Abstract
Dementia is an increasingly prevalent disorder as the world population ages, with Alzheimer disease the most common cause. Twin studies find concordance to be substantially higher among monozygotic as compared with dizygotic twin pairs. While a few genes have been identified that are responsible for early-onset Alzheimer disease, they account for well under 5 % of all cases. Among susceptibility genes for Alzheimer disease identified by association studies, population attributable fraction for the most prominent of these—apolipoprotein E—is estimated to be about 25 %, whereas other genes at best predict another 20 %. There are few strong environmental risk or protective factors, with vascular risks the best established, although with mechanisms still not fully understood. It seems likely that clusters of risk alleles, interactions between risk alleles and environmental exposures, and epigenetic mechanisms play a role in explaining Alzheimer disease, with different combinations of influences culminating in the observed pathophysiology. In particular, environmental exposures early in life could lead to deleterious changes in gene expression in late life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mild Cognitive Impairment
- Dementia With Lewy Body
- Amyloid Precursor Protein
- Population Attributable Fraction
- Genomewide Association Study
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Older adulthood is characterized by normative changes in cognition as described in Chaps. 5 and 6. At the same time, age is also the most important risk factor for nonnormative cognitive changes, or dementia. In this chapter, we briefly review the epidemiology of dementia then turn to behavior genetic research, molecular and genomic studies, environmental risk factors, and interactions of genetic and environmental risk factors. The field is rapidly growing, with new work on biomarkers , ever larger genome-wide association studies (GWAS) consortia, and yet more “omics” approaches; thus, we conclude by pointing to areas where new developments are likely to emerge.
1 Introduction to Dementia
1.1 Defining Dementia
Dementia refers to a group of disorders marked by progressive cognitive deterioration, primarily in old age. Persons with dementia show significant difficulties in performing everyday activities, which eventually lead to complete reliance on others in basic self-care. Current understanding places dementia on a spectrum, where a disease process may start to occur years before symptoms manifest and cause a mild cognitive decline before symptoms become severe enough to meet diagnostic criteria for dementia (Sperling et al. 2011; see Fig. 7.1). In an effort for early detection and intervention, in the past two decades, a large number of studies have been conducted to characterize mild cognitive impairment (MCI) in relation to normative cognitive aging (see Gauthier et al. 2006, for review). Acknowledging the recent advancement in understanding the continuum of dementia and its clinical utility, the new soon-to-be-released fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) revised its definition of dementia and proposed the new terms “minor neurocognitive disorder” and “major neurocognitive disorder.” According to the DSM-5, a neurocognitive disorder may be broadly defined as a decline from a previously attained level of cognitive functioning in one or more domains (Jeste et al. 2010). Cognitive domains that may be affected include complex attention (sustained and divided attention, processing speed , and selective attention), executive ability (planning, decision-making, working memory , and mental flexibility), learning and memory (immediate and recent episodic memory), language (expressive and receptive language), visuoconstructional–perceptual ability (construction and visual perception), and social cognition (emotion recognition and behavioral regulation). Major neurocognitive disorder indicates sufficient severity of impairment in these domains and loss of independence in daily functioning to be consistent with dementia (Reiman et al. 2011). Recognizing the pattern of specific cognitive domains affected may be helpful to further diagnose subtypes of dementia, with the subtypes representing different etiologies.
Model of the clinical trajectory of Alzheimer disease (AD). The stage of preclinical AD precedes mild cognitive impairment (MCI) and encompasses the spectrum of presymptomatic autosomal dominant mutation carriers, asymptomatic biomarker-positive older individuals at risk for progression to MCI due to AD and AD dementia, as well as biomarker-positive individuals who have demonstrated subtle decline from their own baseline that exceeds that expected in typical aging, but would not yet meet criteria for MCI. Note that this diagram represents a hypothetical model for the pathological–clinical continuum of AD but does not imply that all individuals with biomarker evidence of AD-pathophysiological process will progress to the clinical phases of the illness. (Reprinted from Sperling et al. 2011, p. 283, Copyright 2011, with permission from Elsevier)
1.2 Dementia Prevalence
Reports on dementia prevalence use different age classifications and assessment approaches, making comparisons difficult. Further, prevalence reflects a combination of incidence and survival, and survival rates among the nondemented vary widely in different parts of the world. That said, the following is a summary of the most recent, most comprehensive numbers.
As of 2010, the number of people with dementia above 60 years of age worldwide was estimated to be 35.6 million, with a projection of almost twofold increase by 2030 (Ferri et al. 2005; Wimo and Prince 2010). Estimated crude prevalence of dementia among those aged 60 years and older was higher among developed countries, with approximately 7 % in North America and Western Europe, than among developing countries; whereas the rate of increase in prevalence was far higher among developing countries, including Latin American nations, China, and India (Ferri et al. 2005). Across all population-based studies in different regions of the world, prevalence of dementia consistently increases with age, with the highest percentage of affected people in the population aged 85 years or older (Berr et al. 2005; Ferri et al. 2005; Plassman et al. 2007). Thus, the projected increase in number of people with dementia directly reflects increased life expectancy.
1.3 Dementia Subtypes
By far, the most prevalent subtype is dementia due to AD , a degenerative process that accelerates neuronal death in the brain. Population-based studies of the prevalence of dementia show that AD accounted for 70 % of all cases of dementia in the USA (Plassman et al. 2007); 64 % in Canada (Canadian Study of Health and Aging 1994); 54 % across eight European countries (Lobo et al. 2000); and 60 % in developing countries (Kalaria et al. 2008).
Early clinical presentation of typical AD has progressive short-term memory deficits at its core (Cummings and Cole 2002). Histopathological markers of AD observed postmortem include: extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs). The National Institute on Aging and the Alzheimer’s Association (NIA-AA) recently published the new diagnostic criteria for AD (McKhann et al. 2011). To meet diagnostic criteria for “probable dementia due to AD,” an individual should have (1) a clinical diagnosis of dementia, including impairment in multiple domains that interfere with functional independence, (2) gradual cognitive decline with insidious onset, (3) either amnestic (learning and recall) or nonamnestic (language, visuospatial, and executive dysfunction) cognitive deficits, and (4) no prominent features of other dementia subtypes . Additionally, corroborative evidence that improves diagnostic confidence includes documented decline based on informant report and neuropsychological assessments, presence of known AD genetic mutations, and abnormal pathophysiological biomarkers on imaging or cerebrospinal fluid assays. Of note, the new NIA-AA criteria expanded the definition of AD to include nonmemory types of AD, recognizing that some AD cases may not present memory deficits early on and instead show deficits in other cognitive domains (Lopez et al. 2011). With respect to the continuum of dementia, the new NIA-AA criteria specify diagnostic features of “MCI due to AD” and “preclinical AD.” Criteria for MCI due to AD include self- or informant-reported changes in cognition, education- and age-inappropriate cognitive impairment in one or more cognitive domains, and slight decline in performing functional tasks with intact functional independence (Albert et al. 2011). Notably, preclinical AD is proposed as a category for research only, not for clinical use. Criteria for preclinical AD include asymptomatic individuals with positive biomarkers and presumed at risk for developing either MCI due to AD or AD dementia, or individuals with positive biomarkers and subtle age-incongruent cognitive decline (Sperling et al. 2011).
Hypotheses regarding the cause of AD are relevant to possible genetic pathways, environmental exposures, and treatments. The two most characteristic neuropathological features of the disease are extracellular plaques composed of amyloid beta (Aβ) peptide and neurofibrillary tangles (NFTs) composed of abnormal tau protein (Hyman et al. 2012). The amyloid cascade hypothesis (Hardy 2006) has provided major contributions to understanding the pathophysiology of AD. The amyloid hypothesis as it has evolved posits overaccumulation of Aβ in the form of soluble oligomers and insoluble fibrils that aggregate as plaques. Currently, it is thought that the oligomers instigate the sequence of events, including chronic inflammation that results in neuronal injury and death (White et al. 2005). The tau hypothesis postulates that disruptions in tau–microtubule binding by increased phosphorylation promote abnormal aggregation of “free” tau proteins, eventually leading to the formation of NFTs, which facilitate neuronal injury and death (Mudher and Lovestone 2002). The two hypotheses are not independent; it has been observed that an increased Aβ concentration triggers abnormal changes in tau protein and resultant formation of NFTs (Oddo et al. 2006), with measures of tau thus representing a more “downstream” biomarker of neuronal injury (Albert et al. 2011).
Vascular dementia (VaD) is the second most common subtype of dementia in the elderly. Prevalence estimates range from 16 to 24 % of all dementia cases (Canadian Study of Health and Aging 1994; Kalaria et al. 2008; Lobo et al. 2000; Plassman et al. 2007).
A diagnosis of VaD requires (1) clinical symptomatology of dementia, (2) evidence of ischemic or hemorrhagic cerebrovascular disease (CVD) or hypoperfusive ischemic cerebral infarcts resulting from cardiovascular and circulatory disorders, and (3) close temporal association between dementia and vascular etiology (Chui et al. 1992; Román et al. 1993). Several versions of diagnostic criteria for VaD exist to date, with lack of consensus among them (Chui 2006; Wiederkehr et al. 2008). In particular, it involves considerable challenge to characterize the profile of cognitive impairment in VaD because of the heterogeneous nature of underlying cerebral lesions in terms of number, size, and location (Moorhouse and Rockwood 2008). Increasingly, researchers prefer to use the term vascular cognitive impairment (VCI) (Hachinski and Bowler 1993; O’Brien et al. 2003), which incorporates a range of cognitive disorders with presumed vascular implications in order to encompass significant cognitive decline that does not meet criteria for dementia (Moorhouse and Rockwood 2008). Moreover, VCI construct includes recognition of the interplay between vascular disease and neurodegenerative pathology. Postmortem studies often find that mixed neuropathology, including plaques and tangles characteristic of AD and vascular infarcts characteristic of VaD, is common (Kalaria and Ballard 1999; Schneider et al. 2007), and most experts view these pathologies as additive (Schneider and Bennett 2010).
After AD, dementia with Lewy bodies (DLB) is the second most prevalent neurodegenerative dementia. A systematic review concluded that it accounts for 0–22 % of all dementia cases, with the large range reflecting a need for more studies and greater use of consensus diagnostic criteria (Zaccai et al. 2005). Early cognitive features of DLB include decline in attentional, visuospatial, and executive abilities with relative memory preservation, compared with AD (Mrak and Griffin 2007). In addition to a clinical diagnosis of dementia, DLB is characterized by (1) fluctuating cognition with pronounced variation in attention and alertness, (2) recurrent visual hallucinations, and (3) spontaneous features of parkinsonism (McKeith et al. 2005). In terms of pathophysiology, DLB is marked by the presence of abnormally aggregated protein known as Lewy bodies throughout the whole brain, including neocortical areas and paralimbic structures. Progressive cognitive impairment may also occur in patients with Parkinson disease, called Parkinson disease with dementia (PDD) . Both DLB and PDD are Lewy body dementias (LBD), with Lewy bodies comprising clumps of alpha-synuclein protein in the brain (Ballard 2004). The two LBDs are generally distinguished by which symptoms come first, motor (PDD) or cognitive (DLB). Overlapping pathology is not uncommon, with Lewy bodies often observed in patients with AD (Bonifati 2008).
Another subtype is frontotemporal lobar degeneration (FTLD) , of which Pick disease is one rare clinical syndrome. FTLD is characterized by early manifestations of deficits in behavior, personality, executive functioning, and language (Rabinovici and Miller 2010). With respect to neuropathology and pathophysiology, many FTLD cases have tau protein deposits, a portion of which comprises Pick bodies, while a number of cases who were not tau-positive can show ubiquitin inclusions (Forman et al. 2006).
Finally, individuals may develop dementia symptomatology secondary to many diseases that affect the immune or metabolic system (World Health Organization, 2010).
2 Familial Influences and Estimating Heritability
Heritability is defined as the relative percentage of variance in a phenotype explained by genetic influences compared with the percentage of variance explained by environmental influences , within the population under study. The heritability of dementia carries both clinical and scientific importance and must be considered with regard to the context of each study. Clinically, the information helps relatives of dementia patients to understand their own risk for dementia. Most research attention has been given to investigating heritability in studies of AD, with reports typically specifying AD or combining across all dementias.
2.1 Family Studies
Research findings have consistently shown elevated risk for developing AD in first-degree relatives of AD patients. Among AD probands, various studies have reported that 34–42 % had a positive family history of AD (Green et al. 2002; Lautenschlager et al. 1996; Silverman et al. 1994). Taking a different approach to characterizing the importance of family history, cumulative risk for AD among those with a positive family history ranges from 30 to 39 % (Lautenschlager et al. 1996; Silverman et al. 1994). These figures can be compared with a risk for AD of 12 % among first-degree relatives of normal controls (Silverman et al. 1994) or to an overall estimated lifetime risk for AD of 19 % (Plassman and Breitner 1997).
There is some suggestion that African-American first-degree relatives and normal controls may be at higher risk than respective samples of Whites (Green et al. 2002), and that female first-degree relatives are at greater risk of developing dementia than their male counterparts, even after accounting for the difference in longevity (Lautenschlager et al. 1996; Van Duijn et al. 1993).
Some family studies stratified first-degree relatives of AD patients according to the proband’s age of onset (Li et al. 1995; Lautenschlager et al. 1996; Silverman et al. 2003). From these studies, Li et al. (1995) concluded that earlier age of onset in the case may increase risk of earlier onset AD in the relative but not their total lifetime risk. For example, Lautenschlager et al. (1996) reported that relatives of cases with onset before age 72 years had increased risk of developing AD, but only until they themselves reached age 82 years.
In a population-based study with 74 FTLD probands and 561 age- and gender-matched controls, Stevens et al. (1998) reported that the risk for developing dementia before age 80 years among 411 relatives of FTLD probands was 22 %, compared with 11 % among 2,934 relatives of controls.
2.2 Twin Studies
As the most basic descriptive step, comparing monozygotic (MZ) and dizygotic (DZ) twins in their concordance rates of AD provides evidence with respect to genetic influence on liability to AD. Probandwise concordance rates are based on the ratio of the number of affected twin partners of independently ascertained probands to the total number of probands. Four different population-based twin studies were launched in the mid-1990s. Probandwise concordance rates for all dementia and for AD alone are shown in Table 7.1; all four studies report higher concordance for AD among MZ pairs than DZ pairs, although concordance rates and estimates of heritability vary across studies.
Bergem et al. (1997) identified dementia probands in long-term care facilities in Norway and located their cotwins using the Norwegian Twin Registry. Heritability for AD in this study was estimated to be 60 % (Bergem and Lannfelt 1997).
Breitner et al. (1995) ascertained all twins with dementia from the National Academy of Sciences-National Research Council (NAS-NRC) Registry of Aging Twin Veterans, largely from World War II. Twins in this study were relatively young, with their ages ranging from 62 and 73 years at the time of screening, prior to the age at which many would likely develop dementia, hence reducing concordance and heritability. Heritability was estimated to be 28 % (Plassman and Breitner 1997). When liability to disease was modeled using age of onset rather than disease risk, heritability was estimated to be 37 % (Meyer and Breitner 1998). As the cohort has aged, concordance has increased (Plassman et al. 2004; Steffens et al. 2000). Additionally, Steffens et al. found more cases of AD among first-degree relatives of the concordant twin pairs compared with first-degree relatives of the discordant twin pairs.
In the Finnish Twin Registry consisting of all same-sex twin pairs in Finland, Räihä et al. (1996) identified twins with dementia through matching the twin registry to the national hospital discharge database. On the basis of the data in the article, Plassman and Breitner (1997) computed heritability to be 45 % in this sample. Use of the discharge registry to identify cases likely resulted in underascertainment, which would depress heritability (Gatz and Pedersen 1996).
Gatz et al. (1997) identified all cases of dementia in the Swedish Adoption/Twin Study of Aging (SATSA) sample, which comprises a subset of Swedish Twin Registry (STR). Using standard biometrical models, heritability of AD was estimated to be 74 %, and heritability of all dementias, 43 %. However, the difference between age of onset for twins in concordant MZ pairs was as large as 16 years. Pedersen et al. (2001) estimated heritability of AD in this same sample using multiple thresholds reflecting age of onset rather than disease risk. Using this approach, heritability estimated using a threshold fixed to age-based population prevalence was 78 %, whereas using a model that allows for varying thresholds derived from observed data produced an estimate of 57 %, accounting for mortality and likelihood of follow-up to a certain age in the data set.
Subsequently, Gatz et al. (2006) screened all twin pairs in the STR aged 65 years and older for cognitive impairment, and referred those who screened positive and their cotwins for a complete diagnostic work-up. A total of 11,884 twin pairs were included in the study. Heritability estimates for AD were 58 % in an age-adjusted full model, including genetic, shared, and nonshared environmental factors, and 79 % in the age-adjusted best fitting model, excluding shared environmental effects. Probandwise concordance rates for all dementia were 44 % in MZ and 25 % in DZ pairs for men and 58 % in MZ and 45 % in DZ pairs for women. As a point of comparison, Gatz (2007) created unrelated pairs matched by sex and year of birth; the estimated concordance for dementia over a series of random unrelated pairs was 12 % for men and 21 % for women.
Few findings are available for dementias other than AD. In the Norwegian study, concordance for VaD was 29 % among both MZ and DZ twins (Bergem et al. 1997); in the Finnish study, the figures were 31 % for MZ and 12 % for DZ twins. Wang et al. (2009) examined autopsy-confirmed DLB in the NAS-NRC twins. In 17 pairs, only one MZ pair was concordant for DLB. Four additional pairs were concordant for dementia, but only one twin in each pair was diagnosed with DLB. One newer twin study, the Vietnam Era Twin Study of Aging (VETSA) , a longitudinal study of cognitive and brain aging beginning in midlife (Kremen et al. 2006), will soon be able to report on concordance and heritability of MCI .
An important contribution of twin studies to knowledge about AD lies in their indicating the extent to which genes likely play a role in liability to the disease, providing a context for the search for specific risk genes. Although findings show variability, the variability is less in samples of similar age, and it is clear that heritability of liability for AD is substantial. On the basis of MZ twin similarity alone, Roberts et al. (2012) estimated the predictive capacity of knowing an individual’s personal genome. For most diseases, predictive capacity was low. However, for AD, those who hypothetically received a positive genetic test would have a markedly elevated risk of eventually developing the disease, whereas a negative genetic test would indicate a substantially lowered risk.
3 Molecular Studies
Once there is evidence for familial aggregation of a disorder, such as the increased risk in first-degree relatives, differential concordances in MZ and DZ twins, or heritability estimates, the next logical step is to try to identify which genes are contributing to the disorder.
3.1 Family Linkage Studies and Rare Mutations
The earliest attempts to identify genes that could be responsible for dementia in general and AD in particular were classical linkage studies of relatively large pedigrees, in which multiple family members were identified with the disease (often affected sib pairs). Chromosome 21 was long thought to be a likely chromosome with loci that could be important for dementia, as those with Down syndrome often develop AD-typical plaques. The first gene with mutations linked to early-onset AD was amyloid precursor protein (APP) on chromosome 21 (Goate et al. 1991). Identifying mutations in this gene (now up to 29) and subsequent work with understanding the mechanisms by which mutations change the protein product of this gene did much for developing the amyloid cascade hypothesis of AD (Hardy 2006).
Two other genes also have mutations that are linked with familial, early-onset AD: presenilin 1 (PSEN1) on chromosome 14 (Sherrington et al. 1995) and presenilin 2 (PSEN2) on chromosome 1 (Levy-Lahad et al. 1995; Rogaev et al. 1995). Mutations in all three of these genes are completely penetrant, with an autosomal dominant mode of transmission. All are involved in production or processing of APP, hence leading to Aβ deposition and increases in the Aβ42:Aβ40 ratio (Tanzi 2012). Mutations in PSEN1 are the most common (185 to date); nevertheless, mutations in these three genes are relatively rare, and they account for less than 5 % of all AD cases (Cummings and Cole 2002). Notably, the vast majority of AD cases do not carry mutations in any of these genes.
A rare form of VaD called cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) has similarly been attributed to a mutation in a single gene (Chabriat et al. 2009). Mutations in five genes have been associated with autosomal dominant FTLD, accounting for about 10 % of all FTLD (Rabinovici and Miller 2010). At least two genes have been identified in autosomal dominant LBD, implicated in both PD and DLB, but explaining only a small minority of cases (Bonifati 2008; Forman et al. 2005).
3.2 Association Studies of Candidate Genes
Prior to the technological advancements that enabled large-scale GWAS , most other efforts to identify genes for AD or any dementia were candidate gene association studies. Some leads in the late 1990s revolved around linkage findings on chromosomes 10 and 12. However, several dozen loci on other chromosomes were also considered. The majority of studies focused on candidate genes that were hypothesized to infer increased susceptibility due to what was known of their function, such as being part of certain pathogenic pathways. Many were considered because they were involved in APP processing and Aβ production, clearance, and degradation. Others were considered because of potential roles in the formation of NFTs, whereas others because of their role in inflammation, oxidative stress, or cerebrovascular events (Bertram and Tanzi 2008).
Genetic association studies for AD had their most important breakthrough in 1993 when the epsilon 4 (ε4) allele of apolipoprotein E (APOE) was associated to both late-onset familial and sporadic AD (Corder et al. 1993; Saunders et al. 1993; Strittmatter et al. 1993). Meta-analyses of 38 case-control studies indicated that carriers of the ε4 allele had an odds ratio (OR) of 3.68 compared with carriers of the “wild-type” ε3 allele, whereas ε2 carriers are protected (OR = 0.62) (http://www.alzgene.org). Another meta-analysis indicates that ε4 homozygotes have an OR of 14.9 in Caucasian populations and 33.1 among Japanese (Farrer et al. 1997). APOE genotypes are actually haplotypes of two single nucleotide polymorphisms (SNPs) , rs7412 and rs429358. The latter, which is essential for defining the ε4 allele, is solely responsible for the association of APOE and dementia, and this association is mediated predominantly through its effect on Aβ42 levels in the central nervous system (Bennet et al. 2010). APOE as a susceptibility gene for AD is the most robust genetic association for any complex disorder known today. Not only is the association consistently found across studies and ethnicities (although the effect size in African-Americans needs some clarification), the effect size is several orders of magnitude greater than those typically found for most candidate genes and even findings from GWAS studies described later.
Findings from genetic association studies of candidate genes have been systematically catalogued and reviewed by Bertram and others (Bertram 2011; Bertram and Tanzi 2001, 2008) and are publically available with meta-analyses in the AlzGene database (http://www.alzgene.org) (Bertram et al. 2007; Bertram et al. 2011). From a handful of studies in the early 1990s, there has been an explosion in the number of reports. Through 2001, approximately 450 association studies were published (Bertram 2011). In the most recent update of AlzGene 10 years later (as of April 18, 2011), there were nearly 1,400 studies reporting on nearly 3,000 polymorphisms in 700 genes. Despite the large number of reports and genes evaluated, only 40 genes show significant risk effects in meta-analyses. A few genes that reached significance but were not signals in GWAS include SORL1 (sortilin-related receptor), ACE (angiotensin-converting enzyme), IL8 (interleukin 8), and LDLR (low-density lipoprotein receptor) (Tanzi 2012). Many of the studies suffer from the classic perils of gene discovery studies, such as small sample sizes, publication bias, and insufficient attention to appropriate covariates and confounders.
3.3 Genome-Wide Association Studies of AD
Attempts to find genes involved in the pathogenesis of AD have now shifted over to high-density GWAS with the first reports by Coon et al. (2007), Grupe et al. (2007), and Reiman et al. (2007) (see Table 7.2). As of mid-2012, there were 28 published GWAS reported in recent summaries from http://www.alzgene.org and the catalogue available through the National Human Genome Research Institute website (http://www.genome.gov/gwastudies/index.cfm?pageid=26525384#searchForm), with more than 60 loci implicated as potential modifiers of susceptibility to AD or age at onset for AD. In most cases, APOE comes out as the most significant finding and with the largest effect size. Many of the findings from these studies have yet to be replicated in other samples. Nevertheless, nine other genes have sufficient replication or significant meta-analytic results to be considered real associations: BIN1 (bridging integrator 1), CLU (clusterin), ABCA7 (ATP-binding cassette subfamily A member 7), CR1 (complement receptor 1), PICALM (phosphatidylinositol-binding clathrin assembly protein), MS4A6A/MS4A4E (membrane-spanning 4-domains, subfamily A, member 6A/4E), CD33 (myeloid cell surface antigen CD33 isoform 2 precursor), and CD2AP (CD2 associated protein). In contrast to APOE, for which the meta-analytic OR for ε4 versus ε3 is 3.68, the ORs for these loci are much smaller, ranging from 1.11 to 1.23. Another way of putting the importance of APOE into perspective is to consider the population attributable fraction, which is the proportion of disease burden attributable to a factor, in this case, an allele (Levine 2007). Using OR and minor allele frequencies, Bertram (2011) estimated that the population attributable fraction for APOE was 27 %, whereas the combined attributable fraction for BIN1, CLU, CR1, and PICALM was only 19.3 % with no single locus being greater than 6.5 %. Similarly, many of the large consortia with GWAS data have applied genetic risk prediction models to their findings; all report that the addition of these genes minimally improved prediction of incident AD beyond age, sex, and APOE ε4 (see Seshadri et al. 2010, for example). GWAS findings reinforce the futility of using individual genetic risk profiling for AD beyond having information on age, sex, family history, and APOE status (Pedersen 2010).
At the same time, family history and twin heritability studies indicate that there is genetic risk not yet accounted for. Findings of relatively few replicable genes, each with very modest effect sizes (beyond APOE), suggest that there is considerable genetic heterogeneity for a complex disorder such as AD. It is not surprising that GWAS efforts are finding genes with relatively small effect sizes, as this would be compatible with a polygenic model of inheritance. Larger and larger consortia are combining their findings in the hunt for discovering new associations with AD. Power will increase to find genome-wide significant associations, all with effect sizes as small as those previously reported. Some consortia are implementing genome-wide gene-based approaches to find associations (Lambert et al. 2013). Others are focusing on whole-genome sequencing to identify rare variants that may contribute to late-onset AD. Recently, Jonsson et al. (2012) discovered that a rare variant in the APP gene (frequency of 0.2–0.5 %) is protective of AD and cognitive decline in the oldest old. This finding is important as it gives further insight into the role of β-cleavage in APP and may lead to advances in finding therapeutic interventions.
3.4 Gene–Gene interactions
Moving beyond gene identification requires focusing on multiple genes, including additive and interactive effects, and incorporating information on environmental risk and protective factors is required rather than further pursuit of gene identification or replication. Many cohorts that have contributed information to the GWAS analyses have at least some information on selected risk factors other than age, sex, and APOE genotype. Perhaps the greatest challenge for AD geneticists will be to evaluate both gene–gene interactions as well as gene–environment interactions.
Early evidence for potential interactions between genes at different loci (known as epistasis or gene–gene interaction) for AD came from candidate gene studies that found evidence for association of a candidate gene only when APOE was taken into account. Using synergy factor analysis, Combarros et al. (2009) evaluated 100 “claims or suggestions of epistasis” in AD. They found 27 gene–gene interactions in networks involving cholesterol metabolism, β-amyloid metabolism, inflammation, oxidative stress, and other networks. The vast majority of the interactions were with APOE. Most of the interactions were synergistic, such that the effect of another gene was considerably stronger in the presence of APOE ε4. Nevertheless, some interactions were antagonistic, with ε4 presence masking the effect of another gene. The Epistasis Project, a consortium of seven AD research groups with 1,757 AD cases and 6,294 controls, is systematically replicating interactions that have been reported in AD (Bullock et al. 2013; Heun et al. 2012; Kölsch et al. 2012) and has focused on genes involved in inflammation and glucose metabolism.
Given the strong association of APOE with AD, genome-wide studies that have not adjusted for APOE appropriately may find both false-positive and false-negative results (Wijsman et al. 2011). Indeed, the early GWAS finding for GAB2 required post hoc stratification by APOE to reach significance (Reiman et al. 2007). Nevertheless, gene–gene interactions may explain some of the heritability of AD (Heun et al. 2012), although no attempts to quantify the contribution have been made.
4 Environmental Influences and Gene–Environment Interactions
4.1 Environmental Factors
Findings from twin studies provide evidence for a significant role of environmental influences on liability to dementia. Researchers have made vigorous efforts to identify potential environmental factors that can contribute to higher or lower risk of AD or dementia more generally. The focus has largely been directed to lifestyle choices and medical conditions. Recently, a group of experts was commissioned by National Institutes of Health (NIH) to provide an evidence report with regard to risk-modifying factors of AD (Williams et al. 2010). Key findings from that report, including 25 systematic reviews and 250 primary research studies mainly from developed countries, are featured here, while noting additional research evidence for possible underlying mechanisms.
4.1.1 Education, Occupational Complexity, and Cognitive Engagement
One of the most studied variables is level of education and the related factors of cognitively challenging occupational and leisure activities. The preponderance of evidence from prospective cohort studies indicates that fewer years of education is associated with increased risk of AD (Williams et al. 2010). Low education remains a significant risk in discordant MZ twin pairs, and bivariate twin modeling indicates that the association between low education and dementia is not genetically mediated (Gatz et al. 2007).
The Williams et al. (2010) report did not find sufficient evidence for a significant protective effect of occupation beyond the effect of education, but did conclude that more frequent participation during one’s leisure in activities that are cognitively engaging is associated with reduced risk of AD. In twin studies, both complexity of work with people and midlife participation in cognitively engaging activities have been found to be protective (Andel et al. 2005; Carlson et al. 2008). Not yet resolved is the extent to which the mechanism accounting for the association of complex cognitive activities with lower rates of dementia is neuroprotection or improved compensation.
4.1.2 Physical Activity
Williams et al. (2010) reported a significant association between a high level of physical activity and decreased incident AD. On the basis of animal (Cotman and Berchtold 2002) and human (Erickson et al. 2011) studies, researchers posit that exercise induces increased levels of brain-derived neurotrophic factor (BDNF) , important in facilitating neuronal growth.
4.1.3 Diet
Williams et al. (2010) conclude that high adherence to Mediterranean-type diet , typically involving higher consumption of fish, fruits, vegetables, and unsaturated fatty acids (e.g., olive oil), may be beneficial in lowering the risk of AD. Findings also seem to suggest a reliable association between low baseline serum folate levels and increased risk for AD and dementia. No other findings were judged conclusive with respect to demonstrating a role for any other dietary or nutritional factor.
4.1.4 Smoking
Evidence consistently indicates an elevated risk of AD for current smokers, compared with those who never smoked (Cataldo et al. 2010; Williams et al. 2010). The association is somewhat difficult to specify for former smokers because of variability in the length of tobacco use and abstinence (Williams et al. 2010).
4.1.5 Vascular Factors
The preponderance of research evidence establishes an increased risk of AD among persons with diabetes mellitus, with some evidence for increased risk of AD associated with elevated cholesterol in midlife (Williams et al. 2010). Statin use is associated with a moderately reduced risk for AD (Williams et al. 2010). Inconsistencies were found with respect to other vascular factors, including hypertension, antihypertensive use, and obesity. In twins, we find that obesity and overweight in midlife, but not in old age, are risk factors for dementia (Xu et al. 2011), possibly explaining some inconsistencies. One hypothesis posits that the insulin resistance syndrome may selectively affect the hippocampus and medial temporal cortex in the brain, areas affected in the AD patients (Craft 2009). Cerebrovascular changes associated with vascular risk factors may act additively with AD pathology in impairing brain function, giving additional importance to the role of vascular risk profiles for stroke (Gorelick et al. 2011).
4.1.6 Depression
Reviewed studies have found a reliable association between a history of clinical depression and incident AD (Williams et al. 2010). More studies than not, including studies of twins, have found that the association between depression and dementia only holds for depression that occurs for the first time, close in time to the age of onset of dementia (Brommelhoff et al. 2009). These findings are consistent with the hypothesis proposed by Alexopoulos (2005) that, at least for some individuals, there is disruption of frontal-striatal and frontal-limbic brain pathways that potentiates both late-onset depression and dementia.
4.1.7 Traumatic Brain Injury (TBI)
Some evidence suggests a heightened risk of AD for individuals with a history of TBI (Williams et al. 2010). It appears that the risk may be greater for males than for females, although this apparent difference may reflect the greater chance that males will be exposed, or inclusion of more males in studies reviewed.
4.1.8 Estrogen
Prospective cohort studies generally indicate a protective role for estrogen exposure and for estrogen replacement therapy (Williams et al. 2010). In contrast, in clinical trials with estrogen with or without progesterone, there is either no effect on risk of AD, all dementia, or MCI, or a slightly increased risk of AD (Williams et al. 2010).
4.2 Interaction between genes and environments
Environmental risk factors for dementia may have differential effects on individuals as a function of their genetic status, and vice versa. Understanding the interaction between genetic and environmental influences may be important for understanding disease mechanisms, treatment, and prevention. Studies to date focused almost entirely on the interactions with APOE status.
One of the earliest reports described a synergistic effect in which head injury significantly increased risk of AD only in the presence of APOE ε4 (Mayeux et al. 1995). However, a more recent review of subsequent studies determined that evidence for the interactive role of APOE and head injury in the development of AD was inconclusive (Van Den Heuvel et al. 2007).
Much attention has been devoted to whether cerebrovascular risk factors are potentiated in APOE ε4 carriers. Eriksson et al. (2010) found that nonstroke cardiovascular disease increased risk of AD in APOE ε4 carriers, but not in noncarriers. Similarly, previous stroke or transient ischemic attack predicted increased risk of developing AD only in APOE ε4 carriers, and not in noncarriers (Johnston et al. 2000). Peila et al. (2001) showed a synergistic effect of APOE ε4 and midlife hypertension on cognitive impairment in old age, where elevated systolic blood pressure had a greater adverse effect on cognition in APOE ε4 carriers than in noncarriers. Both Peila et al. (2002) and Irie et al. (2008a) reported that APOE ε4 increases the risk for AD in individuals with diabetes mellitus beyond an additive effect of the separate risks.
A minority of research findings suggest the opposite pattern of interaction between APOE ε4 and cerebrovascular risk factors. In population-based studies of African-Americans and Nigerians, researchers found that increased level of cholesterol was associated with increased risk of AD in noncarriers of APOE ε4, but not in carriers (Evans et al. 2000; Hall et al. 2006).
For other risk factors, predominantly nonvascular, the risk factor is more prominent among non-APOE ε4 carriers, or APOE status made no difference. For smoking, the increased risk of AD for current smokers is limited to those with no APOE ε4 alleles, compared with those with one or two ε4 alleles (Aggarwal et al. 2006; Reitz et al. 2007). These findings were similar for African-American and non-African-American respondents. Two studies have looked at fish intake and APOE ε4 status, finding that more than weekly consumption was associated with reduced risk of AD, but only in APOE ε4 noncarriers (Barberger-Gateau et al. 2007; Huang et al. 2005).
Researchers have also looked at the interaction between APOE ε4 and depression in the development of AD. Steffens et al. (1997) found no evidence for an interaction. Synergistic interactions were reported by Irie et al. (2008b) for AD and by Geda et al. (2006) for MCI, with elevated risk among individuals with both depression and APOE ε4.
Results are mixed for the interaction between physical activity and APOE genotype. In one study, low rate of leisure-time physical activity appears more deleterious among APOE ε4 carriers than noncarriers (Rovio et al. 2005). In another study, the relationship between higher physical activity and reduced risk for dementia was limited to noncarriers, and no such relationship was found among carriers (Podewils et al. 2005).
There are either null and inconsistent findings, or an absence of evidence, with respect to interactions between APOE and education or cognitive activity. Carlson et al. (2008), for example, reported that the protective effect of midlife participation in cognitively engaging activities was significant for APOE ε4 carriers but not for noncarriers, whereas Wilson et al. (2002) reported no difference in the protective effect by APOE status. Finally, using a community sample of older women, one study investigated the interaction between APOE ε4 and estrogen in cognitive impairment and found an association between the current estrogen use and attenuated risk of cognitive impairment only in noncarriers (Yaffe et al. 2000).
5 Current Directions
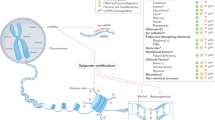
Numerous efforts continue to attempt to identify associations between gene variants and AD, primarily through large GWAS consortia and sequencing efforts to find rare variants (Jonsson et al. 2012). These gene discovery studies are being complemented by replication studies of previous gene candidates, often using additional information about detailed phenotyping, such as that gained through neuroimaging (Meda et al. 2012) or metabolomics . Gene–environment interactions are being pursued through classic epidemiological designs, where specific genes and environmental risk factors are evaluated in the same models, as described above in Sect. 7.4.2. A recent complement to this line of investigation is to evaluate the extent to which epigenetic changes may be induced by environmental risk factors for AD and hence account for gene–environment interactions (Chouliaras et al. 2010).
5.1 Metabolomics and Dementia
Metabolomics refer to the study of small molecules and metabolites in cells, tissues, and body fluids. It is now possible to identify and quantify hundreds to thousands of metabolites simultaneously. Hopes are that these metabolites will represent new biomarkers for disease detection (beyond Aβ and tau in cerebral spinal fluid), disease progression, and identification of networks implicated in disease pathogenesis, as envisioned by the new NIA-AA diagnostic criteria. Complex mathematical models are applied to detect differences in metabolic signatures between diseased and healthy individuals. Like other “omics” approaches, metabolomics is a hypothesis-free method of studying the state of the organism at the global level rather than studying one or a few potential biomarkers (Quinones and Kaddurah-Daouk 2009).
Studies investigating metabolomic changes in dementia are still rather sparse, but the field is rapidly growing. One of the first studies was conducted already in 1995, when Shonk et al. (1995) were able to demonstrate that AD patients had lower levels of N-acetylaspartate (NAA). These results have since been confirmed by several other studies (Adalsteinsson et al. 2000; Block et al. 2002; Rami et al. 2007). More recently, Kaddurah-Daouk et al. (2011) performed a pilot-study to assess the feasibility of identifying AD patients through metabolites in cerebrospinal fluid samples. They found that a model including levels of tryptophan, norepinephrine, and indoleacetic acid was able to completely separate the AD patient group from the control group. Moreover, they were also able to identify important differences between AD patients and controls in the levels of several metabolites related to the norepinephrine and serotonergic pathways. The largest difference was found in the level of norepinephrine, which was significantly decreased in patients with AD.
Focusing on lipidomics , Han et al. (2011) studied the levels of over 800 molecular lipid species in plasma from 26 AD patients and 26 cognitively normal controls. They found significantly reduced levels in eight molecular species of sphingomyelin and significantly increased levels of two ceramide species in AD patients compared with controls. Furthermore, they showed that the ratios of ceramide to sphingomyelin species better discriminated between AD patients and controls compared with either metabolite alone.
Although the field is still in its beginning years, metabolomics is providing dementia research several interesting new directions for further investigations. If new biomarkers for early disease detection and diagnosis can be identified, metabolomics could be of great importance for dementia, since the disease has such a long preclinical phase and is very difficult to diagnose. By using metabolic signatures rather than single biomarkers, it is also possible to capture a more comprehensive picture about the pathology of complex diseases.
5.2 Epigenetics and Dementia
Epigenetics refer to the regulation of gene expression through reversible mechanisms, mainly changes in DNA methylation and chromatin structure (epigenetics is described in more detail in Chap. 6). Several lines of evidence suggest that epigenetic mechanisms are involved in dementia, including the higher frequency of sporadic cases over familial cases, the non-Mendelian inheritance pattern, and the late age of onset (Bihaqi et al. 2012). The following sections provide examples of specific epigenetic mechanisms related to AD risk.
5.2.1 Dysregulation in Epigenetic Mechanisms
Deficient dietary intake of vitamins B6 and B12 and folic acid, which has been implicated in AD (Chouliaras et al. 2010), has been shown to influence the methylation regulatory pathway , specifically through a gene-encoding methylenetetrahydrofolate reductase (MTHFR). In turn, a polymorphism of MTHFR is associated with AD (Wang et al. 2008). Thus, the process by which B6, B12, and folic acid deficiency increases risk for AD may be through dysregulation of this epigenetic mechanism (see Kwok 2010 review).
The methylation status of repetitive elements, such as Line1, Alu, and SAT-α, is also thought to be important for global DNA methylation. Bollati et al. (2011) studied methylation in repetitive elements of AD patients and healthy controls and found a significant increase in methylation status for the transposable element LINE1.
5.2.2 Differences in Methylation of Specific Genes
Tissue-specific methylation patterns (both hypo- and hypermethylation) are associated with cancers, autoimmune diseases, and some neurological disorders, such as DLB (Fernandez et al. 2012), although no significant differences in patterns at 1,505 CpG sites could be detected in a small sample (n = 11) of AD brain tissues. Nevertheless, other studies have found that several genes already implicated in AD show dysregulation in methylation status. APP , the gene most commonly mutated in familial AD, has been shown to be hypomethylated in AD patients compared with healthy controls (West et al. 1995). Cell culture studies have shown that PSEN1, the second gene often mutated in familial AD, is overexpressed in response to alteration in methylation, leading to increase in Aβ production (Wang et al. 2008). Finally, the APOE gene has a hypomethylated CpG-poor promoter and a fully methylated 3′-CpG-island, that contains the sequences for the ε4-haplotype. Aberrant epigenetic control in this CpG-island may contribute to late-onset AD. Wang et al. (2008) showed hypermethylation of the APOE promoter in cells both from postmortem prefrontal cortex and lymphocytes of AD patients compared with controls. Without appropriate longitudinal samples, it is impossible to know whether these differences are a cause or a consequence of the AD pathology (Chouliaras et al. 2010).
5.2.3 Epigenetics as a Mechanism for Environmental Risks and for Gene–Environment Interaction
Environmental risk factors for dementia may act by inducing epigenetic changes, for example, deficiency of vitamin B12, B6, and folate, as discussed above. Head injury is another risk factor for dementia that has been shown to induce epigenetic changes (Chouliaras et al. 2010). Further work is necessary to determine whether epigenetic changes may also underlie gene–environment interactions (Iraola-Guzmán, et al. 2011). For example, it has been suggested that the methylated 3′-CpG-island in APOE may be dysregulated by exposure to environmental triggers, thus lending ε4 carriers more susceptible to developing AD pathology (Wang et al. 2008).
In 1989, Barker et al. proposed the “Fetal Basis of Adult Disease” hypothesis , postulating that many adult diseases actually have fetal origin, where insult at a critical period of development may result in changes in gene expression leading to functional deficits later in life (Barker et al. 1989). Along the same line, Lahiri et al. (2008) proposed the “Latent Early-Life Associated Regulation” (LEARn) model for AD, stating that environmental factors early in life can lead to latent expression of specific genes later in life. According to the model, environmental agents (such as heavy metals, cytokines, or dietary factors) can induce epigenetic changes in a gene, leading to changes in gene expression either immediately or after a period of latency in response to a secondary trigger. Animal studies support this hypothesis. Basha et al. (2005) showed that lead exposure in rodents led to a delayed overexpression of APP 20 months later. In contrast, no change in APP expression could be seen in response to lead exposure during old age. Further work is called for examining longitudinal differences in total methylation and gene-specific epigenetic dysregulation in concert with information about early and midlife exposures.
6 Summary
Dementia is one of the most common disorders in older adults, affecting an estimated 35.6 million people worldwide (or about 5 % of those aged 60 and older). Prevalence increases markedly with age; the number affected will increase as the proportion of the population aged 60 years and older, and especially aged 80 years and older, climbs sharply in both developed and developing countries. The pathophysiology of AD suggests hypotheses about genetic bases for the disorder, that is, that pathways concerning deposition of Aβ may be of importance. Twin and family studies demonstrate that AD is one of the most heritable disorders, with genetic factors accounting for as much as 79 % of the variation in AD.
Mutations in three genes, APP, PSEN1, and PSEN2, all related to Aβ, are highly penetrant, follow Mendelian transmission, but account for a tiny fraction of all AD cases, and mostly those with a relatively early age of onset. APOE continues to be the most important susceptibility gene for AD. Yet, the population attributable fraction for APOE is estimated at approximately 25 %, indicating that a great deal of the heritability for AD must be found in other genes of smaller effect size.
Even with GWAS , we have not succeeded at accounting for all genetic influences. Genes identified through GWAS have very small effect sizes, and little if anything will be gained from further gene discovery efforts. Thus, we are far from the point of personalized genetic risk profiling beyond using information on age, family history, and APOE status.
This situation leads us to pose two possibilities: might it be that AD is not just polygenic but also the result of risk alleles in a cluster of genes (most often including APOE), where some constellations of risk alleles are important in some individuals while other combinations are important in other individuals? Or might different combinations of risk alleles and environmental triggers (manifested as gene–environment interactions) characterize different individuals and thus thwart the ability to predict genetic risk? There are very few strong “environmental” risk (or protective) factors, and there is evidence that many of these work together with genes. The most consistent findings point to the importance of vascular risks in combination with APOE, which is related to cholesterol transport and Aβ42 levels. We urge further work to understand the extent and nature of gene–gene and gene–environment interactions and their role in the pathogenesis of AD. For example, one promising line of research may be in exploring the role of epigenetic mechanisms in explaining how environmental factors may impinge on genetic predisposition and trigger development of the disease.
References
Abraham, R., Moskvina, V., Sims, R., Hollingworth, P., Morgan, A., Georgieva, L., et al. (2008). A genome-wide association study for late-onset Alzheimer’s disease using DNA pooling. BMC Medical Genomics, 1, 44.
Adalsteinsson, E., Sullivan, E. V., Kleinhans, N., Spielman, D. M., & Pfefferbaum, A. (2000). Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet, 355(9216), 1696–1697.
Aggarwal, N. T., Bienias, J. L., Bennett, D. A., Wilson, R. S., Morris, M. C., Schneider, J. A., et al. (2006). The relation of cigarette smoking to incident Alzheimer’s disease in a biracial urban community population. Neuroepidemiology, 26(3), 140–146.
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 270–279.
Alexopoulos, G. S. (2005). Depression in the elderly. Lancet, 365, 1961–1970.
Andel, R., Crowe, M., Pedersen, N. L., Mortimer, J., Crimmins, E., Johansson, B., & Gatz, M. (2005). Complexity of work and risk of Alzheimer’s disease: A population-based study of Swedish twins. Journals of Gerontology: Series B: Psychological Sciences, 60, P251–P258.
Antunez, C., Boada, M., Gonzalez-Perez, A., Gayan, J., Ramirez-Lorca, R., Marin, J., et al. (2011). The membrane-spanning 4-domains, subfamily A (MS4A) gene cluster contains a common variant associated with Alzheimer’s disease. Genome Medicine, 3(5), 33.
Ballard, C. G. (2004). Definition and diagnosis of dementia with Lewy bodies. Dementia and Geriatric Cognitive Disorders, 17, 15–24.
Barberger-Gateau, P., Raffaitin, C., Letenneur, L., Berr, C., Tzourio, C., Dartigues, J. F., & Alpérovitch, A. (2007). Dietary patterns and risk of dementia: the Three-City cohort study. Neurology, 69(20), 1921–1930.
Barker, D. J., Winter, P. D., Osmond, C., Margetts, B., & Simmonds, S. J. (1989). Weight in infancy and death from ischaemic heart disease. Lancet, 2(8663), 577–580.
Basha, M. R., Wei, W., Bakheet, S. A., Benitez, N., Siddiqi, H. K., Ge, Y.-W., et al. (2005). The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. Journal of Neuroscience, 25(4), 823–829.
Beecham, G. W., Martin, E. R., Li, Y. J., Slifer, M. A., Gilbert, J. R., Haines, J. L., & Pericak-Vance, M. A. (2009). Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. American Journal of Human Genetics, 84(1), 35–43.
Bennet, A. M., Reynolds, C. A., Gatz, M., Blennow, K., Pedersen, N. L., & Prince, J. A. (2010). Pleiotropy in the presence of allelic heterogeneity: Alternative genetic models for the influence of APOE on serum LDL, CSF amyloid-β42, and dementia. Journal of Alzheimer’s Disease, 22(1), 129–134.
Bergem, A. L., & Lannfelt, L. (1997). Apolipoprotein E type 4 allele, heritability, and age at onset in twins with Alzheimer’s disease and vascular dementia. Clinical Genetics, 52(5), 408–413.
Bergem, A. L. M., Engedal, K., & Kringlen, E. (1997). The role of heredity in late-onset Alzheimer disease and vascular dementia: A twin study. Archives of General Psychiatry, 54(3), 264–270.
Berr, C., Wancata, J., & Ritchie, K. (2005). Prevalence of dementia in the elderly in Europe. European Neuropsychopharmacology, 15(4), 463–471.
Bertram, L. (2011). Alzheimer’s genetics in the GWAS era: A continuing story of ‘replications and refutations’. Current Neurology and Neuroscience Reports, 11(3), 246–253.
Bertram, L., & Tanzi, R. E. (2001). Of replications and refutations: The status of Alzheimer’s disease genetic research. Current Neurology and Neuroscience Reports, 1(5), 442–450.
Bertram, L., & Tanzi, R. E. (2008). Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nature Reviews Neuroscience, 9(10), 768–778.
Bertram, L., McQueen, M. B., Mullin, K., Blacker, D., & Tanzi, R. E. (2007). Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nature Genetics, 39(1), 17–23.
Bertram, L., Lange, C., Mullin, K., Parkinson, M., Hsiao, M., Hogan, M. F., et al. (2008). Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. American Journal of Human Genetics, 83(5), 623–632.
Bertram, L., McQueen, M., Mullin, K., Blacker, D., & Tanzi, R. (2011, April 18). The AlzGene Database. Retrieved from http://www.alzgene.org. Accessed 23 May 2012.
Bihaqi, S. W., Schumacher, A., Maloney, B., Zhang, Y., Lahiri, D. K., & Zawia, N. H. (2012). Do epigenetic pathways initiate late onset Alzheimer disease (LOAD): Towards a new paradigm. Current Alzheimer Research, 9(5), 574–588.
Block, W., Jessen, F., Traber, F., Flacke, S., Manka, C., Lamerichs, R., et al. (2002). Regional N-acetylaspartate reduction in the hippocampus detected with fast proton magnetic resonance spectroscopic imaging in patients with Alzheimer disease. Archives of Neurology, 59(5), 828–834.
Bollati, V., Galimberti, D., Pergoli, L., Dalla Valle, E., Barretta, F., Cortini, F., et al. (2011). DNA methylation in repetitive elements and Alzheimer disease. Brain, Behavior, and Immunity, 25(6), 1078–1083.
Bonifati, V. (2008). Recent advances in the genetics of dementia with Lewy bodies. Current Neurology and Neuroscience Reports, 8, 187–189.
Breitner, J. C. S., Welsh, K. A., Gau, B. A., McDonald, W. M., Steffens, D. C., Saunders, A. M., et al. (1995). Alzheimer’s disease in the National Academy of Sciences—National Research Council Registry of aging twin veterans: III. Detection of cases, longitudinal results, and observations on twin concordance. Archives of Neurology, 52, 763–771.
Brommelhoff, J. A., Gatz, M., Johansson, B., McArdle, J. J., Fratiglioni, L., & Pedersen, N. L. (2009). Depression as a risk factor or prodomal feature for dementia? Findings in a population-based sample of Swedish twins. Psychology and Aging, 24, 373–384.
Bullock, J. M., Medway, C., Cortina-Borja, M., Turton, J. C., Prince, J. A., Ibrahim-Verbaas, C. A. et al. (2013). Discovery by the Epistasis Project of an epistatic interaction between the GSTM3 gene and the HHEX/IDE/KIF11 locus in the risk of Alzheimer's disease. Neurobiology of Aging, 34(4), 1309e1–7.
Canadian Study of Health and Aging Workshop Group (1994). Canadian study of health and aging: Study methods and prevalence of dementia. Canadian Medical Association Journal, 150, 899–913.
Carlson, M. C., Helms, M. J., Steffens, D. C., Burke, J. R., Potter, G. G., & Plassman, B. L. (2008). Midlife activity predicts risk of dementia in older male twin pairs. Alzheimer’s and Dementia, 4(5), 324–331.
Carrasquillo, M. M., Zou, F., Pankratz, V. S., Wilcox, S. L., Ma, L., Walker, L. P., et al. (2009). Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nature Genetics, 41(2), 192–198.
Cataldo, J. K., Prochaska, J. J., & Glantz, S. A. (2010). Cigarette smoking is a risk factor for Alzheimer’s disease: An analysis controlling for tobacco industry affiliation. Journal of Alzheimers Disease, 19(2), 465–480.
Chabriat, H., Joutel, A., Dichgans, M., Tournier-Lasserve, E., & Bousser, M.-G. (2009). Cadasil. The Lancet Neurology, 8(7), 643–653.
Chouliaras, L., Rutten, B. P., Kenis, G., Peerbooms, O., Visser, P. J., Verhey, F., et al. (2010). Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Progress in Neurobiology, 90(4), 498–510.
Chui, H. C. (2006). Vascular cognitive impairment: Today and tomorrow. Alzheimer’s and Dementia, 2(3), 185–194.
Chui, H. C., Victoroff, J. I., Margolin, D., Jagust, W., Shankle, R., & Katzman, R. (1992). Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology, 42(3), 473–480.
Combarros, O., Cortina-Borja, M., Smith, A. D., & Lehmann, D. J. (2009). Epistasis in sporadic Alzheimer’s disease. Neurobiology of Aging, 30(9), 1333–1349.
Coon, K. D., Myers, A. J., Craig, D. W., Webster, J. A., Pearson, J. V., Lince, D. H., et al. (2007). A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. The Journal of Clinical Psychiatry, 68(4), 613–618.
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science, 261(5123), 921–923.
Cotman, C. W., & Berchtold, N. C. (2002). Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences, 25(6), 295–301.
Craft, S. (2009). The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Archives of Neurology, 66(3), 300–305.
Cummings, J. L., & Cole, G. (2002). Alzheimer disease. JAMA: The Journal of the American Medical Association, 287, 2335–2338.
Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., et al. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of National Academy of Sciences of the United States of America, 108(7), 3017–3022.
Eriksson, U. K., Bennet, A. M., Gatz, M., Dickman, P. W., & Pedersen, N. L. (2010). Non-stroke cardiovascular disease and risk of Alzheimer’s disease and dementia. Alzheimer Disease and Associated Disorders, 24, 213–219.
Evans, R. M., Emsley, C. L., Gao, S., Sahota, A., Hall, K. S., Farlow, M. R., & Hendrie, H. (2000). Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: A population-based study of African Americans. Neurology, 54(1), 240–242.
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kulkull, W. A., Mayeux, R., et al. (1997). Effects of age, sex and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta analysis. Journal of the American Medical Association, 278(16), 1349–1356.
Fernandez, A. F., Assenov, Y., Martin-Subero, J. I., Balint, B., Siebert, R., Taniguchi, H., et al. (2012). A DNA methylation fingerprint of 1628 human samples. Genome Research, 22(2), 407–419.
Ferri, C. P., Prince, M., Brayne, C., Brodaty, H., Fratiglioni, L., Ganguli, M., et al. (2005). Global prevalence of dementia: A Delphi consensus study. Lancet, 366(9503), 2112–2117.
Forman, M. S., Lee, V. M.-Y., & Trojanowski, J. Q. (2005). Nosology of Parkinson’s disease: Looking for the way out of a quackmire. Neuron, 47(4), 479–482.
Forman, M. S., Farmer, J., Johnson, J. K., Clark, C. M., Arnold, S. E., Coslett, H. B., et al. (2006). Frontotemporal dementia: Clinicopathological correlations. Annals of Neurology, 59(6), 952–962.
Gatz, M. (2007). Genetics, dementia and the elderly. Current Directions in Psychological Science, 16, 123–127.
Gatz, M., & Pedersen, N. L. (1996). Use of twin samples to estimate the heritability of Alzheimer’s disease: A methodological note. Alzheimer’s Research, 2, 229–231.
Gatz, M., Pedersen, N. L.., Berg, S., Johansson, B., Johansson, K., Mortimer, J. A., et al. (1997). Heritability for Alzheimer’s disease: The study of dementia in Swedish twins. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences, 52A(2), M117–M125.
Gatz, M., Reynolds, C. A., Fratiglioni, L., Johansson, B., Mortimer, J. A., Berg, S., et al. (2006). Role of genes and environments for explaining Alzheimer disease. Archives of General Psychiatry, 63(2), 168–174.
Gatz, M., Mortimer, J. A. Fratiglioni, L., Johansson, B., Berg, S., Andel, R., et al. (2007). Accounting for the relationship between low education and dementia: A twin study. Physiology & Behavior, 92, 232–237.
Gauthier, S., Reisberg, B., Zaudig, M., Petersen, R. C., Ritchie, K., Broich, K., et al. (2006). Mild cognitive impairment. Lancet, 367(9518), 1262–1270.
Geda, Y. E., Knopman, D. S., Mrazek, D. A., Jicha, G. A., Smith, G. E., Negash, S., et al. (2006). Depression, apolipoprotein E genotype, and the incident of mild cognitive impairment: A prospective cohort study. Archives of Neurology, 63, 435–440.
Goate, A., Chartier-Harlin, M.-C., Mullan, M., Brown, J., Crawford, F., Fidani, L., et al. (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature, 349, 704–706.
Gorelick, P. B., Scuteri, A., Black, S. E., DeCarli, C., Greenberg, S. M., Iadccola, C., et al. (2011). Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 42(9), 2672–2713.
Green, R. C., Cupples, L. A., Go, R., Benke, K. S., Edeki, T., Griffith, P. A. (2002). Risk of dementia among White and African American relatives of patients with Alzheimer disease. JAMA: The Journal of the American Medical Association, 287(3), 329–336.
Grupe, A., Abraham, R., Li, Y., Rowland, C., Hollingworth, P., Morgan, A., et al. (2007). Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Human Molecular Genetics, 16(8), 865–873.
Hachinski, V. C., & Bowler, J. V. (1993). Vascular dementia. Neurology, 43(10), 2159–2160.
Hall, K., Murrell, J., Ogunniyi, A., Deeg, M., Baiyewu, O., Gao, S., et al. (2006). Cholesterol, APOE genotype, and Alzheimer disease: An epidemiologic study of Nigerian Yoruba. Neurology, 66, 223–227.
Han, X., Rozen, S., Boyle, S. H., Hellegers, C., Cheng, H., Burke, J. R., et al. (2011). Metabolomics in early Alzheimer’s disease: Identification of altered plasma sphingolipidome using shotgun lipidomics. PLoS One, 6(7), e21643.
Hardy, J. (2006). Alzheimer’s disease: The amyloid cascade hypothesis: An update and reappraisal. Journal of Alzheimer’s Disease, 9, 151–153.
Harold, D., Abraham, R., Hollingworth, P., Sims, R., Gerrish, A., Hamshere, M. L., et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nature Genetics, 41(10), 1088–1093.
Heinzen, E. L., Need, A. C., Hayden, K. M., Chiba-Falek, O., Roses, A. D., Strittmatter, W. J., et al. (2010). Genome-wide scan of copy number variation in late-onset Alzheimer’s disease. Journal of Alzheimer’s Disease, 19(1), 69–77.
Heun, R., Kölsch, H., Ibrahim-Verbaas, C. A., Combarros, O., Aulchenko, Y. S., Breteler, M., et al. (2012). Interactions between PPAR-α and inflammation-related cytokine genes on the development of Alzheimer’s disease, observed by the Epistasis Project. International Journal of Molecular Epidemiology and Genetics, 3(1), 39–47.
Hollingworth, P., Harold, D., Sims, R., Gerrish, A., Lambert, J. C., Carrasquillo, M. M., et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nature Genetics, 43(5), 429–435.
Hu, X., Pickering, E., Liu, Y. C., Hall, S., Fournier, H., Katz, E., et al. (2011). Meta-analysis for genome-wide association study identifies multiple variants at the BIN1 locus associated with late-onset Alzheimer’s disease. PloS One, 6(2), e16616.
Huang, T. L., Zandi, P. P., Tucker, K. L., Fitzpatrick, A. L., Kuller, L. H., Fried, L. P., et al. (2005). Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology, 65(9), 1409–1414.
Hyman, B. T., Phelps, C. H., Beach, T. G., Bigio, E. H., Cairns, N. J., Carrillo, M. C., et al. (2012). National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s and Dementia, 8(1), 1–13.
Iraola-Guzmán, S., Extivill, X., & Rabionet, R. (2011). DNA methylation in neurodegenerative disorders: A missing link between genome and environment? Clinical Genetics., 80, 1–14.
Irie, F., Fitzpatrick, A. L., Lopez, O. L., Kuller, L. H., Peila, R., Newman, A. B., & Launer, L. J. (2008a). Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE ε4: The Cardiovascular Health Study Cognition Study. Archives of Neurology, 65(1), 89–93.
Irie, F., Masaki, K. H., Petrovitch, H., Abbott, R. D., Ross, G. W., Taaffe, D. R., et al. (2008b). Apolipoprotein E ε4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: The Honolulu-Asia Aging Study. Archives of General Psychiatry, 65(8), 906–912.
Jeste, D., Blacker, D., Blazer, D., Ganguli, M., Grant, I., Paulsen, J., et al. (2010, January 7). Neurocognitive disorders: A proposal from the DSM-5 neurocognitive disorders working group. DSM-5 Neurocognitive Criteria. http://www.dsm5.org/Proposed%20Revision%20Attachments/APA%20Neurocognitive%20Disorders%20Proposal%20for%20DSM-5.pdf. Accessed 21 Oct. 2010.
Johnston, J. M., Nazar-Stewart, V., Kelsey, S. F., Kamboh, M. I., & Ganguli, M. (2000). Relationships between cerebrovascular events, APOE polymorphism and Alzheimer’s disease in a community sample. Neuroepidemiology, 19, 320–326.
Jonsson, T., Atwal, J. K., Steinberg, S., Snaedal, J., Jonsson, P. V., Bjornsson, S., et al. (2012). A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature, 488, 96–99.
Kaddurah-Daouk, R., Rozen, S., Matson, W., Han, X., Hulette, C. M., Burke, J. R., et al. (2011). Metabolomic changes in autopsy-confirmed Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 309–317.
Kalaria, R. N., & Ballard, C. (1999). Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Disease and Associated Disorders, 13(3), S115–S123.
Kalaria, R. N., Maestre, G. E., Arizaga, R., Friedland, R. P., Galasko, D., Hall, K., et al. (2008). Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. The Lancet Neurology, 7(9), 812–826.
Kölsch, H., Lehmann, D. J., Ibrahim-Verbaas, C. A., Combarros, O., van Duijn, C. M., Hammond, N., et al. (2012). Interaction of insulin and PPAR-α genes in Alzheimer’s disease: The Epistasis Project. Journal of Neural Transmission, 119(4), 473–479.
Kremen, W. S., Thompson-Brenner, H., Leung, Y. J., Grant, M. D., Franz, C. E., Eisen, S. A., et al. (2006). Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA). Twin Research and Human Genetics, 9(6), 1009–1022.
Kwok, J. B. J. (2010). Role of epigenetics in Alzheimer’s and Parkinson’s disease. Epigenomics, 2(5), 671–682.
Lahiri, D. K., Zawia, N. H., Greig, N. H., Sambamurti, K., & Maloney, B. (2008). Early-life events may trigger biochemical pathways for Alzheimer’s disease: The “LEARn” model. Biogerontology, 9(6), 375–379.
Lambert, J. C., Heath, S., Even, G., Campion, D., Sleegers, K., Hiltunen, M., et al. (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nature Genetics, 41(10), 1094–1099.
Lambert, J. C., Grenier-Boley, B., Harold, D., Zelenika, D., Chouraki, V., Kamatani, Y., et al. (2013). Genome-wide haplotype association study identifies the FRMD4A gene as a risk locus for Alzheimer’s disease. Molecular Psychiatry, 18(4), 461–470.
Lautenschlager, N. T., Cupples, L. A., Rao, V. S., Auerbach, S. A., Becker, R., Burke, J., et al. (1996). Risk of dementia among relatives of Alzheimer’s disease patients in the MIRAGE study: What is in store for old? Neurology, 46(3), 641–650.
Lee, J. H., Cheng, R., Barral, S., Reitz, C., Medrano, M., Lantigua, R., et al. (2011). Identification of novel loci for Alzheimer disease and replication of CLU, PICALM, and BIN1 in Caribbean Hispanic individuals. Archives of Neurology, 68(3), 320–328.
Levine, B. (2007). What does the population attributable fraction mean? Preventing Chronic Disease, 4(1), 1–5.
Levy-Lahad, E., Wijsman, E. M., Nemens, E., Anderson, L., Goddard, K. A., Weber, J. L., et al. (1995). A familial Alzheimer’s disease locus on chromosome 1. Science, 269(5226), 970–973.
Li, G., Silverman, J. M., Smith, C. J., Zaccario, M. L., Schmeidler, J., Mohs, R. C., & Davis, K. L. (1995). Age at onset and familial risk in Alzheimer’s disease. The American Journal of Psychiatry, 152(3), 424–430.
Li, H., Wetten, S., Li, L., St Jean, P. L., Upmanyu, R., Surh, L., et al. (2008). Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Archives of Neurology, 65(1), 45–53.
Lobo, A., Launer, L. J., Fratiglioni, L., Andersen, K., Di Carlo, A., Breteler, M. M. B., et al. (2000). Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurology, 54(11), S4–S9.
Logue, M. W., Schu, M., Vardarajan, B. N., Buros, J., Green, R. C., Go, R. C., et al. (2011). A comprehensive genetic association study of Alzheimer disease in African Americans. Archives of Neurology, 68(12), 1569–1579.
Lopez, O. L., McDade, E., Riverol, M., & Becker, J. T. (2011). Evolution of the diagnostic criteria for degenerative and cognitive disorders. Current Opinion in Neurology, 24(6), 532–541.
Mayeux, R., Ottman, R., Maestre, G., Ngai, C., Tang, M. X., Ginsberg, H., et al. (1995). Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology, 45, 555–557.
McKeith, I. G., Dickson, D. W., Lowe, J., Emre, M., O’Brien, J. T., Feldman, H., et al. (2005). Diagnosis and management of dementia with Lewy bodies: Third report of the DLB consortium. Neurology, 65(12), 1863–1872.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 263–269.
Meda, S. A., Narayanan, B., Liu, J., Perrone-Bizzozero, N. I., Stevens, M. C., Calhoun, V. D., et al. (2012). A large scale multivariate parallel ICA method reveals novel imaging-genetic relationships for Alzheimer’s disease in the ADNI cohort. Neuroimage, 60(3), 1608–1621.
Meyer, J. M., & Breitner, J. C. S. (1998). Multiple threshold model for the onset of Alzheimer’s disease in the NAS-NRC Twin Panel. American Journal of Medical Genetics (Neuropsychiatric Genetics), 81, 92–97.
Moorhouse, P., & Rockwood, K. (2008). Vascular cognitive impairment: Current concepts and clinical developments. The Lancet Neurology, 7(3), 246–255.
Mrak, R., & Griffin, W. S. T. (2007). Dementia with Lewy bodies: Definition, diagnosis, and pathogenic relationship to Alzheimer’s disease. Neuropsychiatric Diseases and Treatment, 3(5), 619–625.
Mudher, A., & Lovestone, S. (2002). Alzheimer’s disease—Do tauists and baptists finally shake hands? Trends in Neurosciences, 25(1), 22–26.
Naj, A. C., Beecham, G. W., Martin, E. R., Gallins, P. J., Powell, E. H., Konidari, I., et al. (2010). Dementia revealed: Novel chromosome 6 locus for late-onset Alzheimer disease provides genetic evidence for folate-pathway abnormalities. PLoS Genetics, 6(9).
Naj, A. C., Jun, G., Beecham, G. W., Wang, L. S., Vardarajan, B. N., Buros, J., et al. (2011). Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nature Genetics, 43(5), 436–441.
O’Brien, J. T., Erkinjuntti, T., Reisberg, B., Román, G., Sawada, T., Pantoni, L., et al. (2003). Vascular cognitive impairment. The Lancet Neurology, 2, 89–98.
Oddo, S., Caccamo, A., Tran, L., Lambert, M. P., Glabe, C. G., Klein, W. L., & LaFerla, F. M. (2006). Temporal profile of amyloid-beta (Abeta) oligomerization in an in vivo model of Alzheimer disease: A link between Abeta and tau pathology. Journal of Biological Chemistry, 281(3), 1599–1604.
Pedersen, N. L. (2010). Reaching the limits of genome-wide significance in Alzheimer disease: Back to the environment. JAMA: The Journal of the American Medical Association, 303(18), 1864–1865.
Pedersen, N. L., Posner, S. F., & Gatz, M. (2001). Multiple-threshold models for genetic influences on age of onset for Alzheimer disease: Findings in Swedish twins. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 105, 724–728.
Peila, R., White, L. R., Petrovich, H., Masaki, K., Ross, G. W., Havlik, R. J., & Launer, L. J. (2001). Joint effect of the ApoE gene and midlife systolic blood pressure on late-life cognitive impairment: The Honolulu-Asia Aging Study. Stroke, 32, 2882–2889.
Peila, R., Rodriguez, B. L., & Launer, L. J. (2002). Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes, 51, 1256–1262.
Plassman, B. L., & Breitner, J. C. S. (1997). The genetics of dementia in late life. Psychiatric Clinics of North America, 20(1), 60–76.
Plassman, B. L., Steffens, D. C., Burke, J. R., Welsh-Bohmer, K. A., Helms, M. J., & Breitner, J. C. S. (2004). Concordance for Alzheimer’s disease in the NAS-NRC Twin Registry of WWII Veterans. Neurobiology of Aging, 25, S514.
Plassman, B. L., Langa, K. M., Fisher, G. G., Heeringa, S. G., Weir, D. R., Ofstedal, M. B., et al. (2007). Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology, 29(1–2), 125–132.
Podewils, L. J., Guallar, E., Kuller, L. H., Fried, L. P., Lopez, O. L., Carlson, M., & Lyketsos, C. G. (2005). Physical activity, APOE genotype, and dementia risk: Findings from the Cardiovascular Health Cognition Study. American Journal of Epidemiology, 161(7), 639–651.
Poduslo, S. E., Huang, R., Huang, J., & Smith, S. (2009). Genome screen of late-onset Alzheimer’s extended pedigrees identifies TRPC4AP by haplotype analysis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(1), 50–55.
Potkin, S. G., Guffanti, G., Lakatos, A., Turner, J. A., Kruggel, F., Fallon, J. H., et al. (2009). Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PloS One, 4(8), e6501.
Quinones, M. P., & Kaddurah-Daouk, R. (2009). Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiology of Disease, 35(2), 165–176.
Rabinovici, G. D., & Miller, B. L. (2010). Frontotemporal lobar degeneration. CNS Drugs, 24(5), 375–398.
Räihä, I., Kaprio, J., Koskenvuo, M., Rajala, T., & Sourander, L. (1996). Alzheimer’s disease in Finnish twins. Lancet, 347, 573–578.
Rami, L., Gomez-Anson, B., Bosch, B., Sanchez-Valle, R., Monte, G. C., Villar, A., & Molinuevo, J. L. (2007). Cortical brain metabolism as measured by proton spectroscopy is related to memory performance in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 24(4), 274–279.
Reiman, E. M., Webster, J. A., Myers, A. J., Hardy, J., Dunckley, T., Zismann, V. L., et al. (2007). GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron, 54(5), 713–720.
Reiman, E. M., McKhann, G. M., Albert, M. S., Sperling, R. A., Petersen, R. C., & Blacker, D. (2011). Alzheimer’s disease: Implications of the updated diagnostic and research criteria. The Journal of Clinical Psychiatry, 72(9), 1190–1196.
Reitz, C., den Heijer, T., van Duijn, C., Hofman, A., & Breteler, M. M. (2007). Relation between smoking and risk of dementia and Alzheimer disease: The Rotterdam Study. Neurology, 69(10), 998–1005.
Roberts, N. J., Vogelstein, J. T., Parmigiani, G., Kinzler, K. W., Vogelstein, B., & Velculescu, V. E. (2012). The predictive capacity of personal genome sequencing. Science Translational Medicine, 4(133), 1–9.
Rogaev, E. I., Sherrington, R., Rogaeva, E. A., Levesque, G., Ikeda, M., Liang, Y., et al. (1995). Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s type 3 gene. Nature, 376(6543), 775–778.
Román, G. C., Tatemichi, T. K., Erkinjuntti, T., Cummings, J. L., Masdeu, J. C., Garcia, J. H., et al. (1993). Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology, 43(2), 250–260.
Rovio, S., Kåreholt, I., Helkala, E.-L., Viitanen, M., Vinblad, B., Tuomilehto, J., et al. (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. The Lancet Neurology, 4, 705–711.
Saunders, A. M., Strittmatter, W. J., Schmechel, D., St. George-Hyslop, P. H., Pericak-Vance, M. A., Joo, S. H., et al. (1993). Association of apolipoprotein E allele ε4 with late-onset familial and sporadic Alzheimer’s disease. Neurology, 43(1), 1467–1472.
Schneider, J. A., & Bennett, D. A. (2010). Where vascular meets neurodegenerative disease. Stroke, 41(10), S144–S146.
Schneider, J. A., Arvanitakis, Z., Bang, W., & Bennett, D. A. (2007). Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology, 69, 2197–2204.
Seshadri, S., Fitzpatrick, A. L., Ikram, M. A., DeStefano, A. L., Gudnason, V., Boada, M., et al. (2010). Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA: The Journal of the American Medical Association, 303(18), 1832–1840.
Sherrington, R., Rogaev, E. I., Liang, Y., Rogaeva, E. A., Levesque, G., Ikeda, M., et al. (1995). Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature, 375(6534), 754–760.
Sherva, R., Baldwin, C. T., Inzelberg, R., Vardarajan, B., Cupples, L. A., Lunetta, K., et al. (2011). Identification of novel candidate genes for Alzheimer’s disease by autozygosity mapping using genome wide SNP data. Journal of Alzheimer’s Disease: JAD, 23(2), 349–359.
Shonk, T. K., Moats, R. A., Gifford, P., Michaelis, T., Mandigo, J. C., Izumi, J., & Ross, B. D. (1995). Probable Alzheimer disease: Diagnosis with proton MR spectroscopy. Radiology, 195(1), 65–72.
Silverman, J. M., Li, G., Zaccario, M. L., Smith, C. J., Schmeidler, J., Mohs, R. C., & Davis, K. L. (1994). Patterns of risk in first-degree relatives of patients with Alzheimer’s disease. Archives of General Psychiatry, 51, 577–586.
Silverman, J. M., Smith, C. J., Marin, D. B., Mohs, R. C., & Propper, C. B. (2003). Familial patterns of risk in very late-onset Alzheimer disease. Archives of General Psychiatry, 60(2), 190–197.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia, 7(3), 280–292.
Steffens, D. C., Plassman, B. L., Helms, M. J., Welsh-Bohmer, K. A., Saunders, A. M., & Breitner, J. C. (1997). A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer’s disease. Biological Psychiatry, 41, 851–856.
Steffens, D. C., Plassman, B. L., Helms, M. J., Welsh-Bohmer, K. A., Newman, T. T., & Breitner, J. C. (2000). APOE and AD concordance in twin pairs as predictors of AD in first-degree relatives. Neurology, 54(3), 593–598.
Stevens, M., van Duijn, C. M., Kamphorst, W., de Knijff, P., Heutink, P., van Gool, W. A., et al. (1998). Familial aggregation in frontotemporal dementia. Neurology, 50(6), 1541–1545.
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., & Roses, A. D. (1993). Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90(5), 1977–1981.
Tanzi, R. E. (2012). The genetics of Alzheimer disease. Cold Spring Harbor Perspectives in Medicine, 2(10). doi:10.1101/cshperspect.a006296.
Van Den Heuvel, C., Thornton, E., & Vink, R. (2007). Traumatic brain injury and Alzheimer’s disease: A review. Progress in Brain Research, 161, 303–316.
Van Duijn, C. M., Farrer, L. A., Cupples, A., & Hofman, A. (1993). Genetic transmission of Alzheimer’s disease among families in a Dutch population based study. Journal of Medical Genetics, 30(8), 640–646.
Wang, S. C., Oelze, B., & Schumacher, A. (2008). Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS One, 3(7), e2698
Wang, C. S. M., Burke, J. R., Steffens, D. C., Hulette, C. M., Breitner, J. C. S., & Plassman, B. L. (2009). Twin pairs discordant for neuropathologically confirmed Lewy body dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 80(5), 562–565.
West, R. L., Lee, J. M., & Maroun, L. E. (1995). Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. Journal of Molecular Neuroscience, 6(2),141–146.
White, J. A., Manelli, A. M., Holmberg, K. H., Van Eldik, L. J., & LaDu, M. J. (2005). Differential effects of oligomeric and fibrillar amyloid-β1-42 on astrocyte-mediated inflammation. Neurobiology of Disease, 18(3), 459–465.
Wiederkehr, S., Simard, M., Fortin, C., & van Reekum, R. (2008). Comparability of the clinical diagnostic criteria for vascular dementia: A critical review. Part I. Journal of Neuropsychiatry and Clinical Neuroscience, 20(2), 150–161.
Wijsman, E. M., Pankratz, N. D., Choi, Y., Rothstein, J. H., Faber, K. M., Cheng, R., et al. (2011). Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genetics, 7(2), e1001308.
Williams, J. W., Plassman, B. L., Burke, J., Holsinger, T., & Benjamin, S. (2010, April). Preventing Alzheimer’s disease and cognitive decline: Evidence report/technology assessment No. 193. (AHRQ Publication No. 10-E005). Rockville, MD: Agency for Healthcare Research and Quality. http://www.ahrq.gov/downloads/pub/evidence/pdf/alzheimers/alzcog.pdf. Accessed 18 July 2011.
Wilson, R. S., Mendes De Leon, C. F., Barnes, L. L.., Schneider, J. A., Bienias, J. L., Evans, D. A., & Bennett, D. A. (2002). Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA: The Journal of the American Medical Association, 287(6), 742–748.
Wimo, A., & Prince, M. (2010, September 21). World Alzheimer report 2010: The global economic impact of dementia. Alzheimer Disease International website: http://www.alz.co.uk/research/files/WorldAlzheimerReport2010.pdf. Accessed 19 Mar. 2012.
World Health Organization. (2010). International statistical classification of diseases and related health problems 10th revision. http://apps.who.int/classifications/icd10/browse/2010/en. Accessed 6 Apr. 2012.
Xu, W., Atti, A. R., Gatz, M., Pedersen, N. L., Johansson, B., & Fratiglioni, L. (2011). Midlife overweight and obesity increase late-life dementia risk: A population-based twin study. Neurology, 76, 1568–1574.
Yaffe, K., Haan, M., Byers, A., Tangen, C., & Kuller, L. (2000). Estrogen use, APOE, and cognitive decline: Evidence of gene-environment interaction. Neurology, 54(10), 1949–1954.
Zaccai, J., McCracken, C., & Brayne, C. (2005). A systematic review of prevalence and incidence of dementia with Lewy bodies. Age and Ageing, 34(6), 561–566.
Acknowledgments
Grants R01 AG037985 and P50 AG05142.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Gatz, M., Jang, J., Karlsson, I., Pedersen, N. (2014). Dementia: Genes, Environments, Interactions. In: Finkel, D., Reynolds, C. (eds) Behavior Genetics of Cognition Across the Lifespan. Advances in Behavior Genetics, vol 1. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7447-0_7
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7447-0_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7446-3
Online ISBN: 978-1-4614-7447-0
eBook Packages: Behavioral ScienceBehavioral Science and Psychology (R0)