Abstract
In situ chemical reduction (ISCR) is a general term for a suite of in situ groundwater remediation technologies that rely primarily on chemical reduction of contaminants. ISCR has been used for over 15 years for plume treatment, but its use for source treatment is more recent. This chapter provides the first integrated assessment of the entire suite of ISCR technologies, and describes the technical basis, engineering aspects, past experiences and future prospects for using ISCR to treat chlorinated solvent source zones. In situ chemical reduction of contaminants can occur through natural intrinsic biogeochemical processes, as a result of stimulating in situ microbial activity to form reducing minerals, or after direct injection of chemical reductants. The chapter includes case studies of several ISCR technologies and summarizes the lessons learned to date from research and field experience.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 INTRODUCTION
In situ chemical reduction (ISCR) has been defined in various ways since the term first began appearing in the late 1990s (Szecsody et al., 2004; Brown et al., 2006; Brown, 2008; Dolfing et al., 2008; Brown, 2010; Tratnyek, 2010). In general, and for the purposes of this chapter, ISCR refers to the category of in situ groundwater remediation technologies in which treatment occurs primarily by chemical reduction of contaminants. The emphasis of ISCR is on abiotic processes, but contaminant reduction by biogenic minerals is included if the role of microbial activity in the contaminant reduction is indirect. The reducing conditions necessary for ISCR can arise from natural intrinsic biogeochemical processes, can be generated by stimulating in situ microbial activity, or can be created by adding chemical reductants.
The chemical reductants that contribute to ISCR include reduced metal species such as Fe2+, Fe3O4, and Fe0, reduced sulfur species (HS−, S2O4 2−, FeS, FeS2) and other labile, electron-donating substances (natural organic matter [NOM]). The branch of ISCR that relies on intrinsic reductants is limited to minerals that contain FeII, S−II and/or S−I, and NOM, whereas the more highly engineered ISCR technologies may employ stronger reductants, such as dithionite (S2O4 2−) or zero-valent metals. More details on the reductants used for ISCR are given in Section 10.2.2.
The reduction processes responsible for contaminant treatment by ISCR include degradation of organics and sequestration of inorganics. Contaminants that are subject to reduction under ISCR conditions include (1) organic compounds with chloro-, nitro- or other readily reducible functional groups; (2) metal oxyanions that become less mobile upon reduction; and (3) a few nonmetal inorganics such as nitrate and perchlorate. More detail on the contaminants that are treatable by ISCR is given in Section 10.2.1, but the scope of this chapter is limited primarily to chlorinated solvents.
The application of ISCR for chlorinated solvent source remediation is a more recent development than its use for plume containment or treatment (Brown, 2010). This chapter describes the technical basis, engineering aspects, past experiences and future prospects for ISCR treatment of chlorinated solvent source zones. The discussion begins with a summary of the fundamentals, including the chemistry of contaminant reduction reactions and the geotechnical aspects of implementation. The chapter presents case studies for each of the major variations of ISCR in current use and discusses lessons learned from this experience. Finally, the chapter summarizes the overall status of this approach to source zone remediation, with a forecast of likely near-term developments and an assessment of barriers to future progress.
10.1.1 Technology Development
The fact that groundwater and sediment contaminants can be reduced by abiotic pathways (i.e., pathways that do not directly involve microorganisms) has been well documented in the research literature for more than 30 years. Most of the early work on these processes has been summarized in several reviews (Macalady et al., 1986; Tsukano, 1986; Wolfe and Macalady, 1992). More recently, there have been many academic studies of organic contaminant degradation reactions using model systems designed to represent the natural reductants that are most likely to be responsible for abiotic reduction reactions in soils, sediments and groundwaters.
Three types of naturally occurring, abiotic reductants have been studied most thoroughly:
-
1.
Minerals or their amorphous analogs that derive reducing properties from FeII. These include magnetite (Lee and Batchelor, 2002a; Gorski et al., 2010), green rust (Lee and Batchelor, 2002b; Erbs et al., 1999; O’Loughlin and Burris, 2004; Ayala-Luis et al., 2012), ferruginous clays (Cervini-Silva et al., 2002; Elsner et al., 2004), goethite with adsorbed FeII (Amonette et al., 2000; Kenneke and Weber, 2003; Elsner et al., 2004; Butler et al., 2011; Zhang et al., 2011), and possibly minerals commonly associated with basalt (Ingram et al., 2000).
-
2.
Minerals deriving their reducing properties from S−II (or S−I) as well as FeII. Mackinawite (Butler and Hayes, 1999; Butler and Hayes, 2001; Butler et al., 2011) and pyrite (Kriegman-King and Reinhard, 1994; Lee and Batchelor 2002a, b) are the most studied of these minerals, but other FeII and S−II/−I phases of possible significance include greigite, marcasite and amorphous FeS.
-
3.
Redox-active moieties associated with natural organic matter. These are mainly quinones (Tratnyek and Macalady, 1989; Schwarzenbach et al., 1990; Uchimiya and Stone, 2009) but could also include thiol groups and/or complexed metals (Xia et al., 1998; Struyk and Sposito, 2001; Szulczewski et al., 2001).
Few documents have attempted a comprehensive review of the peer-reviewed literature on these intrinsic ISCR reductants; the most thorough and comprehensive review to date is He et al. (2009). However, there are numerous detailed reviews of the recent literature on specific classes of abiotic contaminant reduction pathways (Haderlein and Schwarzenbach, 1995; Haderlein et al., 2000; Tratnyek and Macalady, 2000; Amonette, 2002; Totten and Assaf-Anid, 2003; Adriaens et al., 2004; Elsner and Hofstetter, 2011; O'Loughlin et al., 2011; Strathmann, 2011), and these reactions are attributed to the same putative reductants as listed above.
Despite this extensive background, most in the remediation engineering community have long assumed that in situ abiotic reduction of contaminants in the environment is insignificant as a component of natural attenuation or as a basis for remediation technologies. Recently, however, a growing number of field studies have characterized sites where a significant portion of the overall contaminant degradation appears to be due to direct reactions with reducing mineral phases (Ferrey et al., 2004; Darlington et al., 2008). This trend has created interest in the role these phases play in natural attenuation and in the prospects for manipulating such systems to generate more or better reductants in situ (Becvar et al., 2008; He et al., 2009).
Along with these developments, there has been a rapid growth of remediation technologies that rely on zero-valent iron (ZVI) to serve as the chemical reductant of contaminants (Tratnyek et al., 2003a; Henderson and Demond, 2007; Gillham et al., 2010). This field has become fairly mature in recent years, and considerable competition exists among vendors of ZVI for remediation applications. This competition has led to efforts to engineer better forms of ZVI or to develop other enhancements that will improve ZVI reactivity, longevity or delivery. One major strategy for enhancing ZVI performance involves bimetallic combinations of ZVI and catalytic metals such as Pd or Ni (Grittini et al., 1995; Gui and Gillham, 2003). Another strategy involves nanoscale zero-valent iron (nZVI), where the nano size of these particles provides greater reactive surface area and may impart other nano-size effects that could enhance remediation performance (Li et al., 2006; Tratnyek and Johnson, 2006; Lowry, 2007; Geiger and Carvalho-Knighton, 2009; Comba et al., 2011; Scott et al., 2011; Crane and Scott, 2012; Mueller et al., 2012). Other approaches to engineering better reductants range from manipulation of metallurgical properties (Landis et al., 2001) to replacement of Fe0 with more strongly reducing metals such as Zn0 (Li and Klabunde, 1998; Arnold et al., 1999; Tratnyek et al., 2010).
Even more recently, it has been recognized that all of the above chemical reductants form the basis for a coherent family of remediation technologies, which has come to be known as in situ chemical reduction or ISCR (Brown et al., 2006; Brown, 2008; Brown, 2010). This recognition may allow researchers and practitioners an opportunity to advance all forms of ISCR technologies by utilizing some of the general properties of reductants that determine the strengths, weaknesses, similarities and differences among the options. Furthering this goal is a major objective of this chapter.
10.1.2 State of the Practice
In situ chemical reduction is now widely recognized as a major category of remedial options, encompassing a range of technologies including some that are well established, others that are emerging and a few that are still under development. No consensus has been reached on any one scheme for classification of ISCR technologies, but two approaches seem promising.
The first classification approach distinguishes between technologies based on the relatively natural, intrinsic reductants (including minerals of geological origin or minerals formed by in situ stimulation of otherwise natural biogeochemical processes) and technologies using addition of chemical reductants that do not otherwise occur in nature, such as zero-valent metals. This distinction was made previously in the summary of ISCR reductants in the preceding section. In Figure 10.1, it is represented as a continuum of effective strength, from relatively mild, generally natural, to stronger, typically engineered, reductants.
Map of ISCR technologies in current practice. The horizontal dimension represents the continuum from naturally occurring and/or mild reductants (FeII and SII−/I− containing minerals) to the generally strong chemical reductants used in fully engineered systems (Fe0 and Zn0). The vertical dimension represents the various modes of application, from emplacement of reductants to intercept plumes (PRBs) to injection of reductants that target the source zone (nZVI). Acronyms used to identify the specific technologies are defined in the following subsections.
Another useful way of classifying ISCR technologies distinguishes between those used to create reactive treatment zones that intercept contaminant plumes and those that target source zones directly. In general, plume treatment technologies are designed to be longer lasting, and often can be implemented with relatively less invasive methods. Source zone treatment requires more reductant per unit volume treated, and this is usually accomplished by emplacement into the source zone using comparatively disruptive methods.
Both of these classification schemes are used in Figure 10.1 to provide a summary map of ISCR technologies that currently are established or emerging. The ISCR technologies included in the map are briefly described below, and case studies of each are given in Section 10.3.2. Combined remediation and other variations on these technologies are addressed in Section 10.2.5. A broader perspective on combined remedies is given in Chapter 15 of this volume.
Abiotic Monitored Natural Attenuation .Contaminant degradation during monitored natural attenuation (MNA) is usually dominated by biodegradation, but recently it has become recognized that abiotic degradation pathways can be significant (Ferrey et al., 2004). In these cases, referred to here as abiotic MNA (Brown et al., 2007; Brown, 2010), significant contaminant degradation occurs by direct reaction with mild reductants, primarily ferrous iron and iron sulfide minerals, that are generated from natural biogeochemical processes. Abiotic MNA applies only to contaminants that are relatively labile to reduction and concentrations of contaminants that are relatively small, e.g., part per billion [ppb] levels of trichloroethene.
Biogeochemical Reductive Dechlorination .The term biogeochemical reductive dechlorination (BiRD) was coined by Kennedy et al. (2006a, b) to describe the process of stimulating abiotic reduction of chlorinated solvents by forming reactive iron sulfides. In this scenario, iron sulfides are created by stimulating microbial sulfate reduction in the presence of iron. The application of BiRD requires the presence of sufficient carbon source, sulfate and iron. Carbon and sulfate generally must be added, and iron may be added although naturally present iron minerals are often sufficient. Biogeochemical reductive dechlorination is primarily employed as a barrier technology, and to date these usually have been biowalls of mulch amended with gypsum and goethite.
In Situ Redox Manipulation.In soil matrices with significant iron (>1 wt%), applications of moderately strong chemical reductants such as sodium dithionite or calcium polysulfide cause reduction of the ferric iron associated with the mineral matrix. The resulting FeII-bearing minerals can then serve as the reducing agent to effect reductive transformation of contaminants. An example of such a process is the technology known as in situ redox manipulation (ISRM) (Fruchter et al., 2000; Szecsody et al., 2004), where dithionite, a soluble chemical reductant, is injected into the subsurface to reduce native ferric iron to adsorbed and structural ferrous iron, which can in turn reduce contaminants such as chromate, carbon tetrachloride, trichloroethene and some munition compounds.
Permeable Reactive Barriers.Technologies that mitigate contaminant plumes by in situ placement of permeable, reactive material transverse to groundwater flow are known as reactive treatment zones (RTZs) or more commonly, permeable reactive barriers (PRBs). A wide range of materials (leaf litter, fish bones, activated carbon, etc.) can be used in PRBs to effect a variety of contaminant removal processes, but the most significant is granular ZVI in various forms (Scherer et al., 2000; Tratnyek et al., 2003a; Comba et al., 2011). Permeable reactive barriers may be placed near a source zone or downgradient, such as before a receptor, depending on site-specific considerations. Emplacement of the early ZVI PRBs was done by trenching, but now they often are constructed by hydraulic or pneumatic fracturing, soil mixing, or direct injection of micron- or nano-sized ZVI. Several other ISCR technologies (ISRM and source zone targeted injection [SZTI]) can have operational characteristics that overlap with PRBs.
Source Zone Targeted Injection.The injection of chemical reductants to directly target source zone contamination is not yet widely utilized, but a growing range of applications is being considered. The reductants that have been studied the most include ZVI (both micron- and nano-sized iron) and polysulfide foam, although other forms of chemical reductants are feasible. In general, the materials are either particulate, such as ZVI types, or liquids and foams. Particulate forms of reductant are attractive because they may remain resident and reactive in the source area for longer times than do liquids/foams and therefore provide residual treatment capacity. Recently, SZTI with nanoscale zero-valent iron (nZVI) has attracted a great deal of attention for these reasons, but the fine particulate nature of this reductant raises other challenges related to longevity and emplacement, as discussed below in Section 10.2.4.6. Calcium polysulfide has been used for the in situ treatment of hexavalent chromium source zones. In this treatment method, calcium polysulfide foam is injected throughout the source zone to reduce CrVI to the less mobile CrIII form (Graham et al., 2006). It has been employed at many locations in the United States and elsewhere (Fruchter, 2002).
In Situ Soil Mixing.Relatively shallow contaminated sites can be treated by mixing with a variety of treatment agents using large-diameter augurs. The most prominent example of in situ soil mixing (ISSM) for ISCR involves both ZVI and clay (Shackelford et al., 2005; Wadley et al., 2005). Typically, a mixture of clay (5–10%) and microscale ZVI (0.5–2%) is mixed into a soil matrix using large-diameter (4–8 feet (ft); 1.2–2.4 meters (m) diameter) augurs or soil mixers (e.g., Lang Tool). The clay has the potential to disperse dense nonaqueous phase liquid (DNAPL) as a Pickering emulsion (Roy-Perreault et al., 2005) and can also inhibit the movement of contaminated groundwater by decreasing overall aquifer permeability. The mixing ensures uniform contact between the emulsion and the ZVI. The main application of this technology thus far has been DNAPL zones (Water Science and Technology Board, 2004), including soils at depths as great as 50 ft (15.2 m).
Catalytic Reductive Dechlorination.Dechlorination by noble metal-catalyzed hydrogenolysis has been adapted for in situ remediation of contaminated groundwater, and this process is being called catalytic reductive dechlorination (CRD). Although CRD has performed well in bench-scale tests (Davie et al., 2008) and at least one extended pilot test (McNab et al., 2000), deactivation of the Pd catalyst occurs, especially when the groundwater contains sulfide (Reinhard et al., 2006; Munakata and Reinhard, 2007). This deactivation is reversible upon treatment with a suitable oxidant such as sodium hypochlorite (Lowry and Reinhard, 2000) or air-saturated water (Munakata and Reinhard, 2007). Improved catalyst formulations, for example, incorporating Au clusters on Pd or using zeolite supports to separate the Pd from constituents in the water that deactivate the catalyst, can improve resistance to deactivation and increase the time needed before regeneration (Schüth et al., 2000; Heck et al., 2009). However, implementation of CRD using in-well recirculating reactors remains promising. The reduction of other common groundwater contaminants including perchlorate and nitrate by Pd-based catalysts has also been studied (Wang et al., 2009; Choe et al., 2010).
Combined ISCR and In Situ Bioremediation.Amendments that combine chemical reductants (especially ZVI) with materials that stimulate microbial activity (organic carbon in various forms) are available as commercial products. The products include EHC® and Daramend® (FMC Environmental Solutions), ABC® + (Redox Tech, LLC), and emulsified zero-valent iron (EZVI) (National Aeronautics and Space Administration). The relative significance of abiotic versus biotic contaminant degradation by these amendments is usually not known, but they are included among the case studies described below. Additional discussion of these technologies is presented in Chapter 12 (In Situ Bioremediation of Sources).
10.2 TECHNICAL BACKGROUND
This section provides an overview of the general aspects of ISCR, including the processes that lead to contaminant removal (Section 10.2.1), conditions and agents that cause reduction (Section 10.2.2), strategies for accomplishing source zone remediation (Section 10.2.3), engineering aspects of implementation (Section 10.3.3) and considerations regarding the compatibility of ISCR in combination with other remediation technologies (Section 10.3.2).
10.2.1 Chlorinated Solvent Degradation Under Reducing Conditions
Dehalogenation can occur by several reductive pathways (Elsner and Hofstetter, 2011). The simplest results in replacement of a C-bonded halogen atom with a hydrogen and is known as hydrogenolysis or reductive dehalogenation. For a generic chlorinated aliphatic compound, RCl, hydrogenolysis is described by the half reaction:
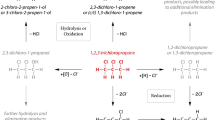
Polychlorinated compounds can undergo sequential hydrogenolysis, resulting in a characteristic sequence of partially dechlorinated products. For polychlorinated methanes, sequential hydrogenolysis via chloroform, dichloromethane, and others is represented by the solid lines in Figure 10.2a. For polychlorinated ethenes, sequential hydrogenolysis results in the dichloroethenes and vinyl chloride, which is shown with solid arrows in Figure 10.2b.
Reaction schemes for reductive transformations of (a) carbon tetrachloride and other chloromethanes and (b) perchloroethene and other chloroethenes. Solid arrows represent hydrogenolysis; finely dashed arrows are for β- and α-reductive elimination; and coarse dashed arrows show hydrogenation, hydrolysis and other steps. The scheme for chloromethanes (a) is synthesized from several sources (McCormick and Adriaens, 2004; Tratnyek, 2010); the scheme for chloroethenes (b) is reproduced from Tratnyek et al. (2003a), which in turn was adapted from Arnold and Roberts (2000).
In both cases, each step in the sequence is much less favorable, thermodynamically and kinetically, than the one before; therefore, sequential hydrogenolysis tends to result in partially dechlorinated products that are persistent and therefore problematic. This is a well-known problem with anaerobic biodegradation of carbon tetrachloride (CT), perchloroethene (PCE) and trichloroethene (TCE). In the case of CT, degradation appears to stall at chloroform and methylene chloride, while degradation of PCE/TCE stalls at the 1,2-dichloroethenes and vinyl chloride. Under conditions where abiotic reduction of contaminants proceeds mainly by sequential hydrogenolysis, as is often observed when CT reacts with ZVI (Matheson and Tratnyek, 1994; Támara and Butler, 2004), the problem of accumulated intermediate dechlorination products may also arise in ISCR.
In contrast to hydrogenolysis, the other major dehalogenation pathway involves eliminating two halogens, leaving behind a pair of electrons that usually forms a carbon–carbon double bond. Where the pathway involves halogens on adjacent carbons, it is known as vicinal dehalogenation or reductive β-elimination, and where both chlorines are eliminated from the same carbon, it is known as gem- or α-elimination. This pathway tends to produce characteristic products: alkenes from vicinal dihaloalkanes (Equation 10.2), alkynes from vicinal dihaloalkenes (Equation 10.3), and fully dehalogenated products from dihalomethanes (Equation 10.4).



After reductive elimination, hydrogenolysis of polychlorinated contaminants can occur, resulting in another series of partially dechlorinated intermediates. On the left side of Figure 10.2b, this process is shown for PCE.
In addition to the two major reductive pathways for dechlorination, two additional reactions must be considered: hydrogenation, which involves addition of hydrogens across a C–C double or triple bond (Equation 10.5), and dehydrohalogenation, which involves elimination of H+ and X– to give a new C–C double bond (Equation 10.6). Hydrogenation has been invoked to explain the distribution of products observed in several studies involving chlorinated alkenes and Fe0 (Arnold and Roberts, 2000) and is particularly important where a noble metal like Pd is present to act as a catalyst for activation of H2. Note that H2(surf) in Equation 10.5 represents all of the various forms of surface-activated hydrogen (e.g., H•) and is not meant to imply that the reaction necessarily involves adsorbed diatomic molecular hydrogen. Dehydrohalogenation (Equation 10.6) has received relatively little attention as a reaction that might contribute to degradation of chlorinated ethenes by Fe0, even though it can be base-catalyzed (Roberts et al., 1993), which might make it favored under alkaline conditions, such as those created by corrosion of zero-valent metals.


The relative significance of hydrogenolysis, reductive elimination, and dehydrohalogenation in the degradation of chlorinated solvents during remediation depends on the contaminant structure, properties of the reductant, and environmental conditions. In general, hydrogenolysis tends to be more important for contaminants that are more nearly per-halogenated (all positions available for substitution are occupied by chlorines) and reductants that are relatively mild and therefore favor 1-electron reduction processes (Equation 10.1). Reductive elimination tends to be most important for contaminants with few chlorines per carbon center and strong reductants that favor two-electron processes (Equations 10.2, 10.3 and 10.4). Dehydrohalogenation is not a reduction reaction and therefore does not require a reductant, but this reaction is base-catalyzed so it is favored at higher pHs.
To improve on the qualitative rules of thumb above, some research has concentrated on developing quantitative models for predicting the relative rates of reduction reactions for the various chlorinated solvents (Tratnyek et al., 2003b). Most of this work has focused on dechlorination by zero-valent iron, and for this reductant—for the limited range of conditions addressed in existing studies—several quantitative structure–activity relationships (QSARs) have been reported (Tratnyek et al., 2003b; Onanong et al., 2007; Cwiertny et al., 2010). However, these relationships do not take into account the reductant type (Fe0 and FeII minerals are likely to produce different relative rates of dechlorination) or the reductant concentrations; thus, they predict only relative rates not absolute rates under specific environmental conditions. In principle, this restriction can be overcome by cross-correlation of reduction rates between two systems (Kenneke and Weber, 2003) or incorporation of site conditions into the QSAR model (Peijnenburg et al., 1991), but neither approach has been applied at the field scale, to date.
10.2.2 Reductants Contributing to ISCR
The dechlorination reactions responsible for most chlorinated solvent degradation during ISCR are written generically as reduction half reactions in Equations 10.1, 10.2, 10.3 and 10.4. To complete these reactions, they must be balanced with oxidation half reactions involving the reducing agents (reductants) that are relevant under ISCR conditions. Identifying the relevant reductants and their corresponding oxidation half reactions is complicated by a number of factors, including equilibrium speciation, kinetically controlled metastable phases, and spatial heterogeneity. This complexity precludes a complete understanding of which reductants are responsible for contaminant degradation during ISCR in any particular field site, but the general principles presented below provide a framework for assessing, predicting, and optimizing the performance of ISCR over all types of sites.
The reductants that play the largest overall role in natural and engineered ISCR processes are the reduced Fe and S mineral phases that were summarized in Section 10.1.1. The relationships between the most stable forms of these phases are shown as redox potential (Eh-pH) diagrams in Figure 10.3a (for Fe minerals) and Figure 10.3c (for Fe/S minerals). From Figure 10.3a, for example, it is evident that the half reaction for contaminant reduction by Fe0 at pH <6 results in Fe2+ (aq) but at pH >7 the thermodynamically preferred oxidation product is Fe3O4 (magnetite). At slightly higher (more positive) potentials, reduction by Fe3O4 favors Fe2O3 (hematite) as the oxidation product. In the presence of S, FeS2 (pyrite) largely replaces Fe3O4 as the most stable intermediate valence phase (Figure 10.3c).
Eh-pH diagrams for iron–water (a, b) and iron–sulfur–water (c, d) systems. The left pair (a, c) shows the most stable species, and the right pair (b, d) shows metastable phases. Blue shading represents solution-phase species and yellow represents solid phases. Calculations were performed using the Geochemist’s Workbench software suite and its default database of thermodynamic stability constants. Red lines represent the conditions with equimolar concentrations of reactants and products.
While the thermodynamically stable phases are favored over the long run, biogeochemical cycling of iron and sulfur or purposeful manipulations of geochemistry for remediation tend to produce metastable phases, which are inclined to be more reactive and therefore can be the dominant reductants of contaminants. To represent these conditions, some of the equilibrium phases in Figure 10.3a, c have been suppressed, resulting in Figure 10.3b, d. In the absence of S, the most important metastable reducing solid phase is probably Fe3(OH)8 (green rust), and in the presence of S, the phase of greatest interest is amorphous FeS.
Where they occur naturally in subsurface environments, the reducing phases represented in Figure 10.3 ultimately arise from and are sustained by microbial activity. This process is mainly due to the metabolism of iron-reducing and sulfate-reducing bacteria, which produce the FeII and SII that go on to form FeII oxides and sulfides as diagramed in Figure 10.4. The four scenarios represented in Figure 10.4 are well supported by laboratory studies of dechlorination in biogeochemical model systems (Lee and Batchelor 2002a, b; Elsner et al., 2004; McCormick and Adriaens, 2004; Butler et al., 2011; Jeong et al., 2011) and are consistent with recent reports of abiotic MNA in the field (Ferrey et al., 2004; Elsner et al., 2010).
However, all of these reducing phases can also be generated in situ through purely abiotic processes by introducing a strong chemical reductant such as dithionite or ZVI. For example, green rust has been detected in and downgradient from ZVI PRBs, and it has been proposed that this phase provides additional opportunity for reduction of contaminants or intermediates of contaminant reduction (Johnson and Tratnyek, 1994; Kamolpornwijit et al., 2004). Recently, it has been shown that green rusts can be synthesized with improved characteristics for remediation (Ayala-Luis et al., 2012; Larese-Casanova et al., 2010), and this might represent a new class of solid-phase reductants engineered for source zone treatment by ISCR.
The reductants emphasized in Figures 10.3 and 10.4 are all solid phases, which implies that contaminant degradation occurs only after direct contact between these reactants. This requires mass transport of the contaminant to the reductant surface and formation of a precursor complex between the two species, either of which can limit the rate of contaminant degradation. In contrast, the analogous steps tend not to be significant barriers to reaction of contaminants with solution-phase reductants; thus, other factors being equal, solution-phase reductants can produce faster degradation rates. Solution-phase reductants that are relevant to ISCR include soluble complexes of FeII (FeII-citrate, FeII-porphyrins), lower-valent forms of S (disulfite, dithionite, thiosulfate, polysulfides), redox-active moieties associated with NOM (quinones) and other redox-active biomolecules such as cobalamins and others.
A selection of these redox couples is summarized on the right side of Figure 10.5 along a redox ladder that represents their standard reduction potentials at pH 7. The figure also shows ranges of potentials of two redox couples involving solid phases (Fe0 nano/FeIII and FeII/FeIII oxides), and comparing these with the data for soluble reductants shows that the two groups overlap in their strength as reductants. Adding the iron sulfide phases and soluble forms of reduced sulfur to Figure 10.5 would not expand the overall range of potentials covered. All of the above are sufficiently strong reductants to make dechlorination thermodynamically favorable (Haas and Shock, 1999; Dolfing, 2003); however, this analysis alone does not ensure that these reactions will be kinetically facile.
Redox ladder of one-electron reduction potentials for dechlorination (left) and standard reduction potentials putative reductants involved in ISCR (right). All potentials are for pH 7. Figure from Elsner and Hofstetter (2011), used with permission.
The link between reductant strength and kinetics of contaminant dechlorination is made in Figure 10.5 by plotting (left of the axis) the one-electron reduction potentials (E 1) for dissociative electron attachment. This step is often the rate-limiting step in dechlorination reactions, and therefore, E 1 is usually a good predictor of dechlorination rates (Tratnyek et al., 2003b; Bylaska et al., 2011). Comparison of the E 1 for dechlorination of various chlorinated solvents to the standard potential for various reductants shows that the initial electron attachment step is only favorable for the more highly chlorinated methanes and ethanes. Even for the less chlorinated alkanes and alkenes, the overall dechlorination reaction (Equations 10.1, 10.2, 10.3 and 10.4) is favorable under most conditions.
Another aspect of contaminant reduction implied by Figures 10.4 and 10.5 is the scenario by which a redox couple of intermediate potential serves to mediate or “shuttle” electrons from the original electron donor to a terminal electron acceptor. This effect can serve to bypass a kinetic barrier to direct reduction of the terminal acceptor by the original donor, if the steps involving the mediator are more facile. It has been shown that the reduction of contaminants can be significantly accelerated by electron transfer mediation involving aqueous complexes of iron, iron oxides, natural organic matter, and model compounds for the quinone moieties associated with natural organic matter (Van der Zee and Cervantes, 2009; Sposito, 2011; Zhang and Weber, 2009). These effects are likely to play a key role in the sustained performance of natural ISCR processes, but they are hard to quantify under field conditions. Electron transfer mediation can also be utilized to achieve enhanced remediation performance in engineered ISCR technologies, although this aspect of ISCR is not yet well developed (Johnson and Tratnyek, 1994).
Throughout the above discussion of contaminant reduction pathways (Section 10.2.1) and the reductants responsible for ISCR processes (this section), half reactions were written that explicitly involve electron transfer (Equations 10.1, 10.2, 10.3 and 10.4), and redox potentials were used to represent relative strength of prospective electron donors and acceptors (Figures 10.3 and 10.5). Most contaminant dechlorination reactions do occur by electron transfer mechanisms, but exceptions exist (e.g., see Equation 10.5 and Smolen et al., 1999), and the potential difference between the electron donor and acceptor does generally correlate with redox reaction rates (Miehr et al., 2004). Furthermore, it is true that faster reduction rates are generally observed in systems that exhibit lower, more negative, overall oxidation–reduction potential (ORP) of the system. However, the latter relationship is weak and indirect, and therefore not a reliable predictor of field scale performance of ISCR, as discussed further in Section 10.3.1.
A full explanation of why the system ORP (as measured by immersing a Pt electrode in a sample of site water or suspension of subsurface sediments) is often not predictive of dechlorination rates and why ORP, per se, should not be interpreted as the cause of contaminant reduction is beyond the scope of this chapter. However, many of the fundamental aspects of this issue have recently been described as they apply to SZTI with nZVI (Shi et al., 2011), and earlier analyses of the general issue of ORP interpretation are cited therein. In the case of SZTI with nZVI, measured ORP has a very complex relationship to the availability of nZVI as an electron donor to effect dechlorination of chlorinated solvents. In ISCR systems dominated by reduced sulfur species, the relationship between ORP and contaminant degradation rates is likely to be even more distant because the mechanism of contaminant reduction by iron sulfides is more complex.
10.2.3 Treatment of Source Zones
As with most other in situ restoration techniques, delivery of reductants to the target treatment zone is critical to the effectiveness of ISCR. In the context of source zone treatment, three types of commonly encountered sources of contaminants are considered here: aqueous plumes (Section 10.2.3.1), entrapped nonaqueous phase liquid (NAPL) sources (Section 10.2.3.2), and diffusional sources (e.g., slow back diffusion of contaminants entrapped in clay lenses) (Section 10.2.3.3). Treating each type of source presents different challenges associated with delivery of materials and will have different requirements in terms of reactivity and reactive lifetime.
10.2.3.1 Treatment of Aqueous Plumes
Although the focus of this book is on treatment of source zones, rather than dissolved groundwater plumes, there are a number of reasons to discuss in situ chemical reduction of aqueous groundwater plumes. Perhaps the most important reason is that many NAPL and diffusion source zones are difficult to access directly because they are located under structures in active use or because the location of the source has not been or cannot be sufficiently characterized to allow for targeted treatment of the source. In these cases, cutting off the source zone with, for example, a permeable reactive barrier may represent the best long-term solution. This approach is practical with ZVI PRBs because they can have very long reactive lifetimes and may require little monitoring, which results in overall favorable life cycle costs when compared with alternatives (Mak and Lo, 2011). Permeable reactive barriers in difficult-to-reach environments (under buildings and in deep groundwater aquifers) may eventually be possible using nZVI. However, challenges with their emplacement (Section 10.2.4.6) will have to be addressed, as will issues related to the relatively short reactive lifetime of nZVI.
10.2.3.2 Treatment of Nonaqueous Phase Liquid Zones
Assuming that the location of the NAPL sources can be determined with sufficient accuracy, delivering reactants to NAPL zones still presents a number of challenges that must be considered if ISCR is to be successful. These challenges are of two general types: hydraulic and partitioning.
Hydraulic challenges arise because ISCR reactive materials are typically delivered using water as the delivery vehicle. Nonaqueous phase liquid zones, and in particular NAPL pools, may have relatively limited contact with flowing groundwater. This situation is common because many sources have been in place for decades, and areas with high NAPL–water contact will have already dissolved away. This limited water–NAPL contact can have significant consequences on the effect of added ISCR reagents. For example, in the nZVI case, the nZVI may not be delivered directly to the NAPL–water interface, but instead will form a reactive barrier in the vicinity of the entrapped NAPL. In this scenario (Figure 10.6), the reaction will be limited by the rate of mass transfer of contaminants from the NAPL phase (present in the layer or in the media or in the intermediate K-layer) into the aqueous phase, where the dissolved contaminants are then transported to the reactive nZVI surfaces (Fagerlund et al., 2012).
Schematic illustrating an entrapped NAPL source of contaminants to an aquifer. Slow dissolution of the entrapped NAPL serves as a long-term source of contamination to the aquifer. Emplacement of nZVI or other particulate reductant in the vicinity of the NAPL or into the NAPL can decrease the source mass, thereby decreasing the mass emission from the source and its lifetime. Challenges lie in providing sufficient reductant to eliminate a significant mass of the entrapped NAPL.
Partitioning challenges arise from the hydrophobic nature of NAPLs, so that water-soluble reagents may move with the groundwater, but will tend to stay in the water phase. A significant amount of research has focused on designing reductants such as nZVI that partition to the NAPL–water interface (Figure 10.7) (Saleh et al., 2005) or completely into the NAPL phase (Ramsburg and Pennell, 2001; Ramsburg and Pennell Kurt, 2002; Ramsburg et al., 2004; Ramsburg et al., 2005). Promoting contact between nZVI and the NAPL will, other factors being equal, increase the rate of reaction because the reaction is proportional to the concentration of dissolved TCE at the nZVI surface (Liu et al., 2007).
Hydrophobic segments (red) of the polymer coating swell in TCE (NAPL) while hydrophilic segments (blue) swell in water. This modification of nZVI could provide thermodynamic affinity of the particles to the NAPL–water interface, causing them to partition to where they can effect maximum NAPL degradation.
However, the approach illustrated in Figure 10.7 still requires that reductants reach the NAPL interface, which can be difficult to achieve because the NAPL may be located in poorly accessible (low conductivity) regions of the aquifer. Furthermore, even if the nZVI can be concentrated at the NAPL–water interface, this will result in rapid oxidation of the nZVI (Fagerlund et al., 2012), the products of which then remain at the NAPL–water interface, thereby preventing attachment of fresh reactive particles (Phenrat et al., 2011). Overall, the concept of targeted delivery of particulate reagents to the NAPL–water interface in situ has proven to be conceptually and practically challenging and has not yet been demonstrated to be a feasible technology for field applications.
10.2.3.3 Treatment of Diffusion Source Zones
Diffusion source zones are created when high-concentration sources such as NAPL remain in contact with lower-permeability media for an extended period of time (years). Such sources can occur at the surfaces of the low-permeability media (beneath pools of NAPL) or deeper within media because of NAPL movement down fractures, and subsequent diffusion. Back diffusion of contaminants from the lower-permeability media such as clay lenses can result in a very long-lived, low concentration sources (Figure 10.8).
Schematic illustrating a diffusional source of contaminants to an aquifer. Slow diffusion of contaminants from clay lenses serves as a long-term source of contamination to the aquifer. nZVI or other particulate reductants can be emplaced in the high K region surrounding the lenses in order to degrade contaminants as they released.
To treat source zones such as those represented in Figure 10.8, the delivered reductant must have sufficient longevity and reactive capacity to sustain adequate degradation rates of contaminants that arrive by diffusion at an interface where groundwater is moving (the surface of the diffusion source). It is necessary in this case to maintain high concentrations of reactant at these interfaces, requiring emplacement of significant quantities of reductant or replenishment of the reductant by repeated or sustained injection via recirculation, for example.
Nanoparticle-sized reagents may ultimately be designed with the desired traits for treating diffusion sources (Khandelwal et al., 1998; Rabideau et al., 1999). For example, particulate reductants could be emplaced into the higher conductivity regions surrounding a clay lens serving as the source (Figure 10.8). The relatively low mobility of these materials (Section 10.2.4.6) could allow them to remain in the region surrounding the lenses and serve as a reactive barrier that degrades contaminants as they are released. This remediation strategy requires reductants that have sufficient capacity and reactivity to produce the required decrease in contaminant concentration, but also have a relatively long reactive lifetime so that they do not need to be replaced frequently.
The process described above primarily treats the mass leaving the diffusion source and has relatively little impact on mass within the diffusion source. If the latter is to be addressed, then the reductant to be delivered must have a number of additional characteristics. For example, it must have a sufficiently large diffusion coefficient that it can penetrate into the diffusion source. Also, it must be sufficiently inert to persist as it diffuses, but also sufficiently reactive to degrade the contaminants when the two come together. Once again, it must have been delivered to the interface with the diffusion source in appropriately high concentrations and for an extended period to control the long-term flux of contaminants from the diffusion source.
This is a challenging set of criteria to meet. Fortunately, the overall process of diffusion and reaction can probably be predicted with reasonable accuracy using numerical modeling (Fagerlund et al., 2012). Thus, if delivery of the reductant to the interface can be accomplished, the effectiveness of the ISCR likely can be assessed.
10.2.4 Strategies for Delivery
Reagents for ISCR are diverse in their chemical form, and some forms involve unique considerations for effective delivery in the subsurface. In situ chemical reduction reagents can include dissolved aqueous species (sodium dithionite), NAPLs (edible oils), emulsions and foams (calcium polysulfide), gases (H2), and particles (ZVI and nZVI). Approaches and challenges for delivery of aqueous solutions of dissolved aqueous species are similar to those for delivery of oxidants for ISCO (Simpkin et al., 2011) (Chapter 9 of this volume). However, delivery of the NAPLs, emulsions, foams, gases, and particulate reagents used in ISCR can present unique challenges. A range of delivery strategies have been utilized for in situ delivery of these various forms of reductants, and they are summarized briefly below.
10.2.4.1 Delivery of Aqueous Solutions of Dissolved Reductants
Perhaps the most common approach for delivery of reductants is by injection of an aqueous solution of a dissolved species via wells into the subsurface. This approach is applicable to moderately permeable media, where the reductants can be emplaced into the formation over distances of meters in time frames of a few days. In high-permeability media, such as gravels, groundwater velocities can be sufficiently large that the residence time of the reductant within the treatment zone may be too small to achieve the required degradation of contaminants or to provide any residual capacity for reduction. In this case, alternate pumping strategies, for example, recirculation and/or injection–extraction (“push–pull”), may be more effective.
In low-permeability materials (clays, tills), solution injection rates can be too low to provide reasonable zones of influence from a given injection well, so hydraulic or pneumatic fracturing has become a common approach to enhance distribution. At least two strategies are used in this type of fracturing. The first is to use the fractures to deliver reductants directly to the source. The second is to use the fractures to focus the groundwater flow through the fractures filled with reductant such that treatment occurs within the fractures. With respect to chlorinated solvent source zones, there has been little evaluation of the relative importance of these mechanisms at the field scale.
10.2.4.2 Improved Delivery of Injected Dissolved Reagents
The delivery of aqueous solutions of reductants is challenging because of the presence of heterogeneity in the porous media and subsequent flow field. In heterogeneous media, targeted delivery of dissolved reductants to source zones is especially difficult if higher flow zones exist that lead the reductant away from the source zone. Several strategies have been developed to improve contact between the reductant and the source. Two of them, shear-thinning fluids and density-enhancing agents, are briefly discussed here.
Shear-thinning fluids have the characteristic that their viscosity decreases as shear velocity increases (Kaplan et al., 1994; Kaplan et al., 1996; Cantrell et al., 1997; Truex et al., 2011a, b). As a result, under high flow rate (injection) conditions, a shear-thinning fluid may be delivered to a high hydraulic conductivity zone, but under lower flow conditions (normal groundwater flow), that fluid will be sufficiently viscous that, once emplaced, flow through that zone will be decreased and as a result increased flow through lower conductivity zones (where the source might be located) will occur. Significant increases in viscosity can be achieved easily with relatively low concentrations (0.1–1 gram per liter [g/L]) of food-grade biopolymers such as xanthan and guar gum. Shear-thinning polymers are commercially available (Truex et al., 2011b). These materials have an added advantage, in the context of ISCR, because they provide readily biodegradable carbon sources that help drive aquifer systems to more strongly reducing conditions.
Shear-thinning fluids have been widely used in the petroleum industry to enhance oil recovery, but they have received less consideration in reductant delivery. One reason may be the compatibility of the viscosity agents and the reductants. For example, the presence of these biopolymers may decrease the reactivity of ZVI or nZVI that is injected along with the shear-thinning fluid (Phenrat et al., 2009a; Tratnyek et al., 2011; Truex et al., 2011b).
Density-driven flow also can be used to deliver aqueous phase dissolved reductants (Henderson et al., 2009; Heiderscheidt et al., 2011). The addition of almost any solute to water increases the density of the resulting solution. For highly soluble solutes, such as inorganic salts, resulting densities can be quite large (>1.2 grams per milliliter (g/mL)). If these solutions are injected into the subsurface, significant downward movement due to gravity can occur. If managed correctly, this enhanced downward movement can be utilized to increase contact between the injected solution and source zones. Several commonly used chemical reductants (sodium dithionite and calcium polysulfide) are very soluble and can be prepared readily at concentrations well suited for density-driven injection.
10.2.4.3 Delivery of Nonaqueous Phase Liquids, Emulsions, and Foams
Nonaqueous phase liquids can be used to emplace reactive materials in the subsurface. The NAPL can be the reactive material itself, or it can be a carrier for other materials. For example, nonaqueous, edible oils have been combined with reductants (especially ZVI) to create biogeochemical zones favorable for ISCR (Quinn et al., 2005). The NAPLs have an important advantage over aqueous liquids because they can become immobilized in the subsurface and as a result can provide a practical approach for maintaining high concentrations of reductants at or near source zones. Furthermore, the low solubility of NAPLs allows them to remain in place and serve as a long-term sources of reductant.
However, it is challenging to deliver nonaqueous fluids in the subsurface in a predictable manner. At least two strategies can improve deliverability of NAPLs: emulsions and fracturing. Emulsions of oil and micron-sized ZVI have been examined in laboratory and field studies (Quinn et al., 2005). The reactivity and transport of oil-in-water emulsions containing nZVI have also been demonstrated in the laboratory (Berge and Ramsburg, 2009). The emulsion may provide multiple benefits:
-
Enhanced transport of the ZVI through the subsurface and to the NAPL interface, thereby requiring less dissolution of NAPL constituents for them to be reduced by the ZVI.
-
Protection of the ZVI from the processes that contribute to natural reductant demand, thereby increasing the ZVI lifetime and possibly its efficiency (proportion of its capacity that goes to reduction of contaminants rather than water and other geochemical oxidants).
-
Provision of organic carbon that can stimulate the biogeochemical conditions that are favorable to microbial dechlorination process.
In practice, the movement of emulsions through the subsurface is problematic for three primary reasons: (1) distribution of emulsions is highly influenced by physical and chemical heterogeneities within the aquifer, (2) emulsions tend to be unstable in porous media (porous media have historically been used to break emulsions created during chemical synthesis) and (3) the emulsions typically used (water–oil–water emulsions) are very viscous (up to 100s of centipoise (cP)) and difficult to inject in porous media, even in relatively high conductivity sand aquifers (Quinn et al., 2005).
Foams are liquid–gas emulsions that have been studied and used to remediate DNAPL and heavy metals from unsaturated soils (Wang and Mulligan, 2004; Shen et al., 2011). The foam is created from mixing a surfactant solution and nonwetting gas. They are very low density and the stability of the foam (time to collapse) can be designed based on surfactant choice to allow for delivery in the subsurface. Foams have been used in at least one field demonstration (Hirasaki et al., 1997) to remove TCE DNAPL. Foams have historically been developed to solubilize and mobilize the contaminants. However, the appropriate choice of surfactants and gas could potentially make foams a viable delivery tool for ISCR reactants in the unsaturated zone.
Movement of NAPL phases, including emulsions and foams, can be enhanced by fracturing. However, precise delivery of these fluids to specific locations (source zones) has proven difficult, even in relatively homogeneous media because it can be difficult to control propagation of the fractures. As discussed above, fracturing can be used to focus groundwater flow and treatment within the fractures, and this may provide the best opportunity for utilizing NAPLs to increase ISCR.
10.2.4.4 Delivery of Gases
Reducing gases, particularly hydrogen, have been used to enhance in situ reduction both in the unsaturated zone (Evans et al., 2011) and in the saturated zone (Newell et al., 1997; Newell et al., 1998; Newell et al., 2000). Delivery of gases in water-saturated media is typically accomplished using in situ sparging, although the use of hollow-fiber membranes also has been investigated (Fang et al., 2002). However, hydrogen solubility in water is only 1–2 mg/L, equivalent to ~800 micromolar (μM) at ambient pressure and groundwater temperature, so delivering significant quantities of dissolved H2 by sparging groundwater is not practical. As with other types of specialized gas sparging, relatively low flow rates and pulsed operations are commonly employed.
In most aquifer materials, especially those with grain sizes less than ~1 millimeter (mm), gases travel almost exclusively in channels, rather than as individual bubbles (Johnson et al., 1993; Clayton, 1998). This limits the contact between water and gas to the flow of water around the gas channel. However, during pulsed operation, significant amounts of gas can remain trapped within the medium, and this trapped gas can provide a long-term low-level source of reductant between pulses. This trapped gas can persist from days to months, depending on the rate of groundwater flow.
One of the important limitations of sparging in source zone treatment is that it can again be difficult to deliver the reactants to the source zone, particularly if those sources are associated with lower-permeability layers (pools on top of those layers or diffusion sources within those layers.) As a result, sparging may be better suited to cutting off plumes from those source zones, rather than in trying to treat the zones directly (Johnson and Johnson, 2012).
10.2.4.5 Emplacement of Solids
Direct in situ mixing of solid-phase reductants and aquifer materials (ISSM) is an attractive approach to ISCR of source zones if conditions allow it (aquifer materials are amenable to auguring and the depth is not prohibitive). If excellent contact between the reductants and the source materials can be achieved, this approach can be quite successful (Olson et al., 2012). However, it does necessitate a significant input of resources. For example, a dense network of augured holes, including significant overlap between the holes, will likely to be necessary if remediation is to be complete. This approach has potential disadvantages, in addition to cost. It may result in mobilization of DNAPLs as part of the auguring process. This mobilization could result in additional downward movement of the DNAPLs, increasing their environmental impact.
As discussed above, in the context of source zone treatment, emplacement of solid reductants as PRBs will probably be useful only when cutoff of the resultant groundwater plume represents the best treatment strategy. In this context, reductant PRBs have been used in conjunction with impermeable barriers (sheet pile, slurry walls) in funnel and gate configurations (Gillham et al., 2010). Fractures propped open with reactants have been proposed for treatment of groundwater plumes (as mini-PRBs) as well as for direct treatment of sources. As discussed above, the focus of source treatment can be either to deliver directly reactants to the source or to capture groundwater flow from the source and treat it within the fractures. In the context of ISCR, emplacement of granular metals or metals mixed with sand, within the fractures, can be an effective approach.
10.2.4.6 Injection of nZVI Suspensions
Of all the in situ approaches discussed here, injection of suspensions containing nZVI has received the most attention from researchers. Nanoscale zero-valent iron has a number of desirable characteristics that have contributed to this interest:
-
It is highly reactive toward a number of important contaminants, including many of the chlorinated solvents.
-
In principle, nZVI or micron-sized ZVI particles can be made mobile in a range of porous media types.
-
The surface properties of these materials can be modified to increase their mobility in the subsurface and to provide NAPL targeting, although the benefits of such targeting are yet to be proven, as discussed above.
-
With sufficient mobility, injection using existing deep wells allows for emplacement of reductants in the deep subsurface, or in other difficult to reach areas, such as under buildings.
-
If the selectivity of nZVI toward the target contaminants can be improved, the emplacement of particulate materials provides excess reductant that will remain in place for extended times (to treat back diffusion sources, for example).
In practice, delivery of nZVI to treat source zones has proven to be more difficult than anticipated. A number of important challenges remain:
-
Magnetic effects of the commonly used nZVI materials are sufficiently large that aggregation (Phenrat et al., 2007) and subsequent straining become an important process limiting nZVI mobility except at very low nZVI concentrations (<<1 g/L) (Phenrat et al., 2009a).
-
Magnetic properties also may contribute to deposition within the subsurface because nZVI particles have a tendency to deposit on previously deposited nZVI particles.
-
Three-dimensional flow pathways tend to cause nZVI transport directions to shift easily as plugging of the formation occurs, particularly in the immediate vicinity of injection wells.
-
Reliance on water as the injection fluid for the dispersion limits the amount of nZVI that can be delivered directly to entrapped DNAPL (Figure 10.9).
Figure 10.9 Images of polymer-coated nZVI targeting entrapped NAPL in situ. A 3 g/L dispersion of nZVI was flowed past the entrapped NAPL, and a fraction of the nZVI remained in the source. (a) A source with low NAPL saturation allows some nZVI to access the entire source. (b) A source with high NAPL saturation diverts water and nZVI above the source and only allows for attachment at the upper surface. Reprinted with permission from Phenrat et al. (2011). Copyright 2011, American Chemical Society.
The combination of these challenges makes it difficult to deliver nZVI in porous media and to deliver the quantities of nZVI necessary to significantly reduce the contaminant masses characteristic of source zones.
As a rule of thumb, travel distances of approximately 1.5 m (5 ft) are required to make nZVI injection an attractive remediation alternative. This spacing allows for an injection gallery with wells approximately 3 m (10 ft) apart. Lower mobility will require too many wells/injection points to distribute the material in the subsurface to create a PRB for plume treatment or to deliver sufficient material to entrapped NAPL. While some laboratory and field data suggest that nZVI transport distances of >2 m (6.5 ft) should be possible (Bennett et al., 2010), there are few field examples of where significant quantities of nZVI have been directly observed at distances of more than 1.5 m (5 ft) from an injection point (Johnson et al., 2013). Within those few examples is evidence that flow was primarily along preferential flow pathways, in agreement with expectations and with laboratory studies (Phenrat et al., 2010a).
It should be noted that travel distance is operationally defined. Sometimes travel distance is considered the distance where nZVI is detected; however, this distance may represent only a small fraction (<1%) of the concentration of nZVI in the injection solution (Johnson et al., 2013). In other cases, it may be defined as the distance where a sizeable fraction (e.g., 25%) of the injected concentration is measured. Inconsistent use of the term travel distance has led to confusion over what is truly achievable in terms of travel distance for nZVI.
These field scale results suggest that it will be difficult to deliver significant quantities of nZVI to NAPL source zones when relying on water as a carrier for the nZVI. These data do, however, suggest that emplacement of nZVI into higher conductivity regions lying above diffusion sources may be possible. This remediation strategy can provide a reactive barrier to degrade contaminants as they diffuse from the low conductivity region to the higher conductivity regions. Progress continues to be made with regard to minimizing aggregation and increasing mobility through the use of surface modification, and the current state of research is summarized below.
A very important limitation of nZVI transport in the subsurface is its aggregation and subsequent filtration by the aquifer materials (Johnson et al., 2013). This leads to pore plugging, changes in flow patterns, and an inability to deliver sufficient material to the source (Phenrat et al., 2010b). The transport of polymer-coated nZVI in porous media can be good at low concentrations (100s of mg/L), but delivery of concentrated nZVI suspensions (~10 g/L) is required to make nZVI a useful remediation alternative, regardless of whether the goal is to emplace a PRB for plume treatment, to target NAPL directly, or to emplace nZVI in high conductivity regions to prevent back diffusion of contaminants. Effective delivery of concentrated solutions may be achieved through the use of surface modifications of nZVI using polymers (polypropylene glycol or carboxymethylcellulose) to prevent aggregation and deposition. It may also be achievable through the use of excess polymer in the injection solution, which can modify the surfaces of porous media as the solution carrying the nZVI moves through. Modification of the porous media further decreases deposition of nZVI, which vastly improves transport (Kim et al., 2012). Shear-thinning fluids for delivery of nZVI may also prove useful, as this method has been used to deliver 2-micron-sized ZVI particles in porous media (Truex et al., 2011a).
Two approaches may be used to modify the surfaces of nZVI. The first is to physically adsorb (physisorb) polymers to the nZVI surface (Phenrat et al., 2008). Polymers readily adsorb to nZVI, and modification is achieved simply by dispersing the nZVI in the presence of the dissolved polymer. This simple procedure allows one to select from a variety of polymer types available. It also allows for the selection of polymers compatible with the site’s geochemical conditions, and polymers that can potentially serve as a biostimulant for microbial degradation of contaminants if free polymer is present in the injection solution. The presence of polyaspartate coating on nZVI increased microbial biomass in an organic carbon limited marine soil by several orders of magnitude (Kirschling et al., 2010). Polymers with average molecular weights greater than ~1,000 g/mol will typically adsorb to nZVI with densities ranging from a 0.25 to 2 mg/m2 (Golas et al., 2010). Adsorption of large molecular weight polymers (e.g., >2,000 g/mole) occurs at multiple points on the nZVI surface and is therefore effectively irreversible over time scales of interest, for example, months to years (Kim et al., 2009).
The second modification approach is to synthesize the nZVI in the presence of the polymeric modifier (carboxymethylcellulose) (He and Zhao, 2007; Liu et al., 2008). In this scenario the polymer is also used to help control the size distribution of the nZVI being synthesized. This process seems to provide surface-modified nZVI that is quite stable against aggregation and has improved transport characteristics over other types of nZVI (Bennett et al., 2010; He et al., 2010).
It should be noted that the adsorption of the polymeric coating onto nZVI will also alter the reactivity of the nZVI (Tratnyek et al., 2011). The coating generally decreases reactivity by a factor of 4 to 10 (Saleh et al., 2007), due to a combination of site blocking and an effective decrease in the surface concentration of the contaminant at the nZVI–water interface (Phenrat et al., 2009b). Typically this reduction in nZVI reactivity will not decrease reactivity to levels that make it impractical, but the reactivity of surface-modified material should be used in design calculations, especially for a PRB scenario where the length of the reactive zone will depend on the reaction rate of the target contaminant with the nZVI.
10.2.5 Combined Remedies
Some of the most significant improvements in remediation efficiency over the last decade have come through combined remedies in which two or more approaches are combined to take advantages of both. Two strategies for enhancing ISCR are the addition of heat and the arrangement of ISCR and other treatment processes into combinations of spatial zones. Further discussion of combined remedies is provided in Chapter 15 of this volume.
10.2.5.1 Heat
Heat represents a straightforward approach for increasing the reactivity of ISCR agents. Since the rates of many abiotic processes increase in a predictable manner with increasing temperature (they show Arrhenius-type behavior), even modest increases in temperature can have a significant effect. Thus, a small amount of electrical resistance heating that raises the temperature 10 or 20°C (50 or 68°F) can result in a several-fold increase in rates. Of course, rates of reactions that compete with the target reaction may also be increased, and as a result the persistence of the reactants may be reduced. However, a number of scenarios have been proposed that are designed to take advantage of increased reaction rates for both oxidants and reductants (La Mori et al., 2010; Truex et al., 2011a, b).
10.2.5.2 Sequential Zones
The deliberate combination of treatment methods into sequential zones has been proposed fairly frequently. Three early efforts of this type were the systematic study of various sequenced reactive barriers for treatment of chlorinated solvents (Fiorenza et al., 2000), a sequential reactive treatment zone proposed for reductive and then oxidative treatment of explosives contaminated groundwater (Tratnyek et al., 2001), and pretreatment zones for protecting ZVI PRBs from premature failure (Kenneke and McCutcheon, 2003). Recently, this approach has been further developed and referred to as multibarrier design (Bastiaens et al., 2005). There are, of course, many possible combinations of treatment zones in a multibarrier design, and it will be some time before the most beneficial combinations are identified and recognized as proven.
Most multibarrier concepts entail combination of a chemical (ISCR) treatment process with some form of enhanced bioremediation. In some cases, the intent is clearly to form distinct zones that isolate treatment processes over a scale of several meters of more (Becvar et al., 2008), whereas other approaches attempt to stimulate abiotic and biotic contaminant degradation in closer proximity (millimeters to centimeters). Examples of the latter (involving products such as EHC®) were summarized in Section 10.1.2. All these scenarios still have uncertainty about whether the abiotic and biotic processes are synergistic or antagonistic: the conditions within a ZVI PRB would seem to be unfavorable for most forms of microbial activity, yet recent work has generally found that the overall effect on remediation performance is positive (Cullen et al., 2011; Kirschling et al., 2010; Van Nooten et al., 2008).
10.3 IMPLEMENTATION
As with any remedy for contaminated groundwater, the implementation of ISCR involves three stages: (1) site delineation and characterization, (2) technology assessment and selection and (3) technology design and implementation. Each of these stages is discussed in the following subsections, with emphasis on aspects unique to ISCR and relevant to source zone remediation.
10.3.1 Site Delineation and Characterization
In addition to the site delineation and characterization requirements that are prerequisite to any sort of remedial action (type and extent of contamination, lithology and hydrology of the contaminated zone, proximity to receptors), several considerations require special attention if ISCR is to be a candidate for source zone treatment.
The first of these requirements is whether the types of contaminants at the site are subject to degradation by reduction. As described in Section 10.2.1, chlorinated solvents, explosives, and pesticides containing nitro groups and a few other types of organic contaminants can be reduced under ISCR conditions. Organic compounds without these functional groups—such as aliphatic and aromatic hydrocarbons (surfactants, most fuel components, polycyclic hydrocarbons [PAHs])—generally will be unaffected by ISCR. Furthermore, with respect to dechlorination, relatively highly chlorinated aliphatic compounds, such as CT and PCE, will be much more labile to reduction than less highly chlorinated aromatic compounds. These trends in susceptibility to ISCR are the inverse of what is expected, and observed, under conditions of ISCO.
The second type of characterization requirement that has aspects unique to ISCR concerns the site geochemistry: specifically, the type, quantity and availability of redox-active materials that will act as sinks for reductants in the treatment zone. The most rigorous approach to this issue would be to inventory all the potentially important materials (reducing minerals, natural organic matter, reducible contaminants, dissolved oxygen) and develop a biogeochemical reactive-transport model for the system based on principles summarized in Section 10.2.2. This, however, is rarely feasible, so a more practical alternative approach is needed. One approach that probably offers the best compromise between rigor and practicality involves standardized assays of reductant demand that are analogous to the assays for oxidant demand that are now widely used to determine the applicability of ISCO to particular sites (Haselow et al., 2003; ASTM, 2007).
The analogy between reductant demand and oxidant demand extends to a range of issues, from the operationally defined nature of these parameters to the variety of qualifiers needed to refine the terminology (natural, total, aquifer, and others). All of these issues have been discussed extensively for oxidant demand (Lee and Batchelor, 2003; Urynowicz et al., 2008; Xu and Thomson, 2008; Xu and Thomson, 2009), but the concept of reductant demand is much less well developed. Few previous publications have explicitly addressed this subject (Ford, 2002; Brown and Robinson, 2004; Johnson et al., 2013), but to be consistent with that work, the term used in this chapter is natural reductant demand (NRD). While the main significance of NRD is to characterize the capacity of the system to consume reductant, any assay for NRD will depend on the reductant used and the time of exposure to the reductant used in the assay (just as NOD depends on the exposure time to a particular oxidant) (Borda et al., 2009).
Currently, there are no validated or widely used protocols for characterizing NRD of an aquifer system. Instead, current practice is to rely on oxidation–reduction potential (ORP) measurements made with an inert working electrode (usually Pt). As a general rule, negative ORPs (vs. the normal hydrogen electrode, NHE) suggest reducing conditions, which means low NRD to be overcome with added reductant, and together these things favor ISCR. Furthermore, treatability by ISCR generally will be more favorable at more negative ORPs.
However, the use of ORP in this way has important caveats. First, ORP measurements generally do not reflect the contribution of solid phases, such as minerals and natural organic matter; these can be significant sinks for reductants. In addition, the ORP response of dissolved-phase redox-active species varies greatly in sensitivity (FeII is high, but ammonia is low), reversibility (electrode reactions involving N and S are irreversible), and electrode material and condition (H2 is electrode active on Pt but not on carbon). Due to these limitations, ORP should be regarded only as a qualitative indicator of the prospects for source zone treatment by ISCR.
Beyond NRD and ORP, other biogeochemical conditions that should be considered in assessing the applicability of ISCR to any particular site are the background concentrations of dissolved species indicative of the major microbial terminal electron-accepting processes (TEAPs). These include O2, iron, nitrate, sulfate, sulfide, methane and hydrogen. Carbonate concentrations (because it can cause precipitation) and pH should also be measured.
10.3.2 Technology Assessment and Selection
Several broad classes of technology are typically considered for chlorinated volatile organic compound (CVOC) sites in addition to ISCR, including physical removal (excavation), containment, extraction (aqueous and volatile; with and without applied heat), chemical destruction and biological treatment. Even within the category of ISCR, the range of technologies (summarized in Section 10.1.2) is rather diverse. To select from among all of these options, four general criteria need to be assessed:
-
1.
Initial Site Conditions: (1) degree of chlorination of the contaminants (number of chlorine atoms per carbon atom), (2) oxidation–reduction state, (3) pH, (4) site permeability and heterogeneity (in lower-permeability and more heterogeneous media, technology selection may be directed toward aggressive emplacement methods, such as mixing or fracturing) and (5) size of treatment zone in relation to the remediation technology (very large sites may be better managed using containment strategies rather than aggressive source treatment).
-
2.
Performance Goals: (1) the treatment goal, (2) the time needed to treat or to achieve the treatment goal and (3) the length of time the agent remains active to address mass transfer issues associated with posttreatment rebound.
-
3.
Implementability: (1) site lithology (permeability, heterogeneity) that can control the distribution of reactants, (2) site conditions/accessibility (e.g., if the CVOCs are located under buildings, in areas of subsurface pipes, one or more technologies may be eliminated from consideration) and (3) depth of contamination may also preclude the use of some technologies.
-
4.
Cost Criteria: (1) the chemical agent(s) to be applied, (2) the application methods, (3) the number of applications and (4) monitoring requirements.
Among these criteria are several aspects of particular importance for ISCR. With regard to site conditions, the aquifer should, in general, be in a reduced state prior to ISCR to minimize reductant demand and mineral precipitation. Ideally, the pH should be slightly acidic (4 < pH <7) and ISCR approaches will, in general, be more effective if the contaminants are highly chlorinated (at least one carbon atom with >50% maximum chlorination).
Since ISCR can be applied in a number of ways (recirculation of reactants, containment using PRBs, direct mixing in source zones), it is difficult to evaluate the strengths and weaknesses of ISCR in a general way. Of the ISCR approaches currently in use, application of granular zero-valent metals (ZVMs) represents a technology that fills a niche without many direct competitors. This is due in part to the comparatively long useful lifetime of granular ZVMs in the subsurface as PRBs. Since transport of ZVMs, including nano- and micro-sized materials, is difficult, the emplacement techniques should be considered carefully. These can include trenching (PRB construction), mechanical mixing, and movement in induced fractures. At the same time, ZVMs are not well suited for distribution via groundwater wells, and application of ISCR in this context utilizes divalent metal such as FeII species and/or reductant anions (S2O4 2−).
10.3.3 Technology Design and Implementation
For many ISCR applications, design and implementation are intimately coupled to the technology itself. This is particularly the case for ZVM applications, where the mode of delivery will often be chosen simultaneously with the reductant. The situation is less defined for dissolved species delivered by some form of aqueous injection such as recirculation wells. In this case, tradeoffs between several candidate reductants and delivery options may need to be evaluated.
The objective of the design process is to provide detailed engineering parameters for implementing the technology. The design process may employ both bench scale and pilot scale testing in order to identify these aspects:
-
The dosage of reactive agent required to reach the treatment goal. The beneficial and nonbeneficial (reductant demand) consumption of the reactive agents.
-
An estimate of the number of sequential treatments necessary to achieve cleanup goals.
-
The construction and spacing of application points, if used, including permanent points, temporary points, in situ mixing design (if used) and monitoring system.
-
Design of the monitoring program including frequency, duration, and density of monitoring points necessary to characterize the performance of the technology. In the context of ISCR, this program should include both a strategy to monitor the delivery and persistence of the reactants as well as to monitor the destruction of the CVOCs.
Of particular design relevance in the context of ISCR are reductant demand, persistence of the reductant in the medium due to competitive reactions, efficacy of the degradation process under site-specific conditions, and the impacts of the reductants on biohydrogeochemical conditions in the aquifer (precipitate formation, pH, dissolved metals concentration).
In situ chemical reduction technologies, like ISCO, come with some inherent level of risk because large quantities of reactive materials are being handled and/or injected under pressure. As a consequence, health and safety aspects of technology implementation are important.
The most common concern of ISCR implementation almost certainly deals with the mobility of nano- and micrometer-sized ZVM. As discussed above, historical interpretation of ZVM transport has, in general, been overly optimistic. Design and implementation of ZVM injection systems, excluding fracturing, should be undertaken with the idea that transport distances will likely be less than 1 m (3.28 ft), except in exceptional circumstances, and as a result, target areas must be small or the number of injection points must be large.
10.3.4 Case Studies
Well documented and characterized sites provide case studies for most of the ISCR remediation technologies. Most ISCR technologies (abiotic MNA, BiRD or ISRM) have only a few such sites, so selection of which case studies to feature below was straightforward. However, for the ZVI-based approaches, a plethora of informative case studies exist. In these cases, the sites with the longest assessment history have been featured to provide evidence regarding longevity.
10.3.4.1 Abiotic Monitored Natural Attenuation Case Study
In 2003, at the Air Force Center for Environmental Excellence (AFCEE) Technology Transfer Workshop, John T. Wilson presented a paper titled “Abiotic reactions may be the most important mechanism in natural attenuation of chlorinated solvents” (Wilson, 2003). This presentation was one of the first public discussions of the role of reduced minerals in the attenuation of chlorinated solvents. This observation was based on the study of a cis-1,2- dichloroethene (DCE) plume at the Twin Cities Army Ammunition Plant (TCAAP) in Arden Hills, Minnesota, USA. It is of note that the TCAAP site was studied as a biologically mediated MNA site (Wilson et al., 2001).
Ferrey and co-workers discussed the analysis of abiotic MNA of the site (Ferrey et al., 2004), comparing laboratory microcosm results to 13 years of monitoring data. They concluded that the reductions in measured groundwater concentrations at the site were consistent with dechlorination rates measured in the microcosms. The rates of dechlorination for autoclaved and live microcosms were observed to be similar, and they attributed the abiotic degradation to magnetite, which was detected in aquifer solids from the site. This result was consistent with their modeling of dechlorination by magnetite using surface-area normalized rate constants derived sample data and literature sources (Lee and Batchelor, 2002a, b).
10.3.4.2 BiRD Case Study
At Dover Air Force Base, Delaware, USA, a portion of a solvent plume was treated with Epson salt (MgSO4•7H2O) and sodium lactate (Kennedy et al., 2006a). The solution was injected into a contaminated groundwater plume in five wells positioned 3 m (10 ft) apart and perpendicular to groundwater flow. Sediment was sampled prior to and 8 months postinjection. Significant iron sulfide minerals were developed in the sandy aquifer matrix. Trichloroethene reduction was observed a few weeks after injection, and 95% reductions in PCE, TCE and cis-DCE concentrations were achieved in less than 1 year.
10.3.4.3 ISRM Case Study
In Fort Lewis, Washington, USA, an extensive treatability study was performed to determine optimal conditions for ISRM (Szecsody et al., 2000), and then a buffered solution of sodium dithionite (Na2S2O4) was injected at the site to reduce the iron in the phyllosilicate clays of the aquifer sediments (Vermeul et al., 2000). After four injections (Figure 10.10), more than 40% of the ferric iron was reduced to ferrous iron, and TCE degradation was observed. Downgradient from the treated, reduced zone, TCE concentrations were decreased by as much as 92%.
Conceptual model and treatment plan for ISRM proof-of-principle test site at the Fort Lewis Logistics Center, WA. From Vermeul et al. (2000), used with permission.
10.3.4.4 Conventional ZVI PRB
Case studies of ZVI PRBs are so numerous that they have been reviewed many times (U.S. Environmental Protection Agency, 1999; Vidic, 2001; Wilkin and Puls, 2003; Wilkin et al., 2003) and there even have been several meta-analyses of data across ZVI PRB case study sites (Powell et al., 2002; Henderson and Demond, 2007). The original pilot test of a ZVI PRB by Gillham and co-workers at Canadian Forces Base Borden in Ontario, Canada, was studied extensively and documented (O’Hannesin and Gillham, 1998; Wadley et al., 2005), and the first full-scale commercial application of a ZVI PRB by Geosyntec Consultants in Sunnyvale, California, USA, was also described in detail and monitored over a considerable time period (Sorel et al., 2003; Warner and Sorel, 2003). However, these efforts were soon followed by several even more thorough and comprehensive assessments of in situ performance of ZVI PRBs. Three of the most impactful early case studies include:
-
1.
Denver Federal Center in Denver, Colorado, USA, led by U.S. Geological Survey investigators (McMahon et al., 1999; Paul et al., 2003; Wilkin et al., 2003)
-
2.
Naval Air Station Moffett Field in Mountain View, California, USA, led by Battelle investigators (Gavaskar et al., 1998; ESTCP, 1999; Gavaskar, 1999; Yabusaki et al., 2001; Jeen et al., 2011)
-
3.
Elizabeth City, New Jersey, USA, led by USEPA investigators (Puls et al., 1996; Blowes et al., 1999a, b; Blowes and Mayer, 1999; Puls et al., 1999a, b; Mayer et al., 2001; Furukawa et al., 2002; Paul et al., 2003; Wilkin et al., 2005; Lee and Wilkin, 2010)
Conventional ZVI PRBs are not applied directly to source zones, although they are frequently used for source zone containment. Direct application of ZVI to source zones is mainly through targeted injection or mixing into source zones, and case studies for each of these are presented in the two sections that follow.
10.3.4.5 Source Zone Targeted Injection of nZVI
Since 2000, at least 34 uses of nZVI injection have been documented, primarily for remediation of chlorinated solvents. These have included direct injection of aqueous slurries of nZVI, injection of emulsions (EZVI) and the use of shear-thinning fluids (for 1 micron-sized ZVI). Pressure injection is the most commonly used injection method, but some cases of gravity fed (low pressure) injections have also been documented. The most comprehensive list of these sites can be found at the U.S. Environmental Protection Agency (USEPA) CLU-IN site (http://clu-in.org/download/remed/nano-site-list.pdf; accessed January 27, 2014).
In some cases cleanup goals were met using the technology, but in many cases cleanup goals were not achieved. Reasons for missing cleanup targets vary by site, but it is likely that the distribution of iron at most sites was insufficient to meet cleanup objectives. Unlike PRBs, very few cases of nZVI injection are documented sufficiently with respect to the distribution of the injected iron and the longevity of treatment to fully assess the potential of these technologies. Some of the best-documented studies are presented here to shed light on the state of the technology with respect to the ability to emplace the nZVI using injection wells. Injections of either nZVI emulsions (EZVI) or stabilized nZVI slurries have shown limited success for emplacing the nZVI uniformly in the subsurface at reasonable distances from the injection wells.
In the most prominent case study of EZVI emplacement (Krug et al., 2010), pneumatic fracturing and direct-push injection was used at a site that consisted of fine to medium sand. With coring they found evidence of EZVI emplaced 1.8 and 0.8 m (6 and 2.6 ft) from the injection well for pneumatic fracturing and direct-push injection, respectively. They also observed significant variability in the vertical distribution of the EZVI in those soil cores. A second example used a recirculating well system to inject an aqueous dispersion of nZVI; Henn and Waddill (2006) showed evidence of nZVI at one monitoring well approximately 1.2 m (4 ft) from an injection well. He et al. (2010) conducted a field test using nZVI that was prepared in the field by borohydride reduction of FeSO4 in carboxymethylcellulose (CMC). With this material, they achieved transport distances of 1.5 m (5 ft); however, breakthrough at that distance occurred more rapidly than was expected based on a simple geometric calculation, probably because of preferential flow in a high hydraulic conductivity layer present at the site (F. He, Oak Ridge National Laboratory, personal communication, 2011). As a consequence, the nZVI was likely delivered into only a small fraction of the vertical injection interval. All of these documented cases suggest that transport of significant quantities of nZVI in the subsurface has been limited using existing injection methods and available nZVI formulations.
Emplacement of injected ZVI (~2 ± 1 micron-sized particles) in a TCE source zone, and the subsequent effect on TCE concentrations in monitoring wells, was documented by Truex et al. (2011a). They injected approximately 190 kg (420 lbs) of these mZVI particles at Joint Base Lewis-McChord (formerly Fort Lewis) near Tacoma, Washington, USA, using a shear-thinning polymer (SlurryPro™) present at 0.019 wt%. They injected a total volume of 13,660 L (3,610 gallons) at approximately 83 L/minute (22 gallons per minute) over a 2.5-hour period. Unlike previous reports for injections of nZVI, this injection method in this very high-permeability formation did not cause undue increases in pressure at the injection well (about 1.5 m head). The presence of ZVI was measured at a monitoring well as far as 4 m (13 ft) away from the injection well; however, the maximum concentration of ZVI found at any well away from the injection well was only 26% of the injection concentration at a distance of 1.2 m (4 ft). Most measurements were less than 10% of the injected ZVI concentration, and only 2.6% of the injected ZVI concentration was found at a distance of 4 m (13 ft). The lack of a trend in measured concentration of ZVI versus distance from the injection point suggests that heterogeneity likely influenced the distribution of ZVI in the subsurface.
Despite the nonuniform distribution of nZVI and an initial increase in TCE concentrations in monitoring wells due to the displaced water during injection, TCE concentrations decreased over a 44-day period after injection in all monitoring wells to levels below those observed before treatment, and the formation of reduction products (ethane, acetylene and 1,2-DCE) was observed. Electrical resistance heating of the site to approximately 50°C further enhanced TCE degradation and mass removal. The ZVI kept aqueous phase concentrations of TCE low during the heating which minimized migration of TCE in the vapor phase (Truex et al., 2011a). Overall, this demonstrates the potential to emplace particulate phase reductants for ISCR in a source zone, but highlights the issues surrounding their use, notably the difficulty in obtaining uniform emplacement at significant distances away from an injection well.
10.3.4.6 In Situ Soil Mixing Case Study
A number of in situ soil mixing demonstrations have been presented in the literature (Shackelford et al., 2005; Fjordboge et al., 2012a, b; Olson et al., 2012). The results from Camp Lejeune, North Carolina, USA, are representative of those observed elsewhere. A total of 22,900 m3 (30,000 cubic yards) of soils were treated to an average depth of 7.6 m (25 ft) with 2% ZVI and 3% sodium bentonite (dry-weight basis, Olsen et al., 2012). After one year the total concentrations of CVOCs in soil samples decreased by site-wide average and median values of 97% and >99%, respectively. Because of the addition of the bentonite, pre- and post-mixing average hydraulic conductivity values were 1.7 × 10−5 and 5.2 × 10−8 meters per second (m/s), respectively, indicating a reduction of about 2.5 orders of magnitude. To control volatile organic compound (VOC) volatilization during mixing, a 4-m diameter “hood” was used (Figure 10.11).
Apparatus used for ISSM at Camp Lejeune, NC. (a) Crane-mounted rotary table and hollow kelly bar, (b) 3.0 m diameter augers. From Olsen et al. (2012), used with permission.
10.4 SYNTHESIS AND RECOMMENDATIONS
10.4.1 Advantages and Limitations: Lessons Learned
The remediation technologies grouped under ISCR, as defined here, are diverse in many respects, including the two classification criteria emphasized in Figure 10.1 (treatment volume and reductant strength) and other considerations such as expected longevity, compatibility with other treatment technologies, and track record of successful full-scale implementations. Diversity with respect to the first two criteria is an advantage of ISCR, in that specific ISCR technologies can be applied to a wide range of scales and contaminants. However, diversity with respect to the latter considerations reflects the relative immaturity of ISCR as a family of technologies, and this means the full potential value of ISCR applications probably has not yet been realized.
The background and experience currently available on remediation of contaminated groundwater with ISCR, as summarized in this chapter, lead to the following specific conclusions and recommendations:
-
Treatability of Contaminants: In situ chemical reduction treatment processes range from mild to very strong, so a wide range of contaminants are potentially treatable from CT with dissolved forms of FeII to chlorinated aromatics with H2/Pd. The rates and products of these processes can be favorable, but results may vary.
-
Deployment: In many cases, successful treatment can be achieved by influencing the natural biogeochemical conditions to favor contaminant reduction, and this approach is usually more straightforward to deploy. In cases where deployment of strong, artificial reductants is justified, the natural reductant demand should also be considered.
-
Delivery: Effective delivering of reactants as fluids to source zones is challenging because of aquifer heterogeneity and risk of NAPL displacement and must be considered a limitation in the context of ISCR implementation. In some cases delivery may be improved using amendments such as viscous fluids. There has also been considerable interest in delivery of nZVI with surface treatments designed to favor partitioning into NAPL. However, delivery of sufficient quantities of those materials through aquifers to source zones will require delivery strategies beyond well injections primarily used today.
-
Complementary Processes: Other processes may be stimulated or inhibited by ISCR treatment. In particular, biodegradation is an important component of many ISCR activities (as either the primary or a secondary mode of action) and should be considered in the design of most ISCR systems. The addition of organic and/or inorganic reductants to directly or indirectly facilitate biodegradation represents an important component of long-term treatment.
-
Longevity: The reductant lifetime is an important consideration. As with other in situ chemical treatment approaches, chemical reductants added to the subsurface may have a relatively short lifetime during which the contaminant treatment goal must be achieved, or sufficiently reducing conditions must be created so that contaminant degradation will continue after the added reductant is exhausted.
Of the ISCR approaches discussed above, those involving granular zero-valent iron are the only ones that have been applied at a sufficient number of sites to be considered proven. Zero-valent iron PRBs have been shown to remain active for many years in most cases. In the context of source zone treatment, in situ soil mixing with granular ZVI has been shown to be reliably effective.
10.5 FUTURE PROSPECTS AND NEEDS
10.5.1 Near-Term Prospects Without Additional Research
In situ chemical reduction has developed over the past decade from a plume containment technology to a technique that is being used to treat and/or contain source zones. Despite some limitations, it is a promising technology that can help practitioners manage source zones more effectively and efficiently in the future (Stroo et al., 2012). It is likely that use of ISCR will continue to grow (just as with ISCO some years ago) based on pooling of available technology and information alone, even without or before any new technology development.
Increased adoption of ISCR techniques for source zone treatment is expected as an outgrowth of increased awareness and acceptance of ISCR. The technology can provide rapid mass destruction in situ, potentially with continued long-term treatment of any residual contamination remaining after treatment. In situ chemical reduction also offers remarkable flexibility, with different formulations and products for different objectives and site conditions. Practitioners have learned how best to deploy the different reductants and how to combine them with other processes such as biodegradation, so increased use of existing products and development of improved materials is likely.
10.5.2 Longer-Term Research Needs
Despite the increased use and attention, ISCR still has several important limitations as a source zone treatment technology. Research and development in this area are necessary to overcome or mitigate these limitations and to expand the use of the ISCR technologies. Key areas for future research include the following:
-
Better understanding of the interactions between chemical and biological processes. The emphasis should be on the interactions, because the processes are linked. For example, a more refined distinction between biotic and abiotic is not necessarily that useful, but a more quantitative understanding of the biotic–abiotic interactions should lead to more efficient use of the combined processes.
-
Better methods to characterize in situ reducing conditions. Effective diagnosis and design requires tools that can distinguish intensity from capacity. The current methods are not adequate for characterizing field sites to select or design and ISCR technique.
-
A method to predict dechlorination rates from a readily measurable or controllable system property. This has been the stated goal of many research studies over the last decade, but a variety of factors have prevented the emergence of a practical method of quantitatively assessing the performance of ISCR at any particular field site based on general characteristics of the site and contaminants.
-
More emphasis on improving the overall efficiency of treatment rather than alternative reductants. Most of the most viable candidate reductants are already known, but there are many ways to improve their performance after deployment (catalysts or other activation strategies, innovative delivery techniques or longevity enhancements).
-
Methods to deliver reductants in situ in a controlled manner and with a more uniform distribution are needed.
REFERENCES
Adriaens P, Gruden C, McCormick ML. 2004. Biogeochemistry of halogenated hydrocarbons. Treatise Geochem 9:511–539.
Amonette JE. 2002. Iron redox chemistry of clays and oxides: Environmental applications. In Fitch A, ed, Electrochemistry of Clays. Clay Minerals Society, Aurora, CO, USA. CMS Work Lect 10:89–147.
Amonette JE, Workman DJ, Kennedy DW, Fruchter JS, Gorby YA. 2000. Dechlorination of carbon tetrachloride by Fe(II) associated with goethite. Environ Sci Technol 34:4606–4613.
Arnold WA, Roberts AL. 2000. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environ Sci Technol 34:1794–1805.
Arnold WA, Ball WP, Roberts AL. 1999. Polychlorinated ethane reaction with zero-valent zinc: Pathways and rate control. J Contam Hydrol 40:183–200.
ASTM (American Society for Testing and Materials). 2007. Standard Test Method for Estimating the Permanganate Natural Oxidant Demand of Soil and Aquifer Solids. D7262-07. ASTM International, West Conshohocken, PA, USA. Vol 04.09.
Ayala-Luis KB, Cooper NGA, Koch CB, Hansen HCB. 2012. Efficient dechlorination of carbon tetrachloride by hydrophobic green rust intercalated with dodecanoate anions. Environ Sci Technol 46:3390–3397.
Bastiaens L, Dries J, Vos J, Simons Q, De Smet M, Diels L. 2005. Comparison of different multibarrier concepts designed for treatment of groundwater containing mixed pollutants. IAHS Publ 298:45–51.
Becvar E, Evans P, Lebron C, Stroo H, Wilson JT, Wymore R. 2008. Workshop on In Situ Biogeochemical Transformation of Chlorinated Solvents. AFD-080429–058. Brooks City Base, TX, USA. 65 p.
Bennett P, He F, Zhao D, Aiken B, Feldman L. 2010. In situ testing of metallic iron nanoparticle mobility and reactivity in a shallow granular aquifer. J Contam Hydrol 116:35–46.
Berge ND, Ramsburg CA. 2009. Oil-in-water emulsions for encapsulated delivery of reactive iron particles. Environ Sci Technol 43:5060–5066.
Blowes DW, Mayer KU. 1999. An in situ permeable reactive barrier for the treatment of hexavalent chromium and trichloroethylene in ground water, Volume 3-Multicomponent Reactive Transport Modeling. EPA/600/R-99/095c. U.S. Environmental Protection Agency, Ada, OK, USA. 39 p.
Blowes DW, Gillham RW, Ptacek CJ, Puls RW, Bennett TA, O’Hannesin SF, Hanton-Fong CJ, Bain JG. 1999a. An in situ permeable reactive barrier for the treatment of hexavalent chromium and trichloroethylene in ground water, Volume 1-Design and Installation. EPA/600/R-99/095a. U.S. Environmental Protection Agency, Ada, OK, USA. 111 p.
Blowes DW, Puls RW, Gillham RW, Ptacek CJ, Bennett TA, Bain JG, Hanton-Fong CJ, Paul CJ. 1999b. An in situ permeable reactive barrier for the treatment of hexavalent chromium and trichloroethylene in ground water, Volume 2-Performance Monitoring. EPA/600/R-99/095b. U.S. Environmental Protection Agency, Ada, OK, USA. 207 p.
Borda MJ, Venkatakrishnan R, Gheorghiu F. 2009. Status of nZVI technology: Lessons learned from North American and international implementations. In Geiger Cherie L, Carvalho-Knighton KM, eds, Environmental Applications of Nanoscale and Microscale Reactive Metal Particles. Symposium Series 1027. American Chemical Society, Washington, DC, USA, pp 219–232.
Brown RA. 2008. Developments in in situ chemical reduction (ISCR) technology. Proceedings, 6th International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 19–22. Battelle Press, Columbus, OH, USA, Paper C-066.
Brown RA. 2010. Chemical oxidation and reduction for chlorinated solvent remediation. In Stroo HF, Ward CH, eds, In Situ Remediation of Chlorinated Solvent Plumes. Springer, New York, NY, USA, pp 481–535.
Brown RA, Robinson D. 2004. Response to naturally occurring organic material: Permanganate versus persulfate. Proceedings of the 4th International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27. Battelle Press, Columbus, OH, USA. 2A.06/1-2A.06/8.
Brown RA, Lewis RL, Fiacco RJ, Jr, Leahy MC. 2006. The technical basis for in situ chemical reduction (ISCR). Proceedings, 5th International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 22–25. Battelle Press, Columbus, OH, USA. d 04 ppr/1-d 04 ppr/8.
Brown RA, Wilson JT, Ferrey M. 2007. Monitored natural attenuation forum: The case for abiotic MNA. Remediat J 17:127–137.
Butler EC, Hayes KF. 1999. Kinetics of the transformation of trichloroethylene and tetrachloroethylene by iron sulfide. Environ Sci Technol 33:2021–2027.
Butler EC, Hayes KF. 2001. Factors influencing rates and products in the transformation of trichloroethylene by iron sulfide and iron metal. Environ Sci Technol 35:3884–3891.
Butler EC, Dong Y, Krumholz LR, Liang X, Shao H, Tan Y. 2011. Rate controlling processes in the transformation of tetrachloroethylene and carbon tetrachloride under iron reducing and sulfate reducing conditions. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:519–538.
Bylaska EJ, Salter-Blanc AJ, Tratnyek PG. 2011. One-electron reduction potentials from chemical structure theory calculations. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA. ACS Symp Ser 1071:37–64.
Cantrell KJ, Kaplan DI, Gilmore TJ. 1997. Injection of colloidal Fe0 particles in sand with shear-thinning fluids. J Environ Eng 123:786–791.
Cervini-Silva J, Larson RA, Wu J, Stucki JW. 2002. Dechlorination of pentachloroethane by commercial Fe and ferruginous smectite. Chemosphere 47:971–976.
Choe JK, Shapley JR, Strathmann TJ, Werth CJ. 2010. Influence of rhenium speciation on the stability and activity of Re/Pd bimetal catalysts used for perchlorate reduction. Environ Sci Technol 44:4716–4721.
Clayton WS. 1998. A field and laboratory investigation of air fingering during air sparging. Ground Water Monit Remediat 18:134–145.
Comba S, Molfetta A, Sethi R. 2011. A comparison between field applications of nano-, micro-, and millimetric zero-valent iron for the remediation of contaminated aquifers. Water Air Soil Pollut 215:595–607.
Crane RA, Scott TB. 2012. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–125.
Cullen LG, Tilston EL, Mitchell GR, Collins CD, Shaw LJ. 2011. Assessing the impact of nano- and micro-scale zerovalent iron particles on soil microbial activities: Particle reactivity interferes with assay conditions and interpretation of genuine microbial effects. Chemosphere 82:1675–1682.
Cwiertny DM, Arnold WA, Kohn T, Rodenburg LA, Roberts AL. 2010. Reactivity of alkyl polyhalides toward granular iron: Development of QSARs and reactivity cross correlations for reductive dehalogenation. Environ Sci Technol 44:7928–7936.
Darlington R, Lehmicke L, Andrachek RG, Freedman DL. 2008. Biotic and abiotic anaerobic transformations of trichloroethene and cis-1,2-dichloroethene in fractured sandstone. Environ Sci Technol 42:4323–4330.
Davie MG, Cheng H, Hopkins GD, Lebron CA, Reinhard M. 2008. Implementing heterogeneous catalytic dechlorination technology for remediating TCE-contaminated groundwater. Environ Sci Technol 42:8908–8915.
Dolfing J. 2003. Thermodynamic considerations for dehalogenation. In Häggblom MM, Bossert ID, Bossert ID, eds, Dehalogenation: Microbial Processes and Environmental Applications. Kluwer, Boston, MA, USA, pp 89–114.
Dolfing J, van Eekert M, Seech A, Vogan J, Mueller J. 2008. In situ chemical reduction (ISCR) technologies: Significance of low Eh reactions. Soil Sediment Contam 17:63–74.
Elsner M, Hofstetter TB. 2011. Current perspectives on the mechanisms of chlorohydrocarbon degradation in subsurface environments: Insight from kinetics, product formation, probe molecules and isotope fractionation. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:407–439.
Elsner M, Schwarzenbach RP, Haderlein SB. 2004. Reactivity of Fe(II)-bearing minerals toward reductive transformation of organic contaminants. Environ Sci Technol 38:799–807.
Elsner M, Couloume GL, Mancini S, Burns L, Lollar BS. 2010. Carbon isotope analysis to evaluate nanoscale Fe(0) treatment at a chlorohydrocarbon contaminated site. Ground Water Monit Remediat 30:79–95.
Erbs M, Hansen HCB, Olsen CE. 1999. Reductive dechlorination of carbon tetrachloride using iron(II) iron(III) hydroxide sulfate (green rust). Environ Sci Technol 33:307–311.
ESTCP (Environmental Security Technology Certification Program). 1999. Permeable Reactive Wall Remediation of Chlorinated Hydrocarbons in Groundwater. Cost Performance Report for CU-9604. Alexandria, VA, USA. 52 p.
Evans PJ, Fricke RA, Hopfensperger K, Titus T. 2011. In situ destruction of perchlorate and nitrate using gaseous electron donor injection technology. Ground Water Monit Remediat 31:103–112.
Fagerlund F, Illangasekare TH, Phenrat T, Kim HJ, Lowry GV. 2012. PCE dissolution and simultaneous dechlorination by nanoscale zero-valent iron particles in a DNAPL source zone. J Contam Hydrol 131:9–28.
Fang Y, Hozalski RM, Clapp LW, Novak PJ, Semmens MJ. 2002. Passive dissolution of hydrogen gas into groundwater using hollow-fiber membranes. Water Res 36:3533–3542.
Ferrey ML, Wilkin RT, Ford RG, Wilson JT. 2004. Nonbiological removal of cis-dichloroethylene and 1,1-dichloroethylene in aquifer sediment containing magnetite. Environ Sci Technol 38:1746–1752.
Fiorenza S, Oubre CL, Ward CH. 2000. Sequenced Reactive Barriers for Groundwater Remediation. CRC Press, Boca Raton, FL, USA. 730 p.
Fjordboge AS, Lange IV, Bjerg PL, Binning PJ, Riis C, Kjeldsen P. 2012a. ZVI-Clay remediation of a chlorinated solvent source zone, Skuldelev, Denmark: 2. Groundwater contaminant mass discharge reduction. J Contam Hydrol 140–141:67–79.
Fjordboge AS, Riis C, Christensen AG, Kjeldsen P. 2012b. ZVI-Clay remediation of a chlorinated solvent source zone, Skuldelev, Denmark: 1. Site description and contaminant source mass reduction. J Contam Hydrol 140–141:56–66.
Ford RG. 2002. Solid phase redox characterization. In Wilkin RT, Ludwig RD, Ford RG, eds, Workshop on Monitoring Oxidation-Reduction Processes for Ground-Water Restoration. Workshop Summary, Dallas, TX, USA. April 25–27. EPA/600/R-02/002. U.S. Environmental Protection Agency, Cincinnati, OH, USA, pp 105–113.
Fruchter J. 2002. In-situ treatment of chromium-contaminated groundwater. Environ Sci Technol 36:464A–472A.
Fruchter JS, Cole CR, Williams MD, Vermeul VR, Amonette JE, Szecsody JE, Istok JD, Humphrey MD. 2000. Creation of a subsurface permeable treatment zone for aqueous chromate contamination using in situ redox manipulation. Ground Water Monit Remediat 20:66–77.
Furukawa Y, Kim J-W, Watkins J, Wilkin RT. 2002. Formation of ferrihydrite and associated iron corrosion products in permeable reactive barriers of zero-valent iron. Environ Sci Technol 36:5469–5475.
Gavaskar A. 1999. Design and construction techniques for permeable reactive barriers. J Hazard Mater 68:41–71.
Gavaskar A, Sass B, Gupta N, Hicks J, Yoon S, Fox T, Sminchak JR. 1998. Performance Evaluation of a Pilot-Scale Permeable Reactive Barrier at Former Naval Air Station Moffett Field, Mountain View, California. Final Report for ESTCP CU-9604. Battelle Press, Columbus, OH, USA. 173 p.
Geiger CL, Carvalho-Knighton KM, eds. 2009. Environmental applications of nanoscale and microscale reactive metal particles. ACS Symp Ser 1027. 306 p.
Gillham RW, Vogan J, Gui L, Duchene M, Son J. 2010. Iron barrier walls for chlorinated solvent remediation. In Stroo HF, Ward CH, eds, In Situ Remediation of Chlorinated Solvent Plumes. Springer, New York, NY, USA, pp 537–571.
Golas PL, Lowry GV, Tilton RD, Matyjaszewski K. 2010. Designing polymer stabilizers by ATRP for iron nanoparticles used in groundwater remediation. ACS National Meeting, March 21–25, San Francisco, CA, USA. American Chemical Society, Washington, DC, USA. COLL-304.
Gorski CA, Nurmi JT, Tratnyek PG, Hofstetter TB, Scherer MM. 2010. Redox behavior of magnetite: Implications for contaminant reduction. Environ Sci Technol 44:55–60.
Graham MC, Farmer JG, Anderson P, Paterson E, Hillier S, Lumsdon DG, Bewley RJF. 2006. Calcium polysulfide remediation of hexavalent chromium contamination from chromite ore processing residue. Sci Total Environ 364:32–44.
Grittini C, Malcomson M, Fernando Q, Korte N. 1995. Rapid dechlorination of polychlorinated biphenyls on the surface of a Pd/Fe bimetallic system. Environ Sci Technol 29:2898–2900.
Gui L, Gillham RW. 2003. Preparation and regeneration of nickel-iron for reduction of organic contaminants. In Henry SM, Warner SD, eds, Chlorinated Solvent and DNAPL Remediation: Innovative Strategies for Subsurface Cleanup. American Chemical Society, Washington, DC, USA. ACS Symp Ser 837:206–216.
Haas JR, Shock EL. 1999. Halocarbons in the environment: Estimates of thermodynamic properties for aqueous chloroethylene species and their stabilities in natural settings. Geochim Cosmochim Acta 63:3429-3441.
Haderlein SB, Schwarzenbach RP. 1995. Environmental processes influencing the rate of abiotic reduction of nitroaromatic compounds in the subsurface. In Spain JC, ed, Biodegradation of Nitroaromatic Compounds. Plenum, New York, NY, USA, pp 199–225.
Haderlein SB, Hofstetter TB, Schwarzenbach RP. 2000. Subsurface chemistry of nitroaromatic compounds. In Spain JC, Hughes JB, Knackmus HJ, eds, Biodegradation of Nitroaromatic Compounds and Explosives. Lewis, Boca Raton, FL, USA, pp 311–356.
Haselow JS, Siegrist RL, Crimi M, Jarosch T. 2003. Estimating the total oxidant demand for in situ chemical oxidation design. Remediat J 13:5–16.
He F, Zhao D. 2007. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ Sci Technol 41:6216–6221.
He F, Zhao D, Paul C. 2010. Field assessment of carboxymethyl cellulose stabilized iron nanoparticles for in situ destruction of chlorinated solvents in source zones. Water Res 44:2360–2370.
He Y, Su C, Wilson J, Wilkin R, Adair C, Lee TR, Bradley P, Ferrey M. 2009. Identification and characterization methods for reactive minerals responsible for natural attenuation of chlorinated organic compounds in ground water. EPA/600/R-09/115. U.S. Environmental Protection Agency, Ada, OK, USA. 150 p.
Heck KN, Nutt MO, Alvarez P, Wong MS. 2009. Deactivation resistance of Pd/Au nanoparticle catalysts for water-phase hydrodechlorination. J Catal 267:97–104.
Heiderscheidt JL, Illangasekare TH, Borden RC, Thomson NR. 2011. Principles of ISCO related subsurface transport and modeling. In Siegrist RL, Crimi M, Simpkin TJ, eds, In Situ Chemical Oxidation for Groundwater Remediation. Springer, New York, NY, USA, pp 233–284.
Henderson AD, Demond AH. 2007. Long-term performance of zero-valent iron permeable reactive barriers. A critical review. Environ Eng Sci 24:401–423.
Henderson TH, Mayer KU, Parker BL, Al TA. 2009. Three-dimensional density-dependent flow and multicomponent reactive transport modeling of chlorinated solvent oxidation by potassium permanganate. J Contam Hydrol 106:195–211.
Henn KW, Waddill DW. 2006. Utilization of nanoscale zero-valent iron for source remediation – A case study. Remediat J 16:57–77.
Hirasaki GJ, Miller CA, Szafranski R, Tanzil D, Lawson JB, Meinardus HW, Jin M, Londergan J, Jackson RE, Pope GA, Wade WH. 1997. Field demonstration of the surfactant/foam process for aquifer remediation. SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, San Antonio, TX, USA, October 5–8, Paper EPE 39292:1–16.
Ingram JC, Cortez MM, Bates DL, McCurry MO. 2000. Reductive dechlorination of trichloroethylene and carbon tetrachloride at iron oxides and basalt minerals. In Eller PG, Heineman W, eds, Nuclear Site Remediation. American Chemical Society, Washington, DC, USA, ACS Symp Ser 778:267–281.
Jeen SW, Gillham RW, Przepiora A. 2011. Predictions of long-term performance of granular iron permeable reactive barriers: Field-scale evaluation. J Contam Hydrol 123:50–64.
Jeong HY, Anantharaman K, Han Y-S, Hayes KF. 2011. Abiotic reductive dechlorination of cis-dichloroethylene by Fe species formed during iron- or sulfate-reduction. Environ Sci Technol 45:5186–5194.
Johnson RL, Johnson PC. 2012. In situ sparging for delivery of gases in the subsurface. In Kitanidis PK, McCarty PL, eds, Delivery and Mixing in the Subsurface. Springer, New York, NY, USA, pp 193–216.
Johnson RL, Johnson PC, McWhorter DB, Hinchee RE, Goodman I. 1993. An overview of in situ air sparging. Ground Water Monit Remediat 13:127–135.
Johnson RL, Nurmi JT, O’Brien G, Fan D, Shi Z, Salter-Blanc Alexandra J, Tratnyek, PG, Lowry, GV. 2013. Field scale transport and transformation of carboxymethylcellulose stabilized nano zero valent iron. Env Sci Tech 47:1573–1580.
Johnson TL, Tratnyek PG. 1994. A column study of carbon tetrachloride dehalogenation by iron metal. 33rd Hanford Symposium on Health and the Environment. Battelle Pacific Northwest Laboratories, Richland, WA, USA, Vol 2, pp 931–947.
Kamolpornwijit W, Liang L, Moline GR, Hart T, West OR. 2004. Identification and quantification of mineral precipitation in Fe0 filings from a column study. Environ Sci Technol 38:5757–5765.
Kaplan DI, Cantrell KJ, Wietsma TW. 1994. Formation of a barrier to groundwater contaminants by the injection of zero-valent iron colloids: suspension properties. 33rd Hanford Symposium on Health and the Environment, Vol 2. Battelle Pacific Northwest Laboratories, Richland, WA, USA, pp 821–837.
Kaplan DI, Cantrell KJ, Wietsma TW, Potter MA. 1996. Retention of zero-valent iron colloids by sand columns: Application to chemical barrier formation. J Environ Qual 25:1086–1094.
Kennedy LG, Everett JW, Becvar E, DeFeo D. 2006a. Field-scale demonstration of induced biogeochemical reductive dechlorination at Dover Air Force Base, Dover, Delaware. J Contam Hydrol 88:119–136.
Kennedy LG, Everett JW, Gonzales J. 2006b. Assessment of biogeochemical natural attenuation and treatment of chlorinated solvents, Altus Air Force Base, Altus, Oklahoma. J Contam Hydrol 83:221–236.
Kenneke JF, McCutcheon SC. 2003. Use of pretreatment zones and zero-valent iron for the remediation of chloroalkenes in an oxic aquifer. Environ Sci Technol 37:2829–2835.
Kenneke JF, Weber EJ. 2003. Reductive dehalogenation of halomethanes in iron- and sulfate-reducing sediments. 1. Reactivity pattern analysis. Environ Sci Technol 37:713–720.
Khandelwal A, Rabideau AJ, Shen P. 1998. Analysis of diffusion and sorption of organic solutes in soil-bentonite barrier materials. Environ Sci Technol 32:1333–1339.
Kim H-J, Phenrat T, Tilton RD, Lowry GV. 2009. Fe0 nanoparticles remain mobile in porous media after aging due to slow desorption of polymeric surface modifiers. Environ Sci Technol 43:3824–3830.
Kim H-J, Phenrat T, Tilton RD, Lowry GV. 2012. Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media. J Colloid Interf Sci 370:1–10.
Kirschling TL, Gregory KB, Minkley EG, Jr, Lowry GV, Tilton RD. 2010. Impact of nanoscale zero valent iron on geochemistry and microbial populations in trichloroethylene contaminated aquifer materials. Environ Sci Technol 44:3474–3480.
Kriegman-King MR, Reinhard M. 1994. Transformation of carbon tetrachloride by pyrite in aqueous solution. Environ Sci Technol 28:692–700.
Krug T, O'Hara S, Watling M, Quinn J. 2010. Emulsified zero-valent nano-scale iron treatment of chlorinated solvent DNAPL source areas. Final Report. ESTCP ER-0431. Environmental Security Technology Certification Program, Alexandria, VA, USA, 763 p.
La Mori P, Kirkland E, Faircloth H, Bogert R, Kershner M. 2010. Combined thermal and zero-valent iron in situ soil mixing remediation technology. Remediat J 20:9–25.
Landis RL, Gillham RW, Reardon EJ, Fagan R, Focht RM, Vogan JL. 2001. An examination of zero-valent iron sources used in permeable reactive barriers. International Containment and Remediation Technology Conference and Exhibition, Orlando, FL, USA, June 10–13. Abstract 420.
Larese-Casanova P, Cwiertny DM, Scherer MM. 2010. Nanogoethite formation from oxidation of Fe(II) sorbed on aluminum oxide: Implications for contaminant reduction. Environ Sci Technol 44:3765–3771.
Lee TR, Wilkin RT. 2010. Iron hydroxy carbonate formation in zerovalent iron permeable reactive barriers: Characterization and evaluation of phase stability. J Contam Hydrol 116:47–57.
Lee W, Batchelor B. 2002. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 1. Pyrite and magnetite. Environ Sci Technol 36:5147–5154.
Lee W, Batchelor B. 2002. Abiotic reductive dechlorination of chlorinated ethylenes by iron-bearing soil minerals. 2. Green rust. Environ Sci Technol 36:5348–5354.
Lee W, Batchelor B. 2003. Reductive capacity of natural reductants. Environ Sci Technol 37:535–541.
Li WF, Klabunde KJ. 1998. Ultrafine zinc and nickel, palladium, silver coated zinc particles used for reductive dehalogenation of chlorinated ethylenes in aqueous solution. Croat Chem Acta 71:853–872.
Li X-Q, Elliott DW, Zhang W-X. 2006. Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit Rev Solid State Mater Sci 31:111–122.
Liu J, He F, Durham E, Zhao D, Roberts CB. 2008. Polysugar-stabilized Pd nanoparticles exhibiting high catalytic activities for hydrodechlorination of environmentally deleterious trichloroethylene. Langmuir 24:328–336.
Liu Y, Phenrat T, Lowry GV. 2007. Effect of TCE concentration and dissolved groundwater solutes on NZVI-promoted TCE dechlorination and H2 evolution. Environ Sci Technol 41:7881–7887.
Lowry GV. 2007. Nanomaterials for groundwater remediation. In Wiesner MR, Bottero J-Y, eds, Environmental Nanotechnology. McGraw Hill, New York, NY, USA, pp 297–336.
Lowry GV, Reinhard M. 2000. Pd-catalyzed TCE dechlorination in groundwater: solute effects, biological control, and oxidative catalyst regeneration. Environ Sci Technol 34:3217–3223.
Macalady DL, Tratnyek PG, Grundl TJ. 1986. Abiotic reduction reactions of anthropogenic organic chemicals in anaerobic systems. J Contam Hydrol 1:1–28.
Mak MSH, Lo IMC. 2011. Environmental life cycle assessment of permeable reactive barriers: Effects of construction methods, reactive materials and groundwater constituents. Environ Sci Technol 45:10148–10154.
Matheson LJ, Tratnyek PG. 1994. Reductive dehalogenation of chlorinated methanes by iron metal. Environ Sci Technol 28:2045–2053.
Mayer KU, Blowes DW, Frind EO. 2001. Reactive transport modeling of an in situ reactive barrier for the treatment of hexavalent chromium and trichloroethylene in groundwater. Water Resour Res 37:3091–3104.
McCormick ML, Adriaens P. 2004. Carbon tetrachloride transformation on the surface of nanoscale biogenic magnetite particles. Environ Sci Technol 38:1045–1053.
McMahon PB, Dennehy KF, Sandstrom MW. 1999. Hydraulic geochemical performance of a permeable reactive barrier containing zero-valent iron, Denver Federal Center. Ground Water 37:396–404.
McNab WW, Jr, Ruiz R, Reinhard M. 2000. In-situ destruction of chlorinated hydrocarbons in groundwater using catalytic reductive dehalogenation in a reactive well: Testing and operational experiences. Environ Sci Technol 34:149–153.
Miehr R, Tratnyek PG, Bandstra JZ, Scherer MM, Alowitz M, Bylaska EJ. 2004. The diversity of contaminant reduction reactions by zero-valent iron: Role of the reductate. Environ Sci Technol 38:139–147.
Mueller NC, Braun J, Bruns J, Cernik M, Rissing P, Rickerby D, Nowack B. 2012. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558.
Munakata N, Reinhard M. 2007. Palladium-catalyzed aqueous hydrodehalogenation in column reactors: Modeling of deactivation kinetics with sulfide and comparison of regenerants. Appl Catal B Environ 75:1–10.
Newell CJ, Fisher RT, Hughes JB. 1997. Direct hydrogen addition for the in-situ biodegradation of chlorinated solvents. Proceedings, Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection, and Remediation Conference, Houston, TX, USA, November 12–14, pp 791–800.
Newell CJ, Hughes JB, Fisher RT, Haas PE. 1998. Subsurface hydrogen addition for the in-situ bioremediation of chlorinated solvents. Proceedings, 1st International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 21–28. Battelle Press, Columbus, OH, USA, pp 47–52.
Newell CJ, Haas PE, Hughes JB, Khan TA. 2000. Results from two direct hydrogen delivery field tests for enhanced dechlorination. Proceedings, 2nd International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 22–25. Battelle Press, Columbus, OH, USA, pp 21–38.
O’Hannesin SF, Gillham RW. 1998. Long-term performance of an in situ “iron wall” for remediation of VOCs. Ground Water 36:164–170.
O’Loughlin EJ, Burris DR. 2004. Reduction of halogenated ethanes by green rust. Environ Toxicol Chem 23:41–48.
O’Loughlin EJ, Boyanov MI, Antonopoulos DA, Kemner KM. 2011. Redox processes affecting the speciation of technetium, uranium, neptunium, and plutonium in aquatic and terrestrial environments. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071: 477–517.
Olson MR, Sale TC, Shackelford CD, Bozzini C, Skeean J. 2012. Chlorinated solvent source-zone remediation via ZVI-clay soil mixing: 1-year results. Ground Water Monit Remediat 32:63–74.
Onanong S, Comfort SD, Burrow PD, Shea PJ. 2007. Using gas-phase molecular descriptors to predict dechlorination rates of chloroalkanes by zerovalent iron. Environ Sci Technol 41:1200–1205.
Paul CJ, McNeil MS, Beck Jr FP, Clark PJ, Wilkin RT, Puls RW. 2003. Capstone report on the application, monitoring, and performance of permeable reactive barriers for ground-water remediation: Vol 2 – Long-Term Monitoring of PRBs: Soil and Ground Water Sampling. EPA/600/R-03/045b. U.S. Environmental Protection Agency, Washington, DC, USA.
Peijnenburg WJGM, ’t Hart MJ, den Hollander HA, van de Meent D, Verboom HH, Wolfe NL. 1991. QSARs for predicting biotic and abiotic reductive transformation rate constants of halogenated hydrocarbons in anoxic sediment systems. Sci Total Environ 109/110:283–300.
Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV. 2007. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290.
Phenrat T, Saleh N, Sirk K, Kim H-J, Tilton RD, Lowry GV. 2008. Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: Adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J Nanopart Res 10:795–814.
Phenrat T, Kim H-J, Fagerlund F, Illangasekare T, Tilton RD, Lowry GV. 2009a. Particle size distribution, concentration, and magnetic attraction affect transport of polymer-modified Fe0 nanoparticles in sand columns. Environ Sci Technol 43:5079–5085.
Phenrat T, Liu Y, Tilton RD, Lowry GV. 2009b. Adsorbed polyelectrolyte coatings decrease Fe nanoparticle reactivity with TCE in water: Conceptual model and mechanisms. Environ Sci Technol 43:1507–1514.
Phenrat T, Song JE, Cisneros CM, Schoenfelder DP, Tilton RD, Lowry GV. 2010. Estimating attachment of nano- and submicrometer-particles coated with organic macromolecules in porous media: Development of an empirical model. Environ Sci Technol 44:4531–4538.
Phenrat T, Cihan A, Kim H-J, Mital M, Illangasekare T, Lowry GV. 2010. Transport and deposition of polymer-modified Fe0 nanoparticles in 2-D heterogeneous porous media: Effects of particle concentration, Fe0 content, and coatings. Environ Sci Technol 44:9086–9093.
Phenrat T, Fagerlund F, Illangasekare T, Lowry GV, Tilton RD. 2011. Polymer-modified Fe0 nanoparticles target entrapped NAPL in two dimensional porous media: Effect of particle concentration, NAPL saturation, and injection strategy. Environ Sci Technol 45:6102–6109.
Powell RM, Powell PD, Puls DW. 2002. Economic analysis of the implementation of permeable reactive barriers for remediation of contaminated groundwater. EPA/600/R-02/034. U.S. Environmental Protection Agency, Ada, OK, USA. 31 p.
Puls RW, Paul CJ, Powell RM. 1996. Remediation of Chromate-Contaminated Groundwater Using Zero-Valent Iron: Field Test at USCG Support Center, Elizabeth City, North Carolina. Final Report. EPA/600/R-02/034. U.S. Environmental Protection Agency, Washington, DC, USA. 19 p.
Puls RW, Blowes DW, Gillham RW. 1999a. Long-term performance monitoring for a permeable reactive barrier at the U.S. Coast Guard Support Center, Elizabeth City, North Carolina. J Hazard Mater 68:109–124.
Puls RW, Paul CJ, Powell RM. 1999b. The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chromate-contaminated groundwater: a field test. Appl Geochem 14:989–1000.
Quinn J, Geiger C, Clausen C, Brooks K, Coon C, O’Hara S, Krug T, Major D, Yoon W-S, Gavaskar A, Holdsworth T. 2005. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ Sci Technol 39:1309–1318.
Rabideau AJ, Shen P, Khandelwal A. 1999. Feasibility of amending slurry walls with zero-valent iron. J Geotech Geoenviron Eng 125:330–333.
Ramsburg CA, Pennell KD. 2001. Experimental and economic assessment of two surfactant formulations for source zone remediation at a former dry cleaning facility. Ground Water Monit Remediat 21:68–82.
Ramsburg CA, Pennell KD. 2002. Density-modified displacement for DNAPL source zone remediation: density conversion and recovery in heterogeneous aquifer cells. Environ Sci Technol 36:3176–3187.
Ramsburg CA, Pennell KD, Kibbey TC, Hayes KF. 2004. Refinement of the density-modified displacement method for efficient treatment of tetrachloroethene source zones. J Contam Hydrol 74:105–131.
Ramsburg CA, Pennell KD, Abriola LM, Daniels G, Drummond CD, Gamache M, Hsu H-L, Petrovskis EA, Rathfelder KM, Ryder JL, Yavaraski TP. 2005. Pilot-scale demonstration of surfactant-enhanced PCE solubilization at the Bachman Road site. 2. System operation and evaluation. Environ Sci Technol 39:1791–801.
Reinhard M, Hopkins GD, Cunningham J, Lebron CA. 2006. From laboratory study to full-scale application: Treating groundwater for TCE removal using catalyzed reductive dechlorination. Proceedings, 232nd ACS National Meeting, September 10–14, San Francisco, CA, USA. American Chemical Society. ENVR-68.
Roberts AL, Jeffers PM, Wolfe NL, Gschwend PM. 1993. Structure-reactivity relationships in dehydrohalogenation reactions of polychlorinated and polybrominated alkanes. Crit Rev Environ Sci Technol 23:1–39.
Roy-Perreault A, Kueper BH, Rawson J. 2005. Formation and stability of polychlorinated biphenyl pickering emulsions. J Contam Hydrol 77:17–39.
Saleh N, Phenrat T, Sirk K, Dufour B, Ok J, Sarbu T, Matyjaszewski K, Tilton RD, Lowry GV. 2005. Adsorbed triblock copolymers deliver reactive iron nanoparticles to the oil/water interface. Nano Lett 5:2489–2494.
Saleh N, Sirk K, Liu Y, Phenrat T, Dufour B, Matyjaszewski K, Tilton RD, Lowry GV. 2007. Surface modifications enhance nanoiron transport and NAPL targeting in saturated porous media. Environ Eng Sci 24:45–57.
Scherer MM, Richter S, Valentine RL, Alvarez PJJ. 2000. Chemistry and microbiology of permeable reactive barriers for in situ groundwater clean up. Crit Rev Environ Sci Technol 30:363–411.
Schüth C, Disser S, Schüth F, Reinhard M. 2000. Tailoring catalysts for hydrodechlorinating chlorinated hydrocarbon contaminants in groundwater. Appl Catal B Environ 28:147–152.
Schwarzenbach RP, Stierli R, Lanz K, Zeyer J. 1990. Quinone and iron porphyrin mediated reduction of nitroaromatic compounds in homogeneous aqueous solution. Environ Sci Technol 24:1566–1574.
Scott TB, Popescu IC, Crane RA, Noubactep C. 2011. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J Hazard Mater 186:280–287.
Shackelford CD, Sale TC, Liberati MR. 2005. In-situ remediation of chlorinated solvents using zero valent iron and clay mixtures: A case history. Proceedings, 18th GRI Conference held in conjunction with Geo-Frontiers 2005, January 24–26, Austin, TX, USA. Geo-Institute of the American Society of Civil Engineers, Reston, VA, USA, pp 3699–3707.
Shen X, Zhao L, Ding Y, Liu B, Zeng H, Zhong L, Li X. 2011. Foam, a promising vehicle to deliver nanoparticles for vadose zone remediation. J Hazard Mater 186:1773–1780.
Shi Z, Nurmi JT, Tratnyek PG. 2011. Effects of nano zero-valent iron (nZVI) on oxidation-reduction potential (ORP). Environ Sci Technol 45:1586–1592.
Simpkin TJ, Palaia T, Petri BG, Smith BA. 2011. Oxidant delivery approaches and contingency planning. In Siegrist RL, Crimi M, Simpkin TJ, eds, In Situ Chemical Oxidation for Groundwater Remediation. Springer, New York, NY, USA, pp 449–480.
Smolen JM, Weber EJ, Tratnyek PG. 1999. Molecular probe techniques for the identification of reductants in sediments: Evidence for reduction of 2-chloroacetophenone by hydride transfer. Environ Sci Technol 33:440–445.
Sorel D, Warner SY, Longino BL, Honniball JH, Hamilton LA. 2003. Performance monitoring and dissolved hydrogen measurements at a permeable zero valent iron reactive barrier. In Henry SM, Warner SD, eds, Chlorinated Solvent and DNAPL Remediation. American Chemical Society, Washington, DC, USA, ACS Symp Ser 837:278–285.
Sposito G. 2011. Electron shuttling by natural organic matter: Twenty years after. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:113–127.
Strathmann TJ. 2011. Redox reactivity of organically complexed iron(II) species with aquatic contaminants. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:283–313.
Stroo HF, Leeson A, Marqusee J, Johnson PC, Ward CH, Kavanaugh MC, Sale TC, Newell CJ, Pennell KD, Lebron CA, Unger M. 2012. Chlorinated ethene source remediation: Lessons learned. Environ Sci Technol 46:6438–6447.
Stroo HF, Ward CH. 2010. Future directions and research needs for chlorinated solvent plumes. In Stroo HF, Ward CH, eds, In Situ Remediation of Chlorinated Solvent Plumes. Springer, New York, NY, USA, pp 699–725.
Struyk Z, Sposito G. 2001. Redox properties of standard humic acids. Geoderma 102:329–346.
Szecsody JE, Fruchter JS, Sklarew DS, Evans JC. 2000. In Situ Redox Manipulation of Subsurface Sediments from Fort Lewis, Washington: Iron reduction and TCE Dechlorination Mechanisms. Report PNNL-13178. Pacific Northwest National Laboratory, Richland, WA, USA. 89 p.
Szecsody JE, Fruchter JS, Williams MD, Vermeul VR, Sklarew D. 2004. In situ chemical reduction of aquifer sediments: enhancement of reactive iron phases and TCE dechlorination. Environ Sci Technol 38:4656–4663.
Szulczewski MD, Helmke PA, Bleam WF. 2001. XANES spectroscopy studies of Cr(VI) reduction by thiols in organosulfur compounds and humic substances. Environ Sci Technol 35:1134–1141.
Támara M, Butler EC. 2004. Effects of iron purity and groundwater characteristics on rates and products in the degradation of carbon tetrachloride by iron metal. Environ Sci Technol 38:1866–1876.
Totten LA, Assaf-Anid NM. 2003. Abiotic dehalogenation by metals. In Häggblom MM, Bossert ID, Bossert ID, eds, Dehalogenation: Microbial Processes and Environmental Applications. Kluwer, Boston, MA, USA, pp 261–290.
Tratnyek PG. 2010. Chemical reductants for ISCR: The potential for improvement. Proceedings, 7th International Conference on Remediation of Chlorinated and Recalcitrant Compounds, Monterey, CA, USA, May 24–27. Battelle Press, Columbus, OH, USA, D-054.
Tratnyek PG, Johnson RL. 2006. Nanotechnologies for environmental cleanup. NanoToday 1:44–48.
Tratnyek PG, Macalady DL. 1989. Abiotic reduction of nitro aromatic pesticides in anaerobic laboratory systems. J Agric Food Chem 37:248–254.
Tratnyek PG, Macalady DL. 2000. Oxidation-reduction reactions in the aquatic environment. In Mackay D, Boethling RS, eds, Handbook of Property Estimation Methods for Chemicals: Environmental and Health Sciences. Lewis, Boca Raton, FL, USA, pp 383–415.
Tratnyek PG, Johnson RL, Johnson TL, Miehr R. 2001. In Situ Remediation of Explosives Contaminated Ground-Water with Sequential Reactive Treatment Zones. SERDP Final Report. Project CU-1176. Strategic Environmental Research and Development Program, Alexandria, VA, USA. 32 p.
Tratnyek PG, Scherer MM, Johnson TJ, Matheson LJ. 2003a. Permeable reactive barriers of iron and other zero-valent metals. In Tarr MA, ed, Chemical Degradation Methods for Wastes and Pollutants: Environmental and Industrial Applications. Marcel Dekker, New York, NY, USA, pp 371–421.
Tratnyek PG, Weber EJ, Schwarzenbach RP. 2003b. Quantitative structure-activity relationships for chemical reductions of organic contaminants. Environ Toxicol Chem 22:1733–1742.
Tratnyek PG, Salter AJ, Nurmi JT, Sarathy V. 2010. Environmental applications of zerovalent metals: Iron vs. zinc. In Erickson LE, Koodali RT, Richards RM, eds, Nanoscale Materials in Chemistry: Environmental Applications. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1045:165–178.
Tratnyek PG, Salter-Blanc AJ, Nurmi JT, Amonette JE, Liu J, Wang C, Dohnalkova A, Baer DR. 2011. Reactivity of zerovalent metals in aquatic media: Effects of organic surface coatings. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:381–406.
Truex MJ, Macbeth TW, Vermeul VR, Fritz BG, Mendoza DP, Mackley RD, Wietsma TW, Sandberg G, Powell T, Powers J, Pitre E, Michalsen M, Ballock-Dixon SJ, Zhong L, Oostrom M. 2011a. Demonstration of combined zero-valent iron and electrical resistance heating for in situ trichloroethene remediation. Environ Sci Technol 45:5346–5351.
Truex MJ, Vermeul VR, Mendoza DP, Fritz BG, Mackley RD, Oostrom M, Wietsma TW, Macbeth TW. 2011b. Injection of zero-valent iron into an unconfined aquifer using shear-thinning fluids. Ground Water Monit Remediat 31:50–58.
Tsukano Y. 1986. Transformations of selected pesticides in flooded rice-field soils: A review. J Contam Hydrol 1:47–63.
USEPA (U.S. Environmental Protection Agency). 1999. Field Applications of In Situ Remediation Technologies: Permeable Reactive Barriers. Report EPA 542-R-99-002. Washington, DC, USA. 114 p.
Uchimiya M, Stone AT. 2009. Reversible redox chemistry of quinones: Impact on biogeochemical cycles. Chemosphere 77: 451–458.
Urynowicz MA, Balu B, Udayasankar U. 2008. Kinetics of natural oxidant demand by permanganate in aquifer solids. J Contam Hydrol 96:187–194.
Van der Zee FP, Cervantes FJ. 2009. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol Adv 27:256–277.
Van Nooten T, Springael D, Bastiaens L. 2008. Positive impact of microorganisms on the performance of laboratory-scale permeable reactive iron barriers. Environ Sci Technol 42:1680–1686.
Vermeul VR, Williams MD, Evans JC, Szecsody JE, Bjornstad BN, Liikala TL. 2000. In Situ Redox Manipulation Proof-of-Principle Test at the Fort Lewis Logistics Center. Final Report PNNL-13357. Pacific Northwest National Laboratory, Richland, WA, USA. 330 p.
Vidic RD. 2001. Permeable reactive barriers: Case study review. GWRTAC E-Series Technology Evaluation Report TE-01-01. Ground Water Remediation Technologies Analysis Center, Pittsburgh, PA, USA. 49 p.
Wadley SLS, Gillham RW, Gui L. 2005. Remediation of DNAPL source zones with granular iron: Laboratory and field tests. Ground Water 43:9–18.
Wang S, Mulligan CN. 2004. An evaluation of surfactant foam technology in remediation of contaminated soil. Chemosphere 57:1079–1089.
Wang Y, Sakamoto Y, Kamiya Y. 2009. Remediation of actual groundwater polluted with nitrate by the catalytic reduction over copper-palladium supported on active carbon. Appl Catal A: General 361:123–129.
Warner SD, Sorel D. 2003. Ten years of permeable reactive barriers: Lessons learned and future expectations. In Henry SM, Warner SD, eds, Chlorinated Solvent and DNAPL Remediation: Innovative Strategies for Subsurface Cleanup. American Chemical Society, Washington, DC, USA, ACS Symp Ser 837:36–50.
Water Science and Technology Board. 2004. Contaminants in the Subsurface: Source Zone Assessment and Remediation. U.S. National Academies Press, Washington, DC, USA.
Wilkin RT, Puls RW. 2003. Capstone Report on the application, monitoring, and performance of permeable reactive barriers for ground-water remediation: Vol 1 – Performance evaluations at two sites. EPA 600-R-03–045a. U.S. Environmental Protection Agency, Cincinnati, OH, USA.
Wilkin RT, Puls RW, Sewell GW. 2003. Long-term performance of permeable reactive barriers using zero-valent iron: geochemical and microbiological effects. Ground Water 22:165–168.
Wilkin RT, Su CM, Ford RG, Paul CJ. 2005. Chromium-removal processes during groundwater remediation by a zerovalent iron permeable reactive barrier. Environ Sci Technol 39:4599–4605.
Wilson JT. 2003. Abiotic Reactions May be the Most Important Mechanism in Natural Attenuation of Chlorinated Solvents. AFCEE Technology Transfer Workshop, Brooks AFB, San Antonio, TX, USA, February 24–27.
Wilson JT, Kampbell DH, Ferrey M, Estuesta P. 2001. Evaluation of the protocol for the natural attenuation of chlorinated solvents: Case study at the Twin Cities Army Ammunition Plant. Office of Research and Development NRMRL, U.S. Environmental Protection Agency, Ada, OK, USA.
Wolfe NL, Macalady DL. 1992. New perspectives in aquatic redox chemistry: Abiotic transformations of pollutants in groundwater and sediments. J Contam Hydrol 9:17–34.
Xia K, Weesner F, Bleam WF, Bloom PR, Skyllberg UL, Helmke PA. 1998. XANES studies of oxidation states of sulfur in aquatic and soil humic substances. Soil Sci Soc Am J 62:1240–1246.
Xu X, Thomson NR. 2008. Estimation of the maximum consumption of permanganate by aquifer solids using a modified chemical oxygen demand test. J Environ Eng 134:353–361.
Xu X, Thomson NR. 2009. A long-term bench-scale investigation of permanganate consumption by aquifer materials. J Contam Hydrol 110:73–86.
Yabusaki S, Cantrell K, Sass B, Steefel C. 2001. Multicomponent reactive transport in an in situ zero-valent iron cell. Environ Sci Technol 35:1493–1503.
Zhang H, Weber EJ. 2009. Elucidating the role of electron shuttles in reductive transformations in anaerobic sediments. Environ Sci Technol 43:1042–1048.
Zhang H, Colón D, Kenneke JF, Weber EJ. 2011. The use of chemical probes for the characterization of the predominant abiotic reductants in anaerobic sediments. In Tratnyek PG, Grundl TJ, Haderlein SB, eds, Aquatic Redox Chemistry. American Chemical Society, Washington, DC, USA, ACS Symp Ser 1071:539–557.
ACKNOWLEDGEMENTS
The authors would like to recognize the helpful and substantive input from James E. Szecsody (Pacific Northwest National Laboratory) and permission to reproduce figures from Erica Becvar (AFCEE), Patrick Evans (CDM), and Thomas Hofstetter (EAWAG).
Portions of this work were funded by the U.S. Department of Defense, Strategic Environmental Research and Development Program (SERDP), Department of Energy, Subsurface Biogeochemical Research (SBR) Program, and the Environmental Protection Agency and National Science Foundation (Center for Environmental Implications of Nanotechnology (CEINT)). This document has not been reviewed by any of the sponsoring agencies and therefore it does not necessarily reflect their views and no official endorsement should be inferred.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Tratnyek, P.G., Johnson, R.L., Lowry, G.V., Brown, R.A. (2014). IN SITU Chemical Reduction For Source Remediation. In: Kueper, B., Stroo, H., Vogel, C., Ward, C. (eds) Chlorinated Solvent Source Zone Remediation. SERDP ESTCP Environmental Remediation Technology, vol 7. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6922-3_10
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6922-3_10
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6921-6
Online ISBN: 978-1-4614-6922-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)