Abstract
The function of a linker in an antibody–drug conjugate is to covalently connect its effector moiety, the cytotoxic drug, with its targeting moiety, the antibody. In this chapter, we review various linkers, cleavable and non-cleavable, that have been reported, main approaches that have been used to attach the linkers to the antibodies, and the impact of various linkers on the properties of the resulting ADCs, such as their cytotoxic and antitumor activities, stabilities in circulation and tissues, and the extent of killing of bystander cells and of multidrug-resistant cells. Finally, we review clinical experience with ADCs made with different linkers.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
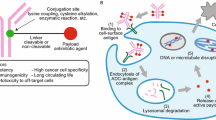
The function of a linker in an ADC is to covalently connect its effector moiety, the cytotoxic drug, with its targeting moiety, the antibody. A conjugate, following its binding to the target cell surface antigen and uptake, degrades in the tumor cell with the release of an active cytotoxic moiety, often called a metabolite. Depending on the design of the linker, this metabolite may consist of either the cytotoxic drug in its original form or that agent with some or all of the linker attached. Linkers are intended to provide sufficient stability to keep the ADC intact during formulation, storage, and in circulation following administration to the patient and yet allow for efficient (i.e., with a high enough yield and fast enough) release of an active, cytotoxic moiety in the tumor. In addition, recently some linkers have been designed to overcome multidrug resistance.
Several types of linkers have been developed that take advantage of differences between the extracellular and intracellular environments, so that the release of the active cytotoxic moiety would happen only following the antigen-mediated internalization of the ADC into a tumor cell. (1) Disulfide-containing linkers are used in ADCs to exploit the abundance of intracellular thiols, which can facilitate the cleavage of their disulfide bonds. The intracellular concentrations of the most plentiful intracellular thiol, reduced glutathione, are typically in the range of 1–10 mM [1], which is about 1,000-fold higher than that of the most abundant low-molecular thiol in the blood, cysteine, at about 5 μM [2]. The thiol group in serum albumin, which has a relatively high concentration in the blood of ∼0.6 mM [3], is buried and relatively inaccessible to thiol–disulfide interchange [4]. The intracellular enzymes of the protein disulfide isomerase family [5] may also contribute to the intracellular cleavage of the disulfide linkers. (2) Hydrazone linkers, which undergo acid-catalyzed hydrolysis, are used with a goal of remaining intact in the near-neutral pH environments in circulation and other extracellular compartments and be cleaved in the acidic environments of the late endosomes and lysosomes [6]. (3) Peptide-based linkers are designed to be cleaved via peptide bond hydrolysis catalyzed by lysosomal and, possibly, by endosomal or cytoplasmic proteases [7]. Conjugates with non-cleavable linkers may also be considered belonging to this category, since the antibody moiety of the ADC undergoes proteolysis inside the cell, presumably in lysosomes, releasing the cytotoxic moiety attached to the linker and the single remaining amino acid derived from the antibody [8].
In the conjugates now in clinical development, two main approaches have been used to attach the linkers to the antibody: (1) conjugation with thiol groups of cysteine residues in the antibody that are generated by reduction of interchain disulfide bonds and (2) conjugation with amino groups of surface lysine residues. These approaches will be covered below. In addition, new approaches to engineer-specific sites of modification, such as introduced cysteine residues, are also being evaluated [9].
In this chapter, we will review various ADC linkers that have been reported and the effects of these linkers on the properties of the resulting ADCs. A separate chapter in this volume discusses the intracellular metabolism of ADCs with alternative linkers.
Linker Structures and Preparations of Antibody–Drug Conjugates
This section describes the structures of linkers and conjugates that have been reported for the various ADCs in clinical and advanced preclinical programs. To prepare ADCs, reactive functional groups are incorporated in the cytotoxic moiety and the antibody molecule for facile conjugation with a linker in aqueous conditions compatible with antibody. Other design requirements for ADCs include a relatively water-soluble cytotoxic moiety and use of heterofunctional reactive groups in the cytotoxic moiety and the antibody to minimize the formation of cytotoxin–cytotoxin and antibody–antibody conjugates. Several types of linkers have been used to make ADCs, including non-cleavable linkers, and cleavable linkages, such as disulfide, cleavable peptide, and hydrazone.
Non-cleavable Linkers
Thioether is the linkage that is most commonly used in non-cleavable linkers. It is prepared by the conjugation of a thiol group on the cytotoxic compound or the antibody with the maleimide or haloacetamide group on antibody or cytotoxic moiety, respectively. Figure 7.1 shows the structures of representative thioether non-cleavable linkers, which include the SMCC–DM1 linkage (also known as MCC–DM1) formed by reaction of N-succinimido 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) with DM1 employed in the trastuzumab emtansine (T-DM1) conjugate [10] and the mc–MMAF linkage, maleimidocaproyl–monomethyl auristatin F, employed in the anti-CD70–mc–MMAF conjugate [11]. Both types of ADCs are currently in clinical trials.
The trastuzumab–SMCC–DM1 conjugate is prepared by the modification of lysine amino groups on the antibody with the N-hydroxysuccinimide ester reactive moiety on the heterobifunctional linker SMCC. This linker also bears a maleimide reactive group, which is conjugated with the thiol-containing maytansinoid, DM1. The trastuzumab–SMCC–DM1 conjugate bears approximately 3.5 maytansinoid molecules per antibody molecule. In target cells, the intracellular cytotoxic metabolite of antibody–SMCC–DM1 is lysine–SMCC–DM1 [12]. An analogous linker where the hydrophobic cyclohexane moiety of SMCC was replaced by a hydrophilic tetraethylene glycol (PEG4) group which results in a hydrophilic link between the antibody and the payload (Fig. 7.1) enhances the potency of the conjugate against pgp-expressing multidrug-resistant cancer cells [13]. The intracellular metabolite derived from the PEG4 thioether-linked conjugate is lysine–PEG4–DM1, which is more hydrophilic than the lysine–SMCC–DM1 metabolite. Another conjugation format has been reported where a cysteine-engineered antibody (termed Thiomab) bearing two nonnative cysteine groups introduced into specific locations on the heavy chain is conjugated with the thiol of DM1 using a PEG4-containing bis-maleimide (1,11-bis-maleimidotetraethyleneglycol) [9].
The maleimidocaproyl–monomethyl auristatin F (mc–MMAF) conjugate contains a thioether linkage derived from conjugation with cysteine residues generated by reduction of native, interchain disulfide bonds in the antibody [14]. Conjugates bearing an average of 4 and 8 MMAF molecules per antibody molecule have been prepared [14], and the anti-CD70–mc–MMAF conjugate in clinical development has an average of about four MMAF molecules per antibody molecule [11]. In lysosomes of target cells, the cysteine-linked mc–MMAF conjugate is processed to the cysteine-linker-MMAF metabolite [14].
Cleavable Disulfide Linkers
A number of antibody–maytansinoid conjugates with sterically hindered disulfide linkers are undergoing clinical evaluation for an array of cancers. The disulfide linker designs shown in Fig. 7.2 incorporate increasing steric hindrance of methyl groups on carbon atoms adjacent to either side of the disulfide linkage. For a simple abbreviation, the hindered disulfide conjugates are denoted by the number of methyl groups on the antibody side and the cytotoxic agent side, respectively, for example, the SPP–DM1 conjugate with monomethyl hindrance on the antibody side and no hindrance on the cytotoxic agent side is abbreviated as 1:0, and the SPDB–DM4 conjugate with no hindrance on antibody side and double methyl hindrance on cytotoxic agent side is abbreviated as 0:2. The rate of cleavage via thiol/disulfide exchange of the different hindered disulfide linkages was first analyzed in vitro using dithiothreitol. The results showed that the 2:2 hindered conjugate was reduced at a rate more than 22,000-fold slower than the unhindered 0:0 conjugate (Fig. 7.2b). The disulfide cleavage rate of the 0:2 hindered SPDB–DM4 conjugate (or the 2:0 hindered conjugate) was about 20-fold slower than that of the 0:0 conjugate, whereas the 1:2 hindered conjugate was reduced at a rate about 1,000-fold slower than that of the 0:0 conjugate. The relative rates of reduction of the hindered disulfide conjugates observed with dithiothreitol in vitro were similar to their relative plasma stabilities in mice [15].
The cytotoxicity in vitro of conjugates made with these diverse disulfide linkers was similar in antigen-expressing cells and comparable to the cytotoxicity of the non-cleavable SMCC–DM1 conjugate, presumably due to their efficient lysosomal processing. A large difference, however, was observed among the in vivo activities of the hindered disulfide-linked conjugates in tumor xenograft studies. In an in vivo study using anti-CanAg maytansinoid conjugates in COLO 205 xenografts (which express CanAg homogenously on all cells) and HT29 xenografts (which express CanAg heterogeneously, only on a fraction of cells), the 0:2 SPDB–DM4 conjugate was the most active. Its activity was greater than that of the 0:1 conjugate, which in turn was greater than that of the 1:0 or 2:0 conjugate. The even more hindered 1:1 and 1:2 conjugates were, however, less active than the 1:0 and 0:2 conjugates. The greater in vivo activities of the less hindered conjugates could be explained by their bystander cytotoxic activities [15, 16], stemming from the abilities of their metabolites DM4 and DM3 and their S-methylated forms to diffuse from target cancer cells in which the conjugates were processed into neighboring tumor cells, irrespective of whether the latter express the target antigen or not, thus enhancing the antitumor activity of the conjugate [16, 17]. In an in vivo study targeting anti-αv integrin in HT29 and A549 xenograft models, the activity trend observed was 0:2 > 1:0 > 1:2 [18], similar to that observed in the anti-CanAg antibody/COLO 205 test system. Both the anti-CanAg conjugate made with the non-cleavable SMCC–DM1 linker and the anti-αv integrin conjugate made with the highly hindered 1:2 disulfide linker were not active, consistent with the predicted lack of the bystander activities of conjugates made with slowly cleavable or uncleavable linkers [15].
Cleavable Peptide Linkers
Figure 7.3 shows the structures of valine–citrulline (often denoted as val–cit or vc) dipeptide-containing protease-cleavable linkers employed in clinical-stage ADCs with both microtubule-targeting (MMAE) and DNA-targeting (a duocarmycin analog) effector molecules. The vc–MMAE linker contains a valine–citrulline dipeptide and a self-immolative p-aminobenzyloxycarbonyl linkage (PABC). Upon endosomal trafficking of the ADC via the lysosomal route, cleavage of the val–cit peptide by lysosomal proteases releases PABC–MMAE that undergoes self-immolation at the PABC site, further releasing the cytotoxic MMAE molecule that has potential bystander effect on neighboring tumor cells [19, 20]. The anti-CD30–vc–MMAE conjugate currently in clinical testing has an average of about 4 linked MMAE molecules per antibody molecule, derived from the reduction of native interchain disulfide bonds to cysteine and its conjugation with maleimide-containing mc–MMAE [20]. Cysteine-engineered antibodies (Thiomabs) have been conjugated with mc–MMAE to generate ADCs with two MMAE molecules per antibody molecule [21]. The duocarmycin analog is an esterase-cleavable prodrug that is attached via a val–cit linker to the antibody. Following esterase-catalyzed cleavage, the prodrug converts into a DNA alkylator (Fig. 7.3b).
Acid-Cleavable Hydrazone Linkers
The hydrazone linkage is designed to be hydrolyzed in the acidic environment of the endosomes. Two types of hydrazone-linked DNA-targeting cytotoxic effector conjugates currently (or formerly) in the clinic are shown in Fig. 7.4. The highly potent DNA-alkylating N-acetyl-γ I1 -calicheamicin is linked via an acid-cleavable hydrazone linkage to antibodies targeting CD33, MUC1, and CD22, of which the CD22 conjugate is currently in clinical trials [22]. Antibody–calicheamicin conjugates were prepared using the N-hydroxysuccinimide ester of N-acetyl-γ-calicheamicin dimethyl hydrazide 4-(4′-acetylphenoxy)butanoic acid, which reacts with lysine residues on the antibody with an average incorporation of 5–7 calicheamicin molecules per antibody molecule. The acid-hydrolyzable 4-(4′-acetylphenoxy)butanoic acid hydrazone linker contains a disulfide linkage, which needs to be metabolically cleaved to release the thiol form of calicheamicin. The latter undergoes a Bergman cyclization reaction generating a p-benzyne biradical that causes sequence-specific double-stranded DNA cleavage in target cancer cells. Another hydrazone-linked ADC in clinical testing consists of the cytotoxic agent doxorubicin conjugated to an anti-CD74 antibody [23]. The doxorubicin containing 4-(N-maleimidomethyl)cyclohexane-1-carboxyhydrazide is conjugated to cysteine residues in the antibody generated by interchain disulfide reduction, with an average incorporation of 6–8 doxorubicin molecules per antibody molecule.
Stability of ADCs with Various Linkers in Circulation and Tissues
The pharmacokinetics of immunoconjugates from the bloodstream is controlled by two concurrent phenomena, clearance of the intact immunoconjugate from circulation and release (cleavage) of the cytotoxic effector moiety from the antibody in the circulation and during the diffusion of the conjugate through tissues from the bloodstream to the tumor cells. The former process has been reviewed previously [24] and is also covered in a separate chapter of this volume. Here, we will focus on the effects of the linker on the rate of decrease of the cytotoxic drug per antibody ratio (DAR) while the immunoconjugate is in circulation or on its way from circulation to the tumor site.
Linkers are designed to hold the conjugate together in circulation for a reasonably long period of time (days) and stable enough not to cleave upon conjugate exposure to tissues on its way from circulation to the tumor while allowing rapid release of the cytotoxic linker in its active form following uptake of the conjugate by the target cell. The accomplishment of these objectives is complicated by several factors: (1) a variety of proteases are present in extracellular matrix, interstitial fluids, on extracellular surface of plasma membranes, and in the blood (although the latter are mostly present as proenzymes) [13, 17, 25, 26]; some of these enzymes may, in principle, degrade the antibody moiety or cleave the linker if it contains a peptide bond; (2) the thiol groups of cysteine and serum albumin which are present in the bloodstream (see above), and of cell surface protein disulfide isomerase [5], may contribute to the cleavage of disulfide-containing linkers; (3) acid-sensitive linkers that cleave at a sufficient rate at pH 5–6 in endosomal or lysosomal compartments in cancer cells will also hydrolyze at neutral pH, for example, just tenfold slower at pH 7 than pH 6; and (4) while in the bloodstream, antibodies continuously recirculate in and out of endothelial cells [8], and as discussed above, while in the endosome, conjugates are exposed to low pH which may enhance hydrolysis of acid-labile linkers, glutathione which may cleave the disulfide bond of the linker, and, possibly, to proteases which may cleave peptide bonds of the linker or degrade the antibody. The relative importance of each of these mechanisms is at present unclear.
A variety of linkers have been designed to keep the conjugate intact in the circulation while affording the release of the cytotoxic effector moiety inside the target cell. These linkers have been described above in more detail: the acid-labile hydrazone functionality [2, 27, 28], lysosomal-protease-cleavable dipeptide-containing linkers [5, 29], thiol-labile hindered (to a varying degree) disulfide, and non-cleavable thioethers. Among antibody–maytansinoid conjugates connected by disulfide-containing linkers, a strong correlation was found between the degree of their steric hindrance, resistance to thiol-mediated cleavage in vitro, and the relative role of cleavage in circulation in mice [15]. The two disulfide linker-maytansinoid combinations used in conjugates in clinical development, SPP–DM1 and SPDB–DM4, were found to release maytansinoids from the ADC slowly in circulation in mice, with the half-degradation periodsFootnote 1 of 2.4 and 6.9 days, respectively [15]. The trastuzumab–SMCC–DM1 conjugate linked via the non-cleavable thioether was found to be stable in circulation in preclinical studies in mice with the half-life being longer than 7 days [10] and similarly stable in patients [30]. The plasma stability of a thioether-linked MMAF conjugate, bromoacetamidocaproyl–MMAF (bc–MMAF), was found to be better than that of the maleimidocaproyl–MMAF (mc–MMAF) in circulation in mice [31, 32], despite the fact that both conjugates have nominally non-cleavable linkers (Fig. 7.1). It appears that the reaction between cysteine thiol of antibody and maleimido group of mc–MMAF can be slowly reversed in circulation [31]. An auristatin dipeptide-linked immunoconjugate (valine–citrulline–MMAE) was reported to be stable with a linker half-life of 6 days and 9.6 days in circulation of mice and cynomolgus monkeys, respectively [33].
There is another phenomenon, in addition to the linker cleavage, that may contribute to the apparent decrease in the DAR value of an ADC in circulation. ADCs typically consist of mixtures of species with different DAR values [34], and, in principle, conjugates with different DAR values may have different circulation lifetimes. Indeed, it was found that the clearance rate of an antibody–auristatin conjugate depended on its DAR. An anti-CD30–vc–MMAE conjugate preparation was separated using hydrophobic interaction chromatography into fractions containing conjugates with approximately two, four, and eight cytotoxic drugs per antibody (E2, E4, and E8, respectively), and the blood clearance rates of these conjugates and of the nonconjugated antibody were investigated in mice. E2 and E4 fractions cleared with rates only modestly faster than that of the nonconjugated antibody, but E8 cleared dramatically faster [35]. In contrast, anti-CD70–mc–MMAF conjugates bearing 4 and 8 MMAF molecules per antibody were reported to have similar clearance rates, with terminal half-lives of 12.8 and 14.1 days in mice, respectively [11]. One caveat here is that interpretation of the terminal half-life of an ADC may, sometimes, reflect the behavior of only a small fraction of initially injected material, not representative of the bulk conjugate. From an unusually low C max reported in Table S1 [11] for the dose injected (in our experience with maytansinoid ADCs, approximately threefold lower than a typical C max), this may well be the case. Then, since according to [35], the conjugate with high DAR would clear faster than that with low DAR, the remaining material might consist mostly of the latter, irrespective of what the initial DAR was.
Effect of Linker Design on the Extent of the Cytotoxicity of ADCs Toward Bystander Cells
In addition to killing antigen-positive cells, some ADCs also kill other cells in their vicinity, irrespective of whether these neighboring cells express the antigen or not, a phenomenon that was termed “bystander cytotoxicity” or “bystander effect” [16]. For example, antibody–maytansinoid conjugates linked via a reducible disulfide-bond-containing linker have the bystander effect, whereas similar conjugates linked via a non-reducible thioether link exhibited no bystander killing [15, 16, 36]. We found that the ability of a given conjugate to induce bystander killing depends on the nature of the maytansinoid derivative(s) into which it is converted inside the target cell. Lysosomal proteases proteolytically degrade the antibody and release maytansinoid attached to the linker which is attached to lysine [12]. The newly formed maytansinoid-linker-lysine derivatives diffuse into cytoplasm where they target microtubules, leading to mitotic arrest and cell death [37]. The maytansinoid-thioether linker-lysine is the terminal metabolite of thioether-linked conjugates, whereas maytansinoid-disulfide linker-lysine is further metabolized to the maytansinoid thiol, which either remains free or is S-methylated [12]. The cytotoxicity of these maytansinoid metabolites, prepared as synthetic compounds, was tested in vitro. The maytansinoid-linker-lysine derivatives were found to be only modestly cytotoxic, presumably due to their hydrophilicity, which likely inhibited their diffusion across the plasma membrane into the cell, while lipophilic maytansinoid thiols and S-methyl maytansinoid compounds were highly cytotoxic, implicating the latter in the bystander killing [12, 17].
We found that the degree of the bystander cytotoxicity of a given antibody–maytansinoid conjugate depended on the steric hindrance of maytansinoid thiol derivatives. The in vitro bystander activity of conjugates of the hindered maytansinoids DM3 or DM4 was found to be superior compared to those of the conjugates of unhindered DM1 [15]. This can be explained by the higher reactivity of the DM1 thiol compared to the DM3 thiol or the DM4 thiol in disulfide interchange with cystine, with the likely enhanced formation of a hydrophilic, poorly cytotoxic mixed disulfide (cysteine-DM1). In addition, the thiols of DM3 and DM4 are readily S-methylated inside the cell, forming stable, lipophilic, and highly cytotoxic S-methyl maytansinoid compounds [17]. The thiol of DM1 appears to be a poor substrate for such S-methyl transferase activity in cancer cells [17].
There is some evidence that these phenomena are not limited to ADCs made with maytansinoids and that lipophilicity of metabolites of other cytotoxic agents released from their respective ADCs may also affect the degree of the bystander activity of these ADCs. A conjugate of an anti-CD30 antibody with auristatin MMAE linked via protease-cleavable linker containing valine–citrulline dipeptide is metabolized to a lipophilic auristatin derivative [19] and induces potent bystander killing [20]. A conjugate of the same antibody with auristatin MMAF linked via a non-cleavable linker is metabolized to a hydrophilic auristatin derivative, which has only modest cytotoxicity [14], and therefore is presumably incapable of the bystander killing. A disulfide-linked conjugate of the CC1065 analog DC1 induced a prominent bystander effect, while a similar “non-cleavable” conjugate of DC1 did not kill bystander cells [16], most likely because the former was metabolized to a lipophilic compound, while the latter to its hydrophilic lysine derivative.
The bystander cytotoxicity can enhance the potency of ADCs against solid tumors. Many tumors express the target cell surface antigen in a heterogeneous fashion and consist of a mixture of antigen-positive and antigen-negative cancer cells [38, 39]. Our experiments with heterogeneous xenograft tumors in mice suggest that ADCs that induce the bystander effect may be more effective in eradicating such tumors [16] than ADCs that lack this activity. ADCs that induce bystander effect may also be more potent in eradicating solid tumors that express the target antigen homogenously. Poor and nonuniform penetration of antibodies into tumors has been reported [40–42], and some cells within the tumor might be relatively inaccessible to ADCs due to the barriers to macromolecule delivery and their slow diffusion. The small cytotoxic molecules released from ADCs inside such tumors may be able to penetrate the solid tumors deeper than the antibodies, killing additional cells. In addition, the bystander activity may effect local damage to the tissues involved in supporting tumor growth, such as endothelial cells and pericytes of the tumor neovasculature, or tumor stromal cells.
The bystander effect may add a degree of nonselective killing activity to the target-cell-restricted cytotoxicity of ADCs. Potentially, this could be a drawback if normal cells in tissues surrounding the tumor are affected. This potential collateral toxicity might, however, be well tolerated if it is limited only to a small number of cells in the immediate proximity of the tumor tissues. Indeed, if active cytotoxic metabolites are released from an accessible cancer cell, the concentration of the released cytotoxin will decrease with distance from the cancer cell (assuming no barriers to free diffusion of the small molecule compounds), and because of this concentration gradient, only the proximal bystander cells are likely to be exposed to the concentration of the cytotoxic agent sufficient for cell killing. Also, the potential toxicities contributed by the bystander effect to normal tissues might be mitigated by the inherent insensitivity of nondividing cells to some cytotoxic compounds, including DNA- and microtubule-targeting agents, in particular, maytansine [43, 44].
Effect of Linker Design on Activity of ADCs Against Multidrug-Resistant Cells
Multidrug resistance (MDR) of cancers is one of the main reasons for clinical failures of chemotherapies. Overexpression of ATP-dependent drug transporters MDR1, MRP1, and BCRP is the best studied and the most commonly observed mechanisms of cancer-related MDR [45]. A majority, if not all cytotoxic drugs that are currently used in ADCs, are substrates of at least one of these three transporters. For example, all three transporters effectively mediate efflux of anthracyclines, such as doxorubicin [45], and accordingly, immunoconjugates of doxorubicin are ineffective in killing MDR cell lines [46]. MDR1 [47], and to a lesser degree MRP1 [48], mediates efflux of the enediyne antibiotic calicheamicin used in gemtuzumab ozogamicin. MDR1 mediates efflux of taxanes [45], dolastatin 10 [49], and CC-1065 [50, 51]. Recently, it was reported that MDR1 mediates resistance of cancer cells to maytansinoids and antibody–maytansinoid conjugates, while MRP1 and BCRP do not [13, 52].
Since MDR1 favors hydrophobic substrates [53], we developed linkers that contained either a polar or a negatively charged group and used these linkers in antibody–maytansinoid conjugates with the hope that the conjugates would escape MDR1-mediated efflux and would be able to kill MDR1-expressing cells. A polar ethylene glycol tetramer (PEG4) was incorporated into a thioether-containing non-cleavable linker. The observed ADC metabolite, lysine-PEG4Mal-DM1, was retained inside MDR1-expressing cells better than the lysine–SMCC–DM1 metabolite from an analogous SMCC-linked conjugate [13], and in accord, PEG4Mal-linked conjugates had a greater antimitotic and cytotoxic potency in vitro against MDR-expressing cells and a greater antitumor activity against MDR1-expressing xenograft tumors in mice [13]. To enhance the potency of disulfide-linked conjugates against multidrug-resistant cells, a negatively charged sulfonate group was added to the SPDB linker (sulfo-SPDB linker). The sulfo-SPDB-linked conjugate was more potent than an analogous SPDB-linked conjugate against MDR1-expressing cells in cell culture and in a xenograft tumor model, while the two conjugates had similar activities in vitro and in vivo toward MDR1-negative cells [54].
The polar nature of the released metabolite possibly contributed to the enhanced cytotoxicity of two non-maytansinoid ADCs to MDR cells. Hamann et al. reported that substitution of a hydrazide group by an amide in the linker of an antibody–calicheamicin conjugate rendered this ADC more efficacious against MDR cells [46]. Although the authors did not explain the mechanism of this phenomenon, we speculate that the conjugate with the pH-sensitive hydrazide linker released a hydrophobic calicheamicin via hydrolysis, whereas the non-cleavable amide-linked conjugate was processed to a polar amino acid-containing derivative that might be a poor MDR1 substrate. In another study, an ADC of a polar version of the cytotoxic compound auristatin was able to kill MDR cells [14]. However, it is not clear if the potency of this conjugate could be attributed to the polarity of its cytotoxic moiety since the potency of ADC of the original, nonpolar auristatin was not reported.
Clinical Experience with ADCs Made with Different Linkers
There are presently over a dozen ADCs in clinical testing, employing a diverse set of linkers and cytotoxic agents. Compounds which have clinical data reported to date, along with their respective linker designs, are summarized in Table 7.1. Several different cleavable linkers, designed to allow release of the payload upon internalization into tumor cells through disulfide reduction, protease activity, or acid hydrolysis, have now been evaluated in cancer patients. Many of these compounds are still in early phases of clinical testing, and the diversity of different targets, antibodies, payloads, and disease indications makes it difficult to attribute particular clinical findings uniquely to the linker component of the ADCs. Nevertheless, the emerging data can provide useful information and important lessons on the clinical performance of these linker designs.
For disulfide-linked maytansinoid conjugates, the contribution of linker design to ADC pharmacokinetics in patients has proven to be predictable from preclinical studies (see above) and reflects the inherent chemical stability of the disulfide bond, with increasing bond resistance to thiol–disulfide exchange reactions extending the half-life of conjugate in circulation. In clinical studies, maytansinoid conjugates incorporating a highly hindered disulfide linkage, SPDB–DM4, with an important exception (see below) have achieved predictably longer circulating half-lives than conjugates with a less hindered disulfide bond, SPP–DM1. In the most direct comparison, two maytansinoid ADCs incorporating the same CanAg-targeting antibody, huC242, conjugated to either SPP–DM1 (cantuzumab mertansine) or SPDB–DM4 (IMGN242; cantuzumab ravtansine) were both evaluated in phase I studies. IMGN242 exhibited a significantly longer terminal half-life of 4–5 days (for patients with low CanAg plasma levels) compared to about 2 days reported for cantuzumab mertansine [55, 56] which mirrors similar differences in the pharmacokinetics of ADCs employing these cleavable linker formats in preclinical studies [24, 57]. A major confounding factor when assessing linker design and its role in pharmacokinetics in patients is the impact of target-mediated clearance of the antibody/conjugate (“antigen sink”). In the case of IMGN242, the clearance of the conjugate (and its huC242 antibody component) was greatly accelerated in a subset of patients with very high levels of circulating shed CanAg antigen resulting in a t ½ of less than 2 days (in some patients down to 0.6 days), whereas in patients with low circulating CanAg antigen levels, the half-life was about 4.6 days [55]. Another maytansinoid conjugate incorporating the SPDB–DM4 design, SAR3419, has a reported half-life of about 7 days in lymphoma patients with minimal target-mediated clearance of the CD19-targeting antibody component [58]. By contrast, an SPDB–DM4 conjugate targeting CD33 (AVE9633) exhibited a shorter half-life, ranging from 1 to 4 days, reflecting the accelerated clearance of the anti-CD33 antibody component of the compound in AML patients with high leukemic cell burdens [59]. Similarly, IMGN901, an SPP–DM1 conjugate that targets CD56, has a half-life of about 1 day in patients, which principally reflects the clearance of the antibody component of the conjugate via antigen-mediated clearance of the entire ADC rather than cleavage of the SPP–DM1 linkage [60].
The design of the linker in an ADC can have important consequences for the tolerability and/or nature of the dose-limiting toxicities ultimately observed in patients. Linker designs with respect to mechanism and rate of release of active payload both within cells and in the extracellular compartment are key parameters that affect the distribution and the pharmacokinetics (exposure) of the conjugate, or potentially the released payload, in patients. As discussed above, the choice of linker can have significant impact on the nature of the active metabolite produced after the conjugate is processed in targeted cells or metabolized through normal clearance mechanisms. By altering linker chemistries, conjugates can be designed to have improved antitumor activity (e.g., better retention of metabolites in multidrug-resistant cells or improved bystander activity in tumors with heterogeneous antigen expression) and/or potentially improved tolerability (e.g., yielding metabolites with less systemic toxicity).
Distinct clinical findings were reported in phase I studies of two maytansinoid ADCs incorporating the same CanAg-targeting huC242 antibody but differing in their disulfide linker design. Cantuzumab mertansine (huC242-SPP-DM1), which incorporated a more labile disulfide linker (1:0 format), reached a maximum tolerated dose (MTD) of 235 mg/m2 (dosed every 3 weeks), with dose-limiting toxicities at 295 mg/m2, associated with reversible elevation of hepatic transaminases [56]. Cantuzumab ravtansine (huC242-SPDB-DM4), with a more hindered disulfide linker (0:2), achieved an MTD of 168 mg/m2 (in patients with low circulating CanAG antigen) with dose-limiting toxicities at 208 mg/m2 associated with reversible ocular toxicity [55]. The significantly longer half-life in circulation of cantuzumab ravtansine relative to cantuzumab mertansine resulted in a greater exposure to the intact ADC at the MTD in patients despite the somewhat lower dose. However, the altered distribution of the payload (ADC vs. small molecule metabolite) and the different nature of the metabolites produced by these two conjugates in targeted (and nontargeted) cells [17] can both have an impact on their toxicity profiles. In general, while different maytansinoid conjugates to different targets made with three different maytansinoid-linker formats exhibit different DLTs at intolerable doses to define their MTDs, nevertheless, the MTDs for the 12 antibody–maytansinoid conjugates evaluated to date are in a similar range, from about 3.6 to 6.4 mg/kg [61]. For example, the maximum tolerated doses of trastuzumab-DM1 (non-cleavable SMCC–DM1) [30] and SAR3419 (hindered disulfide SPDB–DM4) [58] were defined as 3.6 mg/kg and 160 mg/m2 (∼4.3 mg/kg), respectively, in phase 1 studies (dosed every 3 weeks).
Conclusion
Several different linkers have now been validated in clinical testing with respect to their intended performance, yielding ADCs that are stable in circulation and activated upon internalization into tumor cells. Linker design is critical for the optimal performance of an ADC and can impact virtually all key attributes of an ADC, including antitumor efficacy, pharmacokinetics, and tolerability. Understanding the mechanisms of ADC activation and metabolism in cells has provided opportunities to rationally develop new linker chemistries that can significantly alter the properties of the active metabolite (payload) ultimately released in cells. Linker research thus represents an important area for innovation in developing ADCs with improved therapeutic window.
Notes
- 1.
Half-degradation period is defined as the period of the twofold decrease of the average DAR value.
References
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Mills BJ, Lang CA (1996) Differential distribution of free and bound glutathione and cyst(e)ine in human blood. Biochem Pharmacol 52:401–406
Turell L, Carballal S, Botti H, Radi R, Alvarez B (2009) Oxidation of the albumin thiol to sulfenic acid and its implications in the intravascular compartment. Braz J Med Biol Res 42:305–311
Wilson JM, Wu D, Motiu-DeGrood R, Hupe DJ (1980) A spectrophotometric method for studying the rates of reaction of disulfides with protein thiol groups applied to bovine serum albumin. J Am Chem Soc 102:359–363
Appenzeller-Herzog C, Ellgaard L (2008) The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783:535–548
Pillay CS, Elliott E, Dennison C (2002) Endolysosomal proteolysis and its regulation. Biochem J 363:417–429
Ciechanover A (2006) Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Hematol Am Soc Hematol Educ Prog 1–12:505–506
Singh R, Erickson HK (2009) Antibody-cytotoxic agent conjugates: preparation and characterization. Methods Mol Biol 525:445–467, xiv
Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, Bhakta S, Nguyen T, Dugger DL, Li G, Mai E, Lewis Phillips GD, Hiraragi H, Fuji RN, Tibbitts J, Vandlen R, Spencer SD, Scheller RH, Polakis P, Sliwkowski MX (2010) Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res 16:4769–4778
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX (2008) Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68:9280–9290
Oflazoglu E, Stone IJ, Gordon K, Wood CG, Repasky EA, Grewal IS, Law CL, Gerber HP (2008) Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res 14:6171–6180
Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, Lutz RJ, Goldmacher VS, Blattler WA (2006) Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 66:4426–4433
Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, Erickson HK, Sun X, Wilhelm S, Ab O, Lai KC, Widdison WC, Kellogg B, Johnson H, Pinkas J, Lutz RJ, Singh R, Goldmacher VS, Chari RV (2010) Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res 70:2528–2537
Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, Oflazoglu E, Toki BE, Sanderson RJ, Zabinski RF, Wahl AF, Senter PD (2006) Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem 17:114–124
Kellogg BA, Garrett L, Kovtun Y, Lai KC, Leece B, Miller M, Payne G, Steeves R, Whiteman KR, Widdison W, Xie H, Singh R, Chari RV, Lambert JM, Lutz RJ (2011) Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug Chem 22:717–727
Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blattler WA, Goldmacher VS (2006) Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 66:3214–3221
Erickson HK, Widdison WC, Mayo MF, Whiteman K, Audette C, Wilhelm SD, Singh R (2010) Tumor delivery and in vivo processing of disulfide-linked and thioether-linked antibody-maytansinoid conjugates. Bioconjug Chem 21:84–92
Chen Q, Millar HJ, McCabe FL, Manning CD, Steeves R, Lai K, Kellogg B, Lutz RJ, Trikha M, Nakada MT, Anderson GM (2007) Alphav integrin-targeted immunoconjugates regress established human tumors in xenograft models. Clin Cancer Res 13:3689–3695
Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21:778–784
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, Senter PD, Alley SC (2010) Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 16:888–897
Dornan D, Bennett F, Chen Y, Dennis M, Eaton D, Elkins K, French D, Go MA, Jack A, Junutula JR, Koeppen H, Lau J, McBride J, Rawstron A, Shi X, Yu N, Yu SF, Yue P, Zheng B, Ebens A, Polson AG (2009) Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 114:2721–2729
DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, Kunz A, Hamann PR, Gorovits B, Udata C, Moran JK, Popplewell AG, Stephens S, Frost P, Damle NK (2004) Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 103:1807–1814
Stein R, Mattes MJ, Cardillo TM, Hansen HJ, Chang CH, Burton J, Govindan S, Goldenberg DM (2007) CD74: a new candidate target for the immunotherapy of B-cell neoplasms. Clin Cancer Res 13:5556s–5563s
Xie H, Blattler WA (2006) In vivo behaviour of antibody-drug conjugates for the targeted treatment of cancer. Expert Opin Biol Ther 6:281–291
Foidart J-M, Muschel RJ (2002) Proteases and their inhibitors in cancer metastasis. Kluwer Academic Publishers, Dordrecht/Boston
Lavie G, Zucker-Franklin D, Franklin EC (1980) Elastase-type proteases on the surface of human blood monocytes: possible role in amyloid formation. J Immunol 125:175–180
Ciechanover A (2007) Intracellular protein degradation from a vague idea through the lysosome and the ubiquitin-proteasome system and on to human diseases and drug targeting: Nobel Lecture, December 8, 2004. Ann N Y Acad Sci 1116:1–28
Jain RK, Baxter LT (1988) Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res 48:7022–7032
Ciechanover A (2010) Intracellular protein degradation: from a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Medicina (Buenos Aires) 70:105–119
Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, Girish S, Tibbitts J, Yi JH, Sliwkowski MX, Jacobson F, Lutzker SG, Burris HA (2010) Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28:2698–2704
Alley SC, Benjamin DR, Jeffrey SC, Okeley NM, Meyer DL, Sanderson RJ, Senter PD (2008) Contribution of linker stability to the activities of anticancer immunoconjugates. Bioconjug Chem 19:759–765
Fishkin N, Maloney EK, Chari RV, Singh R (2011) A novel pathway for maytansinoid release from thioether linked antibody-drug conjugates (ADCs) under oxidative conditions. Chem Commun (Camb) 47:10752–10754
Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, Senter PD, Wahl AF (2005) In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res 11:843–852
Lazar AC, Wang L, Blattler WA, Amphlett G, Lambert JM, Zhang W (2005) Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Commun Mass Spectrom 19:1806–1814
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 10:7063–7070
Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, Kiziltepe T, Vallet S, Pozzi S, Santo L, Perrone G, Tai YT, Cirstea D, Raje NS, Uherek C, Dalken B, Aigner S, Osterroth F, Munshi N, Richardson P, Anderson KC (2009) The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res 15:4028–4037
Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, Kovtun Y, Chari R, Jordan MA (2010) Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther 9:2700–2713
Christiansen J, Rajasekaran AK (2004) Biological impediments to monoclonal antibody-based cancer immunotherapy. Mol Cancer Ther 3:1493–1501
Greiner JW (1986) Modulation of antigen expression in human tumor cell populations. Cancer Invest 4:239–256
Adams GP, Schier R, McCall AM, Simmons HH, Horak EM, Alpaugh RK, Marks JD, Weiner LM (2001) High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res 61:4750–4755
Rudnick SI, Adams GP (2009) Affinity and avidity in antibody-based tumor targeting. Cancer Biother Radiopharm 24:155–161
Saga T, Neumann RD, Heya T, Sato J, Kinuya S, Le N, Paik CH, Weinstein JN (1995) Targeting cancer micrometastases with monoclonal antibodies: a binding-site barrier. Proc Natl Acad Sci USA 92:8999–9003
Drewinko B, Patchen M, Yang LY, Barlogie B (1981) Differential killing efficacy of twenty antitumor drugs on proliferating and nonproliferating human tumor cells. Cancer Res 41:2328–2333
Rao PN, Freireich EJ, Smith ML, Loo TL (1979) Cell cycle phase-specific cytotoxicity of the antitumor agent maytansine. Cancer Res 39:3152–3155
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234
Hamann PR, Hinman LM, Beyer CF, Greenberger LM, Lin C, Lindh D, Menendez AT, Wallace R, Durr FE, Upeslacis J (2005) An anti-MUC1 antibody-calicheamicin conjugate for treatment of solid tumors. Choice of linker and overcoming drug resistance. Bioconjug Chem 16:346–353
Matsui H, Takeshita A, Naito K, Shinjo K, Shigeno K, Maekawa M, Yamakawa Y, Tanimoto M, Kobayashi M, Ohnishi K, Ohno R (2002) Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 16:813–819
Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML (2003) Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 102:1466–1473
Toppmeyer DL, Slapak CA, Croop J, Kufe DW (1994) Role of P-glycoprotein in dolastatin 10 resistance. Biochem Pharmacol 48:609–612
Butryn RK, Smith KS, Adams EG, Abraham I, Stackpole J, Sampson KE, Bhuyan BK (1994) V79 Chinese hamster lung cells resistant to the bis-alkylator bizelesin are multidrug-resistant. Cancer Chemother Pharmacol 34:44–50
Zsido TJ, Beerman TA, Meegan RL, Woynarowski JM, Baker RM (1992) Resistance of CHO cells expressing P-glycoprotein to cyclopropylpyrroloindole (CPI) alkylating agents. Biochem Pharmacol 43:1817–1822
Tang R, Cohen S, Perrot JY, Faussat AM, Zuany-Amorim C, Marjanovic Z, Morjani H, Fava F, Corre E, Legrand O, Marie JP (2009) P-gp activity is a critical resistance factor against AVE9633 and DM4 cytotoxicity in leukaemia cell lines, but not a major mechanism of chemoresistance in cells from acute myeloid leukaemia patients. BMC Cancer 9:199
Loo TW, Clarke DM (2005) Recent progress in understanding the mechanism of P-glycoprotein-mediated drug efflux. J Membr Biol 206:173–185
Kovtun Y, Jones G, Audette C, Mayo M, Leece B, Zhao R, Clancy L, Sun X, Chari R, Singh R (2010) 235. Negatively-charged sulfonate group in linker improves potency of antibody–maytansinoid conjugates against multidrug-resistant cancer cells. 22nd EORTC-NCI-AACR symposium on molecular targets and cancer therapeutics, Berlin, Germany
Qin A, Watermill J, Mastico RA, Lutz RJ, O’Keeffe J, Zildjian S, Mita AC, Phan AT, Tolcher AW (2008) The pharmacokinetics and pharmacodynamics of IMGN242 (huC242-DM4) in patients with CanAg-expressing solid tumors. ASCO Meet Abstr 26:3066
Tolcher AW, Ochoa L, Hammond LA, Patnaik A, Edwards T, Takimoto C, Smith L, de Bono J, Schwartz G, Mays T, Jonak ZL, Johnson R, DeWitte M, Martino H, Audette C, Maes K, Chari RV, Lambert JM, Rowinsky EK (2003) Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol 21:211–222
Xie H, Audette C, Hoffee M, Lambert JM, Blattler WA (2004) Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. J Pharmacol Exp Ther 308:1073–1082
Younes A, Gordon L, Kim S, Romaguera J, Copeland AR, de Castro Farial S, Kwak L, Fayad L, Hagemeister F, Fanale M, Lambert J, Bagulho T, Morariu-Zamfir R (2009) Phase I multi-dose escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous (IV) infusion every 3 weeks to patients with relapsed/refractory B-Cell non-Hodgkin’s lymphoma (NHL). ASH Annu Meet Abstr 114:585
Lapusan S, Vidriales MB, Thomas X, de Botton S, Vekhoff A, Tang R, Dumontet C, Morariu-Zamfir R, Lambert JM, Ozoux ML, Poncelet P, San Miguel JF, Legrand O, Deangelo DJ, Giles FJ, Marie JP (2012) Phase I studies of AVE9633, an anti-CD33 antibody-maytansinoid conjugate, in adult patients with relapsed/refractory acute myeloid leukemia. Invest New Drugs 30(3):1121–1131
Thompson DS, Patnaik A, Bendell JC, Papadopoulos K, Infante JR, Mastico RA, Johnson D, Qin A, O’Leary JJ, Tolcher AW (2010) A phase I dose-escalation study of IMGN388 in patients with solid tumors. ASCO Meet Abstr 28:3058
Lambert JM (2010) Antibody-maytansinoid conjugates: a new strategy for the treatment of cancer. Drugs of the future 35:471–480
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Goldmacher, V.S., Singh, R., Chittenden, T., Kovtun, Y. (2013). Linker Technology and Impact of Linker Design on ADC Properties. In: Phillips, G. (eds) Antibody-Drug Conjugates and Immunotoxins. Cancer Drug Discovery and Development. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5456-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5456-4_7
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5455-7
Online ISBN: 978-1-4614-5456-4
eBook Packages: MedicineMedicine (R0)