Abstract
This chapter focuses on practical issues, such as suggested protocols for dealing with common conditions, and provides examples of dermatologic sonography reports. The guidance suggestions can help to facilitate the implementation of dermatologic ultrasound techniques at your own work place.
Suggested guidance and protocols for performing the examinations within a practical approach
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The implementation of any medical technique can present difficulties. Thus, many questions can emerge from the sonographer, the referring physician, and of course the patient. In this chapter, recommendations and guidance to facilitate the process are suggested. The suggestions are the result of our experience, but are of course susceptible to modification or improvement according to the reality of your own place of work. Hence, the application of the technique relies mainly on three points: the sonographic anatomy, the technical requirements, and the team of work necessary to optimize the results.
We assume at this point that the reader is been well-informed regarding the sonographic anatomy, and has reviewed the technical requirements and common dermatologic entities that he or she will most often deal with. Moreover, ideally, a team of work between the following departments has been initiated: Imaging (Radiology), Clinical (e.g., Dermatology, Rheumatology, Plastic Surgery, etc.), and Pathology. Therefore, we will focus on practical issues, such as suggested protocols for dealing with common conditions and providing examples of dermatologic reports. We hope that this guide will help to facilitate the implementation of this technique at your work place.
2 Protocol for Studying Skin Lesions
The sonographer is an active part of the examination and it is suggested that before beginning the test, a visual inspection of the lesion and a conversation with the patient be performed. This interactive pre-test is of paramount importance and can provide clinical data that can assist in the final interpretation of the results or help with proper probe placement. Therefore, the sonographer can potentially guess the questions or doubts of the referring physician and the differential diagnoses. An algorithm for studying skin lesions is proposed, which shows ultrasound as the first-line imaging modality for assessing dermatologic entities (Algorithm 23.1).
2.1 Visual Inspection of the Lesion
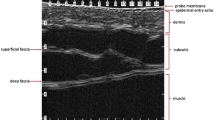
Performing a visual inspection in a well-illuminated room is recommended (Figs. 23.1 and 23.2). A dimmer can be used to regulate the intensity of the light in the room during the different parts of the sonographic examination. The visual inspection should determine such clinical characteristics of the lesion:
-
Location (body region[s])
-
Skin color (erythema, blue or purple colored, pigmented, etc.)
-
Ulceration (yes or no)
-
Palpation (soft, firm, hard)
-
Pain (painful, painless)
-
History (recent, old, slow-growing, fast-growing, without changes, surgeries, biopsies, etc.)
2.2 Tips for the Sonographic Examination of the Skin
-
Use a copious amount of gel (Fig. 23.3).
-
Sedation is used in children (<4 year-old, for e.g. Chloral Hydrate, 50 mg/kg, orally, 30 min before the examination after informed consent has been signed by the parent or guardian). The modified Aldrete scoring system can be used for monitoring the child during the sedation process.
-
When studying lesions in the ear pinna, a small piece of cotton can be used to cover the meatus (Fig. 23.4).
-
Grey scale ultrasound: a sweep in at least two perpendicular axes is recommended.
-
Color Doppler ultrasound: look for the lesional and peri-lesional blood flow
-
Spectral curve analysis: registration of at least three curves in non-vascular and six curves in vascular lesions (three curves per each/axis) is recommended.
3 Protocol for Studying Lesions of the Nail
-
1.
Visual inspection
-
2.
Sonographic examination
Tips for the sonographic study of the nail:
Use the following sequence:
-
1.
Grey scale ultrasound
-
A sweep in at least two different axes (e.g., from medial to lateral and from proximal to distal) including the periungual tissue and the distal interphalangeal joint is recommended.
Set your focus and depth for observing both the complete nail bed and the bony margin of the distal phalanx.
-
-
2.
Color Doppler ultrasound: register the ungual and periungual blood flow
-
3.
Spectral Curve Analysis
At least three spectral curves in non-vascular and six in vascular lesions are suggested.
4 Protocol for Studying the Scalp
-
Visual Inspection
-
Sonographic Examination:
-
Displace the hair tracts to apply the gel (Fig. 23.7).
-
Try to avoid software that softens the images (i.e., median filtering that comes with some machines) because you may lose definition of the hair follicles in the dermis.
-
The rest is similar to the protocol for studying localized skin lesions.
5 Suggested Settings for the Ultrasound Machine
-
Power Doppler to detect slow flow
-
Lowest repetition pulse frequencies and wall filters
-
Color gain below the noise threshold that does not cause artifacts
-
Extended field of view
-
Compound software
-
3D reconstruction (optional)
-
Try to use compact linear probes (hockey stick) that adapt well to the different concavities and convexities of the skin surface and small structures such as the fingers or the nail.
6 Tips for Studying Multiple Skin Lesions
-
1.
Visual Inspection
If necessary, consider scheduling the patient for two or more sessions (Fig. 23.8)
-
2.
Manage the light in the examination room
It will probably be necessary to turn the lights on and off in order to properly position the probe
-
3.
If you are dealing with an inflammatory disease such as morphea or psoriasis, try to position the probe in the transition zone between the lesional and non-lesional tissue and/or perform a comparison with the contralateral region (Fig. 23.9). These observations may provide a clearer view of the abnormalities.
-
4.
Sonographic Examination
Similar for studying localized lesions
Remember that a high-frequency probe does not necessarily mean high definition. In addition to high frequency you will need:
-
A multi-channeled ultrasound machine with a potent processor
-
Focus that goes from superficial to deep, ideally from the skin to the bony margin. If necessary, change the probe (to a lower frequency) for observing deeper structures.
7 Tips for the Examination
-
Use a copious amount of gel including on the lesion and surrounding tissue.
-
If necessary, apply gel on the contralateral side
-
Try to not use stand-off pads and first view the lesion without any artificial compression.
-
During the examination you can try compression maneuvers as a dynamic test
-
Try the nearest position of the lesion to the examiner’s hand and probe
8 Tips on How to Perform Sonographic Locoregional Staging in Melanoma
-
Start from the primary lesion or scar.
-
Follow 10–20 cm around the primary region and study the entire extremity
-
Follow the superficial vein tracts and the distribution of the probable lymphatic drainage
-
Go to the ipsilateral and contralateral nodal stations (axillae-groin)
-
If the primary tumor is located in the head and neck region, also study the supra- and infraclavicular stations.
9 Sonography Report of Cutaneous Lesions
9.1 Key Questions
As in any other imaging technique, the report is of paramount importance and should include relevant data unavailable from the naked eye clinical examination. Thus, the sonography report should ideally provide the following answers of the clinician:
-
1.
Is the lesion originated in the skin?
-
2.
Where is the exact anatomical location?
-
3.
Is there any relevant surrounding anatomical structure that can affect the planning of surgery or the medical treatment?
-
4.
Is it cystic or solid?
-
5.
What are the diameters of the lesion in every axis?
-
6.
Is it a vascular lesion? Does it present high or low flow ? Is it arterial or venous flow? What is the diameter of the vessels? Is there any arterio-venous shunt? Is it a vascular lesion (e.g., a hemangioma) in a proliferative or regression phase?
-
7.
Is it suggestive of a benign or malignant cutaneous lesion?
-
8.
If the lesion is an inflammatory condition, is it in the active, inactive, or atrophic phase?
-
9.
Differential diagnoses?
Once the questions and answers are clear in the mind of the sonographer, the practical outputting of the report can take place.
9.2 Examples of Sonography Reports of Common Dermatologic Lesions
Some practical examples of reports of common dermatologic lesions are provided below. These fictitious examples are based on the most common forms of presentation of cutaneous lesions but it should be kept in mind that there are variants of these presentations that can be reviewed in the other chapters of this book.
9.2.1 Reporting an Epidermal Cyst
9.2.1.1 Clinical History
A 28-year-old male patient presenting with a 6-month history of a painless and occasionally erythematous bump on the left cheek.
9.2.1.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed of the left cheek.
9.2.1.3 Sonography Report
A round-shaped well-defined anechoic cystic structure with internal echoes and posterior acoustic transmission is detected in the dermis and subcutaneous tissue of the left cheek.
The measurements of the cyst are 2.5 mm (longitudinal axis) × 2.3 mm (transverse axis) and 0.8 mm (depth axis).
There is a 0.4 mm (transverse axis) × 1.1 mm (depth axis) anechoic tract that connects the surface of the cyst with the subepidermal region.
No signs of hypervascularity are detected within the lesion area or in the surrounding tissues.
The left parotid gland and masseter muscle appear normal.
The rest of the examination is unremarkable.
9.2.1.4 Impression on Sonography
These findings are suggestive of an intact epidermal cyst.
9.2.2 Reporting a Pilomatrixoma
9.2.2.1 Clinical History
A 8-year-old female patient presenting with an 8-month history of slow-growing, erythematous, firm and painless swelling located in the right arm.
9.2.2.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed of the right arm.
9.2.2.3 Sonographic Report
A round-shaped well-defined solid nodule with a hypoechoic rim and hyperechoic center is detected in the dermis and subcutaneous tissue of the right arm.
The nodule measures 3.4 mm (longitudinal axis) × 3.7 mm (transverse axis) and 3.2 mm (depth axis). There are several 0.5–0.8 mm focal hyperechoic spots suggestive of calcium deposits within the center of the lesion.
Increased echogenicity of the subcutaneous tissue that surrounds the lesion consistent with edema is noted.
On color Doppler ultrasound, thin arterial and venous vessels that measure between 0.4 mm and 0.8 mm (thickness) are detected in the periphery and the center of the tumor.
Spectral curve analysis shows low velocity flow within the vessels. The maximum peak systolic velocity of the arterial vessels is 6.2 cm/s.
The deeper layers are unremarkable.
9.2.2.4 Impression on Sonography
The findings are consistent with Pilomatrixoma.
9.2.3 Reporting a Hemangioma
9.2.3.1 Clinical History
A 4-month-old male infant presenting with a 3-month history of a fast-growing reddish and soft lesion of the right cheek.
9.2.3.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed of the right cheek.
9.2.3.3 Sonography Report
The study shows a heterogeneous ill-defined mass in the dermis and subcutaneous tissue of the left cheek, predominantly hypoechoic in the dermal layer.
The mass also involves the ipisilateral parotid gland and masseter muscle.
The measurements of the lesional tissue are 6.2 cm (longitudinal axis) × 5.1 cm (transverse axis) × 3.2 cm (depth axis).
On color Doppler ultrasound, prominent vascularity is detected within the mass, particularly in the dermal region. The lesion shows high flow arterial vessels, low flow venous vessels, and a few arterio-venous shunts. The thickness of the vessels within the mass ranges between 0.6 and 1.6 mm.
The maximum peak systolic velocity of the arterial vessels is 42.3 cm/s.
The bony margin of the maxillae is unremarkable.
9.2.3.4 Impression on Sonography
The findings are consistent with infantile hemangioma in the proliferative phase with involvement of the ipsilateral parotid gland.
9.2.4 Reporting a Vascular Malformation
9.2.4.1 History
A 5-year-old female patient presenting with a slow-growing, blue-colored painless bump of the lower lip
9.2.4.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed of the lower lip
9.2.4.3 Sonography Report
Multiple, serpiginous, easily compressible, anechoic thin ducts are detected in the submucosal layer of the lower lip that extends into the orbicularis oris muscle. The thickness of the ducts varies between 0.6 and 1.8 mm. The lesion area measures 1.7 cm (longitudinal axis), 3.8 cm (transverse axis), and 0.7 cm (depth axis).
On color Doppler ultrasound, low-velocity venous flow is detected within the vessels.
No signs of thrombosis are seen in the venous tracts on sonography.
There are a few low flow arterial vessels in the periphery of the lesional tissue that seem to correspond to normal stromal vessels of the lower lip.
There are no signs of involvement of the deeper layers or at the oral cavity.
9.2.4.4 Impression on Sonography
The findings are consistent with a low flow venous vascular malformation with involvement of the orbicularis muscle of the lower lip.
9.2.5 Reporting a Basal Cell Carcinoma
9.2.5.1 History
A 75-year-old male patient presenting with a 1-year history of an erythematous and ulcerated lesion located in the right wing of the nasal tip suspicious of basal cell carcinoma (BCC).
9.2.5.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed at the nasal tip.
9.2.5.3 Sonography Report
Ovoid-shaped, well-defined, hypoechoic solid lesion that involves the dermis and the anterior aspect of the right nasal cartilage.
The lesion has multiple hyperechoic spots and measures 3.8 mm (longitudinal axis), 3.5 mm (transverse axis), and 2.5 mm (depth axis). The lesion involves the anterior aspect of the right nasal cartilage through a 2.1 mm (transverse axis) × 1.8 mm (longitudinal axis) and 0.5 mm (depth axis) area.
On color Doppler ultrasound, increased vascularity with low flow arterial vessels is detected at the bottom of the lesion. The thickness of the lesion vessels varies between 0.4 mm and 1.6 mm. The maximum peak systolic velocity of the arterial vessels is 9.8 cm/s.
No satellite extensions are detected in the periphery of the primary lesion.
9.2.5.4 Impression on Sonography
The findings are consistent with a neoplastic lesion, probably BCC that involves the skin and the anterior aspect of the right nasal cartilage.
9.2.6 Reporting a Melanoma
9.2.6.1 History
A 54-year-old female patient presenting with a 4-month history of painless, fast-growing, hyper-pigmented lesion located in the anterior aspect of the distal third of the left lower leg that is suspicious of melanoma.
9.2.6.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed in the left lower extremity and groin regions
9.2.6.3 Sonography Report
In correlation with the cutaneous hyper-pigmented lesion, an ovoid-shaped, well-defined, hypoechoic solid structure is detected. The lesion involves the dermis and measures 2.8 mm (longitudinal axis) × 1.4 mm (transverse axis) and 1.6 mm (depth axis).
The underlying subcutaneous tissue and muscles are unremarkable.
On color Doppler ultrasound, prominent vascularity is detected within the lesion tissue with low flow arterial vessels that present a thickness that varies between 0.5 and 1.1 mm. The maximum peak systolic velocity of the arterial vessels is 9.8 cm/s.
At the ipisilateral popliteal fossa, the subcutaneous tissue shows a hypoechoic well-defined structure with slightly lobulated and irregular margins that measures 1.7 mm (longitudinal axis) × 2.5 mm (transverse axis) × 0.8 mm (depth axis). On color Doppler ultrasound, slow flow arterial and venous vessels are detected within the structure. The maximum peak systolic velocity of the arterial vessels is 8.6 cm/s.
Bilateral enlarged round-shaped inguinal lymph nodes are seen in the subcutaneous tissue. The lymph nodes present thickening and hypoechogenicity of their cortex, lack of the nodal well-defined hyperechoic center, and presence of low flow vessels in the cortex region. Additionally, some lymph nodes have a nodular appearance in the cortex. The lymph nodes located on the left inguinal region vary in their measurements (transverse axis) between 1.5 and 2.8 cm, and the lymph nodes located in the right inguinal region vary between 1.7 and 2.3 cm.
9.2.6.4 Impression on Sonography
The findings are consistent with a melanoma, that shows a sonographic Breslow index type II and a satellite lesion in the ipsilateral popliteal fossa as well as bilateral inguinal nodal involvement as described.
9.2.7 Reporting a Plantar Wart
9.2.7.1 Clinical History
A 38-year old-male patient presenting with a 9-month history of a painful hyperkeratotic lesion located in the sole of the right foot. A foreign body or a plantar wart is suspected clinically.
9.2.7.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed in the sole of the right foot.
9.2.7.3 Sonography Report
The study shows a fusiform-shaped, well-defined, hypoechoic solid structure in the epidermis and dermis in correlation with the visible cutaneous lesion.
The structure measures 2.9 cm (anteroposterior axis) × 2.8 cm (transverse axis) and 1.8 cm (depth axis).
On color Doppler ultrasound, increased vascularity is detected in the dermis underlying the lesion with slow flow arterial vessels that present a thickness that varies between 0.4 and 0.9 mm and a maximum peak systolic velocity of 8 cm/s.
There is a distension of the plantar bursa underlying the lesion located in the subcutaneous tissue and superficial to the flexor tendon at the level of the third metatarsophalangeal joint. No signs of hypervascularity are detected within the bursa.
The plantar fascia, flexor tendons of the foot, and the flexor digitorum brevis muscle are unremarkable.
There are no signs of a foreign body on ultrasound.
The metartasophalangeal joints demonstrate no signs of distension.
The bony margins are well defined with no signs of erosions.
9.2.7.4 Impression on Sonography
-
The findings are consistent with a plantar wart.
-
A mild plantar bursitis underlying the lesion is noted, probably secondary to the inflammation induced by the wart.
9.2.8 Reporting Morphea
9.2.8.1 Clinical History
A 18-year-old female patient presenting with a 6-month history of two painless, indurated, and hyperpigmented plaques located in the abdominal wall and right arm, respectively.
9.2.8.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep is performed in the abdominal wall and right arm in correlation with the lesional tissue. Side-by-side sonography views and comparisons with the non-lesional tissue are also performed.
9.2.8.3 Sonography Report
Focal hypoechoic thickening and decreased echogenicity of the dermis at the lesion areas are detected. The lesion located at the abdominal wall also presents increased echogenicity of the subcutaneous tissue and measures 5.6 cm (longitudinal axis) × 3.8 cm (transverse axis) and 3.5 cm (depth axis). On color Doppler ultrasound there is increased vascularity in the dermis and subcutaneous tissue in the abdominal wall lesion with low flow arterial vessels that measure between 0.6 and 0.9 mm (thickness) with a maximum peak systolic arterial velocity of 6 cm/s.
The lesion located in the right arm measures 3.5 cm (longitudinal axis) × 2.8 cm (transverse axis) and 1.6 cm (depth axis). No signs of involvement of the subcutaneous tissue or hypervascularity at the cutaneous layers are noted in the right arm.
The muscular layer is unremarkable at both lesion sites.
9.2.8.4 Impression on Sonography
The findings are consistent with morphea that shows sonographic signs of activity in the plaque located in the abdominal wall and inactivity in the plaque at the right arm.
9.2.9 Reporting a Glomus Tumor of the Nail
9.2.9.1 Clinical History
A 53-year-old female presenting with a 1-year history of a painful fingernail with ungual dystrophy that affects the right middle finger.
9.2.9.2 Technique
Grey scale and color Doppler multiplanar sonographic sweep of the ungual and periungual region is performed.
9.2.9.3 Sonography Report
The study shows an oval-shaped, well-defined hypoechoic solid nodule in the proximal nail bed that affects the matrix region. The nodule is centrally located and measures 1.2 mm (longitudinal axis) × 1.6 mm (transverse axis) and 0.8 mm (depth axis).
On color Doppler ultrasound, there is increased vascularity within the nodule with low flow arterial vessels that measure between 0.4 and 0.6 mm (thickness) and show a maximum arterial peak systolic velocity of 8 cm/s.
The ungual plate is displaced upward in correlation with the nodule and become thickened and slightly irregular at the distal fingernail in the same axis of the nodule.
Scalloping of the underlying bony margin of the distal phalanx underlying the nodule is detected.
The distal interphalangeal joint and the insertion of the lateral bands of the extensor tendon are unremarkable.
9.2.9.4 Impression on Sonography
-
Hypervascular solid nodule located in the proximal nail bed that shows a benign appearance and is compatible with a glomus tumor.
-
Secondary dystrophic changes of the nail plates.
10 Final Suggestions
The learning curve of the operator can be accelerated if a strong clinical, sonographic, and pathological correlation of the lesions is performed. Thus, ultrasound can be suggested as the first imaging modality in the study of common dermatological lesions, and will probably answer approximately 95 % of the questions of the referring physician that are generated from the clinical examination of these entities. Depending on the primary characteristics of the cutaneous lesion(s), it may be necessary to perform additional imaging studies (See Algorithm 23.1). The imaging studies can include well-known imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), confocal microscopy (CFM), or optical coherence tomography (OCT). The use of additional imaging techniques can complement the information delivered using ultrasound. The imaging techniques that can be performed for the presence of multiple or deeper involvement are MRI and CT, or when dealing with epidermal or subepidermal lesions, CFM and OCT can be performed. As in any field of medicine, the use of these imaging modalities will depend on the availability of the machines and the presence of a well-trained operator.
A major advantage of the ultrasound examination is that it non-invasively provides both anatomical and functional information of the lesions, a concept that we call “Two-for-One”. The latter broad sonography conceptual analysis may support the understanding of the physiopathology of dermatologic entities and improve the final cosmetic prognosis of patients [1–5].
Finally, we encourage the reader to learn, enjoy, and develop this technique, taking advantage of the tools provided in this book and also from the on-line information that can be found on the skin ultrasound educational website www.sonoskin.com, a virtual on-line community dedicated to this type of sonography examination.
References
Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010;62(2):247–56.
Wortsman X. Common applications of dermatologic sonography. J Ultrasound Med. 2012;31(1):97–111.
Wortsman X, Wortsman J, Soto R, Saavedra T, Honeyman J, Sazunic I, et al. Benign tumors and pseudotumors of the nail: a novel application of sonography. J Ultrasound Med. 2010;29(5):803–16.
Wortsman X, Wortsman J, Matsuoka L, Saavedra T, Mardones F, Saavedra D, et al. Sonography in pathologies of scalp and hair. Br J Radiol. 2012;85(1013):647–55.
Wortsman X. Sonography of cutaneous and ungual lumps and bumps. Ultrasound Clin. 2012;7(4):505–23. doi:10.1016/j.cult.2012.08.006.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Wortsman, X. (2013). How to Start on Skin, Nail, and Hair Ultrasound: Guidance and Protocols. In: Wortsman, X. (eds) Dermatologic Ultrasound with Clinical and Histologic Correlations. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-7184-4_23
Download citation
DOI: https://doi.org/10.1007/978-1-4614-7184-4_23
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-7183-7
Online ISBN: 978-1-4614-7184-4
eBook Packages: MedicineMedicine (R0)