Abstract
Pharmacological advances in the treatment of HIV infection have transformed what was once a terminal illness into a chronic illness. Although an increasing number of patients are being placed on antiretroviral therapy, poor adherence to treatment remains one of the leading causes of treatment failure. As such, research assessing factors that predict medication adherence to antiretroviral therapy is highly important from a public health perspective. This chapter will focus on the relationship between cognition and medication adherence among HIV-infected adults, with special emphasis on older HIV+ adults, one of the fasting growing subgroups in the HIV-infected populace. The chapter is intended to provide a broad overview of neuropsychological factors associated with medication adherence to antiretroviral therapy. We will begin with a brief review of some of the many methodological complexities important to consider in the study of medication adherence and cognition. The balance of the chapter will focus on key neurocognitive, psychiatric (including substance use), and psychosocial factors that are related to medication adherence in the HIV population.
Drs. Panos and Patel are supported
By the National Institute of Mental Health Training Grant T32MH19535 (PI: C. H. Hinkin). Dr. Thames is supported National Institute of Mental Health Career Development Award K23MH095661 (PI: A. Thames)
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Medication Adherence
- Prospective Memory
- Adherence Rate
- Adherence Level
- Medication Event Monitoring System
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Pharmacological advances in the treatment of HIV infection—referred to by the lay media as having a “Lazarus effect” due to its success—have transformed what was once a terminal illness into a chronic illness. As a result, individuals with HIV are living longer. But there is a catch. As succinctly captured by the ex-Surgeon General C. Everett Koop, even the most effective advance in pharmacotherapy is of little use if patients fail to adequately adhere to their medication regimen (“Drugs don’t work if people don’t take them”). Nearly 50 % of all adults in the United States are living with at least one chronic illness (Centers for Disease Control 2009) and 50 % of those individuals are poorly adherent to their prescribed medications (Sabaté 2003). The costs of non-adherence are staggering from both personal and public health perspectives. For example, an estimated 86,000 premature deaths can be prevented annually if patients were adherent to their prescribed antihypertensive medications (Cutler et al. 2007). Anywhere between one-third and two-thirds of medication-related emergency visit hospitalizations are related to failures in medication adherence, a cost that is estimated to approximate $100 billion annually (McDonnell and Jacobs 2002; Osterberg and Blaschke 2005).
There exists an extensive body of research across diseases, as well as specific to HIV, that has examined predictors of medication adherence as well as interventions designed to improve adherence. With regard to HIV, medication adherence is associated with a wide range of factors, including: (a) those related to the patient (e.g., demographic factors, psychiatric illness, beliefs about medications and illness); (b) the medications prescribed (e.g., adverse side effects and complex dosing regimens); (c) the illness under treatment (e.g., HIV-associated cognitive dysfunction); and (d) the psychosocial environment (e.g., poor access to care, social support, and the patient-provider relationship) (Ickovics and Meisler 1997). This chapter will primarily focus on the relationship between cognition and medication adherence among HIV-infected adults, with a particular emphasis on older HIV+ adults, one of the fast growing subgroups in the HIV populace.
There are several shared factors that converge to make the study of neurocognition and medication adherence among individuals living with HIV, especially older individuals living with HIV, of considerable interest from a public health perspective. First, older adults are at particular risk for poor medication adherence secondary to cognitive compromise. The normal aging process and HIV infection are associated with decline in an overlapping array of neurocognitive abilities, including executive functions, working memory, attention, learning/memory, and speed of cognitive processing (Heaton et al. 1995; Hinkin et al. 1990, 2002b; Wendelken and Valcour 2012; Wilkie et al. 2000). By the year 2015, an estimated 50 % of the HIV-infected population will be over the age of 50 (Centers for Disease Control 2007) and these ‘older’ HIV+ individuals are three times more likely to develop HIV-associated dementia than younger HIV+ individuals (Valcour et al. 2004).
Second, both older adults and individuals living with HIV are more likely to require self-management of multiple medications for various chronic illnesses. Older individuals in the general population use up to 40 % of all prescribed medications (Vik et al. 2006), though they constitute only approximately 15 % of the population. Antiretroviral therapy for the treatment of HIV typically consists of a combination of at least three different classes of antiretroviral agents. This has traditionally required at minimum three different medications (often more) to be taken multiple times a day, though single pill combinations (typically a combination of two or more medications in one pill) are becoming more prevalent. In addition, it has been demonstrated that 89 % of older HIV+ individuals (age 55 years and older) have at least one comorbid condition (Shah et al. 2002). These individuals are more likely to take medications for a variety of comorbid chronic diseases, such as heart disease, diabetes, hypertension, and hyperlipidemia, to name a few.
Third, relative to other medication regimens, antiretroviral therapy requires meticulous adherence (90–95 % adherence levels) as suboptimal adherence can result in the emergence of treatment resistant viral mutations (Paterson et al. 2000). This dwarfs the level of adherence required for most chronic diseases (e.g., hypertension), and underscores why this issue is of such public health concern. Unfortunately, only up to 50–60 % of HIV+ adults taking antiretroviral therapy maintain these optimal adherence levels (Gifford et al. 2000; Hinkin et al. 2004; Nieuwkerk et al. 2001), leading to what can be a rather precipitous decline in immunocompetence and the advent of untoward clinical consequences.
Finally, due to recent developments, the number of HIV+ individuals prescribed antiretroviral therapy will likely increase. Historically, many HIV+ adults have refused to initiate pharmacotherapy, holding off until absolutely necessary. However, it has just recently been recognized that early initiation of antiretroviral therapy reduces risk of transmission to uninfected sexual partners by 96 % (HIV Prevention Trials Network 2011). Moreover, even HIV- individuals who are at heightened risk for contracting HIV (e.g., those in a HIV sero-discordant relationship) are being placed on antiretroviral therapy given recent approval of Truvada for prophylactic use (US Food and Drug Administration 2012). Given the potential that suboptimal adherence can lead to the development of treatment resistant viral strains, rigorous adherence to antiretroviral therapy is therefore an increasing public health concern.
This chapter is intended to provide a broad overview of factors associated with medication adherence to antiretroviral therapy, with particular emphasis on the most current knowledge regarding the relationship between neuropsychological functioning and medication adherence. We will begin with a brief review of some of the many methodological complexities important to consider in the study of medication adherence and cognition. The balance of the chapter will focus on key neurocognitive, psychiatric (including substance use), and psychosocial factors that have been shown to be related to medication adherence in the HIV-infected population.
Methodology
Medication Adherence Measurement Techniques
Medication adherence involves taking the correct dosage of a medication (e.g., the correct number of pills), at the correct time, while adhering to specific instructions (e.g., with or without food; Gould et al. 1999). A number of methods have been developed to measure medication adherence, each with inherent advantages and disadvantages in terms of measurement accuracy and feasibility. These methodologies fall into two broad categories, subjective (e.g., self-report) and objective (e.g., pill counts, electronic monitoring devices) techniques (see Table 6.1; Castellon et al. 2009). Although a comprehensive review of each of these techniques is beyond the scope of this chapter, we will briefly review several commonly used approaches.
Self-report is the most widely used method to track adherence in both research and clinical settings because of its low cost and ease of administration. Patients are asked to recall how adherent they were (often using a Likert Scale) to their prescribed medication regimen over the course of a specified time period. While the advantages to this approach are clear, there are considerable limitations; most notable is the consistent finding that self-report tends to overestimate actual adherence rates (Arnsten et al. 2001; Liu et al. 2001). When using self-report methodologies, shorter recall intervals (e.g., 4 days) have been found to be more closely related to objective measures (Levine et al. 2006). For obvious reasons, however, relying on self-report of cognitively impaired patients is problematic (Thames et al. 2011a).
As an alternative to subjective measures, the Medication Event Monitoring System (MEMS) (Aprex Corp, Union City, California) was created. In its most basic form, this is a pill bottle with a microchip-embedded cap that records the precise date, time, and duration of each bottle opening. When this is used, medication adherence is typically calculated as the total number of MEMS bottle openings divided by the total number of bottle openings that should have occurred over a specified time period. There are some logistical complexities to this approach, including its cumbersome nature. This may cause patients to remove extra doses from their MEMS for later ingestion. This approach also prevents individuals from placing their medications into organized pillboxes that aid in medication taking behavior. Perhaps as a result of this, researchers have found that MEMS may underestimate medication adherence (Liu et al. 2001). It should also be noted that studies using MEMS often track one medication as opposed to multiple medications (for logistical reasons). Therefore, these more general associations may in fact also underestimate the effect of cognition on adherence rates to multiple medications. There is no gold standard in regards to measuring medication adherence. However, there is evidence to suggest that objective measures (including MEMS) are better approaches compared to self-report, though a multi-measure methodological approach is ideal (Arnsten et al. 2001; Liu et al. 2001). More recently, the MedTracker (Hayes et al. 2006) and the Med-eMonitor devices (www.informedix.com; Harberer et al. 2012) were developed to overcome some of the MEMS limitations.
Pill count is an objective and straightforward technique that has been used to measure adherence rates. Clinicians calculate the number of pills that should remain based on the number of pills initially possessed and the number of pills that should have been ingested. However, this is easy for patients to calculate and they may remove extra doses from their pill bottle prior to appointments in order to appear more adherent. To overcome this limitation, “unannounced pill counts” (Bangsberg et al. 2001) can be condutced. Unannoucned pill counts have been shown to correlate with HIV RNA and medication adherence electronic monitoring devices (Haberer et al. 2012). Unannounced telephonic pill counts is another twist to this technique.
Precise quantification of medication adherence can be ascertained through blood tests. However, one drawback of this technique is that it is not useful for examining adherence rates for medications that metabolize rapidly. Second, blood levels will only detect recent medication behaviors, but do not provide insight into the patient’s typical medication taking behaviors.
Pharmacy refill records can also be used to assess medication adherence. It is assumed that if the patients are refilling their medications on time, it is likely that they are taking them as prescribed. Although this technique is cost-effective, it requires centralized pharmacy records and assumes that patients are taking their medications as prescribed.
Laboratory-based performance measures (analog measures) of medication management have been increasingly used by researchers, who are interested in finer-grained examination of adherence behavior. Medication management refers to select aspects of medication taking abilities, such as the ability to read and understand labels on pill bottles, and the ability to know when to refill their medications, how many pills are required for each dosage, and how to correctly place pills in a pillbox based on the instructions on the labels. Analog measures have been found to correlate with self-reported medication adherence (Albert et al. 1999). To our knowledge, their relationship to other measures of medication adherence has yet to be assessed.
Measurement of Cognition in the Study of Medication Adherence
A number of different techniques have also been used to measure cognition in medication adherence studies. There remains significant methodological variability within the literature assessing cognition and the impact of cognition on adherence to antiretroviral therapy (Lovejoy and Suhr 2009; Robertson et al. 2009). HIV predominantly affects fronto-subcortical regions of the brain, resulting in deficits in executive functions, learning and memory, processing speed, attention, and motor functioning. Researchers must decide whether they will measure a single cognitive domain (e.g., memory) or multiple domains. As we will discuss below, medication taking-behavior is a complex behavior enlisting multiple domains and measuring a single construct (e.g., memory) may not fully capture the manner by which cognition affects medication adherence. Neuropsychological test selection, the number of tests used to assess cognitive domains of choice, and methods used for post-processing neuropsychological test data (e.g., whether to employ demographically adjusted normative data; how to best collapse data for analysis) must also be considered. Since the advent of antiretroviral therapy, milder forms of HAND have become more prevalent and test selection aimed at accurately capturing milder forms of cognitive impairment is crucial. Furthermore, the demographic composition of the HIV-infected populace has changed considerably over time, with the result that researchers must now carefully consider how demographic factors may affect neuropsychological test performance over and above the effects of HIV infection itself. In a recent review of studies examining the impact of HIV-associated neurocognitive dysfunction (HAND) on adherence, Lovejoy and Suhr (2009) found that studies that used multiple measures of cognition were more likely to detect a relationship between cognition and medication adherence than were those that relied upon a single screening tool. They concluded that comprehensive neuropsychological batteries coupled with the use of demographically corrected normative data should be viewed as the ideal approach that should be employed whenever possible unless logistical constraints demand otherwise.
Neuropsychological Functioning and Medication Adherence
Cognitive Dysfunction and Medication Adherence
Although it was long recognized that impaired cognition can be an important barrier to treatment (Geller 1982; Parkin et al. 1976), formal study using objective measures of cognition and their relationship to medication adherence did not emerge in the general medical literature until about two decades ago (Farmer et al. 1990; Meyer and Schuna 1989) and in the HIV literature until about 10 years ago (Avants et al. 2001; Hinkin et al. 2002a). Since then, a large body of research has accrued and converged on the clear conclusion that impaired cognition has an adverse impact on medication adherence. Difficulties with medication adherence have been linked to a wide range of neurocognitive dysfunction, including deficits in executive function, complex attention/working memory, learning and memory, psychomotor processing speed, and motor functioning (for review, see Lovejoy and Suhr 2009). The fact that deficits in a wide array of neurocognitive abilities can lead to compromised adherence is not surprising given that medication adherence is indeed a rather complex behavior that would arguably enlist multiple cognitive functions (e.g., comprehension of instructions and of risks of non-adherence, the ability to develop a systematic plan for pill taking that requires a significant degree of forethought and initiation, the ability to remember to take the medications at the right times in the face of concurrent demands in one’s day-to-day life) as well as relatively more simple behaviors (e.g., the ability to open a pill bottle). Hinkin et al. (2002a) found that HIV+ adults who presented with global cognitive impairment (as assessed by a battery of neuropsychological tests) were more than twice as likely to be non-adherent than those with intact cognition. Among individual cognitive domains, higher order neurocognitive processes (i.e., executive functions, learning/memory, and complex attention) appear to be more robustly related to medication adherence (Hinkin et al. 2002a; Lovejoy and Suhr 2009).
Longitudinal Studies of Cognition and Medication Adherence
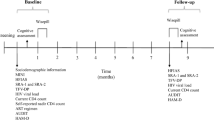
Although the majority of studies assessing the relationship between cognition and medication adherence to antiretroviral therapy have been cross-sectional/correlative in nature, second-generation studies of medication adherence and cognition have begun to focus on “cause and effect” questions. To the extent that medications beneficially impact cognition (as is the case with many medications, including antiretroviral therapy), the relationship between cognition and medication adherence should be conceptualized as bidirectional in nature. As such, we would expect not only that cognitive dysfunction should lead to poor adherence, but also that poor medication adherence should lead to worsening neuropsychological dysfunction. Disentangling the effects of each (cognition and medication adherence) requires sophisticated longitudinal studies that assess both medication adherence and neurocognition at multiple time points. Two longitudinal studies from our laboratory have directly tackled this chicken and egg conundrum. Employing path-analysis in a sample of 91 HIV+ adults, Ettenhofer et al. (2010) measured medication adherence prospectively over the course of 6 months using MEMS caps and assessed cognition at baseline and 6 months later. As seen in Fig. 6.1, baseline cognition was predictive of medication adherence over the course of the 6 month study. Analyses by discrete cognitive sub-domains revealed that this was driven primarily by executive functions and learning/memory. After controlling for the inherent correlation between cognition assessed at both time points (before and end of study), they found that higher levels of medication adherence across the 6 months were predictive of better cognition at the end of the study. Analysis by discrete cognitive domains revealed this was primarily driven by executive functions, complex attention, information processing, and motor functioning.
Model of longitudinal path analysis of global cognition and medication adherence. Reproduced with permission from Wolters Kluwer Health. From Ettenhofer et al. (2010, p. 1219)
Using a different longitudinal methodology, Becker et al. (2011) compared the medication adherence trajectories of HIV+ patients who exhibited cognitive decline and those who did not over the course of 6 months. Those who exhibited cognitive decline over the course of the 6 months also evidenced steeper adherence declines over time. In the final month of the study, those with cognitive decline evidenced 52 % adherence levels compared to the 66 % adherence levels of the cognitively stable group. Follow-up analyses revealed that learning and memory in particular was associated with changes in adherence.
Taken together, these studies suggest that a variety of cognitive functions are related to medication adherence. The relationship between cognition and medication adherence is bidirectional, with higher order cognitive functions (executive functions and learning/memory) appearing to be more robust predictors of medication adherence. Declines in cognition over time result in corresponding declines in medication adherence. Conversely, medication adherence is predictive of a wider range of neurocognitive changes. Executive functioning in particular appears to be involved both directions of the relationship between cognition and medication adherence and is therefore a prime target for focused intervention.
Interactions Between Cognition and Other Variables
Age
In general, Dr. Koop would be most pleased with older HIV+ adults, as they have been shown to be far more adherent than are younger patients. Assessing medication adherence over the course of a month, Hinkin et al. (2004) found that older HIV+ individuals (individuals 50 years of age and olderFootnote 1) demonstrated significantly better adherence rates (87.5 %) to antiretroviral therapy compared to younger HIV+ individuals (78.3 %). Using adherence cutoff scores to classify patients as “good adherers” (95 % adherence or better) and “poor adherers” (less than 95 % adherence levels), they found that older individuals were three times more likely to be classified as good adherers. This beneficial “effect” of older age on medication adherence has been consistently replicated (Becker et al. 2002; Halkitis et al. 2008). A variety of explanations have been advanced, including the possibility that older individuals are more accustomed to taking medications for other age-related illnesses, are less burdened by lifestyle alterations necessary for successful adherence, and have an increased appreciation of their own mortality and therefore increased motivation to be medically compliant.
However, while medication adherence levels are typically higher among older adults, older adults appear to be more susceptible to the deleterious impact of cognitive dysfunction on medication adherence. Finer-grained analyses conducted as part of the abovementioned study performed by our group found that an overwhelming majority of older patients who were classified as “poor adherers” were cognitively compromised. In contrast, only 35 % of those classified as “good adherers” were found to demonstrate cognitive dysfunction (Hinkin et al. 2004). In a follow-up study, we assessed the relationship between age and cognition on medication adherence more directly (Ettenhofer et al. 2009). Using a large sample (N = 431) and sophisticated statistical analyses (structural equation modeling), we found that neurocognitive impairment was associated with poorer medication adherence among older individuals, but not among their younger counterparts (see Fig. 6.2).
Latent model of cognition and medication adherence among younger and older HIV+ adults. Reproduced with permission from American Psychiatric Publishing, Inc. From Ettenhofer et al. (2009, p. 287). Notes Mem.: memory; Attent.: attention; Exec.: executive; Qual.: qualitative self-report; 30 day: 30 day self-report; 1 day: 1 day self-report.Standardized values shown from model C0. Factor loadings of 1 day self-report have been reversed for ease of interpretation
As mentioned previously, researchers are increasingly employing laboratory-based analog tasks to examine aspects of medication taking abilities. One such measure, the medication management task (MMT; Albert et al. 1999), has been refined by Heaton et al. (2004) in their work with HIV-infected patients. Our group employed the MMT to further assess the relationship between cognition, age, and medication management and found that medication management abilities correlated with a wide range of cognitive abilities, including executive functioning, attention/working memory, verbal fluency, and spatial processing (Thames et al. 2011b). As seen in Fig. 6.3, cognitively impaired individuals, particularly older patients, performed significantly worse on the medication management task than did younger patients. These findings suggest that cognition can impact medication adherence via some of the more basic aspects of medication management as well as some of the more complex aspects (e.g., planning ahead, remembering to take the medications at the right times in the face of concurrent demands in one’s day-to-day life). This suggests that the MMT has potential for providing clinicians with a tool for identifying patients who need support with some of the more concrete aspects of medication management.
Age and group differences on medication management task (mean scores represented for ease of interpretation). GDS Global deficit score. Reproduced with permission from Taylor and Francis, Ltd. From Thames et al. (2011b, p. 204)
Regimen Complexity
Regimen complexity (e.g., dosing frequency, number of medications) has long been recognized as a factor to adversely impact medication adherence across a variety of illnesses, including HIV (for reviews, see Ammassari et al. 2002; Claxton et al. 2001; Ingersoll and Cohen 2008; Iskedjian et al. 2002). As the number of medications and/or dosing frequency increases, medication adherence tends to decline. From a neurocognitive standpoint, select populations may be at particular risk for poor medication adherence secondary to regimen complexity. Assessing the effects of regimen complexity (once or twice daily vs. three or more times daily) and cognition on MEMS cap adherence in an HIV+ sample, Hinkin et al. (2002a) found main effects for cognition and regimen complexity as well as an interaction between cognition and regimen complexity. Individuals who were cognitively compromised and who were prescribed a more complex dosing regimens evidenced the lowest adherence rates (see Fig. 6.4). Complex dosing regimens did not pose disproportionate difficulty for cognitively intact adults. Analyses by discrete neurocognitive domains revealed that this interaction was primarily driven by higher order cognitive processes (executive functions and higher order attentional processes), but not memory. These findings suggest that medication-specific factors such as complex dosing regimens can play an important role, particularly if individuals are experiencing significant executive dysfunction.
Relationship between cognitive status, regimen complexity, and medication Adherence among HIV-infected adults. Reproduced with permission from Wolters Kluwer Health. From Hinkin et al. (2002a, p. 1947)
Relationship Between Broader Aspects of Cognition and Medication Adherence
Prospective Memory
Prospective memory (i.e., “remembering to remember”) also appears to play a critical role in medication adherence. This refers to one’s ability to execute a future intention (e.g., remembering to take one’s medication at lunchtime) and is dependent on a variety of cognitive processes (as opposed to discrete areas of neurocognitive functioning that have been traditionally assessed in neurocognitive studies of medication adherence). In order to execute a future intention, individuals must be able to pair their intention with a retrieval cue (i.e., a specific time or event) and be able to maintain this intention-cue pairing—via automatic and strategic monitoring- while they are engaged in other tasks. They must subsequently be able to detect the cue and then follow through with the intention (McDaniel and Einstein 2007; Woods et al. 2008). The process of “remembering to remember” is dependent on the prefrontal cortex (Simons et al. 2006). Woods et al. found prospective memory to be a powerful predictor of self-reported medication adherence (2008) and objective measures of medication adherence (2009) in HIV that independently accounted for predictive variance over and beyond other cognitive, psychiatric, psychosocial, and environmental predictors of adherence. As discussed by these researchers, poor medication adherence may result from an inability to strategically allocate cognitive resources.
Health Literacy
Low health literacy has also emerged as a critical factor associated with a variety of adverse health outcomes, including medication-taking behavior. Broadly defined, health literacy includes the ability to understand instructions on prescription drug bottles, appointment slips, medical education brochures, doctor’s directions and consent forms, and the ability to negotiate complex health care systems (US Department of Health and Human Services Office of Disease Prevention and Health Promotion 2010). While we have previously discussed how these skills rely heavily on intact cognitive functions, even basic literacy skills contribute to the successful management of medications (Kalichman et al. 1999; Waldrop-Valverde et al. 2010). Brief interventions that increase HIV/AIDS and medication knowledge and self-efficacy have shown to be fruitful in increasing adherence in a sample of HIV+ individuals with low health literacy (Kalichman et al. 2005). This is an important finding, as the US immigrant population is rapidly growing with the number of individuals with limitations in health literacy increasing as well. The current challenge, both scientifically and clinically, is to continue to identify methods for delivering effective health communications to immigrants and other marginalized groups with low literacy skills to help recognize, minimize, and respond effectively to potential health problems.
Psychiatric/Psychological Functioning and Medication Adherence
Up until this point, we have focused on the effects of cognitive dysfunction on medication adherence. However, medication adherence is related to a far wider variety of factors. Two such factors that have a relationship with both cognition and medication adherence are psychiatric disturbance and substance use and abuse.
Psychiatric Functioning
In the HIV population, there is a high prevalence of psychiatric disorders, with approximately 50 % of individuals screening positive for one or more disorders, including depression and anxiety (Bing et al. 2001). These rates are considerably higher than rates found in the general population (Blazer et al. 1994). Bing et al. (2001) found that rates of depression were five times higher than the general population. The adverse effect of psychiatric disorders on medication adherence has been documented in a wide range of illnesses and has received considerable attention in HIV. Similar to the effects of cognition on medication adherence to antiretroviral therapy, individuals who are depressed are up to three times more likely to be non-adherent than non-depressed HIV+ individuals (Ammassari et al. 2004). There are a variety of possible mechanisms that may account for this. For example, symptoms associated with depression (e.g., sadness, lack of motivation, fatigue, and hopelessness) can often be debilitating for individuals, which can lead to self-neglect and poor adherence behaviors (e.g., not getting their prescriptions filled). Furthermore, depressed individuals may be more likely to drive away their social support through isolative behaviors, thereby not being able to rely on their support for assistance with medications.
A complicating element, however, is the fact that psychiatric disorders are often accompanied by a variety of cognitive difficulties. Determining the source of poor medication adherence, therefore, is critical for the selection of successful treatment interventions. Two psychiatric conditions that warrant particular attention in this regard are apathy and irritability. These neuropsychiatric conditions have emerged as critical co-morbid conditions that are believed to be direct manifestations of underlying HIV-associated neuropathology rather than a psychological reaction to a life-threatening illness (Castellon et al. 1998; Cole et al. 2007). They have begun to receive increased attention as they relate to deficits in executive dysfunction, including working memory deficits, learning efficiency, and cognitive flexibility (Bungener et al. 1996; Castellon et al. 2000; Cole et al. 2007; Paul et al. 2005). Patients presenting with these psychiatric symptoms (compared to other psychiatric disturbances such as depression) may be at particular risk for experiencing difficulty adhering to medication regimen as a result of neurocognitive dysfunction. Formal study assessing the relationship between these symptoms and medication adherence has yet to be conducted, however. Preliminary work from our laboratory suggests that both apathy and irritability are predictive of longitudinal medication adherence even after controlling for the effects of depression and cognition (Panos et al. 2011). Given these emerging findings, further study is clearly warranted.
A critical factor that warrants attention for clinicians treating individuals with both HIV and psychiatric dysfunction is accuracy of self-report of medication adherence. Decisions made by clinicians about treatment efficacy largely rely on patient self-report. If patients report they are adhering to their regimen and continue to evidence precipitous decline in immunocompetence, this is taken as evidence that their current regimen is not effective and alterations in their regimen are made, perhaps unnecessarily. As we mentioned previously, there are only a limited number of antiretroviral medications available for use, limiting the number of possible regimen alterations available to the treating clinician. We have found that HIV+ individuals with psychiatric disturbance are not as accurate in identifying reasons for non-adherence (Thames et al. 2011a). In this study, depressed individuals were more likely to report that cognitive difficulties interfered with their abilities to take their medications. However, upon formal testing, they did not exhibit any difficulty managing their medications. Conversely, individuals who were cognitively impaired were more likely to be unaware of any difficulty managing their medications, and thus denied having any difficulty in this regard. However, upon formal testing, the cognitively compromised subjects evidenced considerable difficulty managing their medications. Taken together, this highlights the importance of considering depression and cognitive ability when assessing self-reported medication behaviors. Furthermore, these findings implicate the use of objective instrumental activities of daily living measures (versus the use of self-report) in order to accurately detect declines in medication behaviors, and subsequently guide treatment interventions.
Substance Use
In the HIV-infected population, rates of substance abuse/dependence are particularly high, with nearly 50 % reporting the use of illicit substances over the previous 12 months, and 12 % screening positive for current dependence (Bing et al. 2001). Lifetime diagnoses of drug and alcohol abuse/dependence are yet higher (Dew et al. 1997; Rabkin 1996). The negative impact of substance use/abuse on medication adherence, especially stimulants, has received considerable attention (Fogarty et al. 2002; Hinkin et al. 2007; Howard et al. 2002; Meade et al. 2011). In a study from our laboratory assessing medication adherence prospectively over the course of 6 months, individuals with recent drug use were four times more likely to be non-adherent to antiretroviral therapy than were those who were drug-free (Hinkin et al. 2007). Subsequent analyses in this study found that stimulant use (cocaine and amphetamine) was particularly deleterious on medication adherence, with participants who tested positive for stimulants at least once over the course of the 6 month study being seven times more likely to be classified as “poor adherers.” A number of mechanisms have been proposed to explain the manner by which drug use may impede medication adherence. These include stable psychological traits that characterize individuals using drugs, the interruption of daily routines during drug use that serve as cues for taking medications, and the effect of drug-induced cognitive dysfunction (Gorman et al. 2009).
As mentioned throughout this chapter, determining the specific basis for poor adherence in crucial for the development of successful treatment interventions. There has been a limited amount of work aimed at directly assessing which of the abovementioned factors may account for poor adherence among drug users in the HIV populace. Meade et al. (2011) compared adherence rates and neurocognitive performance between HIV+ individuals who met diagnostic criteria for current cocaine dependence and HIV+ individuals who were not dependent on any drugs. They found that individuals who were cocaine-dependent exhibited both lower medication adherence rates and poorer neurocognitive functioning. Subsequent analyses revealed that neurocognition partially mediated the relationship between stimulant use and medication adherence in this sample.
There is evidence to suggest that the relationship between substance use and medication adherence may also be mediated by other factors. In the above-mentioned study from our laboratory, we compared medication adherence rates for individuals who had recently used stimulants (within 3 days of testing) with their adherence rates when they had not used drugs within the past 3 days (as indexed by urine toxicology assays). Despite the fact that all of these subjects had used stimulants at some point during the 6 month study, we found that during periods of even brief abstinence their adherence rates returned to normal (Hinkin et al. 2007). This lends support for the contention that drug use may adversely impact adherence by altering routines that serve as cues for taking medications. This also suggests that the adverse effects of drug use on adherence should be conceptualized as a transient and reversible “state” rather than some immutable “trait” that defines drug users (Malta et al. 2008, 2010). For individuals who use drugs, therefore, interventions aimed at improving adherence during times of drug use may be warranted.
Psychosocial Factors
We would be remiss not to discuss at least briefly some of psychosocial theories that have been set forth to describe medication taking behavior. It is within this broader psychosocial context that the aforementioned factors interact. In general, psychosocial theories focus on larger internal (e.g., beliefs about illness and treatment) and external (e.g., social support, access to resources) factors that affect health behavior. Several theories have been developed over the course of the last several decades to explain health behavior. In the context of medication adherence, for example, the Health Belief Model (HBM) (Rosenstock 1974) posits that adherence is optimal when individuals perceive that they are susceptible to illness (e.g., the belief that they will get sick should they not take their medications), perceive that the consequences of this illness can be severe, believe that the prescribed treatment will reduce risk of illness, and do not perceive there to be significant barriers to treatment adherence (e.g., they perceive that the benefits of adherence outweigh the costs). Moreover, this theory contends that a variety of other variables (e.g., patient demographics, mood factors, etc.) may influence these perceptions. In the HIV literature, various components of the HBM have been shown to be related to medication adherence and influenced by a variety of factors, including patient demographics and disease severity (Barclay et al. 2007; Gao et al. 2000). Barclay et al. (2007) assessed a variety of psychosocial, psychiatric, and drug use predictors in concert and found that components of HBM and self-efficacy were important predictors of antiretroviral medication adherence among younger participants. Among older participants, however, cognition remained the sole predictor of medication adherence.
Researchers have begun to develop theoretical models of social support, which has long been recognized to have a positive influence on medication adherence in a variety of illnesses (Becker and Maiman 1980; Greene et al. 1982), including HIV (Catz et al. 2000; Gonzalez et al. 2004). Studies aimed at delineating the manner by which social support influences medication adherence suggest that the relationship is indirect. Higher levels of social support are associated with reduced negative affect (e.g., depression, anxiety, and stress), increased spirituality, and increased medication-taking self-efficacy (either directly or indirectly through negative affect and/or spirituality). An important finding in these models is that medication-taking self-efficacy is directly related to medication adherence (Cha et al. 2008; Simoni et al. 2006). A positive relationship between the patient and health care provider also appears to have a beneficial impact on medication adherence and self-efficacy appears to mediate this relationship as well (Johnson et al. 2006). Ethnicity appears to be an important demographic variable that influences the relationship between social support and medication adherence. In a sample of HIV+ adults, a recent study from our laboratory found that patient satisfaction with healthcare provider was the strongest predictor of medication adherence among African–Americans beyond common factors (e.g., current drug use) that have been traditionally linked to medication adherence. Among Caucasians, however, depressive symptoms and treatment related social support (e.g., friends assisting them with HIV-related care) were the strongest predictors of medication adherence (Thames et al. 2012). These findings lead us to conclude that strengthening and promoting social support (including the physician-patient bond) should be an absolute priority for increasing and maintaining optimal medication adherence. It is logical to opine that social support (e.g., receiving illness related support) may be an important mediating variable for individuals who present with cognitive dysfunction. These individuals may particularly benefit from having significant others around them reminding them to take their medications. Furthermore, the interaction between cognition and self-efficacy—now emerging as having a direct relationship to medication adherence—is also unknown. Individuals who are cognitively impaired may not be accurate in their reports of self-efficacy, and therefore this variable may be a poor predictor of adherence behavior for them. Preliminary work from our laboratory suggests that psychosocial variables (e.g., treatment related support) may buffer against the adverse effects of poor cognition on medication adherence (Arentsen et al. 2012).
More recently, a new theoretical framework, temporal self-regulation theory (TST; Hall and Fong 2007), has been developed. This model integrates social cognitive and neurocognitive factors. TST posits that individuals perceive a temporal dispersion between costs and benefits of health behavior. For example, for some unhealthy behaviors (e.g., smoking or drinking behavior), the benefits are more proximal than the costs. Individuals’ ability to regulate behavior (self-regulatory capacity) in the context of this temporal dispersion of costs and benefits is a key and unique component in this theoretical model. Self-regulatory capacity refers to specific neurocognitive abilities (i.e., executive functions) and is thought to moderate the relationship between intention and action in health behavior. Hall and Fong (2007 ) found that frontal functioning moderated the relationship between intention and action for physical activity and dietary behavior (Hall et al. 2008). To our knowledge, this is the first integrative psychosocial model to include a neurocognitive component. Although this model has yet to be evaluated in the context of medication adherence to antiretroviral therapy, this may be a fruitful area of future research given the deleterious effect of HIV on executive functioning and the temporal dispersion of costs and benefits associated with adherence.
Conclusion
In summary, remarkable advances in antiretroviral therapy have led to a renewal of hope and a reconceptualization of HIV as a chronic disease rather than one that is inexorably terminal. Not unlike diseases such as diabetes or prostate cancer, increasingly patients will ultimately die with HIV rather than from HIV. But challenges remain. Comorbid conditions that initially placed some individuals at risk for contracting HIV, such as substance abuse and psychiatric illness, also pose considerable problems with regard to adherence. Cognitive disorders caused by the virus compromise treatment adherence, which in turn aggravates those very cognitive difficulties. Concurrent factors such as normal and pathological aging, socioeconomic impoverishment, and systemic barriers impeding access to care yet further hinder attempts to understand and improve adherence behavior. Considerable progress has been made, particularly with regard to understanding risk factors associated with poor adherence, though interventions to address these risks remain understudied and insufficiently deployed. The challenge now is to implement translational, tailored interventions that incorporate knowledge gained through research into the clinical care of the multitude of patients who remain HIV infected.
Notes
- 1.
Readers familiar with gerontology research, or who themselves are on the far side of 50, might balk at the use of age 50 to define “older” adults. This cut point was not arrived at arbitrarily but instead has its roots in the proceedings of several working groups convened by the NIH in the late 1990s/early 2000s on this topic when the “graying” of the HIV epidemic was first recognized. Now that increasing numbers of HIV+ adults are living into their 70s and beyond, it may be time to soon revisit and revise this convention.
References
Albert, S. M., Weber, C. M., Todak, G., Polanco, C., Clouse, R., McElhiney, M., et al. (1999). An observed performance test of medication management ability in HIV: Relation to neuropsychological status and medication adherence outcomes. AIDS and Behavior, 3(2), 121–128. doi:10.1023/a:1025483806464.
Ammassari, A., Trotta, M. P., Murri, R., Castelli, F., Narciso, P., Noto, P., et al. (2002). Correlates and predictors of adherence to highly active antiretroviral therapy: Overview of published literature. Journal of Acquired Immune Deficiency Syndromes, 31(Suppl 3), S123–S127.
Ammassari, A., Antinori, A., Aloisi, M. S., Trotta, M. P., Murri, R., Bartoli, L., et al. (2004). Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics, 45(5), 394–402. doi:10.1176/appi.psy.45.5.394.
Arentsen, T. J., Panos, S. E., Thames, A. D., Streiff, V., Arbid, N., & Hinkin, C. H. (2012). Psychosocial indicators of medication adherence among cognitively impaired HIV-infected individuals [Abstract]. Proceedings of the 92nd Annual Western Psychological Association Convention, San Francisco, CA.
Arnsten, J. H., Demas, P. A., Farzadegan, H., Grant, R. W., Gourevitch, M. N., Chang, C. J., et al. (2001). Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Diseases, 33(8), 1417–1423. doi:10.1086/323201.
Avants, S. K., Margolin, A., Warburton, L. A., Hawkins, K. A., & Shi, J. (2001). Predictors of nonadherence to HIV-related medication regimens during methadone stabilization. The American Journal On Addictions/American Academy of Psychiatrists in Alcoholism and Addictions, 10(1), 69–78.
Bangsberg, D. R., Hecht, F. M., Clague, H., Charlebois, E. D., Ciccarone, D., Chesney, M., et al. (2001). Provider assessment of adherence to HIV antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes, 26(5), 435–442.
Barclay, T. R., Hinkin, C. H., Castellon, S. A., Mason, K. I., Reinhard, M. J., Marion, S. D., et al. (2007). Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology, 26(1), 40–49.
Becker, M. H., & Maiman, L. A. (1980). Strategies for enhancing patient compliance. Journal of Community Health, 6(2), 113–135.
Becker, S. L., Dezii, C. M., Burtcel, B., Kawabata, H., & Hodder, S. (2002). Young HIV-infected adults are at greater risk for medication nonadherence. Medscape General Medicine, 4(3), 21.
Becker, B. W., Thames, A. D., Woo, E., Castellon, S. A., & Hinkin, C. H. (2011). Longitudinal change in cognitive function and medication adherence in HIV-infected adults. AIDS and Behavior, 15(8), 1888–1894. doi:10.1007/s10461-011-9924-z.
Bing, E. G., Burnam, M. A., Longshore, D., Fleishman, J. A., Sherbourne, C. D., London, A. S., et al. (2001). Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Archives of General Psychiatry, 58(8), 721–728.
Blazer, D. G., Kessler, R. C., McGonagle, K. A., & Swartz, M. S. (1994). The prevalence and distribution of major depression in a national community sample: The National Comorbidity Survey. The American Journal of Psychiatry, 151(7), 979–986.
Bungener, C., Le Houezec, J. L., Pierson, A., & Jouvent, R. (1996). Cognitive and emotional deficits in early stages of HIV infection: an event-related potentials study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 20(8), 1303–1314.
Castellon, S. A., Hinkin, C. H., Wood, S., & Yarema, K. T. (1998). Apathy, depression, and cognitive performance in HIV-1 infection. The Journal of Neuropsychiatry and Clinical Neurosciences, 10(3), 320–329.
Castellon, S. A., Hinkin, C. H., & Myers, H. F. (2000). Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. Journal of the International Neuropsychological Society, 6(3), 336–347.
Castellon, S. A., Hinkin, C. H., Wright, M., & Barclay, T. R. (2009). Neuropsychological Function and Adherence to Medical Treatments. In I. Grant & K. Adams (Eds.), Neuropsychological Assessment Of Neuropsychiatric And Neuromedical Disorders (pp. 688–712).
Catz, S. L., Kelly, J. A., Bogart, L. M., Benotsch, E. G., & McAuliffe, T. L. (2000). Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology, 19(2), 124–133.
Centers for Disease Control. (2007). HIV/AIDS Surveillance Report, 2005. (Vol. 17, Rev ed., pp. 1–54). Atlanta, GA: U.S. Department of Health and Human Services.
Centers for Disease Control and Prevention. (2009). Chronic Diseases: The Power to Prevent, The Call to Control-At a Glance 2009. Retrieved March 9, 2013, from http://www.cdc.gov/nccdphp/publications/AAG/chronic.htm.
Cha, E., Erlen, J. A., Kim, K. H., Sereika, S. M., & Caruthers, D. (2008). Mediating roles of medication-taking self-efficacy and depressive symptoms on self-reported medication adherence in persons with HIV: A questionnaire survey. International Journal of Nursing Studies, 45(8), 1175–1184.
Claxton, A. J., Cramer, J., & Pierce, C. (2001). A systematic review of the associations between dose regimens and medication compliance. Clinical Therapeutics, 23(8), 1296–1310.
Cole, M. A., Castellon, S. A., Perkins, A. C., Ureno, O. S., Robinet, M. B., Reinhard, M. J., et al. (2007). Relationship between psychiatric status and frontal-subcortical systems in HIV-infected individuals. Journal of the International Neuropsychological Society, 13(3), 549–554. doi:10.1017/S135561770707066X.
Cutler, D. M., Long, G., Berndt, E. R., Royer, J., Fournier, A. A., Sasser, A., et al. (2007). The value of antihypertensive drugs: A perspective on medical innovation. Health Aff (Millwood), 26(1), 97–110. doi:10.1377/hlthaff.26.1.97.
Dew, M. A., Becker, J. T., Sanchez, J., Caldararo, R., Lopez, O. L., Wess, J., et al. (1997). Prevalence and predictors of depressive, anxiety and substance use disorders in HIV-infected and uninfected men: a longitudinal evaluation. Psychological Medicine, 27(2), 395–409.
Ettenhofer, M. L., Hinkin, C. H., Castellon, S. A., Durvasula, R., Ullman, J., & Lam, M. (2009). Aging, neurocognition, and medication adherence in HIV infection. The American Journal of Geriatric Psychiatry, 17(4), 281–290. doi:10.1097/JGP.0b013e31819431bd.
Ettenhofer, M. L., Foley, J., Castellon, S. A., & Hinkin, C. H. (2010). Reciprocal prediction of medication adherence and neurocognition in HIV/AIDS. Neurology, 74(15), 1217–1222.
Farmer, M. E., Kittner, S. J., Abbott, R. D., Wolz, M. M., Wolf, P. A., & White, L. R. (1990). Longitudinally measured blood pressure, antihypertensive medication use, and cognitive performance: The Framingham study. Journal of Clinical Epidemiology, 43(5), 475–480.
Fogarty, L., Roter, D., Larson, S., Burke, J., Gillespie, J., & Levy, R. (2002). Patient adherence to HIV medication regimens: A review of published and abstract reports. Patient Education and Counseling, 46(2), 93–108.
Gao, X., Nau, D. P., Rosenbluth, S. A., Scott, V., & Woodward, C. (2000). The relationship of disease severity, health beliefs and medication adherence among HIV patients. AIDS care, 12(4), 387–398. doi:10.1080/09540120050123783.
Geller, J. L. (1982). State hospital patients and their medication–do they know what they take? The American Journal of Psychiatry, 139(5), 611–615.
Gifford, A. L., Bormann, J. E., Shively, M. J., Wright, B. C., Richman, D. D., & Bozzette, S. A. (2000). Predictors of self-reported adherence and plasma HIV concentrations in patients on multidrug antiretroviral regimens. Journal of Acquired Immune Deficiency Syndromes, 23(5), 386–395.
Gonzalez, J. S., Penedo, F. J., Antoni, M. H., Duran, R. E., McPherson-Baker, S., Ironson, G., et al. (2004). Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychology, 23(4), 413–418. doi:10.1037/0278-6133.23.4.413.
Gorman, A. A., Foley, J. M., Ettenhofer, M. L., Hinkin, C. H., & van Gorp, W. G. (2009). Functional consequences of HIV-associated neuropsychological impairment. Neuropsychology Review, 19(2):186–203. doi: 10.1007/s11065-009-9095-0
Gould, O. N., McDonald-Miszczak, L., & Gregory, J. (1999). Prediction accuracy and medication instructions: Will you remember tomorrow. Aging, Neuropsychology, and Cognition, 6, 141–154.
Greene, J. Y., Weinberger, M., Jerin, M. J., & Mamlin, J. J. (1982). Compliance with medication regimens among chronically ill, inner city patients. Journal of Community Health, 7(3), 183–193.
Halkitis, P., Palamar, J., & Mukherjee, P. (2008). Analysis of HIV medication adherence in relation to person and treatment characteristics using hierarchical linear modeling. AIDS Patient Care and STDs, 22(4), 323–335. doi:10.1089/apc.2007.0122.
Hall, P. A., & Fong, G. T. (2007). Teporal self-regulation theory: A model for individual health behavior. Health Psychology Review, 1(1), 6–52. doi:10.1080/17437190701492437.
Hall, P. A., Fong, G. T., Epp, L. J., & Elias, L. J. (2008). Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychology and Health, 23(3), 309–326. doi:10.1080/14768320701212099.
Harberer, J. E., Robbins, G. K., Ybarra, M., Monk, A., Ragland, K., Weiser, S. D., et al. (2012). Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behavior, 16(2), 375–382.
Hayes, T. L., Hunt, J. M., Adami, A., & Kaye, J. A. (2006). An electronic pillbox for continuous monitoring of medication adherence. Proceedings of the 27th Annual International Conference of the IEE Engineering in Medicine and Biology Society, New York.
Heaton, R. K., Grant, I., Butters, N., White, D. A., Kirson, D., Atkinson, J. H. et al. (1995). The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society, 1(3), 231–251.
Heaton, R. K., Marcotte, T. D., Mindt, M. R., Sadek, J., Moore, D. J., Bentley, H., et al. (2004). The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society, 10(3), 317–331. doi:10.1017/s1355617704102130.
Hinkin, C. H., Cummings, J. L., van Gorp, W. G., Satz, P., Mitrushina, M., & Freeman, D. (1990). Frontal/subcortical features of normal aging: an empirical analysis. Canadian Journal on Aging, 9, 104–119.
Hinkin, C. H., Castellon, S. A., Durvasula, R. S., Hardy, D. J., Lam, M. N., Mason, K. I., & Stefaniak, M. (2002a). Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology, 59(12), 1944–1950.
Hinkin, C. H., Hardy, D. J., Mason, K. I., Castellon, S. A., Lam, M. N., Stefaniak, M., & Zolnikov, B. (2002b). Verbal and spatial working memory performance among HIV-infected adults. Journal of the International Neuropsychological Society, 8(4), 532–538.
Hinkin, C. H., Hardy, D. J., Mason, K. I., Castellon, S. A., Durvasula, R. S., Lam, M. N., et al. (2004). Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS, 18(Suppl 1), S19–S25.
Hinkin, C. H., Barclay, T. R., Castellon, S. A., Levine, A. J., Durvasula, R. S., Marion, S. D., et al. (2007). Drug use and medication adherence among HIV-1 infected individuals. AIDS and Behavior, 11(2), 185–194. doi:10.1007/s10461-006-9152-0.
HIV Prevention Trials Network. (2011). Initiation of antiretroviral treatment protects uninfected sexual partners from HIV Infection (HPTN Study 052) [Press Release]. Retrieved from http://www.hptn.org/web%20documents/PressReleases/HPTN052PressReleaseFINAL5_12_118am.pdf.
Howard, A. A., Arnsten, J. H., Lo, Y., Vlahov, D., Rich, J. D., Schuman, P., et al. (2002). A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS, 16(16), 2175–2182.
Ickovics, J. R., & Meisler, A. W. (1997). Adherence in AIDS clinical trials: A framework for clinical research and clinical care. Journal of Clinical Epidemiology, 50(4), 385–391.
Ingersoll, K. S., & Cohen, J. (2008). The impact of medication regimen factors on adherence to chronic treatment: A review of literature. Journal of Behavioral Medicine, 31(3), 213–224. doi:10.1007/s10865-007-9147-y.
Iskedjian, M., Einarson, T. R., MacKeigan, L. D., Shear, N., Addis, A., Mittmann, N., et al. (2002). Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: Evidence from a meta-analysis. Clinical Therapeutics, 24(2), 302–316.
Johnson, M. O., Chesney, M. A., Goldstein, R. B., Remien, R. H., Catz, S., Gore-Felton, C., et al. (2006). Positive provider interactions, adherence self-efficacy, and adherence to antiretroviral medications among HIV-infected adults: A mediation model. AIDS Patient Care and STDs, 20(4), 258–268.
Kalichman, S. C., Ramachandran, B., & Catz, S. (1999). Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine, 14(5), 267–273.
Kalichman, S. C., Cherry, J., & Cain, D. (2005). Nurse-delivered antiretroviral treatment adherence intervention for people with low literacy skills and living with HIV/AIDS. The Journal of the Association of Nurses in AIDS Care, 16(5), 3–15.
Levine, A. J., Hinkin, C. H., Marion, S., Keuning, A., Castellon, S. A., Lam, M. M., et al. (2006). Adherence to antiretroviral medications in HIV: Differences in data collected via self-report and electronic monitoring. Health Psychology, 25(3), 329–335. doi:10.1037/0278-6133.25.3.329.
Liu, H., Golin, C. E., Miller, L. G., Hays, R. D., Beck, C. K., Sanandaji, S., & Wenger, N. S. (2001). A comparison study of multiple measures of adherence to HIV protease inhibitors. Annals of Internal Medicine, 134(10), 968–977.
Lovejoy, T. I., & Suhr, J. A. (2009). The relationship between neuropsychological functioning and HAART adherence in HIV-positive adults: A systematic review. Journal of Behavioral Medicine, 32(5), 389–405. doi:10.1007/s10865-009-9212-9.
Malta, M., Strathdee, S. A., Magnanini, M. M., & Bastos, F. I. (2008). Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: A systematic review. Addiction, 103(8), 1242–1257. doi:10.1111/j.1360-0443.2008.02269.x.
Malta, M., Magnanini, M. M., Strathdee, S. A., & Bastos, F. I. (2010). Adherence to antiretroviral therapy among HIV-infected drug users: A meta-analysis. AIDS and Behavior, 14(4), 731–747. doi:10.1007/s10461-008-9489-7.
McDaniel, M. A., & Einstein, G. O. (2007). Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks: Sage Publications.
McDonnell, P. J., & Jacobs, M. R. (2002). Hospital admissions resulting from preventable adverse drug reactions. The Annals of Pharmacotherapy, 36(9), 1331–1336.
Meade, C. S., Conn, N. A., Skalski, L. M., & Safren, S. A. (2011). Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal of Behavioral Medicine, 34(2), 128–138. doi:10.1007/s10865-010-9293-5.
Meyer, M. E., & Schuna, A. A. (1989). Assessment of geriatric patients’ functional ability to take medication. DICP, 23(2), 171–174.
Nieuwkerk, P. T., Sprangers, M. A., Burger, D. M., Hoetelmans, R. M., Hugen, P. W., Danner, S. A., et al. (2001). Limited patient adherence to highly active antiretroviral therapy for HIV-1 infection in an observational cohort study. Archives of Internal Medicine, 161(16), 1962–1968.
Osterberg, L., & Blaschke, T. (2005). Adherence to medication. The New England Journal of Medicine, 353(5), 487–497. doi:10.1056/NEJMra050100.
Panos, S. E., Del Re, A., Thames, A. D., Levine, A. J., Streiff, V., Castellon, S. A., et al. (2011). Predictors of longitudinal medication adherence: Evidence from an integrative model [Abstract]. The Clinical Neuropsychologist, 25(4), 556.
Parkin, D. M., Henney, C. R., Quirk, J., & Crooks, J. (1976). Deviation from prescribed drug treatment after discharge from hospital. British Medical Journal, 2(6037), 686–688.
Paterson, D. L., Swindells, S., Mohr, J., Brester, M., Vergis, E. N., Squier, C., et al. (2000). Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine, 133(1), 21–30.
Paul, R., Flanigan, T. P., Tashima, K., Cohen, R., Lawrence, J., Alt, E., et al. (2005). Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. The Journal of Neuropsychiatry and Clinical Neurosciences, 17(1), 114–118. doi:10.1176/appi.neuropsych.17.1.114.
Rabkin, J. G. (1996). Prevalence of psychiatric disorders in HIV illness. International Review of Psychiatry, 8(2–3), 157–166. doi:10.3109/09540269609046300.
Robertson, K., Liner, J., & Heaton, R. (2009). Neuropsychological assessment of HIV-infected populations in international settings. Neuropsychology Review, 19(2), 232–249. doi:10.1007/s11065-009-9096-z.
Rosenstock, I. M. (1974). Historical origins of the health belief model. Health Education Monographs, 2, 1–8.
Sabaté, E. (2003). Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization.
Shah, S. S., McGowan, J. P., Smith, C., Blum, S., & Klein, R. S. (2002). Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clinical Infectious Diseases, 35(10), 1238–1243. doi:10.1086/343048.
Simoni, J. M., Frick, P. A., & Huang, B. (2006). A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychology, 25(1), 74–81.
Simons, J. S., Schölvinck, M. L., Gilbert, S. J., Frith, C. D., & Burgess, P. W. (2006). Differential components of prospective memory? Evidence from fMRI. Neuropsychologia, 44, 1388–1397. doi:10.1016/j.neuropsychologia.2006.01.005.
Thames, A. D., Becker, B. W., Marcotte, T. D., Hines, L. J., Foley, J. M., Ramezani, A., & Hinkin, C. H. (2011a). Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal versus objective performance. The Clinical Neuropsychologist, 25(2), 224–243. doi: 10.1080/13854046.2010.539577.
Thames, A. D., Kim, M. S., Becker, B. W., Foley, J. M., Hines, L. J., Singer, E. J., Hinkin, C. H. (2011b). Medication and finance management among HIV-infected adults: The impact of age and cognition. Journal of Clinical and Experimental Neuropsychology, 33(2), 200–209. doi: 10.1080/13803395.2010.499357.
Thames, A. D., Moizel, J., Panos, S. E., Patel, S. M., Byrd, D. A., Myers, H. F.,…Hinkin, C. H. (2012). Differential predictors of medication adherence among African American and Caucasian HIV+ adults. AIDS Patient Care and STDs, 26(10), 621–630. doi:10.1089/apc.2012.0157.
US Department of Health and Human Services Office of Disease Prevention and Health Promotion. (2010). National Action Plan to Improve Health Literacy. Washington: Author.
US Food and Drug Administration. (2012). FDA Approves first drug for reducing the risk of sexually acquired HIV infection [Press Release]. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
Valcour, V., Shikuma, C., Shiramizu, B., Watters, M., Poff, P., Selnes, O., et al. (2004). Higher frequency of dementia in older HIV-1 individuals: The Hawaiian aging with HIV-1 Cohort. Neurology, 63(5), 822–827.
Vik, S. A., Hogan, D. B., Patten, S. B., Johnson, J. A., Romonko-Slack, L., & Maxwell, C. J. (2006). Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs and Aging, 23(4), 345–356.
Waldrop-Valverde, D., Jones, D. L., Gould, F., Kumar, M., & Ownby, R. L. (2010). Neurocognition, health-related reading literacy, and numeracy in medication management for HIV infection. AIDS Patient Care and STDs, 24(8), 477–484. doi:10.1089/apc.2009.0300.
Wendelken, L. A., & Valcour, V. (2012). Impact of HIV and aging on neuropsychological function. Journal of Neurovirology, 18(4), 256–263. doi:10.1007/s13365-012-0094-1.
Wilkie, F. L., Goodkin, K., van Zuilen, M. H., Tyll, M. D., Lecusay, R., & Edwin, T. (2000). Cognitive effects of HIV-1 infection. CNS Spectrums, 5(5), 33–51.
Woods, S. P., Moran, L. M., Carey, C. L., Dawson, M. S., Iudicello, J. E., Gibson, S., et al. (2008). Prospective memory in HIV infection: Is “remembering to remember” a unique predictor of self-reported medication management? Archives of Clinical Neuropsychology, 23(3), 257–270. doi:10.1016/j.acn.2007.12.006.
Woods, S. P., Dawson, M. S., Weber, E., Gibson, S., Grant, I., & Atkinson, J. H. (2009). Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. Journal of the International Neuropsychological Society, 15(1), 42–52. doi:10.1017/s1355617708090012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Highlights
Highlights
-
Those living with HIV face the prospect of dealing with complex medication regimens and disease-related cognitive dysfunction.
-
Cognitive dysfunction has been shown to disrupt medication adherence in HIV+ individuals.
-
Executive function appears to be most centrally implicated in non-adherence.
-
The consequences of non-adherence among HIV+ individuals is of significant concern to affected individuals and public health as a whole.
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Panos, S.E., Patel, S.M., Thames, A.D., Hinkin, C.H. (2013). Neurocognition and Medication Adherence in HIV-Infected Adults. In: Hall, P. (eds) Social Neuroscience and Public Health. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6852-3_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6852-3_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6851-6
Online ISBN: 978-1-4614-6852-3
eBook Packages: MedicineMedicine (R0)