Abstract
The incidence of erectile dysfunction (ED) after blunt pelvic trauma is between 11 and 30 % and increases greatly with concomitant urethral injury and/or bilateral pubic rami. Most patients with ED after pelvic trauma respond to intracavernosal injection therapy, suggesting that there is a neurogenic component. Only a very select group of patients are candidates for a revascularization procedure. Potential candidates are young (usually less than 55 years old), have a focal occlusive disease of the penile or cavernosal artery on arteriography, no vascular risk factors, no evidence of neurologic erectile dysfunction, and a history of acute or chronic perineal or pelvic trauma. Specifics as to the penile revascularization surgical technique of inferior epigastric artery to dorsal artery of the penis anastomosis and as an alternative to the dorsal vein are detailed within. Overall reported success rates are from 50 to 60 %.
Venous leak erectile dysfunction typically results from atrophy of the intracorporal muscles or of the tunica albuginea. These patients are not amenable to penile venous surgery as they have an uncorrectable physiology. A minority of patients have either congenital or acquired isolated proximal penile venous anomalies. Congenital venous leakage results in large ectopic, superficial dorsal veins or large crural veins. Blunt perineal trauma may result in structural changes in crural erectile tissue resulting in isolated crural venous leak. Although initial published series with 1-year follow-up demonstrated poor outcomes, highly select patients with isolated crural venous leak treated by crural ligation may have significant improvement in erectile function. The Report on the Treatment of Organic Erectile Dysfunction, however, does not recommend venous leak surgery and considers it experimental.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Summary
The incidence of erectile dysfunction (ED) after blunt pelvic trauma is between 11 and 30 % and increases greatly with concomitant urethral injury and/or bilateral pubic rami. Most patients with ED after pelvic trauma respond to intracavernosal injection therapy, suggesting that there is a neurogenic component. Only a very select group of patients are candidates for a revascularization procedure. Potential candidates are young (usually less than 55 years old), have a focal occlusive disease of the penile or cavernosal artery on arteriography, no vascular risk factors, no evidence of neurologic erectile dysfunction, and a history of acute or chronic perineal or pelvic trauma. Specifics as to the penile revascularization surgical technique of inferior epigastric artery to dorsal artery of the penis anastomosis and as an alternative to the dorsal vein are detailed within. Overall reported success rates are from 50 to 60 %. Venous leak erectile dysfunction typically results from atrophy of the intracorporal muscles or of the tunica albuginea. These patients are not amenable to penile venous surgery as they have an uncorrectable physiology. A minority of patients have either congenital or acquired isolated proximal penile venous anomalies. Congenital venous leakage results in large ectopic, superficial dorsal veins or large crural veins. Blunt perineal trauma may result in structural changes in crural erectile tissue resulting in isolated crural venous leak. Although initial published series with 1-year follow-up demonstrated poor outcomes, highly select patients with isolated crural venous leak treated by crural ligation may have significant improvement in erectile function. The Report on the Treatment of Organic Erectile Dysfunction, however, does not recommend venous leak surgery and considers it experimental.
Introduction
Atherosclerotic or traumatic arterial occlusive disease can decrease perfusion to the corpora cavernosa, resulting in increase in the time to maximal erection and decreasing rigidity of the erect penis. Arteriogenic erectile dysfunction is most commonly a part of systemic atherosclerosis, and its onset is similar to coronary arterial disease [1]. Risk factors for arteriogenic ED include smoking, hypertension, hyperlipidemia, diabetes, and perineal or pelvic trauma [2]. In patients with atherosclerosis and erectile dysfunction, arteriography demonstrates bilateral diffuse disease affecting the internal pudendal, common penile, and cavernous arteries.
Levine et al. described focal occlusion of the common penile or cavernous artery, which was found in young men who had sustained blunt pelvic or perineal trauma [3]. The incidence of erectile dysfunction after blunt pelvic trauma is between 11 and 30 % and increases up to 62 % when there is a concomitant urethral injury [4–6]. Bilateral pubic rami fractures with urethral injury are highly associated with concomitant erectile dysfunction [6]. Up to 89 % of patients with erectile dysfunction after pelvic trauma respond to intracavernosal injection therapy, suggesting that there is a neurogenic component [6].
Venogenic erectile dysfunction is thought to result from failure of sufficient venous occlusion [7]. Degenerative changes or traumatic injury to the tunica albuginea may result in impaired compression of the subtunical and emissary veins [8, 9]. Acquired venous leak may result from perineal trauma, surgical treatment of priapism, or congenital anomalous penile venous drainage [10–12].
Penile Revascularization Surgery
Patient Selection and Indications
Penile revascularization is the only surgical procedure to date that is capable of restoring natural penile erections without the need for vasoactive medication, external mechanical devices, or surgical placement of a prosthetic device. Michal et al. first described penile arterial revascularization surgery for erectile dysfunction in 1973 with direct arterial anastomosis of the inferior epigastric artery to the corpus cavernosa (Michal I). This group reported excellent flow rates of greater than 100 ml/s, however with also about 100 % stenosis rates [13]. The technique was later modified and the inferior epigastric artery was anastomosed end to side with the dorsal penile artery (Michal II). The modification was reported to have a 56 % success rate [14].
Further modifications were employed by Virag et al. with anastomosing of the inferior epigastric artery to the deep dorsal vein to allow for retrograde penile perfusion [15]. Improvements in the arterialization of the dorsal vein were achieved by ligating the deep dorsal vein distal to anastomosis and its circumflex branches to avoid hyperemia of the glans [16]. Other variations in the surgical technique have included anastomosing the penile artery and vein and then subsequently anastomosing the inferior epigastric artery [17].
Lack of standardization in patient selection, hemodynamic evaluation, surgical technique, and limited long-term outcome data using validated instruments have resulted in this surgery being considered experimental. The Erectile Dysfunction Clinical Guideline Panel published in the Report on the Treatment of Organic Erectile Dysfunction in 1996, is an evidence-based guideline for the diagnosis and treatment of erectile dysfunction [18]. The original report was updated in 2005, and the current guidelines for penile revascularization are based on this report [19]. The panel recommendations were based on an Index Patient that represents the most common presentation of erectile dysfunction. The Arterial Occlusive Disease Index Patient was defined as an otherwise healthy man, 55 years old or younger with recently acquired erectile dysfunction due to focal arterial occlusive disease, who is the most likely patient to benefit from vascular reconstruction. These strict inclusion criteria eliminated other risk factors associated with diffuse vascular disease or chronic ischemia such as smoking, diabetes, and coronary arterial disease. This definition was used to evaluate the efficacy of the treatment of arterial occlusive disease. Of 31 papers initially identified in the world literature on vascular reconstruction, only 4 studies were included in the outcomes evaluation as the other 27 papers did not meet criteria for the Arterial Occlusive Disease Index Patient or for lack of objective outcome data. The four studies included a total of 50 patients of which 42 had inferior epigastric artery to dorsal penile artery and 8 had inferior epigastric artery to dorsal penile vein anastomosis with a range of 36–91 % satisfactory outcome [20–23]. Due to the small patient population who met criteria, the Panel’s recommendation could not objectively confirm satisfactory outcome and stated that Arterial reconstructive surgery is a treatment option only in healthy individuals with recently acquired erectile dysfunction secondary to a focal arterial occlusion and in the absence of any evidence of generalized vascular disease. Based on the Panel’s recommendations, only a very select group of patients are considered to be candidates for a revascularization procedure. A patient is a potential candidate for arterial revascularization if they are young (usually less than 55 years old), have a focal occlusive disease of the penile or cavernosal artery on arteriography, no vascular risk factors, no evidence of neurologic erectile dysfunction, and report of acute or chronic perineal or pelvic trauma [24].
The association of erectile dysfunction with pelvic fracture urethral injuries is well documented with multiple etiologies, including multiple sites of proximal venous leak in 62 % of patients as well as multiple sites of arterial occlusion, most commonly in the penile and cavernosal arteries. Isolated arterial occlusion was only noted in about 30 % of patients [25]. This select subset of patients with purely arterial occlusion resulting in erectile dysfunction may be good candidates for arterial revascularization.
Evaluation
Initial evaluation requires a thorough medical, sexual, and psychosocial history. The goal is to determine any underlying medical conditions that may predispose to arteriogenic erectile dysfunction such as cardiovascular disease, diabetes mellitus, smoking, or history of pelvic/perineal trauma. A history includes assessment of sexual interest, performance, and satisfaction, which can be done with a variety of patient self-administered questionnaires. The International Index of Erectile Function (IIEF) is the most commonly used questionnaire, which has been validated across various cultures and languages [26]. These questionnaires do not only provide information about sexual function and quality of life, but they also serve as baseline metrics by which the patient can be followed with longitudinally for assessment of outcome after treatment. A focused physical exam is performed aimed at evaluating for signs of hypogonadism, sensory abnormality, and Peyronie’s plaque.
Diagnostic Evaluation
There are no specific guidelines for the diagnostic recommendation prior to proceeding with any penile revascularization procedure. However, noninvasive evaluation with penile color Doppler ultrasound in combination with pharmacologic stimulation with intracavernosal injection of a vasodilating agent is used for initial assessment. Peak systolic velocity (PSV) <25 cm/s is considered suggestive of arterial insufficiency erectile dysfunction and has been shown to correspond with 100 % sensitivity and 95 % specificity with abnormal internal pudendal arteriography (Fig. 45.1) [27]. Velocities between 25 and 30 cm/s are considered indeterminate although there are age-related changes to PSV that should be taken into account [28]. Changes in the diameter of the cavernous artery of less than 75 % and less than 0.7 mm after vasodilator injection are found in patients with severe vascular disease [29]. However, changes in cavernous arterial vasodilation have not been found to correlate with abnormalities in arteriography [30]. One must also be aware that variations in the penile arterial anatomy may result in alterations in the measurement of PSV and interpretation of the study is dependent on the expertise of the ultrasonographer [30]. Additionally one must rule exclude coexisting veno-occlusive dysfunction with a normal resistive index of less than 0.9 [31].
Some advocate routine dynamic infusion or pharmacologic cavernosometry and cavernosography or as an additional step if Doppler ultrasound findings are indeterminate in excluding veno-occlusive disease [32]. With complete smooth muscle relaxation after infusion of a vasodilator with possible redosing, findings should be consistent with normal veno-occlusive function. Specifically, infusion flow of <5 cc/min to maintain an intracavernosal erection pressure of >100 mmHg and pressure decrease of less than <45 mmHg over 30 s should be present [33]. Cavernosography identifies any abnormal drainage to the glans, corpus spongiosum, superficial dorsal veins, cavernous veins, and crural veins [7, 34, 35].
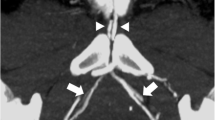
Lastly, selective internal pudendal artery angiography is used to evaluate the penile vascular system and document the location of the focal arterial occlusion. Simultaneous intracavernosal injection of a vasodilating agent is given to achieve maximal vasodilation. Additionally, the angiogram will provide information about patency and anatomy of the donor and recipient vessels for surgical planning (Fig. 45.2).
Surgical Technique
Preparation
After induction of general anesthesia, the patient is placed supine on the operating room table. Pressure points of the upper and lower extremity are padded. The patient’s abdomen and genitalia are shaved. Once the patient is draped, a 16 French Foley catheter is placed with sterile technique. One weight-based dose of a first-generation cephalosporin (e.g., cefazolin) or vancomycin is given if the patient is penicillin allergic.
Harvesting of Inferior Epigastric Artery
The senior author of this chapter prefers to harvest the donor inferior epigastric artery through a laparoscopic approach for improved cosmesis and faster recovery time. An infraumbilical incision is made, and the subcutaneous tissue is dissected down to the level of the anterior rectus sheath off one side of midline. After an incision is made in the anterior rectus sheath and a window is created through the rectus muscle, a 10 mm balloon trocar is placed and preperitoneal insufflation is achieved. Two 5 mm trocars are placed on the contralateral side of the donor inferior epigastric artery, lateral to the artery. The inferior epigastric artery is identified at its origin from the external iliac artery, and the dissection is carried caudal to cephalic, to the point of bifurcation near the umbilicus. The artery and accompanying vein are dissected together to avoid injury to the artery. Once the inferior epigastric bundle is clipped and divided, a small longitudinal incision is made at the base of the penis. A 10 mm dilating trocar is placed through the Hesselbach’s triangle through this incision, and the epigastric bundle is pulled through the trocar sheath under direct visualization to prevent torsion. Transperitoneal laparoscopic- or robotic-assisted technique can also be employed as a minimally invasive approach [36, 37].
Alternatively, a lower midline, transverse, or paramedian abdominal incision is made and carried down to the level of the rectus fascia. The fascia is divided longitudinally and the rectus muscle is mobilized medially or laterally. The inferior epigastric artery is identified in the preperitoneal space and dissected from its origin from the external iliac artery distally to the level of the umbilicus with its surrounding fat and veins. It should be of sufficient length to have a tension-free anastomosis. Bipolar cautery or small titanium clips are used to control and divide any arterial branches. Topical papaverine is applied to the artery during the dissection to prevent vasospasm.
Microvascular Anastomosis
Various penile or peri-penile incisions can be used for the vascular anastomosis including a curvilinear anterior scrotal peri-penile incision two fingerbreadths inferior to the base of the penis on the opposite side of where the inferior epigastric artery will be harvested [38]. If a lower midline incision is used to harvest the inferior epigastric artery, it can be extended to the proximal penile shaft. A longitudinal incision over the dorsal aspect of the penis or a transverse incision at the base of the penis can be used for laparoscopic- or robotic-assisted harvesting of the inferior epigastric artery.
After making the penile skin incision and carrying it down through dartos fascia, the tunica albuginea is bluntly dissected as proximally as possible and the paired dorsal arteries and dorsal vein are identified. If a laparoscopic- or robotic-assisted technique is used to harvest the donor artery, the trocar used to deliver the inferior epigastric artery is placed in Hesselbach’s triangle. If the inferior epigastric artery was harvested in an open fashion, a window is made in the fundiform ligament and extended to the external inguinal ring. A Schnidt clamp is placed through this window to transfer the artery to the dorsal aspect of the penis.
An operating microscope is brought into the surgical field. The chosen dorsal artery is circumferentially dissected from its attachment to the tunica albuginea, taking care to avoid injury to any branches of the cavernosal artery. The adventitia of the dorsal artery and inferior epigastric artery are excised to prevent thrombosis of the anastomosis, and the distal end of the inferior epigastric artery is spatulated. Vascular clamps such as bulldogs or aneurysm clips are placed on the donor and recipient vessels for hemostasis during the anastomosis. A 9-0 Prolene suture is placed through a 1 mm segment of the anterior surface of the dorsal artery and excised after tension is placed on the suture to make an arteriotomy. An end-to-side anastomosis is performed with interrupted 9-0 Prolene sutures after a suture is placed at each apex (Fig. 45.3). The vascular clamps are removed and the donor and recipient artery are inspected for good pulsatile flow as well as good hemostasis. Doppler ultrasound can also be used to document arterial patency. The subcutaneous tissues are close in two layers with 5-0 Vicryl and the skin in closed with 5-0 interrupted horizontal mattress sutures. Xeroform and Coban dressing are placed on the penis for hemostasis and the urethral catheter is left overnight.
Alternative anastomoses include end-to-end arterial revascularization where the proximal dorsal artery is ligated and divided. Dividing the proximal aspect of the artery is thought to decrease turbulence. Dorsal vein arterialization can be achieved with either side-to-end or end-to-end anastomosis. A valvulotome or Fogarty balloon is used to disrupt the valves. The branches of the deep dorsal vein near the glans are ligated with absorbable suture to prevent glans hyperemia.
Complications
The most common complications after penile revascularization surgery are penile edema and ecchymosis. A Coban dressing applied to the penile shaft can help control postoperative edema and ecchymosis. Rates of decreased penile sensation and penile shortening are reported as high as 25 and 28 %, respectively, in one series [32]. Preservation of the suspensory and fundiform ligaments during the penile dissection is thought to help preserve penile length; nevertheless scar formation may still result in penile shortening. Glans hyperemia may occur in dorsal vein arterialization if communicating veins to the glans have not been ligated. Disruption of the anastomosis may occur weeks after the operation due to blunt trauma from intercourse, masturbation, or accidents [33].
Outcomes
Many retrospective studies have reported outcome data for penile revascularization surgery for patients with any cause of arteriogenic erectile dysfunction. These studies are limited by variable inclusion and exclusion criteria, short length of follow-up, and lack of objective follow-up data. Young men who have sustained traumatic arterial lesions appear to have better outcomes compared to elderly patients. No comparative prospective, randomized studies have assessed the outcome of penile revascularization surgery for arteriogenic erectile dysfunction.
Historically, long-term success rates have ranged from 50 to 60 %. As the patient population in these series has been heterogeneous and has not met the current patient criteria of the Arterial Occlusive Disease Index Patient, outcomes are not applicable, nor generalizable.
In 2005, the Erectile Dysfunction Clinical Guideline Panel based their recommendations for revascularization surgery on four studies that had been published to date. Grasso et al. published their outcomes of 22 patients in who underwent inferior epigastric artery to dorsal vein arterialization procedures in 1992 [23]. They reported that 55 % of patients were able to have erections sufficient for intercourse at one-year follow-up. Another published series by De Palma and colleagues reported 60 % success rate of spontaneous erections or with the aid of intracavernosal vasodilating medication or a vacuum constriction device in 11 patients undergoing inferior epigastric to dorsal artery anastomosis [21]. The series with the longest follow-up is that of Jarow and DeFranzo [22]. Of 11 men who underwent penile revascularization, 9 had inferior epigastric artery to dorsal artery anastomosis and 2 had dorsal vein procedures. At an average of 50-month follow-up, 91 % of patients had improved erections from their preoperative baseline with 27 % requiring intracavernosal injections as an adjunct. Ang et al. described the outcomes of six patients with pure arteriogenic or mixed arteriogenic-vasculogenic erectile dysfunction who underwent dorsal vein arterialization [20]. At a follow-up of 20 months, they reported an overall 66 % success, where half of those patients required intracavernosal vasodilator medication.
Since the Erectile Dysfunction Clinical Guideline Panel published their recommendation report in 2005, Munarriz et al. has published the largest series of vascular reconstruction using validated outcomes in 2009 [24]. In this series, inferior epigastric artery to dorsal artery microarterial bypass surgery was performed. Their patient selection criteria include the following: (1) age 55 years or younger, (2) absence of vascular risk factors, (3) no evidence of neurologic ED, (4) no hormonal abnormalities, (5) no evidence of active psychiatric disorders, (6) no evidence of Peyronie’s disease, (7) absence of premature ejaculation, (8) report of acute or chronic perineal/pelvic trauma, (9) no evidence of corporo-occlusive dysfunction by duplex Doppler ultrasound and cavernosometry, and (10) focal occlusive disease of the common penile or cavernosal arteries documented by selective internal pudendal arteriography. This study included 71 men, with an average age of 30.5 years old with a mean follow-up of 34.5 months.
The main outcome measured was preoperative total International Index of Erectile Function (IIEF) score, EF domain of the IIEF, and questions 3 and 4. The preoperative and postoperative outcomes were 35.5, 13.7, 2.2, and 2.1 compared to 56.2, 16.6, and 23.8, respectively. Fifty-five percent of patients had an IIEF-EF domain score of 26, and overall, 73 % of patients had an IIEF-EF score of equal to or greater than 21.
Penile Venous Surgery
Patient Selection Indications
Venous leak erectile dysfunction typically results from atrophy of the intracorporal muscles or of the tunica albuginea. These patients are not amenable to penile venous surgery as they have an uncorrectable physiology. A minority of patients have either congenital or acquired isolated proximal penile venous anomalies. Congenital venous leakage results in large ectopic, superficial dorsal veins or large crural veins [12]. Blunt perineal trauma may result in structural changes in crural erectile tissue resulting in isolated crural venous leak [39].
Typically, these patients report primary erectile dysfunction dating back to the first sexual encounter with the inability to penetrate or with an erection that subsides in seconds. Additionally, patient may report partial rigidity with nocturnal erections or masturbation [40]. In the case of venous leak from perineal trauma, patients typically report normal erectile function prior to the traumatic event with erectile dysfunction similar to the individuals with congenital venous leakage after the trauma.
Although some patients have erectile dysfunction from focal congenital venous anomalies or from perineal trauma, in most patients it is difficult to distinguish functional abnormalities of the corporal smooth muscle from anatomical defects such as tunical abnormalities [41]. It is also difficult to differentiate and diagnose what percentage of erectile dysfunction is due to veno-occlusive disease or general arterial hypoperfusion. Additionally, there is no standardized approach to diagnosis nor evidence from randomized clinical trials indicating the efficacy of surgical management for veno-occlusive disease. As a result, the Erectile Dysfunction Clinical Guideline Panel published in the Report on the Treatment of Organic Erectile Dysfunction in 1996 with a revision in 2005 concluded that surgeries performed with the intent to limit the venous outflow of the penis are not recommended [18, 19]. However, as indicated above, there are a few selected patients with failure of medical management with PDE5 inhibitors or intracavernosal injection with a vasoactive agent, normal cavernous arteries, and with a clearly identifiable location of posterior venous leak that might benefit from penile venous surgery [42].
Evaluation
As stated in the previous section on penile arterial revascularization, the initial evaluation includes thorough medical, sexual, and psychosocial history. Sexual history includes one or self-administered questionnaires such as the IIEF [26]. If a trial of PDE5 inhibitors or intracavernosal injection with a vasoactive agent does not produce any results, the patient can then be further evaluated with color duplex ultrasound, cavernosometry, and cavernosography.
In a patient with a history suggestive of venous leak erectile dysfunction and who has failed medical management, one can proceed with color duplex ultrasound with intracavernosal injection of a vasodilating agent. Duplex ultrasound demonstrates peak flow velocity greater than 35 cm/s, ruling out arteriogenic cause of erectile dysfunction. In addition, there is persistent end-diastolic velocity of greater than 10 cm/s in the cavernous arteries (Fig. 45.4, panel a) [43].
Patient with lifelong history of erectile dysfunction. (a) Doppler penile ultrasound shows high peak systolic flow velocity (normal arterial flow) of the cavernosal arteries after injection of 0.5 ml of Trimix solution and self-stimulation. High end-diastolic flow velocity of >10 cm/s is indicative of venous leak. (b) Pharmacologic cavernosogram after injection of 0.5 ml of Trimix solution (shows crural leakage arrow)
If the color Doppler ultrasound is suggestive of a venous leak, we proceed with further penile hemodynamic evaluation with a pharmacologic cavernosometry and cavernosography with 0.5 ml of Trimix (papaverine 30 mg/ml, phentolamine 1 mg/ml, prostaglandin E1 10 mcg/ml) with redosing if necessary to achieve smooth muscle relaxation [42]. Infusion flow to maintain intracorporal pressure greater than 100 mmHg at greater 5 ml/s and a decrease in intracorporal pressure of greater than 45 mmHg over 30 s is suggestive of venous leak on cavernosometry [44]. Cavernosography with diluted iodinated contrast is done with intracavernosal pressure of less than 100 mmHg to visualize abnormal venous channels while images are taken with fluoroscopy to monitor the drainage of the corpora cavernosa [42]. Abnormal venous channels can be seen draining to the glans, corpus spongiosum, superficial dorsal veins, cavernous veins, and crural veins (Fig. 45.4, panel b) [7, 34, 35].
Surgical Technique
Preparation
After induction of general anesthesia, the patient is placed supine on the operating room table. Alternatively, if crural ligation is being performed, the patient is placed in the dorsal lithotomy position. Pressure points of the upper and lower extremity are padded. The patient’s genitalia are shaved. Once the patient is draped, a 16 French Foley catheter is placed with sterile technique. One weight-based dose of a first-generation cephalosporin (e.g., cefazolin) or vancomycin is given if the patient is penicillin allergic.
Crural Ligation
A 6 cm inguinoscrotal incision 2 cm lateral to the root of the penis is made and carried down to the base of the penis without entering the tunica vaginalis (Fig. 45.5, panel a). Sharp and blunt dissection is used to free the dorsum of the penis from the subcutaneous tissue. The fundiform and suspensory ligaments of the penis are incised which detaches the base of the penis from the pubic bone. After incising Buck’s fascia and identifying the deep dorsal vein, branches are ligated, followed by dissection of the dorsal vein from the tunica. The deep dorsal vein is suture ligated proximally with a 2-0 nonabsorbable suture such as silk or TiCron (braided polyester). A 4 cm segment of the deep dorsal vein is excised from just under the pubic bone to the midshaft of the penis.
Crural ligation surgery. (a) One-sided inguinoscrotal incision is used to perform bilateral crural ligation and ligation/excision of deep dorsal veins. (b) After the deep dorsal vein was resected, the hilum was entered to isolate the neurovascular structures. The three vessel loops go around the patient’s right dorsal nerve and artery, the right and left cavernous arteries, and the left dorsal nerve and artery, respectively. (c) The polyester umbilical tapes were passed between the corpus spongiosum and the corpora cavernosa and were placed under the neurovascular bundles mentioned in (b) before they were tied
The dorsal artery and nerve are carefully reflected off of the tunica albuginea bilaterally. The cavernous artery and nerves are also dissected to identify the entrance of the artery into the corpus cavernosum (Fig. 45.5, panel b). A 16 Fr Foley catheter is passed into the bladder to help locate the bulbar urethra (if not already placed at the beginning of the operation). The crus is then dissected off the bulbar portion of the corpus spongiosum. A right-angle clamp is passed between the crus and bulbar spongiosum. The tip of the clamp is passed dorsally proximal to the entrance of the cavernous artery into the corpus cavernosum. Four umbilical tapes are passed between the spongiosum and crus (Fig. 45.5, panel c). The other ends of the two tapes are passed under the dorsal and cavernous arteries and the nerves on the dorsolateral aspect of the crus and then tied. The same procedure is performed on the opposite crus. The tunica albuginea is reattached to the periosteum of the pubis with a zero silk suture. Careful skin closure is performed in layers to prevent postoperative penile shortening. A compression dressing is applied to the penis and scrotum after surgery. The Foley catheter is removed the following day.
Flores et al. [45] describe a variation to crural ligation, which does not include ligation of the deep dorsal vein or dissection of the dorsal arteries and veins. A transverse scrotal incision is made and subcutaneous tissues are dissected until the crura are exposed. The crura are carefully separated from the corpus spongiosum, and the plane between the two structures is developed. A right-angle clamp was used to pass two umbilical tapes around the crus from medial to lateral. The umbilical tapes are tied around each crus starting 1 cm from the posterior crural end and separated by 1 cm from each other. The same procedure is performed on the opposite crura. A drain is not placed and the patient goes home the same day.
Complications
Common immediate complications include penile and scrotal ecchymosis, hematoma, penile edema, pain from nocturnal erections, and rarely infection. Long-term complications are penile shortening and decreased penile sensation. Penile shortening can occur in as many as 20–30 % of patients; however, it is rarely clinically significant [45, 46]. The use of a vacuum erection device starting 1 month postoperatively for 3 months can help with penile shortening [40]. Numbness of the glans or penile shaft is common and occasionally can affect the ability to achieve orgasm [51]. Typically, loss of penile sensation is temporary and completely returns in 7–9 months [51]. Penile numbness can be minimized by not degloving the penis [40]. Rarely, a patient can experience scar contraction that leads to penile tethering and curvature that requires subsequent release of scar tissue and scar revision [47]. Austoni et al. have reported rare occurrences of priapism after venous leak surgery [48].
Outcomes
Similar to outcomes data for penile arterial revascularization, retrospective studies include a heterogeneous population with variable inclusion and exclusion criteria and lack of objective measures and short follow-up. Immediately postoperatively, multiple series in the 1980s–1990s reported initial success rates up to 85 %; however, with longer than 12-month follow-up, success rates decrease to as low as 25 % [47, 49, 50].
More contemporary series with highly selective groups of younger patients and with greater than a year follow-up report between 45 and 66 % success of achieving excellent unassisted erections with crural ligation surgery. Rahman et al. reported outcomes data on a select cohort of men with congenital crural venous leak erectile dysfunction who underwent crural ligation with ligation of the dorsal vein as described above [42]. This study included 11 men younger than 40 years with a history of lifelong erectile dysfunction and venous leakage from abnormal crural veins on cavernosography. Men with psychogenic or mixed vasculogenic erectile dysfunction were excluded as well as Doppler ultrasound demonstrating peak flow velocity less than 25 cm/s or negative end-diastolic pressure and diffuse venous leakage on cavernosography. With a mean age of 28 years, overall 82 % of patient had improvement in their erectile function. Of those men, 45 % reported improved unassisted erectile function with 36 % requiring intermittent PDE5 inhibitor treatment at a mean follow-up of 34 months. Mean IIEF scores increased from 8.9 to 17.5 postoperatively and 64 % had IIEF score greater than 19.
In a similar selected cohort of men with isolated crural venous leak on dynamic infusion cavernosometry and cavernosography, in 2011, Flores et al. reported 66 % success rate of achieving unassisted erections at a mean follow-up of 16 months in 15 patients with crural ligation alone using a scrotal incision [44]. Overall, 93 % of patients had improved erections with or without PDE5 inhibitor treatment. There was an increase in IIEF score from 18 to 24 postoperatively.
Although initial series publishing longer than 12-month outcomes have demonstrated poor outcomes, highly selected patients with isolated crural venous leak can have significant improvement in erectile function. Even though the Report on the Treatment of Organic Erectile Dysfunction does not recommend venous leak surgery and considers it experimental, improvement in erectile function, even with the assistance of PDE5 inhibitors, greatly improves the quality of life, especially when erectile dysfunction was refractory to medical management prior to crural ligation surgery.
Surgical Pearls and Pitfalls
Arterial Surgery
-
Either laparoscopic or open approach can be used to harvest the donor artery, the inferior epigastric artery.
-
The epigastric vascular bundle is passed via the external ring to the base of the penis.
-
The recipient vessel, the dorsal artery, or the deep dorsal vein is isolated.
-
The adventitia of the donor and recipient vessels is carefully stripped off the vessel to prevent thrombosis of the anastomosis.
-
End-to-side or end-to-end anastomosis is made with fine sutures under operative microscope.
Potential Problems
-
Inadequate length of the donor artery: The anatomy and branching pattern of the epigastric artery on the angiogram must be studied before surgery and appropriate length determined.
-
Glans hyperemia: The deep dorsal vein and its branches distal to the epigastric artery-dorsal vein anastomosis need to be carefully ligated to prevent the problem.
-
Inadequate size of dorsal artery: Change to epigastric artery-dorsal vein anastomosis.
-
Early thrombosis of anastomosis: Mini-heparin at the conclusion of anastomosis and baby aspirin for 3 months.
-
Penile shortening: Avoid dissection of the fundiform ligament.
Venous Surgery (Crural Ligation)
Dorsal Approach
-
An inguinoscrotal incision is made to approach the crura from above.
-
The fundiform and suspensory ligaments are cut to allow detachment of the penile base from the pubic bone.
-
Buck’s fascia is opened to isolate and resect the proximal portion of the deep dorsal vein.
-
The dorsal artery and dorsal nerve are dissected off the tunica.
-
The cavernous arteries are traced to the entrance to the corpora cavernosa.
-
The urethral bulb is separated from the crura to allow passage of umbilical tapes.
-
The crural ligation should be performed proximal to the entrance of the cavernous arteries with at least two separate polyester (not cotton) umbilical tapes.
Ventral Approach
-
A vertical perineal incision is made to identify the urethral bulb and both crura.
-
The ischiocavernosus muscle overlying the crura is dissected to expose the tunica of the crura.
-
At least two umbilical tapes are passed between the crura and the pubic bone and separated tied to secure closure of the crura.
Potential Problems
-
Ligation of the cavernous artery: Intraoperative Doppler ultrasound must be used to positively identify the entrance of the cavernous artery to the corpora to avoid ligation of the cavernous artery.
-
Injury to the urethra: A Foley catheter must be inserted before passing of the umbilical tape to avoid injury.
-
Foreign body reaction/infection of the umbilical tapes: Use polyester, not cotton umbilical tapes.
-
Penile shortening: The dorsal tunica should be sutured to the periosteum of the pubic bone to substitute the severed suspensory ligament, and the layers of tissue in the infrapubic region should be carefully closed to avoid excessive scar and penile shortening.
Editorial Comment
Most of my experience with vascular surgery for erectile dysfunction is with penile revascularization for pelvic fracture patients with erectile dysfunction. Venous leak surgery never really worked in my hands, because with time, alternative drainage channels developed and the venous leak impotence uniformly recurred. At one time early in my career, I was performing many dorsal vein ligations as well as sending patients to interventional radiology for transvenous embolizations of the dorsal vein and branches. Success rates were very poor at 1-year follow-up (<20 %) and thus we stopped performing such surgeries. Lue seems to have had reasonable success with crural ligation for highly select congenital venous leak patients that are refractory to oral and intracavernosal injection therapies. However, the reported study populations are very small and complications rates are not trivial. Clearly a well-performed cavernosography is the key to getting a proper road map, so to properly select the best surgical candidate.
As to penile revascularization, it is key to limit such surgeries to young patients who are impotent after pelvic fracture and have no associated comorbidities, in particular, hypertension smoking, diabetes, etc. The other key is to do a properly performed arteriogram and to speak with your interventional radiologist. Key aspects are to first obtain a penile Doppler with cavernosal injection (Trimix) that demonstrates arteriogenic impotence. Once this is demonstrated, the key technical aspects of the arteriogram are to inject the cavernosa with vasoactive substances 10–15 min before the onset of the study. We prefer 0.2 ml of Trimix. It is important that each pudendal artery is cannulated and intravascular nitroglycerin injected to help dilate the vessels. We would like to see distal reconstitution of the dorsal artery of the penis to confirm its patency, as the potential recipient of an end-to-side inferior epigastric artery anastomosis. I ask the radiologist to also squirt the epigastric arteries at the same time, in order to demonstrate presence and patency on each side. I have always harvested the epigastric arteries via a midline abdominal incision, but after reading this chapter, I will change my practice to a laparoscopic extraperitoneal dissection method. Unless you have extensive microvascular surgical skills and experience, I would suggest you have your plastic surgical colleague who has good microsurgical skills to assist you with the surgery. I would not tackle such surgery alone. My overall success rates with such surgery improving erectile function have been similar to published series, at roughly 50 %. Of those who responded, about half needed to use oral PDE5 meds and the other half use intracavernosal injection therapy. My best success comes with patients who complained of cold glans penis syndrome. Typically, such patients state that after revascularization the cold feeling is gone and the glans and penis feel warm immediately postoperatively – stating this in the recovery room. I send patients home on just aspirin. Early in my career I would send patients home fully anticoagulated on Coumadin, but I had one patient who got into a fight 1 month after surgery and was struck in the suprapubic region with a cue stick. He bled profusely and required ligation of the epigastrics. Aspirin is sufficient to help prevent epigastric artery thrombosis.
Lastly, it has been suggested that caution is warranted in patients who fail posterior urethroplasty for urethral injury from pelvic fracture, concomitant hypospadias, or penile stricture. As anastomotic urethroplasty is urethral advancement surgery, based on bipedal blood supply through the penis, it makes intuitive sense that ED, hypospadias, and synchronous penile stricture patients have compromised urethral retrograde blood supply. Thus, for posterior urethroplasty patients, I would suggest that you evaluate the penile vascular flow in these patients who suffer from ED and penile numbness or prior failed posterior urethroplasty. Those with arteriogenic impotence and distal reconstitution of the vessels would be reasonable candidates for revascularization before the urethroplasty, in order to prevent proximal urethral ischemia.
—Steven B. Brandes
References
Michal V. Arterial disease as a cause of impotence. Clin Endocrinol Metab. 1982;11:725–48.
El-Sakka AI, Morsy AM, Fagih BI, et al. Coronary artery risk factors in patients with erectile dysfunction. J Urol. 2004;172:251–4.
Levine FJ, Greenfield AJ, Goldstein I. Arteriographically determined occlusive disease within the hypogastric-cavernous bed in impotent patients following blunt perineal and pelvic trauma. J Urol. 1990;144:1147–53.
Machtens S, Gänsslen A, Pohlemann T, et al. Erectile dysfunction in relation to traumatic pelvic injuries or pelvic fractures. BJU Int. 2001;87:441–8.
Malavaud B, Mouzin M, Tricoire JL, et al. Evaluation of male sexual function after pelvic trauma by the international index of erectile function. Urology. 2000;55:842–6.
Mark SD, Keane TE, Vandemark RM, et al. Impotence following pelvic fracture urethral injury: incidence, aetiology and management. Br J Urol. 1995;75:62–4.
Rajfer J, Rosciszewski A, Mehringer M. Prevalence of corporeal venous leakage in impotent men. J Urol. 1988;140:69–71.
Iacono F, Barra S, de Rosa G, et al. Microstructural disorders of tunica albuginea in patients affected by impotence. Eur Urol. 1994;26:233–9.
Dalkin BL, Carter MF. Venogenic impotence following dermal graft repair for Peyronie’s disease. J Urol. 1991;146:849–51.
Ebbehoj J, Wagner G. Insufficient penile erection due to abnormal drainage of cavernous bodies. Urology. 1979;13:507–10.
Tsao CW, Lee SS, Meng E, et al. Penile blunt trauma induced veno-occlusive erectile dysfunction. Arch Androl. 2004;50:151–4.
Stief CG, Gall H, Scherb W, et al. Erectile dysfunction due to ectopic penile vein. Urology. 1988;31:300–3.
Michal V, Kramar R, Pospichal J, et al. Direct arterial anastomosis on corpora cavernosa penis in the therapy of erective impotence. Rozhl Chir. 1973;52:587–90.
Michal V, Kramer R, Hejhal L. Revascularization procedures of the cavernous bodies. In: Zorgniotti AW, Rossi G, editors. Vasculogenic impotence: proceedings of the first international conference on corpus cavernosum revascularization. Springfield: CC Thomas; 1980.
Virag R, Zwang G, Dermange H, et al. Vasculogenic impotence – a review of 92 cases with 54 surgical operations. Vasc Surg. 1981;15:9–17.
Furlow WL, Fisher J. Deep dorsal vein arterialization: clinical experience with a new technique for penile revascularization. J Urol. 1988;139:298A.
Hauri D. Possibilities in revascularization for vasculogenic impotence. Aktuel Urol. 1984;15:350–4.
Montague DK, Barada JH, Belker AM, et al. Clinical guidelines panel on erectile dysfunction: summary report on the treatment of organic erectile dysfunction. The American Urological Association. J Urol. 1996;156:2007–11.
Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174:230–9.
Ang LP, Lim PH. Penile revascularisation for vascular impotence. Singapore Med J. 1997;38:285–8.
DePalma RG, Olding M, Yu GW, et al. Vascular interventions for impotence: lessons learned. J Vasc Surg. 1995;21:576–84; discussion 584–5.
Jarow JP, DeFranzo AJ. Long-term results of arterial bypass surgery for impotence secondary to segmental vascular disease. J Urol. 1996;156:982–5.
Grasso M, Lania C, Castelli M, et al. Deep dorsal vein arterialization in vasculogenic impotence: our experience. Arch Ital Urol Nefrol Androl. 1992;64:309–12.
Munarriz R, Uberoi J, Fantini G, et al. Microvascular arterial bypass surgery: long-term outcomes using validated instruments. J Urol. 2009;182:643–8.
Munarriz RM, Yan QR, Nehra A, et al. Blunt trauma: the pathophysiology of hemodynamic injury leading to erectile dysfunction. J Urol. 1995;153:1831–40.
Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30.
Quam JP, King BF, James EM, et al. Duplex and color Doppler sonographic evaluation of vasculogenic impotence. AJR Am J Roentgenol. 1989;153:1141–7.
Chung WS, Park YY, Kwon SW. The impact of aging on penile hemodynamics in normal responders to pharmacological injection: a Doppler sonographic study. J Urol. 1997;157:2129–31.
Lue TF, Hricak H, Marich KW, et al. Vasculogenic impotence evaluated by high-resolution ultrasonography and pulsed Doppler spectrum analysis. Radiology. 1985;155:777–81.
Jarow JP, Pugh VW, Routh WD, et al. Comparison of penile duplex ultrasonography to pudendal arteriography. Variant penile arterial anatomy affects interpretation of duplex ultrasonography. Invest Radiol. 1993;28:806–10.
Naroda T, Yamanaka M, Matsushita K, et al. Clinical studies for venogenic impotence with color Doppler ultrasonography – evaluation of resistance index of the cavernous artery. Nihon Hinyokika Gakkai Zasshi. 1996;87:1231–5.
Munarriz R. Penile microvascular arterial bypass surgery: indications, outcomes, and complications. Sci World J. 2010;10:1556–65.
Hatzichristou D, Goldstein I. Penile microvascular arterial bypass: indications and surgical considerations. Surg Annu. 1993;25(Pt 2):207–29.
Shabsigh R, Fishman IJ, Toombs BD, et al. Venous leaks: anatomical and physiological observations. J Urol. 1991;146:1260–5.
Lue TF, Hricak H, Schmidt RA, et al. Functional evaluation of penile veins by cavernosography in papaverine-induced erection. J Urol. 1986;135:479–82.
Lund GO, Winfield HN, Donovan JF. Laparoscopically assisted penile revascularization for vasculogenic impotence. J Urol. 1995;153:1923–6.
Raynor MC, Davis R, Hellstrom WJ. Robot-assisted vessel harvesting for penile revascularization. J Sex Med. 2010;7:293–7.
Mueller SC, Lue TF. Evaluation of vasculogenic impotence. Urol Clin North Am. 1988;15:65–76.
Kim SH, Kim SH. Post-traumatic erectile dysfunction: Doppler US findings. Abdom Imaging. 2006;31:598–609.
Lue TF. Surgery for crural venous leakage. Urology. 1999;54:739–41.
Wespes E, Moreira de Goes P, Sattar AA, et al. Objective criteria in the long-term evaluation of penile venous surgery. J Urol. 1994;152:888–90.
Rahman NU, Dean RC, Carrion R, et al. Crural ligation for primary erectile dysfunction: a case series. J Urol. 2005;173:2064–6.
Virag R, Paul JF. New classification of anomalous venous drainage using caverno-computed tomography in men with erectile dysfunction. J Sex Med. 2011;8:1439–44.
Flores S, Tal R, O’Brien K, et al. Outcomes of crural ligation surgery for isolated crural venous leak. J Sex Med. 2011;8:3495–9.
Kim ED, McVary KT. Long-term results with penile vein ligation for venogenic impotence. J Urol. 1995;153:655–8.
Freedman AL, Costa Neto F, Mehringer CM, et al. Long-term results of penile vein ligation for impotence from venous leakage. J Urol. 1993;149:1301–3.
Berardinucci D, Morales A, Heaton JPW, et al. Surgical treatment of penile veno-occlusive dysfunction: is it justified? Urology. 1996;47:88–92.
Austoni E, Pisani E. Development and progress in the therapy of penile induration: 15 years’ experience. Arch Ital Urol Nefrol Androl. 1988;60:231–57.
Stief CG, Djamilian M, Truss MC, et al. Prognostic factors for the postoperative outcome of penile venous surgery for venogenic erectile dysfunction. J Urol. 1994;151:880–3.
Treiber U, Gilbert P. Venous surgery in erectile dysfunction: a critical report on 116 patients. Urology. 1989;34:22–7.
Rhee AC, Licht MR, Lewis RW. Microvascular arterial bypass surgery for erectile dysfunction. In: Graham SD, Keane T, editors. Glenn’s urologic surgery. 7th ed. Philadelphia/London: Lippincott, Williams & Wilkins; 2009.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Villalta, J.D., Lue, T.F. (2014). Vascular Surgery for Erectile Dysfunction. In: Brandes, S., Morey, A. (eds) Advanced Male Urethral and Genital Reconstructive Surgery. Current Clinical Urology. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7708-2_45
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7708-2_45
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7707-5
Online ISBN: 978-1-4614-7708-2
eBook Packages: MedicineMedicine (R0)