Abstract
Endosomes play key roles in the control and execution of such diverse and spatially restricted processes as cell signaling, epithelial to mesenchymal transitions as well as cell adhesion and migration. The endocytosis of growth factor receptors and adhesion molecules, such as cadherins and integrins, is coming into focus as a major mechanism in the regulation of cellular processes that govern cell growth, differentiation, survival, and motility. Subversion of these pathways accompanies disease progression, especially cancer. We suggest that endosomes are multifunctional dynamic platforms on which unique sets of molecular components are assembled and sorted to adapt to different environmental and cellular cues. A better understanding of how endosomes can function as conduits for the acquisition of oncogenic phenotypes will lead to more specific therapeutic approaches to combat cancer progression.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epidermal Growth Factor Receptor
- Hepatocyte Growth Factor
- Early Endosome
- Epidermal Growth Factor Receptor Signaling
- Signal Transduction Process
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
Although endocytosis has long been regarded as a conduit for the internalization and degradation of nutrients and cell surface receptors, it is now accepted that the endosomal membrane system plays a vital role in the control and execution of spatially restricted functions, such as cell adhesion and motility. Cell adhesion is essential for the maintenance of multicellularity in living organisms. Intercellular adhesion and cell-to-extracellular matrix (ECM) adhesion are a result of the assembly and functional integrity of “adhesion complexes” at sites of cell–cell or cell–ECM contacts, respectively [34]. These complexes consist of transmembrane adhesion molecules coupled to intracellular scaffold or signaling proteins and the cytoskeleton. Cadherin family cell adhesion molecules and their associated scaffold proteins, the catenins, are major components of cell–cell adhesive contacts, namely, the adherens junctions and desmosomes [34]. The major transmembrane protein at cell–ECM adhesive contacts (i.e., focal adhesions and hemidesmosomes) is the heterodimeric integrin receptors [34]. These cell–cell and cell–ECM adhesion complexes are linked to and stabilized by the actin or intermediate filaments but undergo significant remodeling during the acquisition of migratory and invasive phenotypes in tumor cells [93, 94]. In the initial stages of tumor progression, tumor cells sometimes undergo epithelial–mesenchymal transition, which requires the disruption of cell–cell adhesions [5]. The trafficking of cadherins along the endocytic pathway is now accepted as an important mechanism involved in the remodeling of cell–cell adhesions [39]. Some cancer cells maintain or reestablish cell–cell adhesions during metastasis and their collective migration requires that both cell–cell and cell–ECM adhesions stay intact [25]. Mesenchymal tumor cells will migrate and invade into the basement membrane and surrounding stromal tissues [54]. To facilitate migration and invasion, tumor cells continuously form new cell–ECM adhesions at the leading edge, whereas focal adhesions at the trailing edge are disrupted by endocytosis.
Besides serving as carriers and sorting stations for the trafficking of adhesion molecules, emerging evidence suggests that endosomal compartments are also essential sites of signal transduction [53, 77, 84]. Activated receptors can function in endosomes, and certain signaling components are localized, even exclusively, to endosomes. Signals transmitted from endosomes are robust and typically long-lived, different from those that arise from the plasma membrane. Endosomal signaling is widespread across species and regulates essential processes including growth and differentiation in addition to cell adhesion and motility. Subversion of the mechanisms involved is predicted to play an important role in several human diseases and, most especially, cancer. Pharmacological agents that target receptor signaling at the plasma membrane have proved to be effective therapeutics for some cancers [53]. Thus, selective disruption of receptor signaling in endosomes, which can be accomplished by targeting endosomal-specific signaling pathways that are altered in cancers, could also provide novel therapies for tumor progression.
In this review chapter, we discuss current evidence coupling endocytosis and the regulation of signaling pathways in cells and how altered regulation of these pathways can lead to acquisition of oncogenic phenotypes. We also describe how endocytosis and recycling of adhesion molecules, such as cadherins and integrins, is coming into focus as a major mechanism in the regulation of adhesive and migratory properties of cells.
6.2 Endocytosis Regulates Signal Transduction
Endocytosis plays an important role in the duration and extent of signal transduction via controlling the number and accessibility of cell surface receptors [52, 82, 84]. Signal transduction starts at the cell surface when ligands such as growth factors and hormones bind to their cognate receptors, which recruit and activate signaling molecules such as adaptors and enzymes (e.g., kinases) that further activate downstream effectors to amplify the signal transduction processes ultimately leading to regulation of gene expression, cell proliferation, or cell differentiation. Malfunction of the signal transduction processes is a major cause of cancer.
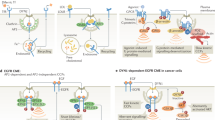
Receptor tyrosine kinases (RTKs) constitute a large family of receptors whose signal transduction processes promote cell proliferation or differentiation. A well-characterized example is the epidermal growth factor receptor (EGFR). Ligand (EGF) binding causes conformational changes, dimerization, and tyrosine phosphorylation of EGFR, which activates phosphoinositide 3-kinase (PI3-K), phospholipase Cγ (PLCγ), and the mitogen-activated protein (MAP) kinase pathways at the cell surface. The ligand also induces endocytosis of EGFR into endosomes and eventually intraluminal vesicles (ILVs) and lysosomes for degradation, which reduces the number of active EGFR molecules and attenuates the signaling in the so-called “downregulation” process. This process is delayed under hypoxia conditions characteristic of cancer cells where hypoxia-inducible factor HIF1α blocks Rabaptin-5 expression, leading to reduced Rab5-mediated endosome fusion and endocytosis [91]. As a result, EGFR signaling is prolonged with increased cell proliferation.
EGFR in endosomes, before delivery to ILVs and lysosomes, may remain engaged to the ligand, and its cytoplasmic domain may continue signaling. Indeed, the first evidence of endosomes serving as a platform for EGFR signaling inside the cell came from a study that showed reduced phosphorylation/activation of MAP kinases in the cells where endocytosis was blocked by a dynamin mutant [90]. This concept was later generalized as the signaling endosome hypothesis [32]. Signaling endosomes not only continue the signaling processes initiated at the cell surface but also gain access to new signaling molecules and start new signaling processes. For example, EGFR can activate the small GTPase Rab5 via RIN1 on endosomes [85]. Rab5-GTP and certain receptors can directly recruit APPL1 to endosomes, which activates Akt and regulates its substrate specificity for GSK-3β, a process critical for cell survival and embryonic development in zebrafish and possibly other vertebrates [76]. On the other hand, an intriguing recent observation suggests that accumulation of activated EGFR on endosomes via blocking ILV formation triggers apoptosis [72], although the signaling pathway is yet to be established. It is clear that the endosomes provide a membrane environment distinct from that of the plasma membrane and their mobility inside cells allows access to additional signaling molecules leading to new functional consequences.
Endosomal transport is critical for retrograde signaling by nerve growth factor (NGF) and its receptor (TrkA) in neuronal survival, migration, axon growth, and target cell innervation [3, 10]. In this case, target-derived NGF binding at the tip of the axon induces endocytosis of the NGF–TrkA complex into the signaling endosomes that start signaling locally inside the axon and undergo retrograde transport along microtubules towards the cell body/soma. The signaling endosomes carry signaling molecules such as the activated TrkA, ERKs, and Akt [28, 32] as well as specific transcription factors (e.g., CREB) translated in the axon [11] to activate specific nuclear gene expression in the soma, including a positive feedback loop of increased expression of TrkA [48]. The functional consequences are the aforementioned neurotrophic effects.
Endosomal membrane is enriched with PI3P, which recruits PI3P-binding signaling molecules such as the FYVE domain-containing proteins important for TGFβ receptor- and G protein-coupled receptor (GPCR)-mediated signal transduction processes. In the case of TGFβ signaling, the TGFβ receptor is endocytosed into endosomes where it interacts with SMAD anchor for receptor activation (SARA), a FYVE domain-containing adaptor protein that recruits SMAD2 to the endosomes for phosphorylation by the TGFβ receptor [88]. The phosphorylated SMAD2 then forms a complex with SMAD4, followed by translocation to the nucleus for activation of gene expression. In the case of GPCR signaling, activated G protein on endosomes can activate the PI3K Vps34 to produce PI3P that in turn recruits FYVE domain-containing proteins to promote MAPK and Cdc42 signaling pathways [84].
Endocytosis also plays an important role in Notch-mediated signal transduction and neuronal cell proliferation [22, 39]. Both Notch and its ligands (the Delta/Serrate/Lag2 (Dsl) domain-containing proteins) are transmembrane proteins on apposing cells. Ligand binding leads to two consecutive proteolytic cleavages (S1 and S2) in the ectodomain of Notch. The C-terminal fragment of Notch is then endocytosed, followed by another cleavage (S3) in the transmembrane domain by γ secretase to release the cytoplasmic intracellular domain that translocates to the nucleus and activates expression of target genes. Interestingly, the ligand itself, e.g., Delta, requires endocytosis and recycling to concentrate at specific regions on the plasma membrane for efficient binding and activation of Notch on the signal-receiving cells [22, 39].
An important mechanism that controls the endocytosis and/or endosomal sorting of the signaling receptors involves ubiquitination at their cytoplasmic domains. While polyubiquitination with the Lys-48 linkage marks the substrate for degradation by the proteosome, polyubiquitination with the Lys-63 linkage and monoubiquitination may regulate other functions of the substrate including endocytosis and endosomal sorting [84]. Ubiquitination is necessary for the endocytosis of Ste2, a GPCR, in yeast [29], but it is not essential for other GPCRs and RTKs in animal cells [20, 79]. In the latter case, ubiquitination may still increase the interaction of these signaling receptors with components of clathrin-coated pits to facilitate their endocytosis [2, 36]. Importantly, ubiquitination is critical for subsequent sorting of signaling receptors into ILVs/MVBs [20, 79], via interaction with the ESCRT complexes, to terminate the signal [35, 81, 82]. Consistently, ubiquitination-deficient EGFR shows increased signaling activity. A well-documented E3 ubiquitin ligase, Cbl, is responsible for the ubiquitination of various RTKs. Mutations that abrogate Cbl ubiquitin ligase activity are known to cause cancer such as myeloid leukemia and lung cancer [74, 86], suggesting that ubiquitination-mediated RTK sequestration in ILVs/MVBs and signal termination are critical for normal signal transduction processes and cell growth. In the case of Notch, non-ligand-bound Notch is ubiquitinated by the E3 ligase ITCH, endocytosed and delivered to lysosomes for degradation [8].
In addition to signaling receptors, ubiquitination of downstream signaling molecules on the plasma membrane, such as the Ras GTPases (H- and N-Ras), leads to endocytosis and signal downregulation in both mammalian cells and Drosophila where the system controls organ development [96, 97]. The E3 ubiquitin ligase responsible for Ras ubiquitination is Rabex-5 [96, 97], which was originally identified as a GEF for activation of the endosome-associated Rab5 and endosome fusion [31]. Rabex-5 targets early endosomes and plasma membrane by binding to Rab22 [99, 100] or by forming a complex with Rabaptin-5 that in turn binds to Rab5 [31]. The K-Ras isoform, on the other hand, is not ubiquitinated, but a recent study shows that a fraction of K-Ras is endocytosed via the clathrin-dependent pathway and follows the conventional endocytic pathway to early endosomes, late endosomes/MVBs, and lysosomes [46]. Interestingly, K-Ras is able to recruit Raf1 to elicit signal transduction on late endosomes [46], which normally degrade endocytosed cargoes and reduce signaling.
Endocytosed receptors are not always destined to degradation in late endosomes and lysosomes; instead they can be recycled to the plasma membrane for reutilization and additional rounds of signaling, depending on the types of ligands and endocytic portals. For example, TGFα also interacts with EGFR but its affinity is lower than EGF and readily dissociates from the receptor in early endosomes [19, 24]. As a result, the receptor is sorted back to the plasma membrane, due to insufficient ubiquitination [45], which likely contributes to the high potency of TGFα in promoting tumor cell growth [56]. In addition, receptors can be endocytosed via clathrin-dependent or clathrin-independent pathways or both [17, 81]. In the case of EGFR, low concentrations of EGF induce clathrin-dependent endocytosis while high concentrations of EGF promote clathrin-independent endocytosis [81]. The former pathway largely recycles EGFR back to the plasma membrane for continual signaling and the latter pathway promotes EGFR traffic to late endosomes and lysosomes for degradation and signal attenuation [81]. In addition to the RTKs, some GPCRs such as the β2 adrenergic receptor (β2AR) require endocytosis and recycling to be resensitized for sustained signaling. In this case, β2AR can be inactivated by phosphorylation at the plasma membrane and endocytosis allows β2AR to gain access to the endosome-associated phosphatase 2A for dephosphorylation and resensitization [66]. The active β2AR is then recycled back to the plasma membrane, via a Rab4-dependent fast recycling pathway, for ligand binding and new rounds of signaling [21, 59, 78].
6.3 EMT in Cancer
Most solid tumors are epithelial in origin. A loss of epithelial cell markers and concomitant acquisition of mesenchymal cell markers have been observed in some epithelial tumors, such as non-small cell lung cancer (NSCLC) and pancreatic, colorectal, and hepatocellular cancers particularly at the invasive front [37, 87]. This profound phenotypic conversion, referred to as epithelial to mesenchymal transition (EMT), is orchestrated by integrated networks of signal transduction pathways that direct marked alterations in cell adhesion and motility. Although EMT is best known for its role in embryonic development, in the adult, several oncogenic pathways (growth factors, Src, Ras, Wnt/beta-catenin, and Notch) may induce EMT [87].
Although there are accumulating data to suggest a critical role for EMT in cancer progression, the demonstration of this process in human cancer has been controversial [67]. Moreover, the acquisition of mesenchymal phenotypes is not a prerequisite for cell migration/invasion and in many cases appears to be characteristic of only a few cells at the invasive fronts in a tumor mass. The evidence to date suggests that the induction of EMT depends on the tumor type and its genetic alterations as well as on its interaction with the extracellular matrix [67]. Over the last decade, however, it’s been shown that some cancer cells reactivate EMT in an effort to escape their normal boundaries [67]. The loss of epithelial cell markers (e.g., E-cadherin) is associated with disease progression and metastatic potential of a tumor. There is accruing evidence that cancer cells can dedifferentiate through activation of specific biological pathways associated with EMT, thereby gaining the ability to migrate and invade. Hence, what has been observed experimentally regarding EMT and normal embryonic development is also thought to apply in the progression of solid tumors—a cellular reprogramming process whereby epithelial tumor cells lose cell polarity and cell junction proteins and at the same time gain signal transduction activities associated with cell invasion and survival in an anchorage-independent environment. Mesenchymal-like tumor cells gain migratory capacity at the expense of proliferative potential. Cellular changes resulting in EMT in cancer are thought to play a major role in disease progression and have been associated with poor prognosis in patients [37].
6.4 Endocytic Trafficking of Cadherins in EMT
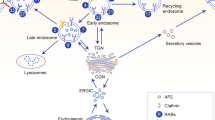
A critical molecular event underpinning the dissolution of cell–cell contacts during EMT is the loss of the cell–cell adhesion molecule, E-cadherin, a key component of the adherens junctions [87]. EMT and metastatic progression are most often associated with a reversible downregulation of E-cadherin (encoded by CDH1) involving either hypermethylation of the CDH1 promoter or repression by EMT-inducing transcription factors [5, 87]. In particular, EMT is accompanied by the activation of two related zinc finger-containing transcription factors, Snail and Slug. The basic helix–loop–helix proteins, Twist 1 and Twist 2, and the ZEB family proteins have also been shown to induce EMT via transcription regulation. Notably, however, in addition to transcriptional downregulation, posttranscriptional regulation of adhesive structures can also markedly influence the progression of EMT [12]. The endocytosis and lysosomal degradation of E-cadherin as described below is one such cellular mechanism that can have a profound impact on the initial stages of EMT.
The cytoplasmic domain of E-cadherin contains a dileucine motif, which is a binding site for clathrin adaptor complexes, and mutations in the motif inhibit E-cadherin endocytosis [50]. The E-cadherin dileucine motif also binds to p120ctn, which in polarized poorly motile epithelia masks the dileucine motif to prevent the endocytosis of E-cadherin [51]. Cellular depletion of p120ctn results in the internalization of cadherins and loss of cell–cell contacts [9, 14, 95]. The binding of an adaptor molecule, Numb, to p120ctn negates the p120ctn-mediated suppression of E-cadherin endocytosis since Numb recruits the AP-2 clathrin adaptor complex promoting endocytosis [75]. Numb can also interact with the NVYYY motif on E-cadherin, which in turn can hinder the p120ctn–E-cadherin interaction [92].
Growth factors such as HGF, as well as oncogenic v-Src, can also induce epithelial–mesenchymal transition (EMT) in part by promoting the endocytosis of cadherin molecules [12, 94]. Src-mediated phosphorylation of the NVYYY motif on E-cadherin induces the dissociation of p120ctn and recruits a c-Cbl-related E3 ubiquitin ligase, Hakai [26]. Hakai induces the ubiquitination of E-cadherin and subsequently its endocytosis and lysosomal degradation, which require the activation of the Rab small GTP-binding proteins, Rab5 and Rab7 [63]. HGF treatment or v-Src expression activates ARF6, an ARF family small GTP-binding protein, which enhances E-cadherin endocytosis [60]. In Madin–Darby Canine Kidney (MDCK) cells, ARF6-GTP recruits the nucleoside diphosphate kinase, Nm23-H1, initiating a downregulation of Rac1 activity and promoting the clathrin-dependent endocytosis of E-cadherin to the early endosome, both of which facilitate the disassembly of the adherens junctions [62]. Furthermore, upon Src-induced loss of cell–cell contacts, internalized E-cadherin is targeted to the lysosome for degradation in an ARF6-dependent manner so that it cannot be recycled to the plasma membrane, thus ensuring that cell–cell contacts will not be reformed. The TBC/RabGAP Armus is thought to play a role in this process, bridging signaling between ARF6, Rac1, and Rab7 [23]. Armus was shown to bind Rac1 and locally facilitate lysosome biogenesis and the degradation of E-cadherin. Inhibition of ARF6 activity, by expression of a dominantly interfering mutant, ARF6(T27N), enhances the epithelial phenotype by preventing the internalization of E-cadherin into endosomal compartments and thereby blocking hepatocyte growth factor (HGF) and Src-induced cell scattering [60, 61]. In addition, the downregulation of ARF6 activity, established by a positive feedback loop between EphA2 and E-cadherin, has been shown to enhance E-cadherin-based adhesion and the maturation of apical–basal polarity in MDCK cells [49]. The formation of stable adherens junctions during epithelial cell polarization is dependent on the spatially regulated activation of ARF6, which is modulated by the formation of a complex between FRMD4A/GRSP-1/PAR3 and the ARF6-GEF cytohesin-1 at cell junctions [33]. HGF also induces Ras-mediated activation of RIN2, an activator for Rab5, which also facilitates the endocytosis of E-cadherin [38].
The aforementioned effects of ARF6 on the internalization of E-cadherin and other cell surface receptors have also been shown to impact epithelial glandular organization in 3D cell cultures [89]. In this regard, sustained ARF6 activation in basement membrane cultures of epithelial cysts, a structural unit of epithelial glandular organs, leads to the internalization of E-cadherin as well as growth factor receptors by ARF6-regulated pathways. Sustained signaling from endosomes (described further below), in turn, leads to the formation of aberrant glandular morphologies, reminiscent of tumorigenic phenotypes seen in vivo [16].
In addition to clathrin-mediated endocytosis, caveolin-mediated endocytosis is also involved in E-cadherin internalization in some cell types [1, 47]. EGF treatment of MCF-7 cells, on the other hand, promotes E-cadherin into endosomes via clathrin-independent pathways [64]. While multiple internalization routes have been implicated in E-cadherin endocytosis, collectively these findings show that the cycling of E-cadherin along the endosomal pathway can markedly impinge on the dynamics of the adherens junctions of epithelial tissues and loss of the epithelial phenotype.
6.5 Endocytic Trafficking of Integrins and Acquisition of Motile Phenotypes
Migrating and invading cells display an increased internalization and recycling of integrins from the retracting edge of the cell to the leading edge and at sites of cell invasion. β1 integrin receptors have been localized to clathrin-coated pits [15] where the endocytic adaptors Numb and Dab2 bind to integrin receptors to facilitate internalization [57]. HS1-associated protein X-1 (HAX-1) has been shown to bind to the cytoplasmic tail of β6 integrin to facilitate the clathrin-mediated internalization of αvβ6 integrin receptors, which in turn enhances migration and invasion of oral sqaumous carcinoma cell lines [69]. While the above is one example, integrin trafficking has been associated with migratory potential of several tumor cells lines [54, 70]. Integrin endocytosis is also thought to facilitate the uptake of ECM proteins such as fibronectin and vitronectin and transport of these molecules to the lysosomes [43, 80].
In actively migrating cells, ARF6 and Rab family GTPases have been linked to the trafficking of integrin receptors. In this regard, β1 integrin has been shown to localize to ARF6-regulated recycling endosomes [6, 68]. Expression of dominant-negative ARF6 inhibits the cell’s ability to recycle this endosomal compartment to the plasma membrane leading to a decrease in receptors at the migrating edge [6, 68]. In addition, the recycling of β1 integrin is regulated by an ARF6-GAP, ACAP1; the inhibition of ACAP1 or its phosphorylation by Akt inhibits integrin recycling and cell migration [41]. The ARF6 GEF, GEP100, which is upregulated in breast cancers, has also been implicated in the trafficking of β1 integrin receptors [18, 73]. The migratory ability of cells is also modulated by engagement with the extracellular matrix and requires ARF6-mediated activation of Rac1 for the formation of protrusive structures such as lamellipodia at the leading edge [13]. The formation of an α4 integrin–paxillin–Arf-GAP complex at the trailing edge of the cell assists in directional migration by inhibiting ARF6 activity, thereby blocking adhesion-dependent Rac1 activation and the extension of lamellipodia [58]. Slit2-Robo signaling can block the ARF6 and Rac1 activation induced by integrin engagement, important in pathologies of endothelial cell protrusive activity [44].
A subset of Rab GTPases on the plasma membrane, early and recycling endosomes control the integrin trafficking, and altered expression levels of these Rabs are often associated with various types of cancer [40]. Rab5 and Rab21 are associated with the plasma membrane and early endosomes and regulate the internalization of β1 integrins via direct interaction with their α subunits [65]. Reduction of Rab5 or Rab21 in carcinoma-associated fibroblasts can decrease α5 integrin at the plasma membrane and remodeling of cell–extracellular matrix interaction, which is required for the invasion of squamous cell carcinoma [30]. Indeed, Rab5 is highly expressed in lung adenocarcinoma and hepatocellular carcinoma [27, 42]. The Rab11 subfamily members (Rab11 and Rab25) are localized in a perinuclear recycling compartment that controls a slow recycling pathway important for trafficking of integrins back to the plasma membrane at the leading edge. Rab11 appears to collaborate with ARF6 to control the exit of integrins from the perinuclear recycling compartment [68]. Importantly, Rab11 controls α6β4 integrin recycling involved in hypoxia-induced breast cancer cell invasion [98]. Rab25 is related to Rab11 and directly binds to the β1 subunit of α5β1 integrin to facilitate its recycling to the leading edge of the plasma membrane, a process implicated in cancer cell invasion and metastasis [7]. Overexpression of Rab25 is well documented in many types of cancers [40] including ovarian cancer, breast cancer, testicular tumor, Wilms tumor, and bladder and hepatocellular carcinomas. Interestingly, Rab25 expression is decreased in colorectal adenocarcinomas, which correlates with poor prognosis of colon cancer patients [55]. In this regard, mouse models of colon cancer show that loss of Rab25 can increase colonic tumor formation [55]. It is not yet clear why different types of cancers require overexpression and loss of Rab25, respectively, to promote cell invasion and aggressiveness. In contrast to Rab11/Rab25-mediated slow recycling, Rab4 is localized in a fast recycling compartment and facilitates the fast recycling of certain integrins (e.g., αvβ3) to the cell surface in response to the platelet-derived growth factor (PDGF) [71].
6.6 Concluding Remarks
Endocytosis has emerged as an important regulatory mechanism in signal transduction/cell proliferation, cell polarity/EMT, and cell adhesion/invasion, in addition to its conventional role in the uptake and digestion of nutrients. The regulatory function of endocytosis in these processes involves internalization and sorting of signaling receptors, cell junction molecules (e.g., cadherins), and integrins to degradative or recycling pathway. Aberrant endocytic machinery and unbalanced endocytosis can contribute to uncontrolled cell proliferation, EMT, and aggressive cell invasion, which are hallmarks of cancer cells. Indeed, altered expression and/or mutations in endocytic genes are frequently found in tumors [82]. One well-documented example is Cbl, an E3 ubiquitin ligase involved in ubiquitination and endocytosis of signaling receptors. Inactivating mutations in Cbl are associated with myeloid malignancies [74]. In addition, mutations in the cytoplasmic domain of signaling receptors may abrogate their endocytosis and reduce degradation, contributing to tumorigenesis. For example, ErbB-2, an endocytosis-defective variant of EGFR, shows strong transforming effect and is highly expressed in breast cancer [4, 83]. A better understanding of the endocytic machinery that regulates the degradation and recycling of signaling receptors, cadherins, and integrins should help develop novel and specific therapeutics against cancer.
References
Akhtar N, Hotchin NA (2001) RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 12:847–862
Arevalo JC, Waite J, Rajagopal R, Beyna M, Chen ZY, Lee FS, Chao MV (2006) Cell survival through Trk neurotrophin receptors is differentially regulated by ubiquitination. Neuron 50:549–559
Ascano M, Bodmer D, Kuruvilla R (2012) Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol 22:266–273
Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G (1996) All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem 271:5251–5257
Berx G, van Roy F (2009) Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1:a003129
Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG (2001) Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol 154:1007–1017
Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC (2007) Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13:496–510
Chastagner P, Israel A, Brou C (2008) AIP4/Itch regulates Notch receptor degradation in the absence of ligand. PLoS One 3:e2735
Chen X, Kojima S, Borisy GG, Green KJ (2003) p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol 163:547–557
Cosker KE, Courchesne SL, Segal RA (2008) Action in the axon: generation and transport of signaling endosomes. Curr Opin Neurobiol 18:270–275
Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR (2008) Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol 10:149–159
D’Souza-Schorey C (2005) Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol 15:19–26
D’Souza-Schorey C, Chavrier P (2006) ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7:347–358
Davis MA, Ireton RC, Reynolds AB (2003) A core function for p120-catenin in cadherin turnover. J Cell Biol 163:525–534
De Deyne PG, O’Neill A, Resneck WG, Dmytrenko GM, Pumplin DW, Bloch RJ (1998) The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J Cell Sci 111(Pt 18):2729–2740
Debnath J, Brugge JS (2005) Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer 5:675–688
Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL (2003) Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol 5:410–421
Dunphy JL, Moravec R, Ly K, Lasell TK, Melancon P, Casanova JE (2006) The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins. Curr Biol 16:315–320
Ebner R, Derynck R (1991) Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul 2:599–612
Eden ER, Huang F, Sorkin A, Futter CE (2011) The role of EGF receptor ubiquitination in regulating its intracellular traffic. Traffic 13:329–337
Filipeanu CM, Zhou F, Lam ML, Kerut KE, Claycomb WC, Wu G (2006) Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes. J Biol Chem 281:11097–11103
Fortini ME, Bilder D (2009) Endocytic regulation of Notch signaling. Curr Opin Genet Dev 19:323–328
Frasa MA, Maximiano FC, Smolarczyk K, Francis RE, Betson ME, Lozano E, Goldenring J, Seabra MC, Rak A, Ahmadian MR, Braga VM (2010) Armus is a Rac1 effector that inactivates Rab7 and regulates E-cadherin degradation. Curr Biol 20:198–208
French AR, Sudlow GP, Wiley HS, Lauffenburger DA (1994) Postendocytic trafficking of epidermal growth factor-receptor complexes is mediated through saturable and specific endosomal interactions. J Biol Chem 269:15749–15755
Friedl P, Hegerfeldt Y, Tusch M (2004) Collective cell migration in morphogenesis and cancer. Int J Dev Biol 48:441–449
Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4:222–231
Fukui K, Tamura S, Wada A, Kamada Y, Igura T, Kiso S, Hayashi N (2007) Expression of Rab5a in hepatocellular carcinoma: possible involvement in epidermal growth factor signaling. Hepatol Res 37:957–965
Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC (1996) Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci 16:7950–7964
Hicke L, Riezman H (1996) Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84:277–287
Hooper S, Gaggioli C, Sahai E (2010) A chemical biology screen reveals a role for Rab21-mediated control of actomyosin contractility in fibroblast-driven cancer invasion. Br J Cancer 102:392–402
Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, Zerial M (1997) A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90:1149–1159
Howe CL, Mobley WC (2004) Signaling endosome hypothesis: A cellular mechanism for long distance communication. J Neurobiol 58:207–216
Ikenouchi J, Umeda M (2010) FRMD4A regulates epithelial polarity by connecting Arf6 activation with the PAR complex. Proc Natl Acad Sci U S A 107:748–753
Juliano RL (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42:283–323
Katzmann DJ, Odorizzi G, Emr SD (2002) Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 3:893–905
Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rodland MS, Traub LM, Stang E, Madshus IH (2009) Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic 10:235–245
Keshamouni VG, Schiemann WP (2009) Epithelial-mesenchymal transition in tumor metastasis: a method to the madness. Future Oncol 5:1109–1111
Kimura T, Sakisaka T, Baba T, Yamada T, Takai Y (2006) Involvement of the Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J Biol Chem 281:10598–10609
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Li G (2011) Rab GTPases, membrane trafficking and diseases. Curr Drug Targets 12:1188–1193
Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW (2005) Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell 9:663–673
Li Y, Meng X, Feng H, Zhang G, Liu C, Li P (1999) Over-expression of the RAB5 gene in human lung adenocarcinoma cells with high metastatic potential. Chin Med Sci J 14:96–101
Lobert VH, Brech A, Pedersen NM, Wesche J, Oppelt A, Malerod L, Stenmark H (2010) Ubiquitination of alpha 5 beta 1 integrin controls fibroblast migration through lysosomal degradation of fibronectin-integrin complexes. Dev Cell 19:148–159
London NR, Li DY (2011) Robo4-dependent Slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Curr Opin Hematol 18:186–190
Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH (2002) Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol 156:843–854
Lu A, Tebar F, Alvarez-Moya B, Lopez-Alcala C, Calvo M, Enrich C, Agell N, Nakamura T, Matsuda M, Bachs O (2009) A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J Cell Biol 184:863–879
Lu Z, Ghosh S, Wang Z, Hunter T (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499–515
Miller FD, Mathew TC, Toma JG (1991) Regulation of nerve growth factor receptor gene expression by nerve growth factor in the developing peripheral nervous system. J Cell Biol 112:303–312
Miura K, Nam JM, Kojima C, Mochizuki N, Sabe H (2009) EphA2 engages Git1 to suppress Arf6 activity modulating epithelial cell-cell contacts. Mol Biol Cell 20:1949–1959
Miyashita Y, Ozawa M (2007) A dileucine motif in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin to the lysosome. J Cell Sci 120:4395–4406
Miyashita Y, Ozawa M (2007) Increased internalization of p120-uncoupled E-cadherin and a requirement for a dileucine motif in the cytoplasmic domain for endocytosis of the protein. J Biol Chem 282:11540–11548
Mosesson Y, Mills GB, Yarden Y (2008) Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer 8:835–850
Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW (2009) Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A 106:17615–17622
Nagano M, Hoshino D, Koshikawa N, Akizawa T, Seiki M (2012) Turnover of focal adhesions and cancer cell migration. Int J Cell Biol 2012:310616
Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, Aronow BJ, Yeatman TJ, Bhartur SG, Calhoun BC, Condie B, Manley NR, Beauchamp RD, Coffey RJ, Goldenring JR (2010) Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest 120:840–849
Nicholson RI, Gee JM, Harper ME (2001) EGFR and cancer prognosis. Eur J Cancer 37(Suppl 4):S9–S15
Nishimura T, Kaibuchi K (2007) Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell 13:15–28
Nishiya N, Kiosses WB, Han J, Ginsberg MH (2005) An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol 7:343–352
Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW 2nd (2004) Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A 101:7082–7087
Palacios F, D’Souza-Schorey C (2003) Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J Biol Chem 278:17395–17400
Palacios F, Price L, Schweitzer J, Collard JG, D’Souza-Schorey C (2001) An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J 20:4973–4986
Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C (2002) ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4:929–936
Palacios F, Tushir JS, Fujita Y, D’Souza-Schorey C (2005) Lysosomal targeting of E-cadherin: a unique mechanism for the down-regulation of cell-cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol 25:389–402
Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS (2003) Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound E-cadherin. J Biol Chem 278:21050–21057
Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol 173:767–780
Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ (1995) The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci U S A 92:8343–8347
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW (2004) Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5:20–36
Ramsay AG, Keppler MD, Jazayeri M, Thomas GJ, Parsons M, Violette S, Weinreb P, Hart IR, Marshall JF (2007) HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin alphavbeta6. Cancer Res 67:5275–5284
Ramsay AG, Marshall JF, Hart IR (2007) Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev 26:567–578
Roberts M, Barry S, Woods A, van der Sluijs P, Norman J (2001) PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol 11:1392–1402
Rush JS, Quinalty LM, Engelman L, Sherry DM, Ceresa BP (2012) Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem 287:712–722
Sabe H, Hashimoto S, Morishige M, Hashimoto A, Ogawa E (2008) The EGFR-GEP100-Arf6 pathway in breast cancer: Full invasiveness is not from the inside. Cell Adh Migr 2:71–73
Sanada M, Suzuki T, Shih LY, Otsu M, Kato M, Yamazaki S, Tamura A, Honda H, Sakata-Yanagimoto M, Kumano K, Oda H, Yamagata T, Takita J, Gotoh N, Nakazaki K, Kawamata N, Onodera M, Nobuyoshi M, Hayashi Y, Harada H, Kurokawa M, Chiba S, Mori H, Ozawa K, Omine M, Hirai H, Nakauchi H, Koeffler HP, Ogawa S (2009) Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature 460:904–908
Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, Kaibuchi K (2011) Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol Biol Cell 22:3103–3119
Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M (2008) The endosomal protein App l1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133:486–497
Scita G, Di Fiore PP (2010) The endocytic matrix. Nature 463:464–473
Seachrist JL, Anborgh PH, Ferguson SS (2000) beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem 275:27221–27228
Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ (2001) Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294:1307–1313
Shi F, Sottile J (2008) Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J Cell Sci 121:2360–2371
Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP (2008) Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15:209–219
Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP (2012) Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev 92:273–366
Sorkin A, Di Fiore PP, Carpenter G (1993) The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene 8:3021–3028
Sorkin A, von Zastrow M (2009) Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10:609–622
Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF (2001) Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell 1:73–82
Tan YH, Krishnaswamy S, Nandi S, Kanteti R, Vora S, Onel K, Hasina R, Lo FY, El-Hashani E, Cervantes G, Robinson M, Hsu HS, Kales SC, Lipkowitz S, Karrison T, Sattler M, Vokes EE, Wang YC, Salgia R (2010) CBL is frequently altered in lung cancers: its relationship to mutations in MET and EGFR tyrosine kinases. PLoS One 5:e8972
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL (1998) SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95:779–791
Tushir JS, Clancy J, Warren A, Wrobel C, Brugge JS, D’Souza-Schorey C (2010) Unregulated ARF6 activation in epithelial cysts generates hyperactive signaling endosomes and disrupts morphogenesis. Mol Biol Cell 21:2355–2366
Vieira AV, Lamaze C, Schmid SL (1996) Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274:2086–2089
Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M (2009) Regulation of endocytosis via the oxygen-sensing pathway. Nat Med 15:319–324
Wang Z, Sandiford S, Wu C, Li SS (2009) Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 28:2360–2373
Weber GF, Bjerke MA, DeSimone DW (2011) Integrins and cadherins join forces to form adhesive networks. J Cell Sci 124:1183–1193
Wu WJ, Hirsch DS (2009) Mechanism of E-cadherin lysosomal degradation. Nat Rev Cancer 9:143; author reply 143
Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP (2003) Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol 163:535–545
Xu L, Lubkov V, Taylor LJ, Bar-Sagi D (2010) Feedback regulation of Ras signaling by Rabex-5-mediated ubiquitination. Curr Biol 20:1372–1377
Yan H, Jahanshahi M, Horvath EA, Liu HY, Pfleger CM (2010) Rabex-5 ubiquitin ligase activity restricts Ras signaling to establish pathway homeostasis in Drosophila. Curr Biol 20:1378–1382
Yoon SO, Shin S, Mercurio AM (2005) Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res 65:2761–2769
Zhu H, Liang Z, Li G (2009) Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol Biol Cell 20:4720–4729
Zhu H, Qian H, Li G (2010) Delayed onset of positive feedback activation of rab5 by rabex-5 and rabaptin-5 in endocytosis. PLoS One 5:e9226
Acknowledgements
The authors’ research programs are supported in part by NIH grants, CA115316 to CDS and GM074692 to GL.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
D’Souza-Schorey, C., Li, G. (2013). Endocytosis and the Regulation of Cell Signaling, Cell Adhesion, and Epithelial to Mesenchymal Transition in Cancer. In: Yarden, Y., Tarcic, G. (eds) Vesicle Trafficking in Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6528-7_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6528-7_6
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6527-0
Online ISBN: 978-1-4614-6528-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)