Abstract
In this work, a carbon membrane was produced from a polymer blend of polyetherimide (PEI)/polyvinylpyrrolidone (PVP). Four samples of the PEI-based carbon membrane were prepared at various concentrations of its blending counterpart, which were 0, 5, 9, and 13 wt.% of PVP. All samples were subjected under similar conditions where the stabilization of their polymeric precursors was performed at 300 °C for 1 h under a continuous flow of purified air. The stabilization was followed by pyrolysis at 400 °C for 1 min under purified nitrogen flow. Sample S3, which was prepared at initial dope solution of 9 wt.% PVP, demonstrated that the decomposition temperature has increased at highest degree compared with the others, and this has brought advantages to its morphology. Single gas permeability tests using CO2 and CH4 gases showed that the polymer-blend-precursor-based carbon membranes possessed molecular sieving properties. Sample with 9 wt.% PVP (S3) was found highest in the delivery of CO2 permeance and CO2/CH4 permselectivity, which were 14.61 and 1.95 GPU, respectively.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The emission of excessive greenhouse gases has been one of the main contributors for global warming. In order to minimize the problem, many countries have regulated the utilization of cleaner fuels such as natural gas for domestic use. In fact, the minimum level of harmful emission by the by-products of natural gas meets the specification implemented under the Environmental Quality Regulation 1996 by Malaysian Government.
The benefits of the natural gas utilization cause this commodity to account for 23 % of the total energy consumed in the developing countries. In 2003 alone, 95 trillion cubic feet of natural gas was consumed worldwide, whereas the number was predicted to increase to 182 trillion cubic feet in 2030 (Xiao et al. 2009). In natural gas processing, the crude natural gas undergoes enrichment to maximize the percentage volume of methane while eliminating carbon dioxide as an impurity. The carbon dioxide content in the crude natural gas may vary from 4 to 50 vol.%, and the typical product after the enrichment is a methane-enriched residue stream containing less than 2 vol.% of carbon dioxide (Safari et al. 2009). The removal of carbon dioxide also increases the calorific value of the natural gas (Datta and Sen 2006). In addition, the elimination of acidic carbon dioxide will also prevent corrosion in the transport line and diminishes the unnecessary total volume of gas, which brings economic advantages for transportation and storage (Hao et al. 2002).
The use of membrane technology in gas processing offers many advantages over other conventional technologies in gas separation such as cryogenic, adsorption, and absorption. The membrane process provides simple and versatile mechanisms that allow smaller space occupancy for relatively larger processing capacity. Its operation requirement only involves a pressure gradient toward the membrane as a driving force for operation supplied by gas compressor or vacuum pump. Maximizing the industrial margin is achievable by optimizing the power to engage the separation process. Two-thirds of the total components cost is associated with the feed-crude natural gas compressor, while the remaining is associated with the membrane module. Therefore, most of the operating cost is contributed by the consumed energy to power the compressor (Baker 2002). It is possible to pair a highly selective membrane with smaller compressor to achieve greater saving in the operation cost. Therefore, finding new materials or extensive modifications of existed materials to enhance the membrane selectivity and permeability has become current focuses.
Unlike the polymeric membranes, carbon membranes possess highly porous structure that allows the gas separation to be performed at higher permeability which means the separation process can be completed at a higher production rate. Another method in accomplishing high production rate is by applying the hollow fiber as the membrane configuration. The hollow fiber provides higher surface area as well as higher packing density, which leads to large permeability of the product (Saufi and Ismail 2004). In addition, this membrane configuration is simple and possesses easy module assembly (He and Hagg 2011). These advantages have attracted attention to enhance the hollow fiber quality and envision the capability of hollow fiber toward the main membrane configuration in future moment.
The porous structure of the carbon membrane consists of pores that lie within the ultramicropore region. These pores are generated during pyrolysis where the evolved decomposition gases channel their way through to the surface leaving traces of opening or pores throughout the membrane structure. The formation of such ultramicropores would enable the carbon membrane to discriminate the diffusing gases according to their kinetic diameters at relatively high sensitivity (down to 0.1 Å) through molecular sieving mechanism (Jones and Koros 1995).
One of the prominent polymer precursors to produce the CO2-selective carbon membrane is polyetherimide (PEI) due to its inexpensive cost compared with other types of polyimides. Salleh and Ismail (2011) have reported the performance of carbon membrane derived from PEI blended with PVP at various pyrolysis temperatures. It was found that the carbon membrane pyrolyzed at higher temperatures demonstrated a remarkable permselectivity compared to lower pyrolysis temperatures. However, no investigation was reported regarding the effect of varying the dope composition on carbon membrane performance. Thus, this chapter reports a study on the influence of dope composition on the morphology and performance of carbon membrane.
Methodology
Materials
Commercially available PEI and N-methyl-2-pyrrolidone (NMP) (99.5 % purity) were supplied by Sigma-Aldrich, USA. Polyvinylpyrrolidone (PVP) was purchased from Fluka, USA. Ethanol (96 % purity) and n-hexane (60 % purity) were purchased from QReC, Malaysia.
Hollow Fiber Production
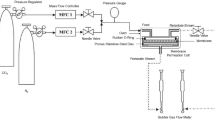
Four types of dope solutions were prepared by varying the input amount of PEI and PVP powder into NMP solvent as shown in Table 1. The dope solutions were stirred using a magnetic stirrer at 110 °C for 24 h. The dope solution was then fed into a spinneret to generate hollow fibers using a well-known method of dry/wet spinning process. The polymeric precursor hollow fibers obtained were then immersed in tap water for 24 h to complete the coagulation process. After that, the polymeric hollow fiber was immersed in ethanol solution for 2 h, followed by another 2 h in n-hexane for solvent exchange procedure to accommodate slow removal of water. Finally, the fibers were dried under normal air atmosphere. The spinning conditions are depicted in Table 2.
Carbon Membrane Production
The high-temperature treatment was performed using Carbolite™ vertical furnace. The various hollow fibers were stabilized at 300 °C for 1 h under purified air flow. Subsequently, the hollow fibers were pyrolyzed by increasing the heating temperature to 400 °C at 3 °C/min with holding time of 1 min. Then, the hollow fibers were naturally cooled to room temperature. Purified nitrogen was supplied at 50 ml/min throughout the processes.
Membrane Characterization
Thermogravimetric analysis of the membranes was characterized via simultaneous thermal analyzer (Model Perkin Elmer STA 6000, USA). The membrane was heated under nitrogen flow from 30 to 500 °C at 3 °C/min. The heating was held for 15 min as the process reached top temperature. The temperature of decomposition (Td) is measured by a tangent line across the curve of the thermal plot. The cross-sectional sample images were observed under scanning electron microscopy (Model FEI Quanta 450 FEG, USA). The membrane permeability toward CO2 and CH4 was evaluated using single gas permeability test method. The permeance, P, and permselectivity, α, were calculated using equations below:
Permeance, P (GPU):
Selectivity, α:
where P/l is the permeance of the hollow fiber, Q i is the volumetric flow rate of gas i at standard temperature and pressure (cm3(STP)/s), Δp is the pressure difference between the feed side and the permeation side of the membrane (cm Hg), A is the effective membrane surface area (cm2), n is the number of fiber in the module, D is an outer diameter of hollow fiber (cm), and l is the effective length of hollow fiber (cm). The pressure difference of 4 bar was applied throughout the experiments, while the outer diameter of the carbon membranes were measured from the SEM images.
Results and Discussion
Scanning Electron Microscopy
The cross sections of the membranes are depicted in Fig. 1. Before thermal treatment, S1 exhibits the fingerlike pore structure, while the S2 possesses the spongelike pore structure. The cross-sectional images of S3 and S4 before the thermal treatment are unavailable since the hollow fibers have been too elastic and unbreakable. It is believed that the presence of PVP has triggered the formation of sponge-like pore. The increase in the PVP concentration would increase the amount of sponge-like pore and may strengthen the membrane matrix. The thermal treatment process toward S1 had caused severe destruction of the hollow fiber membrane structure. The S1 composition could not retain the original shape and melt as the thermal treatment proceeds higher than 300 °C. Thus, no image of S1 after thermal treatment is depicted in Fig. 1. Meanwhile, the S2-based membrane showed extra shrinkage, which was believed to have been caused by insufficient PVP, while S4 exhibited irregular structure of the membrane matrix. The irregularity of the structure possibly originated from the nature of the PVP itself. Excessive amount of PVP has increased the viscosity of dope solution and had caused the nascent fiber to be unstable and slightly non-uniform during spinning. S3 has lesser shrinkage impact on its structure, and the matrix is uniform throughout the membrane wall.
Thermogravimetric Analysis
Figure 2 shows the thermogravimetric analysis of each dope composition sample. The samples with blend polymer indicated two stages of weight loss, contributed by PEI and PVP subgroups release. Salleh and Ismail (2011) have categorized the PEI and PVP as the thermally stable and thermally labile polymer, respectively. Thus, the first weight loss of the thermal curve belonged to the PVP, while the later was belonged to the PEI.
The tangent of the thermal curve of each sample indicated the decomposition temperature (Td) of PVP was slightly increased from S2 to S4. On the other hand, Td of PEI in S2 was significantly higher than that of PEI in S1. The S3 possessed highest PEI Td, while the Td of PEI in S4 was slightly diminished. The increase in Td values indicated that the presence of PVP might improve the thermal stability of the polymeric structure. The enhancement of thermal stability conserves the original shape of hollow fiber during thermal treatment. In fact, severe collapse of S1 during thermal treatment demonstrates the incapability of PEI to retain the original shape of hollow fiber without the presence of PVP. S3 was envisioned as the optimum composition for attaining the highest PEI’s Td. Further addition of PVP (S4) would cause the Td of the PEI to deteriorate.
Single Gas Permeability Test
Since the sample S1 cannot withstand pyrolysis treatment, no permeability data were recorded for that particular sample. The permeabilities of pyrolyzed S2, S3, and S4 toward CO2 and CH4 gases are depicted in Table 3. The CO2 permeance was larger than CH4, which is in accord with their kinetic diameters. This phenomenon confirmed the molecular sieve mechanism might be involved in gas transport throughout the carbon membrane.
Based on Table 3, S3 possesses the highest CO2 permeance and CO2/CH4 permselectivity. The micropore-type structure was believed to be well developed throughout the thermal treatment process. The PVP acted as the pillar to the polymer matrix that prevented the melting of PEI and retained the original shape of the hollow fiber after thermal treatment. The incapability of S1 to retain the original shape of the hollow fiber during thermal treatment has proven the requirement of PVP for structure reinforcement by the formation of sponge-like pore structure. The S2 exhibits the lowest CO2 permeance and CO2/CH4 permselectivity. This was probably due to the collapsing of pores during thermal treatment. The insufficient amount of PVP has triggered the excessive shrinkage of carbon membrane and suppressed the pore development activities. On the other hand, the S4 exhibited low CO2 permeance and CO2/CH4 permselectivity due to excessive amount of PVP in the polymer matrix. The thermally labile PVP would evolve earlier before the PEI. However, excessive amount of PVP has changed the properties of PEI/PVP blend system, which hinders complete decomposition during the pyrolysis. The remaining PVP in the membrane structure would disrupt the arrangement of carbon aggregation that originated from the PEI, which resulted in membrane with less porosity achieved after thermal treatment.
Conclusion
The thermal stability of the polymeric hollow fibers was found to be influenced by the amount of PVP presented in the dope solution. Sample S3 possesses uniform matrix structure with lesser shrinkage. The decomposition temperature of the membrane has increased for S3, indicating better thermal stability and structure preservation. Single gas permeability test has shown that S3 to be an optimum dope composition for production of PEI-/PVP-based hollow fiber carbon membrane.
References
Baker, R. W. (2002). Future direction of membrane gas separation technology. Industrial and Engineering Chemistry Research, 41, 1393–1411.
Datta, A. K., & Sen, P. K. (2006). Optimization of membrane unit for removing carbon dioxide from natural gas. Journal of Membrane Science, 283, 291–300.
Hao, J., Rice, P., & Stern, S. (2002). Upgrading low-quality natural gas with H2S- and CO2-selective polymer membranes: Part I. Process design and economics of membrane stages without recycle streams. Journal of Membrane Science, 209, 177–206.
He, X., & Hagg, M. G. (2011). Optimization of carbonization process for preparation of high performance hollow fiber carbon membranes. Industrial and Engineering Chemistry Research, 50, 8065–8072.
Jones, C. W., & Koros, W. J. (1995). Characterization of ultramicroporous carbon membranes with humidified feeds. Industrial and Engineering Chemistry Research, 34, 158–163.
Safari, M., Ghanizadeh, A., & Montazer-Rahmati, M. M. (2009). Optimization of membrane-based CO2-removal from natural gas using models considering both pressure and temperature effects. International Journal of Greenhouse Gas Control, 3, 3–10.
Salleh, W. N. W., & Ismail, A. F. (2011). Carbon hollow fiber membrane derived from PEI/PVP for gas separation. Separation and Purification Technology, 80, 541–548.
Saufi, S., & Ismail, A. F. (2004). Fabrication of carbon membranes for gas separation: A review. Carbon, 42, 241–260.
Xiao, Y., Low, B. T., Hosseini, S. S., Chung, T. S., & Paul, D. R. (2009). The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas–a review. Progress in Polymer Science, 34, 561–580.
Acknowledgments
The authors wish to thank USM Membrane Cluster Grant and Ministry of Higher Education (MyPhD) for the financial supports.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Harun, W.M.H.F.W., Jaya, M.A.T., Ahmad, M.A. (2013). The Influences of Dope Composition on Gas Permeance of Hollow Fiber Carbon Membrane. In: Pogaku, R., Bono, A., Chu, C. (eds) Developments in Sustainable Chemical and Bioprocess Technology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-6208-8_38
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6208-8_38
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-6207-1
Online ISBN: 978-1-4614-6208-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)