009b; Nair et al. 2013). In keeping, depletion of LSD1 in breast cancer leads to upregulation of the TGF-β pathway, increasing invasiveness and EMT (Wang et al. 2009b). Furthermore, particular acetylation forms of MTA1 may actually convert NuRD into a coactivator (Gururaj et al. 2006; Ohshiro et al. 2010). Additionally, oncogenic fusion proteins, such as EWS-FLI (important in Ewing Sarcoma), recruit NuRD in association with HDACs and LSD1 to repress genes, which helps lead to oncogenesis (Sankar et al. 2012).
Cancer cells often contain aberrant DNA methylation patterns and can bear hypermethylated promoters or “shores” of CpG islands. Here, NuRD may employ associated MBD proteins, like MBD2, to promote gene silencing at these locations, supporting tumorigenesis when the target functions as a tumor suppressor (Magdinier and Wolffe 2001; Sansom et al. 2003). Finally, as mentioned previously, Remodelers cross-talk with master transcriptional regulators for their proper targeting. Lack of specific recruitment of NuRD at genes involved in lymphoid differentiation by the master regulator Ikaros leads to NuRD redistribution to inappropriate genes and their reactivation, resulting in proliferation and progression to a leukemic state (Zhang et al. 2012). Additional links of CHD proteins to cancer include Chd2, an essential gene in mice needed for proper hematopoietic stem cell differentiation, and deficiency in which leads to lymphomas (Nagarajan et al. 2009). Similarly, CHD5, a brain-specific paralog of Mi-2 expressed in neural tissue, is a tumor suppressor that positively regulates genes (i.e., p16 and p19) associated with neuroblastoma (Bagchi et al. 2007). Deletion of CHD5 alters expression of neuronal genes, cell cycle genes, transcription factors, and also brain-specific subunits of SWI/SNF (Potts et al. 2011). Remarkably, the interaction of the PHD finger of CHD5 with the unmodified H3 tail is essential for CHD5 inhibition of proliferation and suppression of tumor growth of neuroblastoma cells in vivo (Paul et al. 2013). Beyond neuroblastoma, the inactivation of CHD5 has been documented in many cancers. Finally, we note that although many links (described above) have been made between INO80-family complexes and both DNA repair and recombination mutations in these Remodelers have not proven common in human cancers.
Overall, SWI/SNF and NuRD subtype Remodelers are well connected to cancer, likely due to their key roles in regulating self-renewal, pluripotency, proliferation, and differentiation. Misregulation of targeting and/or impairment of activity can alter those functions and create or maintain a proliferative progenitor state with epigenetic heterogeneity—states that can promote oncogenesis and metastasis.
3.7 Remodelers and Disease Syndromes
Beyond their roles in cancer, mutations in Remodelers cause a range of developmental disorders, termed syndromes, which relate to their involvement in important developmental decisions.
-
ATRX-syndrome and α-thalassemia myelodysplasia (ATMDS) syndrome: These syndromes are caused by mutations in ATRX (introduced in earlier sections on “orphan” Remodelers and H3.3 variant deposition) (Gibbons et al. 2003). As noted above, ATRX cooperates with Daxx to control H3.3 localization at telomeres and pericentric regions, affecting the maintenance of telomere structural integrity in pluripotent embryonic stem cells, and replication-independent chromatin assembly at telomeres (Xue et al. 2003; Wong et al. 2010; Goldberg et al. 2010; Lewis et al. 2010). Remarkably, half of the disease-causing mutations cluster in the ADD domain of ATRX, rendering it defective in its recognition of H3K9me. Notably, the ADD domain normally binds H3K9me when H3K4me2/me3 is absent (Iwase et al. 2011). Interestingly, an alternative/additional molecular justification for the disease has been recently proposed: ATRX acts as a negative regulator of macroH2A incorporation, and ATRX mutations may cause precocious accumulation of that variant to silence particular genes (Ratnakumar et al. 2012).
-
COFS (cerebro-oculo-facio-skeletal syndrome) and CSB (Cockayne syndrome type B): The COFS and CSB are characterized by growth failure, neurological degeneration, UV sensitivity, and cataracts. CSB is a DNA translocase within the larger SNF2 family that is needed for proper transcription-coupled nucleotide-excision repair (TC-NER) and assists transcription elongation by RNAPII by helping it overcome blocks (such as bulky DNA lesions created by UV irradiation). Alternatively, CSB can help RNAPII release from sites of damage to allow the repair machinery access to the lesion (Woudstra et al. 2002). Interestingly, association of CSB with chromatin requires an ATP hydrolysis-dependent conformational change to overcome an inhibitory effect imposed by its N-terminal region (Lake et al. 2010), a principle that may be similar to chromodomains in yChd1 and to AutoN in dISWI. Moreover, the C-terminal region of CSB contains a ubiquitin-binding domain and the ubiquitination of CSB is required for most functions, including RNAPII recruitment after UV irradiation (Anindya et al. 2010). CSB is stabilized at sites of damage by the proteins UVSSA and USP7, a function needed for TC-NER (Schwertman et al. 2012). Interestingly, CSB and p53 interact and regulate their respective affinities for nucleosomes (Lake et al. 2011). CSB also appears to be involved in the maintenance of telomere length and stability via its interaction with TRF2 and the maintenance of the homeostatic level of TERRA (Batenburg et al. 2012).
-
CHARGE syndrome: CHARGE syndrome is an autosomal dominant disorder characterized by malformations of craniofacial structures and peripheral nervous system leading to deaf-blindness, olfactory dysfunction, balance disorders, and congenital heart malformations. The pleiotropic developmental defects of this syndrome are related to haploinsufficiency of CHD7, encoding the CHD-family ATPase CHD7 (Vissers et al. 2004). Mouse models of CHARGE syndrome suggest that expression levels of CHD7 are critical for developmental pathogenesis, affecting the expression of key genes in development (Hurd et al. 2007). Chromatin remodeling of CHD7 per se has been shown to be impaired by mutations identified in the CHARGE syndrome (Bouazoune and Kingston 2012). Genome-wide analysis implicate CHD7 in temporal and tissue-specific functions, and find CHD7 colocalized with Brg1 and H3K4me1 at active enhancers in mouse ES cells (Schnetz et al. 2009, 2010). This is consistent with recent results showing CHD7 cooperating (in neural crest-like cells) with PBAF at enhancers for neural crest transcriptional programs (Bajpai et al. 2010), as well as genes and enhancers for neurogenesis in the inner ear (Hurd et al. 2010). Taken together, enhancer-mediated gene dysregulation resulting from CHD7 mutations might contribute to the multiple anomalies observed in the CHARGE syndrome pathogenesis (Schnetz et al. 2010). Remarkably, CHD7 interacts with SOX2 during neural stem cell development to activate the genes that encode members (or targets) of the Notch and Sonic Hedgehog signaling pathways—genes, which are mutated in the human genetic diseases Alagille syndrome (JAG1), Feingold syndrome (MYCN) and Pallister–Hall syndrome (GLI3) (Engelen et al. 2011).
-
Coffin–Siris and Nicolaides–Baraitser syndromes: The Coffin–Siris syndrome is a rare autosomal dominant disorder characterized by growth deficiency, intellectual disability, and additional highly variable clinical features. The vast majority of affected individuals display mutations in one of many hBAF Remodeler subunits (hSNF5, BRG1, BRM, BAF250a, BAF250b, or BAF57), but not in the subunits specific for PBAF (Tsurusaki et al. 2012). Interestingly, largely the same spectrums of genes affected in Coffin–Siris syndrome are those mutated in sporadic cancers, consistent with their roles in developmental decisions. The mutations in BAF250a/b involved primarily truncations (Tsurusaki et al. 2012), and those in the ATPase subunits (hBRG1 and hBRM) included mutations in the HELICc subdomain, raising the possibility that these mutations create dominant negative forms of hSWI/SNF complex. The Nicolaides–Baraitser syndrome is a rare dominant disorder only recently defined by sparse hair, typical facial morphology, distal-limb anomalies, and intellectual disability, with marked language impairment. The syndrome is caused by mutations in BRM that are clustered within the ultra-conserved motifs of the catalytic domain of the protein, speculated to create a weak hypomorph due to a reduction in ATPase activity (Van Houdt et al. 2012).
-
Floating-Harbor Syndrome: Floating-Harbor syndrome is a rare condition characterized by a triangular face, thin upper lip, long nose with narrow bridge, and some degree of learning disability, particularly in language. It has been recently linked to mutations clustered in the final exon of SRCAP, the ATPase of the editing Remodeler SRCAP (hSWR1), leading to protein truncations at the C-terminus that remove three small AT-hook domains (Hood et al. 2012), but the impact on SRCAP activity has not been tested.
Abbreviations
- ARP:
-
Actin-related protein
- ACF:
-
ATP-utilizing chromatin assembly and remodeling factor
- ATRX:
-
α-Thalassemia X-linked mental retardation
- CHARGE:
-
Coloboma heart defect, atresia choanae, retarded growth and development, genital hypoplasia, ear anomalies/deafness
- CHRAC:
-
Chromatin accessibility complex
- DCC:
-
Dosage compensation complex
- DSB:
-
Double-strand break
- EMT:
-
Epithelial-to-mesenchymal transition
- ES:
-
Embryonic stem
- HAT:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- HR:
-
Homologous recombination
- MRT:
-
Malignant rhabdoid tumor
- NDR:
-
Nucleosome-depleted region
- NHEJ:
-
Nonhomologous end-joining
- NURF:
-
Nucleosome remodeling factor
- PTM:
-
Posttranslational modification
- RNAPI/II/III:
-
RNA polymerases I, II, or III
- RPA:
-
Replication protein A
- TC-NER:
-
Transcription-coupled nucleotide-excision repair
- TSS:
-
Transcription start site
References
Ahel D, Horejsi Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, Flynn H, Skehel M, West SC, Jackson SP, Owen-Hughes T, Boulton SJ (2009) Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325(5945):1240–1243
Ahmad K, Henikoff S (2002) Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci USA 99(Suppl 4):16477–16484
Aichinger E, Villar CB, Farrona S, Reyes JC, Hennig L, Kohler C (2009) CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet 5(8):e1000605
Aichinger E, Villar CB, Di Mambro R, Sabatini S, Kohler C (2011) The CHD3 chromatin remodeler PICKLE and polycomb group proteins antagonistically regulate meristem activity in the Arabidopsis root. Plant Cell 23(3):1047–1060
Alen C, Kent NA, Jones HS, O'Sullivan J, Aranda A, Proudfoot NJ (2002) A role for chromatin remodeling in transcriptional termination by RNA polymerase II. Mol Cell 10(6):1441–1452
Altaf M, Auger A, Monnet-Saksouk J, Brodeur J, Piquet S, Cramet M, Bouchard N, Lacoste N, Utley RT, Gaudreau L, Cote J (2010) NuA4-dependent acetylation of nucleosomal histones H4 and H2A directly stimulates incorporation of H2A.Z by the SWR1 complex. J Biol Chem 285(21):15966–15977
Anindya R, Mari PO, Kristensen U, Kool H, Giglia-Mari G, Mullenders LH, Fousteri M, Vermeulen W, Egly JM, Svejstrup JQ (2010) A ubiquitin-binding domain in Cockayne syndrome B required for transcription-coupled nucleotide excision repair. Mol Cell 38(5):637–648
Armache KJ, Garlick JD, Canzio D, Narlikar GJ, Kingston RE (2011) Structural basis of silencing: Sir3 BAH domain in complex with a nucleosome at 3.0 A resolution. Science 334(6058):977–982
Armstrong JA, Papoulas O, Daubresse G, Sperling AS, Lis JT, Scott MP, Tamkun JW (2002) The Drosophila BRM complex facilitates global transcription by RNA polymerase II. EMBO J 21(19):5245–5254
Asturias FJ, Chung WH, Kornberg RD, Lorch Y (2002) Structural analysis of the RSC chromatin-remodeling complex. Proc Natl Acad Sci USA 99(21):13477–13480
Awad S, Ryan D, Prochasson P, Owen-Hughes T, Hassan AH (2010) The Snf2 homolog Fun30 acts as a homodimeric ATP-dependent chromatin-remodeling enzyme. J Biol Chem 285(13):9477–9484
Badenhorst P, Voas M, Rebay I, Wu C (2002) Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev 16(24):3186–3198
Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, Bredel M, Vogel H, Mills AA (2007) CHD5 is a tumor suppressor at human 1p36. Cell 128(3):459–475
Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang CP, Zhao Y, Swigut T, Wysocka J (2010) CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature 463(7283):958–962
Bao Y, Shen X (2007) SnapShot: chromatin remodeling complexes. Cell 129(3):632
Batenburg NL, Mitchell TR, Leach DM, Rainbow AJ, Zhu XD (2012) Cockayne Syndrome group B protein interacts with TRF2 and regulates telomere length and stability. Nucleic Acids Res 40(19):9661–9674
Beerens N, Hoeijmakers JH, Kanaar R, Vermeulen W, Wyman C (2005) The CSB protein actively wraps DNA. J Biol Chem 280(6):4722–4729
Bezhani S, Winter C, Hershman S, Wagner JD, Kennedy JF, Kwon CS, Pfluger J, Su Y, Wagner D (2007) Unique, shared, and redundant roles for the Arabidopsis SWI/SNF chromatin remodeling ATPases BRAHMA and SPLAYED. Plant Cell 19(2):403–416
Biswas D, Takahata S, Xin H, Dutta-Biswas R, Yu Y, Formosa T, Stillman DJ (2008) A role for Chd1 and Set2 in negatively regulating DNA replication in Saccharomyces cerevisiae. Genetics 178(2):649–659
Blosser TR, Yang JG, Stone MD, Narlikar GJ, Zhuang X (2009) Dynamics of nucleosome remodelling by individual ACF complexes. Nature 462(7276):1022–1027
Boeger H, Griesenbeck J, Strattan JS, Kornberg RD (2003) Nucleosomes unfold completely at a transcriptionally active promoter. Mol Cell 11(6):1587–1598
Boeger H, Griesenbeck J, Kornberg RD (2008) Nucleosome retention and the stochastic nature of promoter chromatin remodeling for transcription. Cell 133(4):716–726
Bonaldi T, Langst G, Strohner R, Becker PB, Bianchi ME (2002) The DNA chaperone HMGB1 facilitates ACF/CHRAC-dependent nucleosome sliding. EMBO J 21(24):6865–6873
Bouazoune K, Brehm A (2005) dMi-2 chromatin binding and remodeling activities are regulated by dCK2 phosphorylation. J Biol Chem 280(51):41912–41920
Bouazoune K, Kingston RE (2012) Chromatin remodeling by the CHD7 protein is impaired by mutations that cause human developmental disorders. Proc Natl Acad Sci USA 109(47):19238–19243
Bouazoune K, Mitterweger A, Langst G, Imhof A, Akhtar A, Becker PB, Brehm A (2002) The dMi-2 chromodomains are DNA binding modules important for ATP-dependent nucleosome mobilization. EMBO J 21(10):2430–2440
Bourachot B, Yaniv M, Muchardt C (2003) Growth inhibition by the mammalian SWI-SNF subunit Brm is regulated by acetylation. EMBO J 22(24):6505–6515
Bowman GD (2010) Mechanisms of ATP-dependent nucleosome sliding. Curr Opin Struct Biol 20(1):73–81
Boyer LA, Latek RR, Peterson CL (2004) The SANT domain: a unique histone-tail-binding module? Nat Rev Mol Cell Biol 5(2):158–163
Brehm A, Tufteland KR, Aasland R, Becker PB (2004) The many colours of chromodomains. Bioessays 26(2):133–140
Brown SA, Imbalzano AN, Kingston RE (1996) Activator-dependent regulation of transcriptional pausing on nucleosomal templates. Genes Dev 10(12):1479–1490
Brzeski J, Jerzmanowski A (2003) Deficient in DNA methylation 1 (DDM1) defines a novel family of chromatin-remodeling factors. J Biol Chem 278(2):823–828
Brzeski J, Podstolski W, Olczak K, Jerzmanowski A (1999) Identification and analysis of the Arabidopsis thaliana BSH gene, a member of the SNF5 gene family. Nucleic Acids Res 27(11):2393–2399
Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T (2000) A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell 6(6):1287–1295
Bultman SJ, Gebuhr TC, Pan H, Svoboda P, Schultz RM, Magnuson T (2006) Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev 20(13):1744–1754
Burgio G, La Rocca G, Sala A, Arancio W, Di Gesu D, Collesano M, Sperling AS, Armstrong JA, van Heeringen SJ, Logie C, Tamkun JW, Corona DF (2008) Genetic identification of a network of factors that functionally interact with the nucleosome remodeling ATPase ISWI. PLoS Genet 4(6):e1000089
Burrows AE, Smogorzewska A, Elledge SJ (2010) Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc Natl Acad Sci USA 107(32):14280–14285
Cai Y, Jin J, Yao T, Gottschalk AJ, Swanson SK, Wu S, Shi Y, Washburn MP, Florens L, Conaway RC, Conaway JW (2007) YY1 functions with INO80 to activate transcription. Nat Struct Mol Biol 14(9):872–874
Cairns BR (2007) Chromatin remodeling: insights and intrigue from single-molecule studies. Nat Struct Mol Biol 14(11):989–996
Cairns BR (2009) The logic of chromatin architecture and remodelling at promoters. Nature 461(7261):193–198
Cairns BR, Erdjument-Bromage H, Tempst P, Winston F, Kornberg RD (1998) Two actin-related proteins are shared functional components of the chromatin-remodeling complexes RSC and SWI/SNF. Mol Cell 2(5):639–651
Carey M, Li B, Workman JL (2006) RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol Cell 24(3):481–487
Cavellan E, Asp P, Percipalle P, Farrants AK (2006) The WSTF-SNF2h chromatin remodeling complex interacts with several nuclear proteins in transcription. J Biol Chem 281(24):16264–16271
Chaban Y, Ezeokonkwo C, Chung WH, Zhang F, Kornberg RD, Maier-Davis B, Lorch Y, Asturias FJ (2008) Structure of a RSC-nucleosome complex and insights into chromatin remodeling. Nat Struct Mol Biol 15(12):1272–1277
Chai B, Huang J, Cairns BR, Laurent BC (2005) Distinct roles for the RSC and Swi/Snf ATP-dependent chromatin remodelers in DNA double-strand break repair. Genes Dev 19(14):1656–1661
Chambers AL, Ormerod G, Durley SC, Sing TL, Brown GW, Kent NA, Downs JA (2012) The INO80 chromatin remodeling complex prevents polyploidy and maintains normal chromatin structure at centromeres. Genes Dev 26(23):2590–2603
Chatterjee N, Sinha D, Lemma-Dechassa M, Tan S, Shogren-Knaak MA, Bartholomew B (2011) Histone H3 tail acetylation modulates ATP-dependent remodeling through multiple mechanisms. Nucleic Acids Res 39(19):8378–8391
Chen X, Cui D, Papusha A, Zhang X, Chu CD, Tang J, Chen K, Pan X, Ira G (2012) The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature 489(7417):576–580
Cherry CM, Matunis EL (2010) Epigenetic regulation of stem cell maintenance in the Drosophila testis via the nucleosome-remodeling factor NURF. Cell Stem Cell 6(6):557–567
Chi TH, Wan M, Zhao K, Taniuchi I, Chen L, Littman DR, Crabtree GR (2002) Reciprocal regulation of CD4/CD8 expression by SWI/SNF-like BAF complexes. Nature 418(6894):195–199
Chioda M, Vengadasalam S, Kremmer E, Eberharter A, Becker PB (2010) Developmental role for ACF1-containing nucleosome remodellers in chromatin organisation. Development 137(20):3513–3522
Choi K, Park C, Lee J, Oh M, Noh B, Lee I (2007) Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development 134(10):1931–1941
Chou DM, Adamson B, Dephoure NE, Tan X, Nottke AC, Hurov KE, Gygi SP, Colaiacovo MP, Elledge SJ (2010) A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci USA 107(43):18475–18480
Citterio E, Van Den Boom V, Schnitzler G, Kanaar R, Bonte E, Kingston RE, Hoeijmakers JH, Vermeulen W (2000) ATP-dependent chromatin remodeling by the Cockayne syndrome B DNA repair-transcription-coupling factor. Mol Cell Biol 20(20):7643–7653
Clapier CR, Cairns BR (2012) Regulation of ISWI involves inhibitory modules antagonized by nucleosomal epitopes. Nature 492(7428):280–284
Clapier CR, Langst G, Corona DF, Becker PB, Nightingale KP (2001) Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol 21(3):875–883
Clapier CR, Nightingale KP, Becker PB (2002) A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res 30(3):649–655
Clausell J, Happel N, Hale TK, Doenecke D, Beato M (2009) Histone H1 subtypes differentially modulate chromatin condensation without preventing ATP-dependent remodeling by SWI/SNF or NURF. PLoS One 4(10):e0007243
Collins N, Poot RA, Kukimoto I, Garcia-Jimenez C, Dellaire G, Varga-Weisz PD (2002) An ACF1-ISWI chromatin-remodeling complex is required for DNA replication through heterochromatin. Nat Genet 32(4):627–632
Corona DF, Clapier CR, Becker PB, Tamkun JW (2002) Modulation of ISWI function by site-specific histone acetylation. EMBO Rep 3(3):242–247
Corona DF, Siriaco G, Armstrong JA, Snarskaya N, McClymont SA, Scott MP, Tamkun JW (2007) ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLoS Biol 5(9):e232
Cosma MP, Tanaka T, Nasmyth K (1999) Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97(3): 299–311
Costelloe T, Louge R, Tomimatsu N, Mukherjee B, Martini E, Khadaroo B, Dubois K, Wiegant WW, Thierry A, Burma S, van Attikum H, Llorente B (2012) The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 489(7417):581–584
Dang W, Bartholomew B (2007) Domain architecture of the catalytic subunit in the ISW2-nucleosome complex. Mol Cell Biol 27(23):8306–8317
Dang W, Kagalwala MN, Bartholomew B (2007) The Dpb4 subunit of ISW2 is anchored to extranucleosomal DNA. J Biol Chem 282(27):19418–19425
De La Fuente R, Viveiros MM, Wigglesworth K, Eppig JJ (2004) ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev Biol 272(1):1–14
de la Serna IL, Carlson KA, Imbalzano AN (2001) Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat Genet 27(2):187–190
de la Serna IL, Ohkawa Y, Berkes CA, Bergstrom DA, Dacwag CS, Tapscott SJ, Imbalzano AN (2005) MyoD targets chromatin remodeling complexes to the myogenin locus prior to forming a stable DNA-bound complex. Mol Cell Biol 25(10):3997–4009
Deal RB, Kandasamy MK, McKinney EC, Meagher RB (2005) The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. Plant Cell 17(10): 2633–2646
Deal RB, Topp CN, McKinney EC, Meagher RB (2007) Repression of flowering in Arabidopsis requires activation of FLOWERING LOCUS C expression by the histone variant H2A.Z. Plant Cell 19(1):74–83
Dechassa ML, Zhang B, Horowitz-Scherer R, Persinger J, Woodcock CL, Peterson CL, Bartholomew B (2008) Architecture of the SWI/SNF-nucleosome complex. Mol Cell Biol 28(19):6010–6021
Dechassa ML, Sabri A, Pondugula S, Kassabov SR, Chatterjee N, Kladde MP, Bartholomew B (2010) SWI/SNF has intrinsic nucleosome disassembly activity that is dependent on adjacent nucleosomes. Mol Cell 38(4):590–602
Deindl S, Hwang WL, Hota SK, Blosser TR, Prasad P, Bartholomew B, Zhuang X (2013) ISWI remodelers slide nucleosomes with coordinated multi-base-pair entry steps and single-base-pair exit steps. Cell 152(3):442–452
Denslow SA, Wade PA (2007) The human Mi-2/NuRD complex and gene regulation. Oncogene 26(37):5433–5438
Deuring R, Fanti L, Armstrong JA, Sarte M, Papoulas O, Prestel M, Daubresse G, Verardo M, Moseley SL, Berloco M, Tsukiyama T, Wu C, Pimpinelli S, Tamkun JW (2000) The ISWI chromatin-remodeling protein is required for gene expression and the maintenance of higher order chromatin structure in vivo. Mol Cell 5(2):355–365
Dimova D, Nackerdien Z, Furgeson S, Eguchi S, Osley MA (1999) A role for transcriptional repressors in targeting the yeast Swi/Snf complex. Mol Cell 4(1):75–83
Dion V, Shimada K, Gasser SM (2010) Actin-related proteins in the nucleus: life beyond chromatin remodelers. Curr Opin Cell Biol 22(3):383–391
Dorigo B, Schalch T, Bystricky K, Richmond TJ (2003) Chromatin fiber folding: requirement for the histone H4 N-terminal tail. J Mol Biol 327(1):85–96
Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J (2004) Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 16(6):979–990
Doyen CM, An W, Angelov D, Bondarenko V, Mietton F, Studitsky VM, Hamiche A, Roeder RG, Bouvet P, Dimitrov S (2006) Mechanism of polymerase II transcription repression by the histone variant macroH2A. Mol Cell Biol 26(3):1156–1164
Drane P, Ouararhni K, Depaux A, Shuaib M, Hamiche A (2010) The death-associated protein DAXX is a novel histone chaperone involved in the replication-independent deposition of H3.3. Genes Dev 24(12):1253–1265
Drost J, Mantovani F, Tocco F, Elkon R, Comel A, Holstege H, Kerkhoven R, Jonkers J, Voorhoeve PM, Agami R, Del Sal G (2010) BRD7 is a candidate tumour suppressor gene required for p53 function. Nat Cell Biol 12(4):380–389
Durand-Dubief M, Will WR, Petrini E, Theodorou D, Harris RR, Crawford MR, Paszkiewicz K, Krueger F, Correra RM, Vetter AT, Miller JR, Kent NA, Varga-Weisz P (2012) SWI/SNF-like chromatin remodeling factor Fun30 supports point centromere function in S. cerevisiae. PLoS Genet 8(9):e1002974
Eapen VV, Sugawara N, Tsabar M, Wu WH, Haber JE (2012) The Saccharomyces cerevisiae chromatin remodeler Fun30 regulates DNA end resection and checkpoint deactivation. Mol Cell Biol 32(22):4727–4740
Eberharter A, Vetter I, Ferreira R, Becker PB (2004) ACF1 improves the effectiveness of nucleosome mobilization by ISWI through PHD-histone contacts. EMBO J 23(20):4029–4039
Eckey M, Kuphal S, Straub T, Rummele P, Kremmer E, Bosserhoff AK, Becker PB (2012) Nucleosome remodeler SNF2L suppresses cell proliferation and migration and attenuates Wnt signaling. Mol Cell Biol 32(13):2359–2371
Ehrensberger AH, Kornberg RD (2011) Isolation of an activator-dependent, promoter-specific chromatin remodeling factor. Proc Natl Acad Sci USA 108(25):10115–10120
Elsasser SJ, Huang H, Lewis PW, Chin JW, Allis CD, Patel DJ (2012) DAXX envelops a histone H3.3-H4 dimer for H3.3-specific recognition. Nature 491(7425):560–565
Emelyanov AV, Vershilova E, Ignatyeva MA, Pokrovsky DK, Lu X, Konev AY, Fyodorov DV (2012) Identification and characterization of ToRC, a novel ISWI-containing ATP-dependent chromatin assembly complex. Genes Dev 26(6):603–614
Engelen E, Akinci U, Bryne JC, Hou J, Gontan C, Moen M, Szumska D, Kockx C, van Ijcken W, Dekkers DH, Demmers J, Rijkers EJ, Bhattacharya S, Philipsen S, Pevny LH, Grosveld FG, Rottier RJ, Lenhard B, Poot RA (2011) Sox2 cooperates with Chd7 to regulate genes that are mutated in human syndromes. Nat Genet 43(6):607–611
Falbo KB, Alabert C, Katou Y, Wu S, Han J, Wehr T, Xiao J, He X, Zhang Z, Shi Y, Shirahige K, Pasero P, Shen X (2009) Involvement of a chromatin remodeling complex in damage tolerance during DNA replication. Nat Struct Mol Biol 16(11):1167–1172
Farrona S, Hurtado L, March-Diaz R, Schmitz RJ, Florencio FJ, Turck F, Amasino RM, Reyes JC (2011) Brahma is required for proper expression of the floral repressor FLC in Arabidopsis. PloS One 6(3):e17997
Fasulo B, Deuring R, Murawska M, Gause M, Dorighi KM, Schaaf CA, Dorsett D, Brehm A, Tamkun JW (2012) The Drosophila MI-2 chromatin-remodeling factor regulates higher-order chromatin structure and cohesin dynamics in vivo. PLoS Genet 8(8):e1002878
Fazzio TG, Huff JT, Panning B (2008) An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell 134(1):162–174
Ferreira H, Flaus A, Owen-Hughes T (2007a) Histone modifications influence the action of Snf2 family remodelling enzymes by different mechanisms. J Mol Biol 374(3):563–579
Ferreira R, Eberharter A, Bonaldi T, Chioda M, Imhof A, Becker PB (2007b) Site-specific acetylation of ISWI by GCN5. BMC Mol Biol 8:73
Flanagan JF, Peterson CL (1999) A role for the yeast SWI/SNF complex in DNA replication. Nucleic Acids Res 27(9):2022–2028
Flanagan JF, Mi LZ, Chruszcz M, Cymborowski M, Clines KL, Kim Y, Minor W, Rastinejad F, Khorasanizadeh S (2005) Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature 438(7071):1181–1185
Flaus A, Owen-Hughes T (2011) Mechanisms for ATP-dependent chromatin remodelling: the means to the end. FEBS J 278(19):3579–3595
Flaus A, Martin DM, Barton GJ, Owen-Hughes T (2006) Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res 34(10):2887–2905
Flowers S, Nagl NG Jr, Beck GR Jr, Moran E (2009) Antagonistic roles for BRM and BRG1 SWI/SNF complexes in differentiation. J Biol Chem 284(15):10067–10075
Forcales SV, Albini S, Giordani L, Malecova B, Cignolo L, Chernov A, Coutinho P, Saccone V, Consalvi S, Williams R, Wang K, Wu Z, Baranovskaya S, Miller A, Dilworth FJ, Puri PL (2012) Signal-dependent incorporation of MyoD-BAF60c into Brg1-based SWI/SNF chromatin-remodelling complex. EMBO J 31(2):301–316
Ford J, Odeyale O, Shen CH (2008) Activator-dependent recruitment of SWI/SNF and INO80 during INO1 activation. Biochem Biophys Res Commun 373(4):602–606
Fu J, Qin L, He T, Qin J, Hong J, Wong J, Liao L, Xu J (2011) The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Res 21(2):275–289
Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA (2003) MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell 113(2):207–219
Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA (2004) MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell 119(1):75–86
Fyodorov DV, Blower MD, Karpen GH, Kadonaga JT (2004) Acf1 confers unique activities to ACF/CHRAC and promotes the formation rather than disruption of chromatin in vivo. Genes Dev 18(2):170–183
Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z (2008) ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci USA 105(18):6656–6661
Gaspar-Maia A, Alajem A, Polesso F, Sridharan R, Mason MJ, Heidersbach A, Ramalho-Santos J, McManus MT, Plath K, Meshorer E, Ramalho-Santos M (2009) Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature 460(7257):863–868
Gelbart ME, Larschan E, Peng S, Park PJ, Kuroda MI (2009) Drosophila MSL complex globally acetylates H4K16 on the male X chromosome for dosage compensation. Nat Struct Mol Biol 16(8):825–832
Gendrel AV, Lippman Z, Yordan C, Colot V, Martienssen RA (2002) Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science 297(5588):1871–1873
Geng F, Cao Y, Laurent BC (2001) Essential roles of Snf5p in Snf-Swi chromatin remodeling in vivo. Mol Cell Biol 21(13):4311–4320
Gerhold CB, Winkler DD, Lakomek K, Seifert FU, Fenn S, Kessler B, Witte G, Luger K, Hopfner KP (2012) Structure of actin-related protein 8 and its contribution to nucleosome binding. Nucleic Acids Res 40(21):11036–11046
Gibbons RJ, Pellagatti A, Garrick D, Wood WG, Malik N, Ayyub H, Langford C, Boultwood J, Wainscoat JS, Higgs DR (2003) Identification of acquired somatic mutations in the gene encoding chromatin-remodeling factor ATRX in the alpha-thalassemia myelodysplasia syndrome (ATMDS). Nat Genet 34(4):446–449
Gkikopoulos T, Schofield P, Singh V, Pinskaya M, Mellor J, Smolle M, Workman JL, Barton GJ, Owen-Hughes T (2011a) A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science 333(6050):1758–1760
Gkikopoulos T, Singh V, Tsui K, Awad S, Renshaw MJ, Scholfield P, Barton GJ, Nislow C, Tanaka TU, Owen-Hughes T (2011b) The SWI/SNF complex acts to constrain distribution of the centromeric histone variant Cse4. EMBO J 30(10):1919–1927
Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, Dewell S, Law M, Guo X, Li X, Wen D, Chapgier A, DeKelver RC, Miller JC, Lee YL, Boydston EA, Holmes MC, Gregory PD, Greally JM, Rafii S, Yang C, Scambler PJ, Garrick D, Gibbons RJ, Higgs DR, Cristea IM, Urnov FD, Zheng D, Allis CD (2010) Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140(5):678–691
Goldman JA, Garlick JD, Kingston RE (2010) Chromatin remodeling by imitation switch (ISWI) class ATP-dependent remodelers is stimulated by histone variant H2A.Z. J Biol Chem 285(7):4645–4651
Goldmark JP, Fazzio TG, Estep PW, Church GM, Tsukiyama T (2000) The Isw2 chromatin remodeling complex represses early meiotic genes upon recruitment by Ume6p. Cell 103(3):423–433
Gong F, Fahy D, Smerdon MJ (2006) Rad4-Rad23 interaction with SWI/SNF links ATP-dependent chromatin remodeling with nucleotide excision repair. Nat Struct Mol Biol 13(10):902–907
Goodarzi AA, Kurka T, Jeggo PA (2011) KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat Struct Mol Biol 18(7):831–839
Gottschalk AJ, Timinszky G, Kong SE, Jin J, Cai Y, Swanson SK, Washburn MP, Florens L, Ladurner AG, Conaway JW, Conaway RC (2009) Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc Natl Acad Sci USA 106(33):13770–13774
Gottschalk AJ, Trivedi RD, Conaway JW, Conaway RC (2012) Activation of the SNF2 family ATPase ALC1 by poly(ADP-ribose) in a stable ALC1.PARP1.nucleosome intermediate. J Biol Chem 287(52):43527–43532
Grune T, Brzeski J, Eberharter A, Clapier CR, Corona DF, Becker PB, Muller CW (2003) Crystal structure and functional analysis of a nucleosome recognition module of the remodeling factor ISWI. Mol Cell 12(2):449–460
Gururaj AE, Singh RR, Rayala SK, Holm C, den Hollander P, Zhang H, Balasenthil S, Talukder AH, Landberg G, Kumar R (2006) MTA1, a transcriptional activator of breast cancer amplified sequence 3. Proc Natl Acad Sci USA 103(17):6670–6675
Hakimi MA, Bochar DA, Schmiesing JA, Dong Y, Barak OG, Speicher DW, Yokomori K, Shiekhattar R (2002) A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature 418(6901):994–998
Hamiche A, Kang JG, Dennis C, Xiao H, Wu C (2001) Histone tails modulate nucleosome mobility and regulate ATP-dependent nucleosome sliding by NURF. Proc Natl Acad Sci USA 98(25):14316–14321
Han HJ, Russo J, Kohwi Y, Kohwi-Shigematsu T (2008) SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature 452(7184):187–193
Hanai K, Furuhashi H, Yamamoto T, Akasaka K, Hirose S (2008) RSF governs silent chromatin formation via histone H2Av replacement. PLoS Genet 4(2):e1000011
Hang CT, Yang J, Han P, Cheng HL, Shang C, Ashley E, Zhou B, Chang CP (2010) Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466(7302):62–67
Harada A, Okada S, Konno D, Odawara J, Yoshimi T, Yoshimura S, Kumamaru H, Saiwai H, Tsubota T, Kurumizaka H, Akashi K, Tachibana T, Imbalzano AN, Ohkawa Y (2012) Chd2 interacts with H3.3 to determine myogenic cell fate. EMBO J 31(13):2994–3007
Hartlepp KF, Fernandez-Tornero C, Eberharter A, Grune T, Muller CW, Becker PB (2005) The histone fold subunits of Drosophila CHRAC facilitate nucleosome sliding through dynamic DNA interactions. Mol Cell Biol 25(22):9886–9896
Hassan AH, Prochasson P, Neely KE, Galasinski SC, Chandy M, Carrozza MJ, Workman JL (2002) Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111(3):369–379
Hauk G, McKnight JN, Nodelman IM, Bowman GD (2010) The chromodomains of the Chd1 chromatin remodeler regulate DNA access to the ATPase motor. Mol Cell 39(5):711–723
He X, Fan HY, Narlikar GJ, Kingston RE (2006) Human ACF1 alters the remodeling strategy of SNF2h. J Biol Chem 281(39):28636–28647
He X, Fan HY, Garlick JD, Kingston RE (2008) Diverse regulation of SNF2h chromatin remodeling by noncatalytic subunits. Biochemistry 47(27):7025–7033
Henderson A, Holloway A, Reeves R, Tremethick DJ (2004) Recruitment of SWI/SNF to the human immunodeficiency virus type 1 promoter. Mol Cell Biol 24(1):389–397
Hill DA, Imbalzano AN (2000) Human SWI/SNF nucleosome remodeling activity is partially inhibited by linker histone H1. Biochemistry 39(38):11649–11656
Hirochika H, Okamoto H, Kakutani T (2000) Silencing of retrotransposons in arabidopsis and reactivation by the ddm1 mutation. Plant Cell 12(3):357–369
Hirschhorn JN, Brown SA, Clark CD, Winston F (1992) Evidence that SNF2/SWI2 and SNF5 activate transcription in yeast by altering chromatin structure. Genes Dev 6(12A):2288–2298
Ho L, Crabtree GR (2010) Chromatin remodelling during development. Nature 463(7280):474–484
Ho L, Jothi R, Ronan JL, Cui K, Zhao K, Crabtree GR (2009a) An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci USA 106(13):5187–5191
Ho L, Ronan JL, Wu J, Staahl BT, Chen L, Kuo A, Lessard J, Nesvizhskii AI, Ranish J, Crabtree GR (2009b) An embryonic stem cell chromatin remodeling complex, esBAF, is essential for embryonic stem cell self-renewal and pluripotency. Proc Natl Acad Sci USA 106(13):5181–5186
Hochheimer A, Zhou S, Zheng S, Holmes MC, Tjian R (2002) TRF2 associates with DREF and directs promoter-selective gene expression in Drosophila. Nature 420(6914):439–445
Hogan CJ, Aligianni S, Durand-Dubief M, Persson J, Will WR, Webster J, Wheeler L, Mathews CK, Elderkin S, Oxley D, Ekwall K, Varga-Weisz PD (2010) Fission yeast Iec1-ino80-mediated nucleosome eviction regulates nucleotide and phosphate metabolism. Mol Cell Biol 30(3):657–674
Hood RL, Lines MA, Nikkel SM, Schwartzentruber J, Beaulieu C, Nowaczyk MJ, Allanson J, Kim CA, Wieczorek D, Moilanen JS, Lacombe D, Gillessen-Kaesbach G, Whiteford ML, Quaio CR, Gomy I, Bertola DR, Albrecht B, Platzer K, McGillivray G, Zou R, McLeod DR, Chudley AE, Chodirker BN, Marcadier J, Majewski J, Bulman DE, White SM, Boycott KM (2012) Mutations in SRCAP, encoding SNF2-related CREBBP activator protein, cause floating-harbor syndrome. Am J Hum Genet 90(2):308–313
Horn PJ, Carruthers LM, Logie C, Hill DA, Solomon MJ, Wade PA, Imbalzano AN, Hansen JC, Peterson CL (2002) Phosphorylation of linker histones regulates ATP-dependent chromatin remodeling enzymes. Nat Struct Biol 9(4):263–267
Hsiao PW, Fryer CJ, Trotter KW, Wang W, Archer TK (2003) BAF60a mediates critical interactions between nuclear receptors and the BRG1 chromatin-remodeling complex for transactivation. Mol Cell Biol 23(17):6210–6220
Hsu JM, Huang J, Meluh PB, Laurent BC (2003) The yeast RSC chromatin-remodeling complex is required for kinetochore function in chromosome segregation. Mol Cell Biol 23(9):3202–3215
Huang J, Laurent BC (2004) A Role for the RSC chromatin remodeler in regulating cohesion of sister chromatid arms. Cell Cycle 3(8):973–975
Huang X, Gao X, Diaz-Trelles R, Ruiz-Lozano P, Wang Z (2008) Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev Biol 319(2):258–266
Hurd EA, Capers PL, Blauwkamp MN, Adams ME, Raphael Y, Poucher HK, Martin DM (2007) Loss of Chd7 function in gene-trapped reporter mice is embryonic lethal and associated with severe defects in multiple developing tissues. Mamm Genome 18(2):94–104
Hurd EA, Poucher HK, Cheng K, Raphael Y, Martin DM (2010) The ATP-dependent chromatin remodeling enzyme CHD7 regulates pro-neural gene expression and neurogenesis in the inner ear. Development 137(18):3139–3150
Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142(3):375–386
Ishihara K, Oshimura M, Nakao M (2006) CTCF-dependent chromatin insulator is linked to epigenetic remodeling. Mol Cell 23(5):733–742
Ivanov AV, Peng H, Yurchenko V, Yap KL, Negorev DG, Schultz DC, Psulkowski E, Fredericks WJ, White DE, Maul GG, Sadofsky MJ, Zhou MM, Rauscher FJ III (2007) PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol Cell 28(5):823–837
Iwase S, Xiang B, Ghosh S, Ren T, Lewis PW, Cochrane JC, Allis CD, Picketts DJ, Patel DJ, Li H, Shi Y (2011) ATRX ADD domain links an atypical histone methylation recognition mechanism to human mental-retardation syndrome. Nat Struct Mol Biol 18(7):769–776
Jackson EM, Sievert AJ, Gai X, Hakonarson H, Judkins AR, Tooke L, Perin JC, Xie H, Shaikh TH, Biegel JA (2009) Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res 15(6):1923–1930
Jha S, Dutta A (2009) RVB1/RVB2: running rings around molecular biology. Mol Cell 34(5):521–533
Jin C, Zang C, Wei G, Cui K, Peng W, Zhao K, Felsenfeld G (2009) H3.3/H2A.Z double variant-containing nucleosomes mark ‘nucleosome-free regions’ of active promoters and other regulatory regions. Nat Genet 41(8):941–945
Jonsson ZO, Jha S, Wohlschlegel JA, Dutta A (2004) Rvb1p/Rvb2p recruit Arp5p and assemble a functional Ino80 chromatin remodeling complex. Mol Cell 16(3):465–477
Kadam S, Emerson BM (2003) Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol Cell 11(2):377–389
Kaji K, Caballero IM, MacLeod R, Nichols J, Wilson VA, Hendrich B (2006) The NuRD component Mbd3 is required for pluripotency of embryonic stem cells. Nat Cell Biol 8(3):285–292
Kandasamy MK, McKinney EC, Deal RB, Smith AP, Meagher RB (2009) Arabidopsis actin-related protein ARP5 in multicellular development and DNA repair. Dev Biol 335(1):22–32
Kashiwaba S, Kitahashi K, Watanabe T, Onoda F, Ohtsu M, Murakami Y (2010) The mammalian INO80 complex is recruited to DNA damage sites in an ARP8 dependent manner. Biochem Biophys Res Commun 402(4):619–625
Kasten M, Szerlong H, Erdjument-Bromage H, Tempst P, Werner M, Cairns BR (2004) Tandem bromodomains in the chromatin remodeler RSC recognize acetylated histone H3 Lys14. EMBO J 23(6):1348–1359
Kazantseva A, Sepp M, Kazantseva J, Sadam H, Pruunsild P, Timmusk T, Neuman T, Palm K (2009) N-terminally truncated BAF57 isoforms contribute to the diversity of SWI/SNF complexes in neurons. J Neurochem 109(3):807–818
Kehle J, Beuchle D, Treuheit S, Christen B, Kennison JA, Bienz M, Muller J (1998) dMi-2, a hunchback-interacting protein that functions in polycomb repression. Science 282(5395):1897–1900
Kim JH, Saraf A, Florens L, Washburn M, Workman JL (2010) Gcn5 regulates the dissociation of SWI/SNF from chromatin by acetylation of Swi2/Snf2. Genes Dev 24(24):2766–2771
Kitayama K, Kamo M, Oma Y, Matsuda R, Uchida T, Ikura T, Tashiro S, Ohyama T, Winsor B, Harata M (2009) The human actin-related protein hArp5: nucleo-cytoplasmic shuttling and involvement in DNA repair. Exp Cell Res 315(2):206–217
Knizewski L, Ginalski K, Jerzmanowski A (2008) Snf2 proteins in plants: gene silencing and beyond. Trends Plant Sci 13(10):557–565
Konev AY, Tribus M, Park SY, Podhraski V, Lim CY, Emelyanov AV, Vershilova E, Pirrotta V, Kadonaga JT, Lusser A, Fyodorov DV (2007) CHD1 motor protein is required for deposition of histone variant H3.3 into chromatin in vivo. Science 317(5841):1087–1090
Kornberg RD (1974) Chromatin structure: a repeating unit of histones and DNA. Science 184(139):868–871
Krasteva V, Buscarlet M, Diaz-Tellez A, Bernard MA, Crabtree GR, Lessard JA (2012) The BAF53a subunit of SWI/SNF-like BAF complexes is essential for hemopoietic stem cell function. Blood 120(24):4720–4732
Kukimoto I, Elderkin S, Grimaldi M, Oelgeschlager T, Varga-Weisz PD (2004) The histone-fold protein complex CHRAC-15/17 enhances nucleosome sliding and assembly mediated by ACF. Mol Cell 13(2):265–277
Kunert N, Wagner E, Murawska M, Klinker H, Kremmer E, Brehm A (2009) dMec: a novel Mi-2 chromatin remodelling complex involved in transcriptional repression. EMBO J 28(5):533–544
Kusch T, Florens L, Macdonald WH, Swanson SK, Glaser RL, Yates JR III, Abmayr SM, Washburn MP, Workman JL (2004) Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 306(5704):2084–2087
Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR (1994) Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex [see comments]. Nature 370(6489):477–481
Kwon SY, Xiao H, Glover BP, Tjian R, Wu C, Badenhorst P (2008) The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev Biol 316(2):538–547
Kwon SY, Xiao H, Wu C, Badenhorst P (2009) Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities. PLoS Genet 5(7):e1000574
Lai AY, Wade PA (2011) Cancer biology and NuRD: a multifaceted chromatin remodelling complex. Nat Rev Cancer 11(8):588–596
Lake RJ, Geyko A, Hemashettar G, Zhao Y, Fan HY (2010) UV-induced association of the CSB remodeling protein with chromatin requires ATP-dependent relief of N-terminal autorepression. Mol Cell 37(2):235–246
Lake RJ, Basheer A, Fan HY (2011) Reciprocally regulated chromatin association of Cockayne syndrome protein B and p53 protein. J Biol Chem 286(40):34951–34958
Lan L, Ui A, Nakajima S, Hatakeyama K, Hoshi M, Watanabe R, Janicki SM, Ogiwara H, Kohno T, Kanno S, Yasui A (2010) The ACF1 complex is required for DNA double-strand break repair in human cells. Mol Cell 40(6):976–987
Landry JW, Banerjee S, Taylor B, Aplan PD, Singer A, Wu C (2011) Chromatin remodeling complex NURF regulates thymocyte maturation. Genes Dev 25(3):275–286
Larschan E, Bishop EP, Kharchenko PV, Core LJ, Lis JT, Park PJ, Kuroda MI (2011) X chromosome dosage compensation via enhanced transcriptional elongation in Drosophila. Nature 471(7336):115–118
Larsen DH, Poinsignon C, Gudjonsson T, Dinant C, Payne MR, Hari FJ, Rendtlew Danielsen JM, Menard P, Sand JC, Stucki M, Lukas C, Bartek J, Andersen JS, Lukas J (2010) The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J Cell Biol 190(5):731–740
Law MJ, Lower KM, Voon HP, Hughes JR, Garrick D, Viprakasit V, Mitson M, De Gobbi M, Marra M, Morris A, Abbott A, Wilder SP, Taylor S, Santos GM, Cross J, Ayyub H, Jones S, Ragoussis J, Rhodes D, Dunham I, Higgs DR, Gibbons RJ (2010) ATR-X syndrome protein targets tandem repeats and influences allele-specific expression in a size-dependent manner. Cell 143(3):367–378
Lee K, Kang MJ, Kwon SJ, Kwon YK, Kim KW, Lim JH, Kwon H (2007) Expansion of chromosome territories with chromatin decompaction in BAF53-depleted interphase cells. Mol Biol Cell 18(10):4013–4023
Lee HS, Park JH, Kim SJ, Kwon SJ, Kwon J (2010) A cooperative activation loop among SWI/SNF, gamma-H2AX and H3 acetylation for DNA double-strand break repair. EMBO J 29(8):1434–1445
Lemon B, Inouye C, King DS, Tjian R (2001) Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature 414(6866):924–928
Leschziner AE, Lemon B, Tjian R, Nogales E (2005) Structural studies of the human PBAF chromatin-remodeling complex. Structure (Camb) 13(2):267–275
Leschziner AE, Saha A, Wittmeyer J, Zhang Y, Bustamante C, Cairns BR, Nogales E (2007) Conformational flexibility in the chromatin remodeler RSC observed by electron microscopy and the orthogonal tilt reconstruction method. Proc Natl Acad Sci USA 104(12):4913–4918
Lessard J, Wu JI, Ranish JA, Wan M, Winslow MM, Staahl BT, Wu H, Aebersold R, Graef IA, Crabtree GR (2007) An essential switch in subunit composition of a chromatin remodeling complex during neural development. Neuron 55(2):201–215
Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD (2010) Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci USA 107(32):14075–14080
Li J, Santoro R, Koberna K, Grummt I (2005) The chromatin remodeling complex NoRC controls replication timing of rRNA genes. EMBO J 24(1):120–127
Li J, Langst G, Grummt I (2006) NoRC-dependent nucleosome positioning silences rRNA genes. EMBO J 25(24):5735–5741
Li M, Belozerov VE, Cai HN (2010) Modulation of chromatin boundary activities by nucleosome-remodeling activities in Drosophila melanogaster. Mol Cell Biol 30(4):1067–1076
Li X, Wang S, Li Y, Deng C, Steiner LA, Xiao H, Wu C, Bungert J, Gallagher PG, Felsenfeld G, Qiu Y, Huang S (2011) Chromatin boundaries require functional collaboration between the hSET1 and NURF complexes. Blood 118(5):1386–1394
Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, Henkelman RM, Wrana JL, Rossant J, Bruneau BG (2004) Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature 432(7013):107–112
Linder B, Mentele E, Mansperger K, Straub T, Kremmer E, Rupp RA (2007) CHD4/Mi-2beta activity is required for the positioning of the mesoderm/neuroectoderm boundary in Xenopus. Genes Dev 21(8):973–983
Logie C, Peterson CL (1997) Catalytic activity of the yeast SWI/SNF complex on reconstituted nucleosome arrays. EMBO J 16(22):6772–6782
Lorch Y, Maier-Davis B, Kornberg RD (2006) Chromatin remodeling by nucleosome disassembly in vitro. Proc Natl Acad Sci USA 103(9):3090–3093
Luk E, Ranjan A, Fitzgerald PC, Mizuguchi G, Huang Y, Wei D, Wu C (2010) Stepwise histone replacement by SWR1 requires dual activation with histone H2A.Z and canonical nucleosome. Cell 143(5):725–736
Lusser A, Urwin DL, Kadonaga JT (2005) Distinct activities of CHD1 and ACF in ATP-dependent chromatin assembly. Nat Struct Mol Biol 12(2):160–166
Magdinier F, Wolffe AP (2001) Selective association of the methyl-CpG binding protein MBD2 with the silent p14/p16 locus in human neoplasia. Proc Natl Acad Sci USA 98(9):4990–4995
Maier VK, Chioda M, Rhodes D, Becker PB (2008) ACF catalyses chromatosome movements in chromatin fibres. EMBO J 27(6):817–826
Maltby VE, Martin BJ, Schulze JM, Johnson I, Hentrich T, Sharma A, Kobor MS, Howe L (2012) Histone H3 lysine 36 methylation targets the Isw1b remodeling complex to chromatin. Mol Cell Biol 32(17):3479–3485
March-Diaz R, Reyes JC (2009) The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Mol Plant 2(4):565–577
March-Diaz R, Garcia-Dominguez M, Lozano-Juste J, Leon J, Florencio FJ, Reyes JC (2008) Histone H2A.Z and homologues of components of the SWR1 complex are required to control immunity in Arabidopsis. Plant J 53(3):475–487
Marfella CG, Imbalzano AN (2007) The Chd family of chromatin remodelers. Mutat Res 618(1–2):30–40
Marfella CG, Ohkawa Y, Coles AH, Garlick DS, Jones SN, Imbalzano AN (2006) Mutation of the SNF2 family member Chd2 affects mouse development and survival. J Cell Physiol 209(1):162–171
Mathieu EL, Finkernagel F, Murawska M, Scharfe M, Jarek M, Brehm A (2012) Recruitment of the ATP-dependent chromatin remodeler dMi-2 to the transcribed region of active heat shock genes. Nucleic Acids Res 40(11):4879–4891
Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, Kumar R (2001) Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat Cell Biol 3(1):30–37
McConnell AD, Gelbart ME, Tsukiyama T (2004) Histone fold protein Dls1p is required for Isw2-dependent chromatin remodeling in vivo. Mol Cell Biol 24(7):2605–2613
McDowell TL, Gibbons RJ, Sutherland H, O'Rourke DM, Bickmore WA, Pombo A, Turley H, Gatter K, Picketts DJ, Buckle VJ, Chapman L, Rhodes D, Higgs DR (1999) Localization of a putative transcriptional regulator (ATRX) at pericentromeric heterochromatin and the short arms of acrocentric chromosomes. Proc Natl Acad Sci USA 96(24):13983–13988
McKnight JN, Jenkins KR, Nodelman IM, Escobar T, Bowman GD (2011) Extranucleosomal DNA binding directs nucleosome sliding by Chd1. Mol Cell Biol 31(23):4746–4759
Menon T, Yates JA, Bochar DA (2010) Regulation of androgen-responsive transcription by the chromatin remodeling factor CHD8. Mol Endocrinol 24(6):1165–1174
Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C (2004) ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303(5656):343–348
Moreau JL, Lee M, Mahachi N, Vary J, Mellor J, Tsukiyama T, Goding CR (2003) Regulated displacement of TBP from the PHO8 promoter in vivo requires Cbf1 and the Isw1 chromatin remodeling complex. Mol Cell 11(6):1609–1620
Morettini S, Tribus M, Zeilner A, Sebald J, Campo-Fernandez B, Scheran G, Worle H, Podhraski V, Fyodorov DV, Lusser A (2011) The chromodomains of CHD1 are critical for enzymatic activity but less important for chromatin localization. Nucleic Acids Res 39(8):3103–3115
Morillon A, Karabetsou N, O'Sullivan J, Kent N, Proudfoot N, Mellor J (2003) Isw1 chromatin remodeling ATPase coordinates transcription elongation and termination by RNA polymerase II. Cell 115(4):425–435
Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, Shen X (2004) INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 119(6):767–775
Morrison AJ, Kim JA, Person MD, Highland J, Xiao J, Wehr TS, Hensley S, Bao Y, Shen J, Collins SR, Weissman JS, Delrow J, Krogan NJ, Haber JE, Shen X (2007) Mec1/Tel1 phosphorylation of the INO80 chromatin remodeling complex influences DNA damage checkpoint responses. Cell 130(3):499–511
Moshkin YM, Mohrmann L, van Ijcken WF, Verrijzer CP (2007) Functional differentiation of SWI/SNF remodelers in transcription and cell cycle control. Mol Cell Biol 27(2):651–661
Muchardt C, Reyes JC, Bourachot B, Leguoy E, Yaniv M (1996) The hbrm and BRG-1 proteins, components of the human SNF/SWI complex, are phosphorylated and excluded from the condensed chromosomes during mitosis. EMBO J 15(13):3394–3402
Mueller-Planitz F, Klinker H, Ludwigsen J, Becker PB (2013) The ATPase domain of ISWI is an autonomous nucleosome remodeling machine. Nat Struct Mol Biol 20(1):82–89
Murawska M, Kunert N, van Vugt J, Langst G, Kremmer E, Logie C, Brehm A (2008) dCHD3, a novel ATP-dependent chromatin remodeler associated with sites of active transcription. Mol Cell Biol 28(8):2745–2757
Murawska M, Hassler M, Renkawitz-Pohl R, Ladurner A, Brehm A (2011) Stress-induced PARP activation mediates recruitment of Drosophila Mi-2 to promote heat shock gene expression. PLoS Genet 7(7):e1002206
Murawsky CM, Brehm A, Badenhorst P, Lowe N, Becker PB, Travers AA (2001) Tramtrack69 interacts with the dMi-2 subunit of the Drosophila NuRD chromatin remodelling complex. EMBO Rep 2(12):1089–1094
Musselman CA, Ramirez J, Sims JK, Mansfield RE, Oliver SS, Denu JM, Mackay JP, Wade PA, Hagman J, Kutateladze TG (2012) Bivalent recognition of nucleosomes by the tandem PHD fingers of the CHD4 ATPase is required for CHD4-mediated repression. Proc Natl Acad Sci USA 109(3):787–792
Nagaich AK, Walker DA, Wolford R, Hager GL (2004) Rapid periodic binding and displacement of the glucocorticoid receptor during chromatin remodeling. Mol Cell 14(2):163–174
Nagarajan P, Onami TM, Rajagopalan S, Kania S, Donnell R, Venkatachalam S (2009) Role of chromodomain helicase DNA-binding protein 2 in DNA damage response signaling and tumorigenesis. Oncogene 28(8):1053–1062
Nair SS, Li DQ, Kumar R (2013) A core chromatin remodeling factor instructs global chromatin signaling through multivalent reading of nucleosome codes. Mol Cell 49(4):704–718
Nakayama T, Shimojima T, Hirose S (2012) The PBAP remodeling complex is required for histone H3.3 replacement at chromatin boundaries and for boundary functions. Development 139(24): 4582–4590
Natarajan K, Jackson BM, Zhou H, Winston F, Hinnebusch AG (1999) Transcriptional activation by Gcn4p involves independent interactions with the SWI/SNF complex and the SRB/mediator. Mol Cell 4(4):657–664
Neumann FR, Dion V, Gehlen LR, Tsai-Pflugfelder M, Schmid R, Taddei A, Gasser SM (2012) Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev 26(4):369–383
Neves-Costa A, Will WR, Vetter AT, Miller JR, Varga-Weisz P (2009) The SNF2-family member Fun30 promotes gene silencing in heterochromatic loci. PLoS One 4(12):e8111
Nishiyama M, Skoultchi AI, Nakayama KI (2012) Histone H1 recruitment by CHD8 is essential for suppression of the Wnt-beta-catenin signaling pathway. Mol Cell Biol 32(2):501–512
Ogas J, Kaufmann S, Henderson J, Somerville C (1999) PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA 96(24):13839–13844
Ohshiro K, Rayala SK, Wigerup C, Pakala SB, Natha RS, Gururaj AE, Molli PR, Mansson SS, Ramezani A, Hawley RG, Landberg G, Lee NH, Kumar R (2010) Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator. EMBO Rep 11(9):691–697
Okada M, Okawa K, Isobe T, Fukagawa T (2009) CENP-H-containing complex facilitates centromere deposition of CENP-A in cooperation with FACT and CHD1. Mol Biol Cell 20(18):3986–3995
Onishi M, Liou GG, Buchberger JR, Walz T, Moazed D (2007) Role of the conserved Sir3-BAH domain in nucleosome binding and silent chromatin assembly. Mol Cell 28(6):1015–1028
Papamichos-Chronakis M, Peterson CL (2008) The Ino80 chromatin-remodeling enzyme regulates replisome function and stability. Nat Struct Mol Biol 15(4):338–345
Papamichos-Chronakis M, Krebs JE, Peterson CL (2006) Interplay between Ino80 and Swr1 chromatin remodeling enzymes regulates cell cycle checkpoint adaptation in response to DNA damage. Genes Dev 20(17):2437–2449
Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL (2011) Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell 144(2):200–213
Papoulas O, Daubresse G, Armstrong JA, Jin J, Scott MP, Tamkun JW (2001) The HMG-domain protein BAP111 is important for the function of the BRM chromatin-remodeling complex in vivo. Proc Natl Acad Sci USA 98(10):5728–5733
Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M (2008) Cohesins functionally associate with CTCF on mammalian chromosome arms. Cell 132(3):422–433
Parnell TJ, Huff JT, Cairns BR (2008) RSC regulates nucleosome positioning at Pol II genes and density at Pol III genes. EMBO J 27(1):100–110
Paul S, Kuo A, Schalch T, Vogel H, Joshua-Tor L, McCombie WR, Gozani O, Hammell M, Mills AA (2013) Chd5 requires PHD-mediated histone 3 binding for tumor suppression. Cell Rep 3(1):92–102
Percipalle P, Fomproix N, Cavellan E, Voit R, Reimer G, Kruger T, Thyberg J, Scheer U, Grummt I, Farrants AK (2006) The chromatin remodelling complex WSTF-SNF2h interacts with nuclear myosin 1 and has a role in RNA polymerase I transcription. EMBO Rep 7(5):525–530
Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K (2009) Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol 185(3):397–407
Pinskaya M, Nair A, Clynes D, Morillon A, Mellor J (2009) Nucleosome remodeling and transcriptional repression are distinct functions of Isw1 in Saccharomyces cerevisiae. Mol Cell Biol 29(9):2419–2430
Pointner J, Persson J, Prasad P, Norman-Axelsson U, Stralfors A, Khorosjutina O, Krietenstein N, Svensson JP, Ekwall K, Korber P (2012) CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. EMBO J 31(23):4388–4403
Polo SE, Kaidi A, Baskcomb L, Galanty Y, Jackson SP (2010) Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J 29(18):3130–3139
Poot RA, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, Gilbert N, Ferreira J, Varga-Weisz PD (2004) The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat Cell Biol 6(12):1236–1244
Potts RC, Zhang P, Wurster AL, Precht P, Mughal MR, Wood WH III, Zhang Y, Becker KG, Mattson MP, Pazin MJ (2011) CHD5, a brain-specific paralog of Mi2 chromatin remodeling enzymes, regulates expression of neuronal genes. PLoS One 6(9):e24515
Racki LR, Narlikar GJ (2008) ATP-dependent chromatin remodeling enzymes: two heads are not better, just different. Curr Opin Genet Dev 18(2):137–144
Racki LR, Yang JG, Naber N, Partensky PD, Acevedo A, Purcell TJ, Cooke R, Cheng Y, Narlikar GJ (2009) The chromatin remodeller ACF acts as a dimeric motor to space nucleosomes. Nature 462(7276):1016–1021
Radman-Livaja M, Quan TK, Valenzuela L, Armstrong JA, van Welsem T, Kim T, Lee LJ, Buratowski S, van Leeuwen F, Rando OJ, Hartzog GA (2012) A key role for Chd1 in histone H3 dynamics at the 3' ends of long genes in yeast. PLoS Genet 8(7):e1002811
Raisner RM, Hartley PD, Meneghini MD, Bao MZ, Liu CL, Schreiber SL, Rando OJ, Madhani HD (2005) Histone variant H2A.Z marks the 5' ends of both active and inactive genes in euchromatin. Cell 123(2):233–248
Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST (2006) Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev 20(3):282–296
Ratnakumar K, Duarte LF, LeRoy G, Hasson D, Smeets D, Vardabasso C, Bonisch C, Zeng T, Xiang B, Zhang DY, Li H, Wang X, Hake SB, Schermelleh L, Garcia BA, Bernstein E (2012) ATRX-mediated chromatin association of histone variant macroH2A1 regulates alpha-globin expression. Genes Dev 26(5):433–438
Rattner BP, Yusufzai T, Kadonaga JT (2009) HMGN proteins act in opposition to ATP-dependent chromatin remodeling factors to restrict nucleosome mobility. Mol Cell 34(5):620–626
Rea S, Xouri G, Akhtar A (2007) Males absent on the first (MOF): from flies to humans. Oncogene 26(37):5385–5394
Reddy BA, Bajpe PK, Bassett A, Moshkin YM, Kozhevnikova E, Bezstarosti K, Demmers JA, Travers AA, Verrijzer CP (2010) Drosophila transcription factor Tramtrack69 binds MEP1 to recruit the chromatin remodeler NuRD. Mol Cell Biol 30(21):5234–5244
Reinke H, Horz W (2003) Histones are first hyperacetylated and then lose contact with the activated PHO5 promoter. Mol Cell 11(6):1599–1607
Reynolds N, Latos P, Hynes-Allen A, Loos R, Leaford D, O’Shaughnessy A, Mosaku O, Signolet J, Brennecke P, Kalkan T, Costello I, Humphreys P, Mansfield W, Nakagawa K, Strouboulis J, Behrens A, Bertone P, Hendrich B (2012) NuRD suppresses pluripotency gene expression to promote transcriptional heterogeneity and lineage commitment. Cell Stem Cell 10(5):583–594
Roberts CW, Orkin SH (2004) The SWI/SNF complex–chromatin and cancer. Nat Rev Cancer 4(2):133–142
Rodriguez-Paredes M, Ceballos-Chavez M, Esteller M, Garcia-Dominguez M, Reyes JC (2009) The chromatin remodeling factor CHD8 interacts with elongating RNA polymerase II and controls expression of the cyclin E2 gene. Nucleic Acids Res 37(8):2449–2460
Rowbotham SP, Barki L, Neves-Costa A, Santos F, Dean W, Hawkes N, Choudhary P, Will WR, Webster J, Oxley D, Green CM, Varga-Weisz P, Mermoud JE (2011) Maintenance of silent chromatin through replication requires SWI/SNF-like chromatin remodeler SMARCAD1. Mol Cell 42(3):285–296
Ruthenburg AJ, Li H, Milne TA, Dewell S, McGinty RK, Yuen M, Ueberheide B, Dou Y, Muir TW, Patel DJ, Allis CD (2011) Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 145(5):692–706
Ryan DP, Sundaramoorthy R, Martin D, Singh V, Owen-Hughes T (2011) The DNA-binding domain of the Chd1 chromatin-remodelling enzyme contains SANT and SLIDE domains. EMBO J 30(13):2596–2609
Saeki H, Ohsumi K, Aihara H, Ito T, Hirose S, Ura K, Kaneda Y (2005) Linker histone variants control chromatin dynamics during early embryogenesis. Proc Natl Acad Sci USA 102(16):5697–5702
Saha A, Wittmeyer J, Cairns BR (2005) Chromatin remodeling through directional DNA translocation from an internal nucleosomal site. Nat Struct Mol Biol 12(9):747–755
Sala A, La Rocca G, Burgio G, Kotova E, Di Gesu D, Collesano M, Ingrassia AM, Tulin AV, Corona DF (2008) The nucleosome-remodeling ATPase ISWI is regulated by poly-ADP-ribosylation. PLoS Biol 6(10):e252
Sanchez-Molina S, Mortusewicz O, Bieber B, Auer S, Eckey M, Leonhardt H, Friedl AA, Becker PB (2011) Role for hACF1 in the G2/M damage checkpoint. Nucleic Acids Res 39(19):8445–8456
Sang Y, Silva-Ortega CO, Wu S, Yamaguchi N, Wu MF, Pfluger J, Gillmor CS, Gallagher KL, Wagner D (2012) Mutations in two non-canonical Arabidopsis SWI2/SNF2 chromatin remodeling ATPases cause embryogenesis and stem cell maintenance defects. Plant J. doi:10.1111/tpj.12009
Sankar S, Bell R, Stephens B, Zhuo R, Sharma S, Bearss DJ, Lessnick SL (2012) Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene. doi:10.1038/onc.2012.525
Sansom OJ, Berger J, Bishop SM, Hendrich B, Bird A, Clarke AR (2003) Deficiency of Mbd2 suppresses intestinal tumorigenesis. Nat Genet 34(2):145–147
Saravanan M, Wuerges J, Bose D, McCormack EA, Cook NJ, Zhang X, Wigley DB (2012) Interactions between the nucleosome histone core and Arp8 in the INO80 chromatin remodeling complex. Proc Natl Acad Sci USA 109(51):20883–20888
Schmid A, Fascher KD, Horz W (1992) Nucleosome disruption at the yeast PHO5 promoter upon PHO5 induction occurs in the absence of DNA replication. Cell 71(5):853–864
Schnetz MP, Bartels CF, Shastri K, Balasubramanian D, Zentner GE, Balaji R, Zhang X, Song L, Wang Z, Laframboise T, Crawford GE, Scacheri PC (2009) Genomic distribution of CHD7 on chromatin tracks H3K4 methylation patterns. Genome Res 19(4):590–601
Schnetz MP, Handoko L, Akhtar-Zaidi B, Bartels CF, Pereira CF, Fisher AG, Adams DJ, Flicek P, Crawford GE, Laframboise T, Tesar P, Wei CL, Scacheri PC (2010) CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet 6(7):e1001023
Schubert HL, Wittmeyer J, Kasten MM, Hinata K, Rawling DC, Heroux A, Cairns BR, Hill CP (2013) Structure of an actin-related subcomplex of the SWI/SNF chromatin remodeler. Proc Natl Acad Sci USA 110(9):3345–3350
Schultz DC, Friedman JR, Rauscher FJ III (2001) Targeting histone deacetylase complexes via KRAB-zinc finger proteins: the PHD and bromodomains of KAP-1 form a cooperative unit that recruits a novel isoform of the Mi-2alpha subunit of NuRD. Genes Dev 15(4):428–443
Schwabish MA, Struhl K (2004) Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol Cell Biol 24(23):10111–10117
Schwertman P, Lagarou A, Dekkers DH, Raams A, van der Hoek AC, Laffeber C, Hoeijmakers JH, Demmers JA, Fousteri M, Vermeulen W, Marteijn JA (2012) UV-sensitive syndrome protein UVSSA recruits USP7 to regulate transcription-coupled repair. Nat Genet 44(5):598–602
Segal E, Fondufe-Mittendorf Y, Chen L, Thastrom A, Field Y, Moore IK, Wang JP, Widom J (2006) A genomic code for nucleosome positioning. Nature 442(7104):772–778
Sen P, Vivas P, Dechassa ML, Mooney AM, Poirier MG, Bartholomew B (2013) The SnAC domain of SWI/SNF is a histone anchor required for remodeling. Mol Cell Biol 33(2):360–370
Shain AH, Pollack JR (2013) The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One 8(1):e55119
Shen X, Mizuguchi G, Hamiche A, Wu C (2000) A chromatin remodelling complex involved in transcription and DNA processing. Nature 406(6795):541–544
Shen X, Ranallo R, Choi E, Wu C (2003) Involvement of actin-related proteins in ATP-dependent chromatin remodeling. Mol Cell 12(1):147–155
Shen H, Powers N, Saini N, Comstock CE, Sharma A, Weaver K, Revelo MP, Gerald W, Williams E, Jessen WJ, Aronow BJ, Rosson G, Weissman B, Muchardt C, Yaniv M, Knudsen KE (2008) The SWI/SNF ATPase Brm is a gatekeeper of proliferative control in prostate cancer. Cancer Res 68(24):10154–10162
Shim EY, Hong SJ, Oum JH, Yanez Y, Zhang Y, Lee SE (2007) RSC mobilizes nucleosomes to improve accessibility of repair machinery to the damaged chromatin. Mol Cell Biol 27(5):1602–1613
Shimada K, Oma Y, Schleker T, Kugou K, Ohta K, Harata M, Gasser SM (2008) Ino80 chromatin remodeling complex promotes recovery of stalled replication forks. Curr Biol 18(8):566–575
Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL (2006) Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science 311(5762):844–847
Shur I, Solomon R, Benayahu D (2006) Dynamic interactions of chromatin-related mesenchymal modulator, a chromodomain helicase-DNA-binding protein, with promoters in osteoprogenitors. Stem Cells 24(5):1288–1293
Siatecka M, Xue L, Bieker JJ (2007) Sumoylation of EKLF promotes transcriptional repression and is involved in inhibition of megakaryopoiesis. Mol Cell Biol 27(24):8547–8560
Sif S, Stukenberg PT, Kirschner MW, Kingston RE (1998) Mitotic inactivation of a human SWI/SNF chromatin remodeling complex. Genes Dev 12(18):2842–2851
Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, Hartzog GA, Arndt KM (2003) Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J 22(8):1846–1856
Simon JA, Tamkun JW (2002) Programming off and on states in chromatin: mechanisms of Polycomb and trithorax group complexes. Curr Opin Genet Dev 12(2):210–218
Simpson RT (1990) Nucleosome positioning can affect the function of a cis-acting DNA element in vivo. Nature 343(6256):387–389
Sims RJ III, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D (2005) Human but not yeast CHD1 binds directly and selectively to histone H3 methylated at lysine 4 via its tandem chromodomains. J Biol Chem 280(51):41789–41792
Sinha M, Watanabe S, Johnson A, Moazed D, Peterson CL (2009) Recombinational repair within heterochromatin requires ATP-dependent chromatin remodeling. Cell 138(6):1109–1121
Siriaco G, Deuring R, Chioda M, Becker PB, Tamkun JW (2009) Drosophila ISWI regulates the association of histone H1 with interphase chromosomes in vivo. Genetics 182(3):661–669
Sirinakis G, Clapier CR, Gao Y, Viswanathan R, Cairns BR, Zhang Y (2011) The RSC chromatin remodelling ATPase translocates DNA with high force and small step size. EMBO J 30(12):2364–2372
Skiniotis G, Moazed D, Walz T (2007) Acetylated histone tail peptides induce structural rearrangements in the RSC chromatin remodeling complex. J Biol Chem 282(29):20804–20808
Smolle M, Venkatesh S, Gogol MM, Li H, Zhang Y, Florens L, Washburn MP, Workman JL (2012) Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat Struct Mol Biol 19(9):884–892
Soutourina J, Bordas-Le Floch V, Gendrel G, Flores A, Ducrot C, Dumay-Odelot H, Soularue P, Navarro F, Cairns BR, Lefebvre O, Werner M (2006) Rsc4 connects the chromatin remodeler RSC to RNA polymerases. Mol Cell Biol 26(13):4920–4933
Srinivasan S, Armstrong JA, Deuring R, Dahlsveen IK, McNeill H, Tamkun JW (2005) The Drosophila trithorax group protein Kismet facilitates an early step in transcriptional elongation by RNA Polymerase II. Development 132(7):1623–1635
Srinivasan S, Dorighi KM, Tamkun JW (2008) Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA polymerase II. PLoS Genet 4(10):e1000217
Stielow B, Sapetschnig A, Kruger I, Kunert N, Brehm A, Boutros M, Suske G (2008) Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol Cell 29(6):742–754
Stralfors A, Walfridsson J, Bhuiyan H, Ekwall K (2011) The FUN30 chromatin remodeler, Fft3, protects centromeric and subtelomeric domains from euchromatin formation. PLoS Genet 7(3):e1001334
Strohner R, Nemeth A, Jansa P, Hofmann-Rohrer U, Santoro R, Langst G, Grummt I (2001) NoRC – a novel member of mammalian ISWI-containing chromatin remodeling machines. EMBO J 20(17):4892–4900
Strohner R, Nemeth A, Nightingale KP, Grummt I, Becker PB, Langst G (2004) Recruitment of the nucleolar remodeling complex NoRC establishes ribosomal DNA silencing in chromatin. Mol Cell Biol 24(4):1791–1798
Strohner R, Wachsmuth M, Dachauer K, Mazurkiewicz J, Hochstatter J, Rippe K, Langst G (2005) A ‘loop recapture’ mechanism for ACF-dependent nucleosome remodeling. Nat Struct Mol Biol 12(8):683–690
Sugiyama T, Cam HP, Sugiyama R, Noma K, Zofall M, Kobayashi R, Grewal SI (2007) SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128(3):491–504
Szerlong H, Saha A, Cairns BR (2003) The nuclear actin-related proteins Arp7 and Arp9: a dimeric module that cooperates with architectural proteins for chromatin remodeling. EMBO J 22(12):3175–3187
Szerlong H, Hinata K, Viswanathan R, Erdjument-Bromage H, Tempst P, Cairns BR (2008) The HSA domain binds nuclear actin-related proteins to regulate chromatin-remodeling ATPases. Nat Struct Mol Biol 15(5):469–476
Takeuchi JK, Bruneau BG (2009) Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459(7247):708–711
Takeuchi JK, Lickert H, Bisgrove BW, Sun X, Yamamoto M, Chawengsaksophak K, Hamada H, Yost HJ, Rossant J, Bruneau BG (2007) Baf60c is a nuclear Notch signaling component required for the establishment of left-right asymmetry. Proc Natl Acad Sci USA 104(3):846–851
Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguin P, Holloway AK, Mori AD, Wylie JN, Munson C, Zhu Y, Zhou YQ, Yeh RF, Henkelman RM, Harvey RP, Metzger D, Chambon P, Stainier DY, Pollard KS, Scott IC, Bruneau BG (2011) Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nat Commun 2:187
Tamkun JW, Deuring R, Scott M-P, Kissinger M, Pattatucci AM, Kaufmann TC, Kennison JA (1992) brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell 68:561–572
Tea JS, Luo L (2011) The chromatin remodeling factor Bap55 functions through the TIP60 complex to regulate olfactory projection neuron dendrite targeting. Neural Dev 6:5
Thompson BA, Tremblay V, Lin G, Bochar DA (2008) CHD8 is an ATP-dependent chromatin remodeling factor that regulates beta-catenin target genes. Mol Cell Biol 28(12):3894–3904
Tirosh I, Sigal N, Barkai N (2010) Widespread remodeling of mid-coding sequence nucleosomes by Isw1. Genome Biol 11(5):R49
Treand C, du Chene I, Bres V, Kiernan R, Benarous R, Benkirane M, Emiliani S (2006) Requirement for SWI/SNF chromatin-remodeling complex in Tat-mediated activation of the HIV-1 promoter. EMBO J 25(8):1690–1699
Tsai WW, Wang Z, Yiu TT, Akdemir KC, Xia W, Winter S, Tsai CY, Shi X, Schwarzer D, Plunkett W, Aronow B, Gozani O, Fischle W, Hung MC, Patel DJ, Barton MC (2010) TRIM24 links a non-canonical histone signature to breast cancer. Nature 468(7326):927–932
Tsurusaki Y, Okamoto N, Ohashi H, Kosho T, Imai Y, Hibi-Ko Y, Kaname T, Naritomi K, Kawame H, Wakui K, Fukushima Y, Homma T, Kato M, Hiraki Y, Yamagata T, Yano S, Mizuno S, Sakazume S, Ishii T, Nagai T, Shiina M, Ogata K, Ohta T, Niikawa N, Miyatake S, Okada I, Mizuguchi T, Doi H, Saitsu H, Miyake N, Matsumoto N (2012) Mutations affecting components of the SWI/SNF complex cause Coffin-Siris syndrome. Nat Genet 44(4):376–378
van Attikum H, Fritsch O, Hohn B, Gasser SM (2004) Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 119(6):777–788
van Attikum H, Fritsch O, Gasser SM (2007) Distinct roles for SWR1 and INO80 chromatin remodeling complexes at chromosomal double-strand breaks. EMBO J 26(18):4113–4125
Van Houdt JK, Nowakowska BA, Sousa SB, van Schaik BD, Seuntjens E, Avonce N, Sifrim A, Abdul-Rahman OA, van den Boogaard MJ, Bottani A, Castori M, Cormier-Daire V, Deardorff MA, Filges I, Fryer A, Fryns JP, Gana S, Garavelli L, Gillessen-Kaesbach G, Hall BD, Horn D, Huylebroeck D, Klapecki J, Krajewska-Walasek M, Kuechler A, Lines MA, Maas S, Macdermot KD, McKee S, Magee A, de Man SA, Moreau Y, Morice-Picard F, Obersztyn E, Pilch J, Rosser E, Shannon N, Stolte-Dijkstra I, Van Dijck P, Vilain C, Vogels A, Wakeling E, Wieczorek D, Wilson L, Zuffardi O, van Kampen AH, Devriendt K, Hennekam R, Vermeesch JR (2012) Heterozygous missense mutations in SMARCA2 cause Nicolaides-Baraitser syndrome. Nat Genet 44(4):445–449, S441
VanDemark AP, Kasten MM, Ferris E, Heroux A, Hill CP, Cairns BR (2007) Autoregulation of the rsc4 tandem bromodomain by gcn5 acetylation. Mol Cell 27(5):817–828
Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, Bignell G, Butler A, Cho J, Dalgliesh GL, Galappaththige D, Greenman C, Hardy C, Jia M, Latimer C, Lau KW, Marshall J, McLaren S, Menzies A, Mudie L, Stebbings L, Largaespada DA, Wessels LF, Richard S, Kahnoski RJ, Anema J, Tuveson DA, Perez-Mancera PA, Mustonen V, Fischer A, Adams DJ, Rust A, Chan-on W, Subimerb C, Dykema K, Furge K, Campbell PJ, Teh BT, Stratton MR, Futreal PA (2011) Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469(7331):539–542
Varga-Weisz PD, Becker PB (2006) Regulation of higher-order chromatin structures by nucleosome-remodelling factors. Curr Opin Genet Dev 16(2):151–156
Verdaasdonk JS, Gardner R, Stephens AD, Yeh E, Bloom K (2012) Tension-dependent nucleosome remodeling at the pericentromere in yeast. Mol Biol Cell 23(13):2560–2570
Versteege I, Sevenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, Aurias A, Delattre O (1998) Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394(6689):203–206
Vincent JA, Kwong TJ, Tsukiyama T (2008) ATP-dependent chromatin remodeling shapes the DNA replication landscape. Nat Struct Mol Biol 15(5):477–484
Vintermist A, Bohm S, Sadeghifar F, Louvet E, Mansen A, Percipalle P, Ostlund Farrants AK (2011) The chromatin remodelling complex B-WICH changes the chromatin structure and recruits histone acetyl-transferases to active rRNA genes. PLoS One 6(4):e19184
Vissers LE, van Ravenswaaij CM, Admiraal R, Hurst JA, de Vries BB, Janssen IM, van der Vliet WA, Huys EH, de Jong PJ, Hamel BC, Schoenmakers EF, Brunner HG, Veltman JA, van Kessel AG (2004) Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet 36(9):955–957
Walfridsson J, Bjerling P, Thalen M, Yoo EJ, Park SD, Ekwall K (2005) The CHD remodeling factor Hrp1 stimulates CENP-A loading to centromeres. Nucleic Acids Res 33(9):2868–2879
Walfridsson J, Khorosjutina O, Matikainen P, Gustafsson CM, Ekwall K (2007) A genome-wide role for CHD remodelling factors and Nap1 in nucleosome disassembly. EMBO J 26(12):2868–2879
Wang Z, Zhai W, Richardson JA, Olson EN, Meneses JJ, Firpo MT, Kang C, Skarnes WC, Tjian R (2004) Polybromo protein BAF180 functions in mammalian cardiac chamber maturation. Genes Dev 18(24):3106–3116
Wang L, Baiocchi RA, Pal S, Mosialos G, Caligiuri M, Sif S (2005) The BRG1- and hBRM-associated factor BAF57 induces apoptosis by stimulating expression of the cylindromatosis tumor suppressor gene. Mol Cell Biol 25(18):7953–7965
Wang X, Sansam CG, Thom CS, Metzger D, Evans JA, Nguyen PT, Roberts CW (2009a) Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer Res 69(20):8094–8101
Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y (2009b) LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 138(4):660–672
Wang Y, Wong RH, Tang T, Hudak CS, Yang D, Duncan RE, Sul HS (2013) Phosphorylation and recruitment of BAF60c in chromatin remodeling for lipogenesis in response to insulin. Mol Cell 49(2):283–297
Watson AA, Mahajan P, Mertens HD, Deery MJ, Zhang W, Pham P, Du X, Bartke T, Edlich C, Berridge G, Chen Y, Burgess-Brown NA, Kouzarides T, Wiechens N, Owen-Hughes T, Svergun DI, Gileadi O, Laue ED (2012) The PHD and chromo domains regulate the ATPase activity of the human chromatin remodeler CHD4. J Mol Biol 422(1):3–17
Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, Yahata K, Imamoto F, Aburatani H, Nakao M, Imamoto N, Maeshima K, Shirahige K, Peters JM (2008) Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451(7180):796–801
Whitehouse I, Tsukiyama T (2006) Antagonistic forces that position nucleosomes in vivo. Nat Struct Mol Biol 13(7):633–640
Whitehouse I, Rando OJ, Delrow J, Tsukiyama T (2007) Chromatin remodelling at promoters suppresses antisense transcription. Nature 450(7172):1031–1035
Whyte WA, Bilodeau S, Orlando DA, Hoke HA, Frampton GM, Foster CT, Cowley SM, Young RA (2012) Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature 482(7384):221–225
Wilson BG, Roberts CW (2011) SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer 11(7):481–492
Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, Koellhoffer EC, Pomeroy SL, Orkin SH, Roberts CW (2010) Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer CELL 18(4):316–328
Winston F, Carlson M (1992) Yeast SNF/SWI transcriptional activators and the SPT/SIN chromatin connection. Trends Genet 8(11):387–391
Wong LH, McGhie JD, Sim M, Anderson MA, Ahn S, Hannan RD, George AJ, Morgan KA, Mann JR, Choo KH (2010) ATRX interacts with H3.3 in maintaining telomere structural integrity in pluripotent embryonic stem cells. Genome Res 20(3):351–360
Woudstra EC, Gilbert C, Fellows J, Jansen L, Brouwer J, Erdjument-Bromage H, Tempst P, Svejstrup JQ (2002) A Rad26-Def1 complex coordinates repair and RNA pol II proteolysis in response to DNA damage. Nature 415(6874):929–933
Wu WH, Alami S, Luk E, Wu CH, Sen S, Mizuguchi G, Wei D, Wu C (2005) Swc2 is a widely conserved H2AZ-binding module essential for ATP-dependent histone exchange. Nat Struct Mol Biol 12(12):1064–1071
Wu JI, Lessard J, Olave IA, Qiu Z, Ghosh A, Graef IA, Crabtree GR (2007a) Regulation of dendritic development by neuron-specific chromatin remodeling complexes. Neuron 56(1):94–108
Wu S, Shi Y, Mulligan P, Gay F, Landry J, Liu H, Lu J, Qi HH, Wang W, Nickoloff JA, Wu C (2007b) A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair. Nat Struct Mol Biol 14(12):1165–1172
Wykoff DD, O’Shea EK (2005) Identification of sumoylated proteins by systematic immunoprecipitation of the budding yeast proteome. Mol Cell Proteomics 4(1):73–83
Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD (2006) A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442(7098):86–90
Xi R, Xie T (2005) Stem cell self-renewal controlled by chromatin remodeling factors. Science 310(5753):1487–1489
Xiao H, Sandaltzopoulos R, Wang HM, Hamiche A, Ranallo R, Lee KM, Fu D, Wu C (2001) Dual functions of largest NURF subunit NURF301 in nucleosome sliding and transcription factor interactions. Mol Cell 8(3):531–543
Xie W, Ling T, Zhou Y, Feng W, Zhu Q, Stunnenberg HG, Grummt I, Tao W (2012) The chromatin remodeling complex NuRD establishes the poised state of rRNA genes characterized by bivalent histone modifications and altered nucleosome positions. Proc Natl Acad Sci USA 109(21):8161–8166
Xu Y, Ayrapetov MK, Xu C, Gursoy-Yuzugullu O, Hu Y, Price BD (2012) Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol Cell 48(5):723–733
Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, Qin J, Zhou S, Higgs D, Wang W (2003) The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc Natl Acad Sci USA 100(19):10635–10640
Yadon AN, Van de Mark D, Basom R, Delrow J, Whitehouse I, Tsukiyama T (2010) Chromatin remodeling around nucleosome-free regions leads to repression of noncoding RNA transcription. Mol Cell Biol 30(21):5110–5122
Yajima M, Fairbrother WG, Wessel GM (2012) ISWI contributes to ArsI insulator function in development of the sea urchin. Development 139(19):3613–3622
Yamada K, Frouws TD, Angst B, Fitzgerald DJ, DeLuca C, Schimmele K, Sargent DF, Richmond TJ (2011) Structure and mechanism of the chromatin remodelling factor ISW1a. Nature 472(7344):448–453
Yamane K, Mizuguchi T, Cui B, Zofall M, Noma K, Grewal SI (2011) Asf1/HIRA facilitate global histone deacetylation and associate with HP1 to promote nucleosome occupancy at heterochromatic loci. Mol Cell 41(1):56–66
Yamasaki Y, Nishida Y (2006) Mi-2 chromatin remodeling factor functions in sensory organ development through proneural gene repression in Drosophila. Dev Growth Differ 48(7):411–418
Yan Z, Cui K, Murray DM, Ling C, Xue Y, Gerstein A, Parsons R, Zhao K, Wang W (2005) PBAF chromatin-remodeling complex requires a novel specificity subunit, BAF200, to regulate expression of selective interferon-responsive genes. Genes Dev 19(14):1662–1667
Yan Z, Wang Z, Sharova L, Sharov AA, Ling C, Piao Y, Aiba K, Matoba R, Wang W, Ko MS (2008) BAF250B-associated SWI/SNF chromatin-remodeling complex is required to maintain undifferentiated mouse embryonic stem cells. Stem Cells 26(5):1155–1165
Yang X, Zaurin R, Beato M, Peterson CL (2007) Swi3p controls SWI/SNF assembly and ATP-dependent H2A-H2B displacement. Nat Struct Mol Biol 14(6):540–547
Yao H, Brick K, Evrard Y, Xiao T, Camerini-Otero RD, Felsenfeld G (2010) Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev 24(22):2543–2555
Yasui D, Miyano M, Cai S, Varga-Weisz P, Kohwi-Shigematsu T (2002) SATB1 targets chromatin remodelling to regulate genes over long distances. Nature 419(6907):641–645
Yates JA, Menon T, Thompson BA, Bochar DA (2010) Regulation of HOXA2 gene expression by the ATP-dependent chromatin remodeling enzyme CHD8. FEBS Lett 584(4):689–693
Yen K, Vinayachandran V, Batta K, Koerber RT, Pugh BF (2012) Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell 149(7):1461–1473
Yildirim O, Li R, Hung JH, Chen PB, Dong X, Ee LS, Weng Z, Rando OJ, Fazzio TG (2011) Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell 147(7):1498–1510
Yokoyama H, Rybina S, Santarella-Mellwig R, Mattaj IW, Karsenti E (2009) ISWI is a RanGTP-dependent MAP required for chromosome segregation. J Cell Biol 187(6):813–829
Yoo AS, Staahl BT, Chen L, Crabtree GR (2009) MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature 460(7255):642–646
Yoshida T, Hazan I, Zhang J, Ng SY, Naito T, Snippert HJ, Heller EJ, Qi X, Lawton LN, Williams CJ, Georgopoulos K (2008) The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes Dev 22(9):1174–1189
Yoshida T, Shimada K, Oma Y, Kalck V, Akimura K, Taddei A, Iwahashi H, Kugou K, Ohta K, Gasser SM, Harata M (2010) Actin-related protein Arp6 influences H2A.Z-dependent and -independent gene expression and links ribosomal protein genes to nuclear pores. PLoS Genet 6(4):e1000910
Yoshimura K, Kitagawa H, Fujiki R, Tanabe M, Takezawa S, Takada I, Yamaoka I, Yonezawa M, Kondo T, Furutani Y, Yagi H, Yoshinaga S, Masuda T, Fukuda T, Yamamoto Y, Ebihara K, Li DY, Matsuoka R, Takeuchi JK, Matsumoto T, Kato S (2009) Distinct function of 2 chromatin remodeling complexes that share a common subunit, Williams syndrome transcription factor (WSTF). Proc Natl Acad Sci USA 106(23):9280–9285
Yu EY, Steinberg-Neifach O, Dandjinou AT, Kang F, Morrison AJ, Shen X, Lue NF (2007) Regulation of telomere structure and functions by subunits of the INO80 chromatin remodeling complex. Mol Cell Biol 27(16):5639–5649
Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ (2005) Genome-scale identification of nucleosome positions in S. cerevisiae. Science 309(5734):626–630
Yuan X, Feng W, Imhof A, Grummt I, Zhou Y (2007) Activation of RNA polymerase I transcription by cockayne syndrome group B protein and histone methyltransferase G9a. Mol Cell 27(4):585–595
Yudkovsky N, Logie C, Hahn S, Peterson CL (1999) Recruitment of the SWI/SNF chromatin remodeling complex by transcriptional activators. Genes Dev 13(18):2369–2374
Zemach A, Li Y, Wayburn B, Ben-Meir H, Kiss V, Avivi Y, Kalchenko V, Jacobsen SE, Grafi G (2005) DDM1 binds Arabidopsis methyl-CpG binding domain proteins and affects their subnuclear localization. Plant Cell 17(5):1549–1558
Zhang H, Roberts DN, Cairns BR (2005) Genome-wide dynamics of Htz1, a histone H2A variant that poises repressed/basal promoters for activation through histone loss. Cell 123(2):219–231
Zhang H, Stephens LC, Kumar R (2006a) Metastasis tumor antigen family proteins during breast cancer progression and metastasis in a reliable mouse model for human breast cancer. Clin Cancer Res 12(5):1479–1486
Zhang Y, Smith CL, Saha A, Grill SW, Mihardja S, Smith SB, Cairns BR, Peterson CL, Bustamante C (2006b) DNA translocation and loop formation mechanism of chromatin remodeling by SWI/SNF and RSC. Mol Cell 24(4):559–568
Zhang H, Rider SD Jr, Henderson JT, Fountain M, Chuang K, Kandachar V, Simons A, Edenberg HJ, Romero-Severson J, Muir WM, Ogas J (2008) The CHD3 remodeler PICKLE promotes trimethylation of histone H3 lysine 27. J Biol Chem 283(33):22637–22648
Zhang J, Jackson AF, Naito T, Dose M, Seavitt J, Liu F, Heller EJ, Kashiwagi M, Yoshida T, Gounari F, Petrie HT, Georgopoulos K (2012) Harnessing of the nucleosome-remodeling-deacetylase complex controls lymphocyte development and prevents leukemogenesis. Nat Immunol 13(1):86–94
Zhao K, Wang W, Rando OJ, Xue Y, Swiderek K, Kuo A, Crabtree GR (1998) Rapid and phosphoinositol-dependent binding of the SWI/SNF-like BAF complex to chromatin after T lymphocyte receptor signaling. Cell 95(5):625–636
Zhou Y, Grummt I (2005) The PHD finger/bromodomain of NoRC interacts with acetylated histone H4K16 and is sufficient for rDNA silencing. Curr Biol 15(15):1434–1438
Zhou J, Chau CM, Deng Z, Shiekhattar R, Spindler MP, Schepers A, Lieberman PM (2005) Cell cycle regulation of chromatin at an origin of DNA replication. EMBO J 24(7):1406–1417
Zhu Y, Rowley MJ, Bohmdorfer G, Wierzbicki AT (2013) A SWI/SNF chromatin-remodeling complex acts in noncoding rna-mediated transcriptional silencing. Mol Cell 49(2):298–309
Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S (2008) Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature 456(7218):125–129
Zofall M, Persinger J, Kassabov SR, Bartholomew B (2006) Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome. Nat Struct Mol Biol 13(4):339–346
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Clapier, C.R., Cairns, B.R. (2014). Chromatin Remodeling Complexes. In: Workman, J., Abmayr, S. (eds) Fundamentals of Chromatin. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8624-4_3
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8624-4_3
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8623-7
Online ISBN: 978-1-4614-8624-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)