Abstract
Racial minorities are known to have more severe disease and poorer outcomes throughout the US health care system. Accumulating evidence suggests that black race/ethnicity is associated with lower clinical pregnancy and live birthrates following assisted reproductive technology (ART) even after adjusting for many confounders. It is likely that some combination of genetic factors, environmental influences, socioeconomic status, and behavioral differences contributes to such disparities in ART outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Racial disparities are pervasive throughout the US health care system, as evidenced by minorities having more severe disease and poorer outcomes [1, 2]. The obstetric literature reports higher rates of maternal mortality, increased perinatal and neonatal mortality, and increased incidence of low and very low birth weight neonates among African Americans [3–6]. It is therefore reasonable to question the impact of racial disparity in gynecology, and more specifically, infertility and assisted reproductive technology (ART) outcomes.
Infertility is a major public health issue affecting more than six million women in the United States [7]. The number of ART clinics has been increasing steadily (from 300 clinics in 1995 to 361 clinics in 2008) as has the number of ART cycles being performed (from 59,142 cycles in 1995 to 140,795 cycles in 2008) [8, 9]. Despite increasing use of ART, black women in the United States have experienced an increase in the prevalence of infertility at the same time that infertility is decreasing among white women [10]. The population-based rates of 12-month infertility determined by the National Survey of Family Growth in 1982 and 2002 were 7.8 and 11.6 %, respectively, for black women and 11.6 and 7.1 %, respectively, for white women [10]. Among women seeking infertility treatment, black women are significantly different with regard to socioeconomic position [11, 12] and marital status [12]. They have a higher prevalence of some risk factors for infertility such as uterine fibroids [13], tubal disease [11, 13], and excess weight [14, 15]. In addition, the proportion of ART cycles provided for black and white women indicates that there was a racial disparity in the use of ART services in the United States at the beginning of the twenty-first century. The US census data for the general population in the year 2000 showed 12.9 % of the population to be black and 75.1 % to be white [16, 17]. While black and white women made up 7.8 and 72.1 %, respectively, of married, reproductive-age women in the United States during 2002 [10], there were 3,666 (4.6 %) cycles among black women and 68,607 (85.4 %) cycles among white women during 1999–2000, demonstrating underrepresentation of black women in this national dataset [18]. These social, environmental, and anatomic factors most likely play a significant role in infertility disparities among black compared to white women. In addition, these and other factors are likely responsible for the growing evidence in the literature suggesting racial differences in ART outcomes.
Disparities in Infertility Care Access and Utilization
A growing number of studies have investigated the association between race/ethnicity and ART outcomes. The first US study of racial determinants of ART outcomes was published in 2000 by Sharara and McClamrock [15], studying black and white women seeking care at a university-based program in an insurance-mandated state. Women were excluded from analysis if they had specific anatomic predictors of poor outcome (hydrosalpinges and intracavitary lesions) or if they had biochemical evidence of diminished ovarian reserve (FSH of 11 IU/L or greater). Multiple cycles per women were studied. Compared with whites, black women were more likely to have a diagnosis of tubal factor infertility (P < 0.001), had higher mean BMI (P = 0.038), and were more likely to require microdose Lupron Flare protocol during stimulation (P = 0.012). On average, black women had 1.3 more years of infertility before treatment than whites (P = 0.016). The groups were comparable by age, day-3 FSH levels, cycle cancellation rate, and multiple embryologic predictors of pregnancy (number of oocytes retrieved, number of embryos transferred). Black women had significantly lower implantation and clinical pregnancy rates per cycle than white women (implantation rate 9.8 % in blacks vs. 23.4 % in white women, P = 0.0005; clinical pregnancy rate 19.2 % in blacks vs. 42.2 % in white women, P = 0.009). The ongoing pregnancy rate per cycle (a pregnancy beyond 20 weeks gestation or a live birth) was also significantly lower in black than in white women (14.9 % vs. 38.8 %, P = 0.005) [15]. The significant effect of race on ART outcomes in this study was likely mediated in part by differences in BMI and duration of infertility, which were not controlled in the analysis, making it difficult to determine the strength of race as an independent predictor of ART treatments. Conducting the study in a state with an insurance mandate to cover infertility services is a specific strength of this study, minimizing the impact of socioeconomic factors on ART outcomes. Indeed, 28 % of the patients treated in the study were black, significantly more than the reported proportion of black women receiving ART treatments nationwide [13]. However, despite the presence of a mandate, there was still a racial disparity in the duration of infertility before ART was initiated suggesting relative underuse of ART treatments by black women. This difference confirms the growing literature showing that insurance mandates have not been able to completely bridge the racial gap in access to infertility services [9, 11, 19, 20]. For example, a survey of over 500 women attending a fertility clinic in Massachusetts demonstrated that, even in a state with mandated insurance coverage for infertility services, those seeking services are predominantly white, highly educated, and wealthy [19].
To explore the reasons why those populations of women with the greatest need for infertility care do not seek them out even when available, Missmer et al. [9] conducted a survey among 743 women receiving infertility care at a university-based fertility center in a mandated state. Compared with whites, African-American women had been attempting to conceive for 20 months longer (P < 0.0001). African-American women also found it more difficult to find a physician with whom they felt comfortable, to get an appointment with a physician, to take time off from work for their appointment, and to pay for treatment (P < 0.0001). In addition, they reported that it was more difficult to get treatment specifically because of their race or ethnicity (P < 0.0001) or income level (P < 0.0001). Compared with white women, African-American women were three to four times more likely to be concerned about using science to conceive, the social stigma of infertility, and disappointing their spouse. Specifically, the social stigmatization of infertility was of great concern to African-American women as they were 6.6 times more likely to be concerned about friends and family finding out about their infertility treatment compared with white women [9]. Data from this survey suggest that there are cultural factors in addition to known pelvic pathology (i.e., tubal and/or uterine factor) that most likely contribute to lower and/or delayed use by nonwhite women of infertility care.
Feinberg et al. used a unique approach to control for the influence of limited health care access and other social factors on ART outcomes. The investigators compared ART utilization and outcomes in black and white women within the military system, where improved access to care would be expected given the full access to diagnostic modalities and greatly reduced cost of IVF regardless of economic status or military rank. They examined a total of 1,457 patients undergoing first-cycle fresh, nondonor ART [13]. In this equal-access-to-care setting, 17.4 % of the women studied were black: a fourfold increase in use compared with the US ART population. Black and white women were comparable with respect to age, day-3 FSH levels, amount of gonadotropin administered during stimulation, peak estradiol levels, number of mature oocytes retrieved, and number of embryos transferred. However, black women were nearly three times more likely than white women to have leiomyoma as a stated cause of infertility (odds ratio [OR] 2.85, P < 0.0001) and nearly twice as likely to be diagnosed with tubal factor infertility (OR 1.91, P < 0.0001). In an analysis that adjusted for the presence of fibroids (an independent predictor of outcomes in this series), the association between race and ART outcomes (clinical pregnancy rates, spontaneous miscarriage rates, and live birthrates) was not significant [13]. While using a very specialized population such as the military enables to minimize the impact of limited health care access and other social factors on ART outcomes, it compromises the generalizability of findings to other groups of women. The investigators also acknowledge that sample size limitations may have hindered the ability to detect subtle differences in treatment outcomes by race [13]. It is possible that other investigations have also been limited by sample size constraints despite valid methodologic approaches. Of the four studies that found no association between race and ART outcomes the sample sizes ranged from 251 to 1,135 subjects, and none were as large as the Feinberg study, which showed no association [13, 21–23].
Studies Based on Society for Assisted Reproductive Technology (SART) Database
As this area of investigation has evolved, larger datasets have been examined allowing a more thorough evaluation of ART outcomes in multiple racial/ethnic groups. Specifically, studies using data from the national registry of ART cycles in the United States collected by the Society for Assisted Reproductive Technology (SART) and maintained by the Centers for Disease Control and Prevention have circumvented issues of limited sample size and potential type two errors. As these reports have relied on registry data, a unique set of limitations in the interpretation of results must be considered. Specific information regarding socioeconomic status and other potential confounders such as BMI and direct measures of embryo quality is not uniformly available. The units of measure to determine treatment outcome must also be taken into consideration: registries track total number of cycles completed, which allows for individual patients to be represented in the dataset more than once, thus creating potential repeated-measure bias. Women who choose to repeat cycles when previous attempts have failed may be different than women who stop trying in ways that relate both to the risk factor of interest (race/ethnicity) and the outcome (pregnancy). Finally, in a recent systematic review of publications that used SART data from years ranging from 1999 to 2007, it was found that more than 35 % of cycles could not be used for comparisons of racial/ethnic groups and reproductive outcomes because the data on race/ethnicity were lacking [24], raising concern for a potential selection bias.
Five large database studies have recently noted consistent findings indicating racial/ethnic differences in ART outcomes between black and white women [18, 25–28] (Table 5.1). One of the first of these studies examined 80,390 nondonor cycles (both fresh and frozen) from SART for the years 1999 and 2000 [18]. Additional inclusion criteria included cycles from clinics that performed at least 50 ART cycles annually and reported race >95 % of the time. Of the 80,390 cycles evaluated, 3,666 were among black women (4.6 %), 68,607 were among white women (85.4 %), and 8,036 (11.9 %) were among women of other races and ethnicities. Only outcomes in blacks and whites were compared. In this series, black women had a greater duration of infertility before ART than white women (40 vs. 34 months for those having their initial cycle and 48 vs. 36 months for women who had previously undergone ART [P < 0.001]). This translated to older mean ages for black women at treatment than for white women. When analyzing all cycles in the dataset, black women were less likely to experience a live birth per cycle of ART initiated compared with whites after controlling for age, parity, diagnosis, and clinic factors. It was also noted that the overall live birthrate per fresh nondonor cycle for black women (18.7 %) was below the lower 95 % confidence interval (CI) for the rate among all races in the United States during 1999–2000. In contrast, the live birthrate among whites (26.3 %) was above the 95 % CI for all races nationally (P value for difference in live birthrates between blacks and whites, <0.001). When restricting the analysis to patients receiving their first ART cycle, black women were 24 % less likely to experience a live birth than whites when adjusting for the same confounders (P < 0.001). Black women who had been treated with ART previously were 38 % less likely to have a live birth per cycle (P < 0.001). There were no racial differences in live birth when comparing frozen embryo transfer cycles [18].
In a follow-up study, Seifer et al. [25] investigated trends in ART outcomes in black and white women by comparing SART database outcomes for 2004–2006 with previously reported outcomes for 1999–2000. A total of 158,693 nondonor IVF cycles were analyzed. The proportion of cycles in which black women were treated for the first time increased from 5.4 % in the 1999–2000 series to 8.4 % in the 2004–2006 series (P < 0.001) as did the proportion of cycles for black women with previous ART cycles (4.6–7.1 %, P < 0.001). However, trends in ART outcomes were significantly worse for black women over time, representing an expansion of the disparity demonstrated in the analysis of 1999–2000 SART data. This widening of the gap in treatment outcomes may be explained in part by worsening of prognostic indicators in black women over time. The proportion of black women undergoing ART for the first time who were over 35 years old increased in the 2004–2006 assessment (57.9 %) compared with the 1999–2000 assessment (49.9 %) (P < 0.001). In addition, the proportion of black women treated for the first time with the diagnosis of diminished ovarian reserve nearly doubled between these time points (7.5 % in 1999–2000 vs. 14.4 % in 2004–2006, P < 0.001). Significant upward trends were also noted in the diagnosis of unexplained infertility and uterine factors in black patients (P < 0.0001) [25]. In accordance with these trends was a plateau in the likelihood of clinical pregnancy and live birth per cycle of ART in black women. The live birthrate per cycle initiated in black women (first cycle of ART) in 1999–2000 was 20.7 % compared with 22.2 % in 2004–2006 (P = 0.19). In contrast, trends in white women showed improvements in both clinical pregnancy (first-cycle ART clinical pregnancy rate/cycle of 33.6 % in 1999–2000 vs. 38.3 % in 2004–2006, P < 0.001) and live birthrate over time (first-cycle ART live birthrate/cycle 28.4 % in 1999–2000 vs. 32.3 % in 2004–2006). In the 2004–2006 assessment, black women treated for the first time were 31 % less likely to achieve a live birth than white women (adjusted relative risk 1.31, 95 % CI 1.26–1.37). Compared to the adjusted relative risk of first-cycle treatment failure for blacks compared to whites in the 1999–2000 series (1.24) this updated result represents a significant widening gap in treatment outcome. Black women who had ever had prior ART treatment were 33 % less likely than whites to achieve a live birth (P < 0.001), which was comparable to the risk in the 1999–2000 assessment (adjusted relative risk 1.38). Finally, a 10 % lower adjusted odds of live birth after transfer of cryopreserved embryos was noted in black women compared with white women, a difference which was not demonstrated in the 1999–2000 cycle data analysis [18]. This analysis raises concern of a growing disparity in ART outcomes over time.
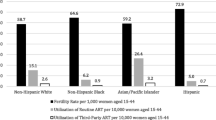
Additional studies have used SART to investigate disparities in ART outcomes. Fujimoto et al. have investigated ART outcomes in multiple racial/ethnic groups based on SART registry data [26]. A total of 139,027 nondonor ART cycles between 2004 and 2006 were assessed. Outcomes were compared between white, black, Asian, and Hispanic women. Compared with the referent group of white women, all other groups had lower live birthrates adjusting for maternal age, number of embryos transferred, and infertility diagnosis (P < 0.0001). Black women (8,903 cycles, 6.5 % of total cycle number) had similar clinical pregnancy rates as white women but were 38 % less likely to achieve a live birth. All ethnic groups studied had significantly higher miscarriage/stillbirth rates than white women (P < 0.0001) [26]. Baker et al. have published the largest evaluation to date of ART outcomes based on SART registry data [28]. Their study population included 225,889 fresh nondonor ART cycles between 2004 and 2006 in multiple racial/ethnic groups. Compared with white women all other racial/ethnic groups were less likely to achieve a clinical intrauterine pregnancy. Black women had significantly lower rates of clinical intrauterine gestation compared with white women (32.2 % vs. 40.5 %, P < 0.0001). Compared with white women, Hispanics and Asians had a significantly greater risk of pregnancy loss in the second and third trimesters, and blacks had a significantly greater risk of pregnancy loss in all trimesters (P < 0.0001). In addition, black women had significantly decreased live birthrate/pregnancy compared with white women (75.1 % vs. 83.7 %, P < 0.0001). In an attempt to eliminate the effects obesity may have on racial disparities in reproductive outcomes following ART, Luke et al. examined a total of 31,672 ART cycles from the SART database in 2007 and stratified them according to patient BMI categories [27]. Within BMI categories, there were substantial racial and ethnic disparities, with black women significantly more likely to have adverse treatment and pregnancy outcomes. Among normal-weight women, the adjusted odds ratio (AOR) of failure to achieve a clinical intrauterine gestation was 1.18 for black women (1,954 cycles, 6.0 % of total cycle number) compared with the reference group of white women, although this difference did not reach statistical significance (P = 0.10). Compared with white women, the AOR of failure to achieve a live birth was 1.45 for black women (P = 0.04). These racial differences in ART outcomes were more pronounced in the obese population. Compared with obese white women (BMI > 30), the AOR of failure to achieve a clinical intrauterine gestation and the AOR of failure to achieve a live birth were 1.47 (P < 0.0001) and 1.84 (P = 0.002), respectively, for obese black women [27].
Despite the acknowledged limitations of these large studies, there is a consistent finding of race/ethnicity as a risk factor for poor ART outcomes after adjusting for many confounders. While environmental, socioeconomic status, behavioral, and anatomic factors are likely contributors to disparities in ART outcomes between black and white women, significant differences still remain even when these factors are controlled for, suggesting that genetic factors may also play an important role.
Possible Genetic Factors and Art Outcome Disparities
Seifer et al. reported a significant difference in the mean level of anti-Mullerian hormone (AMH), a surrogate marker of ovarian aging, as a function of race or ethnicity [29]. After controlling for age, BMI, smoking, and HIV status, black women had lower average AMH values compared with white women (25.2 % lower, P = 0.037). This study presented the first biochemical evidence of a difference in ovarian aging between black and white women. Implications of these findings may have potential broad applications for the life planning of minority women. As such, this information may influence minority women and their physicians to seek/provide infertility treatment earlier if pregnancy is not easily accomplished.
Another genetic risk factor which is likely related to racial disparities in ART outcome is estrogen receptor alpha polymorphism. The estrogen receptor (ER) plays an important role in mediating estrogen action on target tissues. Two subtypes of ER are known, ER-alpha encoded by the ESR1 gene on chromosome 6 [30] and ER-beta encoded by the ESR2 gene on chromosome 14 [31]. ER-alpha, the first identified and the most abundant, is found in all human reproductive tissues. The overall prevalence of the ER-alpha PvuII homozygous (PP) genotype is significantly higher in black women (35 %) than white (13 %) or Hispanic (16 %) women [32]. The higher prevalence of ER-alpha PP genotype in blacks has been associated with the increased occurrence of uterine leiomyoma [32], a well-known poor prognostic factor in ART. Independent of the association with uterine fibroids, Georgiou et al. found ESR1 PvuII polymorphism in women to affect pregnancy rate following IVF [33]. Sundarrajan et al. corroborated their findings [34]. In a study of 200 IVF patients who had normal cycles with unexplained infertility despite extensive workup, PvuII polymorphisms were evaluated and correlated with ART outcomes. The pregnancy rate showed a strong negative correlation to the severity of PvuII polymorphism, being highest in the no polymorphism group (pp) and lowest in the homozygous (PP) group (88.9 % vs. 14.7 %, P < 0.001) [34]. These studies suggest a role for ER-alpha polymorphism in poor ART outcomes in black women. The ER gene could underlie variable responses to estrogens, from fetal to adult follicular growth and differentiation. ESR variability may also suggest a variability in the rate of germ cell depletion throughout reproductive life.
Vitamin D levels have been shown to be lower in black compared with white women [35]. Causes related to lower vitamin D levels include deeper skin pigmentation, decreased exposure to sunlight, and obesity. Low vitamin D levels have been associated with reduced pregnancy rates following IVF [36], suggesting that vitamin D may be a factor contributing to racial disparities in ART outcome. Interestingly, AMH has been shown to correlate with vitamin D levels [37, 38], providing further support for a biological role for vitamin D in ovarian reserve and ART outcome.
Gleicher et al. reported that the distribution of fragile X mental retardation (FMR1) genotypes varies between Caucasian, African, and Asian women [39]. Based on a normal range of 26–34 (median 30) CGG repeats the authors used CGG counts on the two X chromosome alleles to define whether a genotype is normal (norm), heterozygous (het), or homozygous (hom). An individual was defined as norm when both alleles were within range, het by one allele outside, and het-norm/low or het-norm/high, depending on the abnormal count allele being above or below normal range. Both alleles outside range defined hom. The authors reported het-norm/low to be associated with a PCO-like ovarian phenotype [40], rapidly depleting follicles (and ovarian reserve) [41], significantly reduced pregnancy chances in IVF, and high risk towards autoimmunity [40]. In their study, African women demonstrated significantly reduced odds of pregnancy compared to white women after controlling for BMI and age (OR 0.27, 0.10–0.70; P = 0.007) [42]. African women demonstrated a preponderance of abnormally low-count CGG outliers [42], corresponding to het-norm/low, providing a possible explanation for their reduced IVF pregnancy rates.
In summary, accumulating evidence suggests that race/ethnicity is a risk factor for poor ART outcomes after adjusting for many confounders. The impact of race/ethnicity on accessibility to ART centers requires additional study. Such research is needed to better understand if race/ethnicity reflects genetic factors that may influence ART outcomes or if these categories are proxies for socioeconomic status, environmental influences, or behavioral differences that contribute to outcomes. It is likely that some combination of these factors is responsible for the disparities that have been demonstrated.
References
Kelley E, Moy E, Dayton E. Health care quality and disparities: lessons from the first national reports. Med Care. 2005;43(3 Suppl):I1–2. PubMed PMID: 15746585. Epub 2005/03/05. eng.
Butts SF, Seifer DB. Racial and ethnic differences in reproductive potential across the life cycle. Fertil Steril. 2010;93(3):681–90. PubMed PMID: 19939362. Epub 2009/11/27. eng.
Luke B, Murtaugh M. The racial disparity in very low birth weight. N Engl J Med. 1993;328(4):285–6. PubMed PMID: 8418414. Epub 1993/01/28. eng.
Davidson Jr EC, Fukushima T. The racial disparity in infant mortality. N Engl J Med. 1992;327(14):1022–4. PubMed PMID: 1518537. Epub 1992/10/11. eng.
Frisbie WP, Song SE, Powers DA, Street JA. The increasing racial disparity in infant mortality: respiratory distress syndrome and other causes. Demography. 2004;41(4):773–800. PubMed PMID: 15622954. Epub 2004/12/30. eng.
Poole JH, Long J. Maternal mortality—a review of current trends. Crit Care Nurs Clin North Am. 2004;16(2):227–30. PubMed PMID: 15145366. Epub 2004/05/18. eng.
Abma JC, Chandra A, Mosher WD, Peterson LS, Piccinino LJ. Fertility, family planning, and women’s health: new data from the 1995 National Survey of Family Growth. Vital Health Stat 23. 1997;19:1–114. PubMed PMID: 9201902. Epub 1997/05/01. eng.
Jain T, Missmer SA, Hornstein MD. Trends in embryo-transfer practice and in outcomes of the use of assisted reproductive technology in the United States. N Engl J Med. 2004;350(16):1639–45. PubMed PMID: 15084696. Epub 2004/04/16. eng.
Missmer SA, Seifer DB, Jain T. Cultural factors contributing to health care disparities among patients with infertility in Midwestern United States. Fertil Steril. 2011;95(6):1943–9. PubMed PMID: 21420677. Epub 2011/03/23. eng.
Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86(3):516–23. PubMed PMID: 16952500. Epub 2006/09/06. eng.
Jain T. Socioeconomic and racial disparities among infertility patients seeking care. Fertil Steril. 2006;85(4):876–81. PubMed PMID: 16580368. Epub 2006/04/04. eng.
Green JA, Robins JC, Scheiber M, Awadalla S, Thomas MA. Racial and economic demographics of couples seeking infertility treatment. Am J Obstet Gynecol. 2001;184(6):1080–2. PubMed PMID: 11349163. Epub 2001/05/12. eng.
Feinberg EC, Larsen FW, Catherino WH, Zhang J, Armstrong AY. Comparison of assisted reproductive technology utilization and outcomes between Caucasian and African American patients in an equal-access-to-care setting. Fertil Steril. 2006;85(4):888–94. PubMed PMID: 16580370. Epub 2006/04/04. eng.
Nichols Jr JE, Higdon 3rd HL, Crane MM, Boone WR. Comparison of implantation and pregnancy rates in African American and white women in an assisted reproductive technology practice. Fertil Steril. 2001;76(1):80–4. PubMed PMID: 11438323. Epub 2001/07/05. eng.
Sharara FI, McClamrock HD. Differences in in vitro fertilization (IVF) outcome between white and black women in an inner-city, university-based IVF program. Fertil Steril. 2000;73(6):1170–3. PubMed PMID: 10856477. Epub 2000/06/17. eng.
McKinnon J. The black population: 2000. Census 2000 brief. http://www.census.gov/population/www/cen2000/briefs.html (2001). Accessed Aug 2001.
Grieco E. The white population: 2000. Census 2000 brief. http://www.census.gov/population/www/cen2000/briefs.html (2001). Accessed Aug 2001.
Seifer DB, Frazier LM, Grainger DA. Disparity in assisted reproductive technologies outcomes in black women compared with white women. Fertil Steril. 2008;90(5):1701–10. PubMed PMID: 17980873.
Jain T, Hornstein MD. Disparities in access to infertility services in a state with mandated insurance coverage. Fertil Steril. 2005;84(1):221–3. PubMed PMID: 16009188. Epub 2005/07/13. eng.
Bitler M, Schmidt L. Health disparities and infertility: impacts of state-level insurance mandates. Fertil Steril. 2006;85(4):858–65. PubMed PMID: 16580365. Epub 2006/04/04. eng.
Dayal MB, Gindoff P, Dubey A, Spitzer TL, Bergin A, Peak D, et al. Does ethnicity influence in vitro fertilization (IVF) birth outcomes? Fertil Steril. 2009;91(6):2414–8. PubMed PMID: 18691706. Epub 2008/08/12. eng.
Bendikson K, Cramer DW, Vitonis A, Hornstein MD. Ethnic background and in vitro fertilization outcomes. Int J Gynaecol Obstet. 2005;88(3):342–6. PubMed PMID: 15733901. Epub 2005/03/01. eng.
Matalliotakis I, Cakmak H, Arici A, Goumenou A, Fragouli Y, Sakkas D. Epidemiological factors influencing IVF outcome: evidence from the Yale IVF program. J Obstet Gynaecol. 2008;28(2):204–8. PubMed PMID: 18393021. Epub 2008/04/09. eng.
Wellons MF, Fujimoto VY, Baker VL, Barrington DS, Broomfield D, Catherino WH, et al. Race matters: a systematic review of racial/ethnic disparity in Society for Assisted Reproductive Technology reported outcomes. Fertil Steril. 2012;98(2):406–9. PubMed PMID: 22698638. Pubmed Central PMCID: 3409320. Epub 2012/06/16. eng.
Seifer DB, Zackula R, Grainger DA, Society for Assisted Reproductive Technology Writing Group R. Trends of racial disparities in assisted reproductive technology outcomes in black women compared with white women: Society for Assisted Reproductive Technology 1999 and 2000 vs. 2004–2006. Fertil Steril. 2010;93(2):626–35. PubMed PMID: 19368916.
Fujimoto VY, Luke B, Brown MB, Jain T, Armstrong A, Grainger DA, et al. Racial and ethnic disparities in assisted reproductive technology outcomes in the United States. Fertil Steril. 2010;93(2):382–90. PubMed PMID: 19081561. Epub 2008/12/17. eng.
Luke B, Brown MB, Stern JE, Missmer SA, Fujimoto VY, Leach R. Racial and ethnic disparities in assisted reproductive technology pregnancy and live birth rates within body mass index categories. Fertil Steril. 2011;95(5):1661–6. PubMed PMID: 21269616.
Baker VL, Luke B, Brown MB, Alvero R, Frattarelli JL, Usadi R, et al. Multivariate analysis of factors affecting probability of pregnancy and live birth with in vitro fertilization: an analysis of the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertil Steril. 2010;94(4):1410–6. PubMed PMID: 19740463.
Seifer DB, Golub ET, Lambert-Messerlian G, Benning L, Anastos K, Watts DH, et al. Variations in serum mullerian inhibiting substance between white, black, and Hispanic women. Fertil Steril. 2009;92(5):1674–8. PubMed PMID: 18930217. Pubmed Central PMCID: 3037722. Epub 2008/10/22. eng.
Menasce LP, White GR, Harrison CJ, Boyle JM. Localization of the estrogen receptor locus (ESR) to chromosome 6q25.1 by FISH and a simple post-FISH banding technique. Genomics. 1993;17(1):263–5. PubMed PMID: 8406468. Epub 1993/07/01. eng.
Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metabol. 1997;82(12):4258–65. PubMed PMID: 9398750. Epub 1997/12/17. eng.
Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86(3):686–93. PubMed PMID: 16860797. Epub 2006/07/25. eng.
Georgiou I, Konstantelli M, Syrrou M, Messinis IE, Lolis DE. Oestrogen receptor gene polymorphisms and ovarian stimulation for in-vitro fertilization. Hum Reprod. 1997;12(7):1430–3. PubMed PMID: 9262271. Epub 1997/07/01. eng.
Sundarrajan C, Liao W, Roy AC, Ng SC. Association of oestrogen receptor gene polymorphisms with outcome of ovarian stimulation in patients undergoing IVF. Mol Hum Reprod. 1999;5(9):797–802. PubMed PMID: 10460216. Epub 1999/08/25. eng.
Coney P, Demers LM, Dodson WC, Kunselman AR, Ladson G, Legro RS. Determination of vitamin D in relation to body mass index and race in a defined population of black and white women. Int J Gynaecol Obstet. 2012;119(1):21–5. PubMed PMID: 22818533. Epub 2012/07/24. eng.
Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–9. PubMed PMID: 19589516. Pubmed Central PMCID: 2888852. Epub 2009/07/11. eng.
Merhi ZO, Seifer DB, Weedon J, Adeyemi O, Holman S, Anastos K, et al. Circulating vitamin D correlates with serum antimullerian hormone levels in late-reproductive-aged women: women’s interagency HIV study. Fertil Steril. 2012;98(1):228–34. PubMed PMID: 22494925. Pubmed Central PMCID: 3389125. Epub 2012/04/13. eng.
Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS. The level of serum anti-mullerian hormone correlates with vitamin d status in men and women but not in boys. J Clin Endocrinol Metab. 2012;97(7):2450–5. PubMed PMID: 22508713. Epub 2012/04/18. eng.
Gleicher N, Weghofer A, Barad DH. Effects of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. Reprod Biomed Online. 2010;20(4):485–91. PubMed PMID: 20149747. Epub 2010/02/13. eng.
Gleicher N, Weghofer A, Lee IH, Barad DH. FMR1 genotype with autoimmunity-associated polycystic ovary-like phenotype and decreased pregnancy chance. PLoS One. 2010;5(12):e15303. PubMed PMID: 21179569. Pubmed Central PMCID: 3002956. Epub 2010/12/24. eng.
Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19(3):385–90. PubMed PMID: 19778484. Epub 2009/09/26. eng.
Gleicher N, Weghofer A, Lee IH, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS One. 2011;6(4):e18781. PubMed PMID: 21526209. Pubmed Central PMCID: 3078144. Epub 2011/04/29. eng.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Tal, R., Seifer, D.B. (2013). Disparities Between Black and White Women in Assisted Reproductive Technology. In: Sharara, F. (eds) Ethnic Differences in Fertility and Assisted Reproduction. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7548-4_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7548-4_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7547-7
Online ISBN: 978-1-4614-7548-4
eBook Packages: MedicineMedicine (R0)