Abstract
The past decade has witnessed a revolution in the treatment of multiple myeloma as a result of introduction of several new effective drugs, which in conjunction with increased use of autologous stem cell transplantation and improved supportive care strategies have resulted in significantly improved survival outcomes for these patients. The survival of patients with myeloma has more than doubled in the past decade, a success story unparalleled by any other cancer. In addition to the improved armamentarium of therapeutic options, there has been a better understanding of the basic disease biology as well as the heterogeneity seen in the disease, in particular the genetic heterogeneity. This has led to development of risk stratifications systems that is increasingly allowing us to individualize the therapy of patients with multiple myeloma. The general approach to treatment of patients with myeloma can be grouped into seven discrete steps as shown in Table 8.1. A systematic approach to the treatment allows us to judiciously use the available therapeutic options allowing the best possible outcomes for these patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Multiple Myeloma
- Monoclonal Protein
- Lambda Light Chain
- Very Good Partial Response
- Monoclonal Plasma Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The past decade has witnessed a revolution in the treatment of multiple myeloma as a result of introduction of several new effective drugs, which in conjunction with increased use of autologous stem cell transplantation and improved supportive care strategies have resulted in significantly improved survival outcomes for these patients. The survival of patients with myeloma has more than doubled in the past decade, a success story unparalleled by any other cancer. In addition to the improved armamentarium of therapeutic options, there has been a better understanding of the basic disease biology as well as the heterogeneity seen in the disease, in particular the genetic heterogeneity. This has led to development of risk stratifications systems that is increasingly allowing us to individualize the therapy of patients with multiple myeloma. The general approach to treatment of patients with myeloma can be grouped into seven discrete steps as shown in Table 8.1. A systematic approach to the treatment allows us to judiciously use the available therapeutic options allowing the best possible outcomes for these patients.
Diagnosis
The diagnosis of multiple myeloma is essentially a two-step process, the first to establish the presence of a monoclonal plasma cell process and the second to make the determination that it represents active disease requiring therapy. While the first step is more objective based on clear results from a set of laboratory tests, the latter can be more subjective and sometimes challenging.
The diagnosis of a plasma cell proliferative disorder rests on the ability to demonstrate one or more of the following, namely, a monoclonal protein in the serum or urine, and/or the presence of monoclonal plasma cells in the bone marrow, peripheral blood, or discrete soft tissue masses. The demonstration of the monoclonal protein may require one or more of protein electrophoresis performed on serum or urine, immunofixation of serum or urine, and serum free light chain assay. The protein electrophoresis involves charge-based separation of the serum or urine proteins on a gel, which allows detection of the presence of a monoclonal protein. The monoclonal immunoglobulin protein typically migrates to the gamma region, but IgA monoclonal protein and light chains can migrate to the beta region, causing confusion. This test lacks sensitivity and can miss small monoclonal proteins and presence of monoclonal light chain. The next step in the process of monoclonal protein assessment is an immunofixation study, performed on the serum or urine, involving staining with antibodies directed against each of the heavy chains and the kappa and lambda light chains. This allows identification of the type of monoclonal protein in terms of their heavy chains and light chain isotype, as well as detection of small amounts of monoclonal protein otherwise not detected on protein electrophoresis. However, unlike the SPEP or UPEP, IFE is not quantitative. In 0–15 % of patients, both these tests can be negative, a condition previously referred to as nonsecretory myeloma. However, the introduction of the serum free light chain assay allows us to quantitate monoclonal free light chain, kappa or lambda light chain that circulates unbound to the heavy chain, by virtue of its reactivity against epitopes normally hidden when they are bound to the heavy chain. The FLC assay signals the presence of a clonal process when the ratio between the kappa and the lambda FLC is skewed, and more importantly allows quantitation of the clonal chain allowing serial disease monitoring. Between the three tests, over 98 % of patients can be demonstrated to have a monoclonal protein leaving behind a very small minority, who are truly nonsecretory in that they do not secrete any monoclonal protein.
The other component of the diagnosis is demonstration of monoclonal plasma cells, the hallmark of the disease. The plasma cells normally reside in the bone marrow, which is where the clonal plasma cells are typically detected, through a bone marrow aspirate of trephine biopsy. The bone marrow examination gives an estimate of the tumor cell burden in the average patients and can vary anywhere from a normal looking marrow to a marrow almost completely replaced by clonal plasma cells. Unfortunately the marrow involvement in myeloma can be patchy resulting in sampling variations. However, varying numbers of plasma cells can also be detected in circulation in the vast majority of myeloma patients, especially with the use of multiparameter flow cytometry (MFC). Finally, a small proportion of patients will present with soft tissue masses, in association with an area of bone destruction or otherwise, which on biopsy typically shows sheets of monoclonal plasma cells. The demonstration of clonality in the plasma cells depends on their exclusive expression of the kappa or lambda light chain detected by immunohistochemistry, immunofluorescence, or in situ hybridization.
Demonstration of the presence of a monoclonal process is clearly the first step, but even more important is the determination of the need for therapy. MM is but a part of the spectrum of monoclonal disorders that includes MGUS, smoldering multiple myeloma, and symptomatic myeloma. Determination of where it lies in that spectrum determines the course of action, whether to observe or to institute therapy. The entities of MGUS and smoldering myeloma have been described in previous chapters and will not be discussed further. The diagnosis of symptomatic myeloma requiring therapy hinges on the demonstration of end-organ damage from myeloma, which typically includes presence of hyperCalcemia, Renal insufficiency, Anemia, and/or Bone lesions, referred to by the acronym CRAB.
Risk Stratification
Once it has been determined that a patient has myeloma that requires therapy, the next step is to assess the risk status. Risk stratification has become an integral part of the myeloma evaluation as with other cancers and in playing an increasingly important role in the treatment decisions. Various prognostic factors and the different approaches to risk stratification have already been detailed in the previous chapters. From a therapy standpoint, three risk factors play an important role in the selection of treatment; namely the age/performance status, renal function, and the presence or absence of high-risk genetic abnormalities.
Initial Therapy (Induction Therapy)
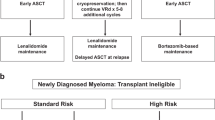
The initial approach to myeloma has seen the most change in the past decade with the advent of the new drugs. While the tools employed have undergone a radical transformation, the basic underlying principles remain same. The goals of the initial therapy are to control the disease process as rapidly as possible and reversing the complications of the disease, while minimizing the toxicity and allowing collection of stem cells for autologous stem cell transplantation when considered appropriate. The early and rapid control of disease without significant toxicity plays an important role in reducing the early mortality that used to be seen previously. Despite the uniform goals, substantial differences exist in terms of the approaches to initial therapy of myeloma, and unfortunately limited data is available from randomized trials to provide firm guidance. We have over the years developed a consensus approach to initial management of myeloma based on a combination of best available data and expert opinion where data is lacking. These guidelines have been published and are freely available on the web at www.msmart.org and are revised several times a year when new and relevant data becomes available (Fig. 8.1).
Risk stratification-based approach to management of myeloma. *Note that a subset of patients with these factors will be classified as high-risk by GEP.†LDH > ULN and beta-2M > 5.5 may indicate worse prognosis. ‡Prognosis is worse when associated with high beta-2M and anemia. **t(11;14) may be associated with plasma cell leukemia. aBortezomib containing regimens preferred in renal failure or if rapid response needed. bIf age >65 or >4 cycles of Rd, consider G-CSF plus cytoxan or plerixafor. cContinuing Rd is optional for patients responding to Rd and with low toxicities; Dex is usually discontinued after first year. dConsider risks and benefits; If used, consider limited duration 12–24 months
Traditionally, the initial therapy of myeloma has been based on whether patients would be considered eligible for autologous stem cell transplantation. This approach was taken to reduce the likelihood of compromised stem cell collection as a result of the use of drugs such as melphalan. However, the determination of transplant eligibility varies significantly across different centers and groups. While the randomized trials have typically included only patients under 65 years of age, there is a wealth of data highlighting the safety and efficacy of SCT in older patients. Over the past decade, the newer drugs have been systematically incorporated into the traditional regimens used in both transplant-eligible and transplant-ineligible patients. In fact, many of the currently used regimens do not significantly impact the ability to collect stem cells and as a result the need to classify patients based on the transplant eligibility has diminished over time. The commonly used regimens along with the response rates and survival outcomes with these regimens are as shown in Table 8.2; the most relevant ones are discussed in more detail below. Results of major randomized trials in transplant-eligible and transplant-ineligible patients are shown in Tables 8.3 and 8.4, respectively.
Lenalidomide/Dexamethasone (Rd)
In previously untreated patients with active MM, initial therapy with Rd results in overall response rates of 91–95 %, with very good partial response (VGPR) or better in 32–38 % [1, 2]. Rajkumar et al. [1] treated 34 patients with lenalidomide 25 mg orally days 1–21 and dexamethasone 40 mg days 1–4, 9–12, and 17–20, both repeated every 28 days. The overall response rate was 91 %, with 6 % achieving complete response and 32 % VGPR. The most common toxicity was neutropenia and fatigue. The 2-year progression-free survival rates for patients proceeding to SCT and patients remaining on Rev-Dex were 83 % and 59 %, respectively; the OS rates were 92 % and 90 % at 2 years and 92 % and 85 % at 3 years, respectively [3]. This was followed by a randomized controlled trial comparing lenalidomide with standard dexamethasone (RD; days 1–4, 9–12, and 17–20 of a 28 day schedule) with lenalidomide with reduced intensity dexamethasone (Rd; weekly dexamethasone) [4]. After 4 months of therapy, 79 % of the RD patients and 68 % of the Rd patients had achieved a partial response or better; however, at 1 year, OS was superior in the Rd arm as compared to the RD arm (92 % versus 87 %, P = 0.0002). The trial was stopped early due to this finding concern, and patients on RD were crossed over to lower dose dexamethasone regimen (Rd). Grade 3–4 AEs and early deaths were higher in the RD group with the most common serious toxicities being DVT, infections, and fatigue.
Based on these trials, Rd has been adopted by as an effective first-line therapy for treatment of newly diagnosed disease. Long-term studies of Rd combination suggest excellent outcomes, with good tolerability and ability to continue on therapy for long periods. The OS of a cohort of 286 patients receiving first-line Rd therapy was nearly 80 % at 5 years. The outcomes among the transplant-eligible patients have been comparable whether or not they proceeded to an early autologous stem cell transplant or chose to have an SCT at the time of their relapse. Moreover, in the non-transplant-eligible patients, the outcomes with Rd as primary therapy have been excellent compared to the historical results.
Bortezomib and Dexamethasone (VD)
Bortezomib was studied as a single agent in the a small phase 2 study by Jagannath et al., with dexamethasone added for lack of adequate response [5]. While Bortezomib as a single agent achieved a 40 % response rate (>PR), the RR further increased to 88 % in combination with dexamethasone. The combination was also compared to VAD as induction therapy prior to SCT in a phase 3 trial, resulting in deeper responses and reduced need for tandem ASCT as well as improved PFS post SCT [6]. In the current era, bortezomib tend to be used more in combination with cyclophosphamide or lenalidomide as described below.
Cyclophosphamide, Bortezomib, and Dexamethasone (CyBorD or VCD)
The new drugs have been combined with alkylators, both cyclophosphamide and melphalan, with excellent results [7, 10, 28, 29]. Reeder et al. treated 33 patients with newly diagnosed MM with four 28 day cycles of bortezomib 1.3 mg/m2 intravenously on days 1, 4, 8, and 11, cyclophosphamide 300 mg/m2 orally on days 1, 8, 15, and 22 and dexamethasone 40 mg orally on days 1–4, 9–12, and 17–20 on a 28-day cycle for four cycles [7]. Responses were rapid with an overall response rate of 88%, and 39 % achieving complete/near complete response. Peripheral neuropathy rate was high at 66 %, with 7 % grade 3. A modified dose schedule of the trial used weekly bortezomib at 1.5 mg/m2 IV on days 1, 8, 15, and 22 and dexamethasone modified to 40 mg once weekly after cycle 2 [28]. Response rates were comparable but with significantly less neuropathy. In another study Kropff et al. treated 30 patients with three 21-day cycles of bortezomib 1.3 mg/m2 on days 1, 4, 8, 11 plus dexamethasone 40 mg on the day of bortezomib injection and the day after plus cyclophosphamide at 900, 1,200, or 1,500 mg/m2 on day 1 [8]. The maximum tolerated dose of cyclophosphamide was defined as 900 mg/m2. Overall response rate was 77 %, with a 10 % CR rate.
Bortezomib, Lenalidomide, and Dexamethasone (VRD)
Richardson studied 66 previously untreated patients in a phase 1/2 study using the combination of bortezomib, lenalidomide, and dexamethasone [9]. Patients received eight 3-week cycles and either proceeded to transplantation or maintenance with bortezomib given at a reduced frequency. All patients responded, with 67 % achieving a VGPR or better. With median follow-up of 21 months, the estimated 18-month PFS and OS for the entire cohort regardless of the use of transplant were 75 % and 97 %, respectively. Sensory neuropathy occurred in 80 % of patients and 32 % reported neuropathic pain.
Another phase 2 study (EVOLUTION) randomized patients to receive either bortezomib, dexamethasone, cyclophosphamide, and lenalidomide (VDCR), bortezomib, dexamethasone, and lenalidomide (VRD), or two different regimens of VCD in 140 previously untreated patients has been reported [10]. A maximum of eight 21-day cycles followed by maintenance bortezomib (1.3 mg/m2 every other week for 24 weeks) was administered. The bortezomib was administered as 1.3 mg/m2 days 1, 4, 8, and 11 and the dexamethasone was administered as 40 mg days 1, 8, and 15 for all patients. The VRD patients received lenalidomide 25 mg days 1–14, whereas the VDCR patients received lenalidomide 15 mg days 1–14 and cyclophosphamide 500 mg/m2 days 1 and 8. The VCD patients received cyclophosphamide 500 mg/m2 days 1 and 8, whereas the VCD-mod patients received cyclophosphamide 500 mg/m2 days 1, 8, and 15. Nearly all patients responded and the VGPR or better (CR) rates were 58 % (25 %), 51 % (24 %), 41 % (22 %), and 53 % (47 %) for patients on VDCR, VDR, VCD, and VCD-mod, respectively. The corresponding 1-year progression-free survival was 86, 83, 93, and 100 %. However, the toxicity was significantly higher in the four-drug arm.
Unfortunately, the different studies have provided therapies for varying durations with or without use of stem cell transplantation making it difficult to compare the survival outcomes associated with specific regimens, and more importantly, the comparison between these regimens. The incorporation of the novel drugs such as IMiDs and the proteasome inhibitors have led to unprecedented response rates and response depth compared to older alkylator and steroid-based therapies. Moreover, combination regimens that include an IMiD and a proteasome inhibitor have led to very high response rates, but at the cost of higher toxicity rates compared to combinations with one or the other. So the debate as to whether to use a combination of both classes of drugs or one or the other, combination versus sequential therapy, continues in the absence of definitive data. One can argue that the endpoints used for assessing the induction therapy should include in addition to the response rates, the associated toxicities and most importantly the benefit in terms of early mortality. However, with the subsequent therapies (such as use of transplant) clouding the long-term outcomes such as overall survival and improvement in short-term outcomes such as avoidance of early death being maximized by any regimen containing at least one of the new drug, it has become difficult to derive conclusion from the available data. Hopefully, as the data matures from the current generation of randomized trials, we will have more definitive answers. In contrast to the question of combination versus sequential therapy, more clarity and consensus exists with respect to use of specific agents in the context of specific high-risk factors (Fig. 8.1). As was discussed in the risk stratification chapter, myeloma can be grouped onto a standard, intermediate, and high-risk categories based primarily on the genetic abnormalities. Our approach, as outlined in the mSMART strategy, is shown in Fig. 8.1.
Transplant-Ineligible Patients
The combination of melphalan and prednisone (MP) has been studied extensively in the non-transplant population and was the standard therapy until the advent of the new drugs [11, 12]. Response rates are from different studies varied from 40 to 60 % and median survival was around 3 years. With the introduction of the new drugs and initial studies showing excellent efficacy when combined with alkylating drugs, a series of phase 3 trials were undertaken examining the impact of adding thalidomide, lenalidomide, or bortezomib to melphalan and prednisone.
Melphalan, Prednisone, and Thalidomide (MPT)
Overall six randomized trials have been reported to date examining the value of adding thalidomide to MP. While all have shown improved response rates and four have shown improved PFS, only three have demonstrated an OS advantage. Meta-analysis of the different trials suggest a clear PFS and OS advantage to the combination; however, the benefit of the combination comes at the cost of considerable increased toxicity.
The initial IFM 99-06 study [13] randomized 447 patients to twelve 6-week cycles of either of MP (melphalan 0.25 mg/kg per day and prednisone 2 mg/kg/day days 1–4 every 6 weeks) or MPT (MP plus 200–400 mg of thalidomide daily) or to two sequential mini-autologous peripheral blood stem cell transplants (MEL100). The thalidomide was not continued past the 12th cycle of therapy. Higher response rates and longer PFS as well as OS were seen with the MPT as compared to either the MP or MEL100 groups. The IFM01-01 [14] in contrast studied patients over the age of 75, who were randomized to twelve 6-week cycles of either of MP (melphalan 0.2 mg/kg per day and prednisone 2 mg/kg/day days 1–4 every 6 weeks) or MPT (MP plus 50–100 mg of thalidomide daily). The combinations resulted in improved PFS and OS, but with increased rates of hematological toxicity as well as neuropathy. In the GIMEMA trial, patients were randomized to either standard dose oral MP for 6 months or to MP for 6 months with concurrent thalidomide, which was then continued indefinitely [15, 16]. Overall response rates were significantly higher with the MPT than the MP, which translated into improved PFS, but long-term results did not confirm the initially observed OS advantage. In the HOVON-49 trial, patients were randomized to either 8 cycles of MP (melphalan 0.25 mg/kg per day and prednisone 2 mg/kg/day days 1–5 every 4 weeks) or MPT (MP plus 200 mg/day thalidomide). The 2 year PFS was higher with MPT (33 % versus 21 %), and OS with MPT was also superior (40 versus 31 months, P < 0.05). In the Nordic study [17], 357 patients were randomized to MP (4 days of melphalan 0.25 mg/kg per day and prednisone 100 mg/day every 6 weeks) or MPT (MP plus 200–400 mg/day thalidomide). Treatment was continued to plateau and the thalidomide was continued until relapse. Although there were superior CR and PR rates in the MPT arm, this did not result in any improvement in PFS or OS between the two groups.
Melphalan, Prednisone, and Lenalidomide (MPR)
MP has also been compared to the combination of melphalan, prednisone, and lenalidomide in a three-arm phase 3 trial [18]: MP versus MP with lenalidomide (MPR) versus MPR with lenalidomide maintenance (MPR-R). Four hundred and fifty-nine patients were randomized to MP (nine 4 week cycles of melphalan 0.18 mg/kg/day and prednisone 2 mg/kg/day days 1–4), MPR (nine 4 week cycles of MP plus lenalidomide 10 mg days 1–21), or nine cycles of MPR with indefinite lenalidomide maintenance (10 mg days 1–21 every 4 weeks). While addition of lenalidomide to MP led to higher response rates, and improved PFS when lenalidomide maintenance was used, there was no difference in the OS between the arms. Toxicity was substantially higher in the lenalidomide arms.
Melphalan, Prednisone, and Bortezomib (VMP)
The VISTA trial [19] compared MP to bortezomib and MP (VMP), with patients receiving nine 6-week cycles of either melphalan (at a dose of 9 mg/m2) and prednisone (60 mg/m2) on days 1–4, alone or in combination with bortezomib (1.3 mg/m2) on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1–4 and on days 1, 8, 22, and 29 during cycles 5–9. Median PFS was 24 months with VMP as compared to 17 months with MP, and 3-year OS was higher for VMP at 68 % compared to 54 % [20]. Grade 3–4 adverse events, however, were more frequent in patients receiving VMP (46 % versus 36 %).
Subsequent trials have sought to build upon the VMP regimen by adding thalidomide to the combination (VMPT) with or without prolonged maintenance therapy. Palumbo and colleagues randomized patients to receive either nine 5-week cycles of VMP or nine 5-week cycles of VMPT, and continued maintenance thalidomide along with alternate week bortezomib. While response rates and PFS were higher in the four-drug combination with maintenance, the OS was not different. Toxicity was significantly higher using the four-drug regimen with more neutropenia, cardiac events, and thromboembolic events. During the course of the trial, the treatment schedule for bortezomib was changed from twice weekly to once weekly, allowing a comparison of the two approaches. It was found that the cumulative dose of bortezomib administered was similar with the two approaches, but with significant reduction in severe sensory peripheral neuropathy from 16 to 3 %. As a result of this study, bortezomib is increasingly being used once weekly as part of different drug combinations.
Consolidation and Maintenance
While the goals of the initial therapy were to rapidly control the disease, reverse the disease-related complications, and ready the patient for stem cell transplantation when indicated, consolidation approaches by definition aim to further build on the gains of the initial therapy. While the concept of consolidation therapy is not as clearly delineated in myeloma as it is with other hematological malignancies like acute leukemia, the broad goals remain the same. Various approaches have been employed as consolidation therapy in myeloma. Traditionally, transplant-eligible patients received 4–6 months of induction therapy with one of the commonly used induction regimens and then received autologous stem cell transplantation, while the transplant-ineligible patients continued on the initial therapy for 12–18 cycles.
For the transplant-eligible patients, SCT has been shown to improve overall survival in several studies when compared to no transplantation. Application of SCT following induction therapy significantly improved the depth of response following the initial therapy, leading to improved progression-free survival as well as overall survival. Based on the results from a series of phase 3 trials, SCT had been considered the standard of care for the younger transplant-eligible patients. Subsequent trials examined the concept of a tandem autologous stem cell transplant compared to a single transplant and showed benefit in a subgroup of patients, where the first transplant failed to achieve a VGPR or better. The results of the various studies and the current concepts regarding SCT in myeloma have been discussed in other chapters.
The distinction between these phases of treatment (induction, consolidation, and maintenance) has increasingly become blurred over the past decade with increasing efficacy of induction regimens with the incorporation of new drugs and more widespread use of maintenance therapy in the post-transplant setting. Prior to the advent of new drugs, the traditional induction regimens, primarily steroid-based, were associated with overall response rates of 40–60 % and complete response rates of less than 10 %, which improved to over 90 % and 30 %, respectively, for overall and complete response with the use of SCT. However, the newer regimens, especially those incorporating both IMiDs and proteasome inhibitors, have led to response rates hitherto only seen in the context on high-dose therapy. Given these results, SCT is increasingly being delayed and used a salvage therapy at the time of disease relapse following initial therapy with various combinations containing the new drugs. These patients, comprising an increasing proportion of patients with myeloma, continue on the initial therapy for prolonged periods reaching the same level of response as would have been seen with a transplant-based consolidation approach with or without maintenance. Based on the data available, this approach has not compromised the overall survival of patients with myeloma, thus shifting the role of SCT from a “consolidation therapy” for all eligible patients to another “treatment regimen” for nearly half of the patients with myeloma who elect to delay the SCT. Along with this, recent trails have shown survival benefit with the use of these new drugs as maintenance approaches following SCT further blurring the lines between these phases of therapy. The pros and cons of maintenance approaches used post SCT have been discussed in depth elsewhere. Finally, the use of prolonged “maintenance approaches both following SCT as well as following non-SCT-based new drug regimens in the transplant-eligible as well as non-transplant-eligible patients have led a remarkable convergence in the treatment approaches across the board for all patients with multiple myeloma in current era.”
Supportive Care
The improvements in the supportive care for MM have significantly contributed towards the improved outcome in patients with myeloma. While this topic is covered more extensively in other sections, it is important to highlight certain aspects of the supportive care approach in myeloma. The most important has been the results of the randomized trials demonstrating a distinct advantage for the use of bisphosphonates in not only reducing the risk of skeletal events, but also improving the overall survival of patients with myeloma. It has become clear that patients with myeloma should be initiated on bisphosphonates at diagnosis irrespective of the presence of bone disease. Aggressive approaches to disease control have led to improvement in renal function early on after diagnosis and clearly contribute to better outcomes. Finally, while randomized trials have failed to demonstrate a benefit for prophylactic antibiotics, aggressive treatment of infections in the early stages after diagnosis is likely to have contributed to better outcomes.
Current Controversies and Critical Questions
One of the most controversial areas with respect to the goals of therapy in myeloma, especially in the context of initial treatment of myeloma, has been the duration of therapy and the depth of response that needs to be attained. While the overall goal is undoubtedly to maximize the survival of patients with myeloma, the optimal way to employ the available tools to reach this goal remains a point of considerable debate supported by limited randomized controlled data and shadowed by a variety of differing “expert” opinions.
The benefit of continued therapy (maintenance or prolonged initial therapy) seen in the recent trials has raised an important question regarding the optimal duration of therapy in patients receiving initial therapy for myeloma. The initial approach had been that a limited duration of therapy is appropriate for these patients, with new regimens as induction followed by transplant in the younger patients, and limited duration of melphalan-based regimen for the older patients. This approach had been primarily driven by the results seen with melphalan-based regimens, where long-term therapy has been associated with leukemogenesis and the potential effects of therapy-related side effects on quality of life has been of concern. With the newer therapies these concerns have been mitigated to a great extent and many of the recent trials have allowed patients to continue on initial therapy until disease progression. In the Mayo Clinic phase 2 trial of lenalidomide in newly diagnosed myeloma [3], long-term therapy with intent to SCT at relapse was associated with increasing depth of response up to 12–18 months ultimately reaching a VGPR rate of 67 %. Arguments in favor of continued therapy till progression is that any let up in therapy may lead to reemergence of disease which then may be more difficult to control, while continuous therapy raise concerns about long-term side effects of the new drugs that we might not be aware of, as well as the possibility of selecting drug-resistant tumor clones. Unfortunately, there is no evidence to support continuous therapy to progression versus repeated therapy based on disease activity.
Another bone of contention has been the goal of therapy with respect to the depth of response to be achieved. Clearly, the new multidrug combinations have contributed to unprecedented response depths as indicated by the high rates of VGPR and CRs seen in the more recent trials. The wealth of available data suggest improved outcomes associated with achievement of complete response, but this has to be viewed in the context of what CR really defines as well as the data linking CR achievement and long-term outcome. CR as defined currently represents only a modest reduction in the tumor burden as is clear from the studies’ inferior outcomes with the presence of residual disease detected by OCR or flow cytometry-based methods. However, the available evidence does not allow us to discern whether the improvement in outcome is related more to the disease biology that allows a patient to get into a CR or whether the therapeutic approach that resulted in the CR is more important. Treatment approaches such as stem cell transplantation in the past have led to increased CR rates and improved survival, and among patients getting the same treatment CR has been associated with improved survival reflecting the impact of disease biology. Similarly in patients with preexisting MGUS and those with an MGUS-like gene expression signature appear to be less likely to obtain a CR with intensive approaches like total therapy [21, 22], with no adverse impact on their outcome. In contrast, the patients who appear to derive the maximum benefit of obtaining a CR with these therapies are those with high-risk disease by gene expression profile. It is likely that a significant proportion of patients with myeloma have a more “indolent” type of disease where achievement of a CR may be difficult with all the current therapies and persisting with this goal will result in unnecessary toxicity, while the patients with more aggressive disease require such a focused approach to maximize clonal eradication and prevention of early relapses and development of resistance.
References
Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–3.
Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111(3):1101–9.
Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82(10):1179–84.
Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37.
Jagannath S, Durie BG, Wolf J, et al. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129(6):776–83.
Harousseau JL, Mathiot C, Attal M, et al. VELCADE/dexamethasone (Vel/D) versus VAD as induction treatment prior to autologous stem cell transplantion (ASCT) in newly diagnosed multiple myeloma (MM): updated results of the IFM 2005/01 trial. ASH Annual Meeting Abstracts. 2007;110(11):450.
Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia. 2009;23(7):1337–41.
Kropff M, Liebisch P, Knop S, et al. DSMM XI study: dose definition for intravenous cyclophosphamide in combination with bortezomib/dexamethasone for remission induction in patients with newly diagnosed myeloma. Ann Hematol. 2009;88(11):1125–30.
Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–86.
Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119(19):4375–82.
Alexanian R, Bonnet J, Gehan E, et al. Combination chemotherapy for multiple myeloma. Cancer. 1972;30(2):382–9.
Costa G, Engle RL, Schilling A, et al. Melphalan and prednisone: an effective combination for the treatment of multiple myeloma. Am J Med. 1973;54:589–99.
Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370(9594):1209–18.
Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22): 3664–70.
Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513): 825–31.
Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized, controlled trial. Blood. 2008;112(8): 3107–14.
Waage A, Gimsing P, Fayers P, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9): 1405–12.
Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–69.
San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17.
Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–66.
Zhan F, Barlogie B, Arzoumanian V, et al. Gene-expression signature of benign monoclonal gammopathy evident in multiple myeloma is linked to good prognosis. Blood. 2007;109(4):1692–700.
Kumar SK, Dingli D, Lacy MQ, et al. Outcome after autologous stem cell transplantation for multiple myeloma in patients with preceding plasma cell disorders. Br J Haematol. 2008;141(2):205–11.
Jagannath S, Durie BG, Wolf JL, et al. Extended follow-up of a phase 2 trial of bortezomib alone and in combination with dexamethasone for the frontline treatment of multiple myeloma. Br J Haematol. 2009;146(6):619–26.
Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27(21):3518–25.
Dispenzieri A, Jacobus S, Vesole DH, Callandar N, Fonseca R, Greipp PR. Primary therapy with single agent bortezomib as induction, maintenance and re-induction in patients with high-risk myeloma: results of the ECOG E2A02 trial. Leukemia. 2010;24(8):1406–11.
Harousseau JL, Attal M, Coiteux V, et al. Bortezomib (VELCADE®) plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: preliminary results of an IFM Phase II Study. ASCO. 2005:#6653.
Ghosh N, Ye X, Ferguson A, Huff CA, Borrello I. Bortezomib and thalidomide, a steroid free regimen in newly diagnosed patients with multiple myeloma. Br J Haematol. 2011;152(5):593–9.
Reeder CB, Reece DE, Kukreti V, et al. Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood. 2010;115(16):3416–7.
Bensinger WI, Jagannath S, Vescio R, et al. Phase 2 study of two sequential three-drug combinations containing bortezomib, cyclophosphamide and dexamethasone, followed by bortezomib, thalidomide and dexamethasone as frontline therapy for multiple myeloma. Br J Haematol. 2010;148(4):562–8.
Wang M, Giralt S, Delasalle K, Handy B, Alexanian R. Bortezomib in combination with thalidomide-dexamethasone for previously untreated multiple myeloma. Hematology. 2007;12(3):235–239.
Kumar SK, Lacy MQ, Hayman SR, et al. Lenalidomide, cyclophosphamide and dexamethasone (CRd) for newly diagnosed multiple myeloma: results from a phase 2 trial. Am J Hematol. 2011;86(8):640–5.
Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9): 1801–9.
Oakervee HE, Popat R, Curry N, et al. PAD combination therapy (PS-341/bortezomib, doxorubicin and dexamethasone) for previously untreated patients with multiple myeloma. Br J Haematol. 2005;129(6): 755–62.
Popat R, Oakervee HE, Hallam S, et al. Bortezomib, doxorubicin and dexamethasone (PAD) front-line treatment of multiple myeloma: updated results after long-term follow-up. Br J Haematol. 2008;141(4):512–6.
Popat R, Oakervee HE, Curry N, et al. Reduced dose PAD combination therapy (PS-341/bortezomib, adriamycin and dexamethasone) for previously untreated patients with multiple myeloma. ASH Annual Meeting Abstracts. 2005;106(11):2554.
Berenson JR, Yellin O, Chen CS, et al. A modified regimen of pegylated liposomal doxorubicin, bortezomib and dexamethasone (DVD) is effective and well tolerated for previously untreated multiple myeloma patients. Br J Haematol. 2011;155(5):580–7.
Hussein MA, Baz R, Srkalovic G, et al. Phase 2 study of pegylated liposomal doxorubicin, vincristine, decreased-frequency dexamethasone, and thalidomide in newly diagnosed and relapsed-refractory multiple myeloma. Mayo Clin Proc. 2006;81(7):889–95.
Offidani M, Corvatta L, Piersantelli MN, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006;108(7):2159–2164.
Zervas K, Dimopoulos MA, Hatzicharissi E, et al. Primary treatment of multiple myeloma with thalidomide, vincristine, liposomal doxorubicin and dexamethasone (T-VAD doxil): a phase II multicenter study. Ann Oncol. 2004;15(1):134–8.
Jakubowiak AJ, Griffith KA, Reece DE, et al. Lenalidomide, bortezomib, pegylated liposomal doxorubicin, and dexamethasone in newly diagnosed multiple myeloma: a phase 1/2 Multiple Myeloma Research Consortium trial. Blood. 2011;118(3): 535–43.
Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354(10):1021–30.
Macro M, Divine M, Uzunhan Y, et al. Dexamethasone+thalidomide (Dex/Thal) compared to VAD as a pre-transplant treatment in newly diagnosed multiple myeloma (MM): a randomized trial. ASH Annual Meeting Abstracts. 2006;108(11):57.
Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005–01 phase III trial. J Clin Oncol. 2010;28(30):4621–9.
Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–85.
Lokhorst HM, van der Holt B, Zweegman S, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115(6):1113–20.
Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–50.
Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–55.
Rosinol L, Oriol A, Teruel AI, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–96.
Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118(22):5752–8; quiz 5982.
Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24(3):431–6.
Zonder JA, Crowley J, Hussein MA, et al. Lenalidomide and high-dose dexamethasone compared with dexamethasone as initial therapy for multiple myeloma: a randomized Southwest Oncology Group trial (S0232). Blood. 2010;116(26):5838–41.
Facon T, Mary JY, Pegourie B, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006;107(4):1292–8.
Ludwig H, Hajek R, Tothova E, et al. Thalidomide-dexamethasone compared to melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113(15):3435–42.
Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–6.
Beksac M, Haznedar R, Firatli-Tuglular T, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2011;86(1):16–22.
Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118(5):1231–8.
Mateos MV, Oriol A, Martinez-Lopez J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010; 11(10):934–41.
Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–9.
Vesole DH, Jacobus S, Rajkumar SV, et al. Lenalidomide plus low-dose dexamethasone (Ld): superior one and two year survival regardless of age compared to lenalidomide plus high-dose dexamethasone (LD). ASH Annual Meeting Abstracts. 2010; 116(21):308.
Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Mayo Foundation for Medical Education and Research
About this chapter
Cite this chapter
Kumar, S., Russell, S.J. (2014). Treatment of Newly Diagnosed Multiple Myeloma. In: Gertz, M., Rajkumar, S. (eds) Multiple Myeloma. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-8520-9_8
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8520-9_8
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-8519-3
Online ISBN: 978-1-4614-8520-9
eBook Packages: MedicineMedicine (R0)