Abstract
Ischemic mitral regurgitation, a late complication of coronary artery disease, develops secondary to changes in the mitral subvalvular apparatus and left ventricular geometry. Surgical treatment of ischemic mitral regurgitation can improve symptoms of congestive heart failure but has not clearly demonstrated a survival benefit. In this chapter, we discuss the pathophysiology of ischemic mitral regurgitation, experimental and clinical evidence underlying current treatment algorithms, and future areas of investigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Functional ischemic mitral regurgitation (FIMR) is a frequent end-stage complication of coronary artery disease which results from the process of negative left ventricular remodeling. Chronic myocardial ischemia and infarction effect an increase in left ventricular size secondary to myocyte loss and lengthening. As left ventricular size increases, the mitral annulus dilates and papillary muscle displacement tethers the mitral valve leaflets, causing FIMR. FIMR causes further impairment of ventricular function through volume overload, leading to the cycle of progressive left ventricular dilatation and worsening mitral regurgitation (MR) known as negative left ventricular remodeling [1–4]. With viable ischemic myocardium, revascularization of significant coronary artery disease may prevent further damage, relieve the contributing ischemia, and stop or reverse the remodeling process. However, the effect of correcting the ischemia alone on valve function has been unpredictable and often transient, leaving the majority of patients with residual, recurrent, or progressive MR [5]. Significant mitral regurgitation is treated by either valve repair or replacement. Mitral repair, while in the distant past was performed by suture annuloplasty, is now primarily performed by reduction annuloplasty (RA) with placement of an undersized annuloplasty device. This strategy increases the coaptive leaflet margin and reduces or eliminates the regurgitation [6]. Current surgical techniques for FIMR have significant procedural risk, and the late survival remains poor [7]. Due to the risk associated with current surgical therapies, the vast majority of patients with FIMR are treated medically [8]. In this chapter we will review current clinical and experimental data on the mechanisms and treatment of FIMR.
Clinical Scope and Consequences of Functional Ischemic MR
Heart failure affects over five million patients in the United States, with nearly 500,000 new cases diagnosed each year [9]. Coronary artery disease is a leading cause of systolic heart failure, affecting 40–60 % of heart failure patients [10]. In these patients, mitral regurgitation frequently coexists with systolic heart failure due to regional and global left ventricular remodeling. This mitral regurgitation, known as FIMR, is distinguished from other organic causes of mitral regurgitation, such as leaflet prolapse due to myxomatous change, leaflet flail secondary to chordal rupture, or leaflet perforation from endocarditis.
Functional ischemic MR can occur with preserved global left ventricular (LV) function (left ventricular ejection fraction [LVEF] > 30 %) or with severe LV dysfunction (LVEF < =30 %). In the former case, regional tethering and restriction of the posterior mitral valve leaflet result in FIMR, while in the latter case, increased LV dimensions and sphericity result in FIMR. Following myocardial infarction, there is a graded independent association between the severity of FIMR and the late development of heart failure. Aronson et al. prospectively studied 1,190 patients with acute myocardial infarction (MI) for the late development of congestive heart failure (CHF) [11]. In this cohort, FIMR, which was quantified echocardiographically during the hospitalization for acute MI, was mild in 39.7 % and moderate or severe in 6.3 %. All grades of FIMR (mild through severe) were associated with a significantly increased risk of CHF and death. Another large cohort study reported a similar relationship between moderate or severe FIMR and the late risk of CHF or death; importantly, this relationship was independent of left ventricular ejection fraction [12]. FIMR occurs in the majority of patients with ischemic cardiomyopathy, and their survival correlates with the degree of FIMR. In a recent observational study, Trichon et al. found an incidence of FIMR of 59 % in a cohort of 1,214 ischemic cardiomyopathy patients at a single center undergoing diagnostic work-up for heart failure (New York Heart Association [NYHA] class 2–4) [13]. The FIMR was mild (grade 1+ or 2+) in 38 % of the patients and moderate or severe (grade 3+ or 4+) in 17 %. Survival rates at 1, 3, and 5 years were significantly lower in patients with moderate to severe MR versus those with mild or no MR, and the degree of mitral regurgitation was an independent predictor of mortality by multivariable analysis.
Grigioni et al. also published their data on long-term outcomes in patients with chronic FIMR. In their series of 303 patients with previous transmural MI, 64 % developed chronic FIMR [14]. Patients with FIMR experienced worse survival rates than those without MR (38 ± 5 % vs. 61 ± 6 % at 5 years). Survival was also affected by FIMR grade (61 ± 6 % at 5 years for no MR; 47 ± 8 % at 5 years for effective regurgitant orifice (ERO) <20 mm2; 29 ± 9 % at 5 years for ERO ≥ 20 mm2).
Because FIMR has been associated with excess cardiac mortality, there has been considerable interest in the impact of its surgical correction on long-term outcomes. It is hypothesized that FIMR itself is an important contributor to the process of negative left ventricular remodeling, and elimination of FIMR, in combination with revascularization and/or medical therapy, may allow for normalization of left ventricular geometry. While FIMR can be reduced or eliminated with valve replacement or repair techniques, there has not been a direct correlation between elimination of FIMR and long-term reverse left ventricular remodeling. Moreover, valve repair techniques may have limited durability in certain clinical circumstances, leading to recurrent FIMR. As a consequence, long-term results of mitral surgery for FIMR have been inconsistent.
Experimental Studies of Functional Ischemic MR: Animal Models
The varied clinical presentation of patients makes it extremely difficult to analyze outcomes of FIMR treatment. Patients have varying amounts of ischemia, infarct, and subvalvular distortion which are both difficult to quantify and impossible to stratify for in the analysis of clinical trials. Fortunately, various animal models have been developed which, despite their limitations, offer insight into this complex disease and its treatment.
The Gorman research group at the University of Pennsylvania has performed a series of ovine myocardial infarct experiments which investigate the complex relationship of ischemic myocardial injury, LV remodeling, and functional mitral regurgitation. Their initial experimental series demonstrated that after a severe ischemic injury, ongoing negative LV remodeling will continue to occur even if the FIMR is “pretreated” or prevented by restrictive annuloplasty performed at the time of ischemic insult [15]. Lateral wall infarcts were induced in two groups of sheep; one group was pretreated with reduction annuloplasty (RA), while the other group was given a sham intervention (control). After 8 weeks, the RA group had successful prevention of FIMR, yet both groups had equal increases in left ventricular end-diastolic dimension (LVEDD; 68 ± 23 % for RA and 89 ± 16 % for control; p = NS) and equal decreases in ejection fraction (36.7 ± 3.7 % to 25.3 ± 2.9 % for RA and 42.4 ± 2.6 % to 32.4 ± 2.0 % for control; p = NS). In a more recent experimental report, restrictive mitral annuloplasty performed 8 weeks after large ischemic injury and myocardial infarction did not alter the ongoing process of LV dilation and negative remodeling, despite “successful” elimination of FIMR. In this model, after a severe infarction, negative remodeling progressed over a period of 6 months (twofold increase in end-diastolic volume and threefold increase in end-systolic volume) and was unaffected by eliminating the volume overload associated with the FIMR. Nevertheless, elimination of FIMR with reduction annuloplasty did significantly reduce pulmonary artery hypertension and was associated with greater forward cardiac output in the treated animals.
These experimental results are in marked opposition to ovine infarct experiments from Levine’s group at Massachusetts General Hospital. In his series [16], all sheep had antero-apical infarcts, and two-thirds were also given controlled MR via a left ventricular to left atrial shunt (creating a 30 % regurgitant fraction). At one month postinfarction, half of the sheep had their shunts closed. The negative remodeling in this “MR-treated” group reversed, such that at 3 months, LV end-diastolic dimensions were the same as infarct alone and significantly better than infarct plus untreated MR. The authors concluded that repair of moderate MR substantially reverses the otherwise progressive remodeling process, with reduced left ventricular volumes, relatively maintained contractility, persistently activated intracellular signals promoting hypertrophy and opposing apoptosis, and reduced matrix proteolytic activity.
These seemingly contradictory results lead us to question the driving mechanisms for ongoing cardiac remodeling in ventricles with large infarcts zones and functional MR. Autopsy series [17] suggest that the remodeling pathway is related to the degree of myocardial mass lost and the amount of scarring, both of which lead to an increase in LV dimension that triggers cardiac myocyte apoptosis in border zones and to a lesser extent in remote regions of the ventricle. Also, there is strong laboratory evidence that mechanical tension (stress/strain) is responsible for activating the apoptotic pathway [18]. Another driving force in cardiac remodeling is the increase in cardiac myocyte length and decrease in end-diastolic wall thickness via chronic volume overload, as well the activation of neurohumoral systems including activation of adrenergic pathways, activation of the renin-angiotensin-aldosterone system, and release of atrial natriuretic peptide. We are left with the unanswered question as to which of the above mechanisms predominates in the remodeling response in these animal models.
Clinical data does provide insight into the ventricular response to the elimination of FIMR in the setting of preexisting damage. Dion demonstrated that when LV end-diastolic dimension is less than 6.5 cm, restrictive annuloplasty can reliably eliminate MR and is associated with sustained reverse ventricular remodeling [19]. This size stratification method may make it possible to identify ventricles in which the activation of cardiac myocyte apoptosis is not overwhelming and in which elimination of FIMR would allow for reversal of the cardiac myocyte lengthening due to chronic volume overload. Similarly, Alfieri reported two different patient responses to reduction annuloplasty for FIMR [20]. In his series, those patients who responded with reverse remodeling had long-term elimination of FIMR, and those who had ongoing negative remodeling had recurrence of their mitral insufficiency. In these patients, the degree of ventricular end-diastolic enlargement had a borderline significance in predicting ongoing negative remodeling.
It is possible to argue that the Gorman laboratory model is congruous with the “nonresponders” and does not reflect the group of patients who are capable of the reverse remodeling response (as demonstrated in the Levine model). Further laboratory work will be necessary to elucidate the above listed factors which control the ultimate fate of the ventricle. With an understanding of this, we will not only be able to clearly identify “responders” to reduction annuloplasty but also be able to develop other strategies to deal with the evolving pattern of ventricular remodeling.
Surgical Techniques for the Treatment of Functional Ischemic MR
While either mitral repair or replacement is acceptable for treating functional mitral regurgitation (FMR), there are two well-known confounding factors which impact the utilization of these therapies. The first factor is that the degree of functional mitral regurgitation is often downgraded during intraoperative transesophageal echocardiography (TEE) evaluation; as a result FMR tends to go untreated. Aklog et al. demonstrated in a population of patients with 3+ FMR documented preoperatively on transthoracic echocardiogram that only 10 % of the patients have 3+ MR on intraoperative TEE [5]. Unfortunately, their postoperative transthoracic echocardiogram reveals that 89 % still have 2+ or worse FMR. The second confounding factor is the misperception that CABG alone will improve MR of ischemic etiology. In patients with preoperative baseline MR of 3+ who underwent CABG alone, 40 % of these patients had no improvement in MR postoperatively, with 86 % having 2+ or worse FMR [5].
Having acknowledged some uncertainty in patient selection, the primary surgical intervention for functional ischemic mitral insufficiency is reduction annuloplasty or mitral valve replacement. While there are multiple factors which influence the decision whether to replace or repair FIMR valves, first we shall review the comparative outcomes. Gillinov et al. presented the Cleveland Clinic series of 397 mitral valve repairs and 85 mitral valve replacements analyzed by propensity case matching [21]. They demonstrated improved survival with valve repair versus valve replacement in “better risk patients.” However, the 5-year survival was 56 % for repair and 36 % for replacement even in these patients. No survival benefit was demonstrated for repair over replacement in NYHA class 4 patients or in patients greater than 70 years of age. At the same time, we reported the experience at New York University. It was clear in our series that there was a significant difference in preoperative risk between those patients who received mitral repair and those that received mitral valve replacement for FIMR. The patients in our series who underwent replacement were more likely to be intubated, have preoperative shock, or have preoperative intra-aortic balloon pump placement. Our repair strategy was downsizing annuloplasty to treat the annular dilation and/or moderate to severe leaflet tethering. Our multivariable analysis demonstrated that hospital death was predicted by NYHA class 4 and a lack of angina. Analysis of death or complication via multiple logistic regression revealed that repair had half the risk compared to replacement. Indeed, when we analyzed the different preoperative risk subgroups, the hazard ratio for death or death and complication was always less than 1. This indicated that there was always a benefit to repair over replacement; the only subgroup in which this was not true was those patients who had previous surgery. Our 5-year complication-free survival was 63 % in our repair patients as compared to 30 % in the replacement patients. While late death was predicted by NYHA class 4 and the presence of prior cardiac surgery, complication-free survival was favored (odds ratio = 0.29) by mitral valve repair.
The above-mentioned datasets have therefore been used to support a preference for repair versus replacement. However, there are some patients in whom mitral repair may not have durability; this is discussed later in this chapter in the “Clinical Results” section.
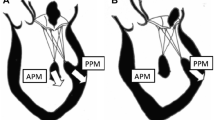
In addition to the standard procedure of reduction annuloplasty, multiple alternative techniques have been advocated to treat FMR. These include the cutting of secondary chordae [22], posterior papillary muscle relocation [23, 24], anterior leaflet augmentation [25], and posterior leaflet patching. Division of secondary chordae releases the downward tented leaflet of the anterior mitral valve and decreases mitral leaflet tenting area. However, it is also argued that this effectively removes support from the papillary muscles, increases the sphericity of the ventricle, and worsens left ventricular performance [26]. The leaflet augmentation strategies with patching have demonstrated success in small series with limited follow-up. Of interest are the papillary muscle relocation procedures. Kron’s group has limited data showing outcomes of placement of a traction suture placed into the posterior papillary muscle which shortens the distance to the right fibrous trigone [22]. Recently, the follow-up results of the “papillary muscle sling” technique have been published. A 4 mm graft is used to bring the papillary muscles together to reduce ventricle distortion. In a patient population at high risk for recurrence of MR (larger LV dimension), 4-year follow-up has been accrued. These results demonstrated good freedom from recurrent MR and improvements in ventricular diameter, volume, ejection fraction, and sphericity index [24].
Clinical Results of Surgical Treatment of Functional Ischemic MR
The strategy for surgical intervention in FIMR is based on four observations. First is the theoretical argument that FIMR imposes an important secondary remodeling stimulus on a ventricle that has already sustained a severe primary injury. Second, there is strong evidence that even mild MR is a poor prognostic sign in acute patients and those who have suffered an MI [14]. Third, there is a dramatic beneficial effect reported with valve repair for structural mitral valve disease. And fourth, there are limited alternative surgical options for FIMR patients with end-stage CHF. Clearly, from retrospective studies, there is little data at this time to support the concept that repairing these valves increases long-term survival [27, 28]. In rough summation, these clinical series show 50–60 % 5-year survival in patients undergoing treatment for their FIMR. This contrasts to Ellis et al.’s follow-up of patients undergoing percutaneous coronary intervention: those with either grade 3 or 4 MR at the time of intervention had only a 50 % survival at 36 months [29].
There is data, however, demonstrating that intervention on moderate or worse mitral insufficiency provides symptomatic benefits in those with patients with heart failure [27]. This has been excellently demonstrated by Dion in a subset of patients [19]. Specifically, in those patients with FIMR and LVEDD <6.5 cm, the 5-year survival for CABG and mitral repair was 80 %. These patients had an improvement in NYHA class from 2.9 to 1.6. Moreover, there was negligible recurrence of MR: mean follow-up MR grade was 0.8 (scale of 0–4), and 85 % of patients had less than grade 2. In contrast, patients with preoperative LVEDD greater than 6.5 cm had a 5-year survival of only 49 %, and there was little evidence of reverse remodeling. The authors concluded that for patients with an end-diastolic dimension of 6.5 cm or less, restrictive annuloplasty with revascularization provides a “cure” for ischemic MR and heart failure. While this may be an optimistic evaluation, their work does demonstrate the dramatic clinical benefit of surgical treatment of FIMR in appropriately selected patients.
Although valve repair is generally believed to be superior over replacement, there are several important technical considerations. First, most authors agree that either a rigid or semirigid remodeling device should be used and aggressive downsizing should be performed. Mitral insufficiency recurs at unacceptable rates when either flexible devices, tissue reinforcement, or suture-only techniques are used [7, 30, 31]. Secondly, multiple authors have noted that in patients with excessive distortion of the subvalvular apparatus, recurrent MR after reduction annuloplasty is not infrequent. Calafiore et al. noted that when the tenting distance was greater than 1 cm, the return of MR was “inevitable” [30]. Similarly, Duran noted that the degree of papillary displacement with respect to depth and angle correlated with return of MR [32].
Therefore, for treatment of FIMR, we do not recommend attempting a repair with reduction annuloplasty alone when the LVEDD is greater than 6.5 cm or the depth of leaflet coaptation is greater than 1 cm. Their results can be unpredictable and disappointing; patients with these dimensions are best served by chordal-sparing tissue valve replacement which reliably provides symptomatic relief.
Acker and colleagues published outcomes of the CorCap study in which patients underwent mitral valve surgery alone as a control arm of a Food and Drug Administration (FDA)-monitored investigational device study [33]. These medically optimized patients with significant functional MR, myopathic hearts, and symptomatic CHF underwent mitral valve surgery alone as control therapy. The patients were NYHA class 3 or 4, had EF < 35 % (mean EF 23 ± 9 %), and had dilated left ventricles (mean LVEDV 270.1 ± 100.3 mL). These patients had a remarkable 1.6 % 30-day mortality and significant improvements in quality of life, exercise performance, and NYHA functional class over the 2-year follow-up. Equally as important, mitral valve operations led to improvements in LV volumes (mean decrease of 45 mL), mass, and shape, all consistent with reverse remodeling. Finally, unlike other reported experiences in the literature, the operations were durable, as recurrence of clinically significant MR was uncommon in this patient cohort. The authors’ concluded that “the improvement in LV structure and clinical function along with a very low mortality rate justifies strong consideration to offering mitral valve (MV) surgery to heart failure patients who are on an optimal medical regimen.” The outcomes do support the hypothesis that these patients with cardiomyopathy benefit from the surgical correction of the functional mitral insufficiency. The results of this study add to a growing experience of clinical improvement with mitral valve repair. There is a significant caveat to this dataset however; 90 % of these FMR patients had a nonischemic etiology. How generalizable this is to the ischemic functional MR population is yet to be determined.
The New York University Experience
Our institutional experience with MV repair in the setting of impaired left ventricular function, including long-term echocardiographic and clinical outcomes, was recently presented at the annual meeting of the American College of Cardiology. Over 14 years, 193 patients with severe mitral regurgitation and EF < 50 % underwent mitral repair alone (reduction annuloplasty) without CABG. Sternotomy was utilized in 56 patients, and a mini-thoracotomy approach was used in 137 patients. Mean age was 63.7 years (range 24–90). Preoperative NYHA class was 2.8 (54.4 % were 3 or 4), and 41 (21.2 %) patients had previous cardiac surgery. Preoperative EF distribution was 40–49 % in 52 patients (26.9 %), 30–39 % in 81(42.0 %), 20–29 % in 37(19.2 %), and <20 % in 23(11.9 %).
Hospital mortality was 5.7 % overall and 3.6 % for mini-thoracotomy. Propensity-adjusted multivariate predictors (odds ratio; p-value) of hospital mortality were ischemic etiology (22.7; p = 0.03), age (p = 0.04), and chronic obstructive pulmonary disease or COPD (6.5; 0.03). The sternotomy approach (4.8; p = 0.10) and peripheral vascular disease (5.8; p = 0.10) were weakly associated with hospital mortality. Freedom from all cause death was 74 % at 5 years (84 % for nonischemic patients and 51 % for ischemic patients; p < 0.001). Predictors of decreased survival were age (p < 0.001), severely impaired ejection fraction (p = 0.01), ischemic etiology (p < 0.04), and cerebrovascular disease (p = 0.06). NYHA class improved 0.9 grades (p = 0.01). At 5 years, freedom from valve reoperation was 92 %; freedom from valve reoperation or severe recurrent mitral insufficiency was 88 %. We concluded that reduction annuloplasty in FMR patients with decreased EF improves late NYHA functional status and is associated with good late survival. Significantly, the predictors of poor survival were age, lower EF, ischemic etiology, and cerebrovascular disease.
We recently published standard outcomes of CABG and reduction annuloplasty for FIMR in a controlled, prospective multicenter series [34]. Seventy patients with coronary artery disease requiring revascularization, severe or symptomatic moderate FIMR, ejection fraction ≥25 %, LVEDD ≤7.0 cm, and >30 days since acute myocardial infarction were treated with CABG and device reduction annuloplasty. Two patients underwent immediate intraoperative conversion to a valve replacement due to inability of reduction annuloplasty to correct MR. The as-treated results included a 30-day mortality of 4.1 %, with the patients receiving an average of 2.8 bypass grafts. Mean follow-up was 24.6 months. MR severity was significantly reduced from 2.54 ± 0.80 at baseline to 0.52 ± 0.66 and 0.35 ± 0.63 at 1 and 2 years, respectively (MR scale was 0 = none, 1 = mild, 2 = moderate, 3 = moderate-severe, 4 = severe). Freedom from death or valve reoperation was 78 ± 5 % at 2 years. Ejection fraction significantly improved from 38 % to 47 % at 2 years. Reverse remodeling was evident with significant decreases in end-diastolic and end-systolic dimensions (Table 4.1). NYHA class was improved one or greater grades in 65.9 % at 1 year and 72.0 % at 2 years. Cox regression analyses suggested that increasing age (p = 0.001; hazard ratio (HR) 1.16/year, 95 % CI 1.06–1.26) and renal disease (p = 0.018; HR = 3.48; 95 % CI 1.25–9.72) were associated with decreased survival.
From these data, we can conclude that CABG with reduction annuloplasty for FIMR predictably reduces MR and relieves symptoms in patients without excessive preexisting ventricular distortion. This operative strategy for the treatment of moderate to severe MR is associated with improved indices of ventricular geometry, improved NYHA functional class, and excellent freedom from recurrent mitral insufficiency. While long-term prognosis and outcomes remain uncertain, this dataset delineates the midterm benefits of such an approach.
Future Approaches in the Treatment of Functional Ischemic MR
Novel Clinical Research
One characteristic of functional MR is the presence of “normal” leaflet structure in the setting of ventricular remodeling which distorts the subvalvular apparatus and impairs valve function. A novel approach to treating this FMR is offered by the Coapsys device (Myocor, Inc., Maple Grove, MI), a ventricular shape change device that can be placed without the need for cardiopulmonary bypass to reduce FMR. This device consists of two pads which are connected by a transventricular “chordal” suture. After echocardiographically assisted placement across the left ventricle, the device is tightened to compress the mitral annulus, thereby reducing FMR and positively reshaping the ventricle [35]. An FDA-monitored investigational device trial was conducted in patients requiring CABG who had severe MR or symptomatic moderate MR, ejection fractions >=25 %, and LVEDD < =7.0 cm. The hypotheses tested were that investigative “off-pump” treatment would have non-inferior efficacy (as measured by MR degree) and superior safety efficacy as compared to standard mitral repair [36]. The trial was terminated prematurely when the recent financial collapse resulted in the bankruptcy of the trial sponsor (Myocor Inc.).
Recruitment had accrued 165 patients, the prespecified value for the “first-look” data analysis. The Coapsys device was associated with greater long-term positive ventricular reshaping, with the LVEDD decreasing from 6.0 ± 0.8 to 5.4 ± 0.8 cm as compared to 5.9 ± 0.7 to 5.6 ± 0.9 cm for the control MV repair (effect of time p < 0.001, repeated measures analysis of variance [ANOVA]; effect of treatment p = 0.02). However, the MR treatment efficacy was not as effective with the Coapsys treatment: the standard mitral repair technique reduced MR (0 = none, 1 = mild, 2 = moderate, 3 = moderate-severe, 4 = severe scale) from 2.54 ± 0.80 to 0.35 ± 0.63 at 24 months, while Coapsys reduced MR from 2.40 ± 0.87 to 1.24 ± 0.97 (both effect of time and treatment p = 0.0001, repeated measures ANOVA). What was totally unanticipated was that the trial discerned a significant survival benefit to the Coapsys treatment; at 24 months, there was nearly half the incidence of death with the Coapsys device as compared to standard mitral repair (Fig. 4.1). Twenty-four-month survival from all cause death was 89 % in the Coapsys randomized group as compared to 78 % in the standard treatment group (adjusted log-rank 4.30; p = 0.038; intent-to-treat analysis); a more powerful benefit to the Coapsys treatment was noted in the as-treated analysis (p = 0.020).
Randomized Coapsys trial demonstrating late superior survival from all cause death for the Coapsys + CABG patients as compared to the control mitral repair + CABG patients (Reprinted from the Journal of the American College of Cardiology, Vol. 56, Grossi EA, Patel N, Woo YJ, et al., Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve), pp. 1984–1993, copyright 2010, with permission from Elsevier)
These findings are very provocative: patients with FIMR requiring revascularization treated with ventricular reshaping rather than standard mitral repair surgery had improved survival and significant reduction of major adverse outcomes. This unique dataset should guide further research in this area towards “ventricular solutions.”
Current Clinical Trials
Currently two topical clinical trials regarding the outcomes of ischemic mitral regurgitation are being conducted by the National Heart, Lung, and Blood Institute (NHLBI)-sponsored Cardiothoracic Surgical Trials Network. The first trial is entitled “Evaluation of Outcomes Following Mitral Valve Repair or Replacement in Severe Chronic Ischemic Mitral Regurgitation.” In this study, patients with severe FIMR will be randomized to either mitral repair or replacement; concomitant CABG will be performed if indicated. Pre- and postoperative evaluation will include cardiopulmonary exercise evaluation. The patients will be followed for 24 months. Interestingly, no restrictions are being applied as to the mitral valve repair technique employed by an individual surgeon.
The second trial is entitled “Surgical Interventions for Moderate Ischemic Mitral Regurgitation.” The purpose of this trial is to determine whether repairing moderate mitral insufficiency at the time of planned CABG will have beneficial effects. Again, cardiopulmonary exercise testing, neurocognitive tests, and quality of life surveys will be conducted over a 2-year period. Unfortunately, patient recruitment has been an issue for both trials. It has been speculated that there is a lack of clinical equipoise when treating “stentable” coronary artery disease in the presence of moderate MR which has limited patient referral. The NHLBI has announced a request for additional investigative sites to correct this issue.
Summary
FIMR is a common end-stage complication of coronary artery disease that develops from myocardial injury and subsequent negative LV remodeling. While various animal models have been developed to offer insight into this complex pathologic process, data inferred from them is conflicting. More sensitive and specific models are warranted to gain insight into patient-specific disease status and treatment outcomes.
FIMR can be eliminated with valve replacement or repair techniques, and this provides documented relief of heart failure symptoms. Notably, in patients with smaller ventricles, a majority will have positive LV remodeling. Mitral repair appears to have benefit over replacement for the majority of patients. However, in those patients who are NYHA class 4 or greater than 70 years of age, there is no advantage to repair over replacement. Extensive valvular distortion is probably best treated with mitral replacement. Novel techniques are being developed not only to treat the valve but also to treat the underlying ventricular disease. The combined approaches of annular repair and ventricular reshaping may offer the best therapy for this very sick patient cohort in the future.
References
Hendren WG, Nemec JJ, Lytle BW, Loop FD, Taylor PC, Stewart RW, et al. Mitral valve repair for ischemic mitral insufficiency. Ann Thorac Surg. 1991;52(6):1246–51.
Sabbah HN, Rosman H, Kono T, Alam M, Khaja F, Goldstein S. On the mechanism of functional mitral regurgitation. Am J Cardiol. 1993;72(14):1074–6.
Komeda M, Glasson JR, Bolger AF, Daughters GT, 2nd, MacIsaac A, Oesterle SN, et al. Geometric determinants of ischemic mitral regurgitation. Circulation. 1997;96(9 Suppl):II-128–33.
Trichon BH, Glower DD, Shaw LK, Cabell CH, Anstrom KJ, Felker GM, et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation. 2003;108 Suppl 1:II103–10.
Aklog L, Filsoufi F, Flores KQ, Chen RH, Cohn LH, Nathan NS, et al. Does coronary artery bypass grafting alone correct moderate ischemic mitral regurgitation? Circulation. 2001;104(12 Suppl 1):I68–75.
Bax JJ, Braun J, Somer ST, Klautz R, Holman ER, Versteegh MI, et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodeling. Circulation. 2004;110(11 Suppl 1):II103–8.
Grossi EA, Bizekis CS, LaPietra A, Derivaux CC, Galloway AC, Ribakove GH, et al. Late results of isolated mitral annuloplasty for “functional” ischemic mitral insufficiency. J Card Surg. 2001;16(4):328–32.
Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Detaint D, Vanoverschelde JL, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28(11):1358–65.
Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–18.
He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161(7):996–1002.
Aronson D, Goldsher N, Zukermann R, Kapeliovich M, Lessick J, Mutlak D, et al. Ischemic mitral regurgitation and risk of heart failure after myocardial infarction. Arch Intern Med. 2006;166(21):2362–8.
Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, et al. Heart failure and death aftery myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111(3):295–301.
Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91(5):538–43.
Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103(13):1759–64.
Guy TSt, Moainie SL, Gorman JH, 3rd, Jackson BM, Plappert T, Enomoto Y, et al. Prevention of ischemic mitral regurgitation does not influence the outcome of remodeling after posterolateral myocardial infarction. J Am Coll Cardiol. 2004;43(3):377–83.
Beeri R, Yosefy C, Guerrero JL, Abedat S, Handschumacher MD, Stroud RE, et al. Early repair of moderate ischemic mitral regurgitation reverses left ventricular remodeling: a functional and molecular study. Circulation. 2007;116(11 Suppl):I288–93.
Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol. 2003;41(5):753–60.
Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res. 2001;89(5):453–60.
Braun J, van de Veire NR, Klautz RJ, Versteegh MI, Holman ER, Westenberg JJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg. 2008;85(2):430–6.
De Bonis M, Lapenna E, Verzini A, La Canna G, Grimaldi A, Torracca L, et al. Recurrence of mitral regurgitation parallels the absence of left ventricular reverse remodeling after mitral repair in advanced dilated cardiomyopathy. Ann Thorac Surg. 2008;85(3): 932–9.
Gillinov AM, Wierup PN, Blackstone EH, Bishay ES, Cosgrove DM, White J, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2001;122(6):1125–41.
Komeda M, Shimamoto T. Cutting secondary chordae and placing dual taut stitches between the anterior mitral fibrous annulus and the heads of each papillary muscle to treat ischemic mitral regurgitation without deteriorating left ventricular function. J Thorac Cardiovasc Surg. 2008;135(1):226–7.
Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74(2):600–1.
Hvass U, Joudinaud T. The papillary muscle sling for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2010;139(2):418–23.
Kincaid EH, Riley RD, Hines MH, Hammon JW, Kon ND. Anterior leaflet augmentation for ischemic mitral regurgitation. Ann Thorac Surg. 2004;78(2):564–8.
Rodriguez F, Langer F, Harrington KB, Tibayan FA, Zasio MK, Liang D, et al. Cutting second-order chords does not prevent acute ischemic mitral regurgitation. Circulation. 2004;110(11 Suppl 1):II91–7.
Harris KM, Sundt TM, 3rd, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thorac Surg. 2002;74(5):1468–75.
Diodato MD, Moon MR, Pasque MK, Barner HB, Moazami N, Lawton JS, et al. Repair of ischemic mitral regurgitation does not increase mortality or improve long-term survival in patients undergoing coronary artery revascularization: a propensity analysis. Ann Thorac Surg. 2004;78(3):794–9.
Ellis SG, Whitlow PL, Raymond RE, Schneider JP. Impact of mitral regurgitation on long-term survival after percutaneous coronary intervention. Am J Cardiol. 2002;89(3):315–8.
Calafiore AM, Gallina S, Di Mauro M, Gaeta F, Iaco AL, D’Alessandro S, et al. Mitral valve procedure in dilated cardiomyopathy: repair or replacement? Ann Thorac Surg. 2001;71(4):1146–52.
McGee EC, Gillinov AM, Blackstone EH, Rajeswaran J, Cohen G, Najam F, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2004;128(6):916–24.
Matsunaga A, Tahta SA, Duran CM. Failure of reduction annuloplasty for functional ischemic mitral regurgitation. J Heart Valve Dis. 2004;13(3):390–7. discussion 397–8.
Acker MA, Bolling S, Shemin R, Kirklin J, Oh JK, Mann DL, et al. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006;132(3):568–77.
Grossi EA, Woo YJ, Patel N, Goldberg JD, Schwartz CF, Subramanian VA, et al. Outcomes of coronary artery bypass grafting and reduction annuloplasty for functional ischemic mitral regurgitation: a prospective multicenter study (Randomized evaluation of a surgical treatment for off-pump repair of the mitral valve). J Thorac Cardiovasc Surg. 2011;141(1):91–7.
Grossi EA, Woo YJ, Schwartz CF, Gangahar DM, Subramanian VA, Patel N, et al. Comparison of Coapsys annuloplasty and internal reduction mitral annuloplasty in the randomized treatment of functional ischemic mitral regurgitation: impact on the left ventricle. J Thorac Cardiovasc Surg. 2006;131(5):1095–8.
Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V, et al. Outcomes of the RESTOR-MV Trial (Randomized evaluation of a surgical treatment for off-pump repair of the mitral valve). J Am Coll Cardiol. 2010;56(24):1984–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Balsam, L.B., Grossi, E.A., Galloway, A. (2013). Mitral Valve Repair for Ischemic Mitral Regurgitation with Advanced Cardiomyopathy. In: Morgan, J., Naka, Y. (eds) Surgical Treatment for Advanced Heart Failure. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6919-3_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6919-3_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6918-6
Online ISBN: 978-1-4614-6919-3
eBook Packages: MedicineMedicine (R0)