Abstract
Physicians who are involved in heart failure treatment are regularly confronted with patients who present with functional mitral regurgitation (MR), occurring in a setting of ischaemic or non-ischaemic cardiomyopathy. For these patients, the current guidelines do not offer clear treatment algorithms, and mitral valve surgery is often not advised. This is however not a proper representation of the currently available literature on this topic, and may lead to patients not being evaluated for an intervention from which they may benefit. This chapter deals with the surgical perspective of functional mitral regurgitation. Topics covered are pathophysiology (with its implications for surgical techniques and annuloplasty ring choice), patient assessment and a critical appraisal of the outcome of different surgical approaches. While the focus lies on the results of undersized restrictive annuloplasty, various additional techniques are discussed in order to provide a tailored medico-surgical approach to this difficult subset of patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mitral Valve
- Mitral Regurgitation
- Cardiac Resynchronization Therapy
- Left Ventricular Remodelling
- Mitral Valve Repair
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Patients with heart failure and functional mitral regurgitation are regularly presented to the heart failure team. Optimal treatment requires an individualized multidisciplinary approach that should also consider surgical options. The cardiac surgeon should therefore always be part of this medico-surgical team to allow a tailor-made solution for each patient. For several reasons the contribution of surgery in the treatment of heart failure patients is still debated. Current guidelines only recommend a marginal role for mitral valve surgery [1, 2]. This chapter will focus on the existing controversies, analyze their backgrounds and provide a structured approach to patients with heart failure and functional mitral regurgitation (MR). The chapter starts with a brief history of functional MR, presents a clinical perspective in terms of prevalence and outcomes, provides recommendations on assessment of functional MR and discusses the different surgical approaches and their outcomes. We present the structured team approach in our clinic, and end with likely future trends in this area.
2 Defining Functional Mitral Regurgitation
Functional mitral regurgitation is a disease condition in which the macroscopically normal mitral valve becomes insufficient as a consequence of left ventricular wall motion abnormalities. As such, it is also referred to as secondary MR. Depending on its cause, functional MR can be classified as ischaemic MR or as MR in non-ischaemic or idiopathic cardiomyopathy. Regardless of aetiology, functional MR carries a poor prognosis and is an independent risk factor for mortality. There are similarities between ischaemic and non-ischaemic functional MR, but there are also distinct differences. In this chapter we will discuss the disease entities together when possible, but separately when necessary.
Ischaemic MR in heart failure patients is always chronic ischaemic MR. Using a proper definition of ischaemic MR is important to differentiate between true chronic ischaemic MR and patients who have organic MR and incidental coronary artery disease (CAD). The latter patient category has a much better prognosis. Basically a definition should appreciate the fact that the mitral insufficiency is caused by CAD. This means that a patient should have CAD that has led to left ventricular (LV) wall motion abnormalities (due to ischaemia, infarction or both) that induce mitral insufficiency. In addition, the mitral valve should be free from organic disease. As such, a definition of chronic ischaemic mitral regurgitation would be insufficiency of an anatomically normal mitral valve in a setting of wall motion abnormalities of the left ventricle that are caused by the sequelae of CAD.
3 Historical Notes on Functional Mitral Regurgitation
3.1 Ischaemic Mitral Regurgitation
In 1963 Burch suggested that the sequelae of ischaemia could cause mitral regurgitation as a functional phenomenon—rather than only following papillary muscle rupture caused by myocardial infarction in a report on two patients [3]. He related the occurrence of a new systolic murmur, appearing shortly after myocardial infarction, to a “syndrome of papillary muscle dysfunction”, and hypothesized that “failure of the infarcted papillary muscle to contract during systole results in mitral regurgitation”, actually suggesting leaflet prolapse. Although experimental studies in dogs showed already in 1971 that damage to the papillary muscle alone does not lead to MR, while MR does occur when this injury also involves the adjacent LV wall [4], papillary muscle dysfunction was considered a major determinant in the development of ischaemic MR for a long time. This gradually changed with the introduction of echocardiography. In the landmark paper by Godley and associates, it was described that most patients who developed MR following myocardial infarction showed that “one or both leaflets were effectively arrested within the cavity of the left ventricle during ventricular systole” [5]. This is a nice description of systolic restrictive motion, which is now considered the echocardiographic signature of functional MR. These patients almost invariably demonstrated dyskinetic wall motion in the region immediately surrounding one of the papillary muscles.
Further insights have gradually replaced the concept of papillary muscle dysfunction. We now realize that all components of the mitral valve complex may play a role in functional MR—regardless of aetiology—which has implications for a therapeutic approach.
3.2 Mitral Regurgitation in Non-ischaemic Dilated Cardiomyopathy
Historically, functional MR in non-ischaemic cardiomyopathy has been less clearly recognized as a distinct disease condition. The presence of MR in idiopathic cardiomyopathy was first quantified by angiography in a series of 36 patients, with MR present in 64 % of patients [6]. The higher LV end-diastolic volume in these patients made the authors conclude that annulus dilatation was involved in causing MR. However, the overlapping range in end-diastolic volumes in patients with and without MR suggested that other factors were also involved, in particular “an altered position of the papillary muscles and their axes of tension”, as hypothesized by Perloff and Roberts [7]. The surgical treatment of functional MR was regarded inappropriate for a long time. In their 1982 review article on cardiomyopathies, Johnson and Palacios state: “Severe mitral regurgitation, present in a small number of patients with idiopathic congestive cardiomyopathy, may tempt the physician to advise replacement of the mitral valve. We believe that this temptation should be resisted: the perioperative mortality is high in patients whose LV ejection fraction is as low as it is in idiopathic cardiomyopathy, and long-term survival is probably unimproved by operation” [8].
3.3 Introduction of Undersized or Restrictive Mitral Annuloplasty as a Surgical Treatment for Functional Mitral Regurgitation
For a very long time, the only surgery considered possible in these patients was cardiac transplantation. The poor reputation of mitral valve surgery in end-stage cardiomyopathy was due to the poor outcome of mitral valve replacement in patients with systolic heart failure [9]. A major concern was that by abolishing MR the poorly functioning left ventricle would lose its low-impedance left atrial “pop-off” and deteriorate further. The case series presented by Bach and Bolling from the University of Michigan in 1995 represented a major breakthrough by demonstrating that mitral valve repair using an annuloplasty ring was feasible with low mortality and fairly good early outcome [10, 11]. In the interesting discussion that followed the presentation of Bolling’s paper at the meeting of the Western Thoracic Surgical Association, he mentions undersizing the trigone-to-trigone distance “perhaps by one size”, and reports a mean ring size of 29. In 1998 the Michigan group reported on 48 patients with end-stage non-ischaemic heart failure with an ejection fraction of 16 %, and severe functional MR with one perioperative death and 2-year survival of 72 % [12]. In the discussion following this manuscript, Bolling mentions that he changed his strategy from undersizing by one ring size to using the “smallest ring possible”. These publications introduced the concept of undersized or restrictive mitral annuloplasty, which is the cornerstone of the surgical treatment of functional MR.
Key Points
-
Patients with functional MR and heart failure require an individualized multidisciplinary approach in which surgery plays a role.
-
Functional MR can occur in ischaemic and non-ischaemic settings.
-
Functional MR is not caused by papillary muscle dysfunction.
4 Pathophysiology of Functional Mitral Regurgitation and Implications for Surgery
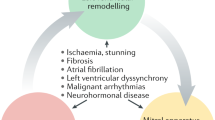
The pathophysiology of functional MR has been the subject of many experimental and clinical studies, and the surgeon should appreciate the basic mechanisms underlying functional MR because of their implications for surgical treatment. Functional MR is a dynamic phenomenon; this has important consequences for patient assessment, which will be discussed in another paragraph. Mitral valve closure is a result of a balance of forces: the closing forces generated by LV contraction and the tethering forces exerted by the (contracting) papillary muscles and chords [13]. In functional MR, the tethering forces are stronger and retain the leaflets in the left ventricular cavity. When LV remodelling sets in, tethering increases and mitral leaflet closure becomes more dependent on closing forces, which are likely to diminish further during the continuing remodelling process. A vicious cycle ensues and MR begets MR. This brings us to the next typical feature of functional MR: it is both a valvular and a ventricular disease. In functional MR, the left ventricle suffers from both the initial disease (ischaemic or other) and from the volume overload resulting from the insufficiency (Fig. 10.1). It is therefore easy to understand that functional MR will have a more profound effect on cardiac function (and therewith on clinical status and outcome) than organic MR of the same severity.
All components of the mitral valve apparatus may contribute to the development of functional MR. The leaflets may demonstrate insufficient adaptation to an increase in annular area [14]. The mitral annulus shows dilatation, flattening (loss of saddle shape) and lack of systolic area reduction with restricted annular motion [15–17]. However, annulus dilatation by itself does not lead to significant MR because of the twofold surplus of mitral valve tissue available to cover the annular cross-sectional area. In chronic ischaemic animal models, the annulus was found to increase both in the septal-to-lateral dimension and in the commissure-to-commissure dimension. However, only septal-to-lateral dilatation was associated with the development of ischaemic MR [18, 19], and reduction of septal-to-lateral dimension by 15–20 % of baseline diameter abolished MR [20]. Necropsy studies showed that the changes in annular dimensions in cardiomyopathy do not only involve the muscular part of the annulus, but also the fibrous part—including the intercommissural area—to a similar extent [21]. In vivo, the pathological increase in annular area (of approximately 60 % in diastole) and perimeter (of approximately 24 %) was confirmed with 3D transthoracic echocardiography (TTE), together with the finding of restricted annular motion [15, 22].
The role of the papillary muscles has received renewed interest with the advent of resynchronization therapy. Papillary muscle dyssynchrony may contribute to the development or worsening of functional MR. Patients with dilated cardiomyopathy and prolonged QRS (>130 ms) show significant MR twice as often as patients with normal QRS duration [23]. This may be explained by geometrical changes induced by the dyssynchrony itself, as well as by delayed LV pressure generation.
Left ventricular changes associated with functional MR include functional changes (wall motion abnormalities and reduced contractility), dimensional changes (LV dilatation with volume increase) and geometrical changes (globally manifested by increased sphericity and locally by papillary muscle displacement). LV dilatation alone is not sufficient to cause functional MR, but increased sphericity has been related to MR through secondary posterolateral displacement of the papillary muscles [24, 25]. Outward displacement of one or both papillary muscles directly influences mitral valve morphology and mechanics by increasing tenting; this can occur in—but is not limited to—a setting of global LV remodelling with increased sphericity.
5 Functional Mitral Regurgitation and the Left Ventricle
The primary injury and the volume overload caused by MR induce changes in global LV size, mass, shape and function that are collectively referred to as “remodelling”. At first, these changes compensate for the loss of pump function resulting from the injury, but over time they become pathological. The heart will maintain stroke volume by increasing its cavity size at the expense of ejection fraction. This causes a right ward shift of the pressure-volume curve with increased end-diastolic volume and end-diastolic pressure. Increased LV wall stress occurs, which can initially be compensated for by secondary hypertrophy. However, the extent of compensatory hypertrophy is limited, and further dilatation will lead to increased wall stress as described by the LaPlace law, which states that wall stress is the product of transmural pressure and radius, divided by wall thickness. These changes increase oxygen demand (which obviously is limited even more in patients with ischaemic disease). Ultimately, myocyte length increases and myofibril content decreases which further leads to contractile dysfunction. In this way a vicious cycle ensues, and dilatation begets dilatation.
A simple mathematical example can demonstrate how a given degree of functional MR influences haemodynamics. Suppose an EF of 50 % and a regurgitant volume of 40 mL. A resting cardiac index of 2 L/min/m2 for an average man is equivalent to a cardiac output of 4 L/min. At a heart rate of 70 mL, this translates into a forward stroke volume of 57 mL. With a regurgitant volume of 40 mL, this adds up to a total stroke volume of approximately 100 mL. The end-diastolic volume then has to be 200 mL to achieve an EF of 50 %, which is about twice the normal size. This example also demonstrates how EF is only a surrogate marker for LV contractility; it is highly load-dependent and can remain more or less normal in MR while LV contractility decreases, due to the low LV impedance in systole created by the mitral insufficiency. It has been demonstrated that end-systolic volume is the most reliable non-invasive marker for LV contractility since it is not dependent of preload and varies directly and linearly with afterload [26].
The term reverse remodelling is applied to a dilated left ventricle that no longer shows progressive dilatation, and instead regresses towards a normal shape and volume. Also in reverse remodelling we can usually only assess global parameters of this process. There is no consensus on what to consider proof of reverse remodelling [27]. Most studies use cut-off values of 10 or 15 % reduction in LV diameters or volumes.
Key Points
-
Functional MR is a dynamic phenomenon, and all components of the mitral valve apparatus may contribute to its development in a patient.
-
Functional MR is both a valvular and a ventricular disease.
-
Functional MR and left ventricular remodelling occur in a vicious cycle.
-
Ejection fraction is not a good marker of LV function in patients with MR.
6 Incidence, Prevalence and Clinical Outcomes of Functional Mitral Regurgitation
Most studies that provide information on incidence and prevalence of functional MR focus on ischaemic MR while some have included both ischaemic and non-ischaemic aetiologies; unfortunately, data are often presented without stratification. While ischaemic MR is relatively common, exact data on its occurrence are hard to provide since studies reporting on this subject are very different. These differences relate to the interval between the ischaemic event and the occurrence of MR (ranging from several hours to months after the infarction); the technique with which MR was diagnosed (angiography vs. echocardiography) and quantified (semi-quantitative vs. quantitative assessment); the severity of MR; and the characteristics of the study population (observational cohorts, patients included in clinical trials, etc.) [28]. Regarding the presence of functional MR in non-ischaemic cardiomyopathy, available studies have similar shortcomings. An important distinction in the development of MR between ischaemic and non-ischaemic MR needs to be pointed out here. In non-ischaemic MR the mitral insufficiency develops rather late in the natural history when considerable remodelling of the left ventricle has taken place. Low EF and the clinical syndrome of heart failure therefore always accompany it. Ischaemic MR can develop in the same way when diffuse ischaemia leads to LV remodelling and thus ischaemic cardiomyopathy. However, more frequently MR results from a local LV wall motion abnormality, following a myocardial infarction or local ischaemia. In this situation the left ventricle (and EF) can be relatively preserved and the syndrome of heart failure may not yet have become manifest (Fig. 10.2).
Table 10.1 provides an overview of the studies that report on this subject [29–39].
It can be seen that the occurrence of post-MR mitral regurgitation of any severity is frequent with a roughly estimated incidence between 20 and 60 % depending on the population studied. Clinically, patients who develop MR following myocardial infarction are often older and more often females, and they show more signs of LV remodelling compared to patients without MR. There is no relationship between infarct size and the occurrence of MR. Several studies indicate a higher prevalence of ischaemic MR following an infarction in the inferior or posterior region, but this is not a consistent finding. Ischaemic MR has a negative effect on prognosis, which is already present in patients with mild MR. This excess mortality is graded, i.e. related to the degree of MR and independent of LVEF. Finally, ischaemic MR is often clinically silent with the absence of systolic murmur, and has a characteristic dynamic phenomenon.
Functional MR in non-ischaemic patients is also frequently present as summarized in Table 10.1. Mortality is high and related to the severity of MR, although evidence regarding its importance as an independent risk factor is ambiguous. There is an important relationship with tricuspid regurgitation, which may reflect the fact that in non-ischaemic cardiomyopathy both ventricles can be injured. Several studies indicate that prognosis in idiopathic cardiomyopathy is somewhat better than in ischaemic cardiomyopathy.
Key Points
-
Functional MR occurs frequently in patients following myocardial infarction, with percentages varying between 20 and 60 %.
-
In ischaemic functional MR, left ventricular function may still be relatively preserved and MR may be the result of only local LV wall motion abnormalities.
-
Regardless of aetiology, functional MR carries an increased risk of death, which is approximately twofold and is related to the severity of MR.
7 Assessment of Functional Mitral Regurgitation
The dynamic nature of functional MR has important diagnostic implications. While structured intraoperative surgical evaluation of the mitral valve—on the arrested heart—is the cornerstone of mitral valve surgery in organic disease, this analysis has no additional value in functional MR since the valve is structurally normal. Therefore, the diagnosis has to be established prior to surgery by looking at restrictive leaflet motion, LV geometric changes and LV wall motion abnormalities.
7.1 Echocardiographic Assessment of Functional MR
Echocardiography is the most appropriate technique to examine patients with functional MR [40, 41]. Proper assessment of the severity of functional MR is important because of its implications for patient prognosis and treatment. Semi-quantitative techniques using colour flow mapping of regurgitant jet area are easily performed but have limited accuracy, since several technical and physiological factors influence results; for instance, a lower driving pressure will make a jet relatively smaller. Determination of the vena contracta width is less sensitive to technical factors. The cross-sectional area of the vena contracta indicates the effective regurgitant orifice area (ERO), which is the narrowest area of actual flow. Size of the vena contracta is independent of flow rate and driving pressure for a fixed orifice. This means that it can change when the regurgitant orifice is dynamic, as is the case in functional MR. Quantitative techniques are therefore preferred. The proximal isovelocity surface area (PISA), or flow convergence technique is used to calculate ERO and regurgitant volume. Another quantitative flow technique is pulsed wave Doppler. Combining pulsed wave Doppler flow velocities with 2D measurements can provide flow rates and stroke volumes and therewith ERO.
Cut-off values for the echocardiographic assessment of MR severity presented in valve disease guidelines are based on the recommendations by Zoghbi et al. [42]. It should be noted that these values are valid for organic MR. Data from the Mayo Clinic suggest, based on patient outcome, that in functional MR lower thresholds should be used for the diagnosis of severe MR: 20 mm2 for ERO and 30 mL for regurgitant volume [30]. For organic MR these values are 40 mm2 and 60 mL, respectively. These adapted criteria for functional MR are mentioned in the European guidelines, although without further recommendation, but not in the American guidelines. Furthermore, the ESC guidelines present separate paragraphs on “ischaemic MR” and “functional MR”; the ESC defines functional MR as MR observed in cardiomyopathy and in ischaemic disease with severe LV dysfunction.
New developments in this area include real-time 3D echocardiography (RT3DE). In a head-to-head comparison, RT3DE proved to be better able to quantify ERO and regurgitant volume in functional MR than 2D TTE, compared to the gold standard of 3D velocity encoded MRI [43]. It was shown that 2D echocardiography underestimates the severity of functional MR.
7.2 Exercise Testing in the Diagnosis of Functional MR
The role of echocardiographic exercise testing in functional MR has been extensively evaluated by colleagues from Liège [44]. Exercise-induced ERO changes in ischaemic MR could be related to local rather than global LV remodelling, more specifically to papillary muscle displacement, tethering and increased coaptation height [45]. In patients with an inferior infarction, reduction in ERO was seen following improvement in wall motion. A study by Lancellotti in patients with LV dysfunction (EF < 45 %) and mild ischaemic MR at rest showed that exercise echocardiography could identify patients with a higher risk of cardiac death [46]. Mortality at 19 months follow-up was higher in patients with a resting ERO > 20 mm2, but also in patients with an ERO that increased during exercise by 13 mm2 or more (value determined using receiver operating characteristic analysis). No mortality occurred in patients with decreasing MR severity during exercise. These results could not be confirmed by others, however [47]. The Lancellotti study underlines the fact that even mild ischaemic MR may be detrimental and therefore warrants additional examinations in such patients to appreciate the true severity of MR.
7.3 Specific Considerations for the Assessment of Functional MR in the Perioperative Setting
The dynamic nature of functional MR also plays a role in the preoperative and intraoperative setting. Patients with functional MR typically show downgrading of MR severity under general anaesthesia [48–50]. Therefore, severity assessment in functional MR should be performed prior to anaesthesia, and the surgical strategy should be determined at that time. For the surgeon it is also important to consider the influence of heart failure therapy and inotropic support on MR severity. Rosario demonstrated how vasodilator and diuretic treatment resulted in reduction of the severity of functional MR [51]. Therapy led to decreased MR by reduction in ERO without reduction in the transmitral gradient. Inotropes can decrease LV volumes and potentially increase LV pressure and lower left atrial pressure and thus raise transmitral pressure, again resulting in decreased MR severity [52].
The reduction of MR severity during general anaesthesia has led to intraoperative simulation of more physiological hemodynamic conditions in patients with mild to moderate ischaemic MR, or MR that is intermittent, to create a situation in which the true severity of MR can be assessed. These so-called loading tests were introduced by Dion et al. [53]. They include a preload test and, if necessary, an afterload test. The preload test is used to increase the pulmonary capillary wedge pressure by 10–15 mmHg by rapid filling through the aortic cannula. An increase of MR severity is considered a positive test and justifies mitral valve repair. A negative test is followed by an afterload test, using a 5 mg IV bolus of phenylephrine (an alpha-agonist that raises systemic vascular resistance without inotropic effects). If MR severity does not increase, mitral valve repair is not performed. In the series presented by Dion, 58 % of patients with intermittent or grade 2+ ischaemic MR showed a significant increase in MR severity following a loading test. Variations of loading tests have been described by others [54–56].
7.4 Geometry of the Mitral Valve Apparatus in Functional MR
With more advanced echocardiographic imaging, more detailed aspects of mitral valve leaflet geometry and the subvalvular apparatus have been described. They can be used in clinical decision making and provide some indication about the feasibility and success of repair, as will be discussed later. For a more detailed description of these parameters and their assessment, the reader is referred to the original publications [22, 57–61]. The most frequently used parameters to describe mitral valve geometry are:
-
Coaptation length: the length of leaflet apposition.
-
Coaptation depth (coaptation distance, tenting height, tenting length): the shortest distance between the point of coaptation on the atrial side and the annular plane.
-
Tenting area: the area enclosed by the mitral leaflets and the annular plane.
7.5 The Value of Cardiac Magnetic Resonance Imaging (MRI) in Functional MR
Cardiac MRI is increasingly used in the evaluation of heart failure patients with cardiomyopathy. MRI provides high spatial resolution and can combine functional and geometrical assessment of the left ventricle in a single examination. A relative disadvantage is the fact that patients with defibrillator/resynchronization therapy devices (a large proportion of the heart failure population) cannot be examined. MRI is currently considered the reference standard for the assessment of ventricular volume and function and for the visualization of scar [62].
Specific use of cardiac MRI in patients with ischaemic cardiomyopathy is found in the assessment of contractile reserve. Late gadolinium enhancement can visualize irreversible myocardial damage. Scar transmurality can predict the likelihood of functional recovery following revascularization, which is high in the absence of scar (78 %) and low if scar is more than 75 % transmural (2 %) [63]. In the intermediate zone predictive value is limited, with a likelihood of recovery of approximately 40 %. In such patients additional low-dose dobutamine stress is advised which can be performed during the same examination; 42 % of segments with an intermediate scar show contractile reserve during stimulation [64]. Predicting functional recovery after revascularization is important, at least in theory, because of its influence on global improvement of LV function, which is related to increased survival [65]. There are no studies that specifically look at the effect of revascularization alone in the setting of ischaemic MR in relation to regional wall motion abnormalities.
The use of MRI in functional MR in non-ischaemic cardiomyopathy is less well defined. Because of the excellent results in volume assessment, which is much less observer-dependent than in echocardiography, MRI can be used as a perfect follow-up tool to observe volumetric and geometric changes in eligible patients.
MRI has also proven its value in the assessment of mitral valve regurgitation, using 3D velocity encoded techniques [66]. MRI can reliably measure regurgitant flow and as such provides a true estimate of ejection fraction, since it can discriminate between true forward flow through the aortic valve and regurgitant flow through the mitral valve. Finally, MRI has demonstrated its usefulness in guiding cardiac resynchronization therapy (CRT). In a comparison with tissue Doppler imaging, MRI was found to have the same accuracy in establishing LV dyssynchrony and LV filling pressures [67].
Key Points
-
The dynamic nature of functional MR has specific implications for MR assessment: it should be preferably performed prior to surgery and induction of anaesthesia and should incorporate different techniques in order to assess severity.
-
Exercise echocardiography should be performed in patients with heart failure symptoms and non-significant MR at rest.
-
Cardiac MRI may have additional value in the assessment of patients with functional MR.
8 Results of Surgery for Ischaemic Mitral Regurgitation
The optimal treatment for ischaemic MR is a subject of ongoing debate in the surgical and cardiology community. Published reports convey ambiguous messages and are essentially difficult to compare because of different patient populations, different definitions of ischaemic MR, different surgical techniques and different follow-up. This controversy is reflected in the guidelines as well. For ischaemic MR, all recommendations have a “C” level of evidence in the European guidelines since randomized trials are absent [41]. For symptomatic patients with severe MR, EF < 30 % and revascularization options, mitral valve surgery has a class IIa recommendation. The same is true for patients with moderate MR undergoing CABG if repair is feasible. Patients with severe MR, EF > 30 %, no revascularization options and low comorbidity have a class IIb recommendation for mitral valve surgery. The American guidelines state that the indication for mitral valve surgery in CABG patients with mild to moderate MR is still unclear, but that there are data indicating benefit of mitral valve repair in such patients [40]. They also state that CABG alone is usually insufficient and leaves many patients with significant residual MR; such patients “would benefit from concomitant mitral valve repair at the time of the CABG”. Finally, it is stated that mitral annuloplasty alone with a downsized annuloplasty ring is often effective at relieving MR.
8.1 Results of Revascularization Only
There has been much discussion whether revascularization alone by improving wall motion abnormalities would suffice to treat ischaemic mitral insufficiency, especially in patients with less than severe MR. This would avoid the supposed increased perioperative morbidity and mortality risk associated with a valvular procedure. In addition, proponents of isolated revascularization claim that valve surgery does not influence long-term outcome and survival.
In Table 10.2, studies reporting on the outcomes of revascularization only in chronic ischaemic MR are summarized [50, 68–78]. Although these studies differ in many aspects, it can be stated that ischaemic MR in patients undergoing revascularization only has a negative influence on survival and functional outcome when compared to results from patients with isolated revascularization for CAD. The effect is graded and can be already observed when only mild MR is present. Furthermore, ischaemic MR treated by revascularization only does not improve in two-thirds of patients and leads to ongoing LV remodelling. There are as yet no predictors that reliably identify patients who will improve with revascularization only. And finally, several studies demonstrate how residual mild MR at rest after revascularization may increase during exercise.
8.2 Results of Mitral Valve Repair with Revascularization
The literature overview presented in Table 10.3 reflects and explains the continuing controversy regarding the true benefit of mitral valve repair in addition to coronary revascularization in ischaemic MR [79–94]. These studies are difficult to compare because of different baseline patient characteristics. More importantly, the technique of mitral valve repair varies widely, especially with respect to the annuloplasty device used and the use of downsizing. Other important differences relate to the grafting strategy (completeness of revascularization and the use of arterial conduits), and to the completeness and time interval of echocardiographic follow-up.
Poor results with regard to MR recurrence can be explained by the use of incomplete and/or flexible rings. Best results seem to be obtained with complete rigid or semi-rigid rings that are downsized by two ring sizes and achieving sufficient leaflet coaptation length during surgery; 8 mm seems an appropriate value. This approach results in consistent and durable reduction of MR severity and LV reverse remodelling in a majority of patients. Nevertheless, at long-term follow-up approximately 15 % of patients have recurrent MR ≥ grade 2+, which is likely related to extensive LV remodelling prior to surgery, which may be clinically reflected by LV dilatation (LV end-diastolic dimension >65–70 mm) or by mitral valve tethering geometry, as will be discussed later. Due to the absence of randomized trials and relatively short follow-up, a survival benefit resulting from additional mitral valve repair has not been demonstrated.
8.3 Studies Comparing Mitral Valve Repair and Mitral Valve Replacement
Obvious differences in patient characteristics and techniques make historical comparisons between mitral valve replacement vs. repair difficult [95–97]. This is nicely addressed in an editorial comment by Miller [98]. Calafiore has advocated to replace the mitral valve in case of excessive coaptation depth exceeding 10 mm, which in his experience occurs in 7 % of cases [99].
9 Results of Surgery for Functional MR in Non-ischaemic Cardiomyopathy
As stated above, the earliest publications from the Michigan group laid the foundation for successful mitral valve repair using downsized annuloplasty rings in patients with functional MR secondary to idiopathic or non-ischaemic cardiomyopathy [10, 11]. It is important to realize that in ischaemic MR the “ventricular component” of the disease can be addressed through coronary revascularization, while this option is absent in non-ischaemic dilating cardiomyopathy. Compared to ischaemic cardiomyopathy, the number of studies that focus on this pathology are less numerous; an overview is presented in Table 10.4 [12, 100–105]. It can be appreciated that 2-year survival is approximately 70–80 %, and 5-year survival 50–70 %. MR recurrence ≥ grade 2+ is approximately 15–20 % at longer follow-up and—similarly to ischaemic MR—seems dependent on the extent of preoperative LV remodelling. More recent studies have focused on external cardiac restraint to address the ventricular component by decreasing transventricular pressure, with promising results.
Key Points
-
Ischaemic MR treated by revascularization only does not improve in two-thirds of patients and leads to ongoing LV remodelling.
-
There are no criteria to identify patients with ischaemic MR who may benefit from revascularization only.
-
Series reporting on the results of mitral valve repair in functional MR are difficult to compare because of different patient populations and different surgical techniques.
-
For ischaemic MR, good and durable results with regard to absence of recurrent MR and reverse LV remodelling are described for complete revascularization and mitral valve annuloplasty with stringent downsizing by two ring sizes, using a semi-rigid or rigid ring and verifying the absence of residual MR with sufficient coaptation length (8 mm).
-
This technique seems insufficient in patients with too advanced LV remodelling, which can be assumed in severe LV dilatation (more than 65 mm end-diastolic dimension and/or severe mitral valve tethering).
-
Although there are less studies available on patients with non-ischaemic cardiomyopathy, similar guidelines seem to apply although the ventricular component of the disease cannot be addressed in a straightforward manner.
10 Choosing an Annuloplasty Ring in Functional Mitral Regurgitation
The available studies on functional MR reflect different views on ring choice: complete or incomplete, flexible or nonflexible, saddle shaped or flat, and even several so-called disease-specific rings have been introduced for functional MR. The concept of the (rigid) remodelling annuloplasty ring was introduced by Carpentier, and proved a major factor in long-term durability of mitral valve repair [106]. Duran introduced a flexible ring to preserve the physiologic annular motion [107]. Theoretical advantages of the flexible ring over (semi-)rigid rings have not been proven clinically, however. The current notion is that after implantation and neo-endothelialization of the ring the benefits of flexibility are greatly diminished as the ring becomes more rigid. Another annular remodelling device is the incomplete flexible band designed by Cosgrove et al. [108]. This device would maintain annular motion because of its flexibility while only providing posterior annulus plication. This was considered sufficient based on the assumption that the intertrigonal distance does not dilate. However, several studies have demonstrated that the anterior part of the annulus shows similar dilatation as the posterior part, at least in dilated cardiomyopathy [15, 21]. Based on these considerations, the use of an incomplete ring in functional MR seems inappropriate, since it will not reduce the anterior annular dimension and, more importantly, it will not sufficiently reduce septal-to-lateral dimension, which is the most important factor on an annular level that contributes to MR. The latter consideration also supports the use of a complete nonflexible ring rather than a complete flexible ring, especially when we consider that we have to undersize. Additional reduction or overcorrection of the septal–lateral dimension of the mitral annulus was found to effectively abolish MR studied in a chronic ischaemic ovine model [20]. Since flexible rings have a variable septal-to-lateral dimension throughout the cardiac cycle, their ability to abolish MR completely in functional MR, at least during the first months after implantation, may be insufficient. In addition, undersizing by two ring sizes will put considerable tension on the annular sutures which might be better compensated for by a (semi-) rigid ring. Finally, a nonflexible ring might be better able to achieve the reduction in circumferential radii of curvature of the left ventricle (remodelling of the LV base, as suggested by Bolling et al. [12]) and therewith reduce wall stress, as described in an acute ischaemic MR model [109].
The Michigan group reviewed their experience in functional MR with regard to ring type [110]. From the year 2000 onwards they used only nonflexible rings. The number of reoperations for recurrent MR as a consequence of ongoing remodelling was significantly higher in the flexible ring group. Although several methodological remarks can be made with regard to the study set-up (confounding effect of different time eras in which both rings were used; reoperation was not examined as a time-related event and is a surrogate endpoint for repair failure), the findings suggest that flexible rings might not be the best option for this pathology. Others have made similar observations [111].
In functional MR, the mitral annulus has become flat. The rationale for saddle shaped rings, reduction of leaflet stress to possibly provide a more durable repair, still has to be proven [112]. Theoretically, these rings could be used in functional MR, although exact data regarding the dimensions of these rings, especially with regard to the ratio of the commissure-to-commissure and the septal-to-lateral dimension, and how this ratio varies per ring size, should be carefully taken into consideration.
The fear of creating a relative mitral stenosis by undersizing with the typically used small ring sizes 24 and 26 prompted the introduction of the so-called disease-specific annuloplasty rings. They are designed to provide further reduction in septal-to-lateral dimension while providing a relatively larger orifice area by maintaining or even increasing the commissure-to-commissure dimension. Evaluating and comparing these rings is highly complex, and actually makes a proper ring choice even harder for the surgeon. A paper by Bothe provides measured dimensions of four disease-specific rings and compares them to those of the Physio ring [113]. Disease-specific rings provide a varying amount of additional septal-to-lateral dimension reduction compared to the Physio ring of the same size (10–25 %), but when compared to a Physio ring that is two sizes smaller (so truly undersized) the septal-to-lateral dimension is actually larger in three out of four rings.
The single intermediate term clinical follow-up study in the English literature performed with a disease-specific ring (Edwards GeoForm) does not reveal better freedom from recurrent MR or more extensive LV remodelling after almost 2 years, but does also not show significant mitral stenosis on exercise [114]. In our opinion, disease-specific rings introduce more ambiguity into the discussion on downsizing while their potential benefit is still unproven.
Based on these considerations, we have always adhered to a complete nonflexible annuloplasty ring (Physio ring) in order to provide a reliable downsizing of the septal-to-lateral dimension of the mitral annulus. Sizing is based on the length of the unfurled anterior leaflet, with the ring then chosen two ring sizes smaller (i.e. size 26 when measuring size 30). The ring is inserted using 14–16 U-shaped stitches. At the end of surgery, repair is considered successful when on intraoperative transoesophageal echocardiography MR is absent and coaptation length is 8 mm or more.
Key Point
-
There is no proven benefit from the so-called disease-specific rings annuloplasty.
11 Recurrence of Mitral Regurgitation After Restrictive Mitral Annuloplasty
Recurrence of mitral regurgitation following mitral annuloplasty negatively influences the results of surgery. Since functional MR is more than a valvular problem, MR recurrence is also related to the course of the underlying ventricular disease which makes it different from recurrence in organic pathologies. Follow-up studies discussed in this chapter present a wide range of MR recurrence. When interpreting these studies, a distinction should be made between residual MR and true recurrent MR. The first is the result of an inappropriate application of a surgical technique, while the latter might be the consequence of disease progression but may also result from inappropriate surgical repair.
Hung examined MR recurrence following annuloplasty for ischaemic MR and concluded that this was related to continued LV remodelling [115]. Thirty patients underwent CABG and mitral annuloplasty with a variety of rings (47 % flexible, mean ring size 30, without marked downsizing). Early results (4 months) showed that 70 % of patients had mild MR and 30 % severe MR; at late follow-up (4 years), 72 % of patients had moderate to severe recurrent MR. Initially patients showed a reduction in LV volumes followed by a late increase, with a similar pattern for LV sphericity. However, one could argue that the simultaneous occurrence of recurrent MR and ongoing LV remodelling does not necessarily imply a causal relationship.
A Japanese study identified increased posterior leaflet tethering after annuloplasty, leading to reduced coaptation length, as an important determinant of persistent MR in ischaemic MR patients [116]. Similar observations were made by the same group in a study involving patients with longer follow-up: late recurrent MR was seen together with augmented posterior leaflet tethering which was not yet present at early follow-up. These patients had an early decrease of end-systolic volume, but showed an increase at late follow-up. Again it should be appreciated that these phenomena are observed simultaneously without proof of a causal relationship.
Results from the Cleveland Clinic are often cited when it comes to MR recurrence following mitral valve annuloplasty in ischaemic MR [80, 84]. Again, we would emphasize that 80 % of patients in these series had an incomplete annuloplasty (20 % with pericardium), and that echo follow-up was performed at a median of only 8 days. The fact that at 6 months follow-up even with a complete semi-rigid ring 25 % of patients had grade 3 or 4 recurrent MR in our opinion does not suggest disease progression with ongoing LV remodelling but rather an imperfect surgical strategy. The median ring size (size 30) suggests insufficient downsizing, and no strategy is provided with respect to the desired intraoperative result in terms of residual MR and minimum coaptation length. More recently this group focused on late MR recurrence, defined as MR ≥ grade 2+ developing at least 6 months after surgery, which occurred in 33 % of patients [117]. Patients with MR recurrence had echocardiographic signs of ongoing LV remodelling, although—again—no temporal relationship between the occurrences of these phenomena is provided.
A properly repaired sufficient mitral valve takes away an important impetus for LV remodelling. MR could then recur secondary to ongoing intrinsic remodelling of the left ventricle. This mechanism is likely present in cases where LV remodelling actually precedes MR recurrence, and can be demonstrated by serial echocardiographic follow-up. De Bonis presented 79 patients who underwent restrictive mitral annuloplasty for end-stage cardiomyopathy with EF ≤ 35 % without residual MR at least 6 months after surgery [118]. In patients with early LV reverse remodelling (15 % decrease of indexed end-systolic volume, present in 52 % of patients) 10 % had grade 2+ MR at late follow-up. Patients without reverse remodelling showed a gradual further increase of end-systolic volume that eventually exceeded preoperative values. MR at late follow-up in this group was 2+ in 42 % of patients and 3+ in 19 %.
11.1 Predictors for MR Recurrence Following Restrictive Mitral Annuloplasty
Several studies have tried to identify preoperative echocardiographic predictors for residual or recurrent MR following undersized annuloplasty. In theory, the extent of remodelling of the left ventricle through its effect on the mitral valve apparatus will determine the extent to which undersizing of the annulus will provide sufficient leaflet coaptation in the acute setting, i.e. intraoperatively and in the short-term. Here, remodelling is expressed as a simple geometric phenomenon. The same extent of remodelling will also determine the potential of recovery of the left ventricle necessary to provide durable reverse remodelling after successful abolishment of mitral regurgitation. Here, remodelling is an epiphenomenon, an expression of changes that occur at a cellular or even molecular level, which remain invisible to our observation.
A rough indicator of the extent of LV remodelling can be found in LV diameters or volumes. Cut-off values for LV end-diastolic diameter (LVEDD) have been related to chances of reverse remodelling [82, 89] and MR recurrence [59, 119]; typically, the upper limit for successful recovery has been identified at 65–70 mm. Others have identified detailed aspects of mitral valve geometry that essentially reflect the degree of leaflet tethering [59–61, 120, 121]. Although these studies provide specific cut-off values for several component of mitral valve geometry, their assessment requires advanced echocardiographic skills and an optimal ultrasound window, and as such they have limited value in everyday practice. Nevertheless, these parameters are important because they provide an indication for which cases adjunctive surgical measures—which will be discussed next—may be indicated or, in less experienced hands, when a mitral valve replacement might be better.
Key Points
-
MR recurrence should be distinguished from residual MR; recurrence within 6 months after surgery should be considered to result from insufficient repair and not be regarded as recurrence secondary to progress of the underlying ventricular disease.
-
MR recurrence may occur in patients without LV reverse remodelling but also in patients with reverse remodelling.
-
Specific indicators to predict MR recurrence are not available, but the extent of preoperative LV remodelling might indicate patients in whom adjunctive measures are necessary to prevent recurrence.
12 Alternative and Adjunct Therapies for Functional Mitral Regurgitation
Several alternative or adjunctive therapies have been described for functional MR. These can either address the mitral valve apparatus, or the left ventricle.
12.1 Addressing the Mitral Valve Leaflets
Valvular adjunctive procedures address loss of coaptation due to excessive tethering. Borger presented results of secondary chord cutting as an adjunctive procedure to reduce tethering in ischaemic MR [122]. MR recurrence ≥ grade 2+ was 15 % at 2 years. Theoretically, this approach may further compromise an already diminished LV function because it disrupts the continuity between the mitral valve and the left ventricle. Long-term results are unknown, and we feel that this technique may only be used, if at all, as a bailout procedure in cases where valve replacement should be avoided at all cost.
The edge-to-edge technique has also been applied successfully in functional MR as an adjunct to undersized annuloplasty in patients with excessive tethering (coaptation depth ≥1 cm) [118, 123].
Another valvular approach is posterior leaflet augmentation. The largest clinical series reports on 44 ischaemic MR patients who underwent posterior leaflet augmentation using bovine pericardium followed by insertion of true-sized annuloplasty rings [124]. Freedom from significant MR is high, and this approach might also be used as an adjunct to restrictive annuloplasty in cases of severe tethering, especially when a small posterior leaflet is present, although this might be technically challenging. A single report on anterior leaflet extension was also published, with very limited follow-up, however [125].
12.2 Addressing the Subvalvular Apparatus
Several techniques applied from inside the ventricle have been described to address tethering by direct action on papillary muscle displacement. Hvass combined the papillary muscle sling technique with mitral annuloplasty [126]. This technique involves positioning of a 4 mm polytetrafluoroethylene (PTFE) tube around the base of both papillary muscles which is then tightened and fixed to approximate the muscles. Mitral annuloplasty with moderately undersized or normally sized rings resulted in good clinical outcomes, low MR recurrence and significant LV reverse remodelling at 1-year follow-up. Theoretically, this approach addresses the ventricular problem by influencing subvalvular anatomy, but also by direct volume reduction of the left ventricle, which makes it an interesting approach for functional MR patients with advanced LV dilatation. Unfortunately, no data on coaptation length are provided, and echocardiographic follow-up is limited to 1 year.
Langer introduced the RING + STRING technique, which adds repositioning of the posterior papillary muscle using a 3-0 PTFE suture that is exteriorized through the aortomitral continuity and then tied in the loaded beating heart under echocardiographic guidance, “achieving the most physiological shape of the anterior mitral leaflet along its entire body and locating the coaptation point as close to the annular plane as possible” [127]. This suture is applied through the aortic valve after the ring (a partial flexible ring with 1–2 sizes downsizing) has been inserted. At 2 years, freedom from recurrent MR ≥ grade 2+ was 94 % in a group of 30 ischaemic MR patients.
Upon addressing the mitral leaflet or subvalvular apparatus one should realize that only the problem of MR recurrence is addressed and not that of LV remodelling. This makes these techniques of limited value. Indeed, when the left ventricle is only moderately dilated, a nonflexible undersized annuloplasty ring will provide adequate results, with low recurrence rates and predictable reverse remodelling [89]. If the left ventricle is grossly dilated and consequently the mitral valve shows severe tethering, these additional techniques may lead to better results regarding MR recurrence but presumably not with respect to LV reverse remodelling.
12.3 External Ventricular Restraint or Reshaping
In cases of advanced LV dilatation, external ventricular restraint or reshaping can provide additional support of the mitral valve repair. The CorCap cardiac support device (CSD) provides circumferential diastolic support, passively reduces LV wall stress and thus counteracts the deleterious changes that occur during the remodelling process. Long-term follow-up has demonstrated that the CSD provides a significantly greater decrease in LV end-diastolic volume compared to controls [128]. In primary ischaemic cardiomyopathy, CABG can be combined with CSD implantation, but the grafts should have their course outside the mesh.
Another externally applied device that addresses LV shape is the Coapsys device. This device reshapes the ventricle and reduces wall stress by compressing the mitral annulus and subvalvular apparatus and as such does not require mitral valve repair. Results in the RESTOR-MV trial were promising [129], showing survival benefit for the first time, but the device is currently not on the market.
12.4 Surgical Ventricular Reconstruction
Patients with functional MR with akinesia or dyskinesia of the LV anterior wall should be considered for additional surgical ventricular reconstruction since this will help to promote reverse remodelling of these often highly enlarged left ventricles. We have described our strategy towards functional MR in this patient category [130]. Basically, we recommend performing restrictive mitral annuloplasty in patients with MR ≥ grade 2+, which may also appear after having performed the surgical LV reconstruction; this technique directly affects papillary muscle displacement and has an unpredictable effect on MR severity.
For idiopathic and ischaemic cardiomyopathy with LV end-diastolic dimension >75 mm and akinesia of the septum with preserved lateral wall function, the septal anterior ventricular exclusion procedure has been successfully used as an adjunct to restrictive mitral annuloplasty [131, 132].
12.5 The Tricuspid Valve
Tricuspid regurgitation should always be evaluated in patients with functional MR, especially in idiopathic cardiomyopathy, which may affect both ventricles [133]. We perform a tricuspid ring annuloplasty in patients with regurgitation ≥ grade 3+, but also in patients without significant tricuspid regurgitation with annular dilatation, which means a tricuspid annulus >40 mm (or 21 mm/m2, indexed to body surface area) on TTE. This approach prevents right ventricular dilatation and tricuspid regurgitation [134], which is commonly seen in heart failure patients.
12.6 Cardiac Resynchronization Therapy
CRT is beneficial in moderate to severe heart failure patients with intraventricular conduction delay with regard to improvements in functional class, exercise capacity and quality of life [135], and leads to a significant mortality reduction [136]. Effects of CRT on functional MR severity and LV reverse remodelling have been demonstrated in both short-term [137] and long-term [138, 139], although approximately 30 % of patients are non-responders. In clinical practice, CRT in heart failure patients is often combined with implantable cardiac defibrillation (ICD) to reduce mortality related to cardiac arrhythmias [140]. Guideline indications overlap to a large extent. CRT-ICD therapy has become an important therapeutic alternative or adjunct therapy in the treatment of heart failure patients with functional MR.
12.7 Other Therapies
The role of pharmacological therapy in functional improvement and mortality reduction in heart failure is beyond the scope of this chapter, but should be evident that it has brought major advances in the treatment of heart failure patients and is the cornerstone of medical therapy, also after surgery has been performed.
Since many patients with heart failure and dilated cardiomyopathy die from ventricular arrhythmias, intraoperative therapies directed at their substrate could improve late outcome. Current knowledge in this field is limited, however, especially for patients with idiopathic cardiomyopathy.
Other surgical alternatives include cardiac transplantation and implantation of ventricular assist devices. Transplantation now has a predictable long-term outcome, but is limited by shortage of donor organs which is not expected to change in the future. A more promising evolution is seen with newer assist devices that show lower thromboembolic and infection rates. Experience with these devices as the so-called destination therapy is increasing. A combination of assist device therapy to unload the ventricle while other therapies aiming to regenerate the damaged myocytes (e.g. stem cell therapy, gene therapy) might be a future solution for patients who do not benefit from what we now address as “conventional heart failure surgery”.
Key Points
-
Patients with increased risk of MR recurrence might benefit from adjunctive measures; these can be directed at the mitral valve leaflets, at the subvalvular apparatus, or at the left ventricle.
-
A tailored surgical approach also involves the tricuspid valve and CRT.
13 A Practical Approach to Patients with Functional MR: The Leiden Experience
Patients with heart failure and functional mitral regurgitation should be treated in a multidisciplinary team so that all possible treatment options can be discussed. Patients should receive optimal pharmacological treatment before considering interventional techniques. Initial evaluation should distinguish between an ischaemic or non-ischaemic cause.
For patients with ischaemic MR, the decision to perform surgery will often be guided by revascularization options; if CABG is warranted, the team should consider whether mitral valve surgery should be performed as well. Since ischaemic MR portends a poor prognosis and revascularization alone does not reliably treat MR, we recommend carefully examining the mitral valve and actively searching for ischaemic mitral regurgitation. In patients with MR grade 3+ or 4+, mitral valve repair should be performed. In patients with MR grade 2+ additional examinations are required. MR severity should then be assessed using quantitative techniques to measure ERO and regurgitant volume (which should then be related to LV function to determine the haemodynamic burden of MR in that particular patient). In addition, exercise echocardiography should be considered to examine an increase in MR severity that would warrant mitral valve repair. In patients who cannot perform exercise for physical or medical reasons (e.g. severe left main disease) an intraoperative loading test should be performed.
In patients with non-ischaemic functional MR without other indication for surgery (e.g. LV aneurysm) CRT should be considered, especially with less severe MR, and its results on MR evaluated. When patients persist in heart failure symptoms surgical correction of MR should be considered. The surgical treatment of functional MR has several basic principles. First, a complete and thorough echocardiographic evaluation should be performed, as discussed before. Mitral valve repair should be performed with a complete nonflexible undersized ring; intraoperative TEE should confirm the absence of residual MR, sufficient coaptation length (at least 8 mm) and the absence of mitral stenosis. Long-term outcome is dependent of the extent of preoperative LV remodelling; since there are as yet no proper techniques to determine that extent, we use LV end-diastolic dimension as a cut-off parameter. In patients with LVEDD > 65 mm (or >30 mm/m2 indexed to body surface area), additional techniques are required. We prefer external ventricular restraint using a CorCap device, but as discussed other techniques either directed at the valve, the subvalvular apparatus or the ventricle itself have been used by others with good results.
Additional techniques should also be considered to individualize treatment: complete revascularization in ischaemic patients; tricuspid valve repair based on annular dilatation (tricuspid annulus >40 mm or and indexed value >21 mm/m2) which is frequently present in non-ischaemic cardiomyopathy; in all patients with LVEF < 30 % we implant an epicardial LV lead to facilitate future CRT.
Our heart failure team has developed a patient-based flow-chart that outlines the different treatment options for heart failure patients (Fig. 10.3). This chart can also be found on http://www.einthoven.nl/Mission!/professional/flowchart_A4_Engels.pdf.
Key Point
-
This paragraph provides a practical medico-surgical approach to the patient with heart failure and in which both interventional and non-interventional treatment strategies have been incorporated.
14 Future Directions
One of the major challenges in the field of functional MR remains patient selection: who will benefit from an individualized surgical strategy, and who will not. Imaging techniques are rapidly improving and a further integration of techniques will provide better answers to the questions which patients have already too extensive remodelling and thus will not benefit from current techniques.
Currently eight interventional randomized controlled studies for functional MR are registered (http://clinicaltrials.gov), the majority involving ischaemic MR. Setting up surgical trials is difficult, and strict criteria should be set with regard to patient inclusion, surgical therapy and follow-up. Failing to do so will lead to outcomes that are not unequivocally accepted, as seen in the STICH trial [141]. It is somewhat disappointing that most registered studies do not have strict guidance with regard to the surgical procedure that should be followed; this could again lead to studies reporting on a variety of surgical techniques with ambiguous outcomes. Since survival benefit has not yet been proven for the surgical treatment of functional MR, another drawback of these trials is that only two studies include mortality as an endpoint. Nevertheless, since other important outcomes will be studied (e.g. components of LV reverse remodelling, objective functional improvement and quality of life), outcomes of these trials will add to our knowledge.
15 Conclusions
Functional MR has tremendous impacts on functional status and survival of heart failure patients. Surgical techniques have developed over more than 15 years, and our current knowledge indicates that, when properly applied and evaluated, these techniques have an important place in the treatment of heart failure patients with functional MR.
References
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA et al (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29:2388–2442
Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG et al (2009) 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 119:1977–2016
Burch GE, De Pasquale NP, Phillips JH (1963) Clinical manifestations of papillary muscle dysfunction. Arch Intern Med 112:112–117
Mittal AK, Langston M, Cohn KE, Selzer A, Kerth WJ (1971) Combined papillary muscle and left ventricular wall dysfunction as a cause of mitral regurgitation. An experimental study. Circulation 44(2):174–180
Godley RW, Wann LS, Rogers EW, Feigenbaum H, Weyman AE (1981) Incomplete mitral leaflet closure in patients with papillary muscle dysfunction. Circulation 63(3):565–571
Feild BJ, Baxley WA, Russell RO, Hood WP, Holt JH, Dowling JT et al (1973) Left ventricular function and hypertrophy in cardiomyopathy with depressed ejection fraction. Circulation 47(5):1022–1031
Perloff JK, Roberts WC (1972) The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation 46(2):227–239
Johnson RA, Palacios I (1982) Dilated cardiomyopathies of the adult (first of two parts). N Engl J Med 307(17):1051–1058
Phillips HR, Levine FH, Carter JE, Boucher CA, Osbakken MD, Okada RD et al (1981) Mitral valve replacement for isolated mitral regurgitation: analysis of clinical course and late postoperative left ventricular ejection fraction. Am J Cardiol 48(4):647–654
Bach DS, Bolling SF (1995) Early improvement in congestive heart failure after correction of secondary mitral regurgitation in end-stage cardiomyopathy. Am Heart J 129(6):1165–1170
Bolling SF, Deeb GM, Brunsting LA, Bach DS (1995) Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg 109(4):676–682; discussion 682–683
Bolling SF, Pagani FD, Deeb GM, Bach DS (1998) Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 115(2):381–386; discussion 387–388
Levine RA (2005) Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation 112(5):745–758
Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ et al (2008) Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation 118(8):845–852
Ahmad RM, Gillinov AM, McCarthy PM, Blackstone EH, Apperson-Hansen C, Qin JX et al (2004) Annular geometry and motion in human ischemic mitral regurgitation: novel assessment with three-dimensional echocardiography and computer reconstruction. Ann Thorac Surg 78(6):2063–2068
Flachskampf FA, Chandra S, Gaddipatti A, Levine RA, Weyman AE, Ameling W et al (2000) Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction. J Am Soc Echocardiogr 13(4):277–287
Kaplan SR, Bashein G, Sheehan FH, Legget ME, Munt B, Li XN et al (2000) Three-dimensional echocardiographic assessment of annular shape changes in the normal and regurgitant mitral valve. Am Heart J 139(3):378–387
Green GR, Dagum P, Glasson JR, Daughters GT, Bolger AF, Foppiano LE et al (1999) Mitral annular dilatation and papillary muscle dislocation without mitral regurgitation in sheep. Circulation 100(19 suppl):II95–II102
Tibayan FA, Rodriguez F, Zasio MK, Bailey L, Liang D, Daughters GT et al (2003) Geometric distortions of the mitral valvular-ventricular complex in chronic ischemic mitral regurgitation. Circulation 108(suppl 1):II116–II121
Tibayan FA, Rodriguez F, Langer F, Zasio MK, Bailey L, Liang D et al (2004) Does septal-lateral annular cinching work for chronic ischemic mitral regurgitation? J Thorac Cardiovasc Surg 127(3):654–663
Hueb AC, Jatene FB, Moreira LFP, Pomerantzeff PM, Kallás E, de Oliveira SA (2002) Ventricular remodeling and mitral valve modifications in dilated cardiomyopathy: new insights from anatomic study. J Thorac Cardiovasc Surg 124(6):1216–1224
Watanabe N, Ogasawara Y, Yamaura Y, Kawamoto T, Toyota E, Akasaka T et al (2005) Quantitation of mitral valve tenting in ischemic mitral regurgitation by transthoracic real-time three-dimensional echocardiography. J Am Coll Cardiol 45(5):763–769
Erlebacher JA, Barbarash S (2001) Intraventricular conduction delay and functional mitral regurgitation. Am J Cardiol 88(1):A7, 83–86
Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S (1992) Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol 20(7):1594–1598
Sabbah HN, Rosman H, Kono T, Alam M, Khaja F, Goldstein S (1993) On the mechanism of functional mitral regurgitation. Am J Cardiol 72(14):1074–1076
White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ (1987) Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 76(1):44–51
Hauptman PJ, Sabbah HN (2007) Reversal of ventricular remodeling: important to establish and difficult to define. Eur J Heart Fail 9(4):325–328
Bursi F, Enriquez-Sarano M, Jacobsen SJ, Roger VL (2006) Mitral regurgitation after myocardial infarction: a review. Am J Med 119(2):103–112
Lamas GA, Mitchell GF, Flaker GC, Smith SC, Gersh BJ, Basta L et al (1997) Clinical significance of mitral regurgitation after acute myocardial infarction. Survival and ventricular enlargement investigators. Circulation 96(3):827–833
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ (2001) Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 103(13):1759–1764
Blondheim DS, Jacobs LE, Kotler MN, Costacurta GA, Parry WR (1991) Dilated cardiomyopathy with mitral regurgitation: decreased survival despite a low frequency of left ventricular thrombus. Am Heart J 122(3 Pt 1):763–771
Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF (2002) Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J 144(3):524–529
Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM (2003) Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol 91(5):538–543
Robbins JD, Maniar PB, Cotts W, Parker MA, Bonow RO, Gheorghiade M (2003) Prevalence and severity of mitral regurgitation in chronic systolic heart failure. Am J Cardiol 91(3):360–362
Patel JB, Borgeson DD, Barnes ME, Rihal CS, Daly RC, Redfield MM (2004) Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail 10(4):285–291
Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA et al (2005) Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation 111(3):295–301
Aronson D, Goldsher N, Zukermann R, Kapeliovich M, Lessick J, Mutlak D et al (2006) Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med 166(21):2362–2368
Agricola E, Ielasi A, Oppizzi M, Faggiano P, Ferri L, Calabrese A et al (2009) Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail 11(6):581–587
Rossi A, Dini FL, Faggiano P, Agricola E, Cicoira M, Frattini S et al (2011) Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart 97(20):1675–1680
Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al (2008) 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease): Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 52:e1–e142
Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G et al (2007) Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J 28:230–268
Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA et al (2003) Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 16:777–802
Marsan NA, Westenberg JJM, Ypenburg C, Delgado V, van Bommel RJ, Roes SD et al (2009) Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc Imaging 2(11):1245–1252
Lebrun F, Lancellotti P, Pierard LA (2001) Quantitation of functional mitral regurgitation during bicycle exercise in patients with heart failure. J Am Coll Cardiol 38(6):1685–1692
Lancellotti P, Lebrun F, Piérard LA (2003) Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. J Am Coll Cardiol 42(11):1921–1928
Lancellotti P, Troisfontaines P, Toussaint A-C, Piérard LA (2003) Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 108(14):1713–1717
Ennezat PV, Maréchaux S, Huerre C, Deklunder G, Asseman P, Jude B et al (2008) Exercise does not enhance the prognostic value of Doppler echocardiography in patients with left ventricular systolic dysfunction and functional mitral regurgitation at rest. Am Heart J 155(4):752–757
Bach DS, Deeb GM, Bolling SF (1995) Accuracy of intraoperative transesophageal echocardiography for estimating the severity of functional mitral regurgitation. Am J Cardiol 76(7):508–512
Grewal KS, Malkowski MJ, Piracha AR, Astbury JC, Kramer CM, Dianzumba S et al (2000) Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol 85(2):199–203
Aklog L, Filsoufi F, Flores KQ, Chen RH, Cohn LH, Nathan NS et al (2001) Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation 104(12 suppl 1):I68–I75
Rosario LB, Stevenson LW, Solomon SD, Lee RT, Reimold SC (1998) The mechanism of decrease in dynamic mitral regurgitation during heart failure treatment: importance of reduction in the regurgitant orifice size. J Am Coll Cardiol 32(7):1819–1824
Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA (1999) Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol 33(2):538–545
Dion R, Benetis R, Elias B, Guennaoui T, Raphael D, van Dyck M et al (1995) Mitral valve procedures in ischemic regurgitation. J Heart Valve Dis 4(suppl 2):S124–S129; discussion S129–S131
Byrne JG, Aklog L, Adams DH (2000) Assessment and management of functional or ischaemic mitral regurgitation. Lancet 355(9217):1743–1744
Mihalatos DG, Gopal AS, Kates R, Toole RS, Bercow NR, Lamendola C et al (2006) Intraoperative assessment of mitral regurgitation: role of phenylephrine challenge. J Am Soc Echocardiogr 19(9):1158–1164
Shiran A, Merdler A, Ismir E, Ammar R, Zlotnick AY, Aravot D et al (2007) Intraoperative transesophageal echocardiography using a quantitative dynamic loading test for the evaluation of ischemic mitral regurgitation. J Am Soc Echocardiogr 20(6):690–697
Otsuji Y, Kumanohoso T, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A et al (2002) Isolated annular dilation does not usually cause important functional mitral regurgitation: comparison between patients with lone atrial fibrillation and those with idiopathic or ischemic cardiomyopathy. J Am Coll Cardiol 39(10):1651–1656
Kumanohoso T, Otsuji Y, Yoshifuku S, Matsukida K, Koriyama C, Kisanuki A et al (2003) Mechanism of higher incidence of ischemic mitral regurgitation in patients with inferior myocardial infarction: quantitative analysis of left ventricular and mitral valve geometry in 103 patients with prior myocardial infarction. J Thorac Cardiovasc Surg 125(1):135–143
Magne J, Pibarot P, Dagenais F, Hachicha Z, Dumesnil JG, Senechal M (2007) Preoperative posterior leaflet angle accurately predicts outcome after restrictive mitral valve annuloplasty for ischemic mitral regurgitation. Circulation 115(6):782–791
Lee AP-W, Acker M, Kubo SH, Bolling SF, Park SW, Bruce CJ et al (2009) Mechanisms of recurrent functional mitral regurgitation after mitral valve repair in nonischemic dilated cardiomyopathy: importance of distal anterior leaflet tethering. Circulation 119(19):2606–2614
Ciarka A, Braun J, Delgado V, Versteegh M, Boersma E, Klautz R et al (2010) Predictors of mitral regurgitation recurrence in patients with heart failure undergoing mitral valve annuloplasty. Am J Cardiol 106(3):395–401
Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde J-L, Nagel E (2012) Imaging in the management of ischemic cardiomyopathy special focus on magnetic resonance. J Am Coll Cardiol 59(4):359–370
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O et al (2000) The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 343(20):1445–1453
Kaandorp TAM, Bax JJ, Schuijf JD, Viergever EP, van der Wall EE, de Roos A et al (2004) Head-to-head comparison between contrast-enhanced magnetic resonance imaging and dobutamine magnetic resonance imaging in men with ischemic cardiomyopathy. Am J Cardiol 93(12):1461–1464
Bax JJ, Van Der Wall EE, Harbinson M (2004) Radionuclide techniques for the assessment of myocardial viability and hibernation. Heart 90(suppl 5):v26–v33
Westenberg JJM, Roes SD, Ajmone Marsan N, Binnendijk NMJ, Doornbos J, Bax JJ et al (2008) Mitral valve and tricuspid valve blood flow: accurate quantification with 3D velocity-encoded MR imaging with retrospective valve tracking. Radiology 249(3):792–800
Marsan NA, Westenberg JJM, Tops LF, Ypenburg C, Holman ER, Reiber JHC et al (2008) Comparison between tissue Doppler imaging and velocity-encoded magnetic resonance imaging for measurement of myocardial velocities, assessment of left ventricular dyssynchrony, and estimation of left ventricular filling pressures in patients with ischemic cardiomyopathy. Am J Cardiol 102(10):1366–1372
Ellis SG, Whitlow PL, Raymond RE, Schneider JP (2002) Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol 89(3):315–318
Tolis GA, Korkolis DP, Kopf GS, Elefteriades JA (2002) Revascularization alone (without mitral valve repair) suffices in patients with advanced ischemic cardiomyopathy and mild-to-moderate mitral regurgitation. Ann Thorac Surg 74(5):1476–1480; discussion 1480–1481
Trichon BH (2003) Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation 108(90101):103II–110II
Mallidi HR, Pelletier MP, Lamb J, Desai N, Sever J, Christakis GT et al (2004) Late outcomes in patients with uncorrected mild to moderate mitral regurgitation at the time of isolated coronary artery bypass grafting. J Thorac Cardiovasc Surg 127(3):636–644
Lam B-K, Gillinov AM, Blackstone EH, Rajeswaran J, Yuh B, Bhudia SK et al (2005) Importance of moderate ischemic mitral regurgitation. Ann Thorac Surg 79(2):462–470; discussion 462–470
Wong DR, Agnihotri AK, Hung JW, Vlahakes GJ, Akins CW, Hilgenberg AD et al (2005) Long-term survival after surgical revascularization for moderate ischemic mitral regurgitation. Ann Thorac Surg 80(2):570–577
Grossi EA, Crooke GA, DiGiorgi PL, Schwartz CF, Jorde U, Applebaum RM et al (2006) Impact of moderate functional mitral insufficiency in patients undergoing surgical revascularization. Circulation 114(1 suppl):I573–I576
Di Mauro M, Di Giammarco G, Vitolla G, Contini M, Iacò AL, Bivona A et al (2006) Impact of no-to-moderate mitral regurgitation on late results after isolated coronary artery bypass grafting in patients with ischemic cardiomyopathy. Ann Thorac Surg 81(6):2128–2134
Calafiore AM, Mazzei V, Iacò AL, Contini M, Bivona A, Gagliardi M et al (2008) Impact of ischemic mitral regurgitation on long-term outcome of patients with ejection fraction above 0.30 undergoing first isolated myocardial revascularization. Ann Thorac Surg 86(2):458–464; discussion 464–465
Fattouch K, Sampognaro R, Speziale G, Salardino M, Novo G, Caruso M et al (2010) Impact of moderate ischemic mitral regurgitation after isolated coronary artery bypass grafting. Ann Thorac Surg 90(4):1187–1194
Kang D-H, Sun BJ, Kim DH, Yun SC, Song J-M, Choo SJ et al (2011) Percutaneous versus surgical revascularization in patients with ischemic mitral regurgitation. Circulation 124(11 suppl 1):S156–S162
Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MIM et al (2004) Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation 110(11 suppl 1):II103–II108
Mc Gee Jr E, Gillinov A, Blackstone E, Rajeswaran J, Cohen G, Najam F et al (2004) Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 128(6):916–924
Glower DD, Tuttle RH, Shaw LK, Orozco RE, Rankin JS (2005) Patient survival characteristics after routine mitral valve repair for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 129(4):860–868
Braun J, Bax JJ, Versteegh MIM, Voigt PG, Holman ER, Klautz RJM et al (2005) Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 27(5):847–853
Geidel S, Lass M, Schneider C, Groth G, Boczor S, Kuck KH et al (2005) Downsizing of the mitral valve and coronary revascularization in severe ischemic mitral regurgitation results in reverse left ventricular and left atrial remodeling. Eur J Cardiothorac Surg 27(6):1011–1016
Mihaljevic T, Lam B-K, Rajeswaran J, Takagaki M, Lauer MS, Gillinov AM et al (2007) Impact of mitral valve annuloplasty combined with revascularization in patients with functional ischemic mitral regurgitation. J Am Coll Cardiol 49(22):2191–2201
Gelsomino S, Lorusso R, De Cicco G, Capecchi I, Rostagno C, Caciolli S et al (2007) Five-year echocardiographic results of combined undersized mitral ring annuloplasty and coronary artery bypass grafting for chronic ischaemic mitral regurgitation. Eur Heart J 29(2):231–240
Gelsomino S, Lorusso R, Capecchi I, Rostagno C, Romagnoli S, Billè G et al (2008) Left ventricular reverse remodeling after undersized mitral ring annuloplasty in patients with ischemic regurgitation. Ann Thorac Surg 85(4):1319–1330
Ngaage DL, Daly RC, Rosales G, Sundt TM, Dearani JA, Mullany CJ et al (2008) Mitral regurgitation surgery in heart failure due to ischemic cardiomyopathy: a 24-year experience. J Heart Valve Dis 17(3):251–259; discussion 259–260
Crabtree TD, Bailey MS, Moon MR, Munfakh N, Pasque MK, Lawton JS et al (2008) Recurrent mitral regurgitation and risk factors for early and late mortality after mitral valve repair for functional ischemic mitral regurgitation. Ann Thorac Surg 85(5):1537–1542; discussion 1542–1543
Braun J, Van de Veire NR, Klautz RJM, Versteegh MIM, Holman ER, Westenberg JJM et al (2008) Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 85(2):430–437
Onorati F, Santarpino G, Marturano D, Rubino AS, Pasceri E, Zinzi S et al (2009) Successful surgical treatment of chronic ischemic mitral regurgitation achieves left ventricular reverse remodeling but does not affect right ventricular function. J Thorac Cardiovasc Surg 138(2):341–351
Williams ML, Daneshmand MA, Jollis JG, Horton JR, Shaw LK, Swaminathan M et al (2009) Mitral gradients and frequency of recurrence of mitral regurgitation after ring annuloplasty for ischemic mitral regurgitation. Ann Thorac Surg 88(4):1197–1201
Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E et al (2009) POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg 138(2):278–285
Pocar M, Passolunghi D, Moneta A, Di Mauro A, Bregasi A, Mattioli R et al (2010) Baseline left ventricular function and surgical annular stiffening to predict outcome and reverse left ventricular remodeling after undersized annuloplasty for intermediate-degree ischemic mitral regurgitation. J Thorac Cardiovasc Surg 139(6):1529–1538
Grossi EA, Woo YJ, Patel N, Goldberg JD, Schwartz CF, Subramanian VA et al (2011) Outcomes of coronary artery bypass grafting and reduction annuloplasty for functional ischemic mitral regurgitation: a prospective multicenter study (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Thorac Cardiovasc Surg 141(1):91–97
Gillinov AM, Wierup PN, Blackstone EH, Bishay ES, Cosgrove DM, White J et al (2001) Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 122(6):1125–1141
Grossi EA, Goldberg JD, LaPietra A, Ye X, Zakow P, Sussman M et al (2001) Ischemic mitral valve reconstruction and replacement: comparison of long-term survival and complications. J Thorac Cardiovasc Surg 122(6):1107–1124
Maltais S, Schaff HV, Daly RC, Suri RM, Dearani JA, Sundt TM et al (2011) Mitral regurgitation surgery in patients with ischemic cardiomyopathy and ischemic mitral regurgitation: factors that influence survival. J Thorac Cardiovasc Surg 142(5):995–1001
Miller D (2001) Ischemic mitral regurgitation redux—to repair or to replace? J Thorac Cardiovasc Surg 122(6):1059–1062
Calafiore AM, Iacò AL, Bivona A, Varone E, Scandura S, Greco P et al (2011) Echocardiographically based treatment of chronic ischemic mitral regurgitation. J Thorac Cardiovasc Surg 141(5):1150–1156.e1
Gummert JF, Rahmel A, Bucerius J, Onnasch J, Doll N, Walther T et al (2003) Mitral valve repair in patients with end stage cardiomyopathy: who benefits? Eur J Cardiothorac Surg 23(6):1017–1022; discussion 1022
Romano MA, Bolling SF (2004) Update on mitral repair in dilated cardiomyopathy. J Card Surg 19(5):396–400
Horii T, Suma H, Isomura T, Nomura F, Hoshino J (2006) Left ventricle volume affects the result of mitral valve surgery for idiopathic dilated cardiomyopathy to treat congestive heart failure. Ann Thorac Surg 82(4):1349–1354; discussion 1354–1355
Acker MA, Bolling S, Shemin R, Kirklin J, Oh JK, Mann DL et al (2006) Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg 132(3):568–577, 577.e1–4
Mann DL, Kubo SH, Sabbah HN, Starling RC, Jessup M, Oh JK et al (2012) Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn Trial. J Thorac Cardiovasc Surg 143:1036–1042
Braun J, Ciarka A, Versteegh MIM, Delgado V, Boersma E, Verwey HF et al (2011) Cardiac support device, restrictive mitral valve annuloplasty, and optimized medical treatment: a multimodality approach to nonischemic cardiomyopathy. J Thorac Cardiovasc Surg 142:e93–e100
Carpentier A (1969) Reconstructive valvuloplasty. A new technique of mitral valvuloplasty. Presse Med 77(7):251–253
Duran CG, Ubago JL (1976) Clinical and hemodynamic performance of a totally flexible prosthetic ring for atrioventricular valve reconstruction. Ann Thorac Surg 22(5):458–463
Cosgrove DM, Arcidi JM, Rodriguez L, Stewart WJ, Powell K, Thomas JD (1995) Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg 60(3):499–503; discussion 503–504
Tibayan FA, Rodriguez F, Langer F, Liang D, Daughters GT, Ingels NB et al (2004) Undersized mitral annuloplasty alters left ventricular shape during acute ischemic mitral regurgitation. Circulation 110(11 suppl 1):II98–II102
Spoor MT, Geltz A, Bolling SF (2006) Flexible versus nonflexible mitral valve rings for congestive heart failure: differential durability of repair. Circulation 114(1 suppl):I67–I71
Silberman S, Klutstein MW, Sabag T, Oren A, Fink D, Merin O et al (2009) Repair of ischemic mitral regurgitation: comparison between flexible and rigid annuloplasty rings. Ann Thorac Surg 87(6):1721–1726; discussion 1726–1727
Mahmood F, Gorman JH, Subramaniam B, Gorman RC, Panzica PJ, Hagberg RC et al (2010) Changes in mitral valve annular geometry after repair: saddle-shaped versus flat annuloplasty rings. Ann Thorac Surg 90(4):1212–1220
Bothe W, Swanson JC, Ingels NB, Miller DC (2010) How much septal-lateral mitral annular reduction do you get with new ischemic/functional mitral regurgitation annuloplasty rings? J Thorac Cardiovasc Surg 140(1):117–121, 121.e1–3
De Bonis M, Taramasso M, Grimaldi A, Maisano F, Calabrese MC, Verzini A et al (2011) The GeoForm annuloplasty ring for the surgical treatment of functional mitral regurgitation in advanced dilated cardiomyopathy. Eur J Cardiothorac Surg 40(2):488–495
Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM et al (2004) Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation 110(11 suppl 1):II85–II90
Zhu F, Otsuji Y, Yotsumoto G, Yuasa T, Ueno T, Yu B et al (2005) Mechanism of persistent ischemic mitral regurgitation after annuloplasty: importance of augmented posterior mitral leaflet tethering. Circulation 112(9 suppl):I396–I401
Shiota M, Gillinov AM, Takasaki K, Fukuda S, Shiota T (2011) Recurrent mitral regurgitation late after annuloplasty for ischemic mitral regurgitation. Echocardiography 28(2):6
De Bonis M, Lapenna E, Verzini A, La Canna G, Grimaldi A, Torracca L et al (2008) Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg 85(3):932–939
Onorati F, Rubino AS, Marturano D, Pasceri E, Santarpino G, Zinzi S et al (2009) Midterm clinical and echocardiographic results and predictors of mitral regurgitation recurrence following restrictive annuloplasty for ischemic cardiomyopathy. J Thorac Cardiovasc Surg 138(3):654–662
Magne J, Pibarot P, Dumesnil JG, Sénéchal M (2009) Continued global left ventricular remodeling is not the sole mechanism responsible for the late recurrence of ischemic mitral regurgitation after restrictive annuloplasty. J Am Soc Echocardiogr 22(11):1256–1264
Gelsomino S, van Garsse L, Lucà F, Lorusso R, Cheriex E, Rao CM et al (2011) Impact of preoperative anterior leaflet tethering on the recurrence of ischemic mitral regurgitation and the lack of left ventricular reverse remodeling after restrictive annuloplasty. J Am Soc Echocardiogr 24(12):1365–1375
Borger MA, Murphy PM, Alam A, Fazel S, Maganti M, Armstrong S et al (2007) Initial results of the chordal-cutting operation for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 133(6):1483–1492
De Bonis M, Lapenna E, La Canna G, Ficarra E, Pagliaro M, Torracca L et al (2005) Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the “edge-to-edge” technique. Circulation 112(9 suppl):I402–I408
de Varennes B, Chaturvedi R, Sidhu S, Cote AV, Shan WLP, Goyer C et al (2009) Initial results of posterior leaflet extension for severe type IIIb ischemic mitral regurgitation. Circulation 119(21):2837–2843
Kincaid EH, Riley RD, Hines MH, Hammon JW, Kon ND (2004) Anterior leaflet augmentation for ischemic mitral regurgitation. Ann Thorac Surg 78(2):564–568; discussion 568
Hvass U, Joudinaud T (2010) The papillary muscle sling for ischemic mitral regurgitation. J Thorac Cardiovasc Surg 139(2):418–423
Langer F, Kunihara T, Hell K, Schramm R, Schmidt KI, Aicher D et al (2009) RING+STRING: Successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation 120(11 suppl):S85–S91
Starling RC, Jessup M, Oh JK, Sabbah HN, Acker MA, Mann DL et al (2007) Sustained benefits of the CorCap Cardiac Support Device on left ventricular remodeling: three year follow-up results from the Acorn clinical trial. Ann Thorac Surg 84(4):1236–1242
Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V et al (2010) Outcomes of the RESTOR-MV trial (randomized evaluation of a surgical treatment for off-pump repair of the mitral valve). J Am Coll Cardiol 56(24):1984–1993
Klein P, Braun J, Holman ER, Versteegh MIM, Verwey HF, Dion RAE et al (2012) Management of mitral regurgitation during left ventricular reconstruction for ischemic heart failure. Eur J Cardiothorac Surg 41:74–80
Suma H, Isomura T, Horii T, Nomura F (2006) Septal anterior ventricular exclusion procedure for idiopathic dilated cardiomyopathy. Ann Thorac Surg 82(4):1344–1348
Suma H, Tanabe H, Uejima T, Isomura T, Horii T (2009) Surgical ventricular restoration combined with mitral valve procedure for endstage ischemic cardiomyopathy. Eur J Cardiothorac Surg 36(2):280–284; discussion 284–285
De Bonis M, Lapenna E, Sorrentino F, La Canna G, Grimaldi A, Maisano F et al (2008) Evolution of tricuspid regurgitation after mitral valve repair for functional mitral regurgitation in dilated cardiomyopathy. Eur J Cardiothorac Surg 33(4):600–606
Van de Veire NR, Braun J, Delgado V, Versteegh MIM, Dion RA, Klautz RJM et al (2011) Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J Thorac Cardiovasc Surg 141(6):1431–1439
Abraham WT, Fisher WG, Smith AL, DeLurgio DB, Leon AR, Loh E et al (2002) Cardiac resynchronization in chronic heart failure. N Engl J Med 346(24):1845–1853
Cleland JGF, Daubert J-C, Erdmann E, Freemantle N, Gras D, Kappenberger L et al (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352(15):1539–1549
Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A et al (2003) Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol 41(5):765–770
St John-Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR et al (2003) Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107(15):1985–1990
Ghio S, Freemantle N, Scelsi L, Serio A, Magrini G, Pasotti M et al (2009) Long-term left ventricular reverse remodelling with cardiac resynchronization therapy: results from the CARE-HF trial. Eur J Heart Fail 11(5):480–488
Ellenbogen KA, Wood MA, Klein HU (2005) Why should we care about CARE-HF? J Am Coll Cardiol 46(12):2199–2203
Conte J (2010) An indictment of the STICH trial: “True, true, and unrelated”. J Heart Lung Transplant 29(5):491–496
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, New York
About this chapter
Cite this chapter
Braun, J., Klautz, R.J.M. (2013). Functional Mitral Regurgitation: The Surgeons’ Perspective. In: Bartunek, J., Vanderheyden, M. (eds) Translational Approach to Heart Failure. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7345-9_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7345-9_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7344-2
Online ISBN: 978-1-4614-7345-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)