Abstract
This chapter describes the neuroanatomy and common lower cranial neuropathies, including clinical presentation, evaluation, diagnosis, and treatment of the following cranial nerves: trigeminal (fifth), facial (seventh), glossopharyngeal (ninth), vagus (tenth), spinal accessory (eleventh), and hypoglossal (twelfth).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Trigeminal nerve
- facial nerve
- glossopharyngeal nerve
- vagus nerve
- spinal accessory nerve
- and hypoglossal nerve
- fifth cranial nerve
- seventh cranial nerve
- ninth cranial nerve
- tenth cranial nerve
- eleventh cranial nerve
- twelfth cranial nerve
Introduction
The enumeration and naming of the cranial nerves represent a curious example of evolution in anatomical classification. The earliest classification was recorded by Galen during the second century A.D., and it included 7 pairs: optic (I); oculomotor (II); trigeminal (III); motor root of trigeminal (IV); facial and auditory (V); glossopharyngeal, vagus, and accessory (VI); and hypoglossal (VII) [1]. Progress in both anatomy and physiology resulted in numerous revisions of the nomenclature of cranial nerves. The current classification of 12 cranial nerves was developed by Soemmerring in 1778 as part of a doctoral thesis in medicine: olfactory (I), optic (II), oculomotor (III), trochlear (IV), trigeminal (V), abducens (VI), facial (VII), vestibulocochlear or acoustic(VIII), glossopharyngeal (IX), vagus (X), spinal accessory (XI), and hypoglossal (XII).
Trigeminal Nerve
Neuroanatomy
The fifth or trigeminal nerve is the largest cranial nerve and functions as a mixed motor and sensory nerve. Its fibers exit through the ventral surface of the mid-pons. The large trigeminal (gasserian) ganglion is positioned in the tip of the petrous bone, where the nerve divides into its three sensory divisions – ophthalmic, maxillary, and mandibular. These branches exit the skull through the superior orbital fissure, foramen rotundum, and foramen ovale, respectively. The motor fibers of the trigeminal nerve form a separate fascicle, which passes underneath the gasserian ganglion, merges with the mandibular division, and terminates in the muscles of mastication (Figs. 42.1 and 42.2).
The multiple subdivisions of the trigeminal nuclei occupy a significant rostrocaudal extent in the midbrain, pons, and medulla. Each branch of the trigeminal nerve (V1 ophthalmic, V2 maxillary, and V3 mandibular) innervates a distinct area of the face, as indicated by the different shaded areas in the illustration. Note that the cornea is supplied by the ophthalmic division
Trigeminal Neuralgia (“Tic Douloureux”)
The incidence of trigeminal neuralgia (TN) is approximately 4–5 in 100,000 [2]. TN manifests as episodes of severe unilateral paroxysmal facial pain, often described as stabbing, shock-like, or electrical in the distribution of one or more branches of the trigeminal nerve. The typical pain is lancinating or shooting. Atypical facial pain is usually constant and aching, burning, throbbing, or stinging. The most common pain location is along the nasolabial fold, innervated by the maxillary branch. It is followed in frequency by symptoms along the lower jaw, in the area of the mandibular branch, and the least common pain location is around the eye and forehead, areas innervated by the ophthalmic branch. Painful episodes may have a duration as brief as a few seconds or extend to several minutes in a continuous crescendo. They are often triggered by chewing, swallowing, talking, or light tactile stimuli. Patients may lose considerable weight due to impaired nutrition.
Classic TN should not be associated with any clinically evident neurologic deficits and can be either idiopathic or associated with vascular compression of cranial nerve V [3]. When a vascular source is identified, often an arterial loop from the superior cerebellar artery compresses the trigeminal nerve’s sensory roots. Less often, vascular compression can occur from branches of the internal carotid artery, basilar artery, a primitive trigeminal artery, or rarely from an arteriovenous malformation or saccular aneurysm.
Symptomatic TN is due to a structural lesion other than vascular compression [3]. Demyelination of the proximal portion of the trigeminal sensory nerve root and ephaptic spread of excitation can cause TN in patients with multiple sclerosis [4]. Two to four percent of patients with TN have multiple sclerosis, and one to five percent of patients with multiple sclerosis will develop trigeminal neuralgia, although the symptoms of multiple sclerosis often precede the onset of TN [5]. Cerebellopontine angle tumors, schwannomas of the trigeminal or vestibular nerve, meningiomas, and arachnoid cysts can less commonly cause trigeminal neuralgia. Younger patient’s age, the presence of sensory deficits, or bilateral symptoms are thought to increase the risk of symptomatic TN.
Imaging in patients with TN identifies structural causes in up to 15 % of patients and should be pursued for evaluation. MRI of the skull base with gadolinium is preferred, and angiography can be helpful in the appropriate clinical context. Measurement of the blink reflexes, in which the latency and amplitude of ipsilateral and contralateral facial muscle contractions are recorded following the stimulation of the supraorbital branch of the trigeminal nerve, identifies symptomatic TN with a sensitivity of 59–100 % and specificity of 93–100 % [6]. With symptomatic trigeminal neuralgia, the ipsilateral R1 and R2 responses and contralateral R2 response are delayed or absent with ipsilateral trigeminal stimulation, while all three responses are normal with contralateral stimulation.
Medical treatment of TN usually involves anticonvulsant medications. Carbamazepine or oxacarbamazepine are effective in 60–80 % of patients and are recommended as first line treatment. Typical doses used include carbamazepine 200–1,200 mg/day or oxacarbamazepine 600–1,800 mg/day. Other medications including gabapentin, phenytoin, clonazepam, baclofen, lamotrigine, and valproate have been tried with variable success [7].
In patients who are refractory to medical treatment, several surgical options have been utilized including percutaneous Gasserian lesions, gamma knife surgery, and microvascular decompression. Methods of percutaneous Gasserian lesions include radiofrequency thermocoagulation, glycerol rhizotomy, and balloon compression. Although variance is noted between different publications, long-term satisfactory pain control in ablative Gasserian ganglion procedures and gamma knife is about 50 % [8]. Microvascular decompression may be superior to other procedures, with 70 % of patients being free of pain after 10 years [9]. The presence of typical lancinating pain (versus atypical and constant) is the only significant and independent predictor of outcome following microvascular decompression in patients with TN [10]. In this procedure, surgeons perform a suboccipital craniotomy, dissect the offending arterial loop, and separate the loop from the trigeminal root with Teflon. The mortality rate of this procedure is 0.2–0.4 %, and morbidity varies based on institutional experience and volume. Other complications include aseptic meningitis, hearing loss, CSF leaks, infarcts, and hematomas.
Trigeminal Sensory Neuropathy
This is an uncommon clinical condition that results in facial paresthesias in the distribution of the fifth cranial nerve. The diagnosis of idiopathic trigeminal neuropathy can be made only after evaluation and exclusion of other causes, including drug intoxication from drugs such as oxaliplatin, trichloroethylene, and hydroxystilbamidine. Isolated chronic trigeminal sensory neuropathy may be a manifestation of connective tissue disorders such as Sjögren’s syndrome, systemic lupus erythematosus, scleroderma, or mixed connective tissue disease. It is frequently bilateral and is not confined to individual nerve branches [11]. It affects patients of all age groups, independent of the etiology. Although a number of series report a benign course without other associated neurological defects and preserved corneal reflexes [12], other series describe frequent associations with tumors of the cerebellopontine angle, skull base, nasopharyngeal region, multiple sclerosis, or vasculitis [13]. Furthermore, sensory deficits in the face should always elicit inquiries about previous facial skin cancer because of the well-known tendency of squamous cell carcinomas to infiltrate along the perineurial sheathing of the cutaneous nerves [14]. Treatment with corticosteroids has limited efficacy in this condition.
Numb Chin and Numb Cheek Syndromes
Persistent numbness in the lower lip and chin (numb chin syndrome) may result from lesions of the mental nerve or the mandibular division of the fifth cranial nerve. This is also referred to as mental neuropathy and is often seen with metastatic cancer; it may be part of disease progression in advanced cases or the initial manifestation of a relapse in patients with known systemic cancer. Sometimes, it is the first symptom of an underlying malignancy. This occurs classically with nasopharyngeal carcinoma but is in fact most common with metastatic breast cancer, lymphoma, prostatic cancer, and lung cancer [15]. In more than half of the patients, this is due to invasion of the mandible and infiltration of the inferior alveolar nerve. In the rest, it is due to lesions of the base of the skull or leptomeningeal seeding [15]. Hence, the work-up should include imaging of the mandible by orthopantomogram or CT, and if negative, imaging of brain, base of skull, and CSF analysis. The prognosis is poor with a very high mortality in 6–12 months [15, 16]. In elderly patients with mandibular atrophy, impingement of the mental nerve at the mental foramen has been described [17] (Fig. 42.2).
On the other hand, numbness in the malar region (numb cheek syndrome) may be the initial manifestation of epithelial tumors (e.g., basal or squamous cell carcinomas of the face) or associated with incompletely treated neoplasms. Thus, persistent or progressive sensory deficits in the maxillary or mandibular divisions require thorough neuroimaging studies, laryngologic surgical evaluation, and careful clinical follow-up, as some neoplasms may initially be too small for detection.
Herpes Virus Infections
Infection by the herpes zoster virus is probably the most common lesion affecting the trigeminal ganglion. The ophthalmic division is involved in a ratio of 4:1 compared to lesions to the maxillary or the mandibular divisions. Clinically, patients describe a relatively rapid onset, with severe, constant, and disabling facial pain. Small vesicles often appear along the forehead and the eyebrow between the third and fifth days, followed by pronounced facial edema.
In contrast, infection caused by the herpes simplex virus (HSV), which results from persistent presence of HSV in the trigeminal ganglion, has a more indolent course. It affects all three trigeminal divisions equally and manifests as lifetime recurring episodes of mucous membrane lesions in the oral cavity and, most commonly, as “cold sores” in the lips.
Facial Nerve
Neuroanatomy
The seventh or facial nerve is a mixed motor and sensory nerve. It innervates the muscles of facial expression, the salivary and lacrimal glands, and the mucous membrane of the oral and nasal cavities. In addition, it conveys taste sensation from the anterior two-thirds of the tongue via the lingual and chorda tympani nerves and provides tactile sensation to a small but distinct area in the posterior external auditory canal (Hitzelberger’s sign). The motor fibers originate in the facial nucleus located in the pons, sweep medially around the nucleus of the abducens nerve, and exit the ventral surface of the pons in the cerebellopontine angle. The facial nerve, along with the nervus intermedius and vestibulocochlear nerves, then passes from the brainstem into the temporal bone through the inner acoustic meatus. The seventh cranial nerve follows a tortuous course within the facial canal of the temporal bone exiting the skull through the stylomastoid foramen to supply the muscles of facial expression. The facial nerve also supplies the submandibular and sublingual glands (Fig. 42.3).
The facial nerve contains fibers from the facial, solitary tract, and superior salivatory nuclei. It exits the skull through the inner acoustic meatus, in conjunction with the vestibular and cochlear nerves. Within each designated segment (labyrinthine, tympanic, and mastoid) of its complex trajectory, the facial nerve gives off a branch that can help in the clinical identification of the site of lesion
Given the close proximity of the genu of the facial nerve and the nucleus of the abducens nerve within the pons, facial palsies secondary to intrapontine lesions are almost always also associated with gaze or sixth nerve impairment, thus distinguishing them from more peripheral lesions. The anatomical branching pattern of the facial nerve sometimes allows for the localization of the lesion in the temporal bone (Fig. 42.3) [18]. Lesions of the most distal mastoid (vertical) segment affect the chorda tympani nerve, thus impairing taste sensation in the ipsilateral anterior two-thirds of the tongue. Lesions involving the tympanic (horizontal) segment within the middle ear result also in hyperacusis due to damage to the nerve to the stapedius muscle. Lesions of the most proximal labyrinthine segment result also in impaired ipsilateral lacrimation because of the involvement of the greater superficial petrosal nerve. Distal lesions of the facial nerve may affect individual motor branches, thus causing focal paresis of individual facial mimetic muscles. Such lesions can usually be confirmed by needle electromyographic examination.
Bell’s Palsy
Idiopathic facial nerve palsy, or Bell’s palsy (BP), is the most common lesion of the seventh cranial nerve. Clinically, patients frequently describe a dull pain in or behind the ipsilateral ear, suggestive of an ear infection as the initial manifestation. This is thought to be due to dysfunction of the sensory supply to portions of the external ear canal. A peripheral-type facial paralysis quickly follows and usually achieves maximum impairment in 48–72 h. In addition to facial paresis, patients may also describe impairment of taste and hyperacusis.
The site of injury to the facial nerve in BP is often in the proximal (and most narrow) portion of the facial canal [19]. The initial lesion is inflammatory in nature, causes segmental demyelination of motor axons, and disrupts the conduction of electric impulses. More severe lesions will result in direct injury to the axons, which can be detected on facial motor nerve conduction studies: there is a significant decrease of amplitude in the compound motor action potential (CMAP) recording the facial muscles in the involved side. The facial CMAP, performed after 7–10 days from the onset of facial weakness (time to complete Wallerian degeneration if any), has an important prognostic value [20]: If the CMAP of the involved side is 30 % or greater of that on the normal side, 84 % of the patients will have complete recovery. However, when the CMAP is 25 % or less of the normal side, there is an 88 % chance of incomplete recovery. In a small percentage of patients, delayed recovery with axonal loss can lead to aberrant regeneration of the remaining motor fibers.
The most common neurological sequelae of aberrant facial nerve regeneration results in synkinesis or contraction of the lower facial muscles on the previously involved side whenever there is an eye blink. Two very uncommon examples of abnormal regeneration patterns are so-called crocodile tears, i.e., lacrimation of the ipsilateral eye during chewing, and Marin-Amat syndrome, or “jaw-winking,” manifested as closure of the ipsilateral eyelid when the jaw opens.
Although BP was classically defined as a unilateral peripheral facial palsy of unknown etiology, many studies support an association of acute facial palsy with herpes simplex virus (HSV). Latent HSV type-1 is isolated in the majority of the geniculate ganglia from autopsy samples [21]. Furthermore, HSV-1 genome was detected in 79 % of facial nerve endoneurial fluid in patients with BP, but not in normal controls [22]. Others propose an immune etiology to BP, and in fact, recent studies suggest that treatment of BP with steroids alone provides the most benefit for facial muscle recovery and is superior to immunotherapy with antiviral medications. Treatment with both steroids and antiviral medications has not been clearly shown to confer additional benefit over treatment with steroids alone [23].
Herpes zoster infection of the geniculate ganglion (Ramsay-Hunt syndrome, herpes zoster oticus with facial palsy) is also increasingly recognized as a cause of facial palsy. Its incidence is 5/100,000 of the United States population with higher incidence in subjects older than 60 years. With zoster infections, in addition to pain, tinnitus, and decreased hearing, the appearance of vesicles in the external auditory canal and soft palate is characteristic [24]. Antibody titers against varicella-zoster virus (VZV) provide a useful adjuvant to the clinical diagnosis of Ramsay-Hunt syndrome. In a prospective study of 43 patients with facial nerve palsy, 75 % of patients with typical vesicular lesions (8 of 12) presented with antibody titers >1/10. In contrast, only 29 % (9 of 31) of patients without vesicular lesions presented with such high titers [25]. Oral antiviral agents are effective in patients with herpes zoster infection in general, by speeding up rash healing and decreasing pain duration. Although corticosteroids are mostly helpful in reducing the acute pain and the incidence of postherpetic neuralgia, their use in the Ramsay-Hunt syndrome remains anecdotal with no available controlled trials [26].
Bilateral Facial Palsy
Bilateral and simultaneous facial weakness (facial diplegia) is an uncommon event and often a diagnostic challenge. Although Guillain-Barré syndrome (GBS) is associated with facial diplegia in up to 60 % of the cases, bilateral facial weakness is a dominant neurological finding in the atypical forms of GBS such as the descending or the pharyngeal-cervical-brachial forms. Another rare GBS variant is characterized by facial diplegia only but is often associated with distal limb paresthesias, abnormal limb nerve conduction studies, and positive serology for a recent cytomegalovirus infection [27]. Neurosarcoidosis and accidental or intentional ingestion of the toxin, ethylene glycol (“antifreeze”), may also cause facial diplegia. The combination of facial diplegia, aseptic meningitis, and a slightly erythematous indurated face resembling painless cellulitis should trigger an investigation for Lyme disease. In Keane’s seminal review of bilateral facial palsies, the most common cause was bilateral idiopathic BP (10 of 43 patients), followed closely by brainstem tumors (9 patients) and Guillain-Barre syndrome (5 patients) [28]. Less common causes of bilateral facial palsy include malignant lymphoma [29] and gelsolin-related familial amyloidosis, an autosomal dominant condition also associated with corneal lattice dystrophy and a generalized polyneuropathy [30].
Congenital Disorders
Unilateral or bilateral facial palsies in neonates are usually due to traumatic injuries versus congenital abnormalities. Traumatic facial nerve damage is usually associated with difficult forceps delivery and presents with periauricular ecchymosis, hemotympanum, and facial swelling. In contrast, congenital facial disorders often present with other systemic stigmata, e.g., microtia, external auditory atresia, limb deformity, or hypoplasia of the pectoral muscle. In particular, Möbius syndrome, which results from bilateral hypogenesis or agenesis of the nucleus of the sixth and seventh cranial nerves, is often associated with limb abnormalities. In the cardiofacial (Cayler’s) syndrome (“asymmetric crying facies”), the neonate exhibits facial asymmetry when crying but not at rest. The cause of this motor deficit is isolated weakness of the depressor anguli oris and depressor labii inferioris [31].
Glossopharyngeal Nerve
Neuroanatomy
The ninth cranial or glossopharyngeal nerve receives contribution from several brainstem nuclear complexes (tractus solitarius [gustatory nucleus], nucleus ambiguus, inferior salivatory nucleus). Distinct subsets of the glossopharyngeal nerve fibers emerge from the medulla between the VIII and X cranial nerves. The nerve leaves the skull through the jugular foramen and reaches the lateral wall of the pharynx by following the inferior border of the stylopharyngeus muscle.
Isolated lesions of the IX nerve are very uncommon. Thus, multiple syndromes are described involving combined injuries to the nucleus or the peripheral fibers of the glossopharyngeal nerve and the other three lower cranial nerves (vagus, spinal accessory, and hypoglossal). However, isolated lesions of the glossopharyngeal nerve can manifest as slight difficulties with swallowing due to weakness of the stylopharyngeus muscle, taste disturbance in the posterior third of the tongue, reduced or absent gag reflex, or decreased parotid gland secretion.
Glossopharyngeal Neuralgia
Glossopharyngeal neuralgia is a relatively rare neuralgia which presents as severe, sharp, and lancinating pain located in the lateral portion of the throat or tonsillar region, producing extreme discomfort. The pain often is referred to the ipsilateral ear and external auditory canal. Trigger zones are usually located in the posterior and lateral pharyngeal and tonsillar fossa. Thus, talking, chewing, or swallowing can initiate the pain. Autonomic dysfunction (salivation, lacrimation, bradycardia, and syncope) may be associated with glossopharyngeal neuralgia. This condition may be idiopathic or secondary to a local structural lesion, such as a tumor of the peripheral nerve or a cerebellopontine angle tumor. Other reported causes include enlarged tortuous vertebral or posterior cerebellar arteries, oropharyngeal carcinoma, paratonsillar abscess, or elongated styloid process. Patients often lose considerable weight before treatment is initiated.
Treatment is targeted toward the underlying cause of secondary neuralgias. Medical treatment is similar to that of trigeminal neuralgia, and carbamazepine has been used as the initial line of treatment. Stereotactic radiosurgery and neurosurgical intervention may be effective in medically refractory cases [32, 33]. Surgical exploration often reveals aberrant vessels across the nerve, neurofibromas, or cholesteatomas adjacent to the nerve. The overall immediate success rate exceeded 90 % in patients treated with microvascular decompression, and long-term patient outcomes were best for typical glossopharyngeal neuralgia with pain restricted to the throat and palate. Nerve sectioning can be curative [34].
Glomus Jugulare Tumors
These are highly vascular erosive tumors that arise from the chemoreceptor cells in proximity to the jugular foramen. Their clinical manifestations result from compression of the adjacent IX, X, and XI cranial nerves. Patients often present with difficulties in swallowing, hoarseness in voice, and pulsatile tinnitus followed by conductive hearing loss. Patients may also present with symptoms of increased intracranial pressure due to compression of the jugular vein which results in impairing venous drainage. Treatment options for glomus jugulare tumors include subtotal resection with or without adjuvant postoperative radiosurgery, gross-total resection, or stereotactic radiosurgery alone [35].
Vagus Nerve
Neuroanatomy
The tenth cranial or vagus nerve has the longest and most widely distributed extracranial trajectory of all cranial nerves. The brainstem nuclear connections are similar to those of the glossopharyngeal nerve. The motor fibers originate from the nucleus ambiguus and the dorsal motor nucleus of the vagus. The sensory fibers are collected mostly at the jugular and the nodose ganglia. The vagus nerve, as do the glossopharyngeal and spinal accessory cranial nerves, exits the cranial vault through the jugular foramen.
Lesions of the vagus nerve lead to weakness of the palate and pharynx. Unilateral lesions cause defective elevation of the palate during phonation with deviation of the uvula to the contralateral side. Bilateral lesions result in nasal speech, nasal regurgitation with swallowing, and snoring. Pharyngeal paralysis results in varying degrees of dysphagia.
Clinically, two branches of the vagus nerve are of particular importance. The superior laryngeal nerve originates in the proximity of the nodose ganglion, and its branches innervate the cricothyroid and the inferior pharyngeal constrictor muscles, as well as the mucosa of the larynx and the various laryngeal glands. The recurrent laryngeal nerve controls all the intrinsic muscles of the larynx responsible for vocalizations. The right and left recurrent laryngeal nerves have different courses: On the right side, the nerve descends caudally with the main vagal trunk, loops underneath the subclavian artery, and redirects rostrally to innervate the larynx. The left recurrent laryngeal nerve loops underneath the aortic arch, thus following a longer course, and is therefore more vulnerable to lesions along its trajectory. The recurrent laryngeal nerves may be damaged by carcinoma of the thyroid, tumors of the mediastinum and lung, trauma, or iatrogenically in procedures such as thyroidectomy. Recurrent laryngeal neuropathy may also be a rare manifestation of idiopathic brachial plexopathy.
Recurrent laryngeal and superior laryngeal nerve lesions can be minimally symptomatic or lead to persistent hoarseness and dyspnea. They can also cause autonomic dysfunction with impairment of esophageal, gastric, and intestinal motility, as well as vagal cardiac activity. Intraoperative nerve monitoring has been utilized in attempts to reduce the risk of recurrent laryngeal nerve injury, but the benefits of these techniques remain uncertain [36].
Brainstem Lesions
Because of significant crossover of the supranuclear pathways to the nucleus ambiguus, only bilateral lesions are of clinical significance. Patients with such lesions, which can be caused by motor neuron disease, poliomyelitis, or primary brainstem tumors, have a pseudo-bulbar clinical presentation manifested as dysphagia and dysarthria.
Nuclear or intramedullary lesions can result from vascular infarcts (lateral medullary or Wallenberg syndrome), neoplasias, inflammatory lesions, syringobulbia, motor neuron disease, or poliomyelitis. Infranuclear lesions often occur as the vagus nerve exits the skull through the jugular foramen and are often associated with lesions of the glossopharyngeal and spinal accessory nerves. Peripheral lesions affecting the recurrent laryngeal nerve, usually the most common type of neurogenic laryngeal dysfunction, can result from trauma, surgical procedures in the neck region, and tumors of the mediastinum and lung. Patients with acute unilateral injury to the recurrent laryngeal nerve often complain of difficulties in coughing forcefully (“bovine cough”) and a hoarse voice.
Extramedullary Lesions
Posterior fossa or jugular foramen tumors can cause compression of the vagus nerve. Vagus nerve damage can also result from carotid artery aneurysm or dissection. Inflammatory and infectious processes responsible, such as sarcoidosis, Lyme disease, and Guillain-Barre syndrome, can affect the vagus nerve in conjunction with other cranial and peripheral neuropathies. Toxic causes and nutritional causes, such as alcohol-induced neuropathy, Beriberi or thiamine deficiency [37], and vincristine-induced nerve toxicity, have been implicated as causes of vagus neuropathies [38].
Spinal Accessory Nerve
Neuroanatomy
The eleventh or spinal accessory nerve is a purely motor cranial nerve and is composed of a smaller cranial part (“accessory” to the vagus nerve) and a larger spinal portion (C2-C6 ventral roots). The cranial part of the XI cranial nerve, in conjunction with the vagus and glossopharyngeal nerves, innervates muscles of the pharynx and larynx. The larger spinal portion innervates the sternocleidomastoid and upper portions of the trapezius muscles. The lower portions of the trapezius receive innervation from the ventral ramus of spinal roots C2-4.
Clinical Syndromes
A great diversity of lesions can injure the nerve fibers of the spinal accessory nerve, because the nerve follows a long course in the spinal canal, inside the cranial vault, and peripherally. Intraspinal lesions can result from motor neuron disease, poliomyelitis, syringomyelia, tumors, and external cervical trauma. Intracranially, masses such as meningiomas and neuromas may damage the neurons or the axons by direct compression. Arguably the most common type of lesion occurs in the periphery, between the jugular foramen and the nerve terminations in the trapezius muscle. Iatrogenic lesions, such as those resulting from radical neck dissections or lymph node biopsies in the neck region, are very common causes of injury (Fig. 42.4). Surgical exploration and nerve repair can be effective in the restoration of function in cases of nerve transection, and early operative intervention yields the best functional results [39]. Other etiologies include carotid endarterectomy [40], coronary artery bypass surgery, radiation damage, shoulder dislocation, blunt trauma, and stretch and biting injuries [41]. In Vernet’s syndrome, the spinal accessory nerve is injured in conjunction with the glossopharyngeal and vagus nerves as these three cranial nerves pass through the jugular foramen (Fig. 42.5). Neuralgic amyotrophy with involvement of cranial nerves IX, X, XI, and XII has been reported [42]. In addition, isolated unexplained lesions of cranial nerve XI can occur with spontaneous recovery. Motor nerve conduction studies of the spinal accessory nerve, particularly when asymmetry is noted in latency and amplitude with a unilateral lesion, and electromyography of the trapezius or sternocleidomastoid can be useful in confirming the diagnosis for CN XI lesions.
Patients with lesions of the spinal accessory nerve proximal to the branching point to the sternocleidomastoid muscle have weakness in turning the head toward the unaffected side. Furthermore, attempts of neck flexion in the supine position may show a head deviation to the affected side. Lesions of the motor branches to the trapezius muscle result in significant wasting of muscle mass, and the affected shoulder is notably lower than the healthy counterpart. At rest the scapula is displaced laterally, with the inferior angle rotated medially. Scapular winging is further accentuated by attempted abduction of the arm. In contrast, in scapular winging associated with weakness of the serratus anterior muscle, which can result from injuries to the long thoracic nerve, the scapula is displaced medially, and winging is accentuated by forward flexion or protraction against resistance.
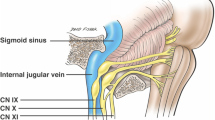
Clinical Anatomy of the Jugular Foramen
There is a unique anatomical relationship between the last four cranial nerves (glossopharyngeal, vagus, spinal accessory, and hypoglossal) and the jugular foramen (JF) which explains the observed clinical syndromes (Table 42.1). Thus, a more detailed examination of this anatomical region is warranted (Fig. 42.5). Three cranial nerves (IX, X, and XI) exit the base of the skull through the JF. The hypoglossal nerve passes through the hypoglossal canal, which lies immediately medial to the JF. The JF is divided in two compartments by a fibrous or bony septum: pars nervosa (anteromedial) and pars vascularis (posterolateral). Despite the nomenclature, only the glossopharyngeal nerve goes through the smaller pars nervosa together with the inferior petrosal sinus. The pars vascularis contains the vagus and the accessory nerves, as well as the jugular vein.
The close proximity of the last four cranial nerves often results in combined nerve lesions. Clinical presentations of such lesions generally include hoarseness or loss of voice strength, nasal speech, and difficulties in swallowing.
The Vernet’s syndrome manifests as deficits of the cranial nerves (IX, X, XI) that traverse the jugular foramen. Sparing of the hypoglossal nerve and the cervical sympathetic chain often suggests the intracranial nature of the lesion causing this syndrome. In contrast, all four lower cranial nerves are impaired in the Collet-Sicard syndrome (Table 42.1). Although an intracranial lesion can result in this combination of cranial nerve deficits, a more parsimonious explanation is an extracranial lesion just outside of the jugular foramen affecting the nerves that pass through the JF (IX, X, XI) and the nearby hypoglossal nerve as it exits through the separate hypoglossal canal. An intracranial lesion extensive enough to cause a Collet-Sicard syndrome would very likely distort the lower brainstem and the cerebellum, thus causing pyramidal and cerebellar signs. The inclusion of a Horner’s syndrome to the impairment of the last four cranial nerves constitutes the clinical presentation of the Villaret’s syndrome. This always indicates an extracranial lesion, usually in the area of the posterior nasopharynx, as it affects the sympathetic chain, which does not traverse the jugular foramen, and the extracranial portion of the lower cranial nerves.
Hypoglossal Nerve
Neuroanatomy
The twelfth or hypoglossal nerve is a purely motor nerve that controls the extrinsic and intrinsic muscles of the tongue. The hypoglossal nucleus is located in the floor of the fourth ventricle, close to the midline, and the fibers traverse the cross-section of the caudal medulla before exiting through the ventral surface of the brainstem, between the pyramidal tract and the olivary eminence. The nerve leaves the skull base through the hypoglossal canal and courses briefly with the IX, X, and XI cranial nerves before it separates at the mastoid level and innervates the tongue. A unilateral lesion of the hypoglossal nerve results in ipsilateral paresis and wasting of the tongue.
Clinical Syndromes
A common cause of isolated hypoglossal nerve damage occurs as a complication of carotid endarterectomies [43], particularly due to variability in location between the hypoglossal nerve and the carotid bifurcation [44]. However, peripheral lesions may also result from radiation therapy, local tumor (e.g., salivary gland neoplasm), carotid artery aneurysm and dissection, internal jugular vein cannulation, glomus jugulare resection, and trauma to the face (Fig. 42.6). Clinically, patients with unilateral injuries to the hypoglossal nerve have unilateral atrophy of the tongue and mild difficulties manipulating food. The tongue deviates toward the side of the lesion due to the unopposed action of the healthy contralateral genioglossus muscle (see Fig. 42.6). There is tremendous difficulty moving the tongue from side to side. However, only with bilateral nerve injuries will patients encounter significant difficulties in swallowing, talking, and respiration, because the tongue falls backward and obstructs the upper airways.
Motor neuron disease, poliomyelitis, syringomyelia, tumors [45], and infarcts may cause lesions to the neurons of the hypoglossal nucleus. An infarct may assume the presentation of the medial medullary syndrome [46] (i.e., ipsilateral tongue paresis associated with a contralateral corticospinal tract lesion causing paresis of the arm and leg and medial lemniscus involvement leading to diminished proprioceptive and tactile sensation). Basilar meningitis or intracranial metastases can occasionally affect CN XII but most commonly will do so in conjunction with other lower cranial nerve lesions. Hypoglossal nerve palsy has also been reported in association with infectious mononucleosis [47] and due to subluxation of the odontoid process with rheumatoid arthritis [48].
References
Rucker CW. History of the numbering of the cranial nerves. Mayo Clin Proc. 1966;41:453–61.
Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945–1984. Neuroepidemiology. 1991;10(5–6):276–81.
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1Suppl 1:9–160.
Abhinav K, Love S, Kalantzis G, Coakham HB, Patel NK. Clinicopathological review of patients with and without multiple sclerosis treated by partial sensory rhizotomy for medically refractory trigeminal neuralgia: a 12-year retrospective study. Clin Neurol Neurosurg. 2012;114(4):361–5.
Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45(7):1294–6.
Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71(15):1183–90.
Sindrup SH, Jensen TS. Pharmacotherapy of trigeminal neuralgia. Clin J Pain. 2002;18(1):22–7.
Meyer B, Lehmberg J. Treatment options for refractory trigeminal neuralgia. World Neurosurg. 2011;77(2):275–6.
Sarsam Z, Garcia-Finana M, Nurmikko TJ, Varma TR, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. BR J Neurosurg. 2010;24(1):18–25.
Miller JD, Magill ST, Acar F, Burchiel KJ. Predictors of long-term success after microvascular decompression for trigeminal neuralgia. J Neurosurg. 2009;110:620–6.
Lecky BRF, Hughes RAC, Murray NMF. Trigeminal sensory neuropathy. Brain. 1987;110:1463–85.
Blau JN, Harris M, Kennett S. Trigeminal sensory neuropathy. N Engl J Med. 1969;281:873–6.
Horowitz SH. Isolated facial numbness. Clinical significance and relation to trigeminal neuropathy. Ann Intern Med. 1974;80:49–53.
Clouston PD, Sharpe DM, Corbett AJ, Kos S, Kennedy PJ. Perineural spread of cutaneous head and neck cancer. Its orbital and central neurologic complications. Arch Neurol. 1990;47:73–7.
Galán Gil S, Peñarrocha Diago M, Peñarrocha Diago M. Malignant mental nerve neuropathy: systematic review. Med Oral Patol Oral Cir Bucal. 2008;13:E616–21.
Lossos A, Siegal T. Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology. 1992;42:1181–4.
Furukawa T. Numb chin syndrome in the elderly. J Neurol Neurosurg Psychiatry. 1990;53:173.
Keane JR, Baloh RW. Posttraumatic cranial neuropathies. Neurol Clin. 1992;10(4):849–67.
Esslen E. The acute facial palsies – investigations on the localization and pathogenesis of Meato-labyrinthine facial palsies. New York: Springer; 1977.
May M, Blumenthal F, Klein SR. Acute Bell’s palsy: prognostic value of evoked electromyography, maximal stimulation, and other electrical tests. Am J Otol. 1983;5(1):1–7.
Furuta Y, Takasu T, Sato KC, Fukuda S, Inuyama Y, Nagashima K. Latent herpes simplex virus type 1 in human geniculate ganglia. Acta Neuropathol. 1992;84:39–44.
Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124(1):27–30.
Quant EC, Jeste SS, Muni RH, Cape AV, Bhussar MK, Peleg AY. The benefits of steroids versus steroids plus antivirals for treatment of Bell’s palsy: a meta-analysis. BMJ. 2009;339:b3354.
Adour KK. Otological complications of herpes zoster. Ann Neurol. 1994;35(Suppl):S62–4.
Shigeta S, Baba M, Ogata M, Nozaki H, Okuaki A, Nakamura S. Importance of anticomplement immunofluorescence antibody titration for diagnosing varicella-zoster virus infection in Bell’s palsy. J Clin Pathol. 1986;39:1254–8.
Uscategui T, Doreee C, Chamberlain IJ, et al. Corticosteroids as adjuvant to antiviral treatment in Ramsay Hunt Syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev, 2008;(3):CD006852.
Susuki K, Koga M. A Guillain-Barré syndrome variant with prominent facial diplegia. J Neurol. 2009;256:1899–905.
Keane JR. Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature. Neurology. 1994;44:1198–202.
Ozaki K, Irioka T, Ishida S, Mizusawa H. Bilateral facial nerve palsy caused by a metastatic malignant lymphoma. Intern Med. 2011;50(19):2247.
Hiltunen T, Kiuru S, Hongell V, Heliö T, Palo J, Peltonen L. Finnish type of familial amyloidosis: cosegregation of Asp187––Asn mutation of gelsolin with the disease in three large families. Am J Hum Genet. 1991;49(3):522–8.
Bawle EV, Conard J, Van Dyke DL, Czarnecki P, Driscoll DA. Seven new cases of Cayler cardiofacial syndrome with chromosome 22q11.2 deletion, including a familial case. Am J Med Genet. 1998;79(5):406–10.
Pollock BE, Boes CJ. Stereotactic radiosurgery for glossopharyngeal neuralgia: preliminary report of 5 cases. J Neurosurg. 2011;115(5):936–9.
Gaul C, Hastreiter P, Duncker A, Naraghi R. Diagnosis and neurosurgical treatment of glossopharyngeal neuralgia: clinical findings and 3-D visualization of neurovascular compression in 19 consecutive patients. J Headache Pain. 2011;12(5):527–34.
Patel A, Kassam A, Horowitz M, Chang YF. Microvascular decompression in the management of glossopharyngeal neuralgia: analysis of 217 cases. Neurosurgery. 2002;50(4):705–10; discussion 710–711.
Ivan ME, Sughrue ME, Clark AJ, Kane AJ, Aranda D, Barani IJ, et al. A meta-analysis of tumor control rates and treatment-related morbidity for patients with glomus jugulare tumors. J Neurosurg. 2011;114(5):1299–305.
Higgins TS, Gupta R, Ketcham AS, Sataloff RT, Wadsworth JT, Sinacori JT. Recurrent laryngeal nerve monitoring versus identification alone on post-thyroidectomy true vocal fold palsy: a meta-analysis. Laryngoscope. 2011;121(5):1009–17.
Koike H, Sobue G. Alcoholic neuropathy. Curr Opin Neurol. 2006;19(5):481–6.
Tarlaci S. Vincristine-induced fatal neuropathy in non-Hodgkin’s lymphoma. Neurotoxicology. 2008;29(4):748–9.
Chandawarkar RY, Cervino AL, Pennington GA. Management of Iatrogen Injury to The Spinal Acce Nerve. Plast Reconstr Surg. 2003;111(2):611–17.
Sweeney PJ, Wilbourn AJ. Spinal accessory (11th) nerve palsy following carotid endarterectomy. Neurology. 1992;42:674–5.
Paljarvi LP. Biting palsy of the accessory nerve. J Neurol Neurosurg Psychiatry. 1980;43:744–6.
Pierre PA, Laterre CE, Van den Bergh PY. Neuralgic amyotrophy with involvement of cranial nerves IX, X, XI and XII. Muscle Nerve. 1990;13(8):704–7.
Maniglia AJ, Hans DP. Cranial nerve injuries following carotid endarterectomy: an analysis of 336 procedures. Head Neck. 1991;13:121–4.
Kim T, Chung S, Lanzino G. Carotid artery-hypoglossal nerve relationships in the neck: an anatomical work. Neurol Res. 2009;31(9):895–9.
Keane JR. Twelfth-nerve palsy. Analysis of 100 cases. Arch Neurol. 1996;53:561–6.
Kim JS, Kim HG, Chung CS. Medial medullary syndrome: report of 18 new patients and a review of the literature. Stroke. 1995;26:1548–52.
Parano E, Giuffrida S, Restivo D, Saponara R, Greco F, Trifiletti RR. Reversible palsy of the hypoglossal nerve complicating infectious mononucleosis in a young child. Neuropediatrics. 1998;29(1):46–7.
Konishi A, Higo R, Nito T, Masumoto T, Seichi A. A case report of unilateral hypoglossal neuroparalysis resulting from horizontal subluxation in the atlanto-occipital joint due to rheumatoid arthritis. ORL J Otorhinolaryngol Relat Spec. 2003;65(5):306–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kesner, V.G., Fournier, C. (2014). Cranial Nerve Palsies. In: Katirji, B., Kaminski, H., Ruff, R. (eds) Neuromuscular Disorders in Clinical Practice. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6567-6_42
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6567-6_42
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6566-9
Online ISBN: 978-1-4614-6567-6
eBook Packages: MedicineMedicine (R0)