Abstract
Rice is a model plant for genomics and is now also becoming a model for plant epigenomics and epigenetics. Rice epigenomic landscapes including genome-wide DNA methylation and histone modifications are emerging. Studies of rice chromatin modification regulators have revealed a number of specific functions in gene expression, transposon repression, and plant development. Epigenomic variations are being identified among different rice subspecies and varieties, which may be related to stable or heritable epialleles implicated in the production of important agronomical traits and may have significance in the hybrid vigor in rice. Thus, studying rice epigenomic variation and epigenetic mechanism may provide novel strategies for rice genetic improvement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
In eukaryotes, the genomic DNA is tightly compacted into chromatin, the structure of which plays essential roles in genome function and gene expression [1]. The primary unit of chromatin is the nucleosome. The nucleosome core particle is comprised of histone H2A, H2B, H3, and H4, and is wrapped around by a segment of 147 bp DNA. Chromatin modifications include DNA methylation and histone modifications. DNA methylation in eukaryotes consists of the addition of a methyl group at position five of the pyrimidine ring of cytosine [2]. Histone modifications include acetylation, methylation, phosphorylation, and monoubiquitination, etc. DNA methylation and histone modifications are reversible and are recognized and bound by different chromatin protein complexes that usually have chromatin remodeling activities to alter chromatin structure [3, 4]. Chromatin modification profiles define distinct epigenomes which are reflected by specific gene expression patterns of different cell types and/or responses to variable environmental conditions. Epigenetic regulations involving variation of DNA methylation and histone modifications and histone variant deposition, etc., control transcriptional activity of genes, repetitive sequences and transposable elements, as well as DNA replication and repair [5]. In this chapter, we will describe recent advances in studies of rice chromatin modification, regulation and recognition mechanisms, and their function in controlling rice gene expression and plant growth.
1 DNA Methylation in Rice
DNA methylation is a hall mark of epigenetic inactivation of repetitive sequences and transposable elements and heterochromatin formation in plants. In plant genomes, cytosine methylation occurs in CG, CHG, and CHH contexts (where H is A, C, or T). DNA methylation is highest within pericentromeric regions that are enriched for transposable elements and repetitive sequences including ribosomal DNA (rDNA). However a significant proportion of genes (15–20 %) also contain methylated cytosines in Arabidopsis and rice [6–9]. Methylation of CG sequences is commonly found within gene bodies, whereas methylation of non-CG (CHG and CHH) sequences is enriched in transposons and repetitive sequences. In genes, DNA methylation is distributed over the transcribed regions or gene bodies but in most cases is depleted from the 5′ and 3′ ends of the genes (Fig. 9.1). It is suggested that moderately expressed genes are more likely to be methylated than those with low or high expression [10]. Gene body methylation (i.e., CG methylation) is conserved between plants and animals and is hypothesized to suppress spurious initiation of transcription within active genes [7, 11]. About 5 % genes show DNA methylation within promoter regions, which has a repressive function on promoter activity. DNA methylation can inhibit transcription or lead to silent chromatin either by physically impeding the binding of transcription factors to the promoter or by interacting with methylcytosine-binding proteins that can recruit additional chromatin proteins to the locus to modify histones or remodel the chromatin thereby forming compact heterochromatin.
In Arabidopsis, CG methylation is maintained by the DNMT1 (DNA MethylTransferase 1) homologue, MET1 (Methyltransferase1), CHG methylation primarily by the plant-specific DNA methyltransferase CMT3 (ChroMomeThylase 3), and CHH methylation by DMR2 (Domains Rearranged Methyltransferase 2), a homologue of mammalian DNMT3 [12] (Fig. 9.2). In addition, DRM2 is responsible for de novo methylation of all three sequence contexts, which is directed by small interfering RNAs (siRNAs) called RNA-dependent DNA methylation (RdDM) [13]. While a general concept is that distinct DNA methyltransferases are responsible for either maintenance or de novo methylation in different sequence contexts, an emerging view is that different enzymes may cooperate to catalyze both steps. Rice genes encoding putative DNA methyltransferases and the siRNA machinery have been identified (Table 9.1). Loss-of-function mutations of rice DNA methyltransferase genes lead to reduction of DNA methylation of repetitive sequences (unpublished). DNA methylation can be lost by passive (non-maintenance during DNA replications) and active (enzymatic removal) mechanisms. Arabidopsis DNA demethylase DME (Demeter) and ROS1 (Repressor Of Silencing 1) have combined DNA glycosylase and AP lyase activities [14, 15]. The rice DNG701 protein that is closely related to Arabidopsis ROS1 has been shown to display 5-methylcytosine DNA glycosylase and lyase activities in vitro [16]. Knockout or knockdown of DNG701 in rice leads to DNA hypermethylation and reduced expression of the retrotransposon Tos17 [16].
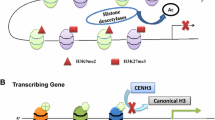
Schematic representation of chromatin structures over repetitive sequence, repressed, and active genic regions. In repetitive sequence regions, cytosines are methylated at CG, CHG, and CHH sequence contexts. H3K9me2 and H3K27me1 are enriched. These modification marks are bound by proteins associated with heterochromatin such as McBP (methylated Cystosine Binding Proteins, etc.) and nucleosomes are highly condensed. In repressed genic regions histones are deacetylated and H3K27me3 is enriched which is bound by LHP1. In active genic regions, histone acetylation level is high and H3K4me3 is enriched at 5′ end of genes. Acetylated histone lysines are recognized by bromodomain-containing proteins (such as HAT) that have transcription coactivator function. H3K4me3 can be recognized by PHD or CHD proteins that facilitate transcription by RNA polymerase II. In plants, DNA methylation (DNAme) is catalyzed by three types of enzymes: MET1, CMT3 and DRM2. De novo DNA methylation mediated by DRM2 is trigged by siRNA. DNAme and H3K9me2 that is regulated by the SUVH (Su(var) homologue) class of histone methyltransferases (HMT, i.e., SDG714/718 in rice) and histone demethylase (HDM, i.e., JMJ706 in rice) are mutually agonistic. H3K27me3 is mediated by the E(Z) type of HMT which is the key component of the polycomb group (PcG) complexes and demethylated by HDM (probably JMJ706 in rice). H3K27me3 is antagonistic to H3K4me3 and DNAme. H3K4me3 is catalyzed by the trithorax homologue (TXH) proteins and demethylated by JMJ703 in rice. H3K4me3 inhibits DNAme and H3K27me3. Histone (mostly H3 and H4) lysines are acetylated by HAT (histone acetyltransferases) and deacetylated by HDAC (histone deacetylases, such as SRT701, HDAC1, and HDT701 in rice). Histone acetylation facilitates H3K4me3, while histone deacetylation facilitates H3K9me2. H3K27me1 is catalyzed primarily by TXH-related (TXR5, 6) proteins
2 Histone Modifications in Rice
2.1 Histone Acetylation/Deacetylation
Strong acetylation of histones induces relaxation of chromatin structure and is associated with transcriptional activation, whereas weak or no acetylation leads to chromatin compaction and gene repression [17] (Fig. 9.2). The dynamic modulation of histone acetylation in plants has been shown to be important for plant gene expression in responding to environmental conditions including light, temperature, biotic, and abiotic stresses [18, 19]. In rice, acetylation of H3 lysine 9 (H3K9) and H4 lysine 12 (H4K12) is elevated in genes located in euchromatic regions [20], suggesting that these markers are associated with active genes. Dynamic and reversible changes in histone H3 acetylation occur at submergence-inducible genes in rice [21]. Recent results have revealed a function of histone acetylation in circadian regulation of rice gene expression [22].
Histone acetylation homeostasis is regulated by antagonistic actions of histone acetyltransferases (HAT) and histone deacetylases (HDAC) (Fig. 9.2). Although HAT function in rice has not been reported, several rice HDAC genes have been studied [23]. The rice genome contains at least 19 HDAC genes belonging to three classes [24]. Among them, two have primary homology to yeast HDAC groups: RPD3 (Reduce Potassium Dependency 3), and SIR2 (Silent mating-type Information Regulation 2). The third group known as the HD2 class is only found in plants [25]. Expression and functional studies suggest that individual rice HDAC genes have specific development functions that may be divergent from the Arabidopsis homologues. Expression of rice HDAC genes shows tissue/organ-specificity. Most of the HDAC genes are responsive to drought or salt stresses and some of them display diurnal expression. Over-expression of OsHDAC1 (also called HDA702), a RPD3 class member, leads to increased growth rate and altered architecture in transgenic rice [26, 27]. OsHDAC1 deacetylates histone H3 lysine 9 (H3K9), lysine 14 (H3K14) and lysine 18 (H3K18) and histone H4 lysine 5 (H4K5), lysine 12 (H4K12) and lysine 16 (H4K16). However, over-expression of several other rice RPD3 members does not produce any visible phenotypes. In contrast, down-regulation of a few RPD3 members causes different developmental defects [23].
SIR2 proteins are NAD+-dependent HDACs, some of which have been found to be involved in metabolic regulation and in increasing lifespan in yeast and animals [28]. In rice or Arabidopsis only two SIR2 genes have been identified [24]. Because the expression pattern of the two rice genes (SRT701 and SRT702) and the subcellular localization of the proteins are different [23, 29, 30], the two genes are likely to have distinct functions. Down-regulation of SRT701 by RNAi induces H3K9 acetylation, but reduces H3K9 dimethylation (see below for histone methylation) on many loci including transposable elements [30]. Transcription of many transposable elements and some of the hypersensitive response-related genes is activated in the RNAi plants, indicating that in wild-type rice plants transposons and cell death-related genes might be amongst the primary targets of SRT701, suggesting that SRT701-mediated histone deacetylation is an important component for transposon repression in rice.
Rice HDT701 (OsHDT1) belongs to the plant-specific HD2 class of HDACs. Its expression displays a circadian rhythm [22]. Over-expression or down-regulation of the gene does not affect plant growth in an elite hybrid rice parent but the over-expression leads to early flowering of the hybrid under long day conditions [22]. Increased OsHDT1 levels repress the long day flowering repressors OsGI and Hd1 whose expression is increased in the hybrid (a so-called “nonadditive” effect), likely due to increased acetylation levels over the genes. OsHDT1 over-expression promotes histone H4 deacetylation over OsGI and Hd1 during their peak expression phases in the hybrid and has an effect on nonadditive expression of many other genes in the hybrid [22]. It is possible that OsHDT1 is involved in epigenetic control of parental genome interaction for differential gene expression.
2.2 Histone Methylation and Histone Lysine Methyltransferases in Rice
Histone lysine methylation is an important epigenetic modification with both activating and repressive roles in gene expression [31]. Histone lysine residues can be mono- di- or trimethylated. For instance, H3K9 can be found at mono (H3K9me1)-, di (H3K9me2)-, or trimethylated (H3K9me3) state. Each methyl state may have a different function for genome activity. In plants, H3K9me2 is almost exclusively associated with heterochromatin regions (Fig. 9.2), while H3K9me3 is associated with genes. Trimethylated H3 lysine 27 (H3K27me3) is negatively correlated with gene expression, whereas trimethylated H3 lysine 4 (H3K4me3) and lysine 36 (H3K36me3) are associated with active genes [32] (Figs. 9.1 and 9.2).
2.2.1 H3K4 Methylation
In Arabidopsis, H3K4 methylation is found over about two-thirds of genes and is underrepresented in repeats and transposon-rich regions of the genome [33]. While H3K4 monomethylation (H3K4me1) and dimethylation (H3K4me2) are associated with both active and inactive genes, H3K4me3 is mostly correlated with active genes. H3K4me3 and H3K4me2 are detected mostly at the promoter and the 5′ end of genes (Fig. 9.1). In rice about half of the protein-coding genes have di- and/or trimethylated H3K4 based on the analysis of two chromosomes [34]. Rice genes with predominant H3K4me3 methylation are actively transcribed, whereas genes with predominant H3K4me2 methylation are transcribed at moderate levels [9, 34]. It has been shown that H3K4me3 increases over inducible genes in plants upon application of inductive signals [21].
Enzymes involved in histone methylation usually contain a motif called a SET domain, which is named after 3 Drosophila genes: Su(var)3-9, Enhancer of zeste (E(Z)), and Trithorax, the mutation of which either enhance or suppress epigenetic mutations [35] (Fig. 9.2). A large number of SET-domain genes are identified in rice and Arabidopsis genomes (Table 9.1). Trithorax proteins are a group of methyltransferases for H3K4 methylation. Arabidopsis TRITHORAX-RELATED1, 2 (ATX1, 2) and ATX-Related7 (ATXR7) have been shown to be involved in H3K4 methylation [36–38]. Other SET-domain proteins (SDG2) have been recently shown to be also involved in H3K4 methylation [39]. Rice homologues of ATX have been identified [40] (Table 9.1), while their function remains to be characterized.
2.2.2 H3K9 Methylation
Methylation of H3K9 is important for chromatin structure and gene regulation. H3K9me2 is found to be enriched in heterochromatic repetitive sequence regions, while H3K9me3 is distributed in the 5′ end of genes in euchromatic regions and is considered as a “mild” activating mark of gene transcription [41, 42] (Figs. 9.1 and 9.2). Drosophila Su(var)3-9 protein was the first identified histone lysine methyltransferase specific for H3K9 [43]. Plant genomes encode many SUVH genes [44]. Arabidopsis SUVH, also known as KYP (KRYPTONITE), and SUVH5 and SUVH6 encode activities of H3K9 mono- and dimethyltransferases [45–47]. The rice genome encodes 12 SUVH genes, among which SDG714 is found to be involved in H3K9me2 and DNA methylation of Tos17, a copia-like retrotransposon [48]. A systematic study of rice SUVH genes indicated that different members display distinct function in histone H3K9 methylation, DNA methylation, and transposon silencing [44].
2.2.3 H3K27 Methylation
All three methylated states of H3 lysine 27 are generally associated with repressive chromatin. In Arabidopsis, H3K27me3 is associated with about 10 % of annotated genes that are expressed at low levels or repressed in a tissue-specific manner [42, 49], whereas H3K27me1 is mostly associated with silent transposable elements and repetitive sequences [50]. H3K27me3 is distributed all over the gene body region (Fig. 9.1). About a similar percentage of genes are marked by H3K27me3 in rice [9], which are mostly repressed genes [50a].
E(Z) homologues which are components of polycomb group (PcG) complexes are responsible for trimethylation of H3K27 (Fig. 9.2). Several homologues of E(Z) in Arabidopsis have been shown to behave as essential regulators of plant developmental transitions by maintaining repression of key developmental regulatory genes [51]. Homologues of E(Z) and other PcG genes have been identified in rice [52]. Loss-of-function of rice E(Z) genes does not lead to similar defects found in mutants of Arabidopsis E(Z) homologues [52], suggesting that developmental function of these genes may not be conserved between different plant species. In Arabidopsis, two other ATX-Related genes, ATXR5 and ATXR6, are shown to be responsible for H3K27 monomethylation over repetitive sequences [50] (Fig. 9.2). The rice genome contains a higher proportion of repetitive sequences. Whether homologues of ATXR5 and ATXR6 or additional proteins are involved in H3K27me1 remains to be determined.
2.3 Histone Demethylases
Repression of active genes implies removal of methyl groups from H3K4me3, while activation of repressed genes may require demethylation of H3K27me3 (Fig. 9.2). Histone methylation is reversed by histone demethylases. LSD1 (Lysine specific demethylase 1) was the first histone demethylase to be identified to demethylate H3K4me1 and H3K4me2, in addition to H3K9me1 and H3K9me2 [53]. Four LSD1 homologues are found in rice and Arabidopsis. Arabidopsis LSD1 genes have been shown to be involved in flowering time regulation [54]. The function of rice LSD1 has not yet been determined. In addition, Jumonji C (jmjC) domain-containing proteins are found to function also as histone demethylases by removing di- and trimethyl groups [55, 56]. More than 20 jmjC protein genes are identified in rice and Arabidopsis [57, 58]. Two Arabidopsis jmjC proteins, JMJ14 and JMJ15 are shown to possess specific demethylase activity to reverse di- and trimethylated H3K4 [32, 59, 60]. Closely related homologues in rice have also been shown to demethylate H3K4 [60a].
Rice JMJ706 (Os10g42690) and Arabidopsis Increase in Bonsai Methylation1 (IBM1/JMJ25, At3g07610) have activities to remove methyl groups of di- and trimethylated H3K9 in vitro and/or in vivo [58, 61]. Mutations in the two genes produce severe developmental defects, suggesting that histone H3K9 demethylation is essential for normal plant development. Mutations in rice JMJ706 affect floral organ number and seed development and lead to an increase of H3K9me2/3 [58]. In addition, the phenotype of rice jmj706 mutants can be partially suppressed by RNAi of a few rice SUVH genes [44], indicating that SUVH proteins may form antagonistic couplets with JMJ706 to regulate the homeostasis of H3K9 methylation. It was recently shown that the Arabidopsis REF6 gene that is closely related to rice JMJ705 and JMJ706 is involved in demethylation of H3K27me3 [62]. It remains to be determined whether these rice proteins also have a demethylase activity of H3K27me3.
3 Recognition of Histone Modifications
Histone modification modules are recognized by chromatin proteins that have activities to remodel chromatin structure to regulate gene transcription or induce heterochromatin formation (Fig. 9.2). Acetylated histone lysine residues (e.g., H3K14ac) are bound by bromodomain-containing proteins, such as histone acetyltransferase GCN5 [63]. The different methylated histone lysine residues are recognized by different chromatin protein modules including chromodomains and PHD (Plant HomeoDomain) fingers. For instance, in animal cells the chromodomain of Heterchromatin Protein1 (HP1) binds to H3K9me2 [64], while the chromodomain of the polycomb protein is associated with H3K27me3 [65]. However, in Arabidopsis, the chromodomain of LHP1 (LIKE HETEROCHROMATIN PROTEIN 1) interacts with H3K27me3 [42]. A subset of PHD finger-containing proteins are able to interact with H3K4me3 [66]. In addition, chromodomains can also recognize methylated H3K4. For instance, mammalian chromodomain protein CHD1 binds to H3K4me3 to regulate gene activation [67]. A rice chromodomain protein CHD3 has been shown to be able to interact with both H3K4me2 and H3K27me3 [50a].
4 Interplay Between Chromatin Modifications
Regulation of chromatin remodeling processes involves functional interactions between multiple chromatin modifications (Fig. 9.2). For instance, H3K4me3 is positively associated with H3K9ac. H3K4me3 and H3K9ac are active marks, and represent a chromatin signature of active genes in plants [68]. In addition, H3K9ac is shown to be antagonistic to H3K9me2 in rice. Down-regulation of the HDAC gene SRT701 not only increases H3K9ac but also reduces H3K9me2 leading to transcriptional activation of many transposable element-related genes in rice [30]. It is likely that deacetylation promotes H3K9 methylation required for transposable element silencing in rice. Genome-wide analysis indicates that H3K4me3 and H3K27me3 are mutually repulsive and antagonistic marks in plants [33, 68]. In rice, relatively few genes are co-modified by both marks [50a]. Interplay between the two histone modification marks is likely to play an important role in gene expression in rice [9]. This may imply physical or functional interactions between histone demethylases and other histone modification complexes as revealed in animal cells [31]. It remains to be known whether similar protein complexes exist in plant cells to coordinate methylation/demethylation of H3K4 and H3K27.
DNA methylation plays critical roles in epigenetic processes and is associated with histone methylation. DNA methylation requires unmethylated H3K4 nucleosomes in mammalian cells [69], while H3K27me3 seems to be antagonistic to DNA methylation in plants [70]. De novo DNA methylation can be triggered not only by small RNA but also by H3K9me2. In Arabidopsis, dimethylation of H3K9 by KYP is required for maintenance of CHG methylation by CMT3 [71]. Conversely, the H3K9 demethylase IBM1/JMJ25 has a function to protect active genes from H3K9me2 and CHG methylation [72]. However, mutations of the rice homologue JMJ706 that affect overall H3K9me2 did not affect DNA methylation in gene bodies (unpublished data), suggesting that JMJ706 may be functionally distinct from IBM1. By contrast, loss-of-function of a H3K4 demethylase gene JMJ14 leads to loss of non-CG methylation at loci targeted by RNAi-directed DNA Methylation (RdDM) in Arabidopsis [73]. Therefore histone demethylation on different lysine residues may have distinct roles in DNA methylation regulation [57]. It remains to be known whether similar interactions are conserved in rice that has a larger amount of repetitive sequences and transposable elements than Arabidopsis.
5 Epigeneomic Variations, Inheritance and Epialleles
Closely related species may develop differences in their epigenetic systems during adaptation to different environmental niches. Studies of natural variation of DNA methylation in a number of other plant species have suggested that epigenetic variation among individuals with similar genotype can lead to phenotypic variation in response to varying environmental conditions [74]. Epigenetic adaptive responses to environmental cues can be transmitted to future generations [75, 76].
It has been shown that DNA methylation in genes is extremely polymorphic among 96 natural accessions of Arabidopsis [77]. More recent studies by examining spontaneously occurred variation in DNA methylation in Arabidopsis plants propagated by single seed descent for 30 generations have revealed that transgenerational changes in cytosine methylation occur at a high frequency [78, 79]. Transgenerational variation in DNA methylation that adversely affects gene expression may generate new epigenetic alleles (epialleles) leading to phenotypic variation without DNA sequence change. DNA methylation-induced silencing of protein-coding genes gives rise to epialleles that can be inherited through meiosis [80]. Two examples of meiotically heritable epialleles resulting in morphological variation are the peloric (in Linaria vulgaris) and colorless non-ripening (in Solanum lycopersicum) loci which are spontaneous epigenetic silencing events [81, 82]. In rice, the epiallele of DWARF1 (D1), Epi-d1, causes a metastable dwarf phenotype [83]. The silenced state is correlated with repressive histone and DNA methylation marks in the D1 promoter region. It has been recently shown that the expression level of OsSPL14 (SQUAMOSA PROMOTER BINDING PROTEIN-LIKE14) is important to regulate panicle branching and grain yield in rice [83]. Differences in DNA methylation and histone modifications on the OsSPL14 locus are likely to be responsible for the expression difference of the gene between two japonica rice varieties that differ in grain numbers per panicle. This case demonstrates that epigenetic mutations may be an important source for variation of important agronomic traits in rice.
6 Perspectives
In the long history of rice evolution, domestication and selection, epigenomic variations may have been generated and may have contributed to phenotypic differences and variations in complex traits among different species, subspecies, and cultivars. In addition, epigenomic variation among individuals with similar genotypes can drive phenotypic variation in response to varying physical, biotic, and abiotic environments. Many morphological and adaptive phenotypes may be dependent on different epialleles. Therefore, investigating rice and other Oryza epigenomes will be important to identify specific epigenetic marks and epialleles involved in important agronomic and adaptive traits. Functional characterization of rice chromatin modification regulators (writers, erasers, and readers) has just started and remains to be an important research field to understand the mechanism of establishment, maintenance, recognition and inheritance, or erasure of rice epigenomes. In fact, understanding how established epigenomic marks corresponding to specialized plant cell types or responding to induction by specific environmental cues can be memorized during subsequent cell divisions and inherited to next generations represents an essential research aspect of epigenetics in the future.
References
Horn PJ, Peterson CL (2002) Molecular biology. Chromatin higher order folding–wrapping up transcription. Science 297:1824–1827
Grewal SI, Moazed D (2003) Heterochromatin and epigenetic control of gene expression. Science 301:798–802
Law JA, Jacobsen SE (2010) Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11:204–220
Pfluger J, Wagner D (2007) Histone modifications and dynamic regulation of genome accessibility in plants. Curr Opin Plant Biol 10:645–652
Margueron R, Reinberg D (2010) Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 11:285–296
Cokus SJ, Feng S, Zhang X et al (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452:215–219
Feng S, Cokus SJ, Zhang X et al (2010) Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A 107:8689–8694
Zemach A, Kim MY, Silva P et al (2010) Local DNA hypomethylation activates genes in rice endosperm. Proc Natl Acad Sci U S A 107:18729–18734
He G, Zhu X, Elling AA et al (2010) Global epigenetic and transcriptional trends among two rice subspecies and their reciprocal hybrids. Plant Cell 22:17–33
Gehring M, Henikoff S (2007) DNA methylation dynamics in plant genomes. Biochim Biophys Acta 1769:276–286
Zemach A, McDaniel IE, Silva P et al (2010) Genome-wide evolutionary analysis of eukaryotic DNA methylation. Science 328:916–919
Chan SW, Henderson IR, Jacobsen SE (2005) Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat Rev Genet 6:351–360
Cao X, Aufsatz W, Zilberman D et al (2003) Role of the DRM and CMT3 methyltransferases in RNA-directed DNA methylation. Curr Biol 13:2212–2217
Gong Z, Morales-Ruiz T, Ariza RR et al (2002) ROS1, a repressor of transcriptional gene silencing in Arabidopsis, encodes a DNA glycosylase/lyase. Cell 111:803–814
Choi Y, Gehring M, Johnson L et al (2002) DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110:33–42
La H, Ding B, Mishra GP et al (2011) A 5-methylcytosine DNA glycosylase/lyase demethylates the retrotransposon Tos17 and promotes its transposition in rice. Proc Natl Acad Sci U S A 108:15498–15503
Berger SL (2007) The complex language of chromatin regulation during transcription. Nature 447:407–412
Chen ZJ, Tian L (2007) Roles of dynamic and reversible histone acetylation in plant development and polyploidy. Biochim Biophys Acta 1769:295–307
Servet C, Conde e Silva N, Zhou DX (2010) Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Mol Plant 3:670–677
Yin BL, Guo L, Zhang DF et al (2008) Integration of cytological features with molecular and epigenetic properties of rice chromosome 4. Mol Plant 1:816–829
Tsuji H, Saika H, Tsutsumi N et al (2006) Dynamic and reversible changes in histone H3-Lys4 methylation and H3 acetylation occurring at submergence-inducible genes in rice. Plant Cell Physiol 47:995–1003
Li C, Huang L, Xu C et al (2011) Altered levels of histone deacetylase OsHDT1 affect differential gene expression patterns in hybrid rice. PLoS One 6:e21789
Hu Y, Qin F, Huang L et al (2009) Rice histone deacetylase genes display specific expression patterns and developmental functions. Biochem Biophys Res Commun 388:266–271
Pandey R, Muller A, Napoli CA et al (2002) Analysis of histone acetyltransferase and histone deacetylase families of Arabidopsis thaliana suggests functional diversification of chromatin modification among multicellular eukaryotes. Nucleic Acids Res 30:5036–5055
Lusser A, Brosch G, Loidl A et al (1997) Identification of maize histone deacetylase HD2 as an acidic nucleolar phosphoprotein. Science 277:88–91
Chung PJ, Kim YS, Jeong JS et al (2009) The histone deacetylase OsHDAC1 epigenetically regulates the OsNAC6 gene that controls seedling root growth in rice. Plant J 59:764–776
Jang IC, Pahk YM, Song SI et al (2003) Structure and expression of the rice class-I type histone deacetylase genes OsHDAC1-3: OsHDAC1 overexpression in transgenic plants leads to increased growth rate and altered architecture. Plant J 33:531–541
Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460:587–591
Chung PJ, Kim YS, Park SH et al (2009) Subcellular localization of rice histone deacetylases in organelles. FEBS Lett 583:2249–2254
Huang L, Sun Q, Qin F et al (2007) Down-regulation of a SILENT INFORMATION REGULATOR2-related histone deacetylase gene, OsSRT1, induces DNA fragmentation and cell death in rice. Plant Physiol 144:1508–1519
Mosammaparast N, Shi Y (2010) Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem 79:155–179
Liu C, Lu F, Cui X et al (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Zhang X, Bernatavichute YV, Cokus S et al (2009) Genome-wide analysis of mono-, di- and trimethylation of histone H3 lysine 4 in Arabidopsis thaliana. Genome Biol 10:R62
Li X, Wang X, He K et al (2008) High-resolution mapping of epigenetic modifications of the rice genome uncovers interplay between DNA methylation, histone methylation, and gene expression. Plant Cell 20:259–276
Jenuwein T, Laible G, Dorn R et al (1998) SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol Life Sci 54:80–93
Pien S, Fleury D, Mylne JS et al (2008) ARABIDOPSIS TRITHORAX1 dynamically regulates FLOWERING LOCUS C activation via histone 3 lysine 4 trimethylation. Plant Cell 20:580–588
Saleh A, Alvarez-Venegas R, Yilmaz M et al (2008) The highly similar Arabidopsis homologs of trithorax ATX1 and ATX2 encode proteins with divergent biochemical functions. Plant Cell 20:568–579
Tamada Y, Yun JY, Woo SC et al (2009) ARABIDOPSIS TRITHORAX-RELATED7 is required for methylation of lysine 4 of histone H3 and for transcriptional activation of FLOWERING LOCUS C. Plant Cell 21:3257–3269
Guo L, Yu Y, Law JA et al (2010) SET DOMAIN GROUP2 is the major histone H3 lysine [corrected] 4 trimethyltransferase in Arabidopsis. Proc Natl Acad Sci U S A 107:18557–18562
Zhou DX, Hu Y (2010) Regulatory function of histone modifications in controlling rice gene expression and plant growth. Rice 3:103–111
Charron JB, He H, Elling AA et al (2009) Dynamic landscapes of four histone modifications during deetiolation in Arabidopsis. Plant Cell 21:3732–3748
Turck F, Roudier F, Farrona S et al (2007) Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 lysine 27. PLoS Genet 3:e86
Rea S, Eisenhaber F, O’Carroll D et al (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406:593–599
Qin FJ, Sun QW, Huang LM et al (2010) Rice SUVH histone methyltransferase genes display specific functions in chromatin modification and retrotransposon repression. Mol Plant 3:773–782
Ebbs ML, Bartee L, Bender J (2005) H3 Lysine 9 methylation is maintained on a transcribed inverted repeat by combined action of SUVH6 and SUVH4 methyltransferases. Mol Cell Biol 25:10507–10515
Ebbs ML, Bender J (2006) Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18:1166–1176
Jackson JP, Johnson L, Jasencakova Z et al (2004) Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112:308–315
Ding Y, Wang X, Su L et al (2007) SDG714, a histone H3K9 methyltransferase, is involved in Tos17 DNA methylation and transposition in rice. Plant Cell 19:9–22
Zhang X, Clarenz O, Cokus S et al (2007) Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol 5:e129
Jacob Y, Feng S, LeBlanc CA et al (2009) ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat Struct Mol Biol 16:763–768
Hu Y, Liu D, Zhong X et al (2012) A CHD3 protein recognizes and regulates methylated histone H3 lysines 4 and 27 over a subset of targets in the rice genome. Proc. Natl. Acad. Sci. USA 109:5773–5778
Pien S, Grossniklaus U (2007) Polycomb group and trithorax group proteins in Arabidopsis. Biochim Biophys Acta 1769:375–382
Luo M, Platten D, Chaudhury A et al (2009) Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol Plant 2:711–723
Shi Y, Lan F, Matson C et al (2004) Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119:941–953
Jiang D, Yang W, He Y et al (2007) Arabidopsis relatives of the human lysine-specific Demethylase1 repress the expression of FWA and FLOWERING LOCUS C and thus promote the floral transition. Plant Cell 19:2975–2987
Klose RJ, Kallin EM, Zhang Y (2006) JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 7:715–727
Trewick SC, McLaughlin PJ, Allshire RC (2005) Methylation: lost in hydroxylation? EMBO Rep 6:315–320
Chen X, Hu Y, Zhou DX (2011) Epigenetic gene regulation by plant Jumonji group of histone demethylase. Biochim Biophys Acta 1809(8):421–426
Sun Q, Zhou DX (2008) Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci U S A 105:13679–13684
Jeong JH, Song HR, Ko JH et al (2009) Repression of FLOWERING LOCUS T chromatin by functionally redundant histone H3 lysine 4 demethylases in Arabidopsis. PLoS One 4:e8033
Lu F, Cui X, Zhang S et al (2010) JMJ14 is an H3K4 demethylase regulating flowering time in Arabidopsis. Cell Res 20:387–390
Chen Q, Chen X, Wang Q et al (2013) Structural basis of a histone H3 Lysine 4 demethylase required for stem elongation in rice. Plos Genet. 9(1): e1003239. doi:10.1371/journal.pgen.1003239
Saze H, Shiraishi A, Miura A et al (2008) Control of genic DNA methylation by a jmjC domain-containing protein in Arabidopsis thaliana. Science 319:462–465
Lu F, Cui X, Zhang S et al (2011) Arabidopsis REF6 is a histone H3 lysine 27 demethylase. Nat Genet 43:715–719
Benhamed M, Bertrand C, Servet C et al (2006) Arabidopsis GCN5, HD1, and TAF1/HAF2 interact to regulate histone acetylation required for light-responsive gene expression. Plant Cell 18:2893–2903
Vermaak D, Malik HS (2009) Multiple roles for heterochromatin protein 1 genes in Drosophila. Annu Rev Genet 43:467–492
Min J, Zhang Y, Xu RM (2003) Structural basis for specific binding of polycomb chromodomain to histone H3 methylated at Lys 27. Genes Dev 17:1823–1828
Sanchez R, Zhou MM (2011) The PHD finger: a versatile epigenome reader. Trends Biochem Sci 36:364–372
Flanagan JF, Mi LZ, Chruszcz M et al (2005) Double chromodomains cooperate to recognize the methylated histone H3 tail. Nature 438:1181–1185
Roudier F, Ahmed I, Berard C et al (2011) Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J 30:1928–1938
Ooi SK, Qiu C, Bernstein E et al (2007) DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448:714–717
Weinhofer I, Hehenberger E, Roszak P et al (2010) H3K27me3 profiling of the endosperm implies exclusion of polycomb group protein targeting by DNA methylation. PLoS Genet 6(10):e1001152
Jackson JP, Lindroth AM, Cao X et al (2002) Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416:556–560
Miura A, Nakamura M, Inagaki S et al (2009) An Arabidopsis jmjC domain protein protects transcribed genes from DNA methylation at CHG sites. EMBO J 28:1078–1086
Deleris A, Greenberg MV, Ausin I et al (2010) Involvement of a Jumonji-C domain-containing histone demethylase in DRM2-mediated maintenance of DNA methylation. EMBO Rep 11:950–955
Richards EJ (2011) Natural epigenetic variation in plant species: a view from the field. Curr Opin Plant Biol 14:204–209
Verhoeven KJ, Jansen JJ, van Dijk PJ et al (2010) Stress-induced DNA methylation changes and their heritability in asexual dandelions. New Phytol 185:1108–1118
Boyko A, Blevins T, Yao Y et al (2010) Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of dicer-like proteins. PLoS One 5:e9514
Vaughn MW, Tanurdzic M, Lippman Z et al (2007) Epigenetic natural variation in Arabidopsis thaliana. PLoS Biol 5:e174
Schmitz RJ, Schultz MD, Lewsey MG et al (2011) Transgenerational epigenetic instability is a source of novel methylation variants. Science 334:369–373
Becker C, Hagmann J, Muller J et al (2011) Spontaneous epigenetic variation in the Arabidopsis thaliana methylome. Nature 480:245–249
Paszkowski J, Grossniklaus U (2011) Selected aspects of transgenerational epigenetic inheritance and resetting in plants. Curr Opin Plant Biol 14:195–203
Cubas P, Vincent C, Coen E (1999) An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401:157–161
Manning K, Tor M, Poole M et al (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Miura K, Agetsuma M, Kitano H et al (2009) A metastable DWARF1 epigenetic mutant affecting plant stature in rice. Proc Natl Acad Sci U S A 106:11218–11223
Liu B, Li P, Li X et al (2005) Loss of function of OsDCL1 affects microRNA accumulation and causes developmental defects in rice. Plant Physiol 139:296–305
Song X, Li P, Zhai J et al (2011) Roles of DCL4 and DCL3b in rice phased small RNA biogenesis. Plant J 69(3):462–474
Liu B, Chen Z, Song X et al (2007) Oryza sativa dicer-like4 reveals a key role for small interfering RNA silencing in plant development. Plant Cell 19:2705–2718
Urayama S, Moriyama H, Aoki N et al (2010) Knock-down of OsDCL2 in rice negatively affects maintenance of the endogenous dsRNA virus, Oryza sativa endornavirus. Plant Cell Physiol 51:58–67
Ahmad A, Dong Y, Cao X (2011) Characterization of the PRMT gene family in rice reveals conservation of arginine methylation. PLoS One 6:e22664
Hu Y, Zhu N, Wang X et al (2013) Analysis of rice Snf2 family proteins and their potential roles in epigenetic regulation. Plant Physiology and Biochemistry. 70:33–42
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Zhou, DX., Hu, Y., Zhao, Y. (2013). Epigenomics. In: Zhang, Q., Wing, R. (eds) Genetics and Genomics of Rice. Plant Genetics and Genomics: Crops and Models, vol 5. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7903-1_9
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7903-1_9
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7902-4
Online ISBN: 978-1-4614-7903-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)