Abstract
Atrial fibrillation and heart failure are commonly coexisting conditions with important pathophysiologic interactions impacting patient management. Treatment of atrial fibrillation with impaired ventricular function is focused towards preventing adverse hemodynamic effects that may result in more symptoms and decreased exercise tolerance. While rate control using medications or atrioventricular nodal ablation combined with pacing is the primary emphasis of management, rhythm control using pharmacologic or pulmonary vein isolation remains a feasible alternative strategy for some patients. The prevalence, mechanisms, and management strategies of atrial fibrillation and heart failure are reviewed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Heart Failure
- Atrial Fibrillation
- Cardiac Resynchronization Therapy
- Catheter Ablation
- Atrial Fibrillation Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Atrial fibrillation (AF) and heart failure (HF), two increasingly common and coexisting conditions encountered in the aging population, interact in ways that are distinct from the general population of AF patients without heart failure. In AF, the primary treatment goals focus on control of symptoms and reducing risk of stroke. Additionally, in the patient with AF and impaired ventricular function, treatment is focused towards preventing adverse hemodynamic effects that may result in more symptoms and decreased exercise tolerance.
Patients with heart failure have a higher risk of developing AF compared to the normal population. The prevalence of AF increases with worsening New York Heart Association (NYHA) functional class [1]. Additionally, patients with abnormal diastolic function but no clinical heart failure diagnosis also have an increased risk of developing AF [2].
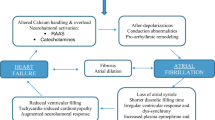
Atrial fibrillation is a disease of the elderly, with 3 out of 4 AF patients between the ages of 65 and 85 years. Interplay between advancing age, comorbidities, and environmental and genetic factors contributes to the development of AF (Fig. 6.1). The prevalence of AF is currently 1–2 %, and is expected to increase with the aging population [3, 4]. Comorbid medical conditions associated with AF including hypertension (HTN), heart failure, valvular heart disease (VHD), cardiomyopathies, coronary artery disease (CAD), obesity, diabetes mellitus, chronic obstructive pulmonary disease (COPD), sleep apnea, and chronic kidney disease are more frequent in the elderly, play a role in propagating AF, and increase morbidity and mortality [5]. Hospitalizations for AF in the United States have increased dramatically (two to threefold) in the last 15 years [6]. The prevalence of heart failure also increases with age, with a lifetime risk of developing heart failure in men and women aged 40 years of 1 in 5 [7].
Atrial fibrillation is a multifactorial condition resulting from an interaction between cardiovascular disease effects, aging, genetics, and environmental factors. CAD coronary artery disease, COPD chronic obstructive pulmonary disease, EtOH alcohol use, HF heart failure, HTN hypertension, OSA obstructive sleep apnea, VHD valvular heart disease
This chapter reviews the current understanding of the pathophysiology of AF in patients with heart failure, providing an in-depth discussion of evidence-based therapies for rhythm versus rate control therapy. Additionally, this chapter will discuss the rationale for pulmonary vein isolation (PVI) versus atrioventricular (AV) nodal ablation and pacing therapies in patients with AF and heart failure. Evidence for benefit of cardiac resynchronization therapy (CRT) in the setting of AF and heart failure will be highlighted.
2 Pathophysiology of Atrial Fibrillation and Heart Failure
2.1 Atrial Fibrillation as a Cause of Heart Failure
2.1.1 Mechanisms
In experimental animal models, it has been observed that chronic tachycardia can result in left ventricular (LV) dilatation with or without systolic dysfunction [8]. Persistent tachycardia depletes cellular high-energy stores in dogs such as creatine, phosphocreatine, and adenosine triphosphate [9]. These changes manifest in a reduced percentage of myocytes and reduced shortening velocity despite a higher LV mass [10]. The depletion of energy stores may be mediated by changes in cellular metabolism with mitochrondrial injury, increased activity of oxidative enzymes, and ischemia [11, 12]. In humans this now well-established entity of reversible congestive heart failure (CHF) in association with chronic tachycardia has been termed tachycardia-mediated cardiomyopathy [13–15].
Atrial fibrillation can also impair myocardial function by its irregular rhythm that produces variable durations of important components of the cardiac cycle, which can impair cardiac output. Furthermore, loss of atrial systole has a negative impact on ventricular filling and cardiac output [16].
The fall in cardiac output associated with AF often results in activation of neurohumoral vasoconstrictors including angiotensin II and norepinephrine, which may further impair ventricular function [17, 18]. Increased sympathetic nerve activity associated with AF is an effect that is partly mediated by the irregular ventricular response [19].
2.2 Heart Failure as a Cause of Atrial Fibrillation
2.2.1 Neurohumoral Activation and Mechanoelectrical Feedback
In CHF, neurohumoral activation of substances including angiotensin II and norepinephrine may promote atrial fibrosis [20, 21] with resultant changes in conduction properties that may predispose to AF. Acute atrial wall stretch is associated with increased dispersion of refractoriness and alterations in anisotropic and conduction properties facilitating AF [22]. Elevated filling pressures that occur in ventricular dysfunction lead to left atrial dilatation, which may stimulate stretch-activated channels and increase vulnerability to AF. Blockade of stretch-activated channels reduces the propensity for AF despite elevated atrial pressure and/or volume [23]. Additionally, left atrial enlargement may facilitate the stability and persistence of atrial fibrillation [24].
Key Points
-
Atrial fibrillation can cause heart failure via tachycardia-mediated cardiomyopathy, impairment of cardiac output due to irregular cycle length and loss of atrial systole, and increased neurohumoral activation.
-
Heart failure contributes to AF by neurohumoral and hemodynamic effects on atrial tissue including fibrosis, acute wall stretch, and chamber dilatation.
3 Prognosis of Atrial Fibrillation and Heart Failure
Several studies have suggested the development of AF is associated with a worse prognosis in patients with preexisting left ventricular (LV) dysfunction. In the Studies of Left Ventricular Dysfunction (SOLVD) prevention and treatment trial [25], AF at baseline was an independent predictor of mortality and morbidity, primarily related to heart failure, death, or rehospitalization for heart failure. In a substudy of the Danish Investigators of Arrhythmia and Mortality ON Dofetilide (DIAMOND) trial [26] of patients with an ejection fraction of 35 % or less, maintenance of sinus rhythm at 1 year was strongly and independently associated with survival, either with placebo or dofetilide. Further evidence that AF causes hemodynamic deterioration in patients with underlying LV dysfunction was provided by an observational study of 344 patients with compensated heart failure who were followed for 19 months [27]. The development of AF in 8 % of these patients was associated with worsening of NYHA functional class, an increase in left atrial size, an increase in both mitral and tricuspid regurgitation, and a reduction in cardiac index and peak oxygen consumption.
4 Current Management
Historically, rate control was considered a “fallback” therapy for AF after failed rhythm control. However, in recent years the practice has shifted from rhythm control to rate control, with rate control being a very feasible alternative therapy for management of AF.
4.1 Rate Control Strategy
The concept of rate control for AF centers on the idea that the primary mechanism for symptoms in AF is tachycardia and the resultant shortening of the diastolic filling period. In addition to symptomatic improvement, many patients with LV dysfunction and AF experience an improvement in ejection fraction following control of the ventricular rate [28, 29], likely reflecting an improvement in tachycardia-mediated ventricular dysfunction.
4.1.1 Medications
Beta-blockers are the preferred agent for rate control in atrial fibrillation, primarily due to their established beneficial effects in heart failure. When a second agent is required, digoxin is often a good choice, with the consideration that patients with impaired renal dysfunction are at higher risk for digoxin toxicity and require closer monitoring. Heart rate should be evaluated both at rest and with activity to determine if control is adequate. In patients with decompensated heart failure and rapid AF, increasing beta-blocker doses is contraindicated and digoxin can be used in this setting. When beta-blockers and digoxin are ineffective, amiodarone can be used alone or in combination with other rate-slowing agents to achieve rate control. Dronedarone slows the heart rate by 10 bpm [30] and should be avoided in any patients with NYHA class III or IV symptoms of heart failure due to its association with increased mortality [31]. Non-dihydropyridine calcium channel blockers carry a risk of exacerbating CHF and thus are generally avoided for this population.
Key Points
-
Effective rate control medications for patients with AF and heart failure include beta-blockers, digoxin, and amiodarone.
-
Rate control drugs to avoid in AF and symptomatic heart failure include dronedarone and calcium channel blockers.
4.1.2 Trials of Rate Control
Potential benefit of rate control in patients with heart failure was observed in a retrospective analysis of the US Carvedilol Congestive Heart Failure trial where 136 of 1,094 patients with heart failure due to systolic dysfunction had AF [28]. In this study, patients treated with carvedilol had a significant increase in the LV ejection fraction (from 23 to 33 % compared with 24 to 27 % with placebo), demonstrating a beneficial effect of carvedilol in this setting. There was also a trend towards a reduction in the primary endpoint of death or CHF hospitalization (p = 0.06). An important caveat is that the study did not prove that the benefit seen was due solely to rate control as opposed to the other neurohumoral effects of beta blockade.
The AF-CHF trial randomized patients with heart failure and paroxysmal AF to medical therapy with either rhythm (amiodarone, sotalol, or dofetilide) or rate control (beta-blockers) [32]. After a 3-year follow-up period, there was no difference in cardiovascular mortality between the two groups (Fig. 6.2). This study supports the concept that a rate control strategy is a more reasonable initial approach for the majority of patients with AF and heart failure due to the increased cost, complexity of medical regimen, and potential adverse affects associated with antiarrhythmic therapy.
Kaplan Meier estimates for death from cardiovascular causes for patients with atrial fibrillation and heart failure treated with either rate or rhythm control [32]. Permission obtained from The Massachusetts Medical Society
The RACE II trial compared strict (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm) versus lenient (resting heart rate <110 bpm) rate control in the AF population [33]. In this study 10 % of patients also had a history of heart failure, and there was no significant difference in the outcome of death from cardiovascular causes, hospitalization for heart failure, stroke, embolism, bleeding, and life-threatening arrhythmic events between the two groups. Based on this trial and other data in the literature (Table 6.1), a goal of average resting heart rate <110 bpm may be a reasonable starting point. However, more data on degree of rate control are needed in the heart failure population.
Key Points
-
Rate control of AF in patients with heart failure is associated with improved clinical outcomes.
-
Available evidence suggests that rate control has similar benefits as rhythm control in AF and heart failure.
4.1.3 Effect of Pacemaker Therapy on Risk of Atrial Fibrillation and Heart Failure
The choice of dual chamber pacing versus single chamber pacing in patients who require a permanent pacemaker may have an impact on their subsequent risk of AF and heart failure. In 2002, the MOST study randomized 2,010 patients with sinus node dysfunction requiring a pacemaker to either dual chamber or single chamber ventricular pacing to determine if there was a difference in the primary endpoint of death or nonfatal stroke [34]. The median age of this population was 74 and comorbidities included prior myocardial infarction in 26 %, prior heart failure in 20 %, diabetes in 22 %, and history of AF in 46 %. There was no difference in the primary endpoint (p = 0.48), however a lower incidence of AF and heart failure was observed in the dual chamber pacing group, at almost 3 years of follow-up suggesting a protective effect of dual chamber pacing in this population. This data reinforces that dual chamber pacing is preferred for patients requiring a permanent pacemaker, in order to maintain AV synchrony and reduce the long-term risk of AF and heart failure.
4.1.4 AV Nodal Ablation with Pacing
AV nodal ablation and pacing provides an attractive means to control AF, particularly in patients with drug-refractory AF or in those who cannot tolerate medications due to intolerances or impaired ventricular function. AV nodal ablation is highly effective (>95 % procedural success), but is also a more invasive option that leaves patients pacemaker-dependent. Emerging evidence in the population undergoing AV nodal ablation for AF supports the role of CRT due to the beneficial effects associated with preserved ventricular synchrony (Table 6.2). PVI, although a preferred rhythm-control option for drug-refractory AF patients with normal LV function, has been infrequently used in heart failure population due to a higher prevalence of comorbidities and structural features that are associated with reduced procedural success.
In a prospective, small randomized trial of 81 patients with class II or III heart failure and ejection fraction <40 % who had symptomatic, drug-refractory AF, PVI for rhythm control was compared with AV nodal ablation and biventricular (BiV) pacing for rate control [35]. At 6 months, PVI was associated with statistically significant improvements in left ventricular ejection fraction (35 % versus 28 %), 6-min walk distance (340 versus 297 m), and score on the Minnesota Living with Heart Failure questionnaire. The improvements in ejection fraction and functional capacity were greater for those with nonparoxysmal compared to paroxysmal AF. In addition, approximately 30 % of patients treated with AV node ablation and biventricular pacing had progressive AF (e.g., paroxysmal to persistent AF); such progression was not seen in patients treated with PVI. Although encouraging, this study only provided short-term data, and the long-term efficacy of PVI in the AF and heart failure population is unknown.
The dual-chamber and VVI implantable defibrillator (DAVID) trial randomized over 5,000 patients with ejection fraction <40 % and indication for an implantable cardioverter defibrillator to either ventricular back-up pacing at 40/min or dual-chamber rate-responsive pacing at 70/min [36]. Patients in the dual chamber pacing group had an increased combined endpoint of mortality and hospitalization for CHF. The increased heart failure and mortality was believed to be due to the maladaptive features of RV stimulation, where ventricular electrical activation proceeds from the right ventricular apex instead of through the existing conduction system, leading to ventricular desynchronization. Although this mechanism was not proven to be the cause of worse outcome, it supported the concept that patients with heart failure requiring frequent ventricular pacing would benefit from CRT.
Observational studies and small randomized trials support the value of CRT for improving symptoms and left ventricular function in patients with poorly controlled AF who have reduced LV systolic function or heart failure [37, 38]. In a small randomized control trial of patients with symptomatic, medically refractory, chronic, rapid AF assigned to AV nodal ablation with either RV pacing or CRT, the group with CRT showed greater improvement in exercise tolerance and greater preservation of ejection fraction [39]. A meta-analysis of three randomized CRT AF trials [37, 40–42] showed a trend towards improved survival among patients randomized to CRT but the difference in survival among patients randomized to CRT versus RV pacing was not statistically significant [43].
A recent observational cohort study of patients with AF and heart failure who received CRT-D showed that AV nodal ablation for definitive biventricular pacing provided a greater improvement in NYHA class and survival benefit compared with drug therapy for rate control [44]. In 154 patients with a median follow-up of 274 days, the median (Q1, Q3) percentage of biventricular pacing after CRT was 99.0 % (95–100 %) in the AV nodal ablation group compared to 96.0 % (85.5–99.0 %) in the drug-treated group (p = 0.05). After CRT, both groups had significant improvements in NYHA class, LV ejection fraction, and LV end diastolic dimension.
Improvement in NYHA class was significantly greater in the AV nodal ablation group compared to the drug-treated group (0.7 ± 0.8 versus 0.4 ± 0.8, p = 0.04), while improvement in echocardiographic parameters was not significantly different between the two groups.
Key Points
-
Dual chamber pacing helps maintain AV synchrony and reduces the long-term risks of AF and heart failure in patients requiring a permanent pacemaker.
-
Radiofrequency ablation of the AV node combined with permanent right ventricular endocardial pacing is a highly effective treatment for controlling the ventricular response of AF.
-
The elderly population is particularly suited to AV nodal ablation and permanent pacing for treatment of AF due to higher frequency of comorbidities, risks of medication intolerance, and the relative safety and simplicity of the procedure.
-
CRT is beneficial for patients with AF and reduced left ventricular systolic function who require frequent pacing.
4.2 Rhythm Control Strategy
Rhythm control may be a reasonable approach in patients with heart failure who are hemodynamically unstable or who are persistently symptomatic despite adequate rate control [45]. Several factors impact the likelihood of successful restoration and long-term maintenance of sinus rhythm, including how long a patient has been in persistent AF, their age, the presence of associated structural heart disease, and left atrial size. Antiarrhythmic drug therapy and radiofrequency catheter ablation are the two primary therapies for rhythm control.
Direct current electrical cardioversion is a useful therapy for patients with new onset AF alone or in combination with antiarrhythmic therapy, and can also be helpful for patients with symptoms that are not clearly attributable to AF. In such patients, when symptoms and functional status improve after cardioversion to sinus rhythm, AF is probably an important factor. In this way cardioversion is helpful in the diagnostic approach to symptoms. Cardioversion is also useful in the management of hemodynamically unstable patients with AF and LV dysfunction. In this group, cardioversion can rapidly improve hemodynamics via restoration of normal cardiac cycle, atrial systole, and decreasing heart rate thus improving diastolic filling time. Cardioversion is more likely to result in sustained maintenance of sinus rhythm in this population when combined with an antiarrhythmic drug.
4.2.1 Antiarrhythmic Therapy
Amiodarone and dofetilide are the first-line therapies for maintenance of sinus rhythm in patients with AF and heart failure recommended by the ACC/AHA/HRS guidelines [46]. Amiodarone has the advantage of being a potassium channel blocker with both beta-blocking and calcium channel-blocking effects. As a result, it has a negative inotropic effect and tends to control the ventricular rate when in atrial fibrillation. Furthermore, amiodarone has been shown to have a low incidence of QT prolongation and less pro-arrhythmia when used in low doses (400 mg per day or less) in patients with heart failure [47]. Compared with dofetilide, additional advantages of amiodarone include its once daily dosing, reduced cost, and ability to start therapy as an outpatient.
4.2.2 Pulmonary Vein Isolation
Although not commonly used as a treatment strategy in the AF population with heart failure, catheter ablation of AF can be successful in patients with concomitant heart failure. In a small observational study of 58 patients undergoing catheter ablation for AF with NYHA class II or greater symptoms and LV ejection fraction <45 %, symptoms, LV function, and exercise capacity were all improved at 12 months [48]. In another observational study 94 patients with impaired LV systolic function (mean ejection fraction 36 %) underwent PVI [49]. After approximately 1 year of follow-up 73 % of the study patients remained AF-free compared to 87 % in a control group of patients with ejection fraction >50 % (p < 0.001). In this study, there was a nonsignificant trend towards improved ejection fraction following ablation in the study group. These data provide some evidence that PVI can improve clinical outcomes in heart failure patients up to 1 year following ablation. However, the long-term durability of the procedure in this population remains unknown.
Key Points
-
Rhythm control in AF and heart failure is useful in patients who are hemodynamically unstable or patients with persistent symptoms from AF despite adequate rate control.
-
Electrical cardioversion (usually combined with antiarrhythmic medication) is useful for hemodynamically unstable patients and in patients with symptoms that are not clearly attributable to AF.
-
First-line antiarrhythmic medications are amiodarone and dofetilide for AF and heart failure.
-
Catheter ablation of AF can be successful in patients with heart failure, but long-term durability remains unknown.
5 Future Trends
The CHALLENGE pilot study is currently recruiting patients to test the hypothesis that AV nodal ablation compared to drug therapy improves outcomes in patients with AF and symptomatic heart failure undergoing CRT. This study is based on the idea that intermittent AV nodal concealed penetrance and ventricular conduction during AF can interrupt CRT pacing and ventricular synchrony, especially in situations of increased myocardial demand (i.e., during exercise). Occurrence of fusion or pseudo-fusion beats may overestimate the amount of “effective” CRT pacing. As a result, optimal clinical benefits may not be achieved even when the device records greater than 80–85 % pacing. It is anticipated that the study will offer valuable insight into whether the ability of AV nodal ablation to achieve 100 % CRT pacing provides a superior clinical effect.
6 Conclusions
Atrial fibrillation and heart failure are two increasingly common conditions in the developed world. In patients with underlying structural heart disease and LV dysfunction, AF can precipitate hemodynamic deterioration and adverse clinical events. AF is also a cause of reversible LV dysfunction in patients without structural heart disease (AF-induced cardiomyopathy) and should be considered when patients present with newly recognized heart failure or AF. When rate control of AF is achieved by either medications or AV nodal ablation with pacing, many hemodynamic consequences of tachycardia may be abated, and ventricular function can improve. In patients with AF and heart failure requiring pacing, increasing data supports the use of CRT to optimize ventricular mechanical synchrony. Ongoing studies will help determine whether AV nodal ablation improves response to CRT in this population. Rhythm control with drug therapy or PVI remains an option, but is generally less successful than rate control.
References
Maisel WH, Stevenson LW (2003) Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 91(6A):2D–8D
Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB (2002) Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 40(9):1636–1644
Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285(18):2370–2375
Stewart S, Hart CL, Hole DJ, McMurray JJ (2001) Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 86(5):516–521
Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck-Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Agladze V, Aliot E, Balabanski T, Blomstrom-Lundqvist C, Capucci A, Crijns H, Dahlof B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJ, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12(10):1360–1420
Wattigney WA, Mensah GA, Croft JB (2003) Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 108(6):711–716
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J (2011) Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation 123(4):e18–e209
Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM (1997) Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol 29(4):709–715
Moe GW, Montgomery C, Howard RJ, Grima EA, Armstrong PW (1993) Left ventricular myocardial blood flow, metabolism, and effects of treatment with enalapril: further insights into the mechanisms of canine experimental pacing-induced heart failure. J Lab Clin Med 121(2):294–301
Spinale FG, Holzgrefe HH, Mukherjee R, Arthur SR, Child MJ, Powell JR, Koster WH (1995) LV and myocyte structure and function after early recovery from tachycardia-induced cardiomyopathy. Am J Physiol 268(2 Pt 2):H836–H847
Spinale FG, Hendrick DA, Crawford FA, Smith AC, Hamada Y, Carabello BA (1990) Chronic supraventricular tachycardia causes ventricular dysfunction and subendocardial injury in swine. Am J Physiol 259(1 Pt 2):H218–H229
O’Brien PJ, Ianuzzo CD, Moe GW, Stopps TP, Armstrong PW (1990) Rapid ventricular pacing of dogs to heart failure: biochemical and physiological studies. Can J Physiol Pharmacol 68(1):34–39
Gaasch WH, Zile MR (1984) Evaluation of myocardial function in cardiomyopathic states. Prog Cardiovasc Dis 27(2):115–132
Gillette PC, Smith RT, Garson A Jr, Mullins CE, Gutgesell HP, Goh TH, Cooley DA, McNamara DG (1985) Chronic supraventricular tachycardia. A curable cause of congestive cardiomyopathy. JAMA 253(3):391–392
Packer DL, Bardy GH, Worley SJ, Smith MS, Cobb FR, Coleman RE, Gallagher JJ, German LD (1986) Tachycardia-induced cardiomyopathy: a reversible form of left ventricular dysfunction. Am J Cardiol 57(8):563–570
Linderer T, Chatterjee K, Parmley WW, Sievers RE, Glantz SA, Tyberg JV (1983) Influence of atrial systole on the Frank-Starling relation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation 67(5):1045–1053
Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S (2003) Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res 60(2):315–325
Tisdale JE, Borzak S, Sabbah HN, Shimoyama H, Goldstein S (2006) Hemodynamic and neurohormonal predictors and consequences of the development of atrial fibrillation in dogs with chronic heart failure. J Card Fail 12(9):747–751. doi:S1071-9164(06)00859-1
Wasmund SL, Li JM, Page RL, Joglar JA, Kowal RC, Smith ML, Hamdan MH (2003) Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation 107(15):2011–2015
Cha YM, Dzeja PP, Shen WK, Jahangir A, Hart CY, Terzic A, Redfield MM (2003) Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol 284(4):H1313–H1320
Li D, Fareh S, Leung TK, Nattel S (1999) Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 100(1):87–95
Solti F, Vecsey T, Kekesi V, Juhasz-Nagy A (1989) The effect of atrial dilatation on the genesis of atrial arrhythmias. Cardiovasc Res 23(10):882–886
Bode F, Katchman A, Woosley RL, Franz MR (2000) Gadolinium decreases stretch-induced vulnerability to atrial fibrillation. Circulation 101(18):2200–2205
Shinagawa K, Shi YF, Tardif JC, Leung TK, Nattel S (2002) Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation 105(22):2672–2678
Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW (1998) Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 32(3):695–703
Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C (2001) Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 104(3):292–296
Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L (1998) Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol 32(1):197–204
Joglar JA, Acusta AP, Shusterman NH, Ramaswamy K, Kowal RC, Barbera SJ, Hamdan MH, Page RL (2001) Effect of carvedilol on survival and hemodynamics in patients with atrial fibrillation and left ventricular dysfunction: retrospective analysis of the US Carvedilol Heart Failure Trials Program. Am Heart J 142(3):498–501
Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA (2000) Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc 75(8):790–795
Davy JM, Herold M, Hoglund C, Timmermans A, Alings A, Radzik D, Van Kempen L (2008) Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am Heart J 156(3):e521–e529
Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J (2008) Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 358(25):2678–2687
Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O’Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL (2008) Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 358(25):2667–2677
Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP (2010) Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med 362(15):1363–1373
Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, Marinchak RA, Flaker G, Schron E, Orav EJ, Hellkamp AS, Greer S, McAnulty J, Ellenbogen K, Ehlert F, Freedman RA, Estes NA III, Greenspon A, Goldman L (2002) Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 346(24):1854–1862
Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, Potenza D, Massaro R, Wazni O, Schweikert R, Saliba W, Wang P, Al-Ahmad A, Beheiry S, Santarelli P, Starling RC, Dello Russo A, Pelargonio G, Brachmann J, Schibgilla V, Bonso A, Casella M, Raviele A, Haissaguerre M, Natale A (2008) Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 359(17):1778–1785
Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A (2002) Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA 288(24):3115–3123
Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, Sutton R, Vardas P, Bongiorni MG, Bergfeldt L, Menozzi C, Musso G (2005) Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur Heart J 26(7):712–722
Leon AR, Greenberg JM, Kanuru N, Baker CM, Mera FV, Smith AL, Langberg JJ, DeLurgio DB (2002) Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J Am Coll Cardiol 39(8):1258–1263
Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, Pires LA (2005) Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol 16(11):1160–1165
Leclercq C, Walker S, Linde C, Clementy J, Marshall AJ, Ritter P, Djiane P, Mabo P, Levy T, Gadler F, Bailleul C, Daubert JC (2002) Comparative effects of permanent biventricular and right-univentricular pacing in heart failure patients with chronic atrial fibrillation. Eur Heart J 23(22):1780–1787
Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC (2003) Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am J Cardiol 91(9):1090–1095
Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo JC, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert JC (2002) Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 40(1):111–118
Bradley DJ, Shen WK (2007) Atrioventricular junction ablation combined with either right ventricular pacing or cardiac resynchronization therapy for atrial fibrillation: the need for large-scale randomized trials. Heart Rhythm 4(2):224–232
Dong K, Shen WK, Powell BD, Dong YX, Rea RF, Friedman PA, Hodge DO, Wiste HJ, Webster T, Hayes DL, Cha YM (2010) Atrioventricular nodal ablation predicts survival benefit in patients with atrial fibrillation receiving cardiac resynchronization therapy. Heart Rhythm 7(9):1240–1245
Cha YM, Redfield MM, Shen WK, Gersh BJ (2004) Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 109(23):2839–2843
Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA III, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, Stevenson WG, Tracy CM, Fuster V, Ryden LE, Cannom DS, Le Heuzey JY, Crijns HJ, Olsson SB, Prystowsky EN, Halperin JL, Tamargo JL, Kay GN, Jacobs AK, Anderson JL, Albert N, Hochman JS, Buller CE, Kushner FG, Creager MA, Ohman EM, Ettinger SM, Guyton RA, Tarkington LG, Yancy CW (2011) 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123(1):104–123
Vorperian VR, Havighurst TC, Miller S, January CT (1997) Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol 30(3):791–798
Hsu LF, Jais P, Sanders P, Garrigue S, Hocini M, Sacher F, Takahashi Y, Rotter M, Pasquie JL, Scavee C, Bordachar P, Clementy J, Haissaguerre M (2004) Catheter ablation for atrial fibrillation in congestive heart failure. N Engl J Med 351(23):2373–2383
Chen MS, Marrouche NF, Khaykin Y, Gillinov AM, Wazni O, Martin DO, Rossillo A, Verma A, Cummings J, Erciyes D, Saad E, Bhargava M, Bash D, Schweikert R, Burkhardt D, Williams-Andrews M, Perez-Lugones A, Abdul-Karim A, Saliba W, Natale A (2004) Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired systolic function. J Am Coll Cardiol 43(6):1004–1009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, New York
About this chapter
Cite this chapter
Eleid, M.F., Cha, YM., Shen, WK. (2013). Atrial Fibrillation and Heart Failure: Rate Versus Rhythm Control. In: Bartunek, J., Vanderheyden, M. (eds) Translational Approach to Heart Failure. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7345-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7345-9_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7344-2
Online ISBN: 978-1-4614-7345-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)