Abstract
Despite advances in its treatment, heart failure prevalence continues to increase and this condition remains a major contributor to mortality and morbidity in both industrialized and developing nations throughout the world. Cardiac remodeling that is activated by injury to the myocardium and/or increased wall stress adversely affects cardiac function over time and is known to play a critical role in the progression of heart failure. Excessive accumulation and stiffening of collagen (i.e., fibrosis) is a well-recognized component of the remodeling process, and it contributes to progression of disease and worsening heart failure by perturbing cardiac contractility, relaxation, and electrical conduction. Cardiac fibroblasts are interstitial cells that are responsible for synthesis and turnover of collagen in the myocardium. Current experimental and clinical evidence suggests that cardiac fibroblasts are an important target when inhibitors of the renin–angiotensin system (RAS) are used to treat heart failure. An alternative pathway of the RAS, involving angiotensin-converting enzyme 2 (ACE2), angiotensin-(1–7) (Ang-(1–7)), and the Mas receptor, has been found recently to be cardioprotective. Evidence suggests that Ang-(1–7), acting predominantly through the Mas receptor, can regulate maladaptive growth-promoting effects of angiotensin ll and inhibit both cardiomyocyte hypertrophy and cardiac fibrosis. This chapter highlights the potential regulatory role of the ACE2/Ang-(1–7)/Mas pathway and how it relates to cardiac fibroblasts during the remodeling process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 The Cardiac Fibroblast and Cardiac Remodeling
Almost 6 million people suffer from heart failure in the United States today [1]. Similar high rates of heart failure prevalence are seen in other industrialized countries, and there is evidence that heart failure is growing rapidly as a public health problem in developing nations [2–5]. Current medical and device therapies have reduced morbidity and mortality in patients with heart failure. However, the effects of available treatment strategies have plateaued, and novel targets are needed if improvements in outcome are to continue in the future. In patients with chronic heart failure, the condition develops as a consequence of progressive changes in the structure of the heart that impair its normal function [6]. Hypertrophy of cardiomyocytes is a hallmark of the remodeling process, and there is evidence that the structural changes in these cells are intimately associated with abnormalities in their function. Another change in the structure of the heart that occurs as part of the remodeling process and which plays a critical role in the progression to heart failure is the deposition of collagen within the heart (i.e., cardiac fibrosis). Cardiac fibrosis stiffens the heart wall and alters electrical contractility, leading to diminished diastolic and/or systolic function and increasing the probability of debilitating or even fatal arrhythmias. Activation of the mechanisms that cause fibrosis as well as the extent of fibrous tissue that has been deposited within the failing heart has been associated with worse outcomes in heart failure patients. Thus, minimizing the development of fibrosis is expected to improve cardiac function, slow the development of heart failure, and help reduce morbidity and mortality in the heart failure population.
1.2 ACE2/Ang-(1–7)/Mas Pathway
There are several current treatment strategies for heart failure that target the renin–angiotensin system (RAS). These involve inhibition of the generation of angiotensin II (Ang II) with angiotensin-converting enzyme (ACE) inhibitors or blocking activation of Ang II receptors with angiotensin receptor blockers (ARBs). The importance of the RAS in the progression of heart failure is apparent by the beneficial effects of both ACE inhibitors and ARBs in large-scale, well-designed clinical trials [7–11]. These agents have been shown to inhibit the remodeling process as well as reduce morbidity and mortality across a broad spectrum of patients ranging from those with asymptomatic left ventricular (LV) dysfunction, to MI survivors, to patients with clinically manifested heart failure including those with advanced disease.
Recently, an alternative axis of the RAS, the ACE2/Ang-(1–7)/Mas pathway, has been receiving much attention as it is believed to function as a means of modulating the effects of Ang II and other growth-promoting factors during remodeling. Angiotensin-converting enzyme 2 (ACE2) was identified in 2000 by two independent groups [12, 13]. ACE2 functions as a carboxymonopeptidase and has approximately 40% sequence similarity when compared to ACE. ACE2 can hydrolyze the carboxy-terminal phenylalanine from Ang II to form the heptapeptide angiotensin-(1–7) (Ang-(1–7)), or it can generate angiotensin-(1–9) from angiotensin I, at which point other peptidases can convert angiotensin-(1–9) to Ang-(1–7) [14]. Other substrates for ACE2 include des-Arg9-bradykinin, apelin-13, dynorphin A (1–13), and β-casomorphin [14]. When considering only the angiotensin peptides, ACE2 has two, potentially beneficial functions. (1) It can degrade Ang II and (2) it can generate Ang-(1–7). As opposed to Ang II, which is considered to activate cardiomyocyte hypertrophy and fibrosis and, thus, to be deleterious by promoting remodeling, Ang-(1–7) is considered to have potentially beneficial antigrowth and antifibrotic effects that would act to inhibit the remodeling process. In human myocardium, Ang II has been shown to be the preferred substrate for ACE2. Moreover, ACE2 effects appear to be impervious to the ACE inhibitors that are used in clinical practice.
Some investigators have reported that Ang-(1–7) can act through well-characterized angiotensin receptors such as AT1 or AT2 [15–17]. However, Ang-(1–7) more frequently acts via its own dedicated receptor, which is widely considered to be the Mas receptor [18]. Mas is a G protein-coupled receptor that was originally described as a protooncogene [19, 20]. Mas becomes detectable after birth in the rat, increases its expression, and then plateaus at 4–6 months of age [21]. Mas exhibits a broad tissue expression profile, including the heart, kidney, testis, brain, retinal pigment epithelium, skeletal muscle, liver, and adipose tissue [22–24]. Within the heart, Mas is localized to the vasculature [25], cardiomyocytes [26, 27], and fibroblasts (detected by qRT-PCR, data not shown). Despite the passage of ten years since the initial characterization of Mas as the Ang-(1–7) receptor [18], the signaling pathways involved have not yet been fully delineated.
2 Responses of the Cardiac Fibroblast to Ang-(1–7)
2.1 Direct Responses to the Peptide
Direct activation of signaling pathways by Ang-(1–7) treatment of cardiac fibroblasts isolated from normal hearts has been reported. We have observed weak, transient phosphorylation of ERK1/ERK2 upon stimulation of adult rat cardiac fibroblasts with Ang-(1–7) (unpublished observations). We have also observed reduced endothelin-1 (ET-1) and leukemia inhibitory factor (LIF) mRNA levels between 1 and 2 h after stimulation of adult rat cardiac fibroblasts with Ang-(1–7), presumably via activation of an undetermined signaling pathway [28]. We have observed modest inhibition of Ang II-stimulated increases in phosphorylation of MAPKs (e.g., ERK, JNK, and p38) in fibroblasts which had been pretreated with Ang-(1–7). McCollum et al. also observed that treatment of cardiac fibroblasts with Ang-(1–7) reduced the Ang II- or ET-1-stimulated increase in phospho-ERK1 and phospho-ERK2 [29]. They observed that Ang-(1–7) increased dual-specificity phosphatase DUSP1 immunoreactivity and mRNA, suggesting that the heptapeptide hormone increases DUSP1 to reduce MAP kinase phosphorylation and activity. The mechanism through which Ang-(1–7) increased DUSP1, however, was not elucidated. Increased generation of second messengers by Ang-(1–7) stimulation has been reported, including nitric oxide in cardiomyocytes [26] and aortic endothelial cells [30], cAMP in glomerular mesangial cells [31], and arachidonic acid from Mas-transfected CHO cells [18]. However, we have failed to observe activation of these second messengers in response to Ang-(1–7) in cultured cardiac fibroblasts (data not shown).
2.2 Indirect Responses to the Peptide
As opposed to the direct stimulatory effects of Ang-(1–7) on cardiac fibroblasts, which tend to be mild, indirect effects of the peptide can produce a much greater response. These indirect responses include alteration of other signaling pathways and the influence of second messengers that have been generated by cardiac fibroblasts, their precursors, or other nearby cell types.
2.2.1 Attenuation/Augmentation of Other Receptor Signaling Pathways
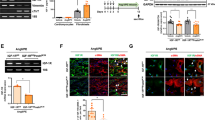
Stimulation of cells with Ang-(1–7) can affect the subsequent activation of other signaling pathways. Stimulation of nitric oxide production by bradykinin in endothelial cells can be augmented by prior exposure of the cells to Ang-(1–7) [32]. There is also evidence that Ang-(1–7) stimulation can prove inhibitory to signaling pathways, such as those activated by Ang II [29, 33, 34], glucose [35], and ET-1 [29]. As noted in the previous section, preincubation with Ang-(1–7) has been reported to reduce subsequent activation of MAP kinases by Ang II and other ligands [29, 33–36]. The mechanism of these effects is not known, although activation of tyrosine phosphatases in proximal tubular cells [35] and upregulation of DUSP1 in neonatal rat cardiac fibroblasts [29] have been implicated. Although DUSP1 upregulation has been implicated in the inhibitory effect of Ang-(1–7) in neonatal rat cardiac fibroblasts [29], we were unable to detect this effect in the adult cells. In adult rat cardiac fibroblasts, we found that Ang-(1–7) preincubation inhibited subsequent Ang II-stimulated upregulation of ET-1 and LIF mRNA. We also found that Ang II-induced secretion by adult rat cardiac fibroblasts of unknown paracrine factors that induced hypertrophy in cultured cardiomyocytes could be significantly inhibited by preincubation of the fibroblasts with Ang-(1–7) (Fig. 30.1 and [28]).
Effects of angiotensins and conditioned media (CM) from adult rat cardiac fibroblasts (ARCFs) treated with angiotensins on [3H]leucine incorporation in cardiomyocytes. Cardiomyo-cytes were stimulated for 24 h with Ang II, Ang-(1–7), both peptides after 1 h pretreatment with Ang-(1–7) or CM from ARCFs to assess cardiomyocyte hypertrophy measured by [3H] leucine incorporation. Ang II-CM, CM from Ang II-treated ARCFs; Ang-(1–7)-CM, CM from Ang-(1–7)-treated ARCFs; Ang-(1–7) + II-CM, CM from ARCFs treated with both peptides after 1 h pretreatment with Ang-(1–7). † P < 0.001 vs. mock CM; ‡ P < 0.001 vs. control CM; § P < 0.05 vs. Ang II-CM (Reproduced with permission [28])
2.2.2 Autocrine/Paracrine Second Messengers
Fibroblasts can originate from other sources in addition to the cardiac interstitium, especially when the heart is actively remodeling. Sources of nonresident fibroblasts in the remodeling heart include circulating fibrocytes and mesenchymal cells generated from epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition (EndMT). It is still not clear whether these additional cells serve some unique purpose or simply elevate the pool of fibroblasts during increased need. However, the possibilities exist that (1) these cells may be affected by Ang-(1–7) differently than the resident fibroblasts and (2) the recruitment and/or differentiation of these cells could be altered by Ang-(1–7). Indeed, Ang-(1–7) has been shown to reduce the number, proliferative capacity, and collagen secretion of human circulating fibrocytes, possibly by increasing nitric oxide production [37]. Also, cGMP-dependent protein kinase (PKG) has been shown previously to disrupt transforming growth factor-β1 (TGF-β1)-induced nuclear translocation of pSmad3 [38]. Considering (1) the involvement of TGF-β1 in EMT (or EndMT) and myofibroblast differentiation and (2) the activation of guanylate cyclase and subsequent cGMP generation after nitric oxide exposure, there is a strong possibility that Ang-(1–7)-induced nitric oxide synthesis could inhibit fibroblast functions in a remodeling tissue. Ang-(1–7) has been shown to directly induce the release of nitric oxide in platelets, cardiomyocytes, and aortic endothelial cells [26, 30, 39], a process which involves activation of Akt and eNos in cardiomyocytes. As noted above, Ang-(1–7) has also been shown to augment the release of nitric oxide from endothelial cells that have been stimulated with bradykinin [32]. Many of these indirect effects can theoretically influence cardiac fibroblasts during cardiac remodeling, but their effects in animal models or human patients have not been studied.
3 Effects of the ACE2/Ang-(1–7)/Mas Pathway on Cardiac Remodeling
Germline ablation of ACE2 results in a phenotype of cardiac dilatation and dysfunction in some [40], but not all [41], mouse models, and it predisposes to cardiac failure when the heart is stressed [42]. We have shown that pharmacologic inhibition of ACE2 in the post-MI rat heart using the selective inhibitor C16 increases infarct size and adversely affects contractile function [43]. Treatment with C16 had no significant effect on the increased level of apoptosis in the infarct and border zones nor did it significantly affect capillary density surrounding the MI. It did, however, significantly reduce the number of c-kit(+) cells in the border region, a finding consistent with the possibility that Ang-(1–7) or some other product of ACE2 helped reduce infarct size by enhancing the viability of cardiomyocytes in the border zone either directly or via paracrine effects of mediators released from the c-kit(+) cells. The observation that C16 inhibited increases in wall thickness and fibrosis in non-infarcted LV, however, raises the possibility that ACE2 activity has diverse (and perhaps even some adverse) effects on post-MI remodeling. Others have reported that loss of ACE2 accelerates maladaptive post-MI LV remodeling [44], while increasing ACE2 levels by gene transfer therapy inhibits post-MI remodeling [45].
In addition to degrading Ang ll, many favorable effects of ACE2 appear to be due to increased Ang-(1–7) levels. Elevated Ang-(1–7) levels are predicted to be inhibitory to both cardiomyocyte hypertrophy and fibrosis. In rats, post-MI remodeling can be inhibited by infusing Ang-(1–7) [46] or ingestion of either AVE 0991 (a nonpeptide Ang-(1–7) analogue) [47], an Ang-(1–7) hydroxypropyl β-cyclodextrin preparation [48], or a stabilized Ang-(1–7) analogue [49]. Angiotensin II-stimulated cardiac hypertrophy in mice is inhibited by cardiac-specific Ang-(1–7) overexpression [50, 51]. Whether Ang-(1–7) cardioprotection is due to effects of the peptide within the heart or effects of the peptide on extracardiac cells/tissues, however, is uncertain as some evidence suggests that circulating rather than cardiac Ang-(1–7) produces the beneficial effects of the peptide on post-MI remodeling [52]. We have shown that ACE2 inhibition increases post-MI infarct expansion [43] so that there is also uncertainty whether Ang-(1–7) inhibits remodeling due to an early reduction in infarct size or due to later effects on the remodeling process. Among strategies for using Ang-(1–7) to treat post-MI remodeling, gene transfer therapy is one of the most appealing [53–55]. Evidence from experiments in which a lentiviral vector encoding an engineered Ang-(1–7) fusion protein was injected directly into the LV wall several weeks prior to coronary ligation supports this possibility [56].
Germline deletion of Mas in mice has been reported to cause decreased cardiac performance, which is believed to be the result of increased levels of extracellular matrix proteins in the right ventricles and AV valves [57]. Pharmacologic stimulation of the Mas receptor has shown promise in animal models of cardiac dysfunction. The nonpeptide Mas agonist, AVE 0991, improves cardiac function in rats with diabetes [58, 59], with isoproterenol treatment [60] and following experimentally induced MI [61]. AVE 0991 has also been shown to ameliorate progression of atherosclerosis in apoE-null mice [62]. Another Mas agonist peptide, CGEN-856S, which has been reported to be more stable than Ang-(1–7), demonstrated anti-arrhythmogenic effects in isolated rat hearts [63], but this peptide has not been extensively studied. To our knowledge, no human data exist regarding these Mas agonists, but their clinical potential as cardioprotective agents is recognized. Based on these considerations, further exploration of the effects of stable Ang-(1–7) analogues or Mas receptor agonists in cardiovascular disease is clearly warranted.
4 Future Considerations
There are many questions that still need to be answered about the relevance of this relatively new axis of the RAS and its potential role in regulating cardiac remodeling. Ang-(1–7)-induced signaling pathways (whether direct or indirect) remain unclear and require further study. Although Mas is widely regarded as the Ang-(1–7) receptor, there are still indications that other receptors are utilized in certain contexts [15–17]. Regarding cardiac fibroblasts, very little study has been devoted to understanding how ACE2/Ang-(1–7)/Mas can influence the recruitment and/or production of other cell types that contribute to the interstitial cell pool in the remodeling heart. Positive outcomes have been obtained when perturbing the ACE2/Ang-(1–7)/Mas system in animal models of disease. However, to our knowledge, no clinical use of such strategies has yet been reported. Since ACE2/Ang-(1–7)/Mas is considered cardioprotective, stimulation of this pathway, rather than inhibition, is expected to be beneficial. Ang-(1–7) infusion would be clinically difficult due to its short half-life. However, synthetic genes have been engineered to enhance Ang-(1–7) secretion from transfected or transduced cells [64]. Theoretically, increased expression of Ang-(1–7) would act cooperatively with existing ACE inhibitor or ARB pharmacologic treatment regimes, but this has yet to be tested. Enhanced ACE2 activity alone could demonstrate considerable improvements over existing therapies since it would simultaneously degrade Ang II and generate the cardioprotective Ang-(1–7). Stimulation of ACE2 enzymatic activity may prove challenging using pharmacology, but overexpression of ACE2 is always possible by transfection or viral transduction. We must remain cautious, however, since ACE2 overexpression by such methods has proven to be detrimental in at least one published work [65]. Also, discrepancies as to whether cardioprotective effects are due to activation of this pathway in the myocardium or in extracardiac tissues demand further studies [52, 56].
5 Conclusions
Although the past several decades have witnessed substantial improvements in heart failure outcomes, morbidity and mortality still remain unacceptably high. This, along with the relentless increase in new cases of heart failure throughout the world, makes it clear that novel treatment strategies are still needed. The RAS has been a pharmacologic target for heart failure treatment for many years. A more recently discovered branch of the RAS, namely, the ACE2/Ang-(1–7)/Mas system, has demonstrated cardioprotection when activated during remodeling in animal models of heart failure. The ACE2/Ang-(1–7)/Mas system can affect many cell types, but the cardiac fibroblast has been shown to be an important target of its action. Effects of Ang-(1–7) on cardiac fibroblasts can involve direct activation of signaling pathways or indirect effects on their function that involve either the paracrine release of second messengers, alterations in the differentiation/recruitment of fibroblast “precursors,” or crosstalk with other signaling pathways. Ultimately, activation of the ACE2/Ang-(1–7)/Mas system is predicted to produce an “antifibrotic” phenotype and should improve cardiac remodeling. Despite favorable results in animal models, however, adaptation of the promising strategies for enhancing the effects of this alternate pathway and proof of efficacy in well-designed human trials are still needed.
References
Roger VL, Go AS, Lloyd-Jones DM et al (2012) Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125:188–197
Schocken DD, Benjamin EJ, Fonarow GC et al (2008) Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 117:2544–2565
Albert MA (2008) Heart failure in the urban African enclave of Soweto: a case study of contemporary epidemiological transition in the developing world. Circulation 118:2323–2325
Shantsila E, Lip GY, Gill PS (2011) Systolic heart failure in South Asians. Int J Clin Pract 65:1274–1282
Khanam MA, Streatfield PK, Kabir ZN et al (2011) Prevalence and patterns of multimorbidity among elderly people in rural Bangladesh: a cross-sectional study. J Health Popul Nutr 29:406–414
Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81:1161–1172
Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 325: 293–302, 1991
Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med 327:685–691, 1992
Greenberg B, Quinones MA, Koilpillai C et al (1995) Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction. Results of the SOLVD echocardiography substudy. Circulation 91:2573–2581
Konstam MA, Kronenberg MW, Rousseau MF et al (1993) Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (Studies of Left Ventricular Dysfunction) Investigators. Circulation 88:2277–2283
Granger CB, McMurray JJ, Yusuf S et al (2003) Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 362:772–776
Tipnis SR, Hooper NM, Hyde R et al (2000) A human homolog of angiotensin-converting enzyme - cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275:33238–33243
Donoghue M, Hsieh F, Baronas E et al (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87:E1–E9
Vickers C, Hales P, Kaushik V et al (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem 277:14838–14843
Lara LS, Cavalcante F, Axelband F et al (2006) Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+−ATPase by Ang-(1–7). Biochem J 395:183–190
Lara LS, Correa JS, Lavelle AB et al (2008) The angiotensin receptor type 1-Gq protein-phosphatidyl inositol phospholipase Cbeta-protein kinase C pathway is involved in activation of proximal tubule Na+−ATPase activity by angiotensin(1–7) in pig kidneys. Exp Physiol 93:639–647
Lopez Verrilli MA, Pirola CJ, Pascual MM et al (2009) Angiotensin-(1–7) through AT receptors mediates tyrosine hydroxylase degradation via the ubiquitin-proteasome pathway. J Neurochem 109:326–335
Santos RAS, Silva ACSE, Maric C et al (2003) Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100:8258–8263
Kostenis E, Milligan G, Christopoulos A et al (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111:1806–1813
Young D, Waitches G, Birchmeier C et al (1986) Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45:711–719
Martin KA, Grant SG, Hockfield S (1992) The mas proto-oncogene is developmentally regulated in the rat central nervous system. Brain Res Dev Brain Res 68:75–82
Kitaoka T, Sharif M, Hanley MR, Hjelmeland LM (1994) Expression of the MAS proto-oncogene in the retinal pigment epithelium of the rhesus macaque. Curr Eye Res 13:345–351
Metzger R, Bader M, Ludwig T et al (1995) Expression of the mouse and rat mas proto-oncogene in the brain and peripheral tissues. FEBS Lett 357:27–32
Munoz MC, Giani JF, Dominici FP (2010) Angiotensin-(1–7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept 161:1–7
Alenina N, Xu P, Rentzsch B et al (2008) Genetically altered animal models for Mas and angiotensin-(1–7). Exp Physiol 93:528–537
Dias-Peixoto MF, Santos RA, Gomes ER et al (2008) Molecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytes. Hypertension 52:542–548
Tallant EA, Ferrario CM, Gallagher PE (2005) Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289:H1560–H1566
Iwata M, Cowling RT, Gurantz D et al (2005) Angiotensin-(1–7) binds to specific receptors on cardiac fibroblasts to initiate antifibrotic and antitrophic effects. Am J Physiol Heart Circ Physiol 289:H2356–H2363
McCollum LT, Gallagher PE, Tallant EA (2012) Angiotensin-(1–7) abrogates mitogen-stimulated proliferation of cardiac fibroblasts. Peptides 34:380–388
Sampaio WO, Santos RA Sd, Faria-Silva R et al (2007) Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49:185–192
Liu GC, Oudit GY, Fang F et al (2012) Angiotensin-(1–7)-induced activation of ERK1/2 is cAMP/protein kinase A-dependent in glomerular mesangial cells. Am J Physiol Renal Physiol 302:F784–F790
Peiro C, Vallejo S, Gembardt F et al (2007) Endothelial dysfunction through genetic deletion or inhibition of the G protein-coupled receptor Mas: a new target to improve endothelial function. J Hypertens 25:2421–2425
Sampaio WO, de Henrique CC, Santos RA et al (2007) Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50:1093–1098
Su Z, Zimpelmann J, Burns KD (2006) Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69:2212–2218
Gava E, Samad-Zadeh A, Zimpelmann J et al (2009) Angiotensin-(1–7) activates a tyrosine phosphatase and inhibits glucose-induced signalling in proximal tubular cells. Nephrol Dial Transplant 24:1766–1773
Giani JF, Gironacci MM, Munoz MC et al (2008) Angiotensin-(1–7) has a dual role on growth-promoting signalling pathways in rat heart in vivo by stimulating STAT3 and STAT5a/b phosphorylation and inhibiting angiotensin II-stimulated ERK1/2 and Rho kinase activity. Exp Physiol 93:570–578
Wang K, Hu X, Du C et al (2012) Angiotensin-(1–7) suppresses the number and function of the circulating fibrocytes by upregulating endothelial nitric oxide synthase expression. Mol Cell Biochem 365:19–27
Li P, Wang D, Lucas J et al (2008) Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res 102:185–192
Fraga-Silva RA, Pinheiro SV, Goncalves AC et al (2008) The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol Med 14:28–35
Crackower MA, Sarao R, Oudit GY et al (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417:822–828
Gurley SB, Allred A, Le TH et al (2006) Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J Clin Invest 116:2218–2225
Yamamoto K, Ohishi M, Katsuya T et al (2006) Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension 47:718–726
Kim MA, Yang D, Kida K et al (2010) Effects of ACE2 inhibition in the post-myocardial infarction heart. J Card Fail 16:777–785
Kassiri Z, Zhong J, Guo D et al (2009) Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail 2:446–455
Zhao YX, Yin HQ, Yu QT et al (2010) ACE2 overexpression ameliorates left ventricular remodeling and dysfunction in a rat model of myocardial infarction. Hum Gene Ther 21:1545–1554
Loot AE, Roks AJ, Henning RH et al (2002) Angiotensin-(1–7) attenuates the development of heart failure after myocardial infarction in rats. Circulation 105:1548–1550
Zeng WT, Chen WY, Leng XY et al (2012) Impairment of cardiac function and remodeling induced by myocardial infarction in rats are attenuated by the nonpeptide angiotensin-(1–7) analog AVE 0991. Cardiovasc Ther 30:152–161
Marques FD, Ferreira AJ, Sinisterra RD et al (2011) An oral formulation of angiotensin-(1–7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension 57:477–483
Durik M, Van VR, Kuipers A et al (2012) The effect of the thioether-bridged, stabilized angiotensin-(1–7) analogue cyclic ang-(1–7) on cardiac remodeling and endothelial function in rats with myocardial infarction. Int J Hypertens 2012:536426
Reudelhuber TL, Mercure C, Jain DB et al (2005) Targeted over-production of angiotensin 1–7 in the heart reverses hypertension-related cardiac hypertrophy. Circulation 112(Supplement II):II-250
Mercure C, Yogi A, Callera GE et al (2008) Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res 103:1319–1326
Wang Y, Qian C, Roks AJ et al (2010) Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail 3:286–293
Kawase Y, Ladage D, Hajjar RJ (2011) Rescuing the failing heart by targeted gene transfer. J Am Coll Cardiol 57:1169–1180
Tilemann L, Ishikawa K, Weber T, Hajjar RJ (2012) Gene therapy for heart failure. Circ Res 110:777–793
Tang T, Gao MH, Hammond HK (2012) Prospects for gene transfer for clinical heart failure. Gene Ther 19:606–612
Qi Y, Shenoy V, Wong F et al (2011) Lentivirus-mediated overexpression of angiotensin-(1–7) attenuated ischaemia-induced cardiac pathophysiology. Exp Physiol 96:863–874
Gava E, de Castro CH, Ferreira AJ et al (2012) Angiotensin-(1–7) receptor Mas is an essential modulator of extracellular matrix protein expression in the heart. Regul Pept 175:30–42
Ebermann L, Spillmann F, Sidiropoulos M et al (2008) The angiotensin-(1–7) receptor agonist AVE0991 is cardioprotective in diabetic rats. Eur J Pharmacol 590:276–280
Benter IF, Yousif MH, Cojocel C et al (2007) Angiotensin-(1–7) prevents diabetes-induced cardiovascular dysfunction. Am J Physiol Heart Circ Physiol 292:H666–H672
Ferreira AJ, Oliveira TL, Castro MC et al (2007) Isoproterenol-induced impairment of heart function and remodeling are attenuated by the nonpeptide angiotensin-(1–7) analogue AVE 0991. Life Sci 81:916–923
Ferreira AJ, Jacoby BA, Araujo CA et al (2007) The nonpeptide angiotensin-(1–7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am J Physiol Heart Circ Physiol 292:H1113–H1119
Toton-Zuranska J, Gajda M, Pyka-Fosciak G et al (2010) AVE 0991-angiotensin-(1–7) receptor agonist, inhibits atherogenesis in apoE-knockout mice. J Physiol Pharmacol 61:181–183
Savergnini SQ, Beiman M, Lautner RQ et al (2010) Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension 56:112–120
Santos RA, Ferreira AJ, Nadu AP et al (2004) Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics 17:292–299
Masson R, Nicklin SA, Craig MA et al (2009) Onset of experimental severe cardiac fibrosis is mediated by overexpression of Angiotensin-converting enzyme 2. Hypertension 53:694–700
Acknowledgment
Funded in part by National Institutes of Health grant 1RO1HL091191 to Dr. Greenberg.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Cowling, R.T., Greenberg, B.H. (2013). The ACE2/Ang-(1–7) Pathway in Cardiac Fibroblasts as a Potential Target for Cardiac Remodeling. In: Jugdutt, B., Dhalla, N. (eds) Cardiac Remodeling. Advances in Biochemistry in Health and Disease, vol 5. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5930-9_30
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5930-9_30
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5929-3
Online ISBN: 978-1-4614-5930-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)