Abstract
Mesenchymal stromal/stem cells (MSCs) were originally isolated from bone marrow (BM) more than 40 years ago. The earliest clinical utilization of these cells was for their bone marrow supportive activity and repair of bone and cartilage. However, over the last decade, MSCs have generated a much higher level of enthusiasm and are now being investigated for a much wider range of clinical indications due to a multitude of tissue regenerative and immunomodulatory properties. For example, in vitro and in vivo data indicate that MSCs are not as immunogenic as other somatic cells, can modulate/suppress immunological responses through interactions with different immune cells, and participate in repair of different types of tissues through their paracrine effects, such as secretion of cytokines and growth factors. Although MSCs have not yet proven to be unequivocally effective for any specific indication, they have been kept at the forefront of cellular and regenerative medicine due to their ease of ex vivo production, their impressive record of safety in a wide variety of clinical scenarios, and their intriguing immunomodulatory properties that allow them to be used as a universal type of cell therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Systemic Lupus Erythematosus
- Hematopoietic Stem Cell
- Allogeneic MSCs
- Autologous MSCs
- Umbilical Cord Blood Unit
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The cells currently known as mesenchymal stromal/stem cells (MSCs), were first described by Friedenstein et al. as an adherent, fibroblast-like population of cells cultured from the bone marrow (BM) of rodents [1]. Through a series of elegant experiments, he showed that following implantation of these cells under the kidney capsule they generated rudimentary bone tissue that was capable of supporting hematopoiesis. Friedenstein’s method was later used to derive similar cells from human BM [2], and then these cells were shown to support hematopoiesis in long-term culture assays [3]. Later Caplan proposed these cells to be stem cells for mesenchymal tissues and proposed “mesenchymal stem cells” could have therapeutic potential [4] in regeneration of many tissues. MSCs are calculated to comprise only a small population (<0.01 %) of adult BM cells [5], and one of the major challenges in this field is the lack of any reliable or widely accepted marker for direct isolation of them from BM aspirates. Thus, although markers such as Stro-1 [6], CD271 [7], and CD146 [8] has been proposed for direct isolation of these cells, there is still no consensus on which marker is the most representative of cells present in their in situ BM environment. The low number of MSCs in BM also means that for any in vivo use, either experimental or clinical, the cells have to be expanded ex vivo through extensive cellular proliferation. MSCs from BM are most commonly isolated by plating BM mononuclear cells in culture plates and passaging the adherent cells, which, after a few passages, leads to expansion of a homogenously fibroblast-like population of cells [9]. According to the widely accepted criteria by International Society for Cellular Therapy (ISCT), such cells can be labeled as MSCs if they (a) express a certain set of markers (i.e., CD105, CD73, CD90) and do not express hematopoietic markers (i.e., CD45, CD34, CD14, CD11b, CD79a, CD19, and HLA-DR) and (b) could differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [10]. Although the end point of this derivation methodology is a seemingly homogenous population of cells, they are functionally heterogeneous and comprised of different subpopulations with different differentiation capabilities at a clonal level [11]. Thus, the term “mesenchymal stem cell” could be only applied to a defined population of MSCs that fulfill the criteria for being true “stem cells,” (i.e., to have the potential not only to self-renew themselves but also generate progenies that could differentiate into osteoblasts, chondroblasts, and adipocytes) [12]. Thus, in addition to the controversies surrounding the true in situ anatomical location and physiological role of these cells, there is also much controversy regarding their true stem cell properties [13]. This chapter discusses the use of ex vivo culture-expanded MSCs in different clinical settings, the rationale behind those approaches including their tissue regenerative and immunomodulatory properties, and unsolved issues and evolving concepts in this highly dynamic field.
Use of MSCs in Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell (HSC) transplantation was the first and is still the most common form of stem cell therapy. It is mainly indicated for the treatment of hematologic malignancies and nonmalignant conditions, such as immunodeficiency syndromes. For HSC transplantation, HSCs are harvested from BM, pharmacologically mobilized into blood followed by their collection, or more recently, HSCs from umbilical cord blood units are used to replace diseased HSCs of recipients (autologous or allogeneic). However, despite decades of clinical experience, HSC transplantation continues to be a high-risk procedure with a high rate of morbidity and mortality related to the toxic effects of pre-transplant preparative regimen, HSC graft failure and/or ejection, and immunologically mediated phenomenon of graft-versus-host disease (GVHD). MSCs are considered to be an essential constituent of the BM stromal microenvironment with an indispensable role in support of hematopoiesis [14]. Thus, not surprisingly, HSC transplant physicians were the first to use MSCs in a clinical setting. The Lazarus team was the first to conduct a phase I trial that showed the safety of intravenous infusion of ex vivo culture-expanded autologous human BM-derived MSCs [15]. These autologous MSCs generated from small-volume BM aspirates and expanded over several weeks were shown to be safe and not causing any adverse reactions. This, in addition to other pioneering studies by HSC transplant physicians, paved the way for the use of MSCs for treatment of a wide variety of other disorders in other disciplines of medicine.
A major goal of transplant physicians has always been to accelerate recovery of hematopoiesis after HSC transplantation. Due to the presumed role of MSCs in supporting hematopoiesis in the BM and evidence from animal studies in which co-transplantation of MSCs with HSCs improved the engraftment of the latter [16], one of the earliest indications for which MSCs were investigated was for their ability to promote hematopoietic engraftment. In a phase I–II clinical trial, ex vivo culture-expanded autologous MSCs were infused into breast cancer patients at the time of autologous HSC transplantation [17]. In another study culture-expanded allogeneic MSCs derived from BM of human leukocyte antigen (HLA)-identical sibling donors were infused to the respective allogeneic HSC transplant recipients [18]. Although these studies could not provide definitive conclusions regarding the engraftment-promoting effect of MSCs, they further reassured investigators that the use of culture-expanded autologous or allogeneic MSCs as a form of cellular therapy is feasible and safe. Since then autologous or allogeneic ex vivo culture-expanded BM-derived MSCs have been used in the context of HSC transplantation in a large number of patients with hematologic and non-hematologic malignancies for different purposes, such as reducing the risk of graft failure, preventing repeat rejections, or rescuing graft failure [19]. Most of these studies have provided encouraging results but have failed to prove their effectiveness in a clinically conclusive manner.
Aside from supporting HSCs, MSCs have also received much attention by HSC transplant physicians due to their effect on immune cells. MSCs have shown to modulate the immune responses in vitro and in vivo via their interactions with a plethora of immune cells. Such interactions lead to suppression of proliferation of activated T lymphocytes, an increase in the number of T regulatory lymphocytes, a decrease in activation and proliferation of B lymphocytes, suppression of cytotoxicity of natural killer cells, suppression of maturation of dendritic cells, modulation of neutrophil activities, and changes in the immunophenotype of macrophages [20–23]. One of the earliest of these effects to be discovered was the suppression of T cell proliferation and activation [23]. Moreover, this immunosuppressive effect of MSCs appears not to depend on the HLA compatibility status of the donor and recipient [24]. Since acute GVHD is a T cell-mediated process [25], years ago it was proposed that MSCs could be potentially used as a therapeutic modality, specifically for treatment-refractory GVHD [26]. Le Blanc et al. were the first to investigate the potential of MSC infusion for the treatment of refractory GVHD in a 9-year-old boy who had received a HLA-matched unrelated donor HSC transplant for leukemia [27]. Infusion of ex vivo expanded MSCs generated from the patient’s mother, not the original donor, resulted in the resolution of GVHD symptoms. This seminal report was followed by larger studies from the same group in which MSCs were given to steroid-refractory GVHD from HLA-identical siblings, haploidentical family donors, and unrelated mismatched donors [28, 29]. These studies showed that infusions of MSCs to this group of very sick patients are safe, resulted in a significantly better survival rate compared to control patients, and the responses were independent of the source and HLA compatibility of MSCs. The latter point is very important, as the generation of patient-specific MSCs is very time consuming, costly, and in many instances impractical due to the urgent nature of the need for their use.
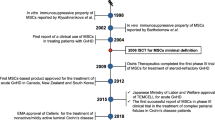
Since these original reports there has been a plethora of studies using BM-derived MSC in different doses and frequencies, made using different methodologies, and used in different age groups [19]. All these studies confirmed the original safety reports but with variably encouraging or successful results. The largest clinical trials performed with MSCs have been two Phase III double-blind, placebo-controlled, randomized trials evaluating (Prochymal) a proprietary formulation of MSCs derived from the marrow of a single third-party donor as a first-line treatment for acute GVHD or for the treatment of refractory acute GVHD. These double-blinded, placebo-controlled (in a 2:1 ratio) trials were designed to assess the safety and efficacy of Prochymal in multicenter international studies. To the surprise of most transplant physicians and investigators in the field, these studies could not show positive results in regard to their primary end points. Some potential confounding factors might have been the differences in treatment regimens administered to patients in conjunction with Prochymal in different centers. However, the use of the same Prochymal in pediatric patients with severe refractory acute GVHD resulted in more promising results [30]; indeed, this product indeed is now approved in Canada, but not yet in the United States, for pediatric patients with GVHD as the first form of “off-the-shelf universal stem cell therapy product.”
While MSCs have largely been used in the field of HSC transplantation for their ability to reconstitute the BM microenvironment and their immunomodulatory functions, another avenue for clinical use of MSCs is to utilize their tissue regenerative properties. Again in the field of hematology, these cells were used in patients who had a different spectrum of tissue and organ toxicities following allogeneic HSC transplantation, such as hemorrhagic cystitis, pneumomediastinum, and perforated colon and peritonitis with overall promising results [31]. Also, one of the earliest reported cases on use of culture-expanded, gene-marked MSCs was in conjunction with BM transplantation in six pediatric patients with osteogenesis imperfecta. This study, done based on the fact that MSCs are progenitors for osteoblasts, showed engraftment in five recipients and an acceleration of growth during the first 6 months post-infusion [32].
Use of MSCs in Non-hematopoietic Stem Cell Transplant Settings
Over the last decade, in addition to the field of HSC transplantation, MSCs have also generated a lot of excitement in other disciplines of medicine and surgery. Originally much of this enthusiasm was due to the assumption that these cells are capable of not only differentiating into mesenchymal tissues, such as bone and cartilage, but also transdifferentiating into many other types of cells, such as cardiomyocytes, hepatocytes, and pancreatic islets [33]. Although there were ample experimental and preclinical models that supported such assumptions, it was later realized that those early observations of such unorthodox plasticity were due to imperfect experimental tools, the use of animal models that were not representative of human biology, or other potential mechanisms such as cell fusion [34]. However, by then hundreds of patients had already been recruited into clinical trials based on those assumptions, many of them with preliminary promising results, albeit on a small scale and in nonrandomized formats. Nevertheless, by this time many new modes of action for these cells were discovered that initially were not appreciated. Indeed, the discovery of these new mechanisms of action was a major paradigm shift in the field and included tropism of MSCs for migration into sites of tissue damage or inflammation, their capability to support and stimulate proliferation and/or survival of resident tissue progenitor cells through secretion of a variety of cytokines and chemokines, and contribution to angiogenesis of tissues [35–38]. However, these newly discovered modes of action, in addition to their previously recognized immunomodulatory and anti-inflammatory properties, kept these cells at the forefront of use in many diverse groups of human pathologies. Indeed, there is a long list of phase I–II trials for a variety of non-hematological indications in which autologous, donor-directed, or third-party allogeneic MSCs had been used, including for treatment of patients with myocardial infarction, chronic obstructive pulmonary disease, amyotrophic lateral sclerosis, stroke, Crohn’s disease, diabetes mellitus, systemic sclerosis, systemic lupus erythematosus, and refractory wounds among others [39–42].
In many of these disorders, allogeneic third-party MSCs were used without any immunosuppression, as MSCs are assumed to escape attack by cytotoxic T cells or NK cells and thus could be transplanted over major histocompatibility complex barriers in humans [43, 44]. This has been the rationale for the use of “off-the-shelf” ex vivo culture-expanded BM-derived MSCs from “third-party” donors, and the myriad of clinical experiences that have confirmed the impressive safety of this product has been reassuring. Also, use of “off-the-shelf,” ready-to-use MSCs as a therapeutic entity, in contrast to production of patient-specific MSCs, is an important concept for large-scale production and commercialization of cellular therapeutics by biopharmaceutical entities [45].
MSCs Derived from Non-bone Marrow Origin
Although MSCs were originally isolated from BM, cells with similar phenotype, differentiation potential, and biological characteristics have now been derived from almost all adult tissues, including adipose tissue [46] and heart [47]; neonatal tissues such as placenta [48]; fetal tissues such as lung, liver, and blood [49]; and even embryonic stem cells [50]. Furthermore, it had been repeatedly shown that MSCs derived from non-BM tissues have immunomodulatory properties very similar to BM-derived MSCs [51–53]. Indeed, based on experimental data showing that AT-derived MSCs possess immunological characteristics similar to BM-derived MSCs, they have been used for treatment of GVHD after HSC transplantation [54]. However, adipose tissue MSCs are expected to find their highest clinical applications in the fields of plastic and reconstructive surgery [55]. Other types of MSCs that have reached the clinic include placenta-derived MSCs for the treatment of GVHD [56] and umbilical cord-derived MSCs for the treatment of severe and refractory systemic lupus erythematosus [57]. It should be no surprise to see other novel sources of MSCs to be tested for specific clinical settings based on their tissue of origin. For example, it could be argued that MSCs derived from pancreatic islets could be of more value for indications such as the protection of transplanted islets after cadaveric transplantation [58].
Unsolved Issues
In clinical medicine a confounding factor in interpreting results of clinical trials of pharmaceuticals is the heterogeneity of patient physiology, which affects the absorption, metabolism, and pharmacokinetics of the drug and the heterogeneity of the targeted diseases, which affects the potential responsiveness of the disease to the administered drug. However, in clinical trials of cellular therapeutics, particularly MSCs, another layer of complexity exists in the enormous heterogeneity in the final product (i.e., MSCs). MSCs are derived from tissues of autologous or allogeneic donors; therefore, from the very early stages of this process, there are numerous reasons for heterogeneity of the final product. The first issue is the appropriate donor source; uncertainties here include the fact that, theoretically and based on some evidence, the MSCs generated from tissues collected from the patients might not be the appropriate source, as these MSCs may be also afflicted by the patient’s disease processes [59]. However, it can be also argued that autologous MSCs could avoid issues such as tissue incompatibility and rejection. However, even in the case of normal healthy allogeneic donors, we do not know if there are donor-specific characteristics that make certain allogeneic donors potentially more suitable for the production of MSCs, such as donors of younger age or special physical attributes. Additionally, in the case of allogeneic MSCs, we still do not know of the appropriate tissue sources of MSCs that could be most effective for specific indications. It is very well known that MSCs from different tissue sources, despite seemingly similar phenotypes, could have significant differences in their functional characteristics. Nevertheless, we do not know the impact of these variables between different preparations of MSCs on the intended clinical outcome.
One other major challenge in the field is the fact that currently there is no standard culture methodology for generating MSCs [60]. It is well known that culturing cells at different densities and using different types of growth media (containing fetal bovine serum, serum-free media, autologous serum, fresh frozen plasma, or human platelet lysates) could affect the phenotype and rate of growth of MSCs. Even the batch of fetal bovine serum used could have a major impact on the end product. All these subtle differences could result in clinically significant changes in the ultimate biological characteristics of the MSCs. Other factors that add to the complexity of interpreting results of clinical trials are the different dosages and frequencies of cells from different passages. MSCs have been used at different doses and frequencies, from different passages, either fresh or frozen, given at different stages of disease, and given alone or with different combinations of other medications and treatments. A major challenge in harmonizing the culture conditions is the fact that although these variations in the culture methodologies could affect the immunomodulatory and regenerative properties of MSCs, there is no clinically applicable potency assay that is widely accepted for MSCs. For example, the same MSCs that are used for treatment of GVHD could be also used for treatment of heart or lung diseases [42]. However, the latter disorders are not T cell-dependent disorders, and other potency assays or functional analyses of MSCs for those diseases are probably more appropriate.
Evolving Concepts
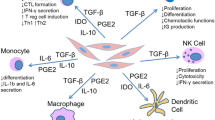
MSCs were originally promoted in the field of regenerative medicine for their potential to differentiate into many different types of cells and to replace lost or damaged cells based on a large body of literature in different animal models. However, these expectations were never realized in human studies. Indeed, despite seemingly encouraging positive results in numerous clinical trials, the durability of the infused MSCs is now a matter of great debate [61], and the new wave of studies report that the extent of MSC engraftment is usually very minimal and could not explain the observed clinical benefits [62]. Indeed, the lack of engraftability of BM MSCs has been well known to HSC transplant physicians for a long time, as most reports have shown that in BM transplant recipients, the MSCs remain of recipient origin and are not replaced by the donor MSCs carried in BM grafts [63, 64]. However, in these BM transplant scenarios, it could be argued that these cells were transplanted in low numbers and therefore were different from ex vivo culture-expanded MSCs. In any circumstance, there have been significant changes in our understanding of the potential mechanisms for MSC to exert their beneficial effects (Fig. 1). This new paradigm proposes that MSCs exert their beneficial effects through mechanisms such as paracrine effects prior to their demise. Thus, instead of “replacing” damaged cells, MSCs contribute to the “regeneration” of damaged cells and tissues indirectly, mainly via indirect paracrine effects. This low level of survival after administration of MSCs could also explain why repeated infusions of MSCs might be needed to achieve a clinical effect. This is in contrast to HSC transplantation, in which the administered HSCs usually engraft fully, and their beneficial effects depend on their persistent engraftment. It is ironic that one of the original attractive properties of MSCs was assumed to be their presumed lack of immunogenicity [43] and thus feasibility of transplanting across HLA barriers without the concern for rejection. However, it could be argued that lack of durability and persistence of MSCs, especially third-party MSCs, could be a desirable property of MSCs, as it could preclude any chance of tumorigenicity in the future.
A major question that has preoccupied the mind of clinicians and basic researchers alike is why these fibroblast-looking cells [65], derived mostly from BM, could have a therapeutic effect in such a wide range of conditions with seemingly unrelated pathophysiology, such as GVHD, myocardial infarction, heart failure, chronic obstructive pulmonary disease, Crohn’s disease, diabetes, osteoarthritis, systemic lupus erythematosus, kidney transplantation, stroke, nonhealing wounds, systemic sclerosis, cirrhosis, and others. A common pathophysiological theme for all these disorders is the contribution of “inflammation.” Now it is well known that a major way that MSCs exert their effects is through their interactions with macrophages, and this could provide a unifying rationale for the continued investigation of these cells in such a wide range of applications [66].
Conclusions
The original clinical trials pioneered by visionary HSC transplant physicians not only provided the initial safety data but also generated much excitement encouraging other disciplines of medicine to take advantage of the potential therapeutic effects of MSCs. A major factor in the expansion of MSC trials is the lack of any documented toxicity or long-term side effects and the unmet need for novel therapies for many degenerative diseases. Due to the inherent shortcomings of these small, nonrandomized clinical trials, a conclusive clinical benefit has been difficult to discern, and important questions remain to be addressed. However, although the final place of MSCs in regenerative biology is not clear, there is no other cell that has been applied to such a wide range of applications. There is a need for carefully designed clinical trials of sufficient size that examine the specific intended mechanism of action and the optimal source, dose, schedule, and route of administration. However, logistical considerations, including the cost for conducting such large clinical trials, are immense so a bedside-to-bench and back-to-bedside approach is also needed. Cell therapists are fascinated by MSCs as they are easy to produce according to good manufacturing guidelines, are not immunogenic but very safe to use, have multifaceted regenerative and immunomodulatory properties, and are therefore expected to remain very attractive to the field of regenerative medicine.
References
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47.
Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301.
Dexter TM. Stromal cell associated haemopoiesis. J Cell Physiol. 1982;1:87–94.
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5): 641–50.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7.
Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78(1):55–62.
Jones EA, Kinsey SE, English A, Jones RA, Straszynski L, Meredith DM, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–60.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Dominici M, Le BK, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10(4):320–30.
Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS One. 2009;4(8):e6498.
Bianco P, Robey PG, Saggio I, Riminucci M. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): a critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21(9):1057–66.
Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol. 2008;15(4):301–6.
Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–64.
Almeida-Porada G, Flake AW, Glimp HA, Zanjani ED. Cotransplantation of stroma results in enhancement of engraftment and early expression of donor hematopoietic stem cells in utero. Exp Hematol. 1999;27(10):1569–75.
Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–16.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–98.
Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11(5):503–15.
Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506.
Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445–53.
Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
Keating A. How do mesenchymal stromal cells suppress T cells? Cell Stem Cell. 2008;2(2):106–8.
Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57(1):11–20.
Barrett AJ, Le Blanc K. Prophylaxis of acute GVHD: manipulate the graft or the environment? Best Pract Res Clin Haematol. 2008;21(2):165–76.
Koc ON, Lazarus HM. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001;27(3):235–9.
Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10): 1390–7.
Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2011;17(4):534–41.
Ringden O, Uzunel M, Sundberg B, Lonnies L, Nava S, Gustafsson J, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21(11):2271–6.
Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5(3):309–13.
Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17(6):939–46.
Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11): 2896–902.
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101(8):2999–3001.
Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs). Clin Pharmacol Ther. 2007;82(3):241–3.
Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7(1):36–45.
Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 2007;109(8):3147–51.
Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211(1):27–35.
Cannon III RO, Dunbar CE. BM-derived cell therapies for cardiovascular disease. Cytotherapy. 2007;9(4):305–15.
Deda H, Inci MC, Kurekci AE, Sav A, Kayihan K, Ozgun E, et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy. 2009;11(1):18–25.
Taupin P. OTI-010 Osiris therapeutics/JCR pharmaceuticals. Curr Opin Investig Drugs. 2006;7(5):473–81.
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003; 31(10):890–6.
McKernan R, McNeish J, Smith D. Pharma’s developing interest in stem cells. Cell Stem Cell. 2010;6(6):517–20.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Lushaj EB, Anstadt E, Haworth R, Roenneburg D, Kim J, Hematti P, et al. Mesenchymal stromal cells are present in the heart and promote growth of adult stem cells in vitro. Cytotherapy. 2011;13(4):400–6.
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45.
In ‘t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur C, Kruisselbrink AB, van Bezooijen RL, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–52.
Hematti P. Human embryonic stem cell-derived mesenchymal progenitors: an overview. Methods Mol Biol. 2011;690:163–74.
Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36(3):350–9.
Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129(1):118–29.
Kim J, Breunig MJ, Escalante LE, Bhatia N, Denu RA, Dollar BA, et al. Biologic and immunomodulatory properties of mesenchymal stromal cells derived from human pancreatic islets. Cytotherapy. 2012;14:925–35.
Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39(10):3358–62.
Hanson SE, Gutowski KA, Hematti P. Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J. 2010;30(6):838–42.
Brooke G, Rossetti T, Pelekanos R, Ilic N, Murray P, Hancock S, et al. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol. 2009;144(4):571–9.
Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, et al. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19: 1502–14.
Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev. 2008;22(4):262–73.
Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, et al. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):741–9.
Samuelsson H, Ringden O, Lonnies H, Le Blanc K. Optimizing in vitro conditions for immunomodulation and expansion of mesenchymal stromal cells. Cytotherapy. 2009;11(2):129–36.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev. 2008;8(9):726–36.
von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicate limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–8.
Koc ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 1999;27(11): 1675–81.
Awaya N, Rupert K, Bryant E, Torok-Storb B. Failure of adult marrow-derived stem cells to generate marrow stroma after successful hematopoietic stem cell transplantation. Exp Hematol. 2002;30(8):937–42.
Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012;14(5):516–21.
Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science + Business Media New York
About this chapter
Cite this chapter
Hematti, P. (2013). Mesenchymal Stromal/Stem Cell Transplantation: From Tissue Regeneration to Immune Modulation. In: Sell, S. (eds) Stem Cells Handbook. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7696-2_27
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7696-2_27
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7695-5
Online ISBN: 978-1-4614-7696-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)