Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are inflammatory diseases whose clinical severity depends on the grade of inflammatory response. Inflammatory cytokines are key elements in the pathogenesis of ALI/ARDS, and the occurrence of an imbalance between pro- and anti-inflammatory cytokines leads to additional non-pulmonary organ dysfunction which contributes to excess mortality rates.
Treatment of these patients includes nutrition support with lipids, usually soybean oil-based lipid emulsions, which are rich in omega (n)-6 polyunsaturated fatty acids (PUFAs) and deficient in n-3 PUFAs; however, too much n-6 PUFAs are detrimental due to their pro-inflammatory effects. Conversely, a large amount of experimental studies and some randomized clinical trials showed the benefits of the n-3 PUFA administration in the context of ALI because of their anti-inflammatory properties. Based on these data, several scientific societies recommended in their guidelines, with an A or B grade of recommendation, the use of n-3 PUFAs in ALI/ARDS patients. However, at present, the issue of lipid therapy in ALI/ARDS is still controversial due, at least in part, to inconclusive or contradicting results in several recent clinical trials using n-3 PUFAs.
You have full access to this open access chapter, Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Acute respiratory distress syndrome (ARDS) is considered a form of acute diffuse lung injury (ALI). According to the Berlin definition (Ferguson et al. 2012), each subcategory of ARDS (mild, moderate, and severe) is defined by mutually exclusive ranges of the ratio between arterial oxygen partial pressure (PaO2) and fractional inspired oxygen (FiO2) (200 mm Hg<PaO2/FIO2≤300 mm Hg, 100 mm Hg<PaO2/FIO2≤200 mm Hg, and PaO2/FIO2≤100 mm Hg, respectively).
The acute phase of this syndrome is manifested by the early onset of respiratory failure (characterized by hypoxemia refractory to oxygen supply within 1 week of a known clinical insult or new/worsening respiratory symptoms) related to several triggers and clinical disorders generally distinguished in pulmonary [pneumonia (bacteria or virus), microbial products (e.g., endotoxins), pulmonary aspiration of gastric contents] and extrapulmonary (trauma/hemorrhagic shock, extensive burns, ischemia/reperfusion injury, acute pancreatitis, cardiopulmonary bypass, transfusions of blood products) (Leaver and Evans 2007).
ARDS is frequently progressive and it is characterized by distinct phases with different clinical, radiographic, and histopathological findings. Radiographic findings are similar to those observed in the case of cardiogenic pulmonary edema, mainly bilateral infiltrates (irregular and asymmetric) and sometimes pleural effusions (Fig. 1).
Histopathological findings of ALI include diffuse alveolar epithelium damage (principally, denudation of the basement membrane of the alveolus and necrosis of type I cells) with activated neutrophils, alveolar macrophages, hyaline membranes, and protein-rich edema fluid in the alveolar spaces (Fig. 2).
Alveolar damage in acute lung injury/acute respiratory distress syndrome. The tissue architecture is disrupted, alveoli are collapsed, alveolar type II (ATII) cells are hyperplastic, and alveolar type I cells are necrotic. Alveoli contain protein-rich edema fluid and macrophages. Collagen deposition in the interstitium has begun. Hematoxylin and eosin staining, 200× magnification. The picture was kindly given by Luisa Delsedime, MD, Anatomia Patologica, AOU Città della Scienza e della Salute, Turin, Italy
The pathophysiology of ALI is complex and multifaceted. The lung inflammatory response involves various interactions among many cell types, and it is characterized by the production and release of several chemical mediators. Figure 3 is a simplified graphic description of how the anatomy and physiology of alveolar space, the main site of inflammatory reactions occurring in ALI, change.
The normal alveolus and the alveolus in acute lung injury. Neutrophil is shown migrating through a gap in the capillary endothelium into the alveolar space (activated neutrophil). In the alveolar space, an alveolar macrophage is secreting cytokines: tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, and IL-10. The alveolar type I cells are damaged or necrotic, while alveolar type II cells are intact and release cytokines. The tissue architecture is disrupted with the formation of hyaline membranes on the denuded basement membrane. The alveolar space is filled by protein-rich edema fluid
Lung and Cytokines
The lung is a complex organ with many different cell types, i.e., pneumocytes (alveolar type I and II), pulmonary capillary endothelial cells, alveolar macrophages, and fibroblasts. Several studies suggest that the lung itself can be an important cytokine-producing organ.
Cytokines are a diverse group of inflammatory mediators, produced by a wide variety of cell types, which initiate and orchestrate the host response to different injuries. Intercellular communication through cytokine networking plays a key role for homeostasis. Cytokines seem to have concentration-dependent effects. At lower concentrations, they modulate tissue response to injuries; at higher concentrations, they induce proportionally more severe local and systemic inflammatory responses. If this cytokine overproduction is not regulated, it may remarkably amplify the release of further pro-inflammatory mediators and, at least, the overall inflammatory response.
Both ALI and ARDS are characterized by an intense inflammatory response. In ARDS early phase, the alveolar space is the site of significant inflammatory reactions (Pugin et al. 1999). ALI/ARDS are characterized by alveolar infiltration with neutrophils and macrophages, both able to release inflammatory cytokines. However, recent theories suggest that it is not the neutrophil and macrophage number but their pro-inflammatory cytokine release that produces lung injury. In this setting, cytokines have a central position as signaling mediators that initiate, increase, and maintain inflammatory responses on a local basis. Indeed, in ALI/ARDS, an accumulation of both pro- and anti-inflammatory cytokines within the alveolar spaces is demonstrated.
Many cytokines were detected at elevated levels in bronchoalveolar lavage (BAL) fluid in patients with ARDS, i.e., tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, and IL-10. TNF-α is the main pro-inflammatory cytokine and it is important in driving the initial lung inflammatory response, principally indirect by the stimulation of other cytokines (i.e., IL-6 and IL-8). IL-6 occupies a crucial place in the ARDS inflammatory response. Chemokine IL-8 is the major neutrophil chemotactic factor into the alveolar space, and it is an early marker for the development of ARDS. Finally, IL-10, which has anti-inflammatory effects, plays a pivotal role in closing down the inflammatory response once it achieves its primary purpose.
The pivotal role of a hyper-inflammatory response, characterized by overproduction of TNF-α, IL-6, and IL-8, in the evolution of the disease from ALI to ARDS is documented. Indeed, many studies report a higher mortality in patients who have increased pro-inflammatory cytokine concentrations (TNF-α, IL-6, and IL-8) in the BAL at the onset of ARDS or persistent increased concentrations in days 3–10.
Physiologically, the critical dependency on the presence of the inflammatory ligand (e.g., platelet-activating factor) and free arachidonic acid (AA) may help to confine the outbreak of lipid-mediator production to sites of initial inflammatory process. Conversely, compartmentalization of alveolar cytokines can be lost in the case of a damaged alveolar barrier. Cytokines generated in the alveoli may go into the systemic circulation leading to the beginning of a systemic inflammatory response syndrome.
The reason why a patient with ALI develops ARDS while another recovers is still not completely clear. In ARDS-developing patients, it is hypothesized that, at some level, regulation of the inflammatory response must be inadequate, but why or when this regulation is insufficient is a matter of debate. The study of the balance between pro- and anti-inflammatory cytokines is of greater importance to understand the pathophysiology of ALI than a single cytokine concentration, because the degree of cytokine imbalance is a contributing element to disease severity (Park et al. 2001).
Persistently high levels of pro-inflammatory cytokines, as well as a reduced production of anti-inflammatory cytokines, have been demonstrated to be correlated with the severity of lung injury. The degree of this cytokine imbalance has a pivotal role in inducing other non-pulmonary organ dysfunctions and increasing mortality rates in ARDS patients. In particular, the pro-inflammatory activity depends on the balance between the cytokine and its inhibitor(s). In ARDS early phase, the finding of low levels of anti-inflammatory cytokines (i.e., IL-10 and IL-1 receptor antagonist) in the BAL is associated with an increase in patient mortality. The ratio TNF-α to IL-10 in the BAL fluid was significantly higher (3.52 vs. 0.85) in patients with ARDS than in those at risk of developing ARDS.
Lipids, PUFAs, and Lung Injury
Among ALI ventilated patients, malnutrition has been associated with adverse outcomes due to increased infection risk, prolonged mechanical ventilation dependency, prolonged intensive care unit (ICU) length of stay (LOS), and higher mortality.
In general, critically ill patients receive nutrition support including lipids. Administration of lipid emulsions offers many advantages in patients with acute respiratory failure, because compared with glucose they provide a richer source of calories in a small volume with a lower carbon dioxide load and less hyperglycemia. Moreover, lipid supply is mandatory to avoid essential fatty acid (FA) deficiency.
Accepted guidelines recommend providing 30–35 % of nonprotein calories as lipids in patients with ALI/ARDS (Mizock 2001). Lipids were introduced into parenteral nutrition (PN) formulations in the 1960s. However, there is a lack of consensus about the optimal source and composition of lipids (long- vs. medium-chain triglycerides, soybean oil vs. olive oil and fish oil) both in parenteral and enteral formulas for the patient with ALI/ARDS.
Commonly used parenteral lipid emulsions (i.e., soybean oil-based fat emulsions) contain large amounts of n-6 polyunsaturated fatty acids (PUFAs) (i.e., more than 50 % of linoleic acid) that form AA [omega (n)-6 PUFA], while only 7 % α-linolenic acid can be metabolically converted to eicosapentaenoic acid (EPA, n-3 PUFA) and docosahexaenoic acid (DHA, n-3 PUFA). Therefore, intravenous fat emulsions have a very low n-3/n-6 PUFA ratio, ranging between 1:5 and 1:7. Likewise, the n-3/n-6 ratio in enteral products may be as low as 1:50. Thus, ALI/ARDS patients – either on standard enteral nutrition (EN) or PN – are generally exposed to a large quantity of linoleic acid. However, administration of high quantities of linoleic acid is not desirable in ALI/ARDS patients because it causes a production of high amounts of AA, whose main functional role is to be a substrate for the synthesis of bioactive pro-inflammatory mediators known as eicosanoids [above all, prostaglandin (PG)-E2, thromboxane (TX)-A2, and leukotriene (LT)-B4] and platelet-activating factor, which modulates severity and duration of inflammatory responses.
The impact of parenteral lipid supply on the lung function of patients with acute respiratory failure has been contradicting. Potential harmful effects have been related to type and/or administration rate of lipid emulsions. In particular, some authors thought that these negative effects might correlate to the infusion of soybean oil-based lipid emulsions – because they are rich in linoleic acid – and suggested that linoleic acid-derived synthesis of eicosanoids may worsen gas exchange and pulmonary hemodynamics in critically ill patients. Eicosanoids are major pathogenetic factors of ARDS, and their negative effects on pulmonary function (e.g., increased permeability, edema formation, polymorphonuclear activation, thrombocyte aggregation, vasoconstriction, and bronchoconstriction) have been widely investigated.
As early as 1942, the immunomodulatory properties of lipids were displayed. Over the past years, there has been an improved understanding of PUFA pathophysiology, and several mechanisms for the interaction between PUFAs and inflammation or immune response have been demonstrated. Indeed, after PUFA supply (diet, as well as EN or PN), many cell properties and related functions are modified, mainly the inflammatory and immunity responses (Calder 2006).
Briefly, n-3 PUFAs are more regarded as anti-inflammatory, whereas n-6 PUFAs as pro-inflammatory. More recent studies suggested that n-3 PUFAs may be involved in the resolution of inflammation (Calder 2013a).
Since a network of factors regulates the relationship between n-3 and inflammation, the mechanisms underlying the effects of n-3 PUFAs on cell event modulation are complex. To summarize the mechanisms of n-3 PUFA modulation of the inflammatory response, it can be said that the main and well-demonstrated anti-inflammatory actions of n-3 PUFAs include the following:
-
(a)
A decreased production of AA-derived mediators such as 2-series PG and LTB4 as a result of a reduction in AA content of cell membranes and of inhibition of AA metabolism.
-
(b)
An increased production of eicosanoid mediators from EPA (e.g., 3-series PG and LTB5), since these mediators have weak biological potency compared with those produced from AA; for example, LTB5 is 10- to 100-fold less potent as a neutrophil chemotactic agent than LTB4, or PGE3 is a less potent inducer of cyclooxygenase-2 gene expression in fibroblasts and of IL-6 production by macrophages compared with PGE2.
-
(c)
A decreased T-cell proliferation and production of IL-2 induced by EPA and DHA providing.
-
(d)
A decreased expression of some adhesion molecules on the surface of monocytes, macrophages, lymphocytes, and endothelial cells induced by EPA and DHA providing.
-
(e)
A decreased production of pro-inflammatory cytokines, due to decreased activation of pro-inflammatory transcription factors such as nuclear factor κ-B and increased activation of anti-inflammatory transcription factors such as peroxisome proliferator-activated receptor-γ.
-
(f)
An increased production of resolvins (E-series from EPA and D-series from DHA) and protectins (from DHA) having potent anti-inflammatory or inflammation-resolving properties.
PUFAs and Lung Injury: Nutrients or Drugs?
Over the last 25 years, the discovered ability of n-3 PUFAs to downregulate many different responses of the inflammatory process has suggested to several researchers that these FAs might have a key role in determining the grade of severity of some inflammatory diseases. Consequently, n-3 PUFAs have been widely used not only as nutrients but mainly as pharmacological agents.
Briefly, the rationale of the therapeutic use of n-3 PUFAs was based on several well-established issues: (1) PUFAs are key structural and functional elements of the phospholipids in cell membranes; (2) cells involved in the immunity and inflammation (neutrophils, monocytes, macrophages, dendritic cells, and endothelial cells) are richer in n-6 PUFAs than n-3, but the contents of AA, EPA, and DHA can be modified by n-3 PUFA (i.e., EPA and DHA) administration (oral, enteral, and parenteral); (3) PUFA challenge, as well as the FA composition of cell membrane of human inflammatory cells, influences the functions of those cells; and, last but more important, (4) n-3 PUFAs and n-6 PUFAs have opposing influences on inflammation.
ALI/ARDS are inflammatory diseases too; therefore, numerous studies have been carried out to evaluate strategies (drugs or interventional procedures) to reduce the severity of lung inflammatory processes by lowering the production of pro-inflammatory mediators (eicosanoids and cytokines). Nonetheless, few studies have found a significant improvement on mortality in those patients.
Since 1997, many editorials have stressed the possibility to manipulate the inflammatory response in ALI/ARDS patients using n-3 PUFAs as drugs (the so-called pharmaconutrition) (Mayer and Seeger 2008). The research on the influence of FAs on immunity started in the 1970s. Subsequently, a great number of works with cell or animal models have been carried out with the aim to demonstrate efficacy in modifying the inflammatory responses of fish oil or their main active components (i.e., EPA and DHA). Likewise, there have been some clinical trials of enteral or parenteral administration of fish oil-enriched nutrition formulas in critically ill patients with ALI/ARDS.
In Vitro Studies
The experimental models used were animal isolated lungs or, mainly, cell line models. Frequently, a human lung carcinoma cell line (A549 cells) was used. A549 are alveolar epithelial cells with type II pneumocyte properties that retain many of the characteristics of normal human type II epithelial cells and could synthesize lecithin and phosphatidylcholine with a high percentage of desaturated FAs. Several authors used alveolar type II cells because it was shown to have a central position in the pathophysiology of the alveolar space.
After in vitro administration, FAs were rapidly incorporated in the phospholipid spectrum of alveolar cell membranes; in fact, as pulmonary surfactant (the surface-active phospholipoprotein complex) producers, alveolar type II cells have a higher lipid metabolism, e.g., a significant increase in DHA concentration in the lung phospholipid fraction was observed 1 h after DHA infusion.
Several experimental studies with isolated lungs and culture of different pulmonary cells clearly showed a pivotal role of AA and its metabolites as mediators of injury. In contrast, supplementation of n-3 PUFAs demonstrated to reduce alveolar release of pro-inflammatory mediators and to reduce organ failure in animal lung models.
Rapid changes in cell membrane phospholipid FA composition and lipid-derived inflammatory mediator generation were observed both after short cell exposure to n-3 PUFAs and short-term intravenous administration of n-3 PUFAs. It was observed that PG3 was produced within 5 min of A549 cell exposure to EPA – with a peak at 4 h – and this rapid increase of PG3 was correlated with the metabolism of EPA by cyclooxygenases in the A549 cells. Experimental data suggested that EPA and DHA are both able to inhibit PGE2 production.
A short-term n-3 PUFA supplementation induced a significant increase in the n-3 PUFA composition of perfusate (after 5 min) and lung tissue (after 3 h). After as little as 3 h of lung perfusion with an n-3 PUFA-enriched emulsion, significant alterations in the spectrum of eicosanoid generation were found in the isolated lung model. Likewise, after 10 min in a model of septic lung injury, it was found that the vascular leakage provoked by bacterial exotoxin in rabbit lungs was differentially influenced by AA versus EPA, related to the generation of 4- versus 5-series LTs.
Early studies demonstrated that EPA and DHA inhibited endotoxin-stimulated production of IL-6 and IL-8 by cultured human endothelial cells and n-3 PUFAs inhibited endotoxin-induced TNF-α production by cultured monocytes. The differential impact of manipulation of cell membrane phospholipid composition due to n-3 PUFA versus n-6 PUFA administration on cytokine production provoked by pro-inflammatory mediators was demonstrated in A549 cells.
Although DHA is not able to give rise to LTs, it has a pivotal role since it can be incorporated unchanged into membrane lipids or retroconverted into EPA. Evidence suggests that the anti-inflammatory and immunosuppressive effects of n-3 PUFA supply may be attributed mainly, or even exclusively, to DHA supplementation.
In the past two decades, many authors have highlighted that the n-3/n-6 PUFA ratio in nutrition support may influence inflammation since optimal n-3 administration was not only dose related but was also independently affected by the n-3/n-6 PUFA ratio. Different n-3/n-6 PUFA ratios from 1:1 to 1:4 have been proposed, but the question of the most favorable n-3/n-6 PUFA ratio in ALI/ARDS patients is still to be defined.
The impact of the n-3/n-6 PUFA ratio on cytokine release by A549 cells exposed to an endotoxin (i.e., lipopolysaccharide – LPS) challenge was investigated in a recent experimental study (Cotogni et al. 2011). As in many studies, the inflammatory process was elicited by administration of LPS – a component of Gram-negative bacteria cell wall – because LPS is an important mediator in the pathogenesis of ARDS and A549 cells are able to produce the acute phase protein LPS-binding protein. In particular, it was determined the time- and dose-dependent effect of LPS on the TNF-α response in A549 cells (Fig. 4). The FA composition in phospholipids of A549 cell membranes was also analyzed, and the n-3/n-6 PUFA ratio was reported to be 1:5, with AA as the most prevalent PUFA (Table 1).
TNF-α release from LPS-stimulated A549 cells. TNF-α concentrations were measured in cell culture media by ELISA assay at the time points indicated. The results were expressed as picograms of released cytokines per 106 adherent cells (pg/106 cells) and as mean ± SD (n = 9 experiments). LPS lipopolysaccharide, TNF-α tumor necrosis factor-α. *P < 0.05 LPS 100 and 200 versus baseline, at 7 and 24 h; **P < 0.01 LPS 400 versus baseline, at 24 h; ***P < 0.005 LPS 400 versus baseline, at 7 h; #P < 0.01 LPS 400 7 h versus LPS 400 24 h
The administration of different ratios of AA and DHA on the LPS-induced cytokine response from A549 cells showed that the supply of 1:1 and 1:2 DHA/AA ratios reversed the baseline predominance of n-6 over n-3 in the n-3/n-6 PUFA ratio of cell membranes. Particularly, the release of pro-inflammatory cytokines (TNF-α, IL-6, and IL-8) was reduced by 1:1 and 1:2 DHA/AA ratios; on the contrary, it was further increased by 1:4 and 1:7 DHA/AA ratios if compared to LPS challenge alone (Fig. 5). Moreover, the 1:1 and 1:2 DHA/AA ratios were effective in increasing anti-inflammatory IL-10 release and in modifying the balance between pro- and anti-inflammatory cytokines (Fig. 6). This finding confirmed previous studies showing that IL-10 protects against the lethal effects of LPS by significantly reducing the production of pro-inflammatory cytokines in human monocytes and murine peritoneal macrophages. These data suggested that inflammatory cytokine release was dependent on the proportion of n-3 in the n-3/n-6 PUFA ratio of alveolar cell membranes, being reduced with the supply of DHA and increased with a high proportion of AA. According to several other data, shifting the PUFA supply from n-6 to n-3 with a ratio of 1:2 may be a good means to dampen pro-inflammatory responses in ALI.
Lipopolysaccharide-stimulated TNF-α, IL-6, IL-8, and IL-10 release from A549 cells treated with different DHA/AA ratios. Interleukin concentrations were measured in cell culture media by ELISA assay at 7 h. The results were expressed as picograms (pg) of released cytokines per 106 adherent cells and as mean ± SD (n = 4 experiments). AA arachidonic acid, bl baseline, C control, DHA docosahexaenoic acid, IL interleukin, TNF-α tumor necrosis factor-α. *P < 0.01 versus control; **P < 0.001 versus all; #P < 0.001 versus control and 1:2
Ratios between pro-inflammatory (TNF-α, IL-6, and IL-8) and anti-inflammatory (IL-10) cytokines released from lipopolysaccharide-stimulated A549 cells treated with different DHA/AA ratios. Interleukin concentrations were measured in cell culture media by ELISA assay at 7 h. The results were expressed as log10 picograms (pg) of released cytokines per 106 adherent cells and as mean ± SD (n = 4 experiments). AA arachidonic acid, bl baseline, C control, DHA docosahexaenoic acid, IL interleukin, TNF-α tumor necrosis factor-α. *P < 0.001 versus control; **P < 0.001 versus all
Animal Studies
Experimental studies with animal models demonstrated that production of AA-derived eicosanoids and cytokines decreases with a diet containing fish oil in ALI and septic murine models.
Macrophages isolated from mice fed with a diet containing fish oil produced less PGE2 and IL-6 in response to LPS stimulation than those isolated from mice fed with other lipid-enriched diets.
Feeding rats with an n-3 PUFA-enriched diet, as compared with an n-6 PUFA-enriched one, was associated to: (1) a reduced severity of lung microvascular protein permeability and hypotension in a model of endotoxin-induced ALI, (2) a reduced synthesis of pro-inflammatory eicosanoid (LTB4, PGE2, and TXB2) released from stimulated alveolar macrophages, and (3) a decrease in AA and an increase in EPA and DHA in cell membrane phospholipids of alveolar macrophages. After intraperitoneal injection of LPS, peak plasma TNF-α, IL-1β, and IL-6 levels were more reduced in mice fed with a fish oil diet than in those fed with a safflower oil diet (i.e., a diet rich in n-6 PUFAs).
Using either a model of ALI, in which LPS was instilled in the trachea, or a model of intraperitoneal inflammation, in which LPS was injected intraperitoneally, it was demonstrated that a 3-day course of intravenous lipid emulsion infusions was sufficient to modify inflammatory responses. Fish oil-based lipid emulsions reduced alveolar leukocyte transmigration, protein leakage, and cytokine production, as well as cytokine concentrations in the intravascular compartment. Conversely, soybean oil-based lipids lead to a further increase in the inflammatory response.
Clinical Studies
N-3 PUFA Administration via Enteral Route
Clinical data showing benefits associated with the administration of nutrition formulas enriched with n-3 PUFAs in ALI/ARDS patients came initially from three randomized controlled trials (RCTs) using the enteral route. These RCTs showed that EN with a study formula containing EPA, γ-linolenic acid, and antioxidants (vitamins C and E, β-carotene, and taurine), as compared with high-fat formula (control formula), reduced alveolar inflammatory mediators and improved clinical outcomes in patients with ALI, ARDS, or sepsis.
The first RCT showed the ability of an enteral formula with a high n-3/n-6 PUFA ratio (1:1) to reduce pulmonary inflammation and improve clinical outcomes, i.e., better oxygenation, shorter requirement for mechanical ventilation, shorter ICU-LOS, and less incidence of new organ failure; however, no difference in mortality was observed in ARDS patients (Gadek et al. 1999). Similarly, an RCT in ALI/ARDS mechanically ventilated patients showed that an n-3 PUFA-enriched enteral diet may be more beneficial for gas exchange, respiratory dynamics, and length of mechanical ventilation if compared with a control formula, but no difference both in ICU or hospital LOS and mortality was found between the two formulas (Singer et al. 2006). Another RCT was able to demonstrate that the administration of the same enteral diet contributed to guarantee an improved oxygenation and an independence from mechanical ventilation, less incidence of new organ dysfunction, and more ICU-free days and, mainly, was associated with lower mortality rates in ARDS mechanically ventilated patients with severe sepsis and septic shock (Pontes-Arruda et al. 2006). In 2008, a meta-analysis of the pooled outcomes from these three RCTs concluded that the n-3 PUFA-enriched enteral formula could reduce mortality, rate of new organ failure, ICU-LOS, and length of ventilation in ALI/ARDS patients (Pontes-Arruda et al. 2008).
Nevertheless, in 2011, three further RCTs were published that addressed the issue of the potential positive effects of an enteral n-3 PUFA-enriched nutrition in ALI/ARDS patients showing mixing results. The first RCT analyzed the effect of an enteral n-3 PUFA-enriched diet in septic patients with ALI or ARDS showing that the administration of the study formula, compared to a control formula with less lipids than in the previous three studies, was associated to a shorter ICU-LOS but not to an improvement in gas exchange or in a lower incidence of novel organ failures (Grau-Carmona et al. 2011). A phase 2 placebo-controlled RCT in ALI patients investigating an enteral administration of fish oil for up to 14 days did not demonstrate a decrease in cytokine or other inflammatory marker concentrations both in BAL fluid from baseline to day 4 or 8 and in plasma, as well as a decrease in ventilator-free days, ICU-free days, organ failure score, or 60-day mortality (Stapleton et al. 2011). The last RCT tested the effects of enteral feeding with n-3 PUFAs, γ-linolenic acid, and antioxidants on clinical outcomes in ALI patients in a phase 3 trial (OMEGA trial) using a twice-daily bolus administration (Rice et al. 2011). Contrary to all other RCTs, the authors reported that enteral administration of this formula was associated to significantly fewer ventilator-free days, ICU-free days, and non-pulmonary organ failure-free days, as well as more days with diarrhea. The trial was stopped early for futility at the first interim analysis. The interpretation of the OMEGA trial presents some difficulties (Lev and Singer 2013). Firstly, it was used a different approach of twice-daily bolus administration of enteral formulas instead of continuous enteral infusion. Secondly, the enteral diets administered to the study group (i.e., megadoses of n-3 PUFAs and lower protein content) and to the control group (i.e., higher protein and carbohydrate content) are notably different from those used in the first three RCTs.
N-3 PUFA Administration via Parenteral Route
Enteral administration of n-3 PUFAs might require more time before effectively influencing the cellular FA composition and the lipid-mediated inflammatory response. Moreover, the daily volume of enteral formula prescribed is calculated such as to meet patients’ requirement of calories rather than a scheduled “pharmacological” dosage of n-3 PUFAs. It is well known that ICU patients exhibit different degrees of tolerance to EN, as it is well documented that the more patients are critically ill, the less they tolerate EN. Thus, an unpredictable number of critical patients might not receive the “pharmacological” dosage of n-3 PUFAs.
An alternative approach to provide n-3 PUFAs to ICU patients unable to meet their nutrition requirements by oral or enteral route is to administer a parenteral formula prepared from fish oil. Differently to oral/enteral feeding, parenteral administration of PUFAs can induce more rapid changes in lipid-derived responses. Indeed, in ALI, there is the need of promptly active treatments to early modulate the net inflammatory response in the alveolar spaces.
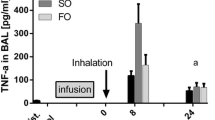
In septic patients, intravenous infusion of n-3 PUFAs modified lipid mediator synthesis and reduced endotoxin-stimulated monocyte pro-inflammatory cytokine production, while cytokine production was markedly amplified by n-6 PUFA administration (Mayer et al. 2003). Conversely, PN administered for 7 days with a 1:1 mixture of medium-chain triglycerides and long-chain triglycerides, with the n-3/n-6 PUFA ratio of 1:2 compared with a same mixture supplemented with fish oil with the n-3/n-6 PUFA ratio of 1:7, did not affect inflammatory marker production or measures of clinical outcomes in an RCT in unselected critically ill medical patients (Friesecke et al. 2008).
In the last 5 years, three RCTs have investigated the effects of parenteral administration of n-3 PUFA-enriched lipid emulsions in ALI/ARDS patients. The first RCT investigated the effects of an n-3 PUFA-enriched lipid emulsion in ARDS patients with EN intolerance showing no significant changes in hemodynamic and gas exchange parameters (Sabater et al. 2008). Previously, the same authors had showed that parenteral administration of an n-3-enriched lipid emulsion was accompanied by the synthesis of a smaller amount of pro-inflammatory lipid mediators compared to long-chain triglyceride 100 % emulsion in ARDS patients. In a second trial, patients with systemic inflammatory response syndrome or sepsis were randomized into two groups to receive an intravenous lipid emulsion enriched or not in fish oil (Barbosa et al. 2010). The authors reported that the PaO2:FiO2 ratio at day 6 was significantly higher and plasma IL-6 concentration significantly decreased in the group treated with fish oil; however, fish oil administration did not improve the length of ventilation or ICU-LOS and mortality. The last RCT investigated the effects of an intravenous administration of n-3 PUFAs with the n-3/n-6 PUFA ratio of 1:2 as supplementation of EN for 14 days in ARDS patients finding that fish oil administration alone did not improve ventilation, ICU-LOS, or survival (Gupta et al. 2011).
Applications to Critical or Intensive Care
ARDS is the most severe life-threatening manifestation of ALI. Both remain a significant health burden for intensivists and critical care physicians because of their considerable morbidity and mortality. In 2005, in the USA, the crude incidence of ALI was 78.9 per 100,000 person-years; each year, there are 190,600 cases of ALI associated with 74,500 deaths and 3.6 million hospital days. In 2004, in 78 ICUs of ten European countries, ALI occurred in 463 (7 %) out of 6,522 admissions, and in the 16 % of the whole mechanically ventilated patients, 65 % cases occurred on ICU admission.
Pathologically, ALI and ARDS are inflammatory diseases; clinically, their severity depends on the grade of inflammatory response. Thus, numerous studies have been carried out investigating new strategies to reduce the grade of this inflammatory response, mainly by reducing the release of pro-inflammatory mediators. However, pharmacological therapies such as corticosteroids, ketoconazole, antioxidants, nitric oxide, prostacyclins, exogenous surfactants, and β2 agonists failed to show positive results in human studies.
Supplementation with n-3 PUFAs was demonstrated to exert beneficial effects in a number of inflammatory diseases. The rationale for their use in ALI/ARDS patients is consistent and based on several issues. Firstly, a large amount of experimental studies in lung models supports the anti-inflammatory properties of n-3 PUFAs. Secondly, patients at risk for ARDS and with established ARDS have plasma EPA concentrations about 25 % and 7 %, respectively, as compared with normal, hypothesizing a potential benefit for n-3 PUFA administration in those patients. Finally, in three level I studies involving patients with ALI, ARDS, and sepsis, the use of an enteral diet enriched with n-3 PUFAs (in the form of EPA), γ-linolenic acid, and antioxidants was shown to significantly reduce ICU-LOS, mechanical ventilation length, organ failure incidence, and mortality if compared to the use of a standard enteral formula.
Based on these data, the European Society for Clinical Nutrition and Metabolism, American Society of Parenteral and Enteral Nutrition, Society of Critical Care Medicine, and Canadian guidelines recommended, with an A or B grade of recommendation, in their guidelines published between 2003 and 2009, the use of n-3 PUFAs in ALI/ARDS patients. However, nowadays, the clinical application of these recommendations has been lagging behind in critical or intensive care, which is due, at least in part, to inconclusive or contradicting results in several recent clinical trials using n-3 PUFAs.
Applications to Other Conditions
Over the last 25 years, the data coming out from a great number of studies have left us an improved knowledge of how n-3 PUFAs and their derivate mediators modulate inflammation/resolution and immunity. Evidence that supports both the anti-inflammatory and antitumor effects of n-3 PUFAs has been gathered; moreover, the molecular pathways underlying these effects have been clarified both in vitro and in vivo. In particular, there is now a better understanding of various mechanisms generating the production of inflammatory cytokines in several inflammatory, metabolic, and neurodegenerative diseases, as well as in inflammation-related cancers.
The capacity of n-3 PUFAs to determine a shift from a strongly pro-inflammatory environment to one of reduced inflammation supports the hypothesis that these FAs might be useful as a component of the therapy in various inflammatory diseases. Thus, EPA and/or DHA have been evaluated in some inflammatory conditions.
For the potential or in-use applications of n-3 PUFAs to clinical conditions different from ALI, see a recent review (Calder 2013b). Briefly, fish oil or its derived EPA and DHA have demonstrated the efficacy in animal models of rheumatoid arthritis, inflammatory bowel diseases, and asthma. There have been a number of clinical trials evaluating the effects of administration of n-3 PUFAs in those diseases with contradicting results. For example, there is some pretty good evidence of the efficacy of n-3 PUFAs in rheumatoid arthritis. Conversely, despite positive results in some studies, there is only weak evidence that n-3 PUFAs have clinical benefits in inflammatory bowel diseases. Finally, clinical studies on fish oil in adult patients with asthma do not show benefit.
Guidelines and Protocols
The “European Society for Clinical Nutrition and Metabolism Guidelines on Enteral Nutrition: Intensive Care” (Kreymann et al. 2006) stated that “Patients with ARDS should receive EN enriched with n-3 fatty acids and antioxidants” as a B grade recommendation. However, this recommendation was not evaluated in the “ESPEN Guidelines on Parenteral Nutrition: Intensive Care” (Singer et al. 2009), although the authors stated that “Addition of EPA and DHA to lipid emulsions has demonstrable effects on cell membranes and inflammatory processes; fish oil-enriched lipid emulsions probably decrease length of stay in critically ill patients” both as B grade recommendations.
The American Society for Parenteral and Enteral Nutrition “Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient” (McClave et al. 2009), also being co-published by the Society of Critical Care Medicine, stated that “Patients with ARDS and severe ALI should be placed on an enteral formulation characterized by an anti-inflammatory lipid profile (i.e., n-3 fish oil, borage oil) and antioxidants” (A grade recommendation). This recommendation was based on the three positive trials published at that time.
The Canadian guidelines (updated June 2013) stated that “Based on 2 level 1 studies and 5 level 2 studies, the use of an enteral formula with fish oils, borage oils and antioxidants in patients with ALI and ARDS should be considered. When PN with intravenous lipids is indicated, lipids that reduce the load of n-6 FA/soybean oil emulsions should be considered. However, there are insufficient data to make a recommendation on the type of lipids to be used that reduce the n-6 FA/soybean oil load in critically ill patients receiving PN.”
Conclusions
ALI/ARDS patients are at high risk of malnutrition; therefore, they need nutrition support, including lipids. Soybean oil-based lipid emulsions, rich in n-6 PUFAs and commonly used for these patients, may aggravate the inflammatory response. In contrast, fish oil-based lipid emulsions, rich in n-3 PUFAs, may exert an anti-inflammatory effect. These data suggest that shifting the PUFA supply from n-6 to n-3 could be an important element affecting the alveolar cytokine and eicosanoid release and consequently ameliorates the clinical outcome in ALI/ARDS patients.
In summary, there are good experimental evidence and convincing rationale according to the n-3 PUFAs used in ALI/ARDS patients. However, recent RCTs using n-3 PUFAs in these patients did not confirm these positive effects. Further studies are necessary to offer more precise indications, timing, and modalities for n-3 PUFA administration.
Summary Points
-
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are inflammatory diseases whose clinical severity depends on the grade of inflammation.
-
Cytokines are key elements in the pathogenesis of ALI/ARDS.
-
Soybean oil-based lipid emulsions, rich in omega (n)-6 polyunsaturated fatty acids (PUFAs), may aggravate the inflammatory response. In contrast, fish oil-based lipid emulsions, rich in n-3 PUFAs, may exert an anti-inflammatory effect.
-
Experimental and clinical data suggested that shifting the PUFA supply from n-6 to n-3 decreases the alveolar cytokine and eicosanoid release and consequently ameliorates the clinical outcome in ALI/ARDS patients.
-
Based on these data, several scientific societies recommended in their guidelines the use of n-3 PUFAs in ALI/ARDS patients.
Abbreviations
- AA:
-
Arachidonic acid
- ALI:
-
Acute lung injury
- ARDS:
-
Acute respiratory distress syndrome
- BAL:
-
Bronchoalveolar lavage
- DHA:
-
Docosahexaenoic acid
- EN:
-
Enteral nutrition
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FiO2:
-
Fractional inspired oxygen
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- LOS:
-
Length of stay
- LPS:
-
Lipopolysaccharide
- LT:
-
Leukotriene
- n:
-
Omega
- PaO2:
-
Arterial oxygen partial pressure
- PG:
-
Prostaglandin
- PN:
-
Parenteral nutrition
- PUFA:
-
Polyunsaturated fatty acid
- RCT:
-
Randomized controlled trial
- TNF:
-
Tumor necrosis factor
- TX:
-
Thromboxane
References
Barbosa VM, Miles EA, Calhau C, et al. Effects of fish oil containing lipid emulsion on plasma phospholipid fatty acids, inflammatory markers, and clinical outcomes in septic patients: a randomized, controlled clinical trial. Crit Care. 2010;14:R5.
Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83 Suppl 6:1505S–19S.
Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. Proc Nutr Soc. 2013a;72:326–36.
Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013b;75:645–62.
Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. http://www.criticalcarenutrition.com. Accessed 1 Nov 2013.
Cotogni P, Muzio G, Trombetta A, et al. Impact of the ω-3 to ω-6 polyunsaturated fatty acid ratio on cytokine release in human alveolar cells. J Parenter Enteral Nutr. 2011;35:114–21.
Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38:1573–82.
Friesecke S, Lotze C, Köhler J, et al. Fish oil supplementation in the parenteral nutrition of critically ill medical patients: a randomised controlled trial. Intensive Care Med. 2008;34:1411–20.
Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med. 1999;27:1409–20.
Grau-Carmona T, Morán-García V, García-de-Lorenzo A, et al. Effect of an enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid and anti-oxidants on the outcome of mechanically ventilated, critically ill, septic patients. Clin Nutr. 2011;30:578–84.
Gupta A, Govil D, Bhatnagar S, et al. Efficacy and safety of parenteral omega-3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15:108–13.
Kreymann KG, Berger MM, Deutz NDP, et al. ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr. 2006;25:210–23.
Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ. 2007;335:389–94.
Lev S, Singer P. n-3 fatty acids and γ-linolenic acid supplementation in the nutritional support of ventilated patients with acute lung injury or acute respiratory distress syndrome. World Rev Nutr Diet. 2013;105:136–43.
Mayer K, Seeger W. Fish oil in critical illness. Curr Opin Clin Nutr Metab Care. 2008;11:121–7.
Mayer K, Gokorsch S, Fegbeutel C, et al. Parenteral nutrition with fish oil modulates cytokine response in patients with sepsis. Am J Respir Crit Care Med. 2003;167:1321–8.
McClave SA, Martindale RG, Vanek VW, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J Parenter Enteral Nutr. 2009;33:277–316.
Mizock BA. Nutritional support in acute lung injury and acute respiratory distress syndrome. Nutr Clin Pract. 2001;16:319–28.
Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–903.
Pontes-Arruda A, Aragão AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–33.
Pontes-Arruda A, Demichele S, Seth A, et al. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. J Parenter Enteral Nutr. 2008;32:596–605.
Pugin J, Verghese G, Widmer MC, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999;27:304–12.
Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, γ-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–81.
Sabater J, Masclans JP, Sacanell J, et al. Effects on hemodynamics and gas exchange of omega-3 fatty acid-enriched lipid emulsion in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Lipids Health Dis. 2008;7:39.
Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–8.
Singer P, Berger MM, Van den Berghe G, et al. ESPEN guidelines on parenteral nutrition: intensive care. Clin Nutr. 2009;28:387–400.
Stapleton RD, Martin TR, Weiss NS, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this entry
Cite this entry
Cotogni, P., Trombetta, A., Muzio, G., Brizzi, M.F., Canuto, R.A. (2015). Polyunsaturated Fatty Acids and Cytokines: Their Relationship in Acute Lung Injury. In: Rajendram, R., Preedy, V.R., Patel, V.B. (eds) Diet and Nutrition in Critical Care. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7836-2_112
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7836-2_112
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7837-9
Online ISBN: 978-1-4614-7836-2
eBook Packages: MedicineReference Module Medicine