Abstract
PTEN is one of the most commonly deleted/mutated tumor suppressor genes in human prostate cancer. As a lipid phosphatase and negative regulator of the PI3K/AKT/mTOR pathway, PTEN controls a number of cellular processes, including survival, growth, proliferation, metabolism, migration, and cellular architecture. Over the past 15 years since its discovery, a number of mechanisms governing PTEN expression and function, including transcriptional and post-transcriptional regulation, post-translational modifications, and protein–protein interactions, have been shown to be altered in human prostate cancer. The functions of PTEN within the cell have been expanded to include phosphatase-independent roles and functions within the nucleus. The generation of genetically engineered mouse models (GEMs) with deletion of Pten has further revealed that varying degrees of Pten loss in combination with other genetic alterations are able to recapitulate all spectrums of human prostate cancer, from tumor initiation to metastasis. With new methods of genomic and transcriptional analysis of human prostate cancer specimens, PTEN loss can potentially be used as a diagnostic and prognostic biomarker for prostate cancer, as well as predict patient responses to emerging PI3K/AKT/mTOR inhibitors. Finally, deeper insight into communication between the PI3K/AKT/mTOR and Ras/MAPK signaling pathways has led to the creation of metastatic murine prostate cancer models that develop lethal metastases, while new understanding of a feedback loop between PTEN and androgen receptor (AR) controlled pathways has unveiled a new mechanism for the development of castration-resistant prostate cancer (CRPC). Our expanded knowledge of PTEN and its role in prostate cancer initiation and progression will inform the rational design of novel therapeutics that target PTEN-controlled pathways alone or in combination with other related pathways for the treatment of metastatic and castration-resistant prostate cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- PTEN Loss
- Castration-resistant Prostate Cancer (CRPC)
- PTEN Expression
- Murine Prostate Cancer Model
- PTEN Allele
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Although partial or complete loss of chromosome 10 in brain, bladder, and prostate cancers was identified as early as 1984 [1], it was not until 1997 that three independent groups, through mapping of mutations on chromosome 10 and cloning of a novel phosphatase, identified a tumor suppressor gene at the 10q23.31 locus named by different laboratories as the phosphatase and tensin homolog (PTEN), mutated in multiple advanced cancers 1 (MMAC1), and TGF-β-regulated and epithelial cell-enriched phosphatase 1 (TEP1) [2–4]. PTEN is a nonredundant phosphatase that antagonizes the phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway, one of the most important and well-studied cancer promoting pathways. As PTEN is the only known 3′ phosphatase counteracting the PI3K/AKT pathway, it is not unexpected that loss of PTEN has a significant impact on prostate cancer progression. Indeed, loss of heterozygosity (LOH) of PTEN occurs frequently in many advanced stage sporadic tumors, including ~60 % of advanced prostate cancers [2]. Germline PTEN gene mutations account for the majority (80 %) of cases of Cowden Syndrome, an autosomal dominant multiple hamartoma syndrome that leads to an increased propensity for patients to develop breast [5], endometrial [5, 6], and thyroid cancers [7–9]. However, prostate cancer has not been associated with Cowden Syndrome and germline PTEN loss [10, 11], perhaps providing credence to the understanding that loss of PTEN is a late event in prostate carcinogenesis [12, 13].
In this chapter, we will review PTEN structure, function, and regulation. The consequences of loss of PTEN regulation and function in different stages of prostate cancer development, as well as the potential use of PTEN loss as a biomarker for prostate cancer prognosis and prediction of patient responses to PI3K/AKT/mTOR pathway inhibitors, will also be addressed. Due to space limitations, some important topics, such as the role of PTEN in prostate stem cell/cancer stem cell maintenance and crosstalk with the tumor microenvironment, cannot be covered. However, these topics have been covered extensively by several outstanding reviews [14–17].
PTEN Structure and Function
PTEN Structure
The PTEN gene comprises nine exons and encodes a protein of 403 amino acids [18]. The amino acid sequence of the PTEN tumor suppressor is considerably homologous to dual-specific protein phosphatases and tensin, a chicken cytoskeletal protein [2]. The crystal structure of PTEN revealed an expanded active site pocket for binding to its substrates and a C2 domain, which mediates membrane attachment of cell signaling proteins. Three other functional domains have also been identified: a short phosphatidylinositol-4,5-bisphosphate (PIP2) binding domain on the N terminus, and PEST sequences and a PDZ interaction motif on the C-terminal tail that regulate protein stability and binding to PDZ domain-containing proteins, respectively [19]. The binding of PIP2 to PTEN produces a conformational change in the enzyme, leading to allosteric activation [20]. The positive charge of PTEN’s substrate binding pocket is also important for accommodating larger acidic substrates such as phosphoinositides. The PTEN phosphatase domain is evolutionarily conserved, and is the recipient of 40 % of its cancer-associated mutations, which occur most commonly through either a C124S mutation that abolishes both lipid and protein phosphatase activity or a G129E mutation that abrogates only its lipid phosphatase activity [21–23]. Although the phosphatase domain is responsible for PTEN’s physiological activity, other PTEN tumorigenic mutations occur on the C-terminal C2 domain and tail sequence, highlighting an important role of the C terminus in maintaining PTEN protein stability [24, 25]. The fact that tumor-associated mutations occur in all PTEN functional domains indicates that each of these regions is biologically relevant to PTEN function. In prostate cancer, PTEN loss most commonly results from a somatic mutation generated through copy number loss rather than point mutation [26], although recent exome sequencing has identified several recurrent mutations in the PTEN gene [27, 28].
PTEN and Regulation of the PI3K/AKT/mTOR Pathway
PI3K/AKT signaling plays a critical role in regulating growth responses, homeostasis, and longevity. At the cellular level, the PI3K/AKT pathway controls cell growth, migration, differentiation, and survival. Activation of the PI3K/AKT pathway is also frequently detected in human cancers [29]. PTEN is a unique lipid phosphatase that removes the phosphate from the D3 position of phosphatidylinositol-3,4,5-triphosphate (PIP3), a product of PI3K, thus directly antagonizing the action of PI3K [23, 30, 31]. PIP3 accumulation at the plasma membrane through PI3K activity results in recruitment and activation of important kinases involved in cell growth and survival, including phosphoinositide-dependent kinase-1 (PDK1) and AKT family members, via their pleckstrin homology (PH) domains [23, 30, 31]. In this manner, PTEN negatively regulates the PI3K/AKT pathway by inhibiting downstream AKT activation.
AKT isoforms (AKT1, AKT2, AKT3) are activated by phosphorylation at two different residues: Thr308 by PDK1 [32] and Ser473 by mammalian target of rapamycin complex 2 (mTORC2) [33]. Activated AKT drives cell survival, proliferation, growth, angiogenesis, and metabolism by phosphorylating downstream signaling proteins, which include inhibitory phosphorylation of GSK3β, FOXO, BAD, p21, p27, and PGC1, and activating phosphorylation of mammalian target of rapamycin complex 1 (mTORC1), IKK-β, MDM2, ENTPD5, SREBP1C, AS160, and SKP2 [33, 34]. AKT promotes cell cycle progression and proliferation by directly inhibiting p21 and p27 and alleviating GSK3β-induced cyclin D1 degradation. Moreover, inhibition of GSK3β has been shown to prevent the degradation of β-catenin, which can further stabilize cyclin D1 mRNA and promote G1-phase/S-phase progression [34, 35]. Activation of AKT also helps evade apoptosis directly by phosphorylation of the pro-apoptotic protein BAD [36]. In this regard, it is not surprising that re-expression of WT PTEN in PTEN null prostate cancer cell lines leads to apoptosis [37].
AKT directly activates the mTOR pathway by phosphorylating TSC2, which dismantles the TSC1/TSC2 complex that normally inhibits Rheb. Rheb, now free from TSC1/TSC2 inhibition, can stimulate the phosphotransferase activity of mTORC1 [38]. AKT may also activate mTORC1 by phosphorylating and inhibiting PRAS40, a negative regulatory subunit of the mTORC1 complex [33, 39]. Active mTORC1 phosphorylates p70 ribosomal protein S6 kinase (S6K) and 4E-binding protein (4EBP1), which in turn initiates cap-dependent protein translation [40]. Therefore, as a consequence of PTEN inactivation, PI3K/AKT/mTOR pathway activation leads to enhanced translation of mRNAs involved in protein synthesis, cell growth, and proliferation.
Interestingly, mTORC1 signaling also triggers a negative feedback loop that inhibits the PI3K/AKT pathway. This occurs through the phosphorylation and degradation of insulin receptor substrate 1 (IRS1), a crucial effector of insulin signaling, by S6K [41, 42]. Conversely, inhibition of mTORC1 results in hyperactivation of the PI3K/AKT pathway, as well as increased signaling through the Ras/MAPK pathway. The growth factor receptor GRB10 is a novel mTORC1 substrate that mediates feedback inhibition of the PI3K/AKT and Ras/MAPK pathways by direct inhibition of IRS proteins [43, 44]. In contrast, PTEN loss can reverse mTORC1-mediated negative feedback inhibition of the PI3K/AKT/mTOR pathway by activating both the upstream and downstream arms of the PI3K/AKT/mTOR pathway. Therefore, effective inhibition of tumors with PTEN loss will require inhibition of both mTORC1 and other signaling molecules upstream in the pathway, including PI3K and AKT.
PTEN and Metabolism
Recent studies have suggested that metabolic reprogramming is a requirement for the rapid cell proliferation of cancer cells. As opposed to differentiated and nonproliferating cells, which primarily utilize mitochondrial oxidative phosphorylation to generate the ATP needed for cellular processes, rapidly proliferating cells, including stem cells and cancer cells, tend to convert most glucose to lactate, even in the presence of oxygen, through aerobic glycolysis and a phenomenon known as the Warburg effect [45]. In this way, cancer cells exhibit high rates of glycolysis with increased glucose and glutamine uptake and lactate production, as well as increased biosynthesis of lipids, amino acids, and nucleic acids, macromolecules that are needed to compensate for anabolic growth [46].
The PI3K/AKT pathway plays a key role in the regulation of glucose metabolism given its position downstream of the insulin receptor. The PI3K/AKT pathway enhances insulin-mediated glucose uptake and membrane translocation of the glucose transporter GLUT1, which has been positively correlated with higher tumor grades and Gleason scores [47], by way of mTORC1 activation and cap-dependent translation [48], and GLUT4, by way of inhibition of AS160 [49]. As PI3K/AKT signaling leads to increased production of HIF1α [50, 51], a transcription factor that regulates the transcription of the Glut-1 gene [52], it is likely that both the PI3K/AKT pathway and HIF1α activation contribute to higher levels of GLUT1 and enhanced glucose uptake [53]. Increased HIF1α expression also upregulates expression of vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis that may further promote tumor metabolism by facilitating access to nutrients in the blood [54]. Conversely, stimulation of the PI3K/AKT pathway blocks gluconeogenesis by preventing both FOXO and PGC1α activation [55, 56]. AKT may indirectly activate glycolysis as well by directly phosphorylating PKF2, whose product, Fru-1,6-P2, is a potent allosteric activator of the glycolysis rate-controlling enzyme PFK1 [32, 57]. A recent study using siRNA-mediated gene silencing in metastatic prostate cancer cell lines revealed that PFKFB4, an isoform of PFK2 that is required for glycolysis, is essential for survival of prostate tumor cells and that ablation of PFKFB4 inhibits tumor growth in a xenograft model [58].
In comparison to other epithelial cancers, primary prostate cancers are less glycolytic and, therefore, not sensitive to FDG-PET imaging until reaching the metastatic stage [59, 60]. On the other hand, prostate cancer is known to be lipogenic, and C-11-acetate and F18-choline have been used, although in limited scale, in prostate cancer imaging [59, 61]. Recent studies suggest that the PI3K/AKT pathway can regulate lipid metabolism as well to further promote anabolic growth through the Warburg effect. Upon PTEN loss and through inhibition of GSK3, the PI3K/AKT axis activates the transcription factor SREBP1C, which in turn transcribes genes involved in cholesterol and fatty acid biosynthesis [62, 63]. PTEN has also been shown to regulate the synthesis of long chain saturated fatty acids by inducing the downregulation of fatty acid synthase (FAS), a lipogenic enzyme overexpressed in many human cancers, including prostate cancer, in a lipid phosphatase-dependent manner [64]. Therefore, PTEN loss in prostate cancer cells may increase FAS protein expression, which is elevated in tumors with a poor prognosis [65]. Collectively, these data indicate that both upstream and downstream components of the PTEN regulated PI3K/AKT/mTOR pathway are involved in the metabolic reprogramming required to sustain the rapid growth and proliferation of tumor cells by (1) increasing glucose metabolism via aerobic glycolysis and (2) promoting macromolecule biosynthesis via lipogenesis.
The recent creation of a mouse model with global PTEN overexpression, the “Super-PTEN” model, has demonstrated that PTEN elevation at the organism level results in diminished glucose and glutamine uptake and increased mitochondrial oxidative phosphorylation, resulting in a reversion to a more healthy metabolism [66]. PTEN elevation in this model coordinates this metabolic shift by negatively regulating both PI3K/AKT-dependent pathways, such as mTORC1 activation of PKM2, a controller of glycolytic flux [67], and PI3K/AKT-independent pathways, such as degradation of PFKFB3, a key regulator of glycolysis [68], through APC/Cdh1 activation [66]. Interestingly, these “Super-PTEN” mutants are resistant to oncogenic transformation, demonstrating that inhibition of the metabolic reprogramming to aerobic glycolysis through PTEN expression or inactivation of the PI3K/AKT pathway may be sufficient to obstruct tumor propagation [66]. These outcomes suggest that PTEN overexpression may indeed be an attractive option for cancer prevention and therapy.
PTEN in the Nucleus
It was initially assumed that PTEN is exclusively localized in the cytoplasm. However, following the discovery that PTEN contains dual nuclear localization signal-like sequences [69], it has been well recognized that PTEN can localize to the nucleus, and recent studies have illustrated the important functions of nuclear PTEN in regulating cell cycle progression and genomic integrity. Indeed, not only is there a marked reduction in nuclear PTEN in rapidly cycling cancer cell lines in comparison to resting or differentiated cells [70–73], but absence of nuclear PTEN has also been associated with reduced overall survival in prostate cancer patients [74].
Oxidative stress is one of the physiological stimuli that regulate the accumulation of nuclear PTEN [75]. Oxidative stress inhibits PTEN nuclear export, a process dependent on phosphorylation at Ser380. Nuclear PTEN, independent of its phosphatase activity, can regulate p53 stability and transcriptional activity [76, 77], leading to p53-mediated G1 growth arrest, cell death, and reduction of reactive oxygen species (ROS) production [75]. Nuclear PTEN is also sufficient to reduce human prostate cancer xenograft growth in vivo in a p53-dependent manner [75], suggesting a unique role of nuclear PTEN to arrest and protect cells following oxidative damage and to regulate prostate cancer development.
Nuclear PIP3, unlike cytoplasmic PIP3, is insensitive to the lipid phosphatase activity of PTEN, implying nuclear functions for PTEN beyond its role as a negative regulator of the PI3K/AKT pathway [78]. This, however, is at odds with another finding that forced nuclear expression of PTEN can reduce nuclear levels of P-AKT, although it was not demonstrated whether this mechanism occurred through a PI3K-dependent or -independent pathway [79]. One proposed function of PTEN in the nucleus is to induce G1 cell cycle arrest in part by reducing cyclin D1 levels through its protein phosphatase activity [80] or through controlling MAPK signaling [81]. Nuclear PTEN maintains chromosomal stability by physically associating with centromeres through docking onto CENP-C, a centromeric-binding protein [82]. Moreover, nuclear PTEN, through a phosphatase-independent mechanism, enhances DNA repair through increasing the activity of RAD51, a protein implicated in double strand break (DSB) repair [82]. Not surprisingly, PTEN-null cells develop spontaneous DNA DSBs at a high rate [82]. Cytoplasmic PTEN can also contribute to DSB repair by inhibiting AKT-dependent sequestration of the cell cycle regulator CHK1 in the cytoplasm [83]. In this fashion, PTEN helps to maintain the G2/S cell cycle checkpoint and likewise prevents genomic instability and DSBs. As PTEN loss leads to homologous recombination defects in human tumor cells through downregulation of RAD51 and CHK1 in the nucleus, tumor cells display increased sensitivity to inhibitors of PARP [84–86]. These findings provide evidence for the use of PARP inhibitors in patients with PTEN-deficient prostate cancer. However, a recent study of clinical prostate cancer specimens suggests that PTEN loss is not associated with reduced RAD51 mRNA or protein expression in primary prostate cancer, and that PTEN-deficient cells only exhibit mild sensitivity to PARP inhibition, casting doubt on whether PTEN is a useful biomarker for response to PARP inhibitors in prostate cancer [87].
Nuclear PTEN directly increases the antitumor and E3 ligase activity of APC/C through a phosphatase-independent mechanism by promoting the association of APC/C with its activator CDH1 [88]. The APC/C-CDH1 complex contains tumor suppressive activities that degrade oncogenic proteins such as PLK1 and Aurora kinases [89, 90]. In this regard, combining PLK and Aurora kinase inhibitors with PI3K/AKT inhibitors may provide increased efficacy in treating PTEN-deficient prostate cancer. Altogether, these findings suggest that the tumor suppressive functions of PTEN are in part due to its functions within the nucleus. New insights into the regulation of PTEN subcellular localization and the functions of PTEN in the nucleus may shed light on novel biomarkers and therapeutics for the treatment of prostate cancer.
PI3K/AKT/mTOR-Independent Functions of PTEN
Although most phenotypes associated with PTEN loss can be accounted for by the activation of the PI3K/AKT pathway, transgenic models with prostate-specific overexpression of p110β or a constitutively active form of AKT develop only localized, precancerous PIN lesions, suggesting that PTEN possesses other tumor suppressor functions independent of the PI3K/AKT pathway [91–93]. Similarly, while conditional deletion of p110β or Rictor, in addition to Pten conditional deletion, prevents the progression of tumor development from PIN to adenocarcinoma, they do not completely prevent prostate cancer initiation [94, 95].
One example of a PI3K/AKT/mTOR-independent mechanism of PTEN regulation is the interaction between PTEN and p53 [96]. PTEN inactivation is known to increase the expression [97] and activation of the p53 repressor MDM2 [98] by a PI3K/AKT-dependent pathway [99] and upregulate p53 through translational mechanisms mediated by mTORC1 [100, 101]. The PTEN C2 domain, which lacks phosphatase activity, can also regulate cell motility [102] and, interestingly, can interact directly with p53 in a phosphatase-independent manner to enhance p53-mediated cell cycle arrest and apoptosis by promoting the stabilization, acetylation, and tetramerization of p53 [75, 76, 103]. Conversely, p53 can also regulate PTEN at the transcription level [104]. In the Pten-null mouse model, deletion of p53 accelerates Pten-null prostate cancer by reducing cellular senescence [105]. Concomitant mutations of PTEN and p53 have been detected within individual human tumors, supporting a selective advantage for combined inactivation of both tumor suppressors. However, whether the cooperation of PTEN and p53 loss in overriding cellular senescence promotes human prostate cancer progression needs to be further investigated.
PTEN can also regulate the expression of other tumor suppressors whose functions are commonly lost as an early event in prostate cancer initiation, such as Nkx3.1 [106]. Not only is Nkx3.1 expression downregulated in the PTEN-null murine prostate cancer model, but forced expression of Nkx3.1 in the PTEN-null prostate epithelium prevents prostate cancer initiation and progression [106]. Moreover, while a transcriptional profiling study has indicated that the JNK pathway is activated following PTEN loss in an AKT-independent manner [107], a recent report elucidated that JNK deficiency collaborates with PTEN loss in promoting CRPC [108].
Though the lipid phosphatase activity of PTEN is central to its role as a tumor suppressor, other, phosphatase-independent functions of PTEN are also important. PTEN is a dual-specificity protein phosphatase with activity toward acidic substrates. PTEN is capable of dephosphorylating phosphorylated serine, threonine, and tyrosine residues on peptide substrates in vitro [109], as well as protein substrates such as FAK [110], CREB [111], eIF2 [112], and SRC [113] in vivo, thereby directly inhibiting cell survival, proliferation, and migration. The activation of these PI3K/AKT-independent pathways after PTEN loss suggests that combining PI3K/AKT inhibitors with inhibitors of these other pathways may improve efficacy in treating patients with PTEN-deficient prostate cancer.
PTEN Regulation
Genetic Regulation
Germline PTEN mutations do not predispose men to prostate cancer [10, 11]. However, the 10q23 gene locus is a frequent target for somatic heterozygous deletion in primary, and, more frequently, in metastatic prostate tumors, where loss of heterozygosity (LOH) is found in 20–60 % of tumors [114]. However, the finding that the rate of PTEN LOH and mutations are far less frequent than the detected rate of PTEN loss at the protein level suggests that other, nongenomic alterations may occur that inactivate the second PTEN allele.
Epigenetic Regulation by DNA Methylation
Supporting its important physiological functions, PTEN is constitutively expressed in normal tissues, including infant and adult human prostates. However, PTEN expression can be downregulated on many levels in various physiological settings. Epigenetic inactivation of the PTEN promoter has been described in prostate cancer xenografts, where loss of PTEN protein is a result of promoter methylation [115]; however, this has yet to be shown in primary prostate cancer specimens. Additionally, the zinc-finger transcription factor SALL4 represses PTEN transcription in embryonic stem cells by recruiting an epigenetic repressor complex called the Mi-2/NuRD complex to the PTEN locus [116]. Despite these discoveries, epigenetic silencing of PTEN in prostate cancer has not been demonstrated in the in vivo and clinical setting.
Transcriptional Regulation
Suppression of PTEN transcription may have an important and understated role in prostate cancer initiation and progression. PTEN was originally cloned as a gene transcriptionally regulated by transforming growth factor β (TGFβ) [4], which both suppresses and induces PTEN expression depending on the activation status of the Ras/MAPK pathway. When Ras/MAPK is activated, as is common in aggressive, late stage disease, TGFβ suppresses PTEN expression through a Smad4-independent pathway [117]. Alternatively, when the Ras/MAPK pathway is blocked, TGFβ induces PTEN expression through its canonical Smad-dependent pathway [118]. The Ras/MAPK pathway also suppresses PTEN levels through the transcription factor c-Jun [119]. Moreover, the MEK–JNK pathway suppresses PTEN transcription via activation of NF-kB, which directly binds to and suppresses the PTEN promoter [120]. Expression of PTEN is also negatively regulated by the epithelial-to-mesenchymal transition (EMT) transcription factor SNAIL, which is itself activated by PI3K/AKT and RAS/MAPK pathways [121–123]. SNAIL competes for binding to the PTEN promoter with p53, which is a transcriptional activator of PTEN and leads to activation of PTEN transcription during p53-mediated apoptosis [96]. Activated NOTCH1 both positively and negatively regulates PTEN expression through MYC and CBF1, respectively [124, 125]. PTEN transcription can also be upregulated through several other transcription factors, including PPARγ [126] and EGR1 [127], as well as downregulated by BMI1 [128], which regulates prostate stem cell self-renewal and malignant transformation [129]. All in all, transcriptional control of PTEN lies within a network of tumor suppressors and oncogenes controlling various signaling and development programs within normal and cancerous prostate cells.
Post-transcriptional Regulation
PTEN mRNA is also post-transcriptionally regulated by PTEN-targeting microRNAs (miRNAs), a class of endogenous 20–25 nucleotide noncoding RNAs that repress mRNA translation through imperfect base pairing between the seed sequence of the miRNA and the complementary seed match sequence in the 3′ untranslated region of the target mRNA [130]. A number of miRNAs have been reported to promote tumorigenesis by downregulating PTEN expression. For example, miR-22 and the miR-106b-25 cluster, both PTEN targeting miRNA loci, are aberrantly overexpressed in human prostate cancer and are capable of initiating prostate tumorigenesis in vitro and in vivo [131]. The identification of these and other prospective PTEN-targeting miRNAs in serum of prostate cancer patients may be valuable as surrogate markers for PTEN status, and hence could correlate with both disease progression and the potential efficacy of PI3K/AKT inhibitor treatment.
In a newly emerging field of research, the PTEN pseudogene 1 (PTENP1) was found to influence PTEN expression through a coding-independent function, uncovering a new mechanism of gene regulation [132]. Since the PTENP1 mRNA transcript shares vast homology with PTEN mRNA, PTENP1 acts as a decoy for PTEN-targeting miRNAs and can thereby sequester and inhibit the negative regulatory effects of miRNAs on PTEN expression [14]. PTENP1 can, therefore, be considered a competing endogenous RNA or ceRNA. Recent research has uncovered a large network of ceRNA transcripts in prostate cancer that can control PTEN expression by blocking the action of PTEN-targeting miRNAs. These discoveries fortify the existence of a large and complex PTEN tumor suppressor network that can be regulated by coding and noncoding RNAs and can be used to explain the observance of partial or incomplete PTEN inactivation in human prostate cancer [133–135].
Post-translational Regulation
PTEN stability is regulated by various post-translational modifications. When inactivated, PTEN is phosphorylated at various serine and threonine residues on its C-terminal tail, which, in turn, increases PTEN stability [136–138]. This C-terminal phosphorylation results in a more stable yet “closed” state of PTEN, which reduces its plasma membrane localization [139] and its ability to form a complex with PDZ domain-containing proteins [138], thereby reducing its PIP3 lipid phosphatase activity [140–142]. As PTEN is activated, dephosphorylation of its C-terminal tail opens its phosphatase domain, increasing PTEN activity and enhancing its interactions with binding partners, but in turn making PTEN increasingly unstable [143]. Also located in the C-terminal tail, Ser370 can be phosphorylated by a downstream effector of SRC, CK2 [144], while Thr366 appears to be phosphorylated by GSK3β [145]; however, the function of phosphorylation at these sites still remains unclear [146]. The targeting of PTEN to the plasma membrane can also be orchestrated through phosphorylation at Ser 229 and Thr321 on its C2 domain by the protein kinase ROCK [147–149]. Tyr336 of PTEN can also be phosphorylated by RAK, which can act as a tumor suppressor in its own right by regulating PTEN stability and function [150]. Future research may unveil other known and unknown kinases that are capable of phosphorylating PTEN and thereby regulate specific PTEN functions.
The open state of PTEN is also more prone to ubiquitin-mediated proteasomal degradation. Lys13 and Lys289 are conserved sites for PTEN ubiquitination, and monoubiquitination is necessary for the movement of PTEN from the cytoplasm to the nucleus [79]. NEDD4-1 is a recently identified E3 ligase of PTEN that induces both PTEN mono- and poly-ubiquitination [151]. However, NEDD4-1 knockout mice contain no differences in the expression level and subcellular localization of PTEN, hinting that other E3 ligases may be involved in the regulation and localization of PTEN [152]. Along these lines, two other E3 ligases, XIAP and WWP2, have been proposed to mediate PTEN ubiquitination [153, 154].
Similar to other phosphatases, the cysteine residues in the bottom of the PTEN catalytic pocket are very sensitive to oxidation [155]. The catalytic activity of PTEN is attenuated by reactive oxygen species (ROS) through the development of a disulphide bond between Cys71 and Cys124 that is induced during oxidative stress [156, 157]. Furthermore, PTEN can also be acetylated at Lys125 through Lys128 by PCAF and at Lys402 by CBP, inhibiting its catalytic activity while facilitating interactions with PDZ domain-containing proteins [158]. Finally, other forms of PTEN redox regulation have been suggested by research demonstrating the inactivation of PTEN through nitrosylation of cysteine residues in its phosphatase domain [159]. Together, these findings highlight the potential to manipulate mechanisms of PTEN post-translational modifications for use as therapeutics to enhance the tumor suppressive functions of PTEN.
Protein–Protein Interactions
A number of PTEN interacting proteins regulate the tumor suppressive abilities of PTEN by altering its conformation, stability, and subcellular localization. PTEN contains a 3 amino acid C-terminal region that binds to PDZ domain-containing proteins, and these PDZ domains are involved in multiprotein complex assembly [137, 160]. Indeed, the PDZ domain of PTEN mediates interactions with NHREF, which binds to and recruits PTEN to PDGFR to inhibit the activation of the PI3K–AKT pathway [161]. The PTEN PDZ-binding domain binds to several other proteins, including MAGI-2 and MAST205, which appear to enhance the stabilization of PTEN [160, 162, 163]. As PTEN can be found in high molecular mass complexes through size-exclusion chromatography, it was hypothesized that the PDZ-binding domain may be required for such complex formation [164]. However, mutagenesis studies demonstrated that neither PTEN’s catalytic activity nor its PDZ binding domain are absolutely required for its complex formation. Instead, PTEN phosphorylation status has a significant role in its complex assembly [165]. Using two-dimensional gel electrophoresis and mass spectrometry analysis, hnRNPC was identified as a novel PTEN-interacting protein [165]. Indeed, PTEN and hnRNPC are colocalized in the nucleus and may be involved in RNA regulation [165, 166].
Additional proteins are capable of binding to other domains on the PTEN protein. PICT-1 interacts with PTEN by binding to and promoting phosphorylation of the C-terminal tail, conferring PTEN stabilization [167]. Through a yeast two-hybrid screen, β-arrestin was identified as a PTEN-binding partner, binding to PTEN’s C2 domain [168]. When PTEN is dephosphorylated at Thr383, this increases the binding affinity of β-arrestin to PTEN, which in turn allows PTEN to negatively regulate cell proliferation through its lipid phosphatase activity, as well as enhance cell migration by reversing the inhibitory effect of the C2 domain [168]. Furthermore, PTEN can directly interact with the regulatory subunit of PI3K, p85, which increases its lipid phosphatase activity and subsequent capability of downregulating the PI3K–AKT pathway [141, 169]. Therefore, p85 can regulate the PI3K/AKT pathway by both negatively regulating PI3K through direct binding to its catalytic subunit, p110, and by positively regulating PTEN activity. Under oxidative stress conditions, DJ-1, which was identified in Drosophila melanogaster, can also directly bind to PTEN, an action that is associated with increased P-AKT levels [170, 171]. Recent screens have identified novel PTEN regulators, including PREX2a [172] and SIPL1 [173], which bind to PTEN directly and inhibit its phosphatase activity against PIP3. MAN2C1 also binds to PTEN and inhibits its function in both prostate cancer cell lines and primary human prostate tumors [174]. Intriguingly, one study found that, of 60 % of primary human prostate tumors that were PTEN-positive, 80 % displayed overexpression of MAN2C1, uncovering a possible new mechanism of PTEN downregulation without genomic loss of PTEN [174]. Despite the discovery of various PTEN protein-binding partners, further investigation is necessary to understand the physiological and clinical relevance of these interactions.
Subcellular Localization
The function of PTEN is also regulated by its subcellular localization. At the plasma membrane, PTEN can regulate directional chemotaxis. PTEN recruitment to the plasma membrane relies on electrostatic interactions with acidic lipids in the membrane, such as phosphatidylserine, PIP2, PIP3, and phosphatidic acid [175], as well as additional protein–protein interactions [143, 176]. PTEN interacts with several membrane-anchored proteins in its dephosphorylated form, including MAGI-2 [160], MAST205 [162], hDLG [138], MVP [177], and PDGFR and NHERF [161], which are thought to be potentially part of a larger PTEN complex, via its C-terminal PDZ domain. NEP has been shown to recruit PTEN to the plasma membrane, which in turn enhances its catalytic activity and subsequently hinders AKT activity [178]. Similarly, the motor protein myosin V regulates the migration of PTEN to the membrane by directly binding to PTEN [179].
PTEN is predominantly localized to the nucleus in differentiated and resting cells in comparison to rapidly cycling cancer cells [72]. The nuclear localization of PTEN is also dependent upon the cell cycle stage, with nuclear PTEN levels highest at the G1 phase and lowest at the S phase [71]. While some studies have shown that PTEN nuclear localization is dependent upon noncanonical nuclear localization sequences on PTEN and major vault protein-mediated nuclear transport [69], others have shown that nuclear localization of PTEN occurs through passive diffusion through the nuclear membrane [180]. It has been further suggested that PTEN contains a type of cytoplasmic localization signal (CLS) in its N-terminal region that, when mutated, induces the nuclear import of PTEN [181]. Other so-called nuclear exclusion motifs and NLS sequences have been identified that control PTEN localization through a RAN-dependent mechanism [182]; however, how they regulate the shuttling of PTEN between the nucleus and the cytoplasm is not understood. More conclusively, PTEN monoubiquitination by the E3 ligase NEDD4-1 induces the nuclear localization of PTEN [151], while the deubiquitinase HAUSP controls PTEN deubiquitination and nuclear exclusion [183]. Oxidative stress induces the accumulation of PTEN in the nucleus, where it associates with p53 to trigger cell cycle arrest and reduce ROS [75]. Nevertheless, mechanisms involved in the nuclear export of PTEN are still waiting to be uncovered. The use of models utilizing PTEN proteins with mutations that disrupt PTEN localization but maintain PTEN phosphatase activity may provide new understandings into the role of nuclear PTEN.
PTEN Loss as a Biomarker for Human Prostate Cancer
Despite recent and past findings firmly establishing loss of PTEN as one of the most common somatic genetic alterations in prostate cancer, prostate cancer specimens are not routinely screened for PTEN loss in the clinical setting. Fluorescence in situ hybridization (FISH) has been used to identify genomic PTEN loss, which is found in 9–23 % of high-grade prostatic intraepithelial neoplasia (PIN) lesions [184, 185] and 10–70 % of prostate cancers [12, 13, 186–190], and is correlated with an overall poor prognosis [26, 74, 190–193]. Loss of PTEN expression in the cytoplasm as well as in the nucleus, as determined by FISH and immunohistochemistry (IHC) analysis, is independently associated with decreased disease-specific survival [74, 194]. Part of the reason for these variations may be due to the subjective nature and tediousness of counting the number of fluorescent signals and positive antibody stains relative to control signals and stains to quantify PTEN expression. FISH analysis also lacks the sensitivity to identify minor mutations/perturbations in the PTEN gene locus, as well as other epigenetic and post-transcriptional changes that may influence PTEN expression [13, 195]. Moreover, current research, through the use of “break apart” FISH technology, has revealed that gross chromosomal rearrangements of the PTEN locus occur in prostate cancer, which could very well explain the absence of PTEN expression in tumors designated as harboring genomic loss of only one PTEN allele using conventional single probe FISH [196].
Despite discrepancies in reported rates of genomic PTEN loss, a general finding of these studies is that loss of one PTEN allele is significantly more frequent than loss of both PTEN alleles, although homozygous deletions are associated with advanced disease and metastasis [197, 198]. Haploinsufficiency for PTEN, as well as inactivation of the second PTEN allele through nongenomic alterations, may explain why heterozygous PTEN deletions outnumber homozygous PTEN deletions in human prostate cancer and also result in poor outcomes [184, 190, 199]. Indeed, nearly 70 % of primary human prostate tumors do not contain inactivation of both copies of PTEN [105]. In terms of disease progression, the frequency of PTEN loss is higher in surgical cohorts enriched for high Gleason grades and aggressive disease stages [192]. PTEN loss is more common in hormone refractory and metastatic prostate cancer than in hormone-dependent primary tumors, with homozygous PTEN loss in 10 and 50 % of hormone-dependent and metastatic/hormone refractory cases, respectively [74, 184, 185, 189–191, 193, 200, 201]. Therefore, PTEN could serve as a prognostic marker for hormone refractory and metastatic disease. PTEN genomic loss is also associated with the TMPRSS2–ERG fusion [26, 185], and recent reports have concluded that these events cooperate to stratify patients with a poorer prognosis in the clinic [192]. A close association between PTEN loss and therapeutic resistance, as demonstrated by a decreased time to biochemical recurrence after radical prostatectomy, adjuvant docetaxel treatment, and radiotherapy, has also been observed [74, 190, 191, 202, 203].
The possibility that FISH and other genomic analyses may fail to detect some cases of PTEN inactivation calls for alternative methods to detect PTEN loss. Considering the role of post-transcriptional and post-translational modifications in PTEN protein expression and subcellular localization as discussed above, quantification of PTEN protein levels using immunohistochemistry (IHC) may be a better indicator of PTEN expression. In a recent study using a rabbit monoclonal antibody against PTEN for IHC analysis, PTEN protein loss was detected in 75–86 % of samples with genomic PTEN loss and was even discovered at times in the absence of genomic PTEN loss [194]. Interestingly, 45 and 37 % of tumors with PTEN protein loss did not show genomic deletions measureable by FISH or SNP microarray analysis, respectively, further suggesting that alternative mechanisms of PTEN inactivation exist beyond the genomic level [194]. Moreover, IHC analysis has correlated PTEN protein loss with high Gleason scores, as well as decreased time to metastasis in a cohort of patients having undergone surgical resection [194]. Other studies using large prostate cancer cohorts combining genomic analysis, through comparative genomic hybridization (CGH) and whole-exome sequencing, and transcriptosome analysis have uncovered frequent alterations of the PI3K/AKT pathway in prostate cancer [26–28], which correlated with 42 and 100 % of human primary and metastatic prostate cancer, respectively, as well as high-risk disease [26]. Using network component analysis, 20 transcription factors have been identified whose activities, as deduced from their target gene expression, are immediately altered upon the re-expression of PTEN in a PTEN-inducible system [204]. Notably, the activity of these transcription factors can be used to predict PTEN functional status in human prostate, breast, and brain tumor samples with increased reliability when compared to basic expression-based analysis [204]. With improved mechanisms for detecting PTEN functional status, PTEN loss could be used not only as a prognostic biomarker for men with prostate cancer but also as a potential predictive marker to identify patients who could benefit from emerging PI3K/AKT pathway therapies.
PTEN in Prostate Cancer Initiation, Progression, CPRC, and Metastasis
Mouse Models of Prostate Cancer
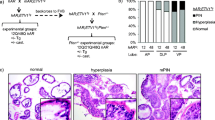
Prostate cancer research has been limited, in part, by the lack of animal models that develop spontaneous prostate tumors in a manner that mimics human prostate cancer. Mouse xenograft models reconstituted from primary human metastatic prostate cancer cells and cell lines have been developed and used extensively in research as preclinical models. However, these xenograft models cannot be used for studying the underlying mechanisms involved in prostate cancer initiation and progression since they are derived from late stage disease. Moreover, many of the key features of the disease, especially the resident tumor microenvironment and the stromal and immune cells that occupy it, are lacking in this immune incompetent system and, therefore, engrafted tumors cannot recapitulate the whole spectrum of human prostate cancer [205]. Genetically engineered mouse (GEM) models of prostate cancer have advanced significantly over the past decade [206–210], and the strong implication of PTEN loss in prostate cancer progression in humans has prompted the expansion of GEM models based on PTEN inactivation. Greater knowledge of the role of PTEN loss as an individual and cooperative agent in prostate cancer development, including initiation, progression and invasion, castration-resistant prostate cancer (CRPC), and metastasis, has been uncovered using mouse models that recapitulate the human disease through genetic loss of the murine homolog of the Pten gene (Fig. 4.1).
Pten knockout mouse models of prostate cancer. Pten heterozygous (Pten +/−) or homozygous (Pten−/−) loss, alone or in combination with other pathway alterations, is able to recapitulate all stages of human prostate cancer, including initiation (PIN), cancer progression/invasion, CRPC, and metastasis. Studies in these murine models provide credence for the use of PI3K/AKT/mTOR, AR, and Ras/MAPK inhibitors for the treatment of metastatic CRPC. In the figure, gray squares represent luminal cells, while green ovals represent basal cells. Cx castration, PIN prostatic intraepithelial neoplasia, LN lymph nodes, Mets metastases, WT wild type, CRPC castration-resistant prostate cancer
PTEN Dosage in Mouse Models of Prostate Cancer
PTEN dosage appears to be an important determinant in the development of many epithelial cancers, as demonstrated in various mouse models of Pten loss [211]. In the prostate, a hypomorphic PTEN allele, which leads to ~20 % reduction of PTEN levels, shows no sign of neoplastic lesions in the prostate epithelium, while conditional or conventional deletion of one Pten allele causes a 50 % reduction of PTEN levels and leads to precancerous PIN lesions but not cancer, indicating that inactivation of one allele of Pten is sufficient to initiate tumorigenesis but not tumor progression [76, 212–216]. Interestingly, by combining a hypomorphic allele with a knockout allele, and thereby reducing PTEN levels by 70–80 %, these mice progress to invasive adenocarcinoma of the prostate [214], indicating that a more profound downregulation of PTEN is needed for cancer progression to occur in the prostate [217]. These findings counter the canonical “two-hit hypothesis” of cancer and suggest that slight variations in PTEN expression, induced through genetic alterations as well as nongenetic changes in PTEN expression, are able to recapitulate varying stages of prostate tumor initiation and progression [218]. Despite the evidence for PTEN haplosufficiency in the mouse, evidence for this in humans still remains to be determined.
Phenotypes Associated with Homozygous Deletion of Pten in the Prostate Epithelium
A number of studies have been performed through the use of conditional mutants with prostate-specific deletion of one or both Pten alleles [211, 213–215, 219–221]. Conditional homozygous Pten deletion (Pten −/−) driven by the PB-Cre4 promoter results in invasive adenocarcinoma in 100 % of mice at 9–12 weeks [215]. Importantly, the Pten −/− prostate cancer model mimics the course of human prostate cancer formation, progressing from hyperplasia to PIN to invasive adenocarcinoma with defined kinetics [215]. Interestingly, homozygous deletion of other tumor suppressors in the murine prostate, including p53 [222], retinoblastoma (Rb) [223], and Nkx3.1 [224], leads to PIN lesions but never an adenocarcinoma phenotype, solidifying the importance of PTEN function in the prostate gland. Moreover, although Pten-null tumors are initially responsive to androgen ablation, eventually mice will develop CRPC, as is commonly seen in human prostate cancer [215]. Pten homozygous deletion driven by other promoters in the mouse, including PSA Cre, MMTV Cre, and Nkx3.1 CreERT2, also results in the development of invasive adenocarcinoma, albeit over a longer latency [225–228].
Compound Pten Knockout Transgenic Mouse Models of Prostate Cancer
Pten Loss Combined with Alterations in Other Tumor Suppressors
Several studies carried out with compound transgenic mice have shown that monoallelic or biallelic deletion of tumor suppressor genes such as Nkx3.1 [229, 230], p27 KIP1 [231], and p53 [105] can cooperate with Pten loss in promoting prostate cancer (Fig. 4.1). While loss of a single allele of Nkx3.1 [224, 232] and p27 KIP1 [233], both of which occurrences have been implicated in advanced stage prostate cancer and poor disease-free survival in humans [234, 235], is sufficient to promote prostate cancer initiation and PIN lesions, concomitant loss of Pten is needed to promote prostate tumorigenesis and cancer progression [211, 230, 231, 236]. Moreover, while the TRAMP mouse model alone, which contains inactivation of the p53 and Rb tumor suppressor genes through expression of the large/small SV40 tumor T antigens under the probasin promoter, is capable of inducing the development of aggressive prostate tumors [209], loss of heterozygosity of Pten in TRAMP mice demonstrated an increased rate of tumor development, with a subsequent decrease in overall survival from 245 days to 159 days [237]. In the same way, conditional ablation of one or two alleles of p53 leads to the development of PIN lesions, while Pten −/−;p53 −/− double mutants exhibit invasive prostate cancer as early as 2 weeks after puberty that is invariably lethal by 7 months of age [105, 238]. Also, deletion of Smad4, a tumor suppressor known to regulate the TGF-β signaling pathway, cooperates with Pten deletion in the prostate to enhance tumor cell proliferation and drive invasion to produce fully penetrant prostate cancer and metastases to the lymph nodes and lungs [239]. Finally, combining Pten and p53 loss with loss of Smad4 or reactivation of murine telomerase (mTert) produces prostate cancer metastases in the bone [240], indicating that additional pathway alterations are necessary to drive prostate tumor cells to form metastases in the microenvironment of the bone, an important feature of human prostate cancer.
Pten Loss Combined with Alterations in Oncogenes and Oncogenic Signaling Pathways
Activation of oncogenes and oncogenic signaling pathways cooperates with PTEN loss to promote invasive prostate cancer. In prostate cancer, the ERG gene is frequently translocated to the TMPRSS2 promoter region, with the resulting TMPRSS2–ERG fusion protein expressed in 50 % of human prostate cancer specimens [241–243]. Whereas mice expressing TMPRSS2-ERGa under the control of the ARR2Pb promoter only develop PIN lesions [242], this translocation collaborates with Pten haplosufficiency to cause invasive adenocarcinoma of the prostate [244]. Similarly, cooperation between FGF8b overexpression and Pten haplosufficiency in a murine model leads to adenocarcinoma of the prostate, as well as lymph node metastases, in comparison to FGF8b overexpression alone, which leads to only hyperplastic and PIN lesions [245]. The 8q24 chromosomal region comprising the MYC oncogene is somatically amplified in a cohort of advanced human prostate tumors [246]. While mice engineered to express high levels of human c-Myc in the prostate (PB-Cre4 Myc hi) develop invasive adenocarcinomas with 100 % penetrance [247], focal expression of c-Myc specifically in luminal epithelial cells of the prostate of mice (PB-Cre4 Z-Myc) results in only a mild pathology [248]. However, when combined with deletion of Pten, PB-Cre4 Z-Myc mice develop high-grade PIN and prostate cancer [248]. Although further investigation is needed to fully understand the synergistic effect of c-Myc activation and Pten loss in prostate cancer, evidence from this study and others suggests that loss of Pten may have differential effects depending on the cell types and regions/lobes/zones of the prostate where genetic deletion occurs. With the advent of cell type specific promoters in the prostate, future murine models will be able to tease out the effects of PTEN loss in specific cells in the prostate. For now, these models confirm that concomitant loss of Pten and genetic activation of oncogenes such as ERG, FGF8b, and Myc accelerate initiation and progression in human prostate cancer (Fig. 4.1).
Pten Loss Combined with Alterations in Inflammatory Pathway Regulators
Various lines of evidence suggest that chronic inflammation is linked to prostate tumorigenesis [249–251]. Indeed, expression of specific cytokines can be used as a prognostic indicator of biochemical recurrence in human prostate cancer [252]. One of the most prevalent inflammatory mediators clearly implicated in prostate cancer is IL-6, a cytokine that has not only been associated with tumor growth, proliferation, and angiogenesis in many cancers [253], but whose high levels in the circulating plasma of prostate cancer patients have also been correlated with advanced stages of the disease, therapeutic resistance, and an overall poor prognosis [254]. Although the foremost effect of IL-6 is activation of the JAK/STAT3 pathway [255], the PI3K/AKT pathway can also directly activate and phosphorylate STAT3 at Ser727 [256], which induces metastatic behavior of prostate cancer cells both in vitro and in vivo through stimulation of angiogenesis and suppression of antitumor immune responses [257]. While transgenic mice that constitutively express Stat3 under the control of the ARR2Pb promoter develop only PIN lesions, when crossed with Pten +/− mice, the subsequent compound mutants develop invasive prostate tumors [258].
Many inflammatory cytokines and chemokines promote tumor progression by converging on and stimulating the IKK2/NF-κB signaling axis [259]. The main function of IKK2 is the phosphorylation of IκB molecules, which act as inhibitors of NF-κB, thus rendering them subject to degradation and allowing NF-κB to remain activated. Constitutive activation of the transcription factor NF-κB in prostate cancer has been correlated with disease progression [260], and inhibition of NF-κB activity in prostate cancer cells can suppress angiogenesis and subsequent tumor invasion and metastasis by downregulating expression of downstream NF-κB targets such as VEGF and MMP9 [261]. Interestingly, while a mouse model containing a constitutively active version of IKK2 alone is insufficient in promoting prostate tumorigenesis, in combination with heterozygous loss of Pten, IKK2 activation leads to an increase in tumor size, accompanied by increased inflammation [262]. These studies demonstrate that inflammatory cytokines secreted from the stromal microenvironment of the prostate cooperate with PTEN loss to drive epithelial prostate tumor cells toward invasive disease.
PTEN and Tumor Cell Migration and Invasion
As demonstrated in various models of conditional Pten deletion in the prostate, homozygous Pten loss leads to progression from PIN lesions to invasive adenocarcinoma, a process that requires disruption of the basement membrane and junctional integrity in epithelial acinar structures to allow the invasion of tumor cells through the surrounding basement membrane and into the stromal microenvironment (Fig. 4.1). PTEN and PIP3 play conserved roles in the determination of cell polarity in diverse cell types. From data first obtained in Dictyostelium discoideum [263–266], a unicellular amoeba, and later from neutrophils undergoing chemotaxis [267, 268], enrichment of PIP3 at the leading edge of migrating cells and localization of PTEN in the lateral and trailing edges of the cell has been observed. The PI3K pathway also promotes membrane ruffling, cell motility, and cellular spreading through downstream effectors such as RHO, RAC1, and CDC42 [269]. Consequently, forced expression of PTEN in tumor cell lines inhibits tumor cell invasiveness in vitro and in xenografts in vivo through both phosphatase-dependent [110, 270] and phosphatase-independent [102] mechanisms. In normal glandular development, PTEN concentrates to the apical plasma membrane during epithelial morphogenesis, where it catalyzes the conversion PIP3 into PIP2, which recruits ANX2, CDC42, and aPKC to the membrane to establish cellular polarity [271]. In this regard, loss of PTEN expression may block the development of the apical surface and lumen of epithelial structures. Therefore, activation of the PI3K/AKT pathway upon PTEN loss may lead to the loss of epithelial features, and thereby increase the likelihood of cells developing the properties of increased motility and invasive capacity through an epithelial-to-mesenchymal transition (EMT) [128]. In all, these findings raise the possibility that the considerable increase in the PTEN mutation/deletion rate in metastatic tumors might result from a selective metastatic advantage acquired through the loss of PTEN regulation of motility and invasion.
Pten Loss in Metastatic Prostate Cancer Mouse Models
It is clear from these models that Pten LOH is required for cancer progression and invasive adenocarcinoma development. Although biallelic Pten deletion, alone or in combination with homozygous deletion of p53 [105, 238], Nkx3.1 [230], or Smad4 [239] or activation of FGF8b [245], does lead to the occurrence of small micrometastases in the lymph nodes and lungs, it fails to produce significant metastatic burden, particularly in the bone [215]. Therefore, other genetic alterations and signaling pathway abnormalities must collaborate with activation of the PI3K/AKT pathway to promote metastatic prostate cancer to the bone.
Although Ras mutations [272–274] and Ras fusion events [275] in prostate cancer are uncommon, strong evidence suggests that Ras/MAPK activation plays a substantial role in human prostate cancer progression, particularly in metastasis and CRPC development. Indeed, the Ras/Raf/MAPK pathway has been recently shown to be altered in 43 and 90 % of primary and metastatic lesions, respectively [26]. P-MAPK levels, as assessed in tumor microarrays (TMAs) from human prostate cancer samples, are significantly elevated in neo-adjuvant treated, recurrent, and CRPC patients as compared to benign prostatic hyperplasia (BPH) specimens, corresponding with a significant reduction in PTEN expression [122]. These findings have prompted the development of two recent murine models of prostate cancer that combine homozygous Pten loss with activation of the Ras/Raf/MAPK pathway: the PbCre;Pten L/L ;Kras G12D/+ model [122], and the Nkx3.1 CE2/+ ;Pten f/f ;Braf CA/+ model [276]. In both models, activation of the MAPK pathway through either Braf or Kras conditional overexpression resulted in overt macrometastases to the lymph nodes, lungs, liver, and, importantly, the bone marrow, in around 30 % [276] and 100 % [122] of cases, respectively. In the PbCre;Pten L/L ;Kras G12D/+ model, treatment with a MEK inhibitor alone was able to fully ablate metastatic spread to the lungs and other distant organs, implicating the RAS/RAF/MAPK pathway as a driver of metastasis in Pten-deficient prostate cancer [122]. Interestingly, an EMT phenotype is also observed at the primary tumor site in the PbCre;Pten L/L ;Kras G12D/+ model [122]. As EMT has been postulated to play a critical role in the process of metastasis [277], this new model provides a unique opportunity to study the impact of EMT in prostate cancer metastasis in vivo in the context of Pten loss and Ras/MAPK activation. With these novel metastatic models of prostate cancer, a better understanding of the contribution of PTEN to the metastatic cascade, including localized invasion, intravasation into the blood stream, survival as circulating tumor cells (CTCs), extravasation out of the blood stream, and metastatic seeding to distant organ sites, can be further uncovered. Overall, past and present murine models of prostate cancer induced by Pten loss have demonstrated that loss of PTEN, to varying degrees and in combination with other genetic alterations, can recapitulate the entire spectrum of prostate cancer, from initiation (heterozygous Pten loss), through progression (homozygous Pten loss), and, finally, to metastasis (homozygous Pten loss and Ras/MAPK activation) (Fig. 4.1).
PTEN and CRPC
Androgens are indispensable for prostatic glandular development and homeostasis and contribute to prostate cancer development through activation of the androgen receptor (AR). Androgen deprivation therapy remains the most common treatment for advanced prostate cancer. However, therapeutic effects are short lived, and patients usually succumb to CRPC within 18–24 months, leaving the disease essentially untreatable [278]. New generation androgen deprivation therapies (ADTs), such as abiraterone [279] and MDV3100 [280], that more effectively ablate androgen production and AR signaling, are rapidly being developed and approved for patients with metastatic CRPC. Similar to human prostate cancers, while castration initially results in massive apoptosis of the prostate epithelium in the Pten-null murine model of prostate cancer, the ki67 proliferation index remains constant, indicating that a select population of cells remains resistant to androgen withdrawal [215].
AR is expressed in CRPC and may function through autocrine signaling or crosstalk with other prosurvival and proliferation pathways [281, 282], including the PI3K/AKT pathway, which has been shown to induce AR expression in the absence of PTEN [283–285]. Multiple studies have found an association between the loss of PTEN and the development of CRPC [201, 215, 286, 287]. Moreover, loss of PTEN and AR expression has been correlated clinically with increased mortality in CRPC patients [193]. aCGH analysis on metastatic prostate cancer samples has also demonstrated frequent amplification of AR (73 %), coinciding with aberrant deletion of PTEN (87 %) [288].
While some studies have proposed that PTEN deletion activates AR through PI3K-mediated stabilization of AR protein levels or AKT-mediated phosphorylation and activation of the AR [231, 289, 290], other reports have revealed that PI3K/AKT pathway stimulation promotes degradation of AR and inhibits AR transcriptional activity [291]. Supporting the later claim, levels of AR are heterogeneous, and, in many cases, absent in late stage, metastatic disease [292–295]. These observations raise the possibility that loss of AR expression and activity may serve as a means of evading androgen withdrawal through simultaneous activation of other signaling pathways. Indeed, two independent laboratories have recently demonstrated that PTEN loss inhibits androgen-responsive gene expression by regulating AR activity [296, 297], indicating that castration-resistant growth is an intrinsic property of Pten null prostate cancer cells regardless of cancer stage [296]. These studies further suggest a reciprocal feedback loop that exists between AR and PTEN in prostate cancer, in which conditional deletion of Ar in the prostate epithelium promotes the proliferation of Pten-deficient cancer cells in PbCre;Pten L/L ;Ar L/Y mice through the downregulation of the androgen-responsive gene Fkbp5 and preventing PHLPP-mediated AKT inhibition [296]. Moreover, inhibition of the PI3K/AKT pathway was shown to upregulate the receptor tyrosine kinase HER3 [297]. As suppression of HER2/HER3 heterodimers has been linked to inhibition of AR transcriptional activity through an AKT-independent mechanism [298], it is plausible that PI3K/AKT inhibition upregulates AR transcriptional activity by increasing HER3 expression.
In all, it is probable that AR suppresses the PI3K/AKT pathway in order to promote differentiation of the prostate epithelium and keep prostate cancer cells sensitive to androgens. When AR activity is downregulated upon ADT treatment, the PI3K/AKT pathway takes over to promote cell proliferation and cell survival in the absence of androgen or AR activity, further driving tumor progression toward metastatic CRPC [296, 297]. These findings may explain why clinical trials that inhibit the activation of the PI3K/AKT signaling axis, as well as its downstream effector mTOR, failed to have a substantial effect on tumor progression in men [299, 300], as inhibition of the PI3K/AKT/mTOR pathway causes an upregulation of AR transcriptional activity that promotes cell survival [297]. Since, in the background of PTEN-deficient prostate cancer, AKT regulates proliferation, while AR regulates survival, inhibition of both signaling pathways is necessary for effective tumor reduction. Indeed, combined therapy targeting both PI3K and AR pathways reduces tumor growth in Pten-null mice [296, 297], suggesting the possible efficacy of combined PI3K/AKT and AR inhibitor treatment in the clinic (Fig. 4.1).
PI3K/AKT/mTOR Pathway Inhibition as a Treatment for Prostate Cancer
Current Prostate Cancer Treatment
Treatment resistance is a major issue in the management of prostate cancer, as it is estimated that 30,000 men in the USA will die in 2012 alone from metastatic and CRPC, for which there is currently no cure. Although androgen-deprivation therapy (ADT) remains the standard treatment of metastatic prostate cancer, progression to castration-resistant disease occurs in the majority of patients [301–303]. Among available therapeutic approaches for the treatment of CRPC, conventional chemotherapy with docetaxel and other agents has limited efficacy and has yet to produce long-term benefits [304, 305]. Although agents that specifically inhibit the AR, androgen synthesis, and/or AR-regulated pathways, such as MDV3100 and Abiraterone, have recently entered the clinic and have shown promising results [279, 280], their therapeutic effects are short lived, and patients eventually develop CRPC [278]. Another novel therapy, sipuleucel-T, which is the first ever FDA-approved therapeutic cancer vaccine for the treatment of metastatic prostate cancer, also only modestly improves the survival of late-stage patients by a few months [306, 307].
The current trend in medicine has been to exercise a personalized treatment approach that is based on molecular and genetic profiling of individual patients to determine the best therapeutic strategy. A considerable number of novel therapeutics are presently undergoing clinical trials, including small molecules that target common genetic or pathway alterations found in human cancers. These inhibitors have been FDA approved for treatment of various solid tumors including, renal, GIST, breast, pancreatic, colorectal, and NSCLC cancer [308–314], and thus hold promise for the treatment of prostate cancer. As it is clear that PI3K/AKT/mTOR pathway activation plays a prominent role in prostate cancer initiation and progression, CRPC, and metastatic disease, the loss of PTEN expression in individuals with prostate cancer could be used as a biomarker to stratify populations of patients that may benefit from treatment with PI3K/AKT/mTOR pathway inhibitors.
PI3K Inhibitors
Class I PI3Ks are heterodimers composed of one catalytic subunit of p110α, p110β, or p110δ, collectively known as p110, and a regulatory subunit, p85. PI3K isoform selectivity may be essential to boost therapeutic efficacy and minimize off-target toxicity. Recent research suggests a dominant role for the PI3K isoform p110β in PTEN-deficient tumors. In the Pten-null prostate cancer model, loss of p110β, but not p110α, decreased PI3K signaling and prevented prostate carcinogenesis [94]. In a similar fashion, inducible depletion of p110β, but not p110α, using shRNA in PTEN-deficient human cancer cell lines quenches PI3K-mediated signaling and inhibits growth both in vitro and in vivo [315].
The most studied PI3K inhibitors to date are the first-generation PI3K inhibitors LY294002 and wortmannin. LY294002 treatment results in cell-cycle arrest and sensitizes the LNCaP prostate cancer cell line to radiation therapy, decreases the invasive properties of LNCaP, PC-3, and DU145 prostate cancer cell lines, and inhibits angiogenesis in PC-3 prostate cancer cells by way of decreased levels of HIF1-α and VEGF. Similarly, wortmannin induces apoptosis and radiosensitizes DU145 cells [316, 317]. However, both wortmannin and LY294004 show limited selectivity for individual PI3K isoforms, nonspecifically target multiple other signaling molecules [318–321], and demonstrate significant toxicity in animals [317, 322], limiting their effectiveness in vivo.
One potential consequence and side effect of PI3K pathway inhibition is the development of insulin resistance in patients. While both p110α and p110β play specific roles in insulin signaling, research suggests that glucose homeostasis is predominantly mediated by p110α [94, 323]. Indeed, p110α inhibitors, but not p110β or p110δ inhibitors, alter insulin-dependent glucose regulation in mice [323]. Thus, in the setting of PTEN-deficient tumors, p110β-specific inhibitors, in contrast to pan-PI3K inhibitors, may offer enhanced efficacy with a reduced likelihood of insulin resistance. Together, these studies suggest that effective treatment of PTEN-deficient prostate tumors may necessitate the use of therapeutic agents that successfully target p110β. However, even in cancers that may be specifically reliant on either p110α or p110β, there remains the possibility that other, noninhibited p110 isoforms may make up for the decreased activity of the targeted isoform. Moreover, not all tumors that are driven by PTEN loss are dependent on p110β, and the presence of other genetic modifications and pathway alterations is likely to change the PI3K-isoform reliance of these tumors. Interestingly, PTEN loss appears to be a predictive marker for sensitivity to PX-866, an oral derivative of wortmannin, despite the fact that PX-866 displays a high efficacy against p110α and p110δ but not p110β [324]. Therefore, although PI3K signaling is an obvious target for therapy, especially in PTEN-deficient prostate cancer, given the redundancy and complex feedback regulation existing in the PI3K/AKT/mTOR pathway, as well as a need for a more in-depth understanding of the pathway, the clinical efficacy of using PI3K inhibitors as single agents is modest (Fig. 4.2).
PI3K/AKT/mTOR, Ras/MAPK, and AR signaling pathways converge to promote prostate cancer development. Although all three pathways promote cell proliferation/growth, AR signaling maintains prostate cells in a differentiated state, while PI3K/AKT/mTOR and Ras/MAPK signaling promotes EMT and cell migration/invasion. Red, blue, and green ovals represent AR, PI3K/AKT/mTOR, and Ras/MAPK signaling molecules, respectively. Orange ovals denote adaptor molecules. Pathway activators are in black letters, and pathway suppressors are in white letters. Solid black lines depict signaling within a pathway, and dotted black lines depict crosstalk between pathways or feedback loops. Red lines denote the drug targets. Signaling molecules in these pathways that are the targets of drug inhibitors are in red letters
AKT Inhibitors
The significance of the individual AKT isoforms in prostate cancer has yet to be fully uncovered, despite findings that AKT-1 isoform expression may be a prognostic marker for biochemical recurrence in patients with prostate cancer [325]. There are several classes of AKT inhibitors currently in development, including isoform-selective AKT catalytic–domain inhibitors and inhibitors of its PH domain, and many have been tested in prostate cancer. Perifosine, an alkylphospholipid that targets the PH domain of AKT and prevents it from binding to PIP3, decreases AKT phosphorylation, inhibits growth, and induces cell-cycle arrest of PC-3 cells [326]. Although there are no published preclinical studies investigating perifosine activity against prostate cancer, perifosine has gone to clinical trials for patients with CRPC. Though generally well tolerated, perifosine showed no evidence of significant inhibitory activity [300, 327]. Genistein, a non-specific AKT inhibitor, causes significant growth inhibition and apoptosis of cancer cells [328, 329]. While genistein has demonstrated significant potential in vivo, decreasing the incidence of lung metastasis in an orthotopic model using PC-3 cells [330] and inhibiting tumor growth when combined with docetaxel in an experimental model of bone metastasis [331], another report claimed that genistein increased the size of metastatic lymph nodes in a PC-3 orthotopic model [326]. Concomitant targeting of AKT-1 and AKT-2 with ATP-competitive inhibitors, such as GSK690693, has been shown to be more effective than inhibition of single isoforms for the induction of apoptosis in tumor cells, suggesting that these Pan-AKT inhibitors are likely to have more promise in the clinic, although increased toxicity may be a potential issue [332]. However, AKT inhibitors will not block the non-AKT effectors downstream of PI3K signaling. Paradoxically, AKT inhibitors could increase upstream receptor tyrosine kinase activities by alleviating downstream negative feedback loops [333]. Therefore, the importance of AKT-independent effectors of PI3K signaling and downstream negative feedback loops in the pathway might considerably affect the clinical effectiveness of AKT inhibitors (Fig. 4.2).
mTOR Inhibitors
mTOR inhibitors have been the most effective among the inhibitors of the PI3K/AKT/mTOR pathway in treating solid tumors and have received the most consideration in the treatment of prostate cancer. Rapamycin, the prototypical mTOR inhibitor, associates with its intracellular receptor, FKBP12, which then binds directly to mTORC1 and suppresses mTOR-mediated phosphorylation of its downstream effectors, S6K and 4EBP1. Rapamycin induces cell-cycle arrest in PC-3 and DU145 prostate cancer cell lines in vitro [334–337] and reduces tumor volume and blocks growth and proliferation in tumors with activated AKT or loss of PTEN in vivo [338, 339]. Although limited, there have been reports on in vitro and preclinical studies demonstrating the efficacy of the rapamycin analogs (rapalogs) CCI-779 and RAD001 in the treatment of prostate cancer. CCI-779 inhibits growth of PC-3 and DU145 cells in vitro, and, in vivo, and reduces tumor volumes in PC-3 and DU145 xenografts [340]. Likewise, RAD001 treatment decreases proliferation of prostate cancer cells in vitro [341, 342] and reverses PIN lesions in AKT-1 transgenic mice [343].
Despite these preclinical findings, mTORC1 inhibitors, including rapamycin and rapalogs, have demonstrated little success as single agent treatments in the clinic [299, 344, 345]. Although rapamycin and rapalogs are effective at inhibiting mTORC1 kinase activity, inhibition of mTORC1 eventually leads to AKT activation and increased P-AKT levels due to the loss of the S6K to IRS-1 feedback loop and reactivation of PI3K signaling [41, 345, 346]. Moreover, while mTORC1 is sensitive to rapamycin treatment, mTORC2 is generally considered to be resistant to rapamycin. In this regard, mTORC2 phosphorylation and activation of AKT may further limit the efficacy of mTORC1 inhibitors like rapamycin [38]. Therefore, the use of rapamycin and rapalogues as single treatments could potentially cause the hyperactivation of the PI3K/AKT pathway (Fig. 4.2).
To achieve a significant clinical effect, mTORC1 inhibition with rapamycin and rapalogs may require the addition of upstream inhibitors, such as insulin-like growth factor signaling or PI3K signaling inhibitors [347–350], or, alternatively, more effective inhibition of both TORC1 and TORC2 activity. mTOR catalytic site inhibitors, which are currently in clinical development, target the kinase domain of mTOR and have the advantage of blocking the activity of both mTORC1 and mTORC2. The additional inhibition of mTORC2 provides the benefit of blocking AKT activation through S473 phosphorylation, and therefore, these catalytic site inhibitors would be expected to inhibit the mTOR pathway more effectively than rapamycin. Current research has described torkinibs and torin1, two selective ATP-competitive inhibitors of mTOR that impede cellular growth and proliferation more effectively than rapamycin [351, 352]. Interestingly, however, the enhanced activity of these mTOR kinase inhibitors seems to be due to more complete inhibition of mTORC1 activity rather than mTORC2 inhibition, as measured by decreased levels of 4EBP1 phosphorylation and cap-dependent translation compared to rapamycin treatment [351, 352]. In support of the efficacy of these mTOR catalytic site inhibitors, a recent preclinical study using the mTOR catalytic site inhibitor INK218 in the Pten-null murine prostate cancer model demonstrated that INK218 is able to inhibit AKT and 4E-BP1 phosphorylation in addition to S6K1 phosphorylation, lead to a 50 % decrease in PIN lesions, reduce overall tumor volume, and promote tumor cell apoptosis, as opposed to RAD0001 treatment, which results in inhibition of S6K1 but not AKT and 4E-BP1 phosphorylation, only partial regression of PIN lesions, and no significant effect on tumor cell apoptosis [353]. Remarkably, treatment with INK218 blocks progression of invasive prostate cancer locally in the prostate and even inhibits the total number and size of distant metastases [353]. Although new generation mTOR catalytic site inhibitors have the capacity to reduce prostate tumor invasion and metastasis by more effectively disabling mTORC1 signaling and inhibiting mTORC2 activation, treatment with these inhibitors alone does not inhibit PI3K activity, and, therefore, would need to be combined with other PI3K antagonists to fully ablate distant metastasis and lead to complete tumor regression (Fig. 4.2).
Dual PI3K–mTOR Inhibitors
The use of multiple inhibitors to target the PI3K/AKT/mTOR pathway may be of particular importance to alleviate the issue of negative-feedback loops in the pathway. As the catalytic domains of the p110 subunits and mTOR are similar in structure, there are a number of small molecule inhibitors currently being tested that can block both PI3K and mTOR. Compared to other PI3K pathway inhibitors, dual PI3K–mTOR inhibitors, which include NVP-BEZ235, BGT-226, XL765, SF1126, PKI-402, and PKI587, have the possible advantage of inhibiting all PI3K isoforms, as well as both mTORC1 and mTORC2. Therefore, these inhibitors should effectively turn off the PI3K/AKT/mTOR pathway completely and overcome feedback inhibition normally observed with mTORC1 inhibitors such as rapamycin and other rapalogs [347]. BEZ235 is capable of simultaneously inhibiting multiple class I PI3K isoforms and mTOR kinase activity by binding to their respective catalytic sites [349]. BEZ235, unlike PI3K inhibitors alone, is able to lower levels of both P-S6 and P-AKT, demonstrating that dual inhibition of both mTOR and PI3K is capable of preventing an increase in P-AKT levels [349, 350]. BEZ235 exhibits greater antiproliferative effects compared with rapamycin treatment in cancer cell lines in vitro and slows tumor growth and vasculature development in PTEN-deficient cell line engrafted mice, where it is well tolerated with no significant changes in body weight [349, 350, 354]. In preclinical studies, SF1126, a conjugate of LY294002, reduces cell growth, proliferation, and angiogenesis, and exhibits lower toxicity than LY294002 [355]. Furthermore, PKI-587, another dual PI3K/mTOR inhibitor, induces tumor regression in several cancer cell line xenograft models, and has a favorable drug safety profile in toxicology studies [356]. Importantly, in contrast to PI3K inhibitors that cause cytostatic effects through tumor cell G0–G1 arrest [357–359], PKI-587 inhibition of PI3K and mTOR can fully ablate AKT activation and cause the induction of apoptosis, the preferred outcome against tumor cells [356]. Despite these preclinical findings, a major issue with dual PI3K/mTOR inhibitors is their efficacies in vivo in the clinical settings.
Combination Therapy with PI3K/AKT/mTOR and Ras/MAPK Inhibitors
One explanation behind the limited success of PI3K/AKT/mTOR pathway inhibition in the clinic is that blockade of PI3K signaling may shift the tumor survival signaling to a Ras/MAPK-dependent pathway [360]. Analyzes of human prostate cancer microarrays have demonstrated that the PI3K/AKT and Ras/MAPK pathways are often coordinately dysregulated during prostate cancer progression in humans [122, 361]. Although BEZ235 is effective against PI3K-driven tumors as a single agent, the inhibitor responds poorly to tumors harboring Kras mutations [350]. Indeed, BEZ235 is only effective against Kras-driven tumors when combined with a MEK inhibitor [362]. Humans with advanced prostate cancer treated with RAD001 experience increased activation of MAPK signaling, probably due to the loss of the S6K-IRS1 feedback loop that leads to Ras activation [360] (Fig. 4.2). In addition, neoadjuvant hormone therapy can lead to increased P-MAPK activation and N-cadherin expression, both of which have been implicated in the induction of the EMT program and metastatic prostate cancer [122, 363]. Ras activation can also play a direct role in moving prostate cancer cell lines toward decreased androgen dependence [364]. Indeed, PI3K/AKT and Braf/ERK pathway activation acts combinatorially in a mouse model of CRPC [365]. These studies suggest the importance for combined PI3K/AKT and Ras/MAPK pathway blockade in the treatment of CRPC and metastatic prostate cancer.
A number of studies conducted with Pten knockout mice have shown that combined pharmacological targeting of mTOR and MEK may lead to reduced primary prostate tumor progression [122, 361]. Combination therapy using the mTORC1 inhibitor rapamycin and the MEK inhibitor PD0325901 inhibits not only growth in prostate cancer cell lines [361] but also reduces tumor burden in castrated, androgen-insensitive prostate tumors in the Nkx3.1 −/−;Pten +/− murine model [365]. Dual mTOR and MEK inhibition also completely ablates the dissemination of distant metastases in the PbCre;Pten L/L ;Kras G12D/+ murine prostate cancer model, which exhibits 100 % penetrable macrometastasis [122], as well as reduces tumor and metastatic burden in Nkx3.1 Cre-ER ;Pten f/f ;Braf CA/+ mice [276]. Thus, in late stage, metastatic prostate cancer, dual PI3K/AKT and Ras/MAPK inhibition may be necessary to reduce metastasis, as well as slow primary tumor growth (Fig. 4.1).
Combination Therapy with PI3K/AKT/mTOR and AR Pathway Inhibitors
Recent studies using the Pten-null murine model of prostate cancer have demonstrated a reciprocal feedback loop that exists between AR and PI3K pathways in the prostate cancer, whereby inhibition of the PI3K pathway in Pten-deficient prostate cancer results in reactivation of AR signaling by modulating AR corepressor activities or through feedback signaling to the receptor tyrosine kinase HER2/HER3 [296, 297]. Therefore, the efficacy of PI3K inhibitors for the treatment of PTEN-deficient prostate cancer may be improved through combined AR pathway inhibition. Another recent study utilizing surgical castration in Pten-null mice to model CRPC demonstrated that dual targeting of both AKT and mTOR with inhibitors MK-2206 and MK-8869, respectively, is highly effective at inhibiting CRPC in vivo [366]. Moreover, the AR agonist MDV3100, which has shown promise in the clinic, has improved efficacy in combination with BEZ235 [350], a dual inhibitor of PI3K and mTORC1/2, in castration-resistant GEM mice [297]. Other laboratories have also documented beneficial effects of combined AR and mTORC1 inhibition with rapamycin in Pten−/− models [296, 367]. Thus, more effective inhibition of the AR signaling axis with new generation inhibitors such as abiraterone and MDV3100 in combination with mTOR or PI3K/mTOR dual inhibitors may prove to be more beneficial in treating CRPC patients displaying alterations in PI3K/AKT pathway signaling (Fig. 4.2).
In all, although dual PI3K/mTOR inhibitors now offer the advantage of complete PI3K/AKT/mTOR pathway inhibition, with signaling feedback loops present in the PI3K/AKT/mTOR pathway that negatively control both Ras/MAPK and AR signaling, it is likely that PI3K inhibition alone will not be able to achieve full regression of tumors in patients with prostate tumors driven by PTEN loss. A better understanding of pathway dynamics gained from recent preclinical studies prompts the rationale for combining inhibition of the PI3K/AKT/mTOR pathway with inhibition of either the Ras/MAPK or AR signaling pathways for the treatment of metastatic CRPC. However, better surrogate biomarkers that predict patient responses to PI3K inhibitors, as well as more high-throughput systems to molecularly profile and detect PTEN loss or PI3K/AKT/mTOR activation in patients, will be needed to accurately assess the efficacy of PI3K/AKT/mTOR inhibition as a treatment in individual patients.
Conclusions and Perspectives
In the 15 years since the discovery of PTEN as a frequently mutated gene in cancer, great progress in understanding the function and regulation of PTEN has been made. While PTEN was first identified as a lipid phosphatase with tumor suppressive activity against the PI3K/AKT pathway, recent studies have revealed that PTEN has additional protein phosphatase and lipid phosphatase-independent activities, as well as functions in the nucleus. Further understanding of the mechanisms behind PTEN post-transcriptional regulation, post-translational modifications, and protein–protein interactions offers novel therapeutic opportunities, as well as explanations of why, clinically, loss of PTEN expression can occur without genetic deletion or mutations at the PTEN locus. With improved methods for detecting PTEN status, such as CGH, whole-exome sequencing, and transcriptome analysis, PTEN loss can more readily and more accurately be used as a prognostic biomarker for men with prostate cancer, as well as a potential predictive marker to identify patients who could benefit from emerging PTEN/PI3K/Akt pathway therapies. Moreover, studies with large human prostate cancer cohorts have revealed that alterations in the PI3K/AKT/mTOR pathway are more common in advanced, metastatic prostate cancer and CRPC compared to primary, androgen-dependent tumors and are associated with a poorer overall prognosis and increased chance of biochemical recurrence. Recent works using mouse models with varying degrees of Pten loss have helped to reveal the role of PTEN dosage in prostate tumorigenesis and the collaborative effects of PTEN loss and other genetic and pathway alterations in prostate cancer initiation, progression, and metastasis. A better understanding of the interactions between the PI3K/AKT pathway and Ras/MAPK, p53, and TGF-β–Smad pathways has facilitated the development of metastatic models of prostate cancer with bone metastasis potential, an important feature of human prostate cancer. As bone tropism of prostate cancer metastasis is not well understood, these mouse models should provide better insights into the cell types and molecular pathways involved in metastasis to the bone. Better systems, via lineage-tracing and cell type-specific deletion, are needed to address which cell types are responsible for different stages of the disease, including prostate cancer progression, castration resistance, and metastasis. As previous studies have suggested that prostate luminal, transit amplifying (TA), and basal cells can serve as a cell-of-origin and as cancer stem cell (CSC) populations in prostate cancer [368], it will be important for future models to employ more restrictive prostate-specific promoters allowing the potential for tumor initiation from basal, TA, neuroendocrine, and luminal cell types. Two recent reports have also elucidated a reciprocal feedback loop between the PI3K/AKT and AR signaling pathways that directly regulates CRPC, offering an explanation for how loss of androgen dependence may further strengthen PI3K/AKT signaling in PTEN-deficient prostate cancer, as well as a rationale for the combined use of AR signaling and PI3K/AKT/mTOR inhibitors in the treatment of CRPC.
Although beyond the scope of this review, emerging research in other solid tumors has demonstrated that the tumor microenvironment itself may play a defined role in tumor propagation and progression, and it will be interesting to see if aberrations in the PI3K/AKT/mTOR signaling in stromal-specific subtypes themselves in the prostate may contribute to tumorigenesis. Moreover, PTEN alterations in the tumor epithelium, which have been demonstrated to induce the release of paracrine signals, including chemokines and cytokines that may attract immune cell types to the prostate and contribute to the development of a tumor-permissive rather than a tumor-suppressive microenvironment, suggest that immunotherapy may be a possible treatment for prostate cancer patients. Again, the specific stromal cells and immune cells that contribute to the prostate tumor microenvironment will need to be further pursued with the use of lineage-specific promoters and tracking systems in immune competent models that preserve the tumor’s native microenvironment.
Finally, while past clinical trials using rapamycin and rapalogues to treat human prostate cancer have shown little efficacy, due in part to an inability to inhibit PI3K and AKT signaling, dual PI3K/mTOR inhibitors have the capacity to completely inhibit all strands of the PI3K/AKT/mTOR pathway, and thus deserve further study in preclinical models of prostate cancer. However, with signaling feedback loops present in the PI3K/AKT/mTOR pathway that negatively control both Ras/MAPK and AR signaling, it is unlikely that PI3K inhibition alone will be able to achieve full regression of PTEN-deficient prostate tumors. Further understanding of pathway dynamics gained from recent preclinical studies prompts the rationale for combining inhibition of the PI3K/AKT/mTOR pathway with inhibition of either the Ras/MAPK or AR signaling pathways for the treatment of metastatic CRPC. In the end, though much progress has been made in understanding the role PTEN and its regulation of the PI3KAKT/mTOR pathway in prostate cancer, in the future, more basic and preclinical mechanistic studies that further elucidate the complexity of the PI3K/AKT/mTOR signaling pathway and can be translated from bench to bedside will help to design better treatment options for patients with metastatic CRPC, for which there is still no cure.
References
Bigner SH, Mark J, Mahaley MS, Bigner DD (1984) Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas 101:103–113
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R (1997) PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–1947
Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV (1997) Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 15:356–362
Li DM, Sun H (1997) TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 57:2124–2129
Gustafson S, Zbuk KM, Scacheri C, Eng C (2007) Cowden syndrome. Semin Oncol 34:428–434
Blumenthal GM, Dennis PA (2008) PTEN hamartoma tumor syndromes. Eur J Hum Genet 16:1289–1300
Lloyd KM II, Dennis M (1963) Cowden's disease. A possible new symptom complex with multiple system involvement. Ann Intern Med 58:136–142
Dahia PL, Marsh DJ, Zheng Z, Zedenius J, Komminoth P, Frisk T, Wallin G, Parsons R, Longy M, Larsson C, Eng C (1997) Somatic deletions and mutations in the Cowden disease gene, PTEN, in sporadic thyroid tumors. Cancer Res 57:4710–4713
Halachmi N, Halachmi S, Evron E, Cairns P, Okami K, Saji M, Westra WH, Zeiger MA, Jen J, Sidransky D (1998) Somatic mutations of the PTEN tumor suppressor gene in sporadic follicular thyroid tumors. Genes Chromosomes Cancer 23:239–243
Xie CC, Lu L, Sun J, Zheng SL, Isaacs WB, Gronberg H, Xu J (2011) Germ-line sequence variants of PTEN do not have an important role in hereditary and non-hereditary prostate cancer susceptibility. J Hum Genet 56:496–502
Cooney KA, Tsou HC, Petty EM, Miesfeldt S, Ping XL, Gruener AC, Peacocke M (1999) Absence of PTEN germ-line mutations in men with a potential inherited predisposition to prostate cancer. Clin Cancer Res 5:1387–1391
Feilotter HE, Nagai MA, Boag AH, Eng C, Mulligan LM (1998) Analysis of PTEN and the 10q23 region in primary prostate carcinomas. Oncogene 16:1743–1748
Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG, Jen J, Isaacs WB, Bova GS, Sidransky D (1997) Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 57:4997–5000
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13:283–296
Visvader JE (2011) Cells of origin in cancer. Nature 469:314–322
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768
Mulholland DJ, Wu H. Genetic and signaling pathway regulations of tumor-initiating cells of the prostate. In S. Cramer (ed.), Stem Cells and Prostate Cancer, DOI 10.1007/978-1-4614-6498-3_5 © Springer Science+Business Media, LLC 2013
Denu JM, Stuckey JA, Saper MA, Dixon JE (1996) Form and function in protein dephosphorylation. Cell 87:361–364
Lee JO, Yang H, Georgescu MM, Di Cristofano A, Maehama T, Shi Y, Dixon JE, Pandolfi P, Pavletich NP (1999) Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323–334
Redfern RE, Redfern D, Furgason ML, Munson M, Ross AH, Gericke A (2008) PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry 47:2162–2171
Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, Eng C, Parsons R (1997) Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet 16:64–67
Tonks NK, Cicirelli MF, Diltz CD, Krebs EG, Fischer EH (1990) Effect of microinjection of a low-Mr human placenta protein tyrosine phosphatase on induction of meiotic cell division in Xenopus oocytes. Mol Cell Biol 10:458–463
Maehama T, Dixon JE (1998) The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273:13375–13378
Waite KA, Eng C (2002) Protean PTEN: form and function. Am J Hum Genet 70:829–844
Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H (2000) Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res 60:7033–7038
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18:11–22
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, Tomlins SA (2012) The mutational landscape of lethal castration-resistant prostate cancer. Nature 487:239–243
Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae SS, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, Macdonald TY, Sheikh K, Vuong T, Guiducci C, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Hussain WM, Ramos AH, Winckler W, Redman MC, Ardlie K, Tewari AK, Mosquera JM, Rupp N, Wild PJ, Moch H, Morrissey C, Nelson PS, Kantoff PW, Gabriel SB, Golub TR, Meyerson M, Lander ES, Getz G, Rubin MA, Garraway LA (2012) Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 44:685–689
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW (1998) Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29–39
Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H (1999) PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 96:6199–6204
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129:1261–1274
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, Buttyan R, Chopin D (2003) Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res 9:1801–1807
Lee HK, Kwak HY, Hur J, Kim IA, Yang JS, Park MW, Yu J, Jeong S (2007) beta-catenin regulates multiple steps of RNA metabolism as revealed by the RNA aptamer in colon cancer cells. Cancer Res 67:9315–9321
Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M (2002) Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res 62:6141–6145
Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL (1998) The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA 95:15587–15591
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Vander Haar E, Lee S, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9:316–323
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318
Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205
Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166:213–223
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332:1317–1322
Yu G, Xiao CL, Lu CH, Jia HT, Ge F, Wang W, Yin XF, Jia HL, He JX, He QY (2011) Phosphoproteome profile of human lung cancer cell line A549. Mol Biosyst 7:472–479
Warburg O (1956) On respiratory impairment in cancer cells. Science 124:269–270
Tong X, Zhao F, Thompson CB (2009) The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev 19:32–37
Stewart GD, Gray K, Pennington CJ, Edwards DR, Riddick AC, Ross JA, Habib FK (2008) Analysis of hypoxia-associated gene expression in prostate cancer: lysyl oxidase and glucose transporter-1 expression correlate with Gleason score. Oncol Rep 20:1561–1567
Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A (1999) Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem 274:33085–33091
Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE (2005) Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab 2:263–272
Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK (2001) Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ 12:363–369
Gordan JD, Simon MC (2007) Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev 17:71–77
Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B (1998) Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J 17:5085–5094
Majumder PK, Sellers WR (2005) Akt-regulated pathways in prostate cancer. Oncogene 24:7465–7474
Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ (2000) Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 14:391–396
Sundqvist A, Bengoechea-Alonso MT, Ye X, Lukiyanchuk V, Jin J, Harper JW, Ericsson J (2005) Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab 1:379–391
Li X, Monks B, Ge Q, Birnbaum MJ (2007) Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447:1012–1016
Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH (1997) Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem 272:17269–17275
Ros S, Santos CR, Moco S, Baenke F, Kelly G, Howell M, Zamboni N, Schulze A (2012) Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov 2:328–343
Plathow C, Weber WA (2008) Tumor cell metabolism imaging. J Nucl Med 49(Suppl 2):43S–63S
Jadvar H (2009) Molecular imaging of prostate cancer with 18 F-fluorodeoxyglucose PET. Nat Rev Urol 6:317–323
Oyama N, Akino H, Kanamaru H, Suzuki Y, Muramoto S, Yonekura Y, Sadato N, Yamamoto K, Okada K (2002) 11C-acetate PET imaging of prostate cancer. J Nucl Med 43:181–186
Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H (2004) Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci USA 101:2082–2087
Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, Naito M, Enomoto K, Watanabe S, Mak TW, Nakano T (2004) Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 113:1774–1783
Van de Sande T, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV (2002) Role of the phosphatidylinositol 3'-kinase/PTEN/Akt kinase pathway in the overexpression of fatty acid synthase in LNCaP prostate cancer cells. Cancer Res 62:642–646
Li JN, Gorospe M, Chrest FJ, Kumaravel TS, Evans MK, Han WF, Pizer ES (2001) Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res 61:1493–1499
Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, Carracedo A, Vander Heiden MG, Cantley LC, Pinton P, Haigis MC, Pandolfi PP (2012) Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149:49–62
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452:230–233
Telang S, Yalcin A, Clem AL, Bucala R, Lane AN, Eaton JW, Chesney J (2006) Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene 25:7225–7234
Chung JH, Ginn-Pease ME, Eng C (2005) Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) has nuclear localization signal-like sequences for nuclear import mediated by major vault protein. Cancer Res 65:4108–4116
Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, Gil E, Hinze R, Delbridge L, Lees JA, Mutter GL, Robinson BG, Komminoth P, Dralle H, Eng C (2000) Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156:1693–1700
Ginn-Pease ME, Eng C (2003) Increased nuclear phosphatase and tensin homologue deleted on chromosome 10 is associated with G0-G1 in MCF-7 cells. Cancer Res 63:282–286
Lachyankar MB, Sultana N, Schonhoff CM, Mitra P, Poluha W, Lambert S, Quesenberry PJ, Litofsky NS, Recht LD, Nabi R, Miller SJ, Ohta S, Neel BG, Ross AH (2000) A role for nuclear PTEN in neuronal differentiation. J Neurosci 20:1404–1413
Perren A, Weng LP, Boag AH, Ziebold U, Thakore K, Dahia PL, Komminoth P, Lees JA, Mulligan LM, Mutter GL, Eng C (1999) Immunohistochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. Am J Pathol 155:1253–1260
McCall P, Witton CJ, Grimsley S, Nielsen KV, Edwards J (2008) Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br J Cancer 99:1296–1301
Chang CJ, Mulholland DJ, Valamehr B, Mosessian S, Sellers WR, Wu H (2008) PTEN nuclear localization is regulated by oxidative stress and mediates p53-dependent tumor suppression. Mol Cell Biol 28:3281–3289
Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H (2003) PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3:117–130
Li Y, Guessous F, Kwon S, Kumar M, Ibidapo O, Fuller L, Johnson E, Lal B, Hussaini I, Bao Y, Laterra J, Schiff D, Abounader R (2008) PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res 68:1723–1731
Lindsay Y, McCoull D, Davidson L, Leslie NR, Fairservice A, Gray A, Lucocq J, Downes CP (2006) Localization of agonist-sensitive PtdIns(3,4,5)P3 reveals a nuclear pool that is insensitive to PTEN expression. J Cell Sci 119:5160–5168
Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP (2007) Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell 128:141–156
Weng LP, Brown JL, Eng C (2001) PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet 10:599–604
Chung JH, Ostrowski MC, Romigh T, Minaguchi T, Waite KA, Eng C (2006) The ERK1/2 pathway modulates nuclear PTEN-mediated cell cycle arrest by cyclin D1 transcriptional regulation. Hum Mol Genet 15:2553–2559
Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y (2007) Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128:157–170
Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, Piwnica-Worms H, Hibshoosh H, Parsons R (2005) Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7:193–204
Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A (2009) Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med 1:315–322
McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S (2010) PTEN loss compromises homologous recombination repair in astrocytes: implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res 70:5457–5464
Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A, Lord CJ, Ashworth A, Reis-Filho JS (2010) PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2:53ra75
Fraser M, Zhao H, Luoto KR, Lundin C, Coackley C, Chan N, Joshua AM, Bismar TA, Evans A, Helleday T, Bristow RG (2012) PTEN deletion in prostate cancer cells does not associate with loss of RAD51 function: implications for radiotherapy and chemotherapy. Clin Cancer Res 18:1015–1027
Hu Z, Gu Y, Han B, Zhang J, Li Z, Tian K, Young CY, Yuan H (2012) Knockdown of AGR2 induces cellular senescence in prostate cancer cells. Carcinogenesis 33:1178–1186
Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP (2011) Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell 144:187–199
Liu XS, Song B, Elzey BD, Ratliff TL, Konieczny SF, Cheng L, Ahmad N, Liu X (2011) Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation. J Biol Chem 286:35795–35800
Blanco-Aparicio C, Renner O, Leal JF, Carnero A (2007) PTEN, more than the AKT pathway. Carcinogenesis 28:1379–1386
Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, Loda M, Sellers WR (2003) Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Natl Acad Sci USA 100:7841–7846
Lee SH, Poulogiannis G, Pyne S, Jia S, Zou L, Signoretti S, Loda M, Cantley LC, Roberts TM (2010) A constitutively activated form of the p110beta isoform of PI3-kinase induces prostatic intraepithelial neoplasia in mice. Proc Natl Acad Sci USA 107:11002–11007
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454:776–779
Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, Mullholland DJ, Magnuson MA, Wu H, Sabatini DM (2009) mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell 15:148–159
Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW (2001) Regulation of PTEN transcription by p53. Mol Cell 8:317–325
Chang CJ, Freeman DJ, Wu H (2004) PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem 279:29841–29848
Mayo LD, Donner DB (2001) A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA 98:11598–11603
Mayo LD, Dixon JE, Durden DL, Tonks NK, Donner DB (2002) PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem 277:5484–5489
Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, Rosivatz E, Woscholski R, Cognetti F, Scher HI, Pandolfi PP (2010) A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 120:681–693
Nardella C, Carracedo A, Alimonti A, Hobbs RM, Clohessy JG, Chen Z, Egia A, Fornari A, Fiorentino M, Loda M, Kozma SC, Thomas G, Cordon-Cardo C, Pandolfi PP (2009) Differential requirement of mTOR in postmitotic tissues and tumorigenesis. Sci Signal 2:ra2
Raftopoulou M, Etienne-Manneville S, Self A, Nicholls S, Hall A (2004) Regulation of cell migration by the C2 domain of the tumor suppressor PTEN. Science 303:1179–1181
Zhou M, Gu L, Findley HW, Jiang R, Woods WG (2003) PTEN reverses MDM2-mediated chemotherapy resistance by interacting with p53 in acute lymphoblastic leukemia cells. Cancer Res 63:6357–6362
Cully M, You H, Levine AJ, Mak TW (2006) Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 6:184–192
Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, Cordon-Cardo C, Pandolfi PP (2005) Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436:725–730
Lei Q, Jiao J, Xin L, Chang CJ, Wang S, Gao J, Gleave ME, Witte ON, Liu X, Wu H (2006) NKX3.1 stabilizes p53, inhibits AKT activation, and blocks prostate cancer initiation caused by PTEN loss. Cancer Cell 9:367–378
Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL (2007) Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell 11:555–569
Hubner A, Mulholland DJ, Standen CL, Karasarides M, Cavanagh-Kyros J, Barrett T, Chi H, Greiner DL, Tournier C, Sawyers CL, Flavell RA, Wu H, Davis RJ (2012) JNK and PTEN cooperatively control the development of invasive adenocarcinoma of the prostate. Proc Natl Acad Sci USA 109:12046–12051
Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK (1997) P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA 94:9052–9057
Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM (1998) Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280:1614–1617
Gu T, Zhang Z, Wang J, Guo J, Shen WH, Yin Y (2011) CREB is a novel nuclear target of PTEN phosphatase. Cancer Res 71:2821–2825
Mounir Z, Krishnamoorthy JL, Robertson GP, Scheuner D, Kaufman RJ, Georgescu MM, Koromilas AE (2009) Tumor suppression by PTEN requires the activation of the PKR-eIF2alpha phosphorylation pathway. Sci Signal 2:ra85
Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, Sahin AA, Esteva FJ, Hortobagyi GN, Yu D (2011) Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med 17:461–469
Sansal I, Sellers WR (2004) The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 22:2954–2963
Whang YE, Wu X, Suzuki H, Reiter RE, Tran C, Vessella RL, Said JW, Isaacs WB, Sawyers CL (1998) Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA 95:5246–5250
Lu J, Jeong HW, Kong N, Yang Y, Carroll J, Luo HR, Silberstein LE, Yupoma, Chai L (2009) Stem cell factor SALL4 represses the transcriptions of PTEN and SALL1 through an epigenetic repressor complex. PLoS One 4:e5577
Mukhopadhyay NK, Cinar B, Mukhopadhyay L, Lutchman M, Ferdinand AS, Kim J, Chung LW, Adam RM, Ray SK, Leiter AB, Richie JP, Liu BC, Freeman MR (2007) The zinc finger protein ras-responsive element binding protein-1 is a coregulator of the androgen receptor: implications for the role of the Ras pathway in enhancing androgenic signaling in prostate cancer. Mol Endocrinol 21:2056–2070
Chow JY, Quach KT, Cabrera BL, Cabral JA, Beck SE, Carethers JM (2007) RAS/ERK modulates TGFbeta-regulated PTEN expression in human pancreatic adenocarcinoma cells. Carcinogenesis 28:2321–2327
Hettinger K, Vikhanskaya F, Poh MK, Lee MK, de Belle I, Zhang JT, Reddy SA, Sabapathy K (2007) c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ 14:218–229
Xia D, Srinivas H, Ahn YH, Sethi G, Sheng X, Yung WK, Xia Q, Chiao PJ, Kim H, Brown PH (2007) Wistuba, II, B.B. Aggarwal, J.M. Kurie, Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF kappa B-dependent pathway. J Biol Chem 282:3507–3519
Escriva M, Peiro S, Herranz N, Villagrasa P, Dave N, Montserrat-Sentis B, Murray SA, Franci C, Gridley T, Virtanen I, Garcia de Herreros A (2008) Repression of PTEN phosphatase by Snail1 transcriptional factor during gamma radiation-induced apoptosis. Mol Cell Biol 28:1528–1540
Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H (2012) Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 72:1878–1889
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Palmero I, Pantoja C, Serrano M (1998) p19ARF links the tumour suppressor p53 to Ras. Nature 395:125–126
Whelan JT, Forbes SL, Bertrand FE (2007) CBF-1 (RBP-J kappa) binds to the PTEN promoter and regulates PTEN gene expression. Cell Cycle 6:80–84
Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH (2001) Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol 11:764–768
Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, de Belle I (2001) The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol 3:1124–1128
Song LB, Li J, Liao WT, Feng Y, Yu CP, Hu LJ, Kong QL, Xu LH, Zhang X, Liu WL, Li MZ, Zhang L, Kang TB, Fu LW, Huang WL, Xia YF, Tsao SW, Li M, Band V, Band H, Shi QH, Zeng YX, Zeng MS (2009) The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 119:3626–3636
Lukacs RU, Memarzadeh S, Wu H, Witte ON (2010) Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 7:682–693
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Poliseno L, Salmena L, Riccardi L, Fornari A, Song MS, Hobbs RM, Sportoletti P, Varmeh S, Egia A, Fedele G, Rameh L, Loda M, Pandolfi PP (2010) Identification of the miR-106b 25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal 3:ra29
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP (2010) A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465:1033–1038
Karreth FA, Tay Y, Perna D, Ala U, Tan SM, Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, Krauthammer M, Halaban R, Provero P, Adams DJ, Tuveson DA, Pandolfi PP (2011) In vivo identification of tumor- suppressive PTEN ceRNAs in an oncogenic BRAF-induced mouse model of melanoma. Cell 147:382–395
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer A, Llobet-Navas D, Rajbhandari P, Bansal M, Guarnieri P, Silva J, Califano A (2011) An extensive microRNA-mediated network of RNA-RNA interactions regulates established oncogenic pathways in glioblastoma. Cell 147:370–381
Tay Y, Kats L, Salmena L, Weiss D, Tan SM, Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, Lieberman J, Rigoutsos I, Pandolfi PP (2011) Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147:344–357
Torres J, Pulido R (2001) The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem 276:993–998
Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H (1999) The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA 96:10182–10187
Vazquez F, Grossman SR, Takahashi Y, Rokas MV, Nakamura N, Sellers WR (2001) Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem 276:48627–48630
Das S, Dixon JE, Cho W (2003) Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 100:7491–7496
Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG (2002) Direct identification of PTEN phosphorylation sites. FEBS Lett 528:145–153
Rabinovsky R, Pochanard P, McNear C, Brachmann SM, Duke-Cohan JS, Garraway LA, Sellers WR (2009) p85 Associates with unphosphorylated PTEN and the PTEN-associated complex. Mol Cell Biol 29:5377–5388
Odriozola L, Singh G, Hoang T, Chan AM (2007) Regulation of PTEN activity by its carboxyl-terminal autoinhibitory domain. J Biol Chem 282:23306–23315
Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382:1–11
Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT, Mills GB, Hortobagyi GN, Mendelsohn J, Hung MC, Fan Z (2010) Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 18:423–435
Maccario H, Perera NM, Davidson L, Downes CP, Leslie NR (2007) PTEN is destabilized by phosphorylation on Thr366. Biochem J 405:439–444
Al-Khouri AM, Ma Y, Togo SH, Williams S, Mustelin T (2005) Cooperative phosphorylation of the tumor suppressor phosphatase and tensin homologue (PTEN) by casein kinases and glycogen synthase kinase 3beta. J Biol Chem 280:35195–35202
Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D (2005) Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7:399–404
Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, Hla T (2005) PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA 102:4312–4317
Papakonstanti EA, Ridley AJ, Vanhaesebroeck B (2007) The p110delta isoform of PI 3-kinase negatively controls RhoA and PTEN. EMBO J 26:3050–3061
Yim EK, Peng G, Dai H, Hu R, Li K, Lu Y, Mills GB, Meric-Bernstam F, Hennessy BT, Craven RJ, Lin SY (2009) Rak functions as a tumor suppressor by regulating PTEN protein stability and function. Cancer Cell 15:304–314
Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X (2007) NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128:129–139
Fouladkou F, Landry T, Kawabe H, Neeb A, Lu C, Brose N, Stambolic V, Rotin D (2008) The ubiquitin ligase Nedd4-1 is dispensable for the regulation of PTEN stability and localization. Proc Natl Acad Sci USA 105:8585–8590
Van Themsche C, Leblanc V, Parent S, Asselin E (2009) X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem 284:20462–20466
Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen J (2011) WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol 13:728–733
Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP (2008) Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene 27:5464–5476
Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem 277:20336–20342
Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT (2008) PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest 118:3762–3774
Okumura K, Mendoza M, Bachoo RM, DePinho RA, Cavenee WK, Furnari FB (2006) PCAF modulates PTEN activity. J Biol Chem 281:26562–26568
Yu CX, Li S, Whorton AR (2005) Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol 68:847–854
Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan XJ, Wood J, Ross C, Sawyers CL, Whang YE (2000) Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA 97:4233–4238
Takahashi Y, Morales FC, Kreimann EL, Georgescu MM (2006) PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 25:910–920
Valiente M, Andres-Pons A, Gomar B, Torres J, Gil A, Tapparel C, Antonarakis SE, Pulido R (2005) Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem 280:28936–28943
Subauste MC, Nalbant P, Adamson ED, Hahn KM (2005) Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein beta-catenin with the scaffolding protein MAGI-2. J Biol Chem 280:5676–5681
Vazquez F, Ramaswamy S, Nakamura N, Sellers WR (2000) Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol 20:5010–5018
Mosessian S, Avliyakulov NK, Mulholland DJ, Boontheung P, Loo JA, Wu H (2009) Analysis of PTEN complex assembly and identification of heterogeneous nuclear ribonucleoprotein C as a component of the PTEN-associated complex. J Biol Chem 284:30159–30166
Mosessian S, Wu H (2010) PTEN-associated complexes: an overview. Curr Top Biochem Res 12:37–42
Okahara F, Ikawa H, Kanaho Y, Maehama T (2004) Regulation of PTEN phosphorylation and stability by a tumor suppressor candidate protein. J Biol Chem 279:45300–45303
Lima-Fernandes E, Enslen H, Camand E, Kotelevets L, Boularan C, Achour L, Benmerah A, Gibson LC, Baillie GS, Pitcher JA, Chastre E, Etienne-Manneville S, Marullo S, Scott MG (2011) Distinct functional outputs of PTEN signalling are controlled by dynamic association with beta-arrestins. EMBO J 30:2557–2568
Chagpar RB, Links PH, Pastor MC, Furber LA, Hawrysh AD, Chamberlain MD, Anderson DH (2010) Direct positive regulation of PTEN by the p85 subunit of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 107:5471–5476
Kim YC, Kitaura H, Taira T, Iguchi-Ariga SM, Ariga H (2009) Oxidation of DJ-1-dependent cell transformation through direct binding of DJ-1 to PTEN. Int J Oncol 35:1331–1341
Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, Binari RC, Manoukian AS, Bray MR, Liu FF, Tsao MS, Mak TW (2005) DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell 7:263–273
Fine B, Hodakoski C, Koujak S, Su T, Saal LH, Maurer M, Hopkins B, Keniry M, Sulis ML, Mense S, Hibshoosh H, Parsons R (2009) Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science 325:1261–1265
He L, Ingram A, Rybak AP, Tang D (2010) Shank-interacting protein-like 1 promotes tumorigenesis via PTEN inhibition in human tumor cells. J Clin Invest 120:2094–2108
He L, Fan C, Kapoor A, Ingram AJ, Rybak AP, Austin RC, Dickhout J, Cutz JC, Scholey J, Tang D (2011) alpha-Mannosidase 2C1 attenuates PTEN function in prostate cancer cells. Nat Commun 2:307
Yeung T, Grinstein S (2007) Lipid signaling and the modulation of surface charge during phagocytosis. Immunol Rev 219:17–36
Vazquez F, Matsuoka S, Sellers WR, Yanagida T, Ueda M, Devreotes PN (2006) Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc Natl Acad Sci USA 103:3633–3638
Yu Z, Fotouhi-Ardakani N, Wu L, Maoui M, Wang S, Banville D, Shen SH (2002) PTEN associates with the vault particles in HeLa cells. J Biol Chem 277:40247–40252
Sumitomo M, Iwase A, Zheng R, Navarro D, Kaminetzky D, Shen R, Georgescu MM, Nanus DM (2004) Synergy in tumor suppression by direct interaction of neutral endopeptidase with PTEN. Cancer Cell 5:67–78
van Diepen MT, Parsons M, Downes CP, Leslie NR, Hindges R, Eickholt BJ (2009) MyosinV controls PTEN function and neuronal cell size. Nat Cell Biol 11:1191–1196
Liu F, Wagner S, Campbell RB, Nickerson JA, Schiffer CA, Ross AH (2005) PTEN enters the nucleus by diffusion. J Cell Biochem 96:221–234
Denning G, Jean-Joseph B, Prince C, Durden DL, Vogt PK (2007) A short N-terminal sequence of PTEN controls cytoplasmic localization and is required for suppression of cell growth. Oncogene 26:3930–3940
Gil A, Andres-Pons A, Fernandez E, Valiente M, Torres J, Cervera J, Pulido R (2006) Nuclear localization of PTEN by a Ran-dependent mechanism enhances apoptosis: Involvement of an N-terminal nuclear localization domain and multiple nuclear exclusion motifs. Mol Biol Cell 17:4002–4013
Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP (2008) The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 455:813–817
Yoshimoto M, Cutz JC, Nuin PA, Joshua AM, Bayani J, Evans AJ, Zielenska M, Squire JA (2006) Interphase FISH analysis of PTEN in histologic sections shows genomic deletions in 68 % of primary prostate cancer and 23 % of high-grade prostatic intra-epithelial neoplasias. Cancer Genet Cytogenet 169:128–137
Han B, Mehra R, Lonigro RJ, Wang L, Suleman K, Menon A, Palanisamy N, Tomlins SA, Chinnaiyan AM, Shah RB (2009) Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol 22:1083–1093
Suzuki H, Freije D, Nusskern DR, Okami K, Cairns P, Sidransky D, Isaacs WB, Bova GS (1998) Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res 58:204–209
Wang SI, Parsons R, Ittmann M (1998) Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res 4:811–815
McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR (1999) Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 59:4291–4296
Verhagen PCMS, van Duijn PW, Hermans KGL, Looljenga LHJ, van Gurp RJHLM, Stoop H, van der Kwast TH, Trapman J (2006) The PTEN gene in locally progressive prostate cancer is preferentially inactivated by bi-allelic gene deletion. J Pathol 208:699–707
Yoshimoto M, Cunha IW, Coudry RA, Fonseca FP, Torres CH, Soares FA, Squire JA (2007) FISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcome. Br J Cancer 97:678–685
Yoshimoto M, Joshua AM, Cunha IW, Coudry RA, Fonseca FP, Ludkovski O, Zielenska M, Soares FA, Squire JA (2008) Absence of TMPRSS2:ERG fusions and PTEN losses in prostate cancer is associated with a favorable outcome. Mod Pathol 21:1451–1460
Reid AH, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, Clark J, Flohr P, Edwards S, Berney DM, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter VE, Scardino PT, Cuzick J, de Bono JS, Cooper CS (2010) Molecular characterisation of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. Br J Cancer 102:678–684
Sircar K, Yoshimoto M, Monzon FA, Koumakpayi IH, Katz RL, Khanna A, Alvarez K, Chen G, Darnel AD, Aprikian AG, Saad F, Bismar TA, Squire JA (2009) PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J Pathol 218:505–513
Lotan TL, Gurel B, Sutcliffe S, Esopi D, Liu W, Xu J, Hicks JL, Park BH, Humphreys E, Partin AW, Han M, Netto GJ, Isaacs WB, De Marzo AM (2011) PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 17:6563–6573
Dong JT, Sipe TW, Hyytinen ER, Li CL, Heise C, McClintock DE, Grant CD, Chung LW, Frierson HF Jr (1998) PTEN/MMAC1 is infrequently mutated in pT2 and pT3 carcinomas of the prostate. Oncogene 17:1979–1982
Reid AH, Attard G, Brewer D, Miranda S, Riisnaes R, Clark J, Hylands L, Merson S, Vergis R, Jameson C, Hoyer S, Sorenson KD, Borre M, Jones C, de Bono JS, Cooper CS (2012) Novel, gross chromosomal alterations involving PTEN cooperate with allelic loss in prostate cancer. Mod Pathol 25:902–910
Di Cristofano A, Pandolfi PP (2000) The multiple roles of PTEN in tumor suppression. Cell 100:387–390
Abate-Shen C, Shen MM (2000) Molecular genetics of prostate cancer. Genes Dev 14:2410–2434
Verhagen PC, van Duijn PW, Hermans KG, Looijenga LH, van Gurp RJ, Stoop H, van der Kwast TH, Trapman J (2006) The PTEN gene in locally progressive prostate cancer is preferentially inactivated by bi-allelic gene deletion. J Pathol 208:699–707
Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N (2007) Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer 120:1284–1292
Mulholland DJ, Dedhar S, Wu H, Nelson CC (2006) PTEN and GSK3beta: key regulators of progression to androgen-independent prostate cancer. Oncogene 25:329–337
Zafarana G, Ishkanian AS, Malloff CA, Locke JA, Sykes J, Thoms J, Lam WL, Squire JA, Yoshimoto M, Ramnarine VR, Meng A, Ahmed O, Jurisca I, Milosevic M, Pintilie M, van der Kwast T, Bristow RG (2012) Copy number alterations of c-MYC and PTEN are prognostic factors for relapse after prostate cancer radiotherapy. Cancer 118:4053–4062
Antonarakis ES, Keizman D, Zhang Z, Gurel B, Lotan TL, Hicks JL, Fedor HL, Carducci MA, De Marzo AM, Eisenberger MA (2012) An immunohistochemical signature comprising PTEN, MYC, and Ki67 predicts progression in prostate cancer patients receiving adjuvant docetaxel after prostatectomy. Cancer 118(24):6063–6071
Tran LM, Chang CJ, Plaisier S, Wu S, Dang J, Mischel PS, Liao JC, Graeber TG, Wu H (2012) Determining PTEN functional status by network component deduced transcription factor activities. PLoS One 7:e31053
De Velasco MA, Uemura H (2012) Preclinical remodeling of human prostate cancer through the PTEN/AKT pathway. Adv Urol 2012:419348
Jeet V, Russell PJ, Khatri A (2010) Modeling prostate cancer: a perspective on transgenic mouse models. Cancer Metastasis Rev 29:123–142
van Weerden WM, Bangma C, de Wit R (2009) Human xenograft models as useful tools to assess the potential of novel therapeutics in prostate cancer. Br J Cancer 100:13–18
van Weerden WM, Romijn JC (2000) Use of nude mouse xenograft models in prostate cancer research. Prostate 43:263–271
Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM (1995) Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA 92:3439–3443
Mimeault M, Batra SK (2011) Animal models relevant to human prostate carcinogenesis underlining the critical implication of prostatic stem/progenitor cells. Biochim Biophys Acta 1816:25–37
Di Cristofano A, De Acetis M, Koff A, Cordon-Cardo C, Pandolfi PP (2001) Pten and p27KIP1 cooperate in prostate cancer tumor suppression in the mouse. Nat Genet 27:222–224
Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP (1998) Pten is essential for embryonic development and tumour suppression. Nat Genet 19:348–355
Podsypanina K, Ellenson LH, Nemes A, Gu J, Tamura M, Yamada KM, Cordon-Cardo C, Catoretti G, Fisher PE, Parsons R (1999) Mutation of Pten/Mmac1 in mice causes neoplasia in multiple organ systems. Proc Natl Acad Sci USA 96:1563–1568
Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, Khoo AS, Roy-Burman P, Greenberg NM, Van Dyke T, Cordon-Cardo C, Pandolfi PP (2003) Pten dose dictates cancer progression in the prostate. PLoS Biol 1:E59
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H (2003) Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 4:209–221
Freeman D, Lesche R, Kertesz N, Wang S, Li G, Gao J, Groszer M, Martinez-Diaz H, Rozengurt N, Thomas G, Liu X, Wu H (2006) Genetic background controls tumor development in PTEN-deficient mice. Cancer Res 66:6492–6496
Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A, Salmena L, Sampieri K, Haveman WJ, Brogi E, Richardson AL, Zhang J, Pandolfi PP (2010) Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42:454–458
Knudson AG Jr (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68:820–823
Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H (2006) Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci USA 103:1480–1485
Luchman HA, Benediktsson H, Villemaire ML, Peterson AC, Jirik FR (2008) The pace of prostatic intraepithelial neoplasia development is determined by the timing of Pten tumor suppressor gene excision. PLoS One 3:e3940
Blando J, Portis M, Benavides F, Alexander A, Mills G, Dave B, Conti CJ, Kim J, Walker CL (2009) PTEN deficiency is fully penetrant for prostate adenocarcinoma in C57BL/6 mice via mTOR-dependent growth. Am J Pathol 174:1869–1879
Elgavish A, Wood PA, Pinkert CA, Eltoum IE, Cartee T, Wilbanks J, Mentor-Marcel R, Tian L, Scroggins SE (2004) Transgenic mouse with human mutant p53 expression in the prostate epithelium. Prostate 61:26–34
Maddison LA, Sutherland BW, Barrios RJ, Greenberg NM (2004) Conditional deletion of Rb causes early stage prostate cancer. Cancer Res 64:6018–6025
Abdulkadir SA, Magee JA, Peters TJ, Kaleem Z, Naughton CK, Humphrey PA, Milbrandt J (2002) Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 22:1495–1503
Ma X, Ziel-van der Made AC, Autar B, van der Korput HA, Vermeij M, van Duijn P, Cleutjens KB, de Krijger R, Krimpenfort P, Berns A, van der Kwast TH, Trapman J (2005) Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res 65:5730–5739
Backman SA, Ghazarian D, So K, Sanchez O, Wagner KU, Hennighausen L, Suzuki A, Tsao MS, Chapman WB, Stambolic V, Mak TW (2004) Early onset of neoplasia in the prostate and skin of mice with tissue-specific deletion of Pten. Proc Natl Acad Sci USA 101:1725–1730
Ratnacaram CK, Teletin M, Jiang M, Meng X, Chambon P, Metzger D (2008) Temporally controlled ablation of PTEN in adult mouse prostate epithelium generates a model of invasive prostatic adenocarcinoma. Proc Natl Acad Sci USA 105:2521–2526
Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM (2009) A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461:495–500
Song H, Zhang B, Watson MA, Humphrey PA, Lim H, Milbrandt J (2009) Loss of Nkx3.1 leads to the activation of discrete downstream target genes during prostate tumorigenesis. Oncogene 28:3307–3319
Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, Abate-Shen C (2002) Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA 99:2884–2889
Gao H, Ouyang X, Banach-Petrosky W, Borowsky AD, Lin Y, Kim M, Lee H, Shih WJ, Cardiff RD, Shen MM, Abate-Shen C (2004) A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci USA 101:17204–17209
Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM (1999) Roles for Nkx3.1 in prostate development and cancer. Genes Dev 13:966–977
Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI (1998) Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst 90:1284–1291
Guo Y, Sklar GN, Borkowski A, Kyprianou N (1997) Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res 3:2269–2274
Bowen C, Bubendorf L, Voeller HJ, Slack R, Willi N, Sauter G, Gasser TC, Koivisto P, Lack EE, Kononen J, Kallioniemi OP, Gelmann EP (2000) Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res 60:6111–6115
Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM (2003) Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res 63:3886–3890
Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M (2001) Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci USA 98:11563–11568
Martin P, Liu YN, Pierce R, Abou-Kheir W, Casey O, Seng V, Camacho D, Simpson RM, Kelly K (2011) Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition. Am J Pathol 179:422–435
Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, Perry SR, Labrot ES, Wu X, Lis R, Hoshida Y, Hiller D, Hu B, Jiang S, Zheng H, Stegh AH, Scott KL, Signoretti S, Bardeesy N, Wang YA, Hill DE, Golub TR, Stampfer MJ, Wong WH, Loda M, Mucci L, Chin L, DePinho RA (2011) SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 470:269–273
Ding Z, Wu CJ, Jaskelioff M, Ivanova E, Kost-Alimova M, Protopopov A, Chu GC, Wang G, Lu X, Labrot ES, Hu J, Wang W, Xiao Y, Zhang H, Zhang J, Zhang J, Gan B, Perry SR, Jiang S, Li L, Horner JW, Wang YA, Chin L, DePinho RA (2012) Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 148:896–907
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648
Tomlins SA, Laxman B, Varambally S, Cao X, Yu J, Helgeson BE, Cao Q, Prensner JR, Rubin MA, Shah RB, Mehra R, Chinnaiyan AM (2008) Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 10:177–188
FitzGerald LM, Agalliu I, Johnson K, Miller MA, Kwon EM, Hurtado-Coll A, Fazli L, Rajput AB, Gleave ME, Cox ME, Ostrander EA, Stanford JL, Huntsman DG (2008) Association of TMPRSS2-ERG gene fusion with clinical characteristics and outcomes: results from a population-based study of prostate cancer. BMC Cancer 8:230
Carver BS, Tran J, Gopalan A, Chen Z, Shaikh S, Carracedo A, Alimonti A, Nardella C, Varmeh S, Scardino PT, Cordon-Cardo C, Gerald W, Pandolfi PP (2009) Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet 41:619–624
Zhong C, Saribekyan G, Liao CP, Cohen MB, Roy-Burman P (2006) Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis. Cancer Res 66:2188–2194
Jenkins RB, Qian J, Lieber MM, Bostwick DG (1997) Detection of c-myc oncogene amplification and chromosomal anomalies in metastatic prostatic carcinoma by fluorescence in situ hybridization. Cancer Res 57:524–531
Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL (2003) Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 4:223–238
Kim J, Eltoum IE, Roh M, Wang J, Abdulkadir SA (2009) Interactions between cells with distinct mutations in c-MYC and Pten in prostate cancer. PLoS Genet 5:e1000542
Haverkamp J, Charbonneau B, Ratliff TL (2008) Prostate inflammation and its potential impact on prostate cancer: a current review. J Cell Biochem 103:1344–1353
Bardia A, Platz EA, Yegnasubramanian S, De Marzo AM, Nelson WG (2009) Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol 9:419–426
De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG (2007) Inflammation in prostate carcinogenesis. Nat Rev Cancer 7:256–269
Blum DL, Koyama T, M’Koma AE, Iturregui JM, Martinez-Ferrer M, Uwamariya C, Smith JA Jr, Clark PE, Bhowmick NA (2008) Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin Cancer Res 14:7790–7797
Tassidis H, Culig Z, Wingren AG, Harkonen P (2010) Role of the protein tyrosine phosphatase SHP-1 in Interleukin-6 regulation of prostate cancer cells. Prostate 70:1491–1500
Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM (2001) Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology 58:1008–1015
Chung TD, Yu JJ, Kong TA, Spiotto MT, Lin JM (2000) Interleukin-6 activates phosphatidylinositol-3 kinase, which inhibits apoptosis in human prostate cancer cell lines. Prostate 42:1–7
Wen Z, Zhong Z, Darnell JE Jr (1995) Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241–250
Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT (2008) Stat3 promotes metastatic progression of prostate cancer. Am J Pathol 172:1717–1728
Blando JM, Carbajal S, Abel E, Beltran L, Conti C, Fischer S, DiGiovanni J (2011) Cooperation between Stat3 and Akt signaling leads to prostate tumor development in transgenic mice. Neoplasia 13:254–265
Schmid JA, Birbach A (2008) IkappaB kinase beta (IKKbeta/IKK2/IKBKB)–a key molecule in signaling to the transcription factor NF-kappaB. Cytokine Growth Factor Rev 19:157–165
Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S (2004) Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 6:390–400
Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH (2007) Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res 67:3310–3319
Birbach A, Eisenbarth D, Kozakowski N, Ladenhauf E, Schmidt-Supprian M, Schmid JA (2011) Persistent inflammation leads to proliferative neoplasia and loss of smooth muscle cells in a prostate tumor model. Neoplasia 13:692–703
Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN (1998) G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95:81–91
Meili R, Ellsworth C, Lee S, Reddy TB, Ma H, Firtel RA (1999) Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J 18:2092–2105
Funamoto S, Meili R, Lee S, Parry L, Firtel RA (2002) Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109:611–623
Iijima M, Devreotes P (2002) Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109:599–610
Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR (2000) Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287:1037–1040
Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D (2003) Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell 114:215–227
Liliental J, Moon SY, Lesche R, Mamillapalli R, Li D, Zheng Y, Sun H, Wu H (2000) Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol 10:401–404
Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E (2001) The lipid phosphatase activity of PTEN is critical for stabilizing intercellular junctions and reverting invasiveness. J Cell Biol 155:1129–1135
Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128:383–397
Gumerlock PH, Poonamallee UR, Meyers FJ, deVere White RW (1991) Activated ras alleles in human carcinoma of the prostate are rare. Cancer Res 51:1632–1637
Cho NY, Choi M, Kim BH, Cho YM, Moon KC, Kang GH (2006) BRAF and KRAS mutations in prostatic adenocarcinoma. Int J Cancer 119:1858–1862
Carter BS, Epstein JI, Isaacs WB (1990) ras gene mutations in human prostate cancer. Cancer Res 50:6830–6832
Wang XS, Shankar S, Dhanasekaran SM, Ateeq B, Sasaki AT, Jing X, Robinson D, Cao Q, Prensner JR, Yocum AK, Wang R, Fries DF, Han B, Asangani IA, Cao X, Li Y, Omenn GS, Pflueger D, Gopalan A, Reuter VE, Kahoud ER, Cantley LC, Rubin MA, Palanisamy N, Varambally S, Chinnaiyan AM (2011) Characterization of KRAS rearrangements in metastatic prostate cancer. Cancer Discov 1:35–43
Wang J, Kobayashi T, Floc’h N, Kinkade CW, Aytes A, Dankort D, Lefebvre C, Mitrofanova A, Cardiff RD, McMahon M, Califano A, Shen MM, Abate-Shen C (2012) Braf activation cooperates with Pten loss to regulate c-Myc activation in advanced prostate cancer. Cancer Res 72:4765–4776
Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, Doglioni C, Beach DH, Hannon GJ (1999) Twist is a potential oncogene that inhibits apoptosis. Genes Dev 13:2207–2217
Attard G, de Bono JS (2011) Translating scientific advancement into clinical benefit for castration-resistant prostate cancer patients. Clin Cancer Res 17:3867–3875
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL (2010) Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 375:1437–1446
Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, Oommen NB, Hawche G, Jameson C, Thompson E, Sipkema R, Carden CP, Parker C, Dearnaley D, Kaye SB, Cooper CS, Molina A, Cox ME, Terstappen LW, de Bono JS (2009) Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 69:2912–2918
Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS (2008) Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 68:4447–4454
Jiao J, Wang S, Qiao R, Vivanco I, Watson PA, Sawyers CL, Wu H (2007) Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res 67:6083–6091
Ha S, Ruoff R, Kahoud N, Franke TF, Logan SK (2011) Androgen receptor levels are upregulated by Akt in prostate cancer. Endocr Relat Cancer 18:245–255
Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC (2000) HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 60:6841–6845
Shen MM, Abate-Shen C (2007) Pten inactivation and the emergence of androgen-independent prostate cancer. Cancer Res 67:6535–6538
Gao H, Ouyang X, Banach-Petrosky WA, Shen MM, Abate-Shen C (2006) Emergence of androgen independence at early stages of prostate cancer progression in Nkx3.1; Pten mice. Cancer Res 66:7929–7933
Friedlander TW, Roy R, Tomlins SA, Ngo VT, Kobayashi Y, Azameera A, Rubin MA, Pienta KJ, Chinnaiyan A, Ittmann MM, Ryan CJ, Paris PL (2012) Common structural and epigenetic changes in the genome of castration-resistant prostate cancer. Cancer Res 72:616–625
Li P, Nicosia SV, Bai W (2001) Antagonism between PTEN/MMAC1/TEP-1 and androgen receptor in growth and apoptosis of prostatic cancer cells. J Biol Chem 276:20444–20450
Nan B, Snabboon T, Unni E, Yuan XJ, Whang YE, Marcelli M (2003) The PTEN tumor suppressor is a negative modulator of androgen receptor transcriptional activity. J Mol Endocrinol 31:169–183
Wang Y, Mikhailova M, Bose S, Pan CX, deVere White RW, Ghosh PM (2008) Regulation of androgen receptor transcriptional activity by rapamycin in prostate cancer cell proliferation and survival. Oncogene 27:7106–7117
Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL (2003) Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol 34:646–653
Heinlein CA, Chang C (2004) Androgen receptor in prostate cancer. Endocr Rev 25:276–308
Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, Macvicar GR, Varambally S, Harwood J, Bismar TA, Kim R, Rubin MA, Pienta KJ (2004) Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 64:9209–9216
Bluemn EG, Nelson PS (2012) The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol 24:251–257
Mulholland DJ, Tran LM, Li Y, Cai H, Morim A, Wang S, Plaisier S, Garraway IP, Huang J, Graeber TG, Wu H (2011) Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19:792–804
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL (2011) Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19:575–586
Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL (2004) HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6:517–527
Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood DP, Creel PA, Mundy K, Davis SL, Wang T, Albadine R, Schultz L, Partin AW, Jimeno A, Fedor H, Febbo PG, George DJ, Gurganus R, De Marzo AM, Carducci MA (2010) A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res 16:3057–3066
Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, Cambio A, Evans CP, Gandara DR, Lara PN Jr (2007) The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer 5:433–437
Kasper S, Cookson MS (2006) Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am 33:201–210, vii
Rini BI, Small EJ (2002) Hormone-refractory prostate cancer. Curr Treat Options Oncol 3:437–446
Singh P, Yam M, Russell PJ, Khatri A (2010) Molecular and traditional chemotherapy: a united front against prostate cancer. Cancer Lett 293:1–14
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115:3670–3679
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, Sridhara R, Pazdur R (2010) FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist 15:428–435
Bukowski RM, Yasothan U, Kirkpatrick P (2010) Pazopanib. Nat Rev Drug Discov 9:17–18
Joensuu H, DeMatteo RP (2012) The management of gastrointestinal stromal tumors: a model for targeted and multidisciplinary therapy of malignancy. Annu Rev Med 63:247–258
Tkaczuk KH (2009) Review of the contemporary cytotoxic and biologic combinations available for the treatment of metastatic breast cancer. Clin Ther 31(Pt 2):2273–2289
Burris H III, Rocha-Lima C (2008) New therapeutic directions for advanced pancreatic cancer: targeting the epidermal growth factor and vascular endothelial growth factor pathways. Oncologist 13:289–298
Eng C (2010) The evolving role of monoclonal antibodies in colorectal cancer: early presumptions and impact on clinical trial development. Oncologist 15:73–84
Cataldo VD, Gibbons DL, Perez-Soler R, Quintas-Cardama A (2011) Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med 364:947–955
Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C (2008) PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 105:13057–13062
Lin J, Adam RM, Santiestevan E, Freeman MR (1999) The phosphatidylinositol 3'-kinase pathway is a dominant growth factor-activated cell survival pathway in LNCaP human prostate carcinoma cells. Cancer Res 59:2891–2897
Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, McKenna WG (2003) Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int J Radiat Oncol Biol Phys 56:846–853
Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, Abraham RT (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15:5256–5267
Stein RC (2001) Prospects for phosphoinositide 3-kinase inhibition as a cancer treatment. Endocr Relat Cancer 8:237–248
El-Kholy W, Macdonald PE, Lin JH, Wang J, Fox JM, Light PE, Wang Q, Tsushima RG, Wheeler MB (2003) The phosphatidylinositol 3-kinase inhibitor LY294002 potently blocks K(V) currents via a direct mechanism. FASEB J 17:720–722
Pasapera Limon AM, Herrera-Munoz J, Gutierrez-Sagal R, Ulloa-Aguirre A (2003) The phosphatidylinositol 3-kinase inhibitor LY294002 binds the estrogen receptor and inhibits 17beta-estradiol-induced transcriptional activity of an estrogen sensitive reporter gene. Mol Cell Endocrinol 200:199–202
Knight ZA, Shokat KM (2007) Chemically targeting the PI3K family. Biochem Soc Trans 35:245–249
Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, Loewith R, Stokoe D, Balla A, Toth B, Balla T, Weiss WA, Williams RL, Shokat KM (2006) A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125:733–747
Ihle NT, Lemos R Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G (2009) Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res 69:143–150
Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F (2006) Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer 94:1906–1912
Floryk D, Thompson TC (2008) Perifosine induces differentiation and cell death in prostate cancer cells. Cancer Lett 266:216–226
Marsh Rde W, Rocha Lima CM, Levy DE, Mitchell EP, Rowland KM Jr, Benson AB III (2007) A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol 30:26–31
Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, Joiner MC, Marples B, Forman JD, Sarkar FH (2004) Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther 3:1271–1279
Li Y, Sarkar FH (2002) Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8:2369–2377
Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC (2008) Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res 68:2024–2032
Li Y, Kucuk O, Hussain M, Abrams J, Cher ML, Sarkar FH (2006) Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res 66:4816–4825
Rhodes N, Heerding DA, Duckett DR, Eberwein DJ, Knick VB, Lansing TJ, McConnell RT, Gilmer TM, Zhang SY, Robell K, Kahana JA, Geske RS, Kleymenova EV, Choudhry AE, Lai Z, Leber JD, Minthorn EA, Strum SL, Wood ER, Huang PS, Copeland RA, Kumar R (2008) Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res 68:2366–2374
Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N (2011) AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19:58–71
Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65:7052–7058
Gao N, Zhang Z, Jiang BH, Shi X (2003) Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun 310:1124–1132
Peffley DM, Sharma C, Hentosh P, Buechler RD (2007) Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch Biochem Biophys 465:266–273
MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, Purcell C, McGurk M, Johnston PG, Waugh DJ (2007) Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res 5:737–748
Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, Sawyers CL, Lichtenstein AK (2004) AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem 279:2737–2746
Podsypanina K, Lee RT, Politis C, Hennessy I, Crane A, Puc J, Neshat M, Wang H, Yang L, Gibbons J, Frost P, Dreisbach V, Blenis J, Gaciong Z, Fisher P, Sawyers C, Hedrick-Ellenson L, Parsons R (2001) An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/- mice. Proc Natl Acad Sci USA 98:10320–10325
Wu L, Birle DC, Tannock IF (2005) Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res 65:2825–2831
Cao C, Subhawong T, Albert JM, Kim KW, Geng L, Sekhar KR, Gi YJ, Lu B (2006) Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res 66:10040–10047
Masiello D, Mohi MG, McKnight NC, Smith B, Neel BG, Balk SP, Bubley GJ (2007) Combining an mTOR antagonist and receptor tyrosine kinase inhibitors for the treatment of prostate cancer. Cancer Biol Ther 6:195–201
Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10:594–601
Amato RJ, Jac J, Mohammad T, Saxena S (2008) Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer 6:97–102
Sarker D, Reid AH, Yap TA, de Bono JS (2009) Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res 15:4799–4805
Park S, Zhao D, Hatanpaa KJ, Mickey BE, Saha D, Boothman DA, Story MD, Wong ET, Burma S, Georgescu MM, Rangnekar VM, Chauncey SS, Habib AA (2009) RIP1 activates PI3K-Akt via a dual mechanism involving NF-kappaB-mediated inhibition of the mTOR-S6K-IRS1 negative feedback loop and down-regulation of PTEN. Cancer Res 69:4107–4111
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26:1932–1940
Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chene P, De Pover A, Schoemaker K, Fabbro D, Gabriel D, Simonen M, Murphy L, Finan P, Sellers W, Garcia-Echeverria C (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther 7:1851–1863
Serra V, Markman B, Scaltriti M, Eichhorn PJ, Valero V, Guzman M, Botero ML, Llonch E, Atzori F, Di Cosimo S, Maira M, Garcia-Echeverria C, Parra JL, Arribas J, Baselga J (2008) NVP-BEZ235, a dual PI3K/mTOR inhibitor, prevents PI3K signaling and inhibits the growth of cancer cells with activating PI3K mutations. Cancer Res 68:8022–8030
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7:e38
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032
Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, Wang S, Ren P, Martin M, Jessen K, Feldman ME, Weissman JS, Shokat KM, Rommel C, Ruggero D (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 485:55–61
Schnell CR, Stauffer F, Allegrini PR, O'Reilly T, McSheehy PM, Dartois C, Stumm M, Cozens R, Littlewood-Evans A, Garcia-Echeverria C, Maira SM (2008) Effects of the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 on the tumor vasculature: implications for clinical imaging. Cancer Res 68:6598–6607
Garlich JR, De P, Dey N, Su JD, Peng X, Miller A, Murali R, Lu Y, Mills GB, Kundra V, Shu HK, Peng Q, Durden DL (2008) A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res 68:206–215
Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, Chen Z, dos Santos O, Ayral-Kaloustian S, Venkatesan A, Hollander I (2011) Antitumor efficacy of PKI-587, a highly potent dual PI3K/mTOR kinase inhibitor. Clin Cancer Res 17:3193–3203
Raynaud FI, Eccles S, Clarke PA, Hayes A, Nutley B, Alix S, Henley A, Di-Stefano F, Ahmad Z, Guillard S, Bjerke LM, Kelland L, Valenti M, Patterson L, Gowan S, de Haven Brandon A, Hayakawa M, Kaizawa H, Koizumi T, Ohishi T, Patel S, Saghir N, Parker P, Waterfield M, Workman P (2007) Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res 67:5840–5850
Dan S, Yoshimi H, Okamura M, Mukai Y, Yamori T (2009) Inhibition of PI3K by ZSTK474 suppressed tumor growth not via apoptosis but G0/G1 arrest. Biochem Biophys Res Commun 379:104–109
Fan QW, Cheng CK, Nicolaides TP, Hackett CS, Knight ZA, Shokat KM, Weiss WA (2007) A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res 67:7960–7965
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118:3065–3074
Kinkade CW, Castillo-Martin M, Puzio-Kuter A, Yan J, Foster TH, Gao H, Sun Y, Ouyang X, Gerald WL, Cordon-Cardo C, Abate-Shen C (2008) Targeting AKT/mTOR and ERK MAPK signaling inhibits hormone-refractory prostate cancer in a preclinical mouse model. J Clin Invest 118:3051–3064
Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14:1351–1356
Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, An J, Horvath S, Gleave M, Rettig MB, Wainberg ZA, Reiter RE (2010) Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med 16:1414–1420
Weber MJ, Gioeli D (2004) Ras signaling in prostate cancer progression. J Cell Biochem 91:13–25
Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C (2006) Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci USA 103:14477–14482
Floc’h N, Kinkade CW, Kobayashi T, Aytes A, Lefebvre C, Mitrofanova A, Cardiff RD, Califano A, Shen MM, Abate-Shen C (2012) Dual targeting of the Akt/mTOR signaling pathway inhibits castration-resistant prostate cancer in a genetically engineered mouse model. Cancer Res 72:4483–4493
Zhang W, Zhu J, Efferson CL, Ware C, Tammam J, Angagaw M, Laskey J, Bettano KA, Kasibhatla S, Reilly JF, Sur C, Majumder PK (2009) Inhibition of tumor growth progression by antiandrogens and mTOR inhibitor in a Pten-deficient mouse model of prostate cancer. Cancer Res 69:7466–7472
Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON (2007) Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci USA 104:181–186
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Mayo Clinic
About this chapter
Cite this chapter
Ruscetti, M.A., Wu, H. (2013). PTEN in Prostate Cancer. In: Tindall, D. (eds) Prostate Cancer. Protein Reviews, vol 16. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6828-8_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6828-8_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6827-1
Online ISBN: 978-1-4614-6828-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)