Abstract
The neuropeptide kisspeptin, encoded by the Kiss1 gene, is required for mammalian puberty and fertility. Examining the development of the kisspeptin system contributes to our understanding of pubertal progression and adult reproduction and sheds light on possible mechanisms underlying the development of reproductive disorders, such as precocious puberty or hypogonadotropic hypogonadism. Recent work, primarily in rodent models, has begun to study the development of kisspeptin neurons and their regulation by sex steroids and other factors at early life stages. In the brain, kisspeptin is predominantly expressed in two areas of the hypothalamus, the anteroventral periventricular nucleus and neighboring periventricular nucleus (pre-optic area in some species) and the arcuate nucleus. Kisspeptin neurons in these two hypothalamic regions are differentially regulated by testosterone and estradiol, both in development and in adulthood, and also display differences in their degree of sexual dimorphism. In this chapter, we discuss what is currently known and not known about the ontogeny, maturation, and sexual differentiation of kisspeptin neurons, as well as their regulation by sex steroids and other factors during development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The status of the neuroendocrine reproductive axis is in flux during various stages of perinatal and pubertal development, ranging from being entirely quiescent to fully active. Additionally, the neuroendocrine reproductive system is anatomically and physiologically differentiated between males and females, and these sex differences originate during key stages of development. Sex differences are also seen in several reproductive health disorders, such as idiopathic hypogonadotropic hypogonadism, constitutional delayed puberty, and precocious puberty [1–5]. Many of these reproductive health disorders have been attributed to known, or in many cases, unknown developmental defects in the brain. Specific neuronal circuits located in the forebrain and hypothalamus have been implicated as control centers responsible for proper development of reproductive physiology, converging on neurons that release gonadotropin releasing hormone (GnRH). GnRH stimulates the release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary, thereby driving the maturation and activation of the gonads. How and when the developmental changes in the neuroendocrine axis are induced is not completely known, but gaining a clearer understanding may help pinpoint the cause and timing of defects in reproductive maturation.
One of the key upstream hypothalamic circuits involved in the control of GnRH secretion consists of neurons expressing the Kiss1 gene and its protein product, kisspeptin. Kisspeptin signaling has been implicated as an essential regulator of fertility and puberty in numerous mammalian species, including humans [6–8]. Alterations in the expression of Kiss1 or kisspeptin over development, along with differences in expression between the sexes, especially during key developmental periods, may be a critical driving force in the maturation of the neuroendocrine reproductive system. Indeed, changes in the Kiss1 system likely contribute to the timing of puberty onset, sex differences in LH secretion, and other facets of reproductive physiology. It is therefore essential to understand how kisspeptin neurons develop, when and how Kiss1 gene and protein expression are modified during development, and what possible regulatory mechanisms govern the development of the kisspeptin system. This chapter discusses the current knowledge for these topics in mammals and also pinpoints several important unanswered questions involving the development of kisspeptin neuronal circuits.
Kiss1 and Kisspeptin Expression in the Adult Brain
In order to study the development of the kisspeptin system, it is essential to first understand the localization and phenotype of kisspeptin neurons in the adult state. Until the recent generation of transgenic mice, which label kisspeptin cells with markers such as GFP [9–11], three techniques were used to examine the localization of kisspeptin neurons in the brain: reverse transcriptase PCR (RT-PCR)/quantitative PCR (qPCR), in situ hybridization (ISH), and immunohistochemistry (IHC). RT-PCR was used in early studies to identify high Kiss1 mRNA expression in large regions of the brain, such as the hypothalamus [12]. However, this technique was weakened by an inability to specifically visualize and separately analyze discrete Kiss1 populations within these large brain areas. This issue was resolved through the subsequent use of ISH and IHC, which allow for precise neuroanatomical mapping of Kiss1- and kisspeptin-synthesizing neurons, respectively. However, in earlier IHC studies, some kisspeptin antibodies were not very specific, as they were shown to cross react with other RFamide family members [13]. The recent use of more specific kisspeptin antibodies has allowed for more precise detection of kisspeptin immunoreactivity in the brain [14–19]. Utilizing these various methods, many studies have confirmed that in adult rodents, Kiss1 (or kisspeptin) is expressed in just a few discrete brain regions, including a small population in the medial amygdala (MeA) [20] and two larger hypothalamic populations in the anteroventral periventricular nucleus and neighboring periventricular nucleus (AVPV/PeN) and the arcuate nucleus (ARC) [21–23]. In non-rodent species, such as sheep and non-human primates, Kiss1 gene expression and kisspeptin immunoreactivity have, for the most part, a similar distribution as in rodents, with expression localized to the pre-optic area (POA) and the ARC/infundibular nucleus (INF) [19, 24–27]. Expression of Kiss1 or kisspeptin in the MeA of non-rodent species has not yet been examined.
In contrast to Kiss1/kisspeptin cell bodies, which are found in just a few discrete brain regions, kisspeptin-immunoreactive (ir) fibers are scattered throughout the brain (discussed in detail in Chap. 3). In adult rodents and sheep, terminals of kisspeptin fibers are found within the POA (and regions containing GnRH neurons), the ARC and medial basal hypothalamus, the paraventricular nucleus, and the median eminence (perhaps targeting GnRH axons/terminals) [14, 15, 28]. In mice, additional regions have been identified containing kisspeptin fibers, including the lateral septum, dorsal-medial nucleus of the hypothalamus, bed nucleus of the stria terminalis (BNST), and the MeA [14]. It also appears that ARC and AVPV/PeN Kiss1 neurons send a number of projections to one another, perhaps allowing these two populations to directly communicate [29], although currently there is no evidence that Kiss1 neurons themselves express Kiss1r [30].
In adult rodents, the AVPV/PeN region displays sex differences in various morphological parameters [31, 32] and is considered the main anatomical site that drives the sexually dimorphic preovulatory luteinizing hormone (LH) surge that occurs in adult females [33]. Mounting evidence supports a critical involvement of AVPV/PeN kisspeptin neurons in the sexually differentiated LH surge. For example, Estradiol (E2) dramatically stimulates Kiss1 expression in the AVPV/PeN, and Kiss1 neurons in this region co-express sex steroid receptors, including ERα [34]. Moreover, Kiss1 neuronal activity (as measured by cfos induction) in the AVPV/PeN is upregulated in a circadian pattern in complete synchrony with the circadian timing of the LH surge [35]. Additionally, as will be discussed in more detail later, the AVPV/PeN Kiss1 population itself is sexually differentiated, just like the preovulatory LH surge, with females expressing greater Kiss1 and kisspeptin expression in this region than males [36]. It is also worth noting that the AVPV/PeN region contains several other sexually dimorphic subpopulations that have been implicated in regulating reproduction, such as dopaminergic neurons, which express the tyrosine hydroxylase (TH) enzyme [37, 38], and cells expressing both GABA and glutamate [39]. Interestingly, most Kiss1 neurons in the AVPV/PeN co-express TH [40, 41], though the functional significance of such co-expression has yet to be determined.
Kisspeptin neurons in the ARC comprise the largest kisspeptin population in the brain [15, 24, 25, 34, 36, 42, 43]. In contrast to the AVPV/PeN population, Kiss1 cells in the ARC are not highly sexually dimorphic in adult rodents, especially when the adulthood sex steroid milieu is similar between the sexes [36, 44]. However, there are sex differences in the regulation of Kiss1 expression in the ARC that are present in mice during the prepubertal period, an intriguing finding that will be discussed more below [44]. Moreover, some species, such as sheep, exhibit sex differences in ARC Kiss1 neurons in adulthood, which may reflect slightly different roles of these ARC kisspeptin neurons between species. While Kiss1 expression is upregulated in the AVPV/PeN by E2 and testosterone (T), the opposite is true in the ARC: sex steroids potently inhibit ARC Kiss1 expression [22, 34, 36, 45], and Kiss1 neurons in this region have a high degree of colocalization with ERα, androgen receptor, progesterone receptor, and to a lesser degree, ERβ [15, 22, 24, 46–49]. It is suggested that the inhibition of Kiss1 expression in the ARC by sex steroids reflects the involvement of ARC kisspeptin neurons in the negative feedback effects of sex steroids on pulsatile GnRH secretion in both sexes [6, 50, 51]. Additionally, ARC Kiss1 cells co-express several other regulatory neuropeptides, neurokinin B (NKB) and dynorphin (DYN), which appear to also play a role in regulating GnRH/LH secretion [52–57]. Moreover, ARC kisspeptin neurons have been shown to have abundant reciprocal connections with each other [58–60] and to highly express the NKB receptor (NK3R) [16, 55, 58, 60], which may allow these neurons to communicate and synchronize with each other [55, 60] (discussed more in Chap. 15).
The Development of the AVPV/PeN Kiss1 Population
Ontogeny of Kiss1 Expression in the AVPV/PeN
In adulthood, Kiss1 is highly expressed in the hypothalamic AVPV/PeN and ARC regions, but this is not always the case earlier in development. In rodents, Kiss1 expression is present in the embryonic brain but is limited to the ARC region [61], with no detectable AVPV/PeN Kiss1 expression at this age. Rather, Kiss1 mRNA and kisspeptin protein expression in the AVPV/PeN of rodents first occurs later in postnatal life. The developmental timing of AVPV/PeN Kiss1 expression has been studied in both mice and rats. However, different studies describing the development of Kiss1 (or kisspeptin) expression sometimes use different nomenclature to describe age of birth: some studies define the day of birth as postnatal day 1 (PND 1), others as PND 0 or P0. For consistency, and to permit for direct comparison between studies, in this chapter the day of birth will be denoted as PND 1; if the day of birth used in the original paper was noted as P0 or PND 0, it will be changed to PND 1 and subsequent ages modified accordingly.
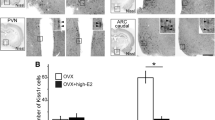
Neither Kiss1 mRNA nor kisspeptin protein expression has been detected in the AVPV/PeN of mice or rats on the day of birth. The earliest documented AVPV/PeN Kiss1 expression in mice was recently shown, using ISH, to be ~PND 10 [40], though this expression was of very low magnitude compared to the next several days examined, PND 12, and PND 14, when moderately higher levels were observed (Fig. 11.1). Another developmental study of kisspeptin protein expression in female mice found no kisspeptin-immunoreactive (ir) cells in the AVPV/PeN at PND 10 and only a small number of kisspeptin cells at PND 15, which was the next chronological age examined [62]. Whether the absence of detectable kisspeptin-ir cells at PND 10 reflects differences in the sensitivities of the techniques used (IHC vs. ISH) or a developmental difference at the level of either post-transcriptional processing or translation is currently unknown.
Development of the Kiss1 sex difference in the anteroventral periventricular nucleus and neighboring periventricular nucleus (AVPV/PeN) of mice. Mean number of Kiss1 neurons in the AVPV/PeN of female and male mice over the course of early postnatal development. Kiss1 cells were first detected in each sex on PND 10. The number of Kiss1 neurons was significantly higher in females than males on PND 12 and later. *Significantly different from males of same age. Modified from Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS 2010 BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817
Looking beyond the first 2 weeks of life, kisspeptin-ir cell number was found to steadily increase in the mouse AVPV/PeN from PND 15 to adulthood (assessed every 5 days of age) [62]. Extending this protein data, our lab recently performed a detailed, day-by-day analysis of Kiss1 mRNA expression in prepubertal and pubertal female mice and found that Kiss1 cell number in the AVPV/PeN steadily and continually increases from PND 15 through PND 28, at which point it resembles adulthood levels [63]. In rats, like mice, AVPV/PeN kisspeptin expression is not detectable on PND 4 or PND 8, but is noticeably present by the next age examined, PND 22 [64]. Developmental changes in Kiss1 mRNA expression have also been documented in the POA of ewes [65] at 25, 30, and 35 weeks of age, and an increase in POA Kiss1 cell number was observed at 30 weeks, corresponding to the time of puberty, with no further increase detectable at 35 weeks [65]. In non-human primates, KISS1 expression also increases in the hypothalamus during puberty, but this was attributed to kisspeptin neurons in the medial basal hypothalamic region (i.e., the ARC) rather than the POA population [25].
In addition to developmental changes in AVPV/PeN kisspeptin cell number, the development of kisspeptin fibers apposing GnRH neurons has been reported to change over development [28]. Detectable kisspeptin fiber appositions to GnRH neurons were absent in female mice younger than PND 25, but such appositions were present on PND 25 and further increased in numbers by PND 31 [28]. The authors postulated that many of these GnRH-apposing kisspeptin fibers were derived from the AVPV/PeN region, though this was not experimentally determined. However, if this assumption is true, then the appearance and subsequent pubertal increase in kisspeptin-GnRH appositions may be a function of the known increase in kisspeptin synthesis in the AVPV/PeN during this age, increasing the visibility and detection of the fibers. Alternatively, it is also possible that a physical increase in the degree of kisspeptin fiber innervation of GnRH neurons occurs peri-pubertally, perhaps affecting GnRH activation around puberty onset.
Sexual Differentiation of AVPV/PeN Kiss1 Expression During Development
A few years ago, it was discovered that Kiss1-expressing neurons in the adult rat AVPV/PeN are sexually differentiated, with adult females possessing more Kiss1 mRNA (and detectable Kiss1 cells) than males (Fig. 11.2) [36]. Similar observations have now been reported for Kiss1 mRNA levels in mice [40] and kisspeptin protein levels in mice and rats [28, 66]. No documented Kiss1 sex differences have yet been reported in the POA of the ewe or monkey, although a sex difference is observed in the ARC Kiss1 population of sheep [67] and is discussed later in further detail.
Sex differences in AVPV Kiss1 cells are organized early in development by perinatal hormones and are unaffected by the activational effects of adult hormones. Representative photomicrographs showing Kiss1 mRNA-expressing cells in the AVPV of adult male, female, and neonatally androgenized female rats. All animals were treated in adulthood with E2. 3V third ventricle; Cast castrated; OVX ovariectomized. Modified from Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783. With permission from The Endocrine Society
Although activational effects of sex steroids play a role in transiently increasing AVPV/PeN Kiss1 expression levels in adulthood [22], the adult sex steroid milieu does not account for the observed sex differences in AVPV/PeN Kiss1 expression [36]. This is evidenced by the fact that male and female rats that are gonadectomized as adults and treated with identical E2 levels still display sexually dimorphic Kiss1 expression in the AVPV/PeN [36, 42]. In fact, the AVPV/PeN Kiss1 sex difference appears to be permanently organized by sex steroid signaling early in postnatal development. In the postnatal “critical period,” which is typically the first week of postnatal life in rodents, males normally secrete elevated gonadal T, whereas females secrete little sex steroids at this time. Experiments manipulating the postnatal sex steroid milieu of rodents support the model that the presence of elevated levels of postnatal sex steroids determines whether many sexually dimorphic traits develop to be male-like in adulthood. Thus, in newborn males, elevated sex steroids act to organize neural circuits to permanently develop a male-like phenotype [68]. In contrast, newborn females are not exposed to sufficient levels of sex steroids, and therefore their brains permanently develop to be female-like [32, 37]. Supporting this “organizational” model of sexual differentiation, castration of newborn males, to remove high postnatal T, results in the permanent development of feminized neural populations. Conversely, sex steroid treatment to newborn females, mimicking elevated T secretion in postnatal males, results in the permanent development of masculinized brain circuitry (reviewed in ref. [6]).
A number of studies have determined that the AVPV/PeN Kiss1 system is organized postnatally by sex steroids. For example, castrating male rats at birth causes a permanent feminization of the developing AVPV/PeN Kiss1 system (Fig. 11.3) [66]. Conversely, neonatal female rats treated once with T or E2 exhibit a permanent reduction of Kiss1- or kisspeptin-expressing cells in the AVPV/PeN in adulthood, similar to what is exhibited in normal males (Figs. 11.2 and 11.3) [36, 66, 69]. The fact that postnatal E2 treatment can, like T, permanently alter the development of the AVPV/PeN Kiss1 system suggests that postnatal masculinization of this system is likely mediated via aromatization of T to E2. In rats, the effects of postnatal E2 on Kiss1 sexual differentiation are likely mediated by ERα and not ERβ, because neonatal treatment with the ERα agonist, PPT, caused a reduction in female AVPV/PeN kisspeptin levels [70], while neonatal administration of the ERβ agonist, DPN, had no significant effect on adulthood Kiss1 levels [71]. In these experiments, however, males and females were not compared, and further studies are needed to determine if PPT can completely masculinize the female Kiss1 AVPV/PeN population to male levels. Additionally, the reduction of AVPV/PeN Kiss1 expression in female rats that were treated neonatally with sex steroids correlates with the inability of these females to generate an E2-mediated LH surge as adults [66], linking the sexually dimorphic AVPV/PeN Kiss1 system and the sexually dimorphic LH surge event.
Kiss1 mRNA and kisspeptin expression in the AVPV of adult rats. Mean level of Kiss1 mRNA expression (a) and kisspeptin immunoreactivity (b) in the AVPV of male and female rats that were either neonatally castrated (Neo Cast), neonatally treated with estradiol benzoate (Neo EB), or untreated. All animals were gonadectomized and treated with E2 or without E2 as adults to equalize hormone levels. Values with same letters are not significantly different within the group with the same adult E2 treatment. Values with asterisks are significantly different from corresponding animals without adult E2 treatment. The number in or on each column indicates the number of animals used. Values are means ± SEM. Modified from Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K-i, Tsukamura H 2009 Significance of Neonatal Testicular Sex Steroids to Defeminize Anteroventral Periventricular Kisspeptin Neurons and the GnRH/LH Surge System in Male Rats. Biology of Reproduction 81:1216–1225. With permission from The Society for the Study of Reproduction
As discussed earlier, Kiss1 mRNA is expressed in the AVPV/PeN as early as PND 10 in mice of both sexes. However, there are no sex differences in AVPV/PeN Kiss1 neuron number or Kiss1 mRNA levels/neuron at this age, even though both of these parameters are well-established sex differences in adulthood [40]. The sex difference in Kiss1 cell number, however, is evident by PND 12 and becomes even more robust on PND 14 and 16 (Fig. 11.1) [40]. Semi-quantitative analysis of Kiss1 mRNA levels/cell revealed a significant sex difference only beginning around PND 16 [40]. Thus, AVPV/PeN Kiss1 mRNA expression first arises at the same time in both sexes (~PND 10), but the sex difference in both cell number and mRNA expression/cell takes several more days to develop, at least as assessed via ISH in mice. As mentioned above, in other rodent studies utilizing IHC, neither sex displays detectable kisspeptin-ir cells in the AVPV/PeN on or before PND 10 [28, 64]. Currently, kisspeptin protein levels have not been directly compared between sexes from PND 11 through PND 21, but the sex difference in kisspeptin-ir cell number was readily apparent in mice on PND 25 [28] and in rats on PND 21 [64].
Possible Mechanisms of Steroid-Mediated Sexual Differentiation of AVPV/PeN Kiss1 Neurons
Sexual differentiation of the AVPV/Pen Kiss1 system is dependent on the postnatal sex steroid milieu, but it is unclear exactly how E2 (aromatized from T) directs this developmental process. Several sex steroid-dependent mechanisms, such as differential neurogenesis, migration, epigenetics, and apoptosis, have been implicated in the sexual differentiation and development of other neuronal populations (Fig. 11.4) [32, 72–74]. E2, for example, can promote neurogenesis in the olfactory bulb and dentate gyrus of the adult rat hippocampus, leading to more newly formed neurons in females [32]. Likewise, in the developing rat hippocampus, higher levels of postnatal sex steroids in males increase the number of new cells, leading to more neurons present in males than in females [75]. However, because the AVPV/PeN as a whole does not undergo differential neurogenesis during the postnatal critical period [32], differential neurogenesis between males and females may not be a major contributor to the sexual differentiation of specific subpopulations within the AVPV/PeN, such as the kisspeptin neurons. However, this assertion has not yet been directly tested.
Schematic of the development of sexually dimorphic AVPV/PeN Kiss1 neurons. Males secrete elevated testosterone (T) at birth, while newborn females secrete negligible levels of sex steroids. Postnatal T converted to estradiol influences the development of neural circuits, leading to their masculinization in adulthood. AVPV/PeN Kiss1 neurons are more abundant in adult females than males. It is currently unknown how the perinatal sex steroid milieu organizes the sexual differentiation of Kiss1 neurons, although the AVPV as a whole is differentiated through Bax-dependent apoptotic mechanisms. However, the sexual differentiation of Kiss1 neurons is likely not due to Bax-dependent apoptosis [40], though other apoptotic mechanisms have not been ruled out. Current data indicate that the Kiss1 gene may be more transcriptionally active in females, pointing to epigenetic alterations as a putative mechanism
One of the primary mechanisms implicated in the sexual differentiation of a number of sexually dimorphic brain populations is programmed cell death (apoptosis) [76–78]. In fact, in rodents, sex differences in the overall size and total cell number of the AVPV region, as well as other brain regions such as the BNST, are induced by apoptosis. Most of these apoptosis-induced sex differences are dependent on the pro-apoptotic gene, Bax [77–79]. BAX is a protein located primarily in the cytosol in a healthy cell. In response to cell death signals, BAX translocates to the mitochondria where it precipitates the release of cytochrome c, thereby triggering caspase pathways that culminate in cell death [80]. Interestingly, in the developing rat AVPV, postnatal males have higher Bax expression than postnatal females, which possibly initiates more cell death in the former sex [81]. Higher Bax expression in postnatal males than females therefore correlates with the presence of fewer AVPV cells in adult males. The sexually dimorphic postnatal Bax levels also coincide with higher sex steroid levels in postnatal males than females, suggesting that sex steroids might affect postnatal Bax expression [81]. Supporting this prediction, E2 treatment of neonatal female rats increases the number of apoptotic AVPV neurons [82]. Importantly, a recent study determined that the sex difference in total number of AVPV neurons is eliminated in Bax knockout mice [76]. Thus, total cell number in the AVPV is sexually differentiated via Bax-dependent apoptotic mechanisms. Despite these findings, the sexual differentiation of Kiss1 neurons in the AVPV/PeN is surprisingly unaltered in Bax knockout mice [40]. When Kiss1 cell numbers are compared between adult male and female Bax KO mice, the Kiss1 sex difference was still incredibly robust [40]. Thus, the Kiss1 population is sexually differentiated either by other apoptotic pathways, such as tumor necrosis α-dependent or -independent mechanisms (as may be the case for AVPV GABA-ergic neurons [78]) or, by non-apoptosis-related mechanisms (Fig. 11.4).
It is likely that the sexual differentiation of AVPV/PeN Kiss1 cells is not induced by mechanisms that affect the physical existence of cells (like apoptosis). Rather, sex differences in Kiss1 cell number may be induced by developmental mechanisms affecting transcriptional activity of the Kiss1 gene (Fig. 11.4) [5, 83]. In fact, epigenetic changes, such as histone modifications and DNA methylation, precipitated by postnatal sex steroids are emerging as critical contributors to alterations in neuronal cell number and gene expression between the sexes [73, 84–88]. Histones are proteins that allow for the packaging of DNA into chromatin. When histones are modified, such as by acetylation, transcriptional activity is altered [89]. Histone acetylation, which is generally associated with increased transcriptional activity, has recently been implicated in the sex steroid-induced sexual differentiation of the BNST [73]. For example, inhibiting histone deacetylase (HDAC) during the early postnatal period in mice blocks the sexual differentiation of the size of the BNST and also alters sexually dimorphic vasopressin fiber projections [73, 88]. Postnatal HDAC inhibition also alters the sexual differentiation of male sexual behavior in rats [87]. It is likely that these postnatal alterations in HDAC activity directly or indirectly affected apoptosis in the BNST, as sex differences in this region are known to be governed specifically by Bax-dependent apoptosis [77]. Other sexual differentiation studies have addressed the role of DNA methylation, which occurs at CpG sites or on CpG islands in a gene or its promoter, and which is generally associated with the repression of gene expression. Studies investigating the involvement of DNA methylation in neural sex differences have found that DNA methylation levels in the hypothalamus correlate with sexually differentiated expression of sex steroid receptor genes in this region [85, 86]. In addition, the expression of DNA methyl transferase 3a (DNMT3a) in newborn rats was found to be sexually dimorphic in the amygdala, which is a known sexually dimorphic brain region [90].
With all the recent evidence implicating the involvement of epigenetics in the sexual differentiation of various brain parameters, we investigated whether either histone deacetylation and/or DNA methylation contributes to the AVPV/PeN Kiss1 sex difference [83]. We pharmacologically blocked histone deacetylation during the postnatal period by administering an HDAC inhibitor, valproic acid (VPA), or vehicle to mice on PND 1 and PND 2 and then analyzed AVPV/PeN Kiss1 expression in adulthood. This postnatal HDAC inhibitor treatment significantly increased the number of detectable Kiss1 cells in the adult AVPV in each sex. However, the sex difference in Kiss1 expression was not eliminated, indicating that histone acetylation is not a key process for inducing the Kiss1 sex difference [83]. Although the sex difference was still robust, the fact that overall Kiss1 levels were higher in mice treated with HDAC inhibitor suggests that the level of histone H3 acetylation during the critical period may be involved in modulating the development of Kiss1 neurons in the AVPV.
Interestingly, we found significant sex differences in the CpG methylation status of the AVPV/PeN Kiss1 gene, predominantly in the putative promoter region (Fig. 11.5). In all cases, these sexually dimorphic Kiss1 CpG sites were more methylated in females than males. Methylation of CpG sites can have multiple modes of affecting gene activity. We tested if methyl-CpG binding protein-2 (MeCP2) was involved in the sex difference by assessing AVPV/PeN Kiss1 levels in male and female Mecp2 mutant mice. The AVPV/PeN sex difference was not eliminated in Mecp2 mutant mice, suggesting that if DNA methylation influences the AVPV/PeN Kiss1 sex difference, it likely does so via non-Mecp2 mechanisms, possibly by blocking the binding of transcriptional repressors [83]. Although these experiments increase our knowledge about the involvement (or lack of involvement) of several epigenetic processes in the development of the AVPV/PeN Kiss1 sex difference, more work is needed to elucidate the exact extent that certain processes, like DNA methylation, are involved. In fact, the AVPV/PeN Kiss1 sex difference may be induced by several epigenetic processes affected by the postnatal sex steroid milieu, causing a silencing of the Kiss1 gene in males while simultaneously allowing increased transcriptional activity in females.
CpG methylation analysis of the putative murine Kiss1 promoter region in the AVPV/PeN. (a) Map of pyrosequenced CpG sites in the putative Kiss1 promoter region using bisulfite-treated DNA derived from AVPV/PeN micropunches of adult males and females that were E2-treated for 1 week before sacrifice. (b) Mean percentage of methylation of sexually dimorphic CpGs (CpG 1, 7, 9) in the Kiss1 promoter. *Significantly different than females (P < 0.05). Modified from Semaan SJ, Dhamija S, Kim J, Ku EC, Kauffman AS 2012 Assessment of epigenetic contributions to sexually dimorphic kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology 153:1875–1886. With permission from The Endocrine Society
Regulation of AVPV/PeN Kiss1 Neurons by Gonadal and Non-gonadal Factors During Peripubertal Development
The steady developmental increase in kisspeptin cell number in the female AVPV/PeN is likely dependent on the presence of ovarian steroids. As mentioned previously, Kiss1 gene expression in the AVPV/PeN increases markedly over the pubertal transition [12, 28, 62, 63, 91]. In adult animals, Kiss1 expression levels in the AVPV/PeN are transiently increased by elevations in the sex steroid milieu and, conversely, decreased by removal of sex steroids [22, 36]. Therefore, it is likely that the observed developmental increase in Kiss1 gene expression during puberty is caused by increased ovarian sex steroid secretion at this time.
Several studies have addressed the effects of sex steroids or their receptors on the developmental increase of kisspeptin cell number in the AVPV/PeN. In one study, female mice were either ovariectomized (OVX) or sham treated on PND 15 and killed on either PND 30 or PND 60. Mice that were OVX on PND 15 had dramatically reduced levels of kisspeptin in the AVPV/PeN later in adulthood, suggesting that the primary cause of the developmental kisspeptin increase is due to ovarian sex steroid secretion [62]. This was supported by the observation that estrogen replacement in OVX animals from either PND 15–30 or PND 22–30 rescues kisspeptin expression when examined at PND 30 [62]. Thus, kisspeptin neurons are sensitive to ovarian steroids peri-pubertally, which is not surprising given their robust regulation by sex steroids in adulthood. It is currently unknown if kisspeptin neurons, however, are responsive to E2 at the first time of visible protein expression (PND 15) or mRNA expression (PND 10), or if OVX at earlier time periods has a more permanent effect on the development of kisspeptin expression.
Several studies have used aromatase knockout (ArKO) mice to examine the effects of E2 signaling on kisspeptin neuron development. One study reported a complete elimination of kisspeptin expression in the AVPV/PeN of adult female ArKO mice [62]. However, E2 was not replaced in these mice prior to sacrifice, and it was therefore unclear if the absence of AVPV/PeN kisspeptin cells in ArKO females mirrored a chronically OVX condition (since removal of E2 via OVX in adulthood reduces AVPV/PeN kisspeptin synthesis). More recently, another study looked at kisspeptin cells in ArKO mice that were given E2 in adulthood. This study found that AVPV/PeN kisspeptin cells were in fact present in adult ArKO mice after E2-treatment. However, surprisingly, the sex difference in kisspeptin cell number was eliminated in these E2-treated ArKO mice [92]. But, instead of ArKO males exhibiting high kisspeptin levels similar to that of wild-type (WT) females, as would be predicted due to the lack of E2-signaling in these males during the postnatal critical period, AVPV/PeN kisspeptin cell number was instead intermediate in level in ArKOs of both sexes, being significantly lower than in normal WT females and significantly higher than in normal WT males [92]. This finding suggests that E2 during development may normally actively contribute to complete feminization of the AVPV/PeN kisspeptin system in females, although when and how this would occur is unknown.
A similar story has emerged concerning the development of the AVPV/PeN kisspeptin system in hypogonadal (hpg) mice. Hypogonadal mice possess a deletion in the Gnrh gene and therefore do not secrete GnRH or gonadal sex steroids [93]. In female hpg mice, AVPV/PeN kisspeptin-ir cell number during development never reaches the level of WT females and is similar to that of hpg males (i.e., the normal kisspeptin sex difference is absent in hpg mice) [94]. Similarly, Kiss1 mRNA expression in pubertal hpg females is decreased compared to that of WT females, and sexually dimorphic Kiss1 expression is eliminated in hpg mice [94]. Hormone replacement was not compared in pubertal hpg females and males in this particular study and may be critical to fully interpret the results. Interestingly though, 1 week of E2 replacement in adult hpg females did not increase AVPV/PeN kisspeptin protein levels to WT female levels, suggesting that gonadal sex steroids may be required at some time during development in order for AVPV/PeN kisspeptin expression to fully mature [94].
Finally, in a different study, ERα was specifically ablated in kisspeptin neurons using cre-lox technology (generating “KERKO” mice) in order to test the role of ERα signaling in kisspeptin neurons [10]. In this model, AVPV/PeN kisspeptin-ir was significantly diminished in adult KERKO females compared to WT females, further implicating the involvement of E2 and ERα in the development of the AVPV/PeN kisspeptin population in females [10]. Likewise, a number of recent studies have implicated endocrine disruptors (which often mimic the effects of E2) in affecting the development of AVPV/PeN Kiss1 gene expression [69, 95, 96], a topic discussed in detail in Chap. 21.
The Development of the ARC Kiss1 Population
Ontogeny of Kiss1 Expression in the ARC
The second major population of kisspeptin neurons in the hypothalamus is located in the ARC, which is equivalent to the INF in humans [7, 14, 21, 62]. The ARC is the most consistently detected kisspeptin population in mammalian species, although its precise distribution and role may vary from species to species [15, 16, 19, 21, 22]. In general, the developmental pattern of Kiss1 expression in the ARC, and the specific factors regulating the development of this neuronal population, are not completely understood and are less well-characterized than the AVPV/PeN.
Unlike in the AVPV/PeN, Kiss1 is expressed in the rodent ARC during embryonic development. Analysis of both kisspeptin-ir and Kiss1 mRNA (via qPCR) in embryonic rats determined that expression in both sexes begins around E14.5 and increases by E18.5, with a sharp drop in levels just prior to birth [61]. Additionally, double-labeling of kisspeptin-ir/BrdU-ir demonstrated that kisspeptin cell neurogenesis in the ARC begins around E12.5 and peaks several days later [97]. In other recent studies, when crossed to reporter mice that permanently mark all cells that ever expressed Kiss1 at any point in development, two Kiss1-Cre transgenic mouse lines were reported to have “extra” cells in the greater ARC region [9, 11]. One of these reports quantified these “extra” Kiss1 cells to comprise ~25 % of the total cells that have ever expressed Kiss1 in the ARC region at some point during development or adulthood [11]. This suggests that the pattern of ARC kisspeptin expression may differ significantly between early developmental and older ages, but it is not clear at what developmental age these “extra” kisspeptin cells become undetectable. Additionally, both male and female adult mice with impaired BAX-mediated apoptosis display “extra” Kiss1 cells in the ARC compared to adult WT mice, particularly in the male, suggesting that the number of ARC Kiss1 neurons is also regulated in early development by apoptosis [40]. The extra Kiss1 cells in the ARC of both the Bax KO and the Kiss1-Cre mice show a similar expression pattern that is located dorsally in relation to the normal ARC Kiss1 population (Fig. 11.6). The ontogeny, developmental pattern, and role, if any, of these extra kisspeptin neurons remain unknown.
Regulation of Kiss1 expression in the ARC by apoptosis. (a) Kiss1 expression in the ARC of GDX adult WT and Bax KO male mice show extra Kiss1 cells in the ARC, suggesting that ARC Kiss1 neurons are regulated in early development by apoptosis. The “extra” Kiss1 cells in the ARC show an expression pattern that is located dorsally in relation to the normal ARC Kiss1 population typically found in WT mice. 3V third ventricle; mARC medial ARC. (b) Mean number of Kiss1 cells in the ARC of adult female (F) and male (M) Bax KO and WT mice that were GDX for 9 day before sacrifice. Kiss1 gene expression in the ARC was significantly higher in Bax KO mice than WT mice (P < 0.01), especially in males. *Significantly different from female mice of same genotype. (a, b) Modified from Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS 2010 BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817. With permission from The Endocrine Society
In rats, initial studies detected Kiss1 expression in the whole hypothalamus on PND 1 using RT-PCR, but it was not determined which specific nuclei (ARC, AVPV/PeN, etc.) were responsible for this neonatal Kiss1 expression [12]. Based on the AVPV/PeN developmental time-course discussed earlier, it is unlikely that the source of this PND 1 Kiss1 expression is the AVPV/PeN. Rather, most, if not all, of the hypothalamic Kiss1 detected in PND 1 rats was probably from the ARC population. Several recent studies have now provided supporting evidence for this likelihood. Using ISH, Kiss1 expression in the ARC was readily detected at PND 1 in mice [98] and rats [47, 99], as well as in rats in another study at the first collected age of PND 4 [64]. Poling and Kauffman provided greater temporal resolution of expression in PND 1 mice: they found notable Kiss1 expression in both sexes at both 0–4 h and 16–20 h after birth (Fig. 11.7), without a significant change in Kiss1 levels between these time-points [98]. Parenthetically, NKB, which is co-expressed in ARC Kiss1 neurons in adulthood, was also readily expressed in the ARC of both sexes on PND 1 [98]. One rat study noted an increase in Kiss1 expression in the ARC between PND 1 and 3 in both sexes [47], with an additional increase at PND 8 in females only. Another study noted similar but non-significant trends in both males and females [64].
Kiss1 gene expression in the murine ARC on PND 1. (a) Representative photomicrographs for Kiss1 expression in newborn females (F) and males (M) that were 0–4 h old. (b) The mean number of Kiss1 neurons in the ARC, as well as the relative level of Kiss1 mRNA/cell, for PND 1 male and female mice that were 0–4 h old. Newborn males had significantly fewer ARC Kiss1 cells and lower mRNA per cell than newborn females (P < 0.05). 3V third ventricle; GPC grains per cell. Modified from Poling MC, Kauffman AS 2012 Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-kiss1r and GnRH signaling. Endocrinology 153:782–793. With permission from The Endocrine Society
The role of Kiss1 in the ARC during early neonatal life is unknown. Because kisspeptin signaling regulates the pubertal and adult reproductive axis, it is possible that kisspeptin plays a similar role earlier in neonatal development. However, since the reproductive axis is typically quiescent at this time, it is not clear what functional significance neonatal kisspeptin signaling would have. The kisspeptin receptor (Kiss1r), also known as Gpr54, is already present in some GnRH neurons at birth and begins appearing in other areas of the brain by PND 6 [100], suggesting the possibility of a functional kisspeptin neuronal network at early postnatal ages. It is possible that neonatal kisspeptin regulates gonadal T secretion, causing the elevation in T that is seen in newborn males but not females [98, 101–105]. Recent evidence has shown that, unlike their WT littermates, newborn Kiss1r KO and hpg mice both have undetectable FSH and LH, demonstrating that kisspeptin and GnRH signaling are each essential for neonatal gonadotropin secretion [98]. However, the same study found that newborn male Kiss1r KO and hpg mice both had elevated serum T levels at birth that were similar to their WT male littermates, indicating that neither GnRH nor kisspeptin signaling is required for neonatal T secretion. These results suggest that, while kisspeptin regulates gonadotropin secretion in neonatal rodents, the neonatal T surge in males is independent of regulation by gonadotropins and kisspeptin.
Prepubertal and Pubertal Changes in Kiss1 Expression in the ARC
Changes in peripubertal Kiss1 expression have been examined in the ARC of rodents, but not as thoroughly as in the AVPV/PeN, and the results are not entirely consistent between studies. Early studies in the whole hypothalamus of the rat showed that Kiss1 levels increased between PND 1–20 and PND 30 in females, and between PND 1–30 and PND 45 in males, followed by decreased expression in adulthood. These studies demonstrated that increases in Kiss1 levels correlate with pubertal timing in males and females [12]. However, since the whole hypothalamus was analyzed, these pubertal changes in Kiss1 expression could be due to changes in AVPV/PeN and/or ARC Kiss1 levels. To get better spatial resolution of the developmental changes in kisspeptin/Kiss1, more recent studies in rodents have used ISH or IHC. Studies using IHC detected kisspeptin-ir in the ARC of juvenile, prepubertal, and peripubertal mice of both sexes, although this kisspeptin-ir was not quantified [28, 94]. An early study using ISH found similar numbers of ARC Kiss1 cells in PND 18 vs. adult male mice [106], suggesting no difference in ARC Kiss1 levels between juvenile and adult stages; however, peripubertal ages in between PND 18 and adulthood were not analyzed. This finding was mirrored by another recent ISH study in female rats that reported no difference in ARC Kiss1 levels between juvenile and late-pubertal animals [107]. Likewise, in male and female mice, Kiss1 expression in the ARC, determined with qPCR, remained virtually unchanged during several stages of postnatal and pubertal development (PND 11–61), and no significant sex differences were noted at any of the ages studied [94].
In contrast to the above studies showing no major peripubertal changes in ARC Kiss1 levels, additional experiments demonstrated increases in ARC Kiss1 expression around the time of early puberty in both female [91] and male rats [108], suggesting that increased ARC Kiss1 expression may be involved in puberty onset. More recently, initial elevations in ARC Kiss1 expression in neonatal/juvenile female rats were reportedly followed by a decrease in Kiss1 levels by 3 weeks of age [47, 64], perhaps concurrent with increased sex steroid production (and negative feedback) at this time. One study also detected another increase in ARC Kiss1 of females between weeks 5 and 8 [64]. Several studies reported that ARC Kiss1 levels in male rats remained essentially unchanged during the first several weeks of life (up to around 4 weeks of age) [47, 64], followed in one case by a small temporary increase in ARC Kiss1 levels around week 5 [64]. Overall, the current data on rodent ARC Kiss1 expression during development, especially at prepubertal and pubertal stages, is incomplete and fairly inconsistent. Thus, full characterization of Kiss1 expression in the ARC during development and puberty is still needed in rodents, as well as other species (see below).
Very few studies on developmental changes in ARC kisspeptin neurons have been conducted in non-rodent species. One study using agonadal male and intact female monkeys described an increase in Kiss1 expression in the ARC during puberty [25]. In peripubertal female lambs with controlled sex steroid levels, a non-significant increase in ARC Kiss1 cell number was seen over time when comparing weeks 25, 30, and 35. When analyzed specifically in lambs that showed increased LH pulse frequency, the number of Kiss1 cells in the middle ARC was found to have a significant linear increase between weeks 25, 30, and 35, possibly implicating the middle ARC in puberty onset [65]. However, another recent report in sheep found that kisspeptin-ir cell number increased in the caudal ARC of females between the prepubertal and postpubertal periods [109]. Thus, the specific location(s) within the ovine ARC where Kiss1 might increase during puberty occurs still needs to be resolved. Collectively, these initial studies in monkeys and sheep suggest that increased levels of Kiss1 in the ARC of pubertal animals correlate with increased activation of the reproductive axis, but these studies surveyed a low resolution of ages over development, necessitating additional data on this subject.
Sex Differences in ARC Kisspeptin Neurons During Development
Most data agree that, unlike the sexually dimorphic AVPV/PeN [31, 47, 70], the number of ARC Kiss1 or kisspeptin cells and the quantity of Kiss1 mRNA per cell are not majorly different between sexes in adult rodents, especially when circulating sex steroid levels are equalized between the sexes [28, 36, 40, 42, 44, 66, 94]. In the few cases where an ARC Kiss1 sex difference was observed, it was typically in gonadally intact adult animals that have unequal sex steroid levels between the sexes. For example, Adachi et al. [42] reported that intact adult male and female rats had equivalent ARC Kiss1 expression at all stages of the female estrous cycle except for at diestrous 2. Moreover, adult males and females exhibit similar increases in Kiss1 levels in the ARC after gonadectomy (GDX) and similar inhibition of ARC Kiss1 expression when treated equivalently with sex steroids [36, 40, 42, 44]. It should be noted that sex differences exist in the ARC in several other parameters, such as the morphology of astroglia, the number of spine and somatic synapses [110–112], the expression of growth hormone-releasing hormone, and the axonal projections of NKB/Dyn neurons [113–115]. Additionally, the kisspeptin fibers visualized in the ARC by IHC are often denser in adult females, but it is likely that some of these fibers are derived from the sexually dimorphic AVPV/PeN kisspeptin population [28, 29, 62, 116]. The ARC population of Kiss1 neurons in rodents is hypothesized to provide tonic stimulatory input to GnRH neurons and to relay negative feedback effects of sex steroids to the GnRH axis [36, 47, 117–119]. These processes occur in both sexes, which is consistent with the lack of a major sexual dimorphism in these neurons in adult rodents.
In contrast to adulthood, recent analysis of Kiss1 expression in the ARC of rodents during development has revealed a different story regarding sexual dimorphism. Work in the Bax KO mouse suggests that male mice may initially have more ARC Kiss1 neurons (or neurons that have the potential to express Kiss1 later in development) than females prior to birth, which is later offset by a higher rate of perinatal apoptosis [40]. However, a recent report in the rat did not find sex differences in the number of kisspeptin-ir cells during prenatal development, but did not look at sex differences in Kiss1 expression levels [97]. A recent study in mice found that newborn PND 1 pups exhibit sexual dimorphism in ARC Kiss1 expression, with newborn females having significantly more Kiss1 and NKB cells in the ARC, as well as higher cellular expression of these mRNAs, than newborn males [98]. Similarly, male rats also have lower ARC Kiss1 levels than females at PND 3 [47] and PND 4 [64]. Studies using both ISH and IHC found that the number of Kiss1 neurons in the ARC of rats is still sexually dimorphic between PND 5 and PND 11 [47, 64, 99, 120], with juvenile females having approximately 2–4 times as many ARC Kiss1 (or kisspeptin) neurons than juvenile males of the same age. This ARC Kiss1 sex difference begins to diminish with the approach of puberty; while males maintain a slight non-significant trend in increasing ARC Kiss1 cell numbers from juvenile life through puberty, Kiss1 expression in the ARC of females decreases around 3 weeks of age to reach levels similar to males [44, 47, 63].
The neonatal and juvenile sex differences in Kiss1 expression in the rodent ARC may be a temporary sex difference due to sexual dimorphism in the circulating sex steroid milieu: higher levels of circulating sex steroids in young males may provide more negative feedback inhibition than in young females, resulting in lower ARC Kiss1 expression in the males. This could certainly be the case in newborn animals, in which males secrete elevated T (to drive sexual differentiation) whereas females do not [98]. However, it is not entirely clear what normal sex steroid levels are in male and female rodents, especially mice, during each phase of neonatal and juvenile development, and most studies that have examined Kiss1 expression early in development have not measured serum sex steroid levels. Moreover, it is also possible that non-steroidal mechanisms also influence the ARC Kiss1 sexual dimorphism, as PND 1 male mice that are 16–20 h old (when circulating T levels are no longer different between the sexes) still display sex differences in ARC Kiss1 levels [98]. Other findings support the role of sex steroid-independent factors in regulating Kiss1/NKB expression in the ARC of juvenile mice in a sexually dimorphic manner [44] (discussed more later). Additionally, the functional significance, if any, of the sexual dimorphism in the ARC Kiss1 neuronal population during neonatal and juvenile development is completely unknown, especially since the reproductive axis is essentially quiescent at this time.
Some non-rodent species, such as the sheep, display clear adulthood sex differences in kisspeptin cells in the ARC. Ewes display a greater number of Kiss1 cells in the ARC than adult males [67]; this may be due to differences in the functional roles of Kiss1 cells in the ARC/INF of rodents vs. sheep. However, as of yet, no direct comparison has been made between adult ewes and rams with equivalent sex steroid levels. Additionally, while prenatal androgen treatment reduced NKB and DYN expression in the ARC in adult sheep, this prenatal treatment did not alter ARC Kiss1 expression, which remained sexually dimorphic [67]. Thus, there may be different critical periods for the sexual differentiation of these ARC genes, but it is also possible that the ovine ARC Kiss1 system is not affected by prenatal sex steroids. Dissimilarities between species may be due to the difference in the role of the ARC; while ARC kisspeptin cells in rodents may mediate negative feedback regulation of GnRH secretion, which is not sexually dimorphic [6, 22, 46, 51, 118], the same kisspeptin population in sheep appears to be key for both negative feedback and positive feedback (i.e., the preovulatory GnRH surge) [51, 121–124]. Similar sex differences in kisspeptin expression have been recently reported in humans in the INF, as well as the POA, with females showing greater number of cells than males in each region [125]. However, note that in these human studies, regulatory factors that are known to alter kisspeptin levels, such as circulating sex steroids, metabolic status, stress hormones, and circadian status, were not controlled for and could differ dramatically between subjects, thereby confounding the results. Whether non-human primates display adult sex differences in ARC/INF kisspeptin levels is currently unexamined [126].
Regulation of ARC Kiss1 Neurons by Gonadal and Non-gonadal Factors During Development
As noted earlier, under normal developmental conditions, the rodent ARC displays a dense network of kisspeptin-ir fibers that likely originate from both the ARC and the AVPV/PeN. Supporting the hypothesis that some of these fibers are projections from the AVPV, data has shown that when gonadal hormones are removed during development, whether by GDX or genetically, as in the case of the hpg and KERKO mice, the number of kisspeptin fibers detected in the ARC are reduced in adulthood [10, 62, 94]. These data also suggest that gonadal steroids during development are necessary for either the physical development of kisspeptin fibers (organizational effects) or for the transient expression of kisspeptin protein within the cells and fibers (activational effects). Considering that the number of kisspeptin fibers in the ARC was restored in adult hpg mice treated with E2, it seems likely that these kisspeptin fibers develop normally but only contain detectable levels of kisspeptin under the correct adult sex steroidal milieu [94]. Thus, the reduction of these fibers in non-E2 treated hpg (and KERKO) mice is likely due to an activational defect (i.e., absence of circulating sex steroids). However, caution must be used in interpreting fiber data since the origin of the fibers cannot always be determined.
The hpg and KERKO mouse models have recently been utilized to shed more light on the role of gonadal sex steroids in the development of ARC Kiss1 neurons. First, kisspeptin cell bodies are more visible in the ARC of adult hpg mice than WT mice [94]. This difference in detectable kisspeptin cells is likely due to the lack of obstruction by dense kisspeptin fibers in hpg mice, as well as a possible increase in ARC kisspeptin synthesis in hpg mice lacking steroid negative feedback (Fig. 11.8). Since differences in ARC kisspeptin-ir expression and cell number were not easily measurable by IHC due to the obstructive overlaying fiber network, Kiss1 mRNA levels were examined instead using ISH. Both the number of Kiss1 neurons and the density of silver grains were increased in the ARC of adult hpg mice compared to WT littermates, suggesting an increased level of kisspeptin synthesis in hpg mice, perhaps due to less steroid negative feedback. Interestingly, at PND 11, only a small difference in Kiss1 levels was present between WT and hpg littermates (as measured by RT-PCR), but by PND 31 this difference was much larger, with dramatically higher ARC Kiss1 expression in hpg mice. This indicates that, at PND 11, there may be non-gonadal hormone factors inhibiting Kiss1 in the ARC, but by PND 31 this gonadal-independent mechanism is reduced (or absent). These hpg data therefore suggest that changes in gonad-independent factors may induce ARC Kiss1 expression around puberty.
Kisspeptin and Kiss1 expression are increased in the ARC of female and male hpg mice. (a) Kisspeptin staining pattern in the ARC of both female and male WT mice (left of dashed line) is dominated by densely stained fibers that obscure kisspeptin-positive cell bodies (white arrowheads). In hpg mice (right of dashed line), kisspeptin immunoreactivity shows reduced fiber staining and increased clusters of large, darkly stained cell bodies (black arrowheads). (b) Representative dark-field images of silver grain Kiss1 mRNA signal as measured by ISH in the ARC of pubertal-aged WT and hpg females (30 days) and males (45 days). Scale bar = 200 mm. Figure modified from Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB 2010 Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5:e11911
The robust ARC Kiss1 expression seen in cell bodies of adult hpg and ArKO mice may be similar to the hypertrophied Kiss1 neurons seen in postmenopausal women [43, 127]; in all cases, the elevated Kiss1 levels may reflect a lack of negative feedback of sex steroids. Finally, female KERKO mice show decreased kisspeptin-ir fibers in the ARC at PND 16, 26, and 35, similar to hpg mice [10]. Surprisingly, unlike hpg mice, adult KERKO mice did not display the high-expressing kisspeptin-ir cell bodies in the ARC, even though the same kisspeptin antibody was used in both studies. However, when mRNA was analyzed by qPCR in the mediobasal hypothalamus (which includes the ARC), Kiss1 levels in KERKO mice were in fact increased relative to WT levels, similar to data from hpg mice. This unexpected difference between protein and mRNA levels in the KERKO mice has not yet been explained.
Recent work has shown that during the prepubertal period of mice, the Kiss1 response to GDX is sexually dimorphic. While prepubertal females display elevated Kiss1 expression in the ARC, along with increased LH levels, several days after GDX, prepubertal male mice do not (Fig. 11.9). Instead, if prepubertal male mice are GDX at PND 14, no increase is seen in ARC Kiss1 cell number or serum LH levels when measured 2–4 days later (PND 16–18) [44]. Because this prepubertal sex difference is only observable after the removal of gonadal hormones (there is no sex differences in ARC Kiss1 cell number at this age in gonadally intact mice), these results suggest a sexually dimorphic difference in the regulation of ARC Kiss1 cells at this point in development: Kiss1/LH levels in PND 16–18 females appear to be controlled primarily by gonadal sex steroid feedback, whereas PND 16–18 males have an additional mechanism of regulation of their ARC Kiss1 neurons by unknown non-gonadal factors. Whether this divergence between the sexes is due to a unique, male-specific mechanism not present in similarly aged females or to sex differences in the rate of pubertal maturation (females initiate puberty earlier than males) remains to be seen. This gonadal hormone-independent regulation of Kiss1 neurons in developing males appears to disappear sometime between PND 18 and 45, as male mice GDX at PND 14 exhibit the expected elevation of ARC Kiss1 and serum LH levels when analyzed at PND 45. The exact function of this sex difference still needs to be elucidated, though it is possible it relates to the sexually dimorphic timing of pubertal progression.
Kiss1 expression in the ARC of prepubertal mice under different sex steroid conditions. Gonadectomized prepubertal female mice have higher ARC Kiss1 expression than similarly aged gonadectomized males. The absence of elevated Kiss1 expression in gonadectomized prepubertal males suggests that some non-gonadal factor(s) acts to suppress the ARC Kiss1 system in males at this developmental stage. 3V third ventricle. Figure modified from Kauffman AS 2010 Gonadal and non-gonadal regulation of sex differences in hypothalamic Kiss1 neurons. J Neuroendocrinol 22:682–691 with permission from John Wiley and Sons
Conclusions and Perspectives
Kiss1 neurons are critical for puberty and reproduction, and this chapter is focused on the development of these neurons in the hypothalamic AVPV/PeN and ARC (Fig. 11.10). In terms of the AVPV/PeN Kiss1 population, which emerges in the second postnatal week of life and is sexually dimorphic, it remains to be answered how this population becomes sexually differentiated. Recent data suggest that epigenetic processes, precipitated by postnatal sex steroid signaling, may induce the AVPV/PeN Kiss1 sex difference [5], especially because a role for the major neuronal pathway of apoptosis was ruled out [40]. This model predicts that the differences in sex steroid exposure between male and female rodent brains early in neonatal life eventually result in permanent changes in the transcriptional activity of the Kiss1 gene in the AVPV/PeN, resulting in greater Kiss1 silencing in males and greater transcriptional activation in females. Supporting this, DNA methylation of the putative promoter of the Kiss1 gene in the AVPV/PeN is sexually differentiated [83], though we still do not know when or how in development this Kiss1 methylation difference first occurs.
Schematic diagram developmental changes in Kiss1 expression in the AVPV/PeN (a) and ARC (b) of rodents. (a) Kiss1 expression in the AVPV/PeN has not been detected on the day of birth or during other days of the “critical period” of perinatal development (light blue shading). Kiss1 mRNA expression is first detected in the AVPV/PeN on postnatal day 10 (PND 10) in both males and females. The sex difference emerges by PND 12. Male Kiss1 gene expression in the AVPV/PeN only slightly increases after that time to adulthood, whereas females have a steady increase in Kiss1 expression that reaches adulthood levels around the time of puberty. (b) 1 Kiss1 is expressed in the ARC before birth. Analysis of combined male and female embryonic rat brains demonstrates increasing levels of Kiss1 in the ARC throughout prenatal development (gray shading), with a slight drop before birth. Comparative analysis of embryonic Kiss1 mRNA levels between sexes has not yet been determined. 2 During the neonatal period, Kiss1 expression in the rodent ARC is sexually dimorphic, with females expressing more Kiss1 than males. This may correlate with differences in the circulating sex steroid milieu, but requires further investigation. 3 Peripubertal Kiss1 levels in the ARC decrease significantly in females to levels similar to males. 4 There may be a slight increase in ARC Kiss1 expression during puberty leading to adulthood levels, though such an increase is controversial at present
Although E2 signaling in males masculinizes the AVPV/PeN Kiss1 system during the neonatal “critical period,” it now appears that exposure to E2 in developing females also shapes and promotes the complete feminization of AVPV/PeN Kiss1 expression [10, 62, 94]. Indeed, several studies in mice have suggested that E2 is required at some time during development in order for AVPV/PeN kisspeptin expression to fully develop a female phenotype [10, 62, 94]. However, the mechanism by which this occurs currently remains a mystery and may also involve epigenetics, neurogenesis, or a combination of these and other processes.
The role of the ARC/INF population of kisspeptin neurons in mediating sex steroid negative feedback in rodents appears to be different than some other mammals, such as sheep, in which ARC kisspeptin cells also play a role in positive feedback. Recent studies suggest that Kiss1 is expressed in the ARC of developing rodents long before the AVPV/PeN, including prenatally (Fig. 11.10). The role of ARC Kiss1 expression during prenatal/neonatal development is currently unknown and requires further investigation. In many species, the regulation of ARC kisspeptin fibers and their projections in development remains under-studied, in part because, until recently, there has not been a good way to distinguish the anatomical origin of kisspeptin-ir fibers. The recent findings that the ARC Kiss1 population co-expresses NKB and DYN (in all species examined to date), whereas AVPV/PeN Kiss1 neurons do not, should facilitate future studies characterizing the development of kisspeptin neuron projections, as should the use of several newly created transgenic Kiss1 mouse models. Additionally, some studies suggest that there may be an increase in Kiss1 expression in the ARC around puberty (Fig. 11.10), but the data is incomplete and inconsistent between studies, and more experiments looking at pubertal Kiss1/kisspeptin expression with higher temporal resolution are needed, including in non-rodent species.
While sex differences in ARC Kiss1 cell number have not been observed by most studies examining adult rodents, such sex differences do exist in the ARC of neonatal and juvenile rodents (Fig. 11.10), as do sex differences in the gonad hormone-independent regulation of ARC Kiss1 neurons in prepubertal life, at least in mice. The functional significance of these early ARC Kiss1 sex differences is unknown, as are the specific factors controlling Kiss1 expression in the ARC at early developmental ages. For example, sex steroids may transiently inhibit ARC Kiss1 neurons (activational effects) during prenatal and postnatal development, as occurs in adulthood, but this has not been directly tested. Indeed, there is a need for examination of normal sex steroid levels in developing rodents, especially mice, during neonatal, juvenile, and prepubertal stages. Interestingly, it appears that in the complete absence of gonadal sex steroids during development, ARC Kiss1 neurons still develop normally and have intact hormone responsiveness later in adulthood, suggesting that this Kiss1 population may only be affected by activational effects of sex steroids, rather than by permanent organizational effects that occur in the AVPV/PeN.
Overall, the development of kisspeptin circuits appears to be complex, region-specific, sex-specific, and influenced by multiple factors. The upstream mechanism(s) driving developmental and pubertal changes in kisspeptin neurons in each hypothalamic region, and how gonadal and non-gonadal factors work together to control development of the Kiss1 system, remain important questions whose answers will provide a better understanding of the regulation of the reproductive neuroendocrine axis at multiple life stages.
References
Cesario SK, Hughes LA (2007) Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs 36:263–274
Fechner A, Fong S, McGovern P (2008) A review of Kallmann syndrome: genetics, pathophysiology, and clinical management. Obstet Gynecol Surv 63:189–194
Bianco SD, Kaiser UB (2009) The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576
Fechner PY (2002) Gender differences in puberty. J Adolesc Health 30:44–48
Semaan SJ, Kauffman AS (2010) Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol 20:424–431
Kauffman AS (2010) Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63
Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE (2008) Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 28:8691–8697
Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M (2009) Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology 151(2):722–730
Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF (2011) Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173:37–56
Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE (2010) Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107:22693–22698
Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA (2011) Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152:4298–4309
Navarro VM, Castellano JM, Fernández-Fernández R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M (2004) Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145:4565–4574
Brailoiu GC, Dun SL, Ohsawa M, Yin D, Yang J, Chang JK, Brailoiu E, Dun NJ (2005) KiSS-1 expression and metastin-like immunoreactivity in the rat brain. J Comp Neurol 481:314–329
Clarkson J, D’Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE (2009) Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 21:673–682
Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A (2006) Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett 401:225–230
Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ (2007) Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148:5752–5760
Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE (2007) Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology 148:1158–1166
Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H (2009) Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21:813–821
Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM (2008) Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149:4387–4395
Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS (2011) Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152:2020–2030
Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077
Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA (2005) Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692
Kauffman AS, Park JH, McPhie-Lalmansingh AA, Gottsch ML, Bodo C, Hohmann JG, Pavlova MN, Rohde AD, Clifton DK, Steiner RA, Rissman EF (2007) The kisspeptin receptor GPR54 is required for sexual differentiation of the brain and behavior. J Neurosci 27:8826–8835
Smith JT, Clay CM, Caraty A, Clarke IJ (2007) KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148:1150–1157
Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM (2005) Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA 102:2129–2134
Kim W, Jessen HM, Auger AP, Terasawa E (2009) Postmenopausal increase in KiSS-1, GPR54, and luteinizing hormone releasing hormone (LHRH-1) mRNA in the basal hypothalamus of female rhesus monkeys. Peptides 30:103–110
Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ (2010) Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod 83:568–577
Clarkson J, Herbison AE (2006) Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825
Yeo SH, Herbison AE (2011) Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 152:2387–2399
Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ (2011) Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 152:1001–1012
Kauffman AS (2009) Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides 30:83–93
Simerly RB (2002) Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536
Herbison AE (2008) Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57:277–287
Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA (2006) Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694
Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS (2009) Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 150:3664–3671
Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M (2007) Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783
Simerly RB (1998) Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav Brain Res 92:195–203
Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS (1997) Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA 94:14077–14082
Ottem EN, Godwin JG, Krishnan S, Petersen SL (2004) Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105
Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS (2010) BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 151:5807–5817
Clarkson J, Herbison AE (2011) Dual phenotype kisspeptin-dopamine neurones of the rostral periventricular area of the third ventricle project to gonadotrophin-releasing hormone neurones. J Neuroendocrinol 23:293–301
Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K (2007) Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev 53:367–378
Rometo AM, Krajewski SJ, Voytko ML, Rance NE (2007) Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750
Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA (2009) Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:E1212–E1221
Smith JT (2008) Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev 57:288–298
Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA (2005) Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984
Cao J, Patisaul HB (2011) Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol 519:2954–2977
Shughrue PJ, Merchenthaler I (2001) Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol 436:64–81
Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95
Oakley AE, Clifton DK, Steiner RA (2009) Kisspeptin signaling in the brain. Endocr Rev 30:713–743
Smith JT (2009) Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102
Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK (2009) TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41:354–358
Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN (2004) Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology 145:2959–2967
Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K (2005) Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 146:4431–4436
Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA (2009) Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866
Sandoval-Guzman T, Rance NE (2004) Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312
Schulz R, Wilhelm A, Pirke KM, Gramsch C, Herz A (1981) Beta-endorphin and dynorphin control serum luteinizing hormone level in immature female rats. Nature 294:757–759
Burke MC, Letts PA, Krajewski SJ, Rance NE (2006) Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498:712–726
Foradori CD, Amstalden M, Goodman RL, Lehman MN (2006) Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol 18:534–541
Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE (2010) Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166:680–697
Desroziers E, Droguerre M, Bentsen AH, Robert V, Mikkelsen JD, Caraty A, Tillet Y, Duittoz A, Franceschini I (2012) Embryonic development of kisspeptin neurones in rat. J Neuroendocrinol 24(10):1284–1295
Clarkson J, Boon WC, Simpson ER, Herbison AE (2009) Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220
Kauffman AS, Semaan SJ (2011) Pubertal changes in gene expression in reproductive neural circuits of female mice. Society for Neuroscience: Abstract #713
Takumi K, Iijima N, Ozawa H (2011) Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J Mol Neurosci 43:138–145
Redmond JS, Baez-Sandoval GM, Spell KM, Spencer TE, Lents CA, Williams GL, Amstalden M (2011) Developmental changes in hypothalamic Kiss1 expression during activation of the pulsatile release of luteinising hormone in maturing ewe lambs. J Neuroendocrinol 23:815–822
Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, K-I M, Tsukamura H (2009) Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81:1216–1225
Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN (2010) The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology 151:301–311
Morris JA, Jordan CL, Breedlove SM (2004) Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039
Bateman HL, Patisaul HB (2008) Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29:988–997
Patisaul HB, Todd KL, Mickens JA, Adewale HB (2009) Impact of neonatal exposure to the ERalpha agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30:350–357
Patisaul HB, Losa-Ward SM, Todd KL, McCaffrey KA, Mickens JA (2012) Influence of ERbeta selective agonism during the neonatal period on the sexual differentiation of the rat hypothalamic-pituitary-gonadal (HPG) axis. Biol Sex Differ 3:2
Forger NG (2009) Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol 21:393–399
McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME (2009) The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823
Murray EK, Hien A, de Vries GJ, Forger NG (2009) Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150:4241–4247
Zhang JM, Konkle AT, Zup SL, McCarthy MM (2008) Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci 27:791–800
Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ (2004) Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101:13666–13671
Gotsiridze T, Kang N, Jacob D, Forger NG (2007) Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol 67:355–362
Krishnan S, Intlekofer KA, Aggison LK, Petersen SL (2009) Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA 106:16692–16697
Holmes MM, McCutcheon J, Forger NG (2009) Sex differences in NeuN- and androgen receptor-positive cells in the bed nucleus of the stria terminalis are due to Bax-dependent cell death. Neuroscience 158:1251–1256
Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26:1324–1337
Tsukahara S, Kakeyama M, Toyofuku Y (2006) Sex differences in the level of Bcl-2 family proteins and caspase-3 activation in the sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neurobiol 66:1411–1419
Waters EM, Simerly RB (2009) Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci 29:9714–9718
Semaan SJ, Dhamija S, Kim J, Ku EC, Kauffman AS (2012) Assessment of epigenetic contributions to sexually-dimorphic kiss1 expression in the anteroventral periventricular nucleus of mice. Endocrinology 153:1875–1886
Nugent BM, McCarthy MM (2010) Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology 93:150–158
Kurian JR, Olesen KM, Auger AP (2010) Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology 151:2297–2305
Schwarz JM, Nugent BM, McCarthy MM (2010) Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 151:4871–4881
Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M (2011) Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152:2760–2767
Murray EK, Varnum MM, Fernandez JL, de Vries GJ, Forger NG (2011) Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J Neuroendocrinol 23(10):906–914
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–638
Kolodkin MH, Auger AP (2011) Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol 23:577–583
Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI (2009) Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol 21:527–537
Bakker J, Pierman S, Gonzalez-Martinez D (2010) Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm Behav 57:390–395
Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G (1977) Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature 269:338–340
Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB (2010) Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One 5:e11911
Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC (2010) Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology 152:581–594
Tena-Sempere M (2010) Kisspeptin/GPR54 system as potential target for endocrine disruption of reproductive development and function. Int J Androl 33:360–368
Desroziers E, Mikkelsen JD, Duittoz A, Franceschini I (2012) Kisspeptin-immunoreactivity changes in a sex- and hypothalamic-region-specific manner across rat postnatal development. J Neuroendocrinol 24:1154–1165
Poling MC, Kauffman AS (2012) Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-kiss1r and GnRH signaling. Endocrinology 153:782–793
Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB (2012) Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology 33:23–36
Herbison AE, de Tassigny X, Doran J, Colledge WH (2010) Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 151:312–321
Corbier P (1985) Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology 116:142–147
Corbier P, Edwards DA, Roffi J (1992) The neonatal testosterone surge: a comparative study. Arch Int Physiol Biochim Biophys 100:127–131
Corbier P, Kerdelhue B, Picon R, Roffi J (1978) Changes in testicular weight and serum gonadotropin and testosterone levels before, during, and after birth in the perinatal rat. Endocrinology 103:1985–1991
Motelica-Heino I, Castanier M, Corbier P, Edwards DA, Roffi J (1988) Testosterone levels in plasma and testes of neonatal mice. J Steroid Biochem 31:283–286
Baum MJ, Brand T, Ooms M, Vreeburg JT, Slob AK (1988) Immediate postnatal rise in whole body androgen content in male rats: correlation with increased testicular content and reduced body clearance of testosterone. Biol Reprod 38:980–986
Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE (2005) Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356
Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, Leon S, Sangiao-Alvarellos S, Castellano JM, Clifton DK, Pinilla L, Steiner RA, Tena-Sempere M (2012) Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci 32:2388–2397
Bentsen AH, Ansel L, Simonneaux V, Tena-Sempere M, Juul A, Mikkelsen JD (2010) Maturation of kisspeptinergic neurons coincides with puberty onset in male rats. Peptides 31:275–283
Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM (2012) Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology 153:2756–2765
Mong JA, Glaser E, McCarthy MM (1999) Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci 19:1464–1472
Mong JA, McCarthy MM (2002) Ontogeny of sexually dimorphic astrocytes in the neonatal rat arcuate. Brain Res Dev Brain Res 139:151–158
Mong JA, Roberts RC, Kelly JJ, McCarthy MM (2001) Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol 432:259–267
Ciofi P, Leroy D, Tramu G (2006) Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 141:1731–1745
Mong JA, Kurzweil RL, Davis AM, Rocca MS, McCarthy MM (1996) Evidence for sexual differentiation of glia in rat brain. Horm Behav 30:553–562
Nurhidayat, Tsukamoto Y, Sasaki F (2001) Role of the gonads in sex differentiation of growth hormone-releasing hormone and somatostatin neurons in the mouse hypothalamus during postnatal development. Brain Res 890:154–161
Losa SM, Todd KL, Sullivan AW, Cao J, Mickens JA, Patisaul HB (2011) Neonatal exposure to genistein adversely impacts the ontogeny of hypothalamic kisspeptin signaling pathways and ovarian development in the peripubertal female rat. Reprod Toxicol 31:280–289
Castellano JM, Bentsen AH, Mikkelsen JD, Tena-Sempere M (2010) Kisspeptins: bridging energy homeostasis and reproduction. Brain Res 1364:129–138
Kauffman AS, Clifton DK, Steiner RA (2007) Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci 30:504–511
Dungan HM, Clifton DK, Steiner RA (2006) Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158
Iijima N, Takumi K, Sawai N, Ozawa H (2011) An immunohistochemical study on the expressional dynamics of kisspeptin neurons relevant to GnRH neurons using a newly developed anti-kisspeptin antibody. J Mol Neurosci 43:146–154
Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A (1998) Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760
Hess DL, Wilkins RH, Moossy J, Chang JL, Plant TM, McCormack JT, Nakai Y, Knobil E (1977) Estrogen-induced gonadotropin surges in decerebrated female rhesus monkeys with medial basal hypothalamic peninsulae. Endocrinology 101:1264–1271
Krey LC, Butler WR, Knobil E (1975) Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology 96:1073–1087
Foster DL, Jackson LM, Padmanabhan V (2006) Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol 254–255:109–119
Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I (2010) The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. Eur J Neurosci 31:1984–1998
Kauffman AS (2010) Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones. J Neuroendocrinol 22:682–691
Rance NE (2009) Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Semaan, S.J., Tolson, K.P., Kauffman, A.S. (2013). The Development of Kisspeptin Circuits in the Mammalian Brain. In: Kauffman, A., Smith, J. (eds) Kisspeptin Signaling in Reproductive Biology. Advances in Experimental Medicine and Biology, vol 784. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6199-9_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6199-9_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6198-2
Online ISBN: 978-1-4614-6199-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)