Abstract
Recurrent brain tumors present a daunting task when recalcitrant to surgical resection, chemotherapy, and radiosurgery. Alternative therapies are desirable when standard neuro-oncologic means are exhausted. Since the 1960s, lasers have been used in medical care at varying levels for the purpose of biomodulation; this includes laser-induced tissue stimulation, coagulation, cutting, and ablation. Here, we discuss a thermography-guided laser ablation technique, representing a novel approach to resistant brain tumors. A growing body of knowledge is accumulating evidence regarding laser-induced thermal ablation techniques for visceral-based tumors. We share our experience employing this novel technique towards recurrent central nervous system tumors with intraoperative MR guidance and discuss current indications and future considerations towards this alternative therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Laser Fiber
- Thermal Ablation
- Burr Hole
- Recurrent Central Nervous System
- Proton Resonance Frequency Shift
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Tumors of the central nervous system may prove resistant to standard of care treatment modalities, including surgical resection and therapeutic ionizing radiation (whole brain or stereotactic radiosurgery). This suggests a niche for therapies that employ a means of novel tissue ablation through minimally invasive approaches. Limitations to open reoperations such as difficult access or eloquent locations may make surgical risks prohibitive. Overall volume and anatomical location as well may also bar radiosurgical approaches because of the risk of significant morbidity.
Minimally invasive techniques with the use of stereotaxy and neuroendoscopy allow surgical access to lesions with these limitations in mind but with presumably less risk compared to open approaches. Unfortunately, new hurdles arise with these methods, such as inadequate tumor excision and limited tumor access. Stereotaxy alone, either via fluoroscopy, computed tomography, or magnetic resonance imaging, provides initial lesion localization. However, after the intracranial space is penetrated, causing brain shift, there is limited control of subarachnoid and ventricular spaces, lack of residual tumor estimation/visualization, and no immediate postoperative imaging prior to surgical closure. Here, intraoperative magnetic resonance imaging (iMRI) allows a minimally invasive approach that overcomes many of these limitations. Such imaging of soft tissue (tumors and brain) allows for better control of brain shift, residual tumor estimation/visualization, improved accuracy of fluid space localization, and immediate postoperative imaging prior to closure.
A combination of thermodynamic ablation through minimal surgical access creates a novel approach to brain tumors that persist or recur after other treatments. Laser interstitial thermal therapy (LITT) delivers light-diffused energy (heat) through a silicon (MR-compatible) tipped fiber. Thermal ablation through LITT enables real-time feedback through a circulating cooling saline solution that allows both the tumor and surrounding parenchyma to be concomitantly imaged with quantitative thermal MR maps. This real-time feedback allows for the safe application of higher levels of energy in shorter amounts of time than has been used in the past through radiofrequency, cryoablation, or neodymium:yttrium-aluminum-garnet laser therapies [1, 2].
History of Thermal Ablation and Neuroscience
“Mod lyset” (towards the light in Danish) models three individuals: two females kneeling next to a man in whom all three were found to be gazing to the sun as if asking for help or taking in as much light could be captured. This statue by Rudolph Tegner was created outside the laboratory in Copenhagen to commemorate Niels Finsen’s (the man in the statue) prominent introduction of phototherapy in medicine, specifically for the treatment of lupus vulgaris (Nobel Lectures, Physiology or Medicine 1901–1921, Elsevier Publishing Company, Amsterdam, 1967). His discovery of tissue destruction after concentrated UV light suggested the possibility of the biomodulation of soft tissue tumors, and for this Finsen received in 1903 the Nobel Prize in Physiology or Medicine.
Since then, several contributions towards the theory and engineering of an operational laser progressed to 1960 when the first working laser was created. In 1983, Bown described laser interstitial thermal therapy (LITT) and its tissue-destroying properties, followed by Jolesz who described this effect in terms of MR imaging in 1988 [3–5]. Currently, a recent report of a commercially available system for real-time thermodynamic ablation in intracranial tumors suggested the approach to be minimally invasive and safe [1]. Here in this chapter, we describe the use of intraoperative MRI guidance in combination with stereotaxy for real-time LITT ablation of CNS tumors and review our initial experience.
Real-Time Magnetic Resonance and Interstitial Laser Therapy
Imaging of in situ tumor ablation is based upon quantitative thermal maps, generated in real time, for the purpose of visualizing tissue destruction and a priori estimation of ablative zones. We have worked with the Visualase system for LITT (Visualase, Houston TX). The therapeutic laser fiber is embedded in a silicone applicator that is surgically placed in the operating theater. The fiber itself is surrounded by cooling cables that run saline solution, continuously providing real-time thermal feedback that can be measured and used for pictorial estimation of tissue ablation. This estimation combines quantitative measure of cooling the fiber itself and adjacent tissue. A thermal map is generated based upon proton resonance frequency shifts and is linearly dependant on change in temperature and time of each gradient echo-based voxel; employment of the Arrhenius model here allows visualization of an estimated cell death [1]. Heat propagated by the laser fiber emanates from the tip in an ellipsoid volume. The fiber is not steerable, and a target volume can be “filled in” by moving the laser fiber in or out through the applicator. Like the use of intraoperative MRI for intraoperative confirmation of adequate applicator positioning, the real-time observation of thermal ablation allows the surgeon to manipulate the laser fiber without applicator adjustment for the purpose of multiple additive ablations. Our goal is to take the guesswork out of the procedure.
Patient Selection
We offer patients LITT therapy when CNS tumors, typically metastatic but certain primary lesions as well, prove recalcitrant to surgical, radiation, and chemotherapy measures. Patients with metastatic tumors should have at most three tumors, with a diameter of 3 cm or less, and a Karnofsky Performance Status of 70 or greater (i.e., they should be capable of self-care). At this point, we have not used LITT to treat patients with high-grade gliomas, as these tumors usually progress despite aggressive local therapies.
Surgical Planning and Operative Procedure
We employ the combination of stereotaxy and intraoperative MRI to ensure accurate placement of the laser fibers before any thermal ablation begins. In the operating room, all patients undergo general endotracheal anesthesia and are positioned supine, prone, or in the lateral decubitus position as needed. Patient positioning is crucial for (1) surgical placement of laser fibers, (2) transport to the MR suite for thermal ablation, and (3) length of time of the overall combination of procedures. Issues such as adequate airway protection and dependant edema associated with positioning must be considered. We also take into account adequate positioning within the magnet bore for best imaging of the surgical target during the LITT procedure itself.
We have championed the use of Medtronic PoleStar N-20 0.15 T intraoperative magnet (Medtronic, Louisville, CO) for image guidance in our intracranial surgical cases. We have found the use of this low-field magnet particularly useful for full operative procedures [6, 7] and more recently for LITT. The system allows for surgical ease by operating with standard surgical tools around this low-field magnet that sits under a regular OR table. The magnet gantry is raised to image the target as needed. While images are not diagnostic quality, they are adequate for such purposes as tumor resection control and for fine adjustments while performing stereotactic biopsies [8]. For laser fiber placement, we prefer to use iMRI so that correct fiber placement can be ensured before the patient is transported for the actual LITT procedure (which cannot be completed in the 0.15 T iMRI, whose field strength is not sufficient for thermography).
After positioning the magnet under the OR table, the patient’s head is secured in the dedicated MRI-compatible clamp. A flexible MRI receiving coil is positioned to allow for imaging during the procedure. A preoperative T1-weighted MRI with contrast is acquired to image the lesion. Image fusion between this scan and a pre-op stereotactic MRI may be done. A navigation plan from the scalp to the lesion was created. Although we prefer to use a single laser fiber to treat the entire tumor volume, when lesion size or geometry mandates, more than one fiber is placed.
After a standard prep and drape, either a navigated twist-drill opening or a burr hole is created. In the former case, a plastic skull bolt is screwed in place, and the laser fiber inserted to the appropriate depth. When a burr hole is chosen, a skull-mounted guide (Navigus, Medtronic Navigation) is placed. The trajectory is aligned and the laser inserted. This latter technique is used when targets in especially critical locations are chosen (e.g., the brainstem). IMRI is repeated to confirm laser fiber location. Skull-mounted system is covered with either bacitracin ointment or Betadine-soaked sponges before transfer to diagnostic MRI for LITT.
Interventional Technique
Here in the MR unit, we transferred patients to the MR table and secured the patients in appropriate positions to fit the closed gantry of the 1.5 T MR unit. At this point test, doses with laser heating was performed with Visualase safety constraint parameters in place. If this was achieved safely, we commenced therapeutic phases. Generally we chose dosing averaged at 7.5 W of energy with 60 °C at the margin of the tumor. The laser is withdrawn as needed to complete growth of the thermal lesion. Real-time thermal maps were constantly generated and viewed during ablation. When the entire tumor appeared to have been treated, a final contrast-enhanced MR was performed. This last sequence typically demonstrated a well-ablated tumor with only a shell/rim of enhancement usually seen after LITT treatment.
In sterile fashion, the fiber(s) was withdrawn and skin incision closed in typical fashion. The patient was reversed from GETA and awoken in the MR suite or returned to the OR for removal of the Navigus device and closure of the burr hole incision. At this point, patients were transported to the Neuro-ICU for observation. After surgery, patients are maintained on glucocorticoid medication until it is clear that vasogenic edema from the tumor and/or the LITT procedure has resolved. Repeat imaging is done 6 weeks after LITT and then at 3 months intervals, barring a clinical change.
Case Illustration
A 42-year-old woman had been diagnosed with breast cancer 8 years before. She had been diagnosed with intracranial metastases nearly 2 years earlier, including a tumor in the pons. She had received whole brain radiation therapy and hypofractionated stereotactic radiosurgery to the pontine tumor.
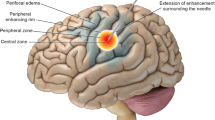
MRI showed progressive enlargement of the brainstem tumor (Fig. 43.1). The patient developed ataxia and diplopia because of this lesion and was referred for LITT. Surgery was done under general anesthesia in the prone position. A left suboccipital burr hole was made, and the tumor was stereotactically targeted. Using a skull-mounted navigation device, a biopsy cannula was placed within the lesion. Figure 43.2 contains images from intraoperative MRI that show placement of the cannula, followed by the fiber, accurately within the lesion.
The patient was then transferred to a 1.5 T MRI for LITT. Tumor ablation maps from the procedure are shown in Fig. 43.3. To complete tumor lysis, the laser fiber was progressively withdrawn until 3 LITT treatments were done. MRI immediately after LITT showed an absence of contrast enhancement, consistent with tumor ablation (Fig. 43.4). The patient initially had a slight increase in her ataxia after surgery but recovered within several weeks. She died of progressive systemic disease 10 months after LITT.
Our Experience
We have used LITT to treat six patients with intracranial tumors and one with a recurrent ependymoma of the conus region (see Table 43.1). In all cases, the patient was seeking alternative opinions after multiple surgical resections had been attempted and lesion recurrence observed after multiple chemotherapy and radiation therapies tried. Nonetheless, most patients referred to our protocol included those with multiple intracranial metastases. However, patients with other recurrent CNS tumors have been referred as well (Table 43.2). Intraoperative MRI was used for surgical navigation and fiber placement confirmation in 5/7 patients (including the lone spinal tumor patient). In 3/5 cases, iMRI was used to improve laser placement. This includes one patient in whom an initial attempt with surgical navigation alone to place two lasers to treat a right parietal metastasis had to be aborted after the lasers were off target when imaged at 1.5 T. The repeat procedure with iMRI proved successful.
Thermal ablation was successfully completed in all patients but one. This 69-year-old man had undergone two prior resections for an ependymoma of the fourth ventricle and medulla and prior radiation therapy. He had slowly worsening lower cranial nerve function and MRI showing tumor progression. Laser fiber placement was difficult because of his prior craniectomies, and a suboptimal amount of laser energy was delivered to the tumor.
Discussion
Intraoperative MR guidance as an adjunct to the placement laser fibers for LITT ablation has allowed for a more precise, minimally invasive approach to refractory intracranial tumors. Laser ablation had been applied to intracranial tumors in the pre-MR era, but the ability to monitor the hyperthermic effects of treatment via real-time thermography has led to a revival in the technique. These thermal generated maps allow for sharper borders and seemingly more efficacious tumor destruction in one attempt as compared to alternative means, including radiofrequency and cryoablation. As LITT remains a surgical procedure, with accurate laser fiber placement as the sine qua non, iMRI is the most accurate current method for ensuring this criterion is met.
The procedure should only improve with the addition of more MR-compatible surgical equipment and the employment of intraoperative high-field MR unit. This would allow for the entire procedure to be completed in the OR, saving the time, hassle, and cost of transport to and use of a diagnostic imaged situated in the radiology department. As the experience with LITT increases and neurosurgeons grow more comfortable with the method, the treatment of patients with functional disorders – epilepsy, pain, and movement disorders – may also become a reality.
Conclusions
Intraoperative MR guidance in combination with stereotaxy ensured adequate placement of laser fibers prior to commencing thermodynamic ablation. This minimally invasive approach has been safe and technically feasible. LITT, a technique of delivering nonionizing energy, may become a regular part of the treatment options for patients with intracranial tumors for whom other methods have not yielded long-term success.
References
Carpentier A, McNichols RJ, Stafford RJ, et al. Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery. 2008;63(1 Suppl 1):ONS21–8; discussion ONS28–9.
Carpentier A, McNichols RJ, Stafford RJ, et al. Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med. 2011;43(10):943–50.
Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700–9.
Jolesz FA, Bleier AR, Jakab P, Ruenzel PW, Huttl K, Jako GJ. MR imaging of laser-tissue interactions. Radiology. 1988;168(1):249–53.
Menovsky T, Beek JF, van Gemert MJ, Roux FX, Bown SG. Interstitial laser thermotherapy in neurosurgery: a review. Acta Neurochir (Wien). 1996;138(9):1019–26.
Schulder M, Salas S, Brimacombe M, et al. Cranial surgery with an expanded compact intraoperative magnetic resonance imager. Technical note. J Neurosurg. 2006;104(4):611–7.
Azmi H, Biswal B, Salas S, Schulder M. Functional imaging in a low-field, mobile intraoperative magnetic resonance scanner: expanded paradigms. Neurosurgery. 2007;60(1):143–8; discussion 148–9.
Quinn J, Spiro D, Schulder M. Stereotactic brain biopsy with a low-field intraoperative magnetic resonance imager. Neurosurgery. 2011;68(1 Suppl Operative):217–24; discussion 224.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Vadivelu, S., Schulder, M. (2014). MRI-Guided Interstitial Laser Therapy of Brain Tumors. In: Jolesz, F. (eds) Intraoperative Imaging and Image-Guided Therapy. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7657-3_43
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7657-3_43
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7656-6
Online ISBN: 978-1-4614-7657-3
eBook Packages: MedicineMedicine (R0)