Abstract
Programmed necrosis or necroptosis is a type of cell death regulated by a specific signaling pathway. Hence it is different from necrosis induced by physical trauma. Receptor-interacting protein kinase (RIPK) 1 and RIPK3 play central roles in programmed necrosis by forming a pro-necrotic signaling complex termed the necrosome. Genetic evidence indicates that in concert with the pro-apoptotic molecules FADD and caspase-8, RIPK1 and RIPK3 regulate embryonic development, T cell clonal expansion, and immune homeostasis. Programmed necrosis contributes to innate immune host defense against certain viruses. The importance of programmed necrosis as a host defense mechanism is highlighted by discovery of viral inhibitors of necrosis. Emerging evidence suggests that programmed necrosis is also involved in bacterial infections and may even directly regulate inflammatory cytokine expression. In this chapter, we discuss how the RIP kinases contribute to different inflammatory diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Necrotic cell death is characterized by extensive organelle and cell swelling and rupture of the plasma membrane. These morphological changes are entirely distinct from those of apoptotic cell death, which show organelle and cell shrinking, nuclear chromatin condensation, and nuclear and cytoplasmic blebbing to form membrane-bound fragments known as apoptotic bodies (Kerr et al. 1972; Schweichel and Merker 1973). Necrosis was once considered to be an accidental and unregulated type of cell injury. However, emerging evidence shows that necrosis can be induced in a regulated manner like apoptosis. Regulated necrosis has been called “programmed necrosis” or “necroptosis” to distinguish it from necrosis induced by physical trauma (Vandenabeele et al. 2010). Programmed necrosis can be induced by plasma membrane-associated death receptors in the TNF receptor (TNFR) superfamily (Laster et al. 1988; Vercammen et al. 1998a, b; Holler et al. 2000), T cell receptor (TCR) (Ch’en et al. 2008, 2011; Cho et al. 2011), and toll-like receptors (TLRs) (He et al. 2011; Fortes et al. 2012; McComb et al. 2012). Necrotic cell death is pro-inflammatory because it releases intracellular contents or the so-called danger-associated molecular patterns (DAMPs) (Kono and Rock 2008). The released DAMPs from necrotic cells such as HMGB1 can activate TLRs on the surface of innate immune effector cells to promote inflammatory cytokine expression (Lamkanfi et al. 2010; Yang et al. 2010). These observations imply that programmed necrosis is an important cell death module in the immune system. In fact, recent studies show that the programmed necrosis is closely associated with infectious and noninfectious inflammatory diseases. In this chapter, we discuss the emerging roles of programmed necrosis in biology with a specific emphasis on its role in immunity and inflammation. For simplicity sake, we will use the term necrosis to refer to regulated programmed necrosis hereafter.
10.2 Molecular Regulation of Necrosis
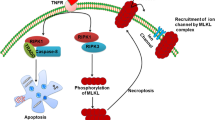
The most extensively characterized pathway leading to necrosis is initiated by ligation of TNF receptor 1 (TNFR-1/TNFRSF1a/CD120a). We will therefore use the pathway regulated by TNFR-1 ligation to illustrate the salient principles that govern necrosis. When TNF binds to TNFR-1, the membrane-associated TNFR-1 signaling complex termed “Complex I” is formed. Complex I comprises multiple protein adaptors including TNFR-associated death domain (TRADD), receptor-interacting protein kinase 1 (RIPK1), cellular inhibitor of apoptosis 1 (cIAP1), cIAP2, TNFR-associated factor 2 (TRAF2), and linear ubiquitin chain assembly complex (LUBAC) (Micheau and Tschopp 2003) (Fig. 10.1). This complex primarily triggers the NF-κB signaling pathway. RIPK1 ubiquitination is an essential event that mediates NF-κB activation (Walczak 2011). Although early reports show that K63 ubiquitination of RIPK1 at K377 is essential for recruitment of NEMO and activation of the IKK complex (Ea et al. 2006; Li et al. 2006), recent studies indicate that ubiquitination at sites other than K377 as well as other types of ubiquitin linkages can also occur (Dynek et al. 2010; Gerlach et al. 2011). RIPK1 ubiquitination prevents assembly of the cytoplasmic death-inducing signaling complex, also known as “Complex II,” through NF-κB-dependent and -independent mechanisms (O’Donnell et al. 2007). Consistent with an inhibitory role for RIPK1 ubiquitination in cell death signaling, ubiquitin hydrolases such as cylindromatosis (CYLD) have been shown to facilitate apoptotic and necrotic responses (Hitomi et al. 2008; Vanlangenakker et al. 2011) (Fig. 10.1).
The necrosis signaling pathway is regulated by protein ubiquitination, phosphorylation, and proteolytic cleavage. The TNFR-1-associated membrane complex (Complex I) is composed of many adaptors. Many of the molecular interactions within this complex require protein ubiquitination. RIPK1 ubiquitination through K63 linkage (U63), which is crucial for downstream NF-κB activation, is highlighted. Removal of ubiquitin chains from RIPK1 by the de-ubiquitinase cylindromatosis (CYLD) is important for transition of Complex I to the cytosol and assembly of Complex II. In the presence of active caspase 8, RIPK1, RIPK3, and CYLD are cleaved and inactivated (only RIPK1 cleavage is shown for simplicity sake). Cleavage of RIPK1 removes the kinase domain, thereby preventing phosphorylation of downstream substrates (e.g., RIPK3) that are important for necrosis induction. When the integrity of RIPK1 and RIPK3 is preserved, they trans-phosphorylate each other. The resulting negative charge may be critical in “opening up” the RHIM domain to facilitate amyloid fibril assembly and recruitment of downstream RIPK3 substrates
Caspase activity is a critical parameter that controls necrosis. Early studies show that the broad caspase inhibitor zVAD-fmk can facilitate RIPK1-dependent necrosis in certain cell types (Vercammen et al. 1998a; Holler et al. 2000). However, RIPK1 does not act alone to drive necrosis. Another serine/threonine kinase, RIPK3, was identified in several RNA interference screens to be a critical partner of RIPK1 in necrosis (Cho et al. 2009; He et al. 2009). Caspase 8 inhibits necrosis by cleaving and inactivating RIPK1, RIPK3, and CYLD (Lin et al. 1999; Chan et al. 2003; Feng et al. 2007; O’Donnell et al. 2011). When the activity of caspase 8 is inhibited or in caspase 8−/− or Fadd−/− cells, the integrity of RIPK1 and RIPK3 is preserved. This allows the two kinases to form a tight and stable complex termed the “necrosome” (Cho et al. 2009; He et al. 2009). Necrosome formation requires the RIP homotypic interaction motif (RHIM) that is present in both RIPK1 and RIPK3 (Sun et al. 2002). Trans-phosphorylation of RIPK1 and RIPK3 appears to be crucial for necrosome assembly, as kinase-inactive RIPK1 or RIPK3, and the RIPK1-specific inhibitor necrostatin-1 potently inhibits TNF-induced necrosis (Degterev et al. 2008). Hence, necrosis is regulated by at least three distinct mechanisms: protein ubiquitination, caspase cleavage, and phosphorylation.
The RHIM is an emerging protein–protein interaction domain found in several other adaptors including TIR domain-containing adaptor molecule 1 (TICAM1/TRIF) and DNA-dependent activator of interferon regulatory transcription factors (DAI/ZBP1) (Moquin and Chan 2010). Thus, the RHIM-containing adaptors all have important functions in innate immune and cell death signaling. The RHIM is defined by a highly conserved tetra-peptide core sequence of mostly hydrophobic residues that are predicted to be β-sheet (IQIG for RIPK1 and VQVG for RIPK3). Recent biophysical studies show that the RHIMs of RIPK1 and RIPK3 assemble in an amyloid-like filamentous fibrillar complex (Li et al. 2012). Mutagenesis of the RHIM core sequences shows that this amyloidal assembly is crucial for activation of RIPK1 and RIPK3 kinase activity, necrosome cluster formation, and necrosis induction (Fig. 10.1).
Although amyloid fibrils are toxic to neurons, the RHIM amyloid fibril appears to be an intermediary that does not directly elicit cell damage. Rather, it has a crucial function in recruitment of downstream RIPK3 substrates. One such substrate is the mixed lineage kinase domain-like (MLKL), which was identified by biochemical purification and by shRNA screen (Sun et al. 2012; Zhao et al. 2012). Phosphorylation of MLKL by RIPK3 is critical for necrosis induction. The significance of MLKL in necrosis is further bolstered by identification of a small-molecule inhibitor called “necrosulfonamide” (NSA). NSA inhibits TNF-induced necrosis by covalently modifying human MLKL. Surprisingly, NSA or siRNA knockdown of MLKL did not interfere with RIPK1–RIPK3 necrosome formation. Hence, MLKL is a key regulator of necrosis downstream of RIPK3 (Sun et al. 2012).
Another RIPK3 substrate is the mitochondrial protein phosphoglycerate mutase family member 5 (PGAM5). Both isoforms of PGAM5, PGAM5S and PGAM5L, were reported to be downstream effectors involved in necrosis induction (Wang et al. 2012). NSA prevented the recruitment of PGAM5S, but not MLKL, to the necrosome. MLKL therefore appears to function as a key adaptor that links the RIPK1–RIPK3 necrosome to downstream effectors. Interestingly, PGAM5 is a phosphatase (Takeda et al. 2009) that can dephosphorylate and activate the mitochondria fission factor Drp-1 (Wang et al. 2012). This raises the interesting possibility that the necrosome can engage the mitochondria fission machinery to execute necrosis. In addition to TNF-induced necrosis, PGAM5 also appears to have broader roles in mediating death receptor-independent necrosis, such as that induced by reactive oxygen species (ROS) or calcium ionophore (Wang et al. 2012). Whether MLKL and PGAM5 are physiologically relevant RIPK3 substrates in vivo will require examination in the relevant mutant animals.
10.3 Role of Necrosis in Innate Inflammatory Responses
10.3.1 Viral Infections
Necrotic cells are characterized by organelle and cell swelling that eventually cumulate in plasma membrane leakage. The release of endogenous adjuvants from necrotic cells is known to be immuno-stimulatory. As we have alluded to in the previous section, many protein adaptors of innate immune signaling pathways contain RHIM domains. This molecular signature suggests that the RIP kinases may have broad roles in innate immunity and inflammation. Further evidence that supports this notion comes from the fact that interferons, which are critical cytokines against viral pathogens, can greatly sensitize cellular necrosis (Kalai et al. 2002).
The first example that highlights this emerging paradigm comes from a study of host defense against vaccinia virus infection. Vaccinia virus, like other poxviruses, encodes many immune evasion genes (Moss and Shisler 2001), including those that inhibit inflammatory cytokines and TLRs (Reading et al. 2002; Harte et al. 2003; Stack et al. 2005). Despite the inhibition of inflammatory signaling, vaccinia virus elicits a strong inflammatory response in infected mice.
One of the immune evasion genes encoded by vaccinia virus is B13R or Spi2, which is a serpin that inhibits caspase 1 and caspase 8 and is functionally similar to the cytokine response modifier A (CrmA) from cowpox virus (Zhou et al. 1997). Despite inhibition of caspase 8 by B13R/Spi2, vaccinia virus-infected cells are still sensitive to the cytotoxic effect of TNF (Li and Beg 2000). TNF-induced cell death of vaccinia virus-infected cells exhibits morphology that resembles necrosis and is dependent on intact RIPK1 and RIPK3 functions (Chan et al. 2003; Cho et al. 2009) (Fig. 10.2). Consistent with results from in vitro infections, RIPK3−/− mice exhibit reduced necrosis and inflammation and greatly increased viral replication in multiple tissues. Eventually, RIPK3−/− mice succumb to the infection 4–5 days post-infection (Cho et al. 2009). In wild-type mice, elevated TNF expression was detected by 24 h post-infection, which coincided with the appearance of RIPK1–RIPK3 complex in the liver (Cho et al. 2009). Because these events occur prior to induction of adaptive T cell responses, which usually peaks at days 7–8 post-infection, we conclude that RIPK3 is critically important for innate immune protection against vaccinia virus. This early control of viral replication is likely to be crucial for host control of the viral factory before virus-specific T and B cells are mobilized in high enough number to fully eradicate the virus.
RIP kinase-dependent necrosis is an important innate immune defense mechanism against vaccinia virus. Vaccinia virus is a large DNA virus that has been shown to activate multiple TLRs. Engagement of TLRs results in expression of inflammatory cytokines including TNF. TNF can elicit the cell death program upon binding to the receptor on the cell surface of an infected cell. Because of the virus-encoded caspase inhibitor B13/Spi2, caspase 8 is inhibited and apoptosis is suppressed. This allows assembly of the RIPK1–RIPK3 necrosome. The induction of necrosis may be advantageous in two ways. First, it serves to limit the viral factory before adaptive immunity is launched. Secondly, the release of DAMPs from the necrotic cells may further promote the antiviral inflammatory reaction
For vaccinia virus, host cell necrosis is an effective innate immune antiviral defense. Could viruses have developed strategies to inhibit necrosis as a means to escape elimination from the host? Murine cytomegalovirus (MCMV) encodes three different types of viral cell death inhibitors, vICA (inhibitor of caspase 8-induced apoptosis), vMIA (mitochondria inhibitor of apoptosis), and vIRA (inhibitor of RIP activation) (reviewed in Mocarski et al. 2012). Productive infection and replication of viral progenies require the action of all three inhibitors. Although no enzymatic activity can be detected (Lembo et al. 2004), vIRA or M45 exhibits homology to ribonucleotide reductase (Brune et al. 2001). Interestingly, M45 contains a RHIM at the amino terminus that is crucial for binding to RHIM-containing cellular adaptors including RIPK1, RIPK3, and DAI (Kaiser et al. 2008; Upton et al. 2008, 2012; Rebsamen et al. 2009). Recombinant virus that encodes a defective vIRA with tetra-alanine substitutions within the core RHIM sequence fails to establish productive infection in cells and in mice due to premature cell death by necrosis. Significantly, productive infection is restored when the RHIM mutant MCMV infects RIPK3−/− mice (Upton et al. 2010). Surprisingly, the necrotic cell death induced upon mutant MCMV infection is not driven by TNF or RIPK1. Instead, RIPK3 pairs with another RHIM-containing adaptor DAI to induce necrosis (Upton et al. 2012). Hence, similar to poxviruses, MCMV infection sensitizes cells to necrosis. However, unlike vaccinia virus, MCMV has developed an effective strategy to inhibit host cell necrosis to ensure productive viral replication within the infected host. It will be of great interest to determine if similar viral inhibition of necrosis also occurs in human CMV infection. The MCMV studies also reveal that other than RIPK1, other RHIM-containing adaptors can partner with RIPK3 to induce necrosis (Fig. 10.3). It is noteworthy that similar RHIM-mediated interactions between RIPK1–TRIF and RIPK1–DAI/ZBP1 have been shown to mediate NF-κB activation (Meylan et al. 2004; Kaiser and Offermann 2005; Rebsamen et al. 2009) (Fig. 10.3). It will be important to determine how the different types of RHIM complexes can mediate cell survival and cell death signaling under different conditions.
Different RHIM domain-containing adaptors can partner with RIPK3 to induce necrosis. The three known RHIM-mediated interactions that lead to necrosis are shown on the left. In the case of TRIF–RIPK3, genetic and pharmacological evidence suggests that RIPK1 is also involved. However, biochemical evidence for RIPK1–RIPK3–TRIF complex is lacking at present. Hence, RIPK1 is not included in the necrotic TRIF–RIPK3 complex. On the right, the different types of RHIM-mediated interactions that regulate NF-κB activation are shown. Note that RIPK3 has been shown to have positive and negative effects on NF-κB activation by different complexes (Kaiser et al. 2008; Rebsamen et al. 2009)
10.3.2 Viral Necrosis Inhibitors
The vIRA/M45 story reveals that active suppression of host cell necrosis can be an important immune evasion mechanism for viruses. In fact, vIRA/M45 is not the first viral necrosis inhibitor identified. Viral FLICE-like inhibitor proteins (FLIPs) are orthologs of cellular caspase 8 and caspase 10. They contain tandem death effector domains but lack the enzymatic domains. Hence, they were first recognized as caspase and apoptosis inhibitors (Bertin et al. 1997; Hu et al. 1997; Thome et al. 1997). In 2003, a subset of vFLIPs, namely, MC159 from molluscum contagiosum virus and E8 from equine herpesvirus, were found to also inhibit TNF-induced necrosis (Chan et al. 2003). In contrast to M45, which inhibits necrosis through RHIM-mediated interaction with RIPK3 (Upton et al. 2010), the molecular basis by which vFLIPs inhibit necrosis is not fully understood. Because vFLIPs and vIRA/M45 are structurally unrelated, these results indicate that viral inhibitors of necrosis can come in different flavors. It will be interesting to see if additional classes of viral necrosis inhibitors will be identified in the future.
10.3.3 Bacterial Infections
Macrophages are sentinels against bacterial infections. Recent studies indicate that in the presence of caspase inhibition, the TLR4 agonist bacterial lipopolysaccharides (LPS) can induce RIPK3-dependent necrosis in macrophages (He et al. 2011). In addition, Smac mimetics, which target cIAP1, cIAP2, and XIAP for proteasomal degradation (Varfolomeev et al. 2007; Vince et al. 2007; Bertrand et al. 2008), can induce RIPK1- and RIPK3-dependent macrophage necrosis (McComb et al. 2012). Besides RIPK1 and RIPK3, another RHIM-containing adaptor TRIF also plays a crucial role in macrophage necrosis (He et al. 2011). TRIF is a TIR domain-containing adaptor that mediates type I interferon expression in response to TLR3 and TLR4 signaling (Yamamoto et al. 2002; Oshiumi et al. 2003). Like RIPK1 and RIPK3, TRIF can induce apoptosis under certain conditions (Kaiser and Offermann 2005; Weber et al. 2010). Treatment with LPS and zVAD-fmk, which mimics bacterial septic shock, causes an inflammatory cytokine storm and extensive macrophage necrosis. These effects were greatly ameliorated in RIP3−/− and TRIFlps2/lps2 mutant mice (He et al. 2011). Moderately reduced inflammatory cytokine production in response to LPS was also observed in RIP3−/− mice treated with LPS alone (Newton et al. 2004), suggesting that necrosis-induced inflammation can occur in vivo without pharmacologic inhibition of caspases.
TNF is a major inflammatory cytokine that mediates the systemic effects of LPS-induced septic shock. Consistent with a role for TNF in bacterial sepsis, RIPK3−/− mice are protected from TNF-induced systemic inflammatory response syndrome (SIRS) (Duprez et al. 2011; Linkermann et al. 2012a) and cecal ligation puncture-induced sepsis (Duprez et al. 2011). However, results obtained using the RIPK1 inhibitor necrostatin-1 (Nec-1) were less definitive than those obtained with RIPK3−/− mice. While one report shows protection by Nec-1, another report indicates that Nec-1 exacerbates TNF-induced SIRS (Duprez et al. 2011; Linkermann et al. 2012a). These opposing observations may be due to off-target effects of Nec-1 (Cho et al. 2011). Unfortunately, genetic model to assess RIPK1 function in these inflammatory diseases is currently not available because RIPK1−/− mice exhibit neonatal lethality (Kelliher et al. 1998). Conditional RIPK1−/− mice will be invaluable tools to dissect the in vivo role of RIPK1 in inflammatory diseases.
10.3.4 Necrosis in Sterile Inflammation
Besides its role in pathogen-induced inflammation, necrosis can also promote sterile inflammation. For example, retinal detachment-induced photoreceptor necrosis is blocked in RIPK3−/− cells (Trichonas et al. 2010). Because caspase inhibition greatly sensitizes cells to necrosis, it is no surprise that a large number of studies on necrosis-induced sterile inflammation have been performed using caspase 8−/− or mice deficient in FADD, an upstream adaptor that is essential for caspase 8 recruitment and activation. Similar to caspase inhibition in tissue culture, caspase 8−/− or FADD−/− mice are highly sensitive to necrosis induction. Most remarkably, germline inactivation of these genes results in extensive necrosis during embryogenesis, which results in lethality on E9.5. Embryonic lethality of caspase 8−/− or FADD−/− mice is rescued by deletion of RIPK1 or RIPK3 (Kaiser et al. 2011; Oberst et al. 2011; Zhang et al. 2011; Dillon et al. 2012). Keratinocyte- or intestinal epithelium-specific deletion of FADD or caspase 8 causes severe spontaneous inflammation in the respective tissues that can be corrected by deletion of RIPK3 (Kovalenko et al. 2009; Bonnet et al. 2011; Gunther et al. 2011; Welz et al. 2011). While the more popular view is that the inflammatory disease is caused by increased necrosis, the possibility that FADD, caspase 8, RIPK1, and RIPK3 can directly regulate innate inflammatory signaling cannot be overlooked (see below) (Rajput et al. 2011a; Wallach et al. 2011).
In addition to caspase 8−/− or FADD−/− mice, necrosis-induced sterile injury and inflammation have also been detected in wild-type animals with normal FADD and caspase 8 functions. For instance, repeated doses of cerulein can cause RIPK3-dependent acinar cell necrosis and acute pancreatitis in wild-type mice (He et al. 2009; Zhang et al. 2009). Administration of the RIPK1 inhibitor Nec-1 significantly ameliorates tissue damage in animal models of myocardial infarction, ischemia-induced brain injury, and renal ischemia/reperfusion injury (Degterev et al. 2005; Lim et al. 2007; Smith et al. 2007; Northington et al. 2011; Linkermann et al. 2012b), indicating that RIPK1-dependent necrosis is activated under these conditions in wild-type animals. Although TNF and other inflammatory cytokines are often elevated in ischemia/reperfusion-induced injury (Watters and O’Connor 2011; Lambertsen et al. 2012), it is not clear if they are the direct triggers for necrosis in these diseases. If necrosis is induced without death receptor engagement in these situations, it will be analogous to “intrinsic” apoptosis induced in response to genotoxic stress.
10.4 Direct Roles for RIPK1 and RIPK3 in Inflammation Signaling
As we have discussed in previous sections, promoting inflammation via NF-κB was the first function ascribed to RIPK1. In addition to TNFR, RIPK1 also mediates NF-κB activation by certain innate immune receptors such as TLR3 (Meylan et al. 2004), TLR4 (Cusson-Hermance et al. 2005; Ermolaeva et al. 2008) and RIG-I (Michallet et al. 2008; Rajput et al. 2011b). In contrast to RIPK1, RIPK3−/− cells exhibit normal NF-κB induction in response to TNFR-1 and several TLR agonists (Newton et al. 2004). However, early reports show that over-expression of RIPK3 can often inhibit or promote NF-κB activation (Sun et al. 1999; Kasof et al. 2000; Meylan et al. 2004; Kaiser and Offermann 2005). Hence, it remains possible that RIPK3 can modulate NF-κB responses in specific scenarios.
Recent evidence suggests that RIPK3 has a surprising function in driving maturation of the pro-inflammatory cytokine IL-1β (Vince et al. 2012). Production of IL-1β requires two signals. The first signal, which can be provided by activation of innate immune receptors such as TNFR-1 or TLR4, activates de novo synthesis of pro-IL-1β in an NF-κB-dependent manner. Release of mature IL-1β requires a second signal that involves activation of the inflammasome and caspase-mediated processing of pro-IL-1β (reviewed in Rathinam et al. 2012a). In most cases, caspase 1 is the enzyme responsible for processing of pro-IL-1β and related cytokines such as pro-IL-18. However, noncanonical activation of the inflammasome can result in activation of caspase 8 or caspase 11 (Kayagaki et al. 2011; Gringhuis et al. 2012; Pierini et al. 2012; Rathinam et al. 2012b). Vince and colleagues show that in LPS-primed macrophages, Smac mimetics induces IL-1β processing and maturation through canonical NLRP3–caspase 1 and noncanonical NLRP3–caspase 8 inflammasome activation. Surprisingly, RIPK3 and ROS production are also required for Smac mimetic-induced IL-1β maturation. Consistent with the effects of Smac mimetics, LPS-primed cIAP1−/−cIAP2−/−XIAP−/− macrophages exhibit spontaneous IL-1β processing (Vince et al. 2012). These results suggest the tantalizing possibility that RIPK3 can promote inflammation through multiple means. On one hand, release of DAMPs from necrotic cells can activate TLRs to promote inflammatory gene expression. On the other hand, RIPK3 can directly engage the inflammasome to promote the expression of IL-1-like inflammatory cytokines.
10.5 Necrosis in Adaptive Immunity
10.5.1 T Cell Tolerance
The maintenance of immune homeostasis is critically dependent on proper cell death regulation. T cells recognize antigenic peptides bound to self major histocompatibility complex (MHC) through their TCRs. Because antigen receptors on T and B cells are generated by random gene rearrangement, T cells that express TCRs of different affinities to MHC are generated. Lymphocytes that express TCR with little affinity for MHC are eliminated through “death by neglect” in a process termed “positive selection.” T cells that survive positive selection are further subjected to “negative selection,” a process that eliminates potentially autoreactive T cells with TCR that bind too strongly to self peptide–MHC complexes. The cumulative effect of positive and negative selection is a TCR repertoire that is largely devoid of autoreactive cells (reviewed in Stritesky et al. 2012). Death receptors in the TNFR superfamily do not appear to play significant roles in the thymic selection processes, since animals deficient in these receptors undergo normal thymic selection.
Once T cells leave the thymus to populate the peripheral organs, additional mechanisms, collectively termed “peripheral tolerance,” are required to prevent activation of any autoreactive T cells that managed to escape thymic negative selection. In contrast to thymic selection, the death receptors Fas/CD95/APO-1 and, to a lesser extent, TNFR-1 and TNFR-2 play key roles in peripheral tolerance (Zheng et al. 1995; Lenardo et al. 1999). Naïve T cells undergo clonal expansion upon TCR engagement. However, repeated TCR stimulation can result in death of the activated T cells (Zheng et al. 1998). This phenomenon is often referred to as “activation-induced cell death” (AICD) or more appropriately as “restimulation-induced cell death” (RICD) (Snow et al. 2009). Both TCR restimulation and T cell trophic factor IL-2 can greatly enhance Fas and Fas ligand (FasL) expression in activated T cells (Zheng et al. 1998). As a result, activated T cells are eliminated through FasL–Fas interaction in a paracrine fashion. As such, deficiency in the receptor or the ligand leads to defective RICD and lymphoproliferative diseases. The well-known mouse models for autoimmunity lpr and gld are caused by mutations in Fas and FasL, respectively (Watanabe-Fukunaga et al. 1992; Lynch et al. 1994). In human, similar mutations lead to similar systemic autoimmune disease termed the autoimmune lymphoproliferation syndromes (ALPS) (Puck and Sneller 1997).
Because Fas–FasL-induced lymphocyte cell death exhibits classical features of apoptosis (e.g., chromatin condensation, caspase activation), it is widely believed that apoptosis is the cell death module that controls peripheral tolerance. However, this notion was challenged when mice with T cell-specific deletion of FADD or caspase 8 were found to be immunodeficient rather than developing lpr-like autoimmune disease (Zhang et al. 1998, 2005; Ch’en et al. 2008). Similarly, human patients with caspase 8 mutations also exhibit immunodeficiency rather than ALPS-like systemic autoimmunity (Chun et al. 2002). Although these defects were originally attributed to defective TCR-induced NF-κB activation (Su et al. 2005), subsequent experiments show that TCR-induced NF-κB activation was normal in caspase 8−/− T cells (Ch’en et al. 2008). Further examination revealed that FADD−/− or caspase 8−/− T cells undergo extensive necrosis-like cell death upon stimulation through the TCR (Walsh et al. 1998; Kennedy et al. 1999; Hueber et al. 2000). Consistent with the notion that necrosis underlies the proliferative defect, treatment with Nec-1 restored normal T cell proliferation (Osborn et al. 2010). Moreover, FADD−/−RIPK1−/− and caspase 8−/−RIPK3−/− T cells show normal TCR-induced proliferation in vitro, virus-induced clonal expansion in vivo, and cytokine expression (Kaiser et al. 2011; Zhang et al. 2011). Results obtained from RIPK3−/− mice expressing a dominant negative FADD also show similar phenotypes (Lu et al. 2011). Most remarkably, mice deficient in FADD/caspase 8 and RIPK3 developed lpr-like autoimmune disease that is more aggressive than lpr itself (Ch’en et al. 2011; Kaiser et al. 2011; Oberst et al. 2011), possibly because both Fas- and TNFR-1-induced cell deaths are inhibited. These results revealed an unexpected pro-survival function for FADD and caspase 8 during T cell clonal expansion. They also highlight the fact that caspase-dependent apoptosis and RIP kinase-dependent necrosis are both required to enforce T cell tolerance and homeostasis.
10.5.2 B Cell Responses
In contrast to T cells, B cell proliferation through the antigen receptor or CD40 is unaffected in FADD−/− and caspase 8−/− B cells (Beisner et al. 2005; Imtiyaz et al. 2006). By contrast, TLR3- and TLR4-induced B cell proliferation, but not B cell proliferation induced by the TLR9 agonist CpG DNA, is impaired in FADD−/− and caspase 8−/− B cells (Beisner et al. 2005; Imtiyaz et al. 2006). Unlike TCR-induced proliferation, defective FADD−/− B cell proliferation was not restored in FADD−/−RIPK1−/− B cells (Zhang et al. 2011). Because TLR3 and TLR4 share the unique signaling adaptor TRIF and that TRIF has been shown to interact with RIPK1 to mediate NF-κB activation (Meylan et al. 2004; Vivarelli et al. 2004), the defective TLR3/4-induced proliferation in FADD−/−RIPK1−/− B cells can be attributed to defective NF-κB signaling. Taken together, these results illustrate that RIPK1 and RIPK3 have differential roles in regulating antigen receptor-induced proliferation in T and B cells.
10.6 Concluding Remarks
Genetic experiments have clearly demonstrated that the RIP kinase-driven necrosis is a biologically relevant cell death module. However, key questions remained to be answered. For example, why are RIPK1 and RIPK3 expression highly induced during T cell activation (Cho et al. 2009, 2011)? It seems counterintuitive that death-promoting molecules are upregulated at a time when lymphocyte expansion is a priority. Similarly, expression of RIPK3 was highly induced during embryogenesis (Zhang et al. 2011). The potential inflammation and damage that necrosis can lead to, such as that seen in FADD−/− and caspase 8−/− animals, is unlikely to be a desired outcome during embryogenesis. In light of these observations, one can envision that RIPK1 and RIPK3 have important biological functions other than necrosis. Discovering and deciphering the non-necrotic or normal physiological functions of the RIP kinases will be of critical relevance as the scientific community ponders the therapeutic potential of manipulating necrosis in the clinics.
References
Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM (2005) Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol 175:3469–3473
Bertin J, Armstrong RC, Ottilie S, Martin DA, Wang Y, Banks S, Wang GH, Senkevich TG, Alnemri ES, Moss B et al (1997) Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci U S A 94:1172–1176
Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA (2008) cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell 30:689–700
Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M (2011) The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35:572–582
Brune W, Menard C, Heesemann J, Koszinowski UH (2001) A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291:303–305
Ch’en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM (2008) Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A 105:17463–17468
Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM (2011) Mechanisms of necroptosis in T cells. J Exp Med 208(4):633–641
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ (2003) A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem 278:51613–51621
Cho Y, McQuade T, Zhang HB, Zhang JK, Chan FKM (2011) RIP1-dependent and independent effects of necrostatin-1 in necrosis and T cell activation. PLoS One 6
Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK (2009) Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG et al (2002) Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419:395–399
Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA (2005) Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem 280:36560–36566
Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G et al (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR (2012) Survival function of the FADD-CASPASE-8-cFLIPL complex. Cell Rep 1(5):401–407
Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P (2011) RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity 35:908–918
Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS et al (2010) c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO J 29:4198–4209
Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ (2006) Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22:245–257
Ermolaeva MA, Michallet MC, Papadopoulou N, Utermohlen O, Kranidioti K, Kollias G, Tschopp J, Pasparakis M (2008) Function of TRADD in tumor necrosis factor receptor 1 signaling and in TRIF-dependent inflammatory responses. Nat Immunol 9:1037–1046
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M (2007) Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal 19:2056–2067
Fortes GB, Alves LS, de Oliveira R, Dutra FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher M, Golenbock D et al (2012) Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood 119:2368–2375
Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW et al (2011) Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471:591–596
Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol 13:246–254
Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF et al (2011) Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477:335–339
Harte MT, Haga IR, Maloney G, Gray P, Reading PC, Bartlett NW, Smith GL, Bowie A, O’Neill LA (2003) The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J Exp Med 197:343–351
He S, Liang Y, Shao F, Wang X (2011) Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108:20054–20059
He S, Wang L, Miao L, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137:1100–1111
Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J (2008) Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135:1311–1323
Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J (2000) Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495
Hu S, Vincenz C, Buller M, Dixit VM (1997) A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis. J Biol Chem 272:9621–9624
Hueber AO, Zornig M, Bernard AM, Chautan M, Evan G (2000) A dominant negative Fas-associated death domain protein mutant inhibits proliferation and leads to impaired calcium mobilization in both T-cells and fibroblasts. J Biol Chem 275:10453–10462
Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, Zhang J (2006) The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol 176:6852–6861
Kaiser WJ, Offermann MK (2005) Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol 174:4942–4952
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–372
Kaiser WJ, Upton JW, Mocarski ES (2008) Receptor-interacting protein homotypic interaction motif-dependent control of NF-kappa B activation via the DNA-dependent activator of IFN regulatory factors. J Immunol 181:6427–6434
Kalai M, Van Loo G, Vanden Berghe T, Meeus A, Burm W, Saelens X, Vandenabeele P (2002) Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ 9:981–994
Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC (2000) The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett 473:285–291
Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P (1998) The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297–303
Kennedy NJ, Kataoka T, Tschopp J, Budd RC (1999) Caspase activation is required for T cell proliferation. J Exp Med 190:1891–1896
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev Immunol 8:279–289
Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D (2009) Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med 206:2161–2177
Lambertsen KL, Biber K, Finsen B (2012) Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 32(9):1677–1698
Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM (2010) Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 185:4385–4392
Laster SM, Wood JG, Gooding LR (1988) Tumor necrosis factor can induce both apoptotic and necrotic forms of cell lysis. J Immunol 141:2629–2634
Lembo D, Donalisio M, Hofer A, Cornaglia M, Brune W, Koszinowski U, Thelander L, Landolfo S (2004) The ribonucleotide reductase R1 homolog of murine cytomegalovirus is not a functional enzyme subunit but is required for pathogenesis. J Virol 78:4278–4288
Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L (1999) Mature T lymphocyte apoptosis–immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol 17:221–253
Li H, Kobayashi M, Blonska M, You Y, Lin X (2006) Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem 281:13636–13643
Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A et al (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350
Li M, Beg AA (2000) Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol 74:7470–7477
Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC (2007) The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther 21:467–469
Lin Y, Devin A, Rodriguez Y, Liu ZG (1999) Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev 13:2514–2526
Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S (2012a) Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor alpha-induced shock. Mol Med 18:577–586
Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S (2012b) Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int 81:751–761
Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS et al (2011) Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A 108:15312–15317
Lynch DH, Watson ML, Alderson MR, Baum PR, Miller RE, Tough T, Gibson M, Davis-Smith T, Smith CA, Hunter K et al (1994) The mouse Fas-ligand gene is mutated in gld mice and is part of a TNF family gene cluster. Immunity 1:131–136
McComb S, Cheung HH, Korneluk RG, Wang S, Krishnan L, Sad S (2012) cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ 19(11):1791–1801
Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J (2004) RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol 5:503–507
Michallet MC, Meylan E, Ermolaeva MA, Vazquez J, Rebsamen M, Curran J, Poeck H, Bscheider M, Hartmann G, Konig M et al (2008) TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity 28:651–661
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181–190
Mocarski ES, Upton JW, Kaiser WJ (2012) Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nat Rev Immunol 12:79–88
Moquin D, Chan FK (2010) The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35:434–441
Moss B, Shisler JL (2001) Immunology 101 at poxvirus U: immune evasion genes. Semin Immunol 13:59–66
Newton K, Sun X, Dixit VM (2004) Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol 24:1464–1469
Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ (2011) Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab 31:178–189
O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT (2007) Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol 17:418–424
O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT (2011) Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol 13:1437–1442
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367
Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci U S A 107:13034–13039
Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T (2003) TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol 4:161–167
Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19(10):1709–1721
Puck JM, Sneller MC (1997) ALPS: an autoimmune human lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Semin Immunol 9:77–84
Rajput A, Kang TB, Bogdanov K, Kim JC, Ben-Moshe T, Kovalenko A, Wallach D (2011a) Anti-inflammatory functions of caspase-8. Adv Exp Med Biol 691:253–260
Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D (2011b) RIG-I RNA helicase activation of IRF3 transcription factor is negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity 34:340–351
Rathinam VA, Vanaja SK, Fitzgerald KA (2012a) Regulation of inflammasome signaling. Nat Immunol 13:333–342
Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA (2012b) TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150(3):606–619
Reading PC, Khanna A, Smith GL (2002) Vaccinia virus CrmE encodes a soluble and cell surface tumor necrosis factor receptor that contributes to virus virulence. Virology 292:285–298
Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J (2009) DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep 10:916–922
Schweichel JU, Merker HJ (1973) The morphology of various types of cell death in prenatal tissues. Teratology 7:253–266
Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM (2007) Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21:227–233
Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH et al (2009) Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest 119:2976–2989
Stack J, Haga IR, Schroder M, Bartlett NW, Maloney G, Reading PC, Fitzgerald KA, Smith GL, Bowie AG (2005) Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 201:1007–1018
Stritesky GL, Jameson SC, Hogquist KA (2012) Selection of self-reactive T cells in the thymus. Annu Rev Immunol 30:95–114
Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M (2005) Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 307:1465–1468
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X et al (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM (1999) RIP3, a novel apoptosis-inducing kinase. J Biol Chem 274:16871–16875
Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM (2002) Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 277:9505–9511
Takeda K, Komuro Y, Hayakawa T, Oguchi H, Ishida Y, Murakami S, Noguchi T, Kinoshita H, Sekine Y, Iemura S et al (2009) Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. Proc Natl Acad Sci U S A 106:12301–12305
Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer JL, Schroter M et al (1997) Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517–521
Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vavvas DG (2010) Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A 107(50):21695–21700
Upton JW, Kaiser WJ, Mocarski ES (2008) Cytomegalovirus M45 cell death suppression requires receptor-interacting protein (RIP) homotypic interaction motif (RHIM)-dependent interaction with RIP1. J Biol Chem 283:16966–16970
Upton JW, Kaiser WJ, Mocarski ES (2010) Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313
Upton JW, Kaiser WJ, Mocarski ES (2012) DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, Vucic D, Fulda S, Vandenabeele P, Bertrand MJ (2011) cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ 18:656–665
Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ et al (2007) IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell 131:669–681
Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P (1998a) Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med 187:1477–1485
Vercammen D, Brouckaert G, Denecker G, Van de Craen M, Declercq W, Fiers W, Vandenabeele P (1998b) Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J Exp Med 188:919–930
Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O’Reilly L, Mason K, Gross O, Ma S, Guarda G et al (2012) Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36:215–227
Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M et al (2007) IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131:682–693
Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS (2004) RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med 200:399–404
Walczak H (2011) TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 244:9–28
Wallach D, Kovalenko A, Kang TB (2011) ‘Necrosome’-induced inflammation: must cells die for it? Trends Immunol 32:505–509
Walsh CM, Wen BG, Chinnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM (1998) A role for FADD in T cell activation and development. Immunity 8:439–449
Wang Z, Jiang H, Chen S, Du F, Wang X (2012) The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148:228–243
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314–317
Watters O, O’Connor JJ (2011) A role for tumor necrosis factor-alpha in ischemia and ischemic preconditioning. J Neuroinflammation 8:87
Weber A, Kirejczyk Z, Besch R, Potthoff S, Leverkus M, Hacker G (2010) Proapoptotic signalling through Toll-like receptor-3 involves TRIF-dependent activation of caspase-8 and is under the control of inhibitor of apoptosis proteins in melanoma cells. Cell Death Differ 17:942–951
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477:330–334
Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 169:6668–6672
Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y et al (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107:11942–11947
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325(5938):332–336
Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471:373–376
Zhang J, Cado D, Chen A, Kabra NH, Winoto A (1998) Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296–300
Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou YJ, Zhang J (2005) Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol 175:3033–3044
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109:5322–5327
Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ (1995) Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377:348–351
Zheng L, Trageser CL, Willerford DM, Lenardo MJ (1998) T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J Immunol 160:763–769
Zhou Q, Snipas S, Orth K, Muzio M, Dixit VM, Salvesen GS (1997) Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J Biol Chem 272:7797–7800
Acknowledgements
We thank members of the Chan Lab and many colleagues for discussion and ideas. This work is supported by grants from the NIH (AI083497 and AI088502). K.M. is supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Moriwaki, K., Chan, F.KM. (2014). Programmed Necrosis in Immunity and Inflammatory Diseases. In: Shen, HM., Vandenabeele, P. (eds) Necrotic Cell Death. Cell Death in Biology and Diseases. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-8220-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-8220-8_10
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-8219-2
Online ISBN: 978-1-4614-8220-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)