Abstract
The immune response against helminths and allergens is generally characterized by high levels of IgE and increased numbers of Th2 cells, eosinophils, and basophils. Basophils represent a relatively rare population of effector cells and their in vivo functions are incompletely understood. Recent studies with basophil-depleting antibodies revealed that these cells might play an important role during the early and late stages of type 2 immune responses. To further characterize the relevance of basophils for protective immunity and orchestration of allergic inflammation, we generated constitutively basophil-deficient mice. We observed a normal Th2 response induced by helminth infections or immunization with alum/OVA or papain/OVA. However, basophils contributed to worm expulsion during secondary helminth infection and mediated an IgE-dependent inflammatory response of the skin. These results argue against a critical role of basophils as antigen-presenting cells for induction of Th2 polarization and highlight their effector cell potential during later stages of a type 2 immune response.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Basophil Development and Homeostasis
Basophils constitute a distinct hematopoietic cell lineage although they are phenotypically and functionally related to mast cells and eosinophils. Mast cells and basophils are the main cell types that express the high-affinity Fc epsilon receptor (FcεRI) with a configuration consisting of the IgE-binding α-chain and the signal-transducing β- and γ2-chains. However, a αβ-configuration of FcεRI can be found in other cell types including eosinophils, monocytes, and subsets of dendritic cells. Mast cells and basophils further express high levels of the IL-3 receptor and produce a similar set of effector molecules including histamine, Th2-associated cytokines, and lipid mediators. However, mast cells can be distinguished from basophils by their expression of the receptor tyrosine kinase c-Kit. On the other hand, basophils share some characteristics with eosinophils including the condensed nuclear morphology, completion of cellular maturation in the bone marrow, and a short lifespan.
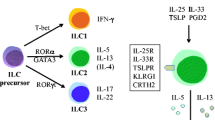
A model has been proposed how the eosinophil, basophil, and mast cell lineages may be connected developmentally [1]. All three lineages seem to originate from a granulocyte/monocyte precursor population which expresses the transcription factor c/EBPα. If in addition the transcription factor GATA-2 is expressed in these precursor cells, they start to develop into eosinophils. In contrast, when c/EBPα is down-regulated while GATA-2 is up-regulated the cells differentiate into a basophil/mast cell common precursor population that can give rise to basophils by secondary up-regulation of c/EBPα or to mast cells if c/EBPα remains down-regulated.
Mature basophils have a lifespan of about 60 h under steady-state conditions [2]. However, the lifespan can increase during inflammatory conditions. Helminth infections of mice have shown that basophilia results from increased de novo production of basophils in the bone marrow and probably a reduced rate of apoptosis [2]. The main cytokine that stimulates basophil development is IL-3. T cell-derived IL-3 has been shown to play a critical role for mobilization of basophils and for their recruitment to lymph nodes [3, 4]. More recently, it has been shown that another cytokine of the hematopoietin family named thymic stroma-derived lymphopoietin (TSLP) can also induce basophilia in mice [5]. IL-3- and TSLP-elicited basophils express different sets of genes and may acquire different effector functions. Further studies are required to determine whether these populations represent distinct subsets of basophils.
4.2 The Role of Basophils for Differentiation of Th2 Cells
Basophils have been proposed to play a crucial role for Th2 cell differentiation in mice. A spontaneous Th2 bias can be observed in mice with enhanced basophil numbers like interferon-response factor 2 (IRF2)-deficient [6] or Lyn tyrosine kinase-deficient mice [7]. In vitro cultures have shown that basophil-derived IL-4 promotes Th2 polarization [8]. However, controversial studies were reported with regard to the role of basophils for Th2 polarization in vivo.
Basophils increase in numbers in draining lymph nodes at early time points after allergen administration or helminth infection of mice [9]. They express large amounts of IL-4 and low levels of MHC-II [10]. Antibody-mediated depletion of basophils resulted in poor Th2 development in response to the pro-allergic protease papain [10]. On the other hand depletion of dendritic cells (DCs) by injection of diphtheria toxin in CD11c-DTR mice had no effect. In addition, selective expression of MHC-II on DCs resulted in a poor Th2 response against the helminth Trichuris muris suggesting that DCs are not important as antigen-presenting cells for initiation of Th2 responses at least under some experimental conditions [11]. However, other studies showed that DCs are critical for Th2 polarization. It was observed that DCs and basophils cooperate during induction of Th2 cells in response to papain [12]. Using a model of allergic inflammation of the lung, it was further shown that depletion of basophils with the MAR-1 antibody which binds to FcεRI also depletes a subset of DCs. These FcεRI+ DCs appeared critical for Th2 polarization in draining lymph nodes [13]. Another study showed that the Th2 response to Schistosoma mansoni eggs required DCs and occurred independently of basophils [14]. We recently generated a genetically basophil-deficient mouse strain (Mcpt8Cre mice) and demonstrated that Th2 polarization in response to papain, alum/OVA, or Nippostrongylus brasiliensis infection occurred independently of basophils [15]. These results were also confirmed by others with another strain of basophil-deficient mice [16].
Taken together, basophils are potent producers of IL-4 but they appear dispensable for Th2 polarization in most experimental models studied so far. However, it remains possible that basophils promote Th2 polarization under certain conditions especially during memory responses [17].
4.3 Basophils as Mediators of Allergic Responses
Basophils can be found in increased numbers in mucosal tissue of patients suffering from allergic rhinitis [18] or atopic asthma [19, 20]. The effector functions of basophils in allergic airway inflammation include the release of vasodilators like histamine or platelet-activating factor and Th2-associated cytokines like IL-4, IL-5, and IL-13. In contrast to mast cells which dominate the early response, basophils appear more important during the late-phase response [21].
Allergic reactions of the skin are also associated with basophil recruitment and activation. A recent study found that basophils were increased in skin biopsies of patients suffering from atopic dermatitis, urticaria, scabies, prurigo, Henoch-Schonlein purpurea, insect bites, eosinophilic pustular folliculitis, and bullous pemphigoid [22]. At present it remains unclear to what extent basophils contribute to inflammation of allergic skin reactions. Murine models have shown that IgE-mediated basophil activation in the skin is essential for a late phase of skin inflammation suggesting that basophils may indeed play a crucial role during the effector phase of allergic responses in the skin [15, 23, 24].
Basophils were also reported to contribute to systemic anaphylactic reactions. In a murine model of anaphylaxis basophils have been shown to release platelet-activating factor (PAF), a potent vasodilator, when stimulated by cross-linking of IgG1 bound to activating Fc gamma receptors on the cell surface [25]. Depletion of basophils with monoclonal antibodies prevented the anaphylactic reaction. However, similar experiments in genetically basophil-deficient mice could not confirm this observation [15]. Furthermore, other cell types like neutrophils or macrophages have also been shown to be important for IgG1-mediated anaphylaxis [26, 27].
4.4 Protective Immunity Against Ticks and Helminths
Ticks of the Ixodid family are ectoparasites that inject their mouth parts through the skin and suck blood from the host. During that process several substances are injected with the tick saliva into the host to avoid blood clotting and inflammation [28]. A study from almost 40 years ago has shown that basophils accumulate in the skin of guinea pigs infested with tick larvae [29]. It was further shown that injection of a basophil-depleting antiserum resulted in loss of resistance against secondary tick infestation [30]. However, it remained unclear whether basophils directly contribute to tick resistance. A recent report demonstrated that depletion of basophils by injection of diphtheria toxin in mice which express the diphtheria toxin receptor exclusively on basophils results in diminished resistance against the tick Haemaphysalis longicornis [23]. The authors further observed that mast cells are also required for tick resistance. However, expression of activating Fc receptors was required on basophils and not on mast cells. Whether basophils are involved in limiting dissemination of tick-transmitted pathogens remains to be determined.
Helminths are parasitic worms and comprise a very diverse spectrum of organisms that are generally well adapted to their hosts and can often persist for months to years. Gastrointestinal helminths are transmitted by the oral route or by penetration of larvae through the skin. They induce a type 2 immune response characterized by Th2 polarization, eosinophilia, and high serum IgE levels. Mast cells and basophils are also mobilized and can increase in numbers in infected tissues. The role of basophils for protective immunity against gastrointestinal helminths can be studied in mouse models. Studies with genetically basophil-deficient Mcpt8Cre mice revealed that basophils are not required for worm expulsion during primary infection with Nippostrongylus brasiliensis [15]. Th2 polarization, eosinophilia, and serum IgE levels were also not affected by the absence of basophils. However, protection against secondary infection was compromised in basophil-deficient mice [15]. Basophils were also reported to contribute to expulsion of Trichuris muris, a gastrointestinal helminth that mainly populates the large intestine [5]. The protective function of basophils is probably dependent on helminth-specific antibodies that can bind to activating Fc receptors on basophils and thereby mediate activation of basophils upon antigen encounter. Further studies are required to unravel the mode(s) of basophil activation and their effector functions against helminths.
4.5 Conclusion
Based on murine models basophils appear important for protective immunity against ticks and helminths. On the other hand they mediate chronic inflammatory skin reactions. The proposed role of basophils as critical Th2-polarizing antigen-presenting cells could not be confirmed with genetically basophil-deficient mice. A better understanding of basophil biology will hopefully lead to improved treatments of allergic skin reactions and development of vaccines against helminths. Unfortunately, most of our current knowledge about basophil in vivo functions is based on murine models. Whether the results can be translated to the human immune system remains to be determined.
References
Iwasaki H, Mizuno S, Arinobu Y, et al. The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev 2006; 20(21):3010–21.
Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood 2009; 113(12):2816–25.
Shen T, Kim S, Do JS, Wang L, Lantz C, Urban JF, Le Gros G, Min B. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol 2008; 20(9):1201–9.
Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol 2010; 184(3):1143–7.
Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011; 477(7363):229–33.
Hida S, Tadachi M, Saito T, Taki S. Negative control of basophil expansion by IRF-2 critical for the regulation of Th1/Th2 balance. Blood 2005; 106(6):2011–7.
Charles N, Watford WT, Ramos HL, et al. Lyn kinase controls basophil GATA-3 transcription factor expression and induction of Th2 cell differentiation. Immunity 2009; 30(4):533–43.
Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood 2007; 109(7):2921–7.
Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol 2008; 9(3):310–8.
Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol 2009; 10(7):713–20.
Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol 2009; 10(7):697–705.
Tang H, Cao W, Kasturi SP, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol 2010; 11(7):608–17.
Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells–not basophils–are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med 2010; 207(10):2097–111.
Phythian-Adams AT, Cook PC, Lundie RJ, et al. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med 2010; 207(10):2089–96.
Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 2010; 33(3):364–74.
Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol 2011; 12(6):527–35.
Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J Exp Med 2004; 200(7):857–70.
Iliopoulos O, Baroody FM, Naclerio RM, Bochner BS, Kagey-Sobotka A, Lichtenstein LM. Histamine-containing cells obtained from the nose hours after antigen challenge have functional and phenotypic characteristics of basophils. J Immunol 1992; 148(7):2223–8.
Macfarlane AJ, Kon OM, Smith SJ, et al. Basophils, eosinophils, and mast cells in atopic and nonatopic asthma and in late-phase allergic reactions in the lung and skin. J Allergy Clin Immunol 2000; 105(1 Pt 1):99–107.
Koshino T, Teshima S, Fukushima N, et al. Identification of basophils by immunohistochemistry in the airways of post-mortem cases of fatal asthma. Clin Exp Allergy 1993; 23(11):919–25.
Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol 1988; 81(3):580–9.
Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy 2011; 66(8):1107–13.
Wada T, Ishiwata K, Koseki H, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 2010; 120(8):2867–75.
Mukai K, Matsuoka K, Taya C, et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 2005; 23(2):191–202.
Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 2008; 28(4):581–9.
Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, Kohl J, Finkelman FD. Peanuts can contribute to anaphylactic shock by activating complement. The Journal of allergy and clinical immunology 2009; 123(2):342–51.
Jonsson F, Mancardi DA, Kita Y, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest 2011; 121(4):1484–96.
Wikel SK. Host immunity to ticks. Annu Rev Entomol 1996; 41:1–22.
Allen JR. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol 1973; 3(2):195–200.
Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. Journal of immunology 1982; 129(2):790–6.
Acknowledgment
This work was supported by an ERC starting grant (PAS_241506) from the European Union FP7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Voehringer, D. (2013). Regulation of Type 2 Immunity by Basophils. In: Katsikis, P., Schoenberger, S., Pulendran, B. (eds) Crossroads Between Innate and Adaptive Immunity IV. Advances in Experimental Medicine and Biology, vol 785. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6217-0_4
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6217-0_4
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6216-3
Online ISBN: 978-1-4614-6217-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)