Abstract
Eicosanoids are signaling molecules made by oxidation of 20 carbon fatty acids. They exert complex control over many bodily systems, mainly in inflammation or immunity, and as messengers in the central nervous system. Eicosanoids control both proinflammatory and anti-inflammatory effectors that are particularly relevant for inflammation. The networks of controls that depend upon eicosanoids are among the most complex in the human body. Lipid mediators are synthesized via cyclooxygenase, lipoxygenase, and cytochrome P450 pathways with fatty acids such as arachidonic acid used as substrate. These mediators include prostaglandins, thromboxanes, leukotrienes, lipoxins, and hydroxyl and epoxy fatty acids – all grouped as eicosanoids – and platelet-activating factor, acting as intercellular signaling molecules. They have an impact on the secretion of immunoregulatory cytokines, on secondary mediators like reactive oxygen species or proteases, and on autocrine eicosanoid regulation loops. Numerous experimental studies suggest that the generation of arachidonate metabolites can play a role in the development of ALI/RDS. In normal conditions, arachidonate is bound to the phospholipids of cell membranes. Following injury and in response to various mediators, free arachidonic acids are released from membrane phospholipids by the action of phospholipases. This arachidonic acid can serve as a substrate for the production of prostaglandins and thromboxanes through a cyclooxygenase enzyme and as a substrate for the production of several hydroxyl fatty acids and leukotrienes through the action of lipoxygenase enzymes. The lung is an important organ in the arachidonate cascade since it possesses the enzymatic capacity to synthesize all the arachidonate derivatives and also responsible in large part for selective catabolism of circulating eicosanoids. The use of diets high in w-3 fatty acids is a means to decrease levels of arachidonic acid in cells, thereby reducing the production of proinflammatory eicosanoids. Clinical investigation with enteral diets enriched in EPA/GLA and antioxidants shows beneficial effect in patients with ALI/ARDS.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Arachidonic Acid
- Acute Lung Injury
- Systemic Inflammatory Response Syndrome
- Enteral Nutrition
- Multiple Organ Dysfunction Syndrome
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The body’s response to illness and injury is extremely complex. The following description is a simplistic description of the inflammatory response to injury and illness (Brun-Buisson 2000).
In response to an injury or insult (trauma, burns, surgery, pancreatitis, infection, etc.), the body attempts to restore homeostasis, and a balance between proinflammatory and anti-inflammatory mediators (various eicosanoids, prostaglandins, and cytokines) occurs. These anti- and proinflammatory mediators play an important role in initiating the healing process by limiting new damage and ameliorating damage that has already occurred. They destroy damaged tissue, promote new tissue growth, and overcome pathogenic organisms, neoplastic cells, and foreign antigens. The localized response can result in a spillover of anti-inflammatory and proinflammatory mediators into the systemic circulation (Table 1).
Ideally, the pro- and anti-inflammatory mediators are in balance. However, when the anti-inflammatory mediators dominate, the immune system is suppressed, creating an increased risk of infection. Conversely, when proinflammatory mediators dominate, a state of uncontrolled inflammation develops, as seen in the systemic inflammatory response syndrome (SIRS), sepsis, or septic shock. In SIRS, the proinflammatory response is overreactive, and the inflammatory mediators are elevated in the systemic circulation, placing all organs of the body at risk. The risk of organ failure, multiple organ dysfunction syndrome (MODS), and death increases dramatically.
Eicosanoids: An Overview
The term eicosanoid is used to embrace biologically active lipid mediators (C20 fatty acids and their metabolites), including prostaglandins, thromboxanes, leukotrienes, and other oxygenated derivatives, which are produced primarily by three classes of enzymes, cyclooxygenases, lipoxygenases, and cytochrome P450 monooxygenases. The key precursor fatty acids are 8c,11c,14c-eicosatrienoic (dihomo-gamma-linolenic or 20:3(w-6)), 5c,8c,11c,14c-eicosatetraenoic (arachidonic or 20:4(w-6)), and 5c,8c,11c,14c,17c-eicosapentaenoic (20:5(w-3) or EPA) acids. However, it is now impossible to discuss these compounds and their biological activities properly without also considering the docosanoids (resolvins and protectins) derived from 4c,7c,10c,13c,16c,19c-docosahexaenoic acid (22:6(w-3) or DHA) and related products formed by nonenzymatic means (isoprostanes). Similarly plant products such as the jasmonates and other oxylipins derived from 9c,12c,15c-octadecatrienoic (alpha-linolenic or 18:3(w-3)) acid have analogous structures and functions. It is noteworthy that the precursor fatty acids for all of these belong to both the omega-6 and omega-3 families (Heller et al. 1998).
Arachidonic acid has been by far the most studied, and it is special in many ways (Fig. 1). It is an essential fatty acid in that it cannot be synthesized de novo in animals, and linoleic acid from the diet is required as the primary precursor. As a major component of phospholipids, and especially of phosphatidylinositol, it is important for the integrity of cellular membranes. The four cis-double bonds mean that the molecule is highly flexible, and this helps to confer the correct degree of fluidity in membranes. Diacylglycerols enriched in arachidonic acid and derived from phosphatidylinositol are important cellular messengers. Anandamide or N-arachidonoylethanolamine is an endogenous cannabinoid or endocannabinoid, which produces neurobehavioral effects similar to those induced by cannabis and may have important signaling roles in the central nervous system, especially in the perception of pain and in the control of appetite. 2-Arachidonoylglycerol has similar properties. There are suggestions that arachidonic acid per se may have some biological importance in animal tissues; for example, the cellular level of unesterified arachidonic acid may be a mechanism by which apoptosis is regulated.
The oxygenated metabolites derived from arachidonic and related fatty acids are rapidly biosynthesized within seconds to minutes of acute challenge by leukocyte from membrane-derived arachidonic acid, using either cyclooxygenase or lipoxygenase, and are produced through a series of complex interrelated biosynthetic pathways sometimes termed the “arachidonate or eicosanoid cascade.” They are so numerous and have such a range of biological activities that they must provide a substantial component of the reason for the essentiality of the latter to the survival and well-being of animals. The prostanoids (prostaglandins, thromboxanes, and prostacyclins) have distinctive ring structures in the center of the molecule. The hydroxyeicosatetraenes are apparently simpler in structure, but are precursors for families of more complex molecules, such as the leukotrienes and lipoxins.
The eicosanoids are considered local hormones. They have specific effects on target cells close to their site of formation. They are rapidly degraded, so they are not transported to distal sites within the body. But in addition to participating in intercellular signaling, there is evidence for involvement of eicosanoids in intracellular signal cascades. Examples of eicosanoids are prostaglandins, prostacyclins, thromboxanes, leukotrienes, and epoxyeicosatrienoic acids. For each, there are two or three separate series, derived either from a w-3 or w-6 EFA. These series of different activities largely explain the health effects of w-3 and w-6 fats. They have various roles in inflammation, fever, regulation of blood pressure, blood clotting, immune system modulation, control of reproductive processes and tissue growth, and regulation of the sleep/wake cycle (Calder 2003).
The w-6 eicosanoids are generally proinflammatory; w-3 are much less so. Cyclooxygenase metabolizes arachidonic acid to 2-series and eicosapentaenoic acid to 3-series prostaglandins. After oxidation by 5-lipoxygenase, 4-series leukotrienes and 5-series leukotrienes are derived from arachidonic acid or eicosapentaenoic acid, respectively. Lipoxins are double-oxygenated mediators from arachidonic acid, whereas the new class of resolvins stems from eicosapentaenoic acid (Fig. 2; Mayer et al. 2006). The amounts and balance of these fats in a person’s diet will affect the body’s eicosanoid-controlled functions, with effects on cardiovascular disease, triglycerides, blood pressure, and arthritis. But with the discovery of lipid mediators that possess both anti-inflammatory and pro-resolution activities (dual action mediators) (Bannenberg et al. 2005), these new families of resolvins and protectins and class of eicosanoids, e.g., lipoxins, constitute a novel genus of pro-resolution mediators (Serhan 2008). Anti-inflammatory drugs such as aspirin and other NSAIDs act by downregulating eicosanoid synthesis.
Applications to Critical Medicine/Intensive Care
ALI and RDS
SIRS is a syndrome caused by increasing amounts of proinflammatory mediators. In this environment, instead of producing a positive healing response, the proinflammatory mediators become destructive and the risk for acute lung injury (ALI), respiratory distress syndrome (RDS), MODS, and death increases dramatically. The lungs are often the first organ affected. Due to the high number of inflammatory cells, they are particularly susceptible to the effects of an overreactive inflammatory response, which results in ALI which may later lead to RDS.
ALI is characterized by pulmonary infiltrates, gas exchange disturbances, poor lung compliance, and hypoxemia. Research suggests that SIRS, triggered by a predisposing condition, accompanies most forms of ALI. Sepsis (SIRS with infection) and pneumonia are the primary predisposing conditions for the development of ALI. As a result of direct or indirect injury to the lung, pulmonary edema and hypoxemia develop, leading to a condition known as respiratory failure and, ultimately, to RDS. ALI is a milder, earlier, and more reversible stage of lung injury, and early interventions after the initial injury to the lung may diminish the likelihood of developing the more severe RDS.
The inflammatory response is regulated by a complex system of endogenous mediators of inflammation capable of having single and multiple functions. The mechanisms that create the inflammatory edema of the lung epithelium are extremely complex. Although a number of triggering factors have been proposed, many of the inflammatory events appear to follow the activation of the complement system. This inflammatory response cascade is illustrated in Fig. 3.
The initial stage of the inflammatory response is characterized by the release of vasoactive mediators from tissue mast cells, platelets, and plasma components, resulting in pulmonary vasodilation. Because of increased vascular permeability, water, salts, and some small proteins from the plasma pass into the damaged area, causing alveolar edema.
This initial stage is followed by the activation of the coagulation system and the complement system and by the generation of plasma- and cell-derived chemotactic factors for inflammatory cells (mainly neutrophils) (Anas et al. 2010). Leukocyte chemotaxis causes white blood cells to gather at the site of injury. Activation of the complement system, in turn, promotes the recruitment and activation of cells such as neutrophils within the lung vasculature. The adhesion of these cells to lung endothelium causes them to aggregate along vessel walls in a process known as leukocyte margination. The neutrophils then migrate through the vessel membrane and pass into the area of tissue damage in a process known as emigration (Lorente and Esteban 2012). Later in the process, other cells such as macrophages also migrate to the damaged area (Serhan 2005).
Eicosanoids as Mediators and Prognostic Markers in ALI/RDS
Experimental studies suggest that the generation of arachidonate metabolites can play a role in the development of ALI/RDS. In normal conditions, arachidonate is bound to the phospholipids of cell membranes. Following injury and in response to various mediators, free arachidonic acids are released from membrane phospholipids by the action of phospholipases. This arachidonic acid can serve as a substrate for the production of prostaglandins and thromboxanes through a cyclooxygenase enzyme and as a substrate for the production of several hydroxyl fatty acids and leukotrienes through the action of lipoxygenase enzymes. The lung is an important organ in the arachidonate cascade since it possesses the enzymatic capacity to synthesize all the arachidonate derivatives and also responsible in large part for selective catabolism of circulating eicosanoids (Leeman and Boeynaems 1984).
Rivkind et al. (1989) studied multiply injured blunt trauma patients at high risk for development of RDS (multisystem trauma including more than one organ or extremity, Injury Severity Score of 26 or more, hypotension and need for 1,500 mL or more blood within the first hour after admission, and PaO2 less than or equal to 70 torr). Mixed venous blood samples were obtained for eicosanoids PGE2, PGF2 alpha, thromboxane B2, PGI2 (6-ketoPGF1 alpha), and leukotriene B4 (LTB4). Platelet and neutrophil counts were also done and plasma elastase was measured. These data were correlated with physiologic measurements of the respiratory index (RI), percent pulmonary shunt (QS/QT), and respiratory compliance measures. Seven patients developed a fulminant posttraumatic RDS within 96 h after injury. Twelve patients without RDS developed sepsis (TS) 4 or more days after injury, and 11 had uncomplicated post-injury courses (TR). Compared to both TR and TS, ARDS had a significant (p < .01) rise in neutrophil superoxide production beginning on day 2 through day 4 after injury. This was preceded by rises in PGE2 and LTB4, which were significantly correlated with subsequent falls in PLAT and WBC and rises in TXB2, PGF1, and superoxide production and followed by increases in RI and QS/QT and a fall in compliance. The significant difference in the pattern and sequence of events in RDS compared to TR and TS patients suggests that in RDS the earliest event may be related to peripheral release of PGE2 and LTB4 due to platelet activation and lung sequestration with release of PGF2 alpha and by aggregation and leukocyte adherence with release of elastase. However, fulminant RDS mortality appears to be related to the subsequent amplification of the LTB4 leukocyte activation with superoxide production that does not achieve significance before the second day after injury and rises to a maximum by day 4 after injury. These data suggest that posttraumatic ARDS follows a different evolutionary pattern than that reported in animal models and is also different from that seen in human TS or TR patients.
Other investigators refer that plasma level of eicosanoids in the RDS patients was higher than reference subjects (p < .05), and no differences were observed between systemic arterial and pulmonary arterial values. The authors’ conclusion was that from all the eicosanoids studied (TXB2, PGF1 alpha, and LTB4), only LTB4 (in both systemic arterial and pulmonary blood) was correlated with the Lung Injury Score (r = 0.49, p < .05, and r = 0.45, p < .05, respectively). Patients who did not survive presented a lower LTB4 systemic-pulmonary arterial gradient than survivors (Masclan et al. 1999).
More recently Amat et al. (2000) described that in both patients at risk of RDS and with RDS, leukotrienes (LTB4, LTC4, LTD4) are elevated during the early phases of RDS, whereas IL-8 increases throughout the study period. The conclusion was that the evaluation of LTB4 and IL-8 may be useful prognostic indexes in patients with early RDS after admission to the intensive care unit.
Dietary Lipids, Eicosanoids, and RDS
Many critically ill patients are admitted with conditions that are associated with an inflammatory response, leading to SIRS. Because the mortality rate increases along the continuum from SIRS to MODS, early and effective intervention is imperative. However, effectively correcting the imbalance of proinflammatory mediators and attenuating the inflammatory response that leads to SIRS have been elusive.
Since inflammation, through the release of arachidonic acid and its proinflammatory metabolites, is considered the key feature of RDS, the use of lipids, as a strategy to modulate the inflammatory response, is gaining acceptance as an important improvement in the daily management of patients with ALI and RDS. The literature suggests that providing specific dietary nutrients may downregulate the overactive inflammatory response and improve cardiopulmonary function in patients with pneumonia or SIRS, ALI, and RDS. These nutrients include gamma-linolenic acid (GLA) , found in oil from seeds of the borage plant, and eicosapentaenoic acid (EPA), found in fish oil, as well as specific dietary antioxidants. These components have been shown to have important metabolic effects by altering the phospholipid content of alveolar macrophages, leading to the production of less proinflammatory mediators and improved pulmonary function while providing general nutrition support (Pontes-Arruda and DeMichele 2009).
Dietary lipids have several important functions, including as source of energy (calories), carrier of fat-soluble vitamins, source of essential fatty acids, and precursors of eicosanoids (prostanoids and leukotrienes), and the relationship between essential fatty acids in nutrition, dietary supplementation, and the biosynthesis of resolvins and protectins is an area of active interest (El Kebir et al. 2012).
The polyunsaturated fatty acids (PUFAs) can be divided into three major families – omega(w)-3, omega(w)-6, and omega(w)-9 fatty acids – based on the location of double bonds. The essential fatty acids consist of the w-6 fatty acids formed from linoleic acid and the w-3 fatty acids formed from alpha-linolenic acid. The intake of PUFAs is especially important for patients with ARDS because fats of the n-6 and n-3 group affect specific and nonspecific immune and inflammatory functions via products from arachidonic acid metabolism. The two families of dietary PUFAs (w-6 and w-3) are desaturated and elongated by the same enzymes. The rate-limiting factor in the conversion of linoleic acid to arachidonic acid is the enzyme delta-6-desaturase. This enzyme is sensitive to feedback inhibition and to competitive inhibition by other PUFAs such as the n-3 fatty acids. Delta-6-desaturase, with the aid of delta-5-desaturase, regulates the metabolism of linoleic acid to GLA and arachidonic acid and of alpha-linolenic acid to EPA, with each having metabolites that cause different immune responses. Therefore, delta-6-desaturase is of major importance in the regulation of immune and inflammatory function.
Arachidonic acid is the major precursor of the eicosanoids (prostanoids and leukotrienes). Eicosanoids are fatty acid metabolites that are synthesized via either a cyclooxygenase or 5-lipoxygenase enzyme system. Arachidonic acid metabolism via the cyclooxygenase pathway leads to the production of the proinflammatory mediators such as thromboxane A2, prostaglandin E2, and prostacyclin I2. Arachidonic acid metabolism via the 5-lipoxygenase pathway mediates the release of leukotrienes (LT). Leukotrienes, specifically LTB4, are potent neutrophil chemotactic factors and also potentiate the respiratory burst and increase cell adhesion processes.
The use of diets high in n-3 fatty acids is a means to decrease levels of arachidonic acid in cells, thereby reducing the production of proinflammatory eicosanoids. For example, the incorporation of n-3 fatty acids (EPA) into cell phospholipids could lead to the production of metabolites with less inflammatory activity than those formed from arachidonic acid. The n-3 fatty acids could compete directly with arachidonic acid as substrates or for enzymes (e.g., delta-6-desaturase) that catalyze the lipoxygenase and cyclooxygenase pathways. Another way to manipulate the inflammatory response by diet is by supplementation with GLA. A common misconception is that the n-6 fatty acid linoleic acid is rapidly converted to intermediates GLA, dihomo-gamma-linolenic acid (DGLA), and arachidonic acid. In fact, desaturation by delta-6-desaturase occurs with difficulty and seems to be rate limited by precursor or product inhibition. Furthermore, metabolic stress, major surgery, and medical disorders also inhibit conversion. GLA is elongated to DGLA, which competes with arachidonic acid for cyclooxygenase binding sites and serves as the precursor of prostaglandins such as PGE1, which have anti-inflammatory properties. DGLA also can be converted to a 15-hydroperoxy derivative that inhibits the conversion of arachidonic acid to the undesirable 4-series of leukotrienes. The supplementation with both GLA and DGLA suppresses acute and chronic inflammation. Thus, dietary supplementation with GLA modulates inflammatory status like the n-3 fatty acids because of its ability to reduce the synthesis of the proinflammatory mediators derived from arachidonic acid metabolism.
Patients with ALI and ARDS have lower circulating antioxidant levels than healthy individuals. During ALI/RDS, the oxidative stress produced from mechanical ventilation and free radical production formed by infiltrating neutrophils can overwhelm circulating antioxidant levels and allow oxygen free radicals to directly damage cellular and tissue structures. Thus, in cases of increased oxidative stress, patients must be supplemented with antioxidants at levels above those recommended to meet the needs of healthy adults because these levels do not reflect nutrient needs in disease.
Manipulating Eicosanoid Synthesis in Patients with ALI/RDS
As has been indicated, ALI/RDS is an inflammatory process with alveolar and vascular endothelial injury in the lung. Neutrophil activation plays a crucial role in the pathogenesis. When activated, it releases harmful mediators including proteases, cytokines, and others that lead to progressive lung damage. An intense inflammatory response within the alveolar spaces, with the accumulation of proinflammatory and anti-inflammatory cytokines, characterizes the ALI/ARDS. The fact that inflammatory mediator production can be modified by lipids has been the basis for investigation about dietary manipulation in ALI/RDS.
Animal models have been investigated to analyze the relation between fat intake and inflammation in lung injury. In an experimental model of ARDS, rats treated with a diet rich in EPA and GLA can modulate the production of inflammatory mediators and improve lung function (Palombo et al. 1996). Another rat model of lung injury (Mancuso et al. 1997) investigated the production of several inflammatory mediators after a diet enriched in fish oil, fish, and borage oil or control diet. Animals that receive fish oil diets have a lower pulmonary synthesis of proinflammatory eicosanoids and cytokines after endotoxin injection as demonstrated in lower levels of leukotriene B4, leukotriene C4/D4, and thromboxane B2 in bronchoalveolar lavage fluid. Lung myeloperoxidase activity, a marker for neutrophil accumulation, was significantly lower with fish oil diets.
Recent investigations are directed to analyze the importance of the resolvin pathway in healing of lung injury and the possible effect of dietary lipids in this mechanism. Resolvin E1 (RvE1), an endogenous lipid mediator derived from EPA, has been shown to promote lung healing via neutrophil apoptosis and their removal by macrophages in “in vitro” or “in vivo” animal models (El Kebir et al. 2012). Resolvin-mediated resolution of inflammation seems to be an important mechanism in lung tissue healing after ALI/ARDS. The clinical relevance of dietary manipulation of RvE1 by increasing EPA in the diet is, at present, not known.
The release of pro- and anti-inflammatory cytokines from human alveolar cells after endotoxin challenge in several w3/w6 conditions has been investigated (Cotogni et al. 2011). These authors demonstrate that omega-3 PUFAs are associated with a decrease in the release of proinflammatory mediators in the cellular culture model.
In the clinical scenario, several authors have investigated the effect of w3–w6 administration in patients with ALI/ARDS. Investigations have been done mainly by increasing EPA and GLA in the enteral formula administered to these patients. In general, these investigations indicate that an increase in the EPA/GLA administration with enteral nutrition results in better outcomes; a decrease in infectious complications and a lower mortality have been described in these studies. As a result of these investigations, dietary manipulation to increase EPA/GLA administration to critically ill patients with ALI/ARDS has been considered beneficial in these patients.
Gadek et al. (1999) recruited 146 patients with ARDS in a randomized, multicenter, prospective, and double-blind study. Compared with control, patients treated with a low-carbohydrate/high-lipid diet enriched in EPA/GLA had a significant improvement in oxygenation parameters and also in mechanical ventilation needs during the study (FiO2, positive end-expiratory pressure (PEEP), minute ventilation, and days on mechanical ventilation). The number of patients who developed a new organ failure during the study was also lesser in the study group. It is interesting to note that, to confirm the hypothesis that EPA/GLA can modulate inflammation, the authors investigate total cell count and neutrophils in bronchoalveolar lavage (BAL) fluid; the number of cells was significantly decreased in patients that received the study diet. This group of investigators published later more information (Pacht et al. 2003) about inflammatory mediators in BAL fluid in a group of 43 patients included in their previous study. Mediators like IL-8 and ceruloplasmin and markers of alveolar protein permeability were reduced in patients treated with the EPA/GLA diet.
Another similar one-center study also compares an EPA-GLA-enriched diet with an isocaloric and isonitrogenous control diet in 95 patients with ALI (Singer et al. 2006). Their results indicate also a significant improvement in oxygenation and pulmonary compliance and a decrease in length of mechanical ventilation in patients treated with EPA-GLA diet. Authors speculate that the cause of the appreciated beneficial effects must be related with an EPA-GLA-induced decrease in the synthesis of interleukin-8 and leukotriene B4, thereby reducing the inflammatory process in the lung. Nevertheless, no investigation about inflammatory components in BAL fluid or in blood was performed in the study.
Various inflammatory mediators that reflect lung injury in ALI/RDS have been investigated as potential biomarkers to diagnosis and outcome prediction. These include IL-6, IL-8, tumor necrosis factor receptor-1 (TNFR-1), von Willebrand factor (VWF), surfactant protein D (SP-D), intercellular adhesion molecule-1 (ICAM-1), protein C, and plasminogen activator inhibitor-1 (PAI-1). As indicated, only few studies with enteral feeding diets enriched in EPA/GLA have been done to analyze the effect of this dietary fat modification on these mediators.
The effect of EPA-GLA-enriched diet has also been investigated in another clinical scenario: patients with sepsis and ALI/ARDS. In this situation, the enriched diet with an isonitrogenous and isocaloric diet in a group of 165 patients was compared (Pontes-Arruda et al. 2006). As in previous works, their results indicate also an increase in oxygenation parameters and in the ventilator-free days in the study group. Mortality and development of new organ failures were also decreased in the group of patients that receive the EPA-GLA-enriched diet. The authors did not investigate inflammatory mediators in their patients. In their opinion, the clinical benefit must be related with diet components.
Also in patients with sepsis and ALI/RDS, a group of Spanish investigators performed a study comparing enteral nutrition with a modified diet (enriched in EPA/GLA) with a control diet. They performed a multicenter study that includes 198 patients. In the “per protocol” analysis (Moran et al. 2006), results indicate a decrease in infectious complications and in ICU stay in the patient that receives the study diet. Nevertheless, in the “intention to treat” analysis for the same study (Grau-Carmona et al. 2011) the effect on length of stay was the unique statistically significant result. Inflammatory mediators were not investigated in this study. Beneficial effect was attributed to the physiological hypothesis that relates EPA-GLA with less proinflammatory eicosanoids.
The abovementioned studies have used EPA/GLA administration in the setting of full nutrition provision. The positive results that, in general, have been appreciated in these studies are in contrast with the negative results appreciated in other recent trials when the fatty acid supplements were administered independently of complete nutrition provision.
In 2011 the OMEGA study was published, a multicenter and randomized study that compares omega-3, GLA, and antioxidant supplement with an isocaloric control diet in 272 ALI patients (Rice et al. 2011). Diet supplements were administered twice daily separately from the enteral nutrition. The main outcome of the study, ventilator-free days to study day 28, was better for patients in the control group. Also, other clinical variables (intensive care unit-free days and non-pulmonary organ failure-free days) were better in the control group. The authors conclude that w-3, GLA, and antioxidant supplements could be harmful in ALI patients. Nevertheless, as indicated by the authors, the study design (twice-daily bolus administration of fat supplement independently of nutrition administration) was selected to facilitate inclusion of patients unable to tolerate full feeding. This is in contrast with clinical practice and would have conditioned the study results. It is of note that the authors investigate the plasma levels of fatty acids and inflammatory mediators in some patients. Levels of EPA were significantly increased in the study group but levels of w-6 (arachidonic acid, leukotriene E4)- or w-3 (leukotriene E5)-derived mediators or IL-6 and IL-8 were similar in both groups during the study. The authors did not report an explanation for this finding.
Also a new controlled study in patients with ALI was performed, evaluating the effect of the administration of enteral omega-3 supplements also dissociated from the enteral feeding (Stapleton et al. 2011). The primary end point was the modification of IL-8 levels in bronchoalveolar lavage fluid. Results indicate that levels of IL-8 in alveolar fluid were similar in the study and control groups. Clinical variables (organ failure score, ventilator-free days, intensive care unit-free days, and 60-day mortality) were similar in both groups. The authors conclude that a fish oil supplement does not reduce biomarkers of inflammation in ALI patients.
Some studies have been done with parenteral fish oil supplementation in patients with ALI/ARDS. Nevertheless, in contrast to enteral EPA/GLA administration, no randomized controlled clinical trials using w-3-enriched parenteral lipid emulsions have shown clear evidence of beneficial effects on clinical end points in ALI/RDS patients.
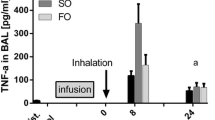
Sabater et al. (2011), in a small number of critically ill patients with ARDS and indication for parenteral nutrition, compared the effect of an omega-3-enriched lipid infusion with a soybean-based emulsion. Inflammatory mediators LTB4, TXB2, and 6-keto prostaglandin F1a were reduced in patients treated with omega-3 lipid infusion. In a previous publication, authors reported that there were no effects on pulmonary variables in the study patients.
Finally a randomized study comparing control enteral diet versus diet supplemented with parenteral fish oil emulsion during 14 days was also published. According to their results there were no effects of w-3 infusion on oxygenation or outcome variables. The authors speculate about the causes of the absence of positive effect (low w-3 dose, need for combination with other lipids, or inadequacy of parenteral administration). No investigation about inflammatory mediators was done in the study (Gupta et al. 2011).
Investigations about inflammatory mediators in BAL fluid are a good support for the hypothesis that implies EPA/GLA intake with inflammatory regulation in patients with ALI/RDS. Nevertheless, investigations in this field are very limited. Modification of plasma levels of inflammatory biomarkers after diet manipulation has been investigated also in a very few works. Clinical investigations have been the main argument favoring the use of EPA-GLA-modified diets in patients with ALI/RDS. Measurement of various proinflammatory and anti-inflammatory bioactive molecules would have given an indication of the products generated during the different stages of the illness and how supplementation of EPA/GLA altered their concentrations and influenced the outcome. These investigations will be of great value to better indicate EPA/GLA supplementation in patients with ALI/RDS in the future.
Applications to Other Conditions
The potential benefit of adding w-3 to enteral nutrition in critically ill septic patients shows non-conclusive results because it is based on studies with diets of different compositions from other substrates, different amounts and percentages of w-3, and different comparative agents. Beneficial effects have been reported in terms of mortality, days on mechanical ventilation, and days of stay at ICUs with the administration of a diet rich in EPA, GLA, and antioxidants, while in other studies, with the same diet, these results could not be confirmed and only reported a reduction in the incidence of nosocomial pneumonia and organ dysfunction (Ortiz et al. 2011).
Guidelines and Protocols
Current guidelines from different scientific societies (ESPEN, SCCM-ASPEN, SEMICYUC-SENPE, etc.) strongly recommend the use of enteral diets rich in EPA, GLA, and antioxidants in the management of RDS.
Summary Points
-
Eicosanoids control both proinflammatory and anti-inflammatory effectors that are particularly relevant for inflammation.
-
The lung is an important organ in the arachidonate cascade since it possesses the enzymatic capacity to synthesize all the arachidonate derivatives and also responsible in large part for selective catabolism of circulating eicosanoids.
-
The generation of arachidonate metabolites can play a role in the development of ALI/RDS.
-
The use of diets high in w-3 fatty acids is a means to decrease levels of arachidonic acid in cells, thereby reducing the production of proinflammatory eicosanoids.
-
Clinical investigation with enteral diets enriched in EPA/GLA and antioxidants shows beneficial effect in patients with ALI/ARDS.
-
Investigation with bolus omega-3 supplements dissociated from the nutritional regimen does not report beneficial effects in patients with ALI/ARDS.
Abbreviations
- ALI:
-
Acute lung injury
- BAL:
-
Bronchoalveolar lavage
- DGLA:
-
Dihomo-gamma-linoleic acid
- EPA:
-
Eicosapentaenoic acid
- GLA:
-
Gamma-linolenic acid
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL:
-
Interleukin
- LT:
-
Leukotriene
- MODS:
-
Multiple organ dysfunction syndrome
- PAI-1:
-
Plasminogen activator inhibitor-1
- PEEP:
-
Positive end-expiratory pressure
- PG:
-
Prostaglandin
- PUFAs:
-
Polyunsaturated fatty acids
- RDS:
-
Respiratory distress syndrome
- SIRS:
-
Systemic inflammatory response syndrome
- TBX:
-
Thromboxane
- TNF:
-
Tumor necrosis factor
References
Amat B, Barcons M, Mancebo J, et al. Evolution of leukotriene B4, peptide leukotrienes, and interleukin-8 plasma concentrations in patients at risk of acute respiratory distress syndrome and with acute respiratory distress syndrome: mortality prognostic study. Crit Care Med. 2000;28:57–62.
Anas A, van der Poll T, de Vos AE. Role of CD14 in lung inflammation and infection. In: Vincent JL, editor. Annual update in intensive care and emergency medicine. Berlin: Springer; 2010. p. 129–40.
Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55.
Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med. 2000;26:S64–74.
Calder PN. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38:343–52.
Cotogni P, Muzio G, Trombetta A, et al. Impact of the omega-3to omega-6 polyunsaturated fatty acid ratio on cytokine release in human alveolar cells. J Parenter Enteral Nutr. 2011;35:114–21.
El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A. 2012;109:14983–8.
Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med. 1999;27:1409–20.
Grau-Carmona T, Morán-García V, García-de-Lorenzo A, et al. Effect of an enteral diet enriched with eicosapentaenoic acid, gamma-linolenic acid and anti-oxidants on the outcome of mechanically ventilated, critically ill, septic patients. Clin Nutr. 2011;30:578–84.
Gupta A, Govil D, Bhatnagar S, et al. Efficacy and safety of parenteral omega 3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15:108–13.
Heller A, Koch T, Schmeck J, van Ackern K. Lipid mediators in inflammatory disorders. Drugs. 1998;55:487–96.
Leeman M, Boeynaems JM. Role of eicosanoids in the development of ARDS. In: Vincent JL, editor. Intensive care and emergency medicine. Berlin: Springer; 1984. p. 14–6.
Lorente JA, Esteban A. Biomarkers of acute lung injury. In: Vincent JL, editor. Annual update in intensive care and emergency medicine. Berlin: Springer; 2012. p. 160–70.
Mancuso P, Whelan J, DeMichele SJ, et al. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med. 1997;25:1198–206.
Masclan JR, Bermejo B, Picó M, et al. Prognostic value of eocosanoids in the acute respiratory distress syndrome. Med Clin (Barc). 1999;112:81–4.
Mayer K, Schafer MB, Seeger W. Fish oil in the critically ill: from experimental to clinical data. Curr Opin Clin Nutr Metab Care. 2006;9:140–8.
Moran V, Grau T, García de Lorenzo A, et al. Effect of an enteral feeding with eicosapentaenoic and gamma-linoleic acids on the outcome of mechanically ventilated critically ill septic patients. Crit Care Med. 2006;34(12 Abstract Supplement):A70.
Ortiz C, Montejo JC, Vaquerizo C. Guidelines for specialized nutritional and metabolic support in the critically-ill patient. Update. Consensus SEMICYUC-SENPE: Septic patient. Nutr Hosp. 2011;26(S2):67–71.
Pacht ER, DeMichele SJ, Nelson JL, et al. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500.
Palombo JD, DeMichele SJ, Lydon E, et al. Rapid modulation of lung and liver macrophage phospholipids fatty acids in endotoxemic rats by continuous enteral feeding with n-3 and gamma-linolenic fatty acids. Am J Clin Nutr. 1996;63:208–19.
Pontes-Arruda A, DeMichele SJ. Enteral nutrition with anti-inflammatory lipids in ALI/ARDS. In: Vincent JL, editor. Annual update in intensive care and emergency medicine. Berlin: Springer; 2009. p. 695–704.
Pontes-Arruda A, Aragao AM, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med. 2006;34:2325–33.
Rice TW, Wheeler AP, Thompson BT, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306:1574–81.
Rivkind AI, Siegel JH, Guadalupi P, Littleton M. Sequential patterns of eicosanoid, platelet, and neutrophil interaction in the evolution of the fulminant post-traumatic adult respiratory distress syndrome. Ann Surg. 1989;210:355–72.
Sabater J, Masclans JR, Sacanell J, et al. Effects of anomega-3 fatty acid-enriched lipid emulsion on eicosanoid synthesis in acute respiratory distress syndrome (ARDS): a prospective, randomized, double-blind, parallel group study. Nutr Metab (Lond). 2011;8:22.
Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–21.
Serhan CN, Chiang N, van Dyke E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61.
Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34:1033–8.
Stapleton RD, Martin TR, Weiss NS, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39:1655–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this entry
Cite this entry
Garcia-de-Lorenzo y Mateos, A., Montejo González, J.C., Quintana Diaz, M. (2015). Eicosanoid Synthesis and Respiratory Distress Syndrome in Intensive Medicine. In: Rajendram, R., Preedy, V.R., Patel, V.B. (eds) Diet and Nutrition in Critical Care. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7836-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7836-2_1
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7837-9
Online ISBN: 978-1-4614-7836-2
eBook Packages: MedicineReference Module Medicine