Abstract
With age, the ability to maintain organ homeostasis and cellular regenerative capacity diminishes. The stem cell theory of aging posits that this decline in homeostasis occurs as a result of functional depletion of the adult stem cell pool by death, injury, senescence, cell cycle arrest, or differentiation. The functionality of the adult stem cell pool depends upon conserved molecular pathways, which ensure the optimal balance between self-renewal, quiescence, and differentiation. In this chapter, we focus on molecular events that converge on a final common path through the tumor suppressor p53 and its downstream target, the cell cycle inhibitor p21Cip1/Waf1 (p21). Both intrinsic, nuclear defects such as unrepaired DNA damage or laminopathies that disturb the integrity of the nuclear membrane and chromatin, and extrinsic defects in the stem cell milieu that are transmitted to the stem cell by receptor-mediated signaling cascades, can activate p53 and turn on p21 transcription in stem cells. The increased load of p21 inhibits the regenerative potential of stem cells, limiting cell turnover, maintenance of tissue function, and, ultimately, life span.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The Stem Cell Theory of Aging
With age, the ability to maintain organ homeostasis and cellular regenerative capacity diminishes. The stem cell theory of aging posits that this decline in homeostasis occurs when stem cells can no longer maintain their functionality, as defined by two characteristics, self-renewal and potency. Self renewal refers to the ability of a stem cell to divide asymmetrically such that one daughter cell is an exact copy that retains stemness, while the other daughter becomes a progenitor cell that gives rise to rapidly dividing precursors, which will eventually differentiate and perform specific functions [1]. Potency refers to the ability of a stem cell to give rise to a range of differentiated cell types. According to the stem cell theory of aging, the functional depletion of the adult stem cell pool by death, injury, senescence, cell cycle arrest, or differentiation results in an inability to regenerate old or injured tissues, leading to the organ dysfunction commonly observed in aged individuals.
The functionality of the adult stem cell pool depends upon conserved molecular pathways, which ensure the optimal balance between self-renewal, quiescence, and differentiation. In this chapter, we focus on molecular events that converge on a final common path through the tumor suppressor p53 and its downstream target, the cell cycle inhibitor p21Cip1/Waf1 (p21). Both intrinsic, nuclear defects such as unrepaired DNA damage or laminopathies that disturb the integrity of the nuclear membrane and chromatin, and extrinsic defects in the stem cell milieu that are transmitted to the stem cell by receptor-mediated signaling cascades, can activate p53 and turn on p21 transcription in stem cells. The increased load of p21 inhibits the regenerative potential of stem cells, limiting cell turnover, maintenance of tissue function, and, ultimately, life span.
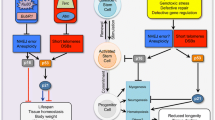
p21 and Stem Cells: The Goldilocks Effect
As the story goes, Goldilocks found the papa bear’s porridge too hot and the mamma bear’s porridge too cold, but the baby bear’s porridge was just right. Eating the porridge that was just right sustained and rejuvenated her. Similarly, stem cells require just the right amount of p21 for long-term regenerative capacity, as illustrated in Fig. 1. In the absence of p21, stem cells fail to maintain quiescence, resulting in hyper-proliferation and expansion of the progenitor cell population, followed by exhaustion of the stem cell pool. We discuss the essential role of p21 in maintaining stem cell quiescence in the next section. When stem cells accumulate excess p21, on the other hand, they can enter a state of prolonged or permanent quiescence, resulting in hypo-proliferation of stem and progenitor cells and exhaustion of differentiated progeny by attrition. We explore the consequences of having too much p21, a far more likely situation to arise during the normal course of aging, in a later section. Together, these examples illustrate how either too little or too much p21 can impair stem cell regenerative capacity and limit healthy life span.
Cell Cycle Arrest and the Maintenance of Stem Cell Quiescence by p21
Over the course of a lifetime, stem cell quiescence is critically important for the maintenance of tissue homeostasis and to prevent premature exhaustion of the stem cell pool under various conditions of stress [2]. p21, a member of the CIP/KIP family of cyclin-dependent kinase inhibitors, appears to play a significant role in the protection of the stem cell pool by ensuring cell cycle arrest during quiescence. In small amounts, p21 acts as a positive cell cycle controller in that it is actually necessary for formation of the cyclinD/CDK4 complex [3, 4] and transit from G0 into G1. When expressed in amounts beyond what is needed for complex formation, however, it has a universally inhibitory role by blocking the activity of cyclin-dependent kinases (CDKs) necessary for cell cycle progression [5–8]. It does this in several ways (reviewed in [9]), as illustrated in Fig. 2. p21 prevents CDKs from working with their respective cyclin to phosphorylate the Rb protein, thus blocking the release of the E2F transcription factor and subsequent transcription of the E2F responsive genes needed for transit from G0 into G1 and from G1 into S phase [10] (Fig. 2a, b, and d). p21 can also function as a cofactor with other DNA binding proteins, such as transcription factors, to control the expression of genes essential for cell cycle progression. For example, p21 can interact with E2F directly and block transcriptional activation of the cyclin A promotor [11] (Fig. 2c). p21 can also bind the mismatch repair factor DNA polδ, inactivating PCNA-mediated DNA replication [12] (Fig. 2e). In addition, p21 indirectly affects transit from G2 into M by binding to and inhibiting CDK-activating kinase (CAK), which phosphorylates CDK1 on Thr161 and activates the CDK1-cyclin B complex (Fig. 2f). Inhibition of CAK is crucial for G2/M checkpoint activation [13].
p21 and the cell cycle. (a) p21 is required for complex formation of Cyclin D/CDK 4/6. This complex participates in the partial phosphorylation of Rb, reducing its binding affinity with E2F and allowing transcription of genes such as Cyclin E. p21 in amounts greater than the minimum for complex formation has an inhibitory effect on the process. (b) p21 inhibits the Cyclin E/CDK2 complex from completing the phosphorylation of Rb. This allows transcription of the Cyclin A gene and the G1/S transition. (c) p21 associates with E2F, directly inhibiting the transcription of Cyclin A. (d) p21 inhibits Cyclin A/CDK2 complex, preventing transcription of E2F responsive genes. (e) p21 inhibits PCNA, blocking polδ from replicating damaged DNA. (f) p21 inhibits phosphorylation and activation of CDK1 by CAK. As a result, transcription of E2F responsive genes is inhibited, blocking the G2/M transition
Cell cycle control by p21 appears to play a particularly important role in stem cells. Studies on the hematopoietic stem cells (HSCs) and neural stem cells (NSCs) of p21-deficient mice provide convincing evidence of the protective role of p21 in the preservation of stem cell pools [14]. In the absence of p21, for example, the profile of hematopoietic cells in the adult mouse is maintained despite decreased cytokine-mediated proliferation of bone marrow progenitor cells [15–18], suggesting that p21 might play a dual role, simultaneously increasing progenitor cell proliferation while preventing stem cell proliferation. In a test of this hypothesis, Cheng et al. compared the time spent in G0 (quiescence) and G1 (cycling) phases of the cell cycle in stem cells from p21-deficient and wild-type mice. They found that p21-deficient HSCs spent less time in G0 and exhibited reduced repopulation capacity following 5-fluorouracil (5-FU) depletion of cycling bone marrow cells, as determined by cobblestone-forming assays (CAFC). Importantly, serial transplantation of p21-deficient bone marrow cells into lethally irradiated recipients resulted in greatly reduced survival compared to that conferred by normal bone marrow cells. Rather than remaining quiescent, p21-deficient stem cells prematurely differentiated, exhausting the pool of self-renewing stem cells and demonstrating the important role of p21 in maintaining HSC quiescence during times of stress.
Under steady state conditions, HSC quiescence appears to be maintained not by p21 but by growth factors, such as angiopoietin or thrombopoietin, which act through AKT or JAK/STAT pathways [19–21]. However, in NSCs, p21 appears to maintain quiescence under both steady state and stressed conditions. There are more NSCs in p21-deficient mice between postnatal days 60–240 than in their wild-type counterparts, and this is due to higher proliferation rates. At 16 months of age, however, NSC numbers drop in p21-deficient mice and display limited self-renewal in vitro, surviving only several passages before exhaustion [22]. This study highlights the contribution of p21 to the relative quiescence of adult NSCs, which might be more important than indefinite proliferation capacity for the life-long maintenance of NSC self-renewal.
By triggering cell cycle arrest and limiting transmission of damaged DNA, p21 can also protect against cell death [111], repressing caspases and other proteins needed for apoptosis [13] [23, 24]. In the case of cancer, this ability of p21 to repress apoptosis can maintain stem cells pools, with obvious deleterious consequences. In leukemogenesis, for example, p21 is actually critical for maintaining the leukemic stem cell pool [24]. A molecular model has been proposed in which the CDKN1A promoter is positively regulated by the tumor suppressors Miz and p53, which promote p21 expression and suppress apoptosis, and negatively regulated by the oncogene Myc (reviewed in [25]). When Myc is activated, it interacts with and suppresses Miz, blocking p53-mediated transactivation of CDKN1A and inducing apoptosis. Point mutations in Myc that render it unable to bind to Miz inhibit the induction of apoptosis in human fibroblasts [26]. When p21 was reexpressed in Myc-transformed cells, apoptosis was inhibited. When Zbtb4, a suppressor of Miz, was depleted in cell culture by siRNA, activation of p53 by vincristine promoted cell cycle arrest over apoptosis [27].
In summary, stem cells lacking p21 fail to maintain quiescence, resulting in expansion of the progenitor cell population and exhaustion of the stem cell pool. Cdkn1a-deficient mice exhibit deficits in HSC and NSC quiescence, leading ultimately to tissue deterioration and loss of function. Several tumor suppressors and oncogenes coordinately regulate the activity of the CDKN1A promoter, with potentially deleterious effects on the ability of the organism to limit the regenerative capacity of tumor stem cells. In the next section, we explore the consequences on stem cell regenerative capacity of having too much p21 and how that can limit healthy life span.
Chronic Activation of p21 and Aging
Events that can result in excess p21 in stem cells fall into two broad classes, nuclear damage and damage to receptor-activated signaling pathways. With age, DNA mutations and epimutations can accumulate, the result of defective repair processes or a compromised nuclear membrane, for example, and cause chronic activation of the p53–p21 axis. Experimental hyperactivation of p53 in mice gives rise to a progeroid syndrome closely resembling normal aging at an accelerated rate. Pharmacologic inhibition of downstream effects of activated p53 returns p21 levels to normal and reverses senescence in fibroblasts derived from these mice. Mouse models of Hutchinson-Gilford progeria, a human progeroid syndrome, which are driven by defects in nuclear lamins, can be rescued by eliminating p53, which simultaneously normalizes p21 levels in fibroblasts and prevents senescence.
Age can also damage the microenvironment resulting in altered intracellular signaling pathways. For example, fluctuating levels of TGFb in the systemic circulation, coupled with defective Notch mobilization in the muscle stem cell, can upset the balance between activation and suppression of p21 and compromise muscle repair. The various effects of age that can chronically activate p21 in stem cells or their environment are represented in Fig. 3 and discussed in more detail in the following sections.
Molecular mechanisms of p21 activation in aging stem cells. Cell intrinsic defects, such as the loss of nuclear architecture, which jeopardize the integrity of the genome or epigenome, activate p53, the principal means by which p21 is induced in stem cells. Surface receptors transduce extrinsic defects that affect the niche, such as a rise in systemic TGFb, to modulate critical intracellular pathways governing p21 expression. Both Wnt and Notch act in part through Myc, a transrepressor of the CDKN1A promoter.  , nuclear (intrinsic) defects.
, nuclear (intrinsic) defects.  , receptor-mediated environmental defects (“niche”)
, receptor-mediated environmental defects (“niche”)
Nuclear Defects and the p53: p21 Pathway in Stem Cells
Nuclear damage, including single- and double-stranded breaks, telomere shortening, chromosome rearrangements, excessive mitogenic signals from oncogenes, and damage by reactive oxygen species [28–32], can trigger activation of the tumor suppressor p53 and induce p21 expression, with powerful consequences on cell proliferation [33]. The p53–p21 pathway is one of the two pathways that can induce cellular senescence (the other is p16–Rb), a cell culture phenomenon first characterized by Hayflick and colleagues, who demonstrated that normal cells had a finite capacity to proliferate in culture [34]. At the end of their proliferative life span, cells permanently halt cell division and become resistant to cell death (reviewed in [35]). Senescent cells can exhibit senescence-associated β-galactosidase activation [36] and senescence-associated DNA damage foci (reviewed in [35, 37]) that can cause the loss of both potency and the ability to self-renew [38, 39]. We will discuss cellular senescence in the context of mouse models of progeroid syndromes that illustrate not only how induction of p21 by p53 can impair cellular regenerative capacity but also how constitutive activation of this pathway can limit organ homeostasis and life span.
Stabilization of p53 by D40p53 Causes Chronic Activation of p21
The first of these models was generated by introducing an ectopic, mutant allele of p53 that codes for a protein missing the first 40 amino acids of full-length p53 into a background of wild-type p53 [40]. This protein, Δ40p53, is one of several naturally occurring isoforms of p53 normally produced by alternate promoter usage or alternative splicing [41]. Δ40p53 is unique in that its primary mode of production is by alternative translation initiation at a start site in exon 4 immediately downstream of an internal ribosome initiation site (IRES) [42]. Full-length p53 initiates at a start site in exon 2, resulting in the addition of 40 amino acids at the N-terminus of the protein, which make up the primary transactivation domain and the overlapping binding site for Mdm2. Other than this, p53 and Δ40p53 are identical, including in the tetramerization domain, where p53 monomers interact to generate the tetrameric form of p53 that binds DNA and functions as a transcription factor. The absence of the N-terminal domain and the loss of the Mdm2 binding site in Δ40p53 contribute to its longer half-life compared to p53 [43] and the increased stability of p53 in heterotetramers with Δ40p53 [44, 45].
p44Tg mice have two normal p53 alleles that code for the full complement of p53 isoforms, as well as the ectopic allele on the transgene that codes for Δ40p53. The increased dosage of Δ40p53 in p44Tg mice leads to a progeroid syndrome characterized by a premature aging phenotype that can be observed as early as 4 months of age and results in an overall reduction in both mean and maximal life span by about 25% [40]. In addition, cells from p44Tg mice exhibit impaired proliferation, which results in fewer than the normal number of cells in adult organs as well as in embryos at all stages of development, and can be attributed at least in part to an increase in cellular senescence [40]. A model was proposed in which the extra dose of Δ40p53 in cells derived from p44Tg mice stabilized p53 and induced high levels of p21 [40], resulting in the state of permanent cell cycle arrest, resistance to apoptosis, and altered gene expression that defines cellular senescence [35].
This model was tested in NSCs and the effect of impaired proliferative capacity on their ability to contribute to the regenerative process of adult neurogenesis. Neurogenesis occurs throughout life in the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus of the mammalian brain [46, 47]. In the SVZ, stem cells generate neural precursor cells, which then go on to migrate along the rostral migratory stream (RMS) until they reach the olfactory bulb (OB) where differentiation into granule cells and periglomerular interneurons occurs [47–49]. In the SGZ, stem cells generate intermediate precursors that eventually undergo differentiation into granule cells in the dentate gyrus (DG) [46, 50–52]. The continuous generation of neurons from these stem cells throughout life is crucial for maintaining odor discrimination and for learning and memory, functions subserved by the OB and DG, respectively [53–56].
Using BrdU incorporation into replicating DNA as a marker of NSC proliferation, p44Tg mice were found to exhibit significantly reduced proliferative capacity in both progenitor cell and stem cell populations with age [57]. At 2–4 months of age, there were no differences in the number of labeled cells in the SVZ, but between 9 and 12 months, there was a significant decrease in the p44Tg mice that did not occur in the NT mice until much later (30 months, Medrano and Scrable, unpublished data). In neurosphere (NS) culture, SVZ cells from p44Tg mice gave rise to fewer and smaller NS that could be serially passaged fewer times compared to cells from age-matched NT mice. NS from both prematurely old p44Tg mice [57] and normally aged NT mice (30 months, Medrano and Scrable, unpublished data) exhibited significantly higher levels of activated (Ser15 phosphorylated) p53 and p21 compared to controls. This can account for the 37 % increase in the length of the cell cycle that characterized p44Tg NSCs.
Consequences of impaired NSC proliferation were seen at all stages of neurogenesis, from reductions in the number of migrating neuroblasts in the RMS and new neurons in the OB to reduced density in the granule cell layer as dead or damaged cells failed to be replaced. The final consequence was a pronounced decrease in olfactory acuity in p44Tg mice relative to normal mice [57]. Thus, the loss of proliferative capacity of neurogenic cells in the SVZ can be linked directly to loss of function in the region of the brain they supply with new neurons, a clear validation of the stem cell theory of aging.
Defects in Nuclear Lamins Activate p53 and Chronically Induce p21
Hutchinson-Gilford progeria syndrome (HGPS) is the result of a mutation in the gene coding for lamin A (LMNA 1824C>T) in 90% of cases [58, 59]. The nuclear lamina is vital to maintaining the shape and size of the nucleus and is instrumental in regulating fundamental processes such as DNA replication, transcription, and repair [60]. These processes are compromised by progressive changes in nuclear lamina morphology that occur as a consequence of normal aging [43] and can explain the inevitable accumulation of cells with unrepaired DNA damage [61]. HGPS has been partially recreated in mice by causing a deficiency in the gene coding for Zmpste24, a homolog of the zinc metallopeptidase STE24 found in yeast. STE24/Zmpste24 post-translationally cleaves prelamin A into mature lamin A, a necessary step in the generation of the nuclear lamina. Predictably, loss of Zmpste24 in mice led to profound abnormalities in the nuclear envelope, resulting in irregularly shaped nuclei with herniation-like blebs [62]. Similar defects have been shown to evoke a p53-mediated response, resulting in apoptosis or senescence [63]. And, in fact, Zmpste24-deficient mice exhibit increased numbers of senescent cells in tissues such as kidney and in cultured adult fibroblasts [64].
A second murine model of HGPS was generated by introducing a transgene expressing the most common LMNA mutation (1824C>T) under the control of a tet-inducible promoter [65]. Here, too, senescence was evident, this time in epidermal skin sections, consistent with depletion of adult epidermal stem cells and loss of regenerative capacity as early as 13 weeks after induction of mutant lamin A expression [66]. In addition to increased SA-b-galactosidase activity [36], isolated keratinocytes exhibited increased numbers of g−H2AX foci, evidence of increased unrepaired DNA double-stranded breaks [66].
In normal cells, recruitment of the DNA repair machinery to sites of double-stranded breaks is facilitated by acetylation of lysine 16 on histone H4 (H4K16), which converts the chromatin to a more relaxed state [67]. This chromatin modification is carried out by the histone acetyltransferase MOF in association with lamin A in the nuclear matrix [68]. Like keratinocytes from mice with inducible mutant lamin A expression, mouse embryonic fibroblasts (MEFs) from Zmpste24-deficient mice exhibit defective DNA repair. In the presence of excess prelamin A, there is reduced binding of MOF to the nuclear matrix and hypoacetylation of H4K16 [69].
The observation that cells from healthy elderly humans also exhibit nuclear abnormalities, prelamin A accumulation, and unrepaired DNA damage links progeroid laminopathies like HGPS and their mouse models to normal aging [70–72]. The same cryptic splice site that is used constitutively in Hutchinson-Gilford progeria to generate mutant lamin A (Δ50 lamin A or progerin)[58, 59] is used sporadically in old cells from normally aging humans [70]. Fibroblasts from individuals ranging in age from 81 to 96 years resembled cells from patients with HGPS, with increased numbers of g-H2AX foci at sites of unrepaired DNA damage, mislocalization of lamin A at the nuclear periphery, and nuclear abnormalities. Among the targets affected by abnormal lamin A processing in cells from normally aging elderly individuals, p21 was significantly increased. Inhibition of the cryptic splice site that gives rise to progerin using a morpholino oligonucleotide reversed these age-related defects, returned p21 levels to normal, and restored proliferative capacity [70].
Other Examples of Nuclear Changes That Increase p21 in Stem Cells
Aging affects processes in the chromatin such as DNA methylation and posttranslational modifications of histones, both of which are now thought to be reversible [73]. The epigenome is greatly affected by extrinsic factors, like diet, and intrinsic factors, such as double-stranded DNA breaks [74–76]. Increased DNA methylation, much of it at CpG islands in gene promoters, has been observed in intestinal, colon, and mesenchymal stem cells (MSCs) from old humans and mice [77–79]. Changes in histone methylation with age appear to be tied closely to the self-renewal and proliferation of stem cells because of their effects on the expression of cell cycle inhibitors, like p21. Polycomb group (PcG) and trithorax group (TrxG) complexes catalyze methylation of specific lysine residues on histones, resulting in repression or activation of gene expression, respectively [80, 81]. PcG and TrxG have been linked to organismal and stem cell aging [82, 83]. One such PcG that has been extensively studied is BMI1 can repress p21. Acute reduction of this protein by shRNA knockdown caused p21-mediated defects in adult mouse NSC self-renewal [82]. In human HSCs, loss of BMI1 affected the ability of HSCs to retain multi-potency by causing premature differentiation [84].
In summary, nuclear changes that occur with normal aging or with premature aging syndromes, such as HGPS, support the stem cell theory of aging. Zmpste 24-deficient mice exhibit osteoporosis, growth retardation, and premature death [62, 85], as well as reduced BrdU incorporation and cell proliferation and defects in cell cycle profiles [64], a phenotype very similar to that of p44Tg mice [40]. As with p44Tg mice, these defects are associated with activation of p53 and upregulation of p53 target genes, such as Cdkn1a [64]. p21 also appears to be a critically important target of aberrant lamin A splicing in normally aged cells, where it is associated with unrepaired DNA damage and reduced proliferation [70–72]. Loss of the chromatin modifier BMI1 in aging stem cells causes derepression of the CDKN1A gene promoter and increased expression of p21, resulting in defects in NSC and HSC self-renewal. Collectively, these examples highlight the central role of the cell cycle inhibitor p21 in mediating the effects of nuclear damage on the ability of cells, particularly stem cells, to maintain tissue homeostasis and healthy aging. That it is, in fact, an axis that acts through p53 to turn on p21 in affected cells is brought into even sharper focus by the finding that the phenotypes of both Zmpste24-deficient and p44Tg mice, including the increase in p21, are significantly rescued in the absence of p53 [64].
Receptor-Mediated Transmission of Defects in the Microenvironment to Stem Cells
In addition to intrinsic changes to stem cells, such as breakdown of the nuclear membrane or impaired chromatin structure, age has important consequences on the availability of soluble ligands for several key signaling pathways, as illustrated in Fig. 3. We focus on one ligand, TGFb, which directly and indirectly controls p21 expression in stem cells, and two major pathways, Wnt and Notch, that modulate this activity of TGFb in stem cells. Notch blocks the binding of the TGFb effector Smad to the CDKN1A promoter, suppressing p21 expression. As Notch levels decrease with age, suppression is lost and p21 levels go up. On the other hand, TGFb counteracts the effects of an age-associated increase in Wnt levels by blocking transactivation of the MYC gene by the Wnt effector β-catenin. As systemic TGFb levels go up, increased suppression of Myc, which transrepresses the CDKN1A promoter, indirectly results in increased p21 expression. Thus, increased TGFb in the systemic “niche,” combined with intrinsic defects in Wnt and Notch pathways in stem cells, results in impaired stem cell proliferation and regenerative capacity as the organism ages.
Decreased Notch Signaling Releases the Block on p21 Transcription Induced by TGFb
Notch is a transmembrane protein that is activated by contact of its extracellular domain with the extracellular domain of a transmembrane protein of the Delta family expressed on the surface of a second cell. Although the Delta–Notch pathway is known primarily for its role in specifying cell fate during development, it is also the critical mediator of muscle regeneration following injury [75, 86–91]. Notch is expressed on the surface of muscle stem cells (satellite cells), and Delta is expressed on both stem cells and myofibers [92], which make up the stem cell niche in adult muscle. With age, regenerative capacity is lost due to decreased Notch signaling in satellite cells, which in turn has been linked to reduced Delta expression in old muscle [86].
The interaction of a cell expressing Delta with a cell expressing Notch leads to cleavage of Notch into a transcriptionally active intracellular domain, which acts as a nuclear transcription factor for a number of genes, including MYC [93]. Myc acts as a pro-proliferative signal in part by suppressing expression of p21 [94]. Thus, in old satellite cells, one consequence of decreased Notch activation is reduced transrepression of the CDKN1A promoter by Myc, and elevated levels of p21. A second, and perhaps more significant consequence is on stem cell proliferation, which requires antagonism of TGFb-dependent upregulation of CDK inhibitors, including p21, by phosphorylated SMAD3. Young satellite cells display high levels of active Notch, which blocks binding of SMAD3 to the p21 promoter [95]. In old satellite cells with reduced Notch activity, this block is removed. Furthermore, p53 synergizes with SMAD3 to coordinately regulate the CDKN1A promoter, which requires p53 for full transcriptional activation [96]. The increase in p53 with age, as described in the previous section, along with systemic increases in TGFb, which occur in older mice and humans [97], would serve to increase promoter activation. In old stem cells, then, decreased Notch activity leads to increased p21 expression by both an indirect mechanism (reduced transrepression by Myc) and a direct mechanism (increased transactivation by SMAD3 and p53), the latter a direct consequence of age-dependent increases in the level of TGFb in the circulation.
In addition to these effects on p21 transcription, Notch and p53 also exert reciprocal effects on each other’s signaling pathways (reviewed in [98]) that are not only sensitive to age but also can help to explain some of their age-associated defects. For example, one of the targets of Notch is MDM2, which binds to and ubiquitinates p53 [99], resulting in its proteasomal degradation. Decreased Notch signaling with age would result in less Mdm2-mediated p53 degradation and stabilization of the protein, which could help to explain the increases in p53 levels seen in aging cells. On the other hand, one of the targets of p53 is PSEN1, the gene encoding the catalytic component of g-secretase. g-secretase is the enzyme that cleaves Notch following its interaction with Delta, releasing the transcriptionally active Notch intracellular domain. p53 can suppress presenilin-1 expression by competing with Ets transcription factors for binding to the PSEN1 promoter [100] or by binding to the CDKN1A promoter and inducing p21 expression [101–103]. Like p53, p21 is a negative regulator of presenilin-1 transcription [103, 104]. One result of age-associated increases in p53 and/or the p53–p21 axis would be decreased expression of presenilin-1, decreased catalytic activity of g-secretase, and decreased nuclear Notch activity. This in turn could account for the relative inactivity of Notch signaling seen in old satellite cells compared to young [86].
TGFb Can Induce p21 Transcription Even in the Presence of Increased Wnt Signaling
Wnt is a glycoprotein signaling molecule that binds to the Frizzled receptor and activates the transcription of cell cycle promoting genes, such as c-Myc, by β-catenin [105]. In an environment of increased Wnt, both intestinal crypt progenitor cells [106] and HSCs [107] are rapidly exhausted, presumably by an intrinsic mechanism of increased suppression of p21 by Myc. As stem cells require some p21 to maintain quiescence, as described in a previous section, too much Wnt, like too little p21, can cause premature reentry into the cell cycle and deplete the stem cell pool.
As the individual ages, however, systemic increases in TGFb exert powerful extrinsic effects on the ability of the Wnt pathway to suppress p21 by Myc. Upon activation of the TGFb receptor, SMAD3 associates with the corepressor p107 in the cytoplasm, and the complex translocalizes to the nucleus, where it binds to and transrepresses the Myc promoter [108]. As Myc is a negative regulator of p21, repression of Myc by increases in systemic TGFb with age would cause p21 levels to go up. This can explain why MSCs exposed to serum from old rats exhibited both higher Wnt signaling and higher levels of p21 compared to cells exposed to young rat serum [109].
Although the upstream mediators of increased Wnt in the stem cell environment are not known, one possibility is suggested by a study of murine embryonic stem cells (ESCs), where genotoxic and non-genotoxic insults induced p53-dependent expression of five different Wnt ligands [110]. The age-associated increased in activated p53 seen in animal models of accelerated aging, such as that described for p44Tg and Zmpste24-deficient mice above, might be one factor contributing to an environment in which stem cells are exposed to increased levels of Wnt. The recent finding that, in an environment of increased Wnt, there is activation of the p53–p21 axis in MSCs [109] suggests another way a niche factor could induce senescence and compromise stem cell function, in this case by a mechanism that is still unknown.
Conclusions
A hallmark of aging is the loss of regenerative potential. The stem cell theory of aging posits that regenerative potential is maintained by stem cells, and that tissue homeostasis is compromised when stem cells fail. p21 is a universal cell cycle inhibitor that appears to be a principal regulator of the stem cell pool throughout adult life. Too little p21 and stem cell quiescence is lost resulting in premature exhaustion of the stem cell pool. Too much p21 and the self-renewal capacity of the stem cell is lost resulting in a state of permanent mitotic arrest that functionally depletes the stem cell pool. Optimal life span requires just the right amount (Fig. 1).
References
Knoblich JA. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol. 2001;2(1):11–20.
Arai F, Suda T. Quiescent stem cells in the niche. Cambridge, MA: Harvard Stem Cell Institute; 2008.
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11(7):847–62.
Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, et al. The p21(Cip1) and p27(Kip1) CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18(6):1571–83.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–16.
Gu Y, Turck CW, Morgan DO. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature. 1993;366(6456):707–10.
Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366(6456):701–4.
Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6(4):387–400.
Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704(1–3):12–20.
Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–12.
Delavaine L, La Thangue NB. Control of E2F activity by p21Waf1/Cip1. Oncogene. 1999;18(39):5381–92.
Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994;369(6481):574–8.
Smits VA, Klompmaker R, Vallenius T, Rijksen G, Makela TP, Medema RH. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 2000;275(39):30638–43.
Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–8.
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377(6549):552–7.
Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82(4):675–84.
Mantel C, Luo Z, Canfield J, Braun S, Deng C, Broxmeyer HE. Involvement of p21cip-1 and p27kip-1 in the molecular mechanisms of steel factor-induced proliferative synergy in vitro and of p21cip-1 in the maintenance of stem/progenitor cells in vivo. Blood. 1996;88(10):3710–9.
Braun SE, Mantel C, Rosenthal M, Cooper S, Liu L, Robertson KA, et al. A positive effect of p21cip1/waf1 in the colony formation from murine myeloid progenitor cells as assessed by retroviral-mediated gene transfer. Blood Cells Mol Dis. 1998;24(2):138–48.
Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–61.
Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–97.
Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1(6):671–84.
Kippin TE, Martens DJ, van der Kooy D. p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 2005;19(6):756–67.
Garner E, Raj K. Protective mechanisms of p53-p21-pRb proteins against DNA damage-induced cell death. Cell Cycle. 2008;7(3):277–82.
Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–14.
Viale A, De Franco F, Orleth A, Cambiaghi V, Giuliani V, Bossi D, et al. Cell-cycle restriction limits DNA damage and maintains self-renewal of leukaemia stem cells. Nature. 2009;457(7225):51–6.
Herkert B, Eilers M. Transcriptional repression: the dark side of myc. Genes Cancer. 2010;1(6):580–6.
Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature. 2002;419(6908):729–34.
Weber A, Marquardt J, Elzi D, Forster N, Starke S, Glaum A, et al. Zbtb4 represses transcription of P21CIP1 and controls the cellular response to p53 activation. EMBO J. 2008;27(11):1563–74.
Itahana K, Zou Y, Itahana Y, Martinez JL, Beausejour C, Jacobs JJ, et al. Control of the replicative life span of human fibroblasts by p16 and the polycomb protein Bmi-1. Mol Cell Biol. 2003;23(1):389–401.
Wahl GM, Carr AM. The evolution of diverse biological responses to DNA damage: insights from yeast and p53. Nat Cell Biol. 2001;3(12):E277–86.
Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602.
Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy JM. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol Cell. 2004;14(4):501–13.
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–25.
Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36.
Campisi J, d’Adda di fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22.
Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7(4):590–8.
Seita J, Asakawa M, Ooehara J, Takayanagi S, Morita Y, Watanabe N, et al. Interleukin-27 directly induces differentiation in hematopoietic stem cells. Blood. 2008;111(4):1903–12.
Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, et al. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18(3):306–19.
Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, et al. p53 Isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19(18):2122–37.
Ray PS, Grover R, Das S. Two internal ribosome entry sites mediate the translation of p53 isoforms. EMBO Rep. 2006;7(4):404–10.
Rovinski B, Munroe D, Peacock J, Mowat M, Bernstein A, Benchimol S. Deletion of 5′-coding sequences of the cellular p53 gene in mouse erythroleukemia: a novel mechanism of oncogene regulation. Mol Cell Biol. 1987;7(2):847–53.
Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U, et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21(44):6722–8.
Yin Y, Stephen CW, Luciani MG, Fahraeus R. p53 stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat Cell Biol. 2002;4(6):462–7.
Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301(3):365–81.
Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149(2):111–4.
Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93(25):14895–900.
Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11(1):173–89.
Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56(2):337–44.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–7.
Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–60.
Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–6.
Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6.
Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–84.
Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–5.
Medrano S, Burns-Cusato M, Atienza MB, Rahimi D, Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2009;30(3):483–97.
De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300(5628):2055.
Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423(6937):293–8.
Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, et al. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22(7):832–53.
Lans H, Hoeijmakers JH. Cell biology: ageing nucleus gets out of shape. Nature. 2006;440(7080):32–4.
Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet. 2002;31(1):94–9.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–10.
Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, et al. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437(7058):564–8.
Sagelius H, Rosengardten Y, Hanif M, Erdos MR, Rozell B, Collins FS, et al. Targeted transgenic expression of the mutation causing Hutchinson-Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. J Cell Sci. 2008;121(Pt 7):969–78.
Rosengardten Y, McKenna T, Grochova D, Eriksson M. Stem cell depletion in Hutchinson-Gilford progeria syndrome. Aging Cell. 2011;10(6):1011–20.
Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Mol Cell Biol. 2007;27(20):7028–40.
Kapoor-Vazirani P, Kagey JD, Powell DR, Vertino PM. Role of hMOF-dependent histone H4 lysine 16 acetylation in the maintenance of TMS1/ASC gene activity. Cancer Res. 2008;68(16):6810–21.
Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proc Natl Acad Sci U S A. 2011;108(30):12325–30.
Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312(5776):1059–63.
McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2(12):e1269.
Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121(20):2200–10.
Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–8.
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273(10):5858–68.
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4.
Fontana L, Partridge L, Longo VD. Extending healthy life span: from yeast to humans. Science. 2010;328(5976):321–6.
Yatabe Y, Tavare S, Shibata D. Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci U S A. 2001;98(19):10839–44.
Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20(3):332–40.
Liu L, Rando TA. Manifestations and mechanisms of stem cell aging. J Cell Biol. 2011;193(2):257–66.
Ringrose L, Paro R. Polycomb/trithorax response elements and epigenetic memory of cell identity. Development. 2007;134(2):223–32.
Buszczak M, Spradling AC. Searching chromatin for stem cell identity. Cell. 2006;125(2):233–6.
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–7.
Siebold AP, Banerjee R, Tie F, Kiss DL, Moskowitz J, Harte PJ. Polycomb repressive complex 2 and trithorax modulate drosophila longevity and stress resistance. Proc Natl Acad Sci U S A. 2010;107(1):169–74.
Iwama A, Oguro H, Negishi M, Kato Y, Morita Y, Tsukui H, et al. Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity. 2004;21(6):843–51.
Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, et al. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci U S A. 2002;99(20):13049–54.
Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–7.
Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122(5):659–67.
Collins CA, Partridge TA. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle. 2005;4(10):1338–41.
Morgan JE, Gross JG, Pagel CN, Beauchamp JR, Fassati A, Thrasher AJ, et al. Myogenic cell proliferation and generation of a reversible tumorigenic phenotype are triggered by preirradiation of the recipient site. J Cell Biol. 2002;157(4):693–702.
Schultz E, Lipton BH. Skeletal muscle satellite cells: changes in proliferation potential as a function of age. Mech Ageing Dev. 1982;20(4):377–83.
Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–10.
Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409.
Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106.
Claassen GF, Hann SR. A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta-induced cell-cycle arrest. Proc Natl Acad Sci U S A. 2000;97(17):9498–503.
Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454(7203):528–32.
Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, Piccolo S. Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with smads. Cell. 2003;113(3):301–14.
Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, et al. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8(6):676–89.
Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9(8):587–95.
Beverly LJ, Felsher DW, Capobianco AJ. Suppression of p53 by Notch in lymphomagenesis: implications for initiation and regression. Cancer Res. 2005;65(16):7159–68.
Pastorcic M, Das HK. Regulation of transcription of the human presenilin-1 gene by ets transcription factors and the p53 protooncogene. J Biol Chem. 2000;275(45):34938–45.
Amson R, Lassalle JM, Halley H, Prieur S, Lethrosne F, Roperch JP, et al. Behavioral alterations associated with apoptosis and down-regulation of presenilin 1 in the brains of p53-deficient mice. Proc Natl Acad Sci U S A. 2000;97(10):5346–50.
Roperch JP, Alvaro V, Prieur S, Tuynder M, Nemani M, Lethrosne F, et al. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat Med. 1998;4(7):835–8.
Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005;19(12):1485–95.
Lohr K, Moritz C, Contente A, Dobbelstein M. p21/CDKN1A mediates negative regulation of transcription by p53. J Biol Chem. 2003;278(35):32507–16.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–80.
Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–6.
Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7(10):1037–47.
Chen CR, Kang Y, Siegel PM, Massague J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell. 2002;110(1):19–32.
Zhang DY, Wang HJ, Tan YZ. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One. 2011;6(6):e21397.
Lee KH, Li M, Michalowski AM, Zhang X, Liao H, Chen L, et al. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci U S A. 2010;107(1):69–74.
van den Bos C, Silverstetter S, Murphy M, Connolly T. p21(cip1) rescues human mesenchymal stem cells from apoptosis induced by low-density culture. Cell Tissue Res. 1998;293(3):463–70.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science + Business Media New York
About this chapter
Cite this chapter
Scrable, H., Ashrafzadeh-Kian, S. (2013). Stem Cells and Aging. In: Sell, S. (eds) Stem Cells Handbook. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4614-7696-2_25
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7696-2_25
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4614-7695-5
Online ISBN: 978-1-4614-7696-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)