Abstract
Yeast, fungal, and mammalian prions determine heritable as well as infectious traits. In mammals, prions cause a group of fatal and rapidly progressive neurodegenerative diseases, originally described as transmissible spongiform encephalopathies (TSEs). Variations in prions, which cause different disease phenotypes, are referred to as strains. Mammalian prion strains are differentiated by qualitative characteristics such as clinical symptoms, brain pathology, targeted brain anatomical areas and cells, or Western blot patterns of glycosylated or deglycosylated pathogenic prion protein (PrPSc). Quantitative prion traits are determined by incubation time, prion dose response, proteolytic sensitivity, and conformational stability of PrPSc. The high degree of fidelity with which prion strains replicate requires a precise molecular mechanism that can account for all these characteristics. Remarkable progress in the past decade produced many lines of evidence arguing that prion traits are encoded in the self-replicating conformation of PrPSc that is unique for each strain. Thus, prions behave like proteinaceous genes. The determination of the full spectrum of human and animal prion strains and the conformational features in the pathogenic human prion protein that govern replication of prion strains is essential for the development of diagnostic as well as therapeutic strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prion strains
- Conformation of prion protein
- Protein misfolding cyclic amplification (PMCA)
- Conformation-dependent immunoassay (CDI)
- Neurodegeneration
12.1 Prion Diversity
Unique characteristics of mammalian prion isolates, which cause distinctive disease phenotypes, are referred to as strains. Prion strains were initially isolated based on distinctive clinical symptoms in goats with scrapie (Pattison and Millson 1961). Subsequently, strains were isolated in rodents based on divergent incubation times and neuropathologic profiles (Fraser and Dickinson 1973; Dickinson and Fraser 1977). New strains have been produced upon passage from one species to another (Kimberlin et al. 1987), from nontransgenic (Tg) mice to mice expressing a foreign or artificial PrP transgene (Scott et al. 1997), or most recently in vitro from recombinant prion protein (Legname et al. 2006; Wang et al. 2010).
For several decades, the existence of several prion strains was offered as an argument for the existence of a scrapie-specific nucleic acid (Bruce and Dickinson 1987; Dickinson and Outram 1988). However, despite numerous attempts to find such a nucleic acid using several approaches and despite mounting evidence against the existence of a strain-coding polynucleotide (Meyer et al. 1991; Kellings et al. 1992, 1994; Safar et al. 2005a), an explanation for prion strains remained a conundrum and a major challenge to basic principles of molecular biology (Safar et al. 2005a; Prusiner 1998a; Weissmann 2004). Moreover, the discovery that different strains of prions can be propagated indefinitely with high fidelity in inbred mouse lines expressing only a single PrP sequence and the finding that prion strains were selective with regard to the cells in which they can replicate raised fundamental questions (a) How many mammalian prion strains exist? (b) How can cells distinguish different prion strains, as reflected in the cells’ ability to propagate them? (c) How are strain-specific characteristics encoded if the prion is composed solely of PrP with the same sequence?
12.2 Distinct Phenotypes of Prion Strains in Bioassay
An important milestone in the history of research on prion strains was the experimental transmission of scrapie from sheep to mice ∼18 months after intracerebral inoculation of brain extracts (Chandler 1961). On second passage, the incubation periods shortened to 4–5 months and remained constant on subsequent passages. The demonstration that scrapie could be transmitted to a small laboratory rodent made possible many new experimental studies that were previously impracticable in sheep or goats and helped to identify and characterize the first prion isolates by distinct clinical symptoms, incubation time, and brain pathology (Fraser and Dickinson 1973; Dickinson et al. 1972). A second milestone occurred with the development of an incubation time bioassay in Syrian hamsters, which reduced the time required to measure prions in samples with high titers by a factor of nearly 6; only 70 days were required instead of the 360 days previously needed. Equally important, four animals could be used instead of the 60 mice that were required for endpoint titrations, and this made possible a large number of parallel experiments (Prusiner et al. 1982, 1999a). However, there were disadvantages to using hamsters instead of mice: (1) the number of inbred hamster strains was small, (2) they we susceptible to only some prion strains, and (3) there were no procedures for transfer and ablation of genes in the hamster. Thus, the third milestone became the production of transgenic (Tg) mice overexpressing prion protein homologous to the original prion host, for example, mouse (Mo), Syrian hamster, or human (Hu) PrP. In contrast to nontransgenic hosts, Tg mouse models of prion diseases produced the original species of prions, and overexpression of the PRNP gene led to significantly shorter incubation times (Carlson et al. 1994a; Scott et al. 1989). Most importantly, the transmission experiments established stable laboratory strains of prions with defined biological characteristics that became standard experimental tools in prion research (Prusiner et al. 1999a, 2004a, b; Scott et al. 2004).
Because of the wealth of data accumulated in animal experiments, the parameters distinguishing distinct mammalian prion isolates fell into qualitative or quantitative categories:
-
1.
Qualitative traits:
-
(a)
Clinical symptoms of the host (Pattison and Millson 1961)
-
(b)
Anatomical distribution and characteristics of brain lesions (Fraser and Dickinson 1973; Dickinson and Fraser 1977)
-
(c)
Anatomical distribution of pathogenic PrPSc in the brain (Gambetti et al. 2003; Taraboulos et al. 1992)
-
(d)
Mass of unglycosylated or deglycosylated rPrPSc on Western blots (WBs) (Parchi et al. 1996; Bessen and Marsh 1994; Telling et al. 1996)
-
(e)
Glycoform pattern of rPrPSc on WBs (Collinge et al. 1996)
-
(f)
Conformational characteristics of PrPSc in conformation-dependent immunoassay (CDI) (Safar et al. 1998)
-
(a)
-
2.
Quantitative traits:
12.3 Prion Species
A prion species is defined by the amino acid sequence of the donor’s (host’s) PrP. Transmission of prions between different animal species frequently results in low transmission rates and long incubation times, which shorten upon repeated transmission to the recipient species (Scott et al. 2004; Safar et al. 2011; Bruce and Dickinson 1979). This so-called species barrier is attributed to differences in the PrP sequences between prion donor and new host that hinder the response of host PrPC to the incoming rPrPSc seed (Scott et al. 2004; Collinge and Clarke 2007). A “species barrier” may also exist within the same animal species; for example, there are two distinct polymorphic PrP alleles in different mouse lines, the Prnpa (108L, 189T) and the Prnpb allele (108F, 189V), and transfer of prions between mice with divergent PrP alleles is subject to a barrier similar to that observed in the transfer between different animal species (Prusiner et al. 2004a; Carlson et al. 1994b; Tremblay et al. 2004).
In the case of interspecies prion transfer to mice, the barrier may be overcome by replacing the murine PrP genes with their counterpart from the donor, for example, Syrian hamster (Prusiner et al. 1990), cattle (Scott et al. 1999), human (Telling et al. 1994), or cervids (Browning et al. 2004). Importantly, in PrP-deficient (Prnp 0/0) mice, neither prion disease nor prion replication has been found (Büeler et al. 1993). But replacement of the murine PrP gene with its homologs from another species does not recreate the physiology of the donor species, and genes other than PrP may play a role in susceptibility to prions, thereby resulting in different incubation times (Tamguney et al. 2008; Stephenson et al. 2000; Prusiner et al. 1999b). From these experiments and those in vitro, several authors have proposed an auxiliary role for an as yet hypothetical host-derived cofactor in prion replication, which could be a polynucleotide, glycosaminoglycan, lipid, or chaperone facilitating conversion (Kaneko et al. 1997; Kim et al. 2010; Deleault et al. 2010, 2012; Piro and Supattapone 2011; Geoghegan et al. 2007).
Cumulatively, the expression of foreign, mutant, or chimeric PrP transgenes in mice has created a wealth of knowledge about prions that was previously unattainable. Most importantly, this knowledge helped to separate the phenomena generated by “species barrier” from true strain characteristics encoded in the prion itself (Scott et al. 2004, 2005; Collinge and Clarke 2007). It has also helped to define the central domain (residues 96–167) in the PrP amino acid sequence determining “species barrier” (Scott et al. 2004), demonstrated an inverse relationship between the level of PrPC expression and the incubation time (Scott et al. 1989), and allowed differentiation of the natural prion isolates from de novo prions generated with mutant and recombinant PrP (Legname et al. 2006; Wang et al. 2010; Tremblay et al. 2004; Safar et al. 2000).
12.4 Cell Tropism of Prion Strains
A few traits, such as clinical symptoms, pathology, and CNS distribution of pathogenic PrPSc, probably indicate distinct susceptibility of different cells to prions (Mahal et al. 2007). Different prion strains are evident in different locations of lesions and PrPSc deposition in the brain and may exhibit different tropism for cell lines (Mahal et al. 2007). Because the uptake of PrPSc by cultured cells appears to be a nonspecific process, the distinct susceptibility of various cells to different prion strains probably reflects the capacity of the cell to replicate prions at a rate exceeding natural clearance (Bergstrom et al. 2006; Mishra et al. 2004).
Some authors studying Western blot patterns of PrP 27-30 proposed that the observed differences in glycosylation specify prion strains (Collinge et al. 1996). However, this proposal is difficult to reconcile with the addition of high mannose oligosaccharides to Asn-linked consensus sites on PrP in the ER and subsequent remodeling of the sugar chains in the Golgi (Endo et al. 1989). Modification of the complex CHOs attached to PrPC is clearly completed prior to the PrPC trafficking to the cell surface (Borchelt et al. 1990; Caughey and Raymond 1991), which indicates that the Asn-linked CHOs of PrPSc do not instruct the addition of such complex-type sugars to PrPC. Mutagenesis of the complex-type sugar attachment sites seemed to increase PrPSc formation in cultured cells (Taraboulos et al. 1990) but resulted in prolonged incubation times in Tg mice and differences in the patterns of PrPC distribution and PrPSc deposition in mice expressing mutant PrPs (DeArmond et al. 1997; Tuzi et al. 2008). Finally, the idea that strain recognition is mediated by the nature of the glycans carried by PrPSc is not supported by the finding that two distinct prion strains could be propagated by PMCA using unglycosylated PrPC (Piro et al. 2009). Cumulatively these studies indicate that Asn-linked glycosylation might alter the stability and susceptibility of PrPC to conversion, thereby resulting in distinctive patterns of PrPSc deposition and glycosylation on WBs.
An important contribution to the understanding of cellular phenomena related to prion strains came from the cell panel assay (CPA) developed by Charles Weissmann and colleagues. Conventionally the distinction between mouse-adapted prion strains requires determination of incubation times in at least two mouse lines extending over 6–10 months. The CPA, which can distinguish between various murine prion strains in less than 2 weeks (Mahal et al. 2007), is based on the standard scrapie cell assay (SSCA), a method for the rapid and sensitive quantification of prions in vitro. The CPA carried out on a set of four cell lines, PK1, R33, CAD5, and LD9, showed different responses to various prions (Mahal et al. 2007; Karapetyan et al. 2009) and allowed reliable distinction of RML, 22L, 301C, and Me7 mouse prion strains. Additionally, when transferred from brain to cultured cells, “cell-adapted” prions outcompeted their “brain-adapted” counterparts, but the opposite occurred when prions were returned from cells to brain. Thus, the authors concluded that prions, although lacking a nucleic acid genome, are subject to mutation and selective amplification (Li et al. 2010).
However, the mechanisms underlying specificity for brain areas and for cultured cell lines in vitro are likely to be somewhat different. Persistent infection requires that the rate of PrPSc synthesis be at least equal to the rate of PrPSc depletion (Weissmann 2004). In cell culture, depletion of PrPSc is caused by degradation, secretion, and cell division, whereas in brain, where PrPSc accumulates predominantly in neurons, depletion does not occur by cell division. Thus, slowing cell division of cultured cells not only increases the accumulation of PrPSc but may also allow cells to become chronically infected by strains to which they are resistant under normal growth conditions (Ghaemmaghami et al. 2007). The fact that many drugs that “cure” chronically infected cell lines are largely ineffective in abrogating prion disease in vivo reflects at least in part the fact that in the brain PrPSc depletion does not occur by cell division (Ghaemmaghami et al. 2007; Collinge et al. 2009; Trevitt and Collinge 2006).
12.5 Conformational Mechanism of Prion Strain Propagation
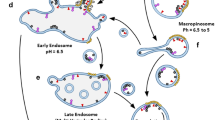
Most researchers now accept the model according to which the infectious pathogen responsible for TSEs is pathogenic PrPSc (Prusiner 1982). This protein is a misfolded, β-sheet-rich isoform of the normal cellular prion protein, PrPC, which is predominantly α-helical (Collinge and Clarke 2007; Prusiner 1998b, 2004; Caughey et al. 2009; Cobb and Surewicz 2009; Morales et al. 2007). The discovery that proteins may be infectious represents a new paradigm of molecular biology and medicine. Although originally deemed heretical, this protein-only model is now supported by a wealth of biochemical, genetic, and animal studies (Collinge and Clarke 2007; Prusiner 1998b, 2004; Caughey et al. 2009; Cobb and Surewicz 2009; Morales et al. 2007), including recent success in generating infectious prions in vitro (Wang et al. 2010; Kim et al. 2010; Legname et al. 2004; Castilla et al. 2005; Barria et al. 2009; Deleault et al. 2007; Geoghegan et al. 2009). The PrPSc conformer is believed to self-replicate by a mechanism which remains poorly understood, but which involves binding to PrPC and causing this protein to convert to the PrPSc state (Fig. 12.1) (Kocisko et al. 1994; Prusiner 1997).
The first suggestion that properties of PrPSc might be distinct in various strains of prions arose from an analysis of two prion isolates from mink that had been passaged in Syrian hamsters and labeled drowsy (DY) and hyper (HY) according dominant clinical symptoms (Bessen and Marsh 1992, 1994). The more pronounced resistance of HY PrPSc to limited proteinase K digestion and distinct sedimentation velocity suggested dissimilar physical properties of PrPSc, but the results did not correlate with other isolates that produced similar incubation times and indistinguishable patterns of PrPSc on WBs (Scott et al. 1997). Only when prion strains generated de novo in humans with inherited prion diseases were passaged in Tg(MHu2M) mice could an argument be made for the distinctive conformation or ligands of PrPSc present in different prion strains (Telling et al. 1996; Prusiner 1997). These studies were fortuitous in the sense that fCJD(E200K) and fatal familial insomnia (FFI) produced different sizes of rPrPSc fragments after limited proteinase K digestion on WBs.
The WB-based studies of PrPSc were limited to the most protease-resistant fraction of PrPSc. It has also been difficult to analyze low levels of PrPSc in the presence of high levels of PrPC. Moreover, the limited digestion by proteinase K resulting in either 19- or 21-kDa bands after deglycosylation of PrP 27-30 could not explain the broad biological diversity observed in more than 30 rodent-adapted prion strains in bioassays. In response to these problems, we developed a rapid, specific, and highly sensitive method for the detection and conformational characterization of PrPSc designated as conformation-dependent immunoassay (CDI) (Safar et al. 1998). After assay calibration with recombinant PrP that has refolded into different conformations, we could distinguish α-helical, β-sheet, and random coil conformations of PrP, either alone or in a mixture. Thus, the assay enabled us to directly measure the amount of PrPSc in brain homogenates without prior digestion with proteinase K to eliminate PrPC. The assay is conformation sensitive, and with selective precipitation of PrPSc before differential immunoassay, PrPSc could be measured in a sandwich format in the presence of ∼10,000-fold excess of PrPC with a sensitivity similar to that of bioassays (Safar et al. 1998, 2002, 2005b, 2008; Kim et al. 2011).
The CDI led to the discovery of a variable fraction of pathogenic prion protein that is actually protease sensitive (sPrPSc) (Fig. 12.1) and allowed us to differentiate all eight strains examined by differently exposed epitopes, response to limited digestion with proteinase K, and stability in a chaotrope guanidine hydrochloride (Gdn HCl) (Safar et al. 1998). Thus, our data provided compelling evidence that eight different strains passaged in the same host (Syrian hamsters) possess at least eight distinct conformations. The differences in conformation of PrPSc detected by CDI in different prion strains in brain homogenates suggested two markedly distinct conformational mechanisms responsible for propagation of different prion characteristics. Under one possibility, each strain would be encoded by the PrPSc molecules in a definite number of conformations, and a specific mixture (ratio) of the same building blocks would replicate itself in the next passage. The second possibility is that each strain characteristic is encoded in a unique conformer of PrPSc, which then replicates with a high degree of fidelity and thus reproduces the strain characteristics.
Thus, in addition to a structure for PrPC that is distinct from PrPSc, our data on prion strains in Syrian hamsters suggested that there may be several PrPSc conformers with distinct stabilities (energies) (Fig. 12.4) (Shirley 1995). This hypothesis represents an obvious departure from earlier work demonstrating that most proteins had a single folded structure that was uniquely encoded in the sequence (Anfinsen 1973). What is the structural basis of these alternative PrPSc conformers? Work on diphtheria toxin identified distinct crystal forms that displayed different tertiary and quaternary structures for a single polypeptide sequence (Bennett et al. 1995). To describe this observation, the notion of domain swapping was introduced whereby a region of one monomer displaced the corresponding region in another monomer to create an interlocking molecular handshake (Cohen and Prusiner 1998). This phenomenon has now been observed in a variety of other protein structures with the swapped elements as small as an isolated α-helix or β-strand and as large as an entire folded domain. We suspect that a similar phenomenon may be responsible for prion strains. The early experimental data obtained with infrared spectroscopy or with mass spectroscopy after hydrogen/deuterium exchange (H/X MS) confirm the conformational plasticity of PrPSc (Cobb and Surewicz 2009; Jones and Surewicz 2005; Caughey et al. 1998). In fact, conformational polymorphism (i.e., ability to form different strains) appears to be a general feature of amyloids and was observed, for instance, in fibrils formed by Aβ peptide associated with Alzheimer’s disease (Paravastu et al. 2008; Petkova et al. 2002).
The data also argue that PrPSc must act as a template in the replication of nascent PrPSc molecules. It seems likely that the binding of PrPC or a metastable intermediate PrP* (Figs. 12.1 and 12.4) (Safar et al. 1994) constitutes the initial step in PrPSc formation and that this is also the rate-limiting step in prion replication (Safar et al. 1998; Kaneko et al. 1997; Cohen and Prusiner 1998; Prusiner et al. 1998). The finding that the rate of PrPSc amplification by PMCA varies considerably for different murine strains supports the view that PrPSc structure is likely rate determining also in vivo (Karapetyan et al. 2009). However, the rate of PrPSc synthesis must also reflect the activation energy required for the conversion process and thus is likely a function of both the conformation of the PrPSc multimer, which is believed to be strain dependent, and of the conformation of the PrPC serving as substrate (Fig. 12.4). The conformational stability of PrPC may depend on posttranslational modifications of PrP such as glycosylation or on association with cellular components which, by favoring certain PrP conformations, could promote preferential propagation of particular strains in different cells. The remarkable affinity of PrPC for nucleic acids (King et al. 2007) and the requirement for polyanions in the PMCA reaction using purified PrPC as substrate (Deleault et al. 2005) together support the view that cell components other than PrPC may play an auxiliary role in prion strain replication (Geoghegan et al. 2007). Thus, the optimal conversion process of different prion strains might require different cofactors, and it is likely that the cofactor content or structure in a particular cell type may contribute to its capacity for propagating a particular strain (Fig. 12.1).
12.6 Human Prion Strains
Although remarkable progress has been made in understanding the pathology, biochemistry, and structure of rodent-adapted prion strains (Prusiner et al. 2004b; Caughey et al. 2009; Cobb and Surewicz 2009; Morales et al. 2007; Watts and Westaway 2007; Telling 2008), understanding of the molecular basis of human prion diseases has lagged behind. The human prion diseases are more complex, and a single pathologic process may present as a sporadic, genetic, or infectious illness (Prusiner 2004). The most common human prion disease is sporadic Creutzfeldt–Jakob disease (sCJD), accounting for ∼85% of cases. Although sCJD was shown to be transmissible to nonhuman primates 40 years ago (Gibbs et al. 1968; Brown et al. 1994), the origin, pathogenesis, and the number of human prion strains causing the disease remain unknown.
Lack of progress in the area of human prions stems from three barriers. First, these diseases present greater variability on complex genetic background; second, experiments with human material are prohibitive; and finally, relatively few investigators focus on human prion diseases. Nevertheless, researchers today generally agree that the genotype at codon 129 of the chromosomal gene PRNP underly the susceptibility to prions and to some degree the phenotypes of diseases (Gambetti et al. 2003; Bishop et al. 2010; Giles et al. 2010). In contrast to the experiments with laboratory rodent prion strains, in which the digestion of brain PrPSc with proteolytic enzyme proteinase K (PK) consistently results in a single protease-resistant domain with mass ∼19 kDa, the outcome in sCJD is more complex. Distinctive glycosylation patterns and up to four PK-resistant fragments of the pathogenic prion protein (rPrPSc) found in sCJD brains are easily distinguishable on Western blot (WB) (Gambetti et al. 2003; Telling et al. 1996; Collinge et al. 1996; Parchi et al. 1997; Wadsworth et al. 1999; Zou et al. 2003) (Fig. 12.2).
Although the disease phenotypes of patients with sCJD are remarkably heterogeneous, the WB findings together with human PRNP gene polymorphism led Parchi, Gambetti, and colleagues to posit a clinicopathologic classification of sCJD into five or six subtypes. Importantly, it has been shown that the WB characteristics of PrPSc breed true upon transmission to susceptible transgenic mice and guinea pigs (Cavia porcellus) (Gambetti et al. 2003; Telling et al. 1996; Safar et al. 2011; Parchi et al. 1997) (Fig. 12.2). Subsequently, Collinge and collaborators (Collinge et al. 1996; Collinge and Clarke 2007; Wadsworth et al. 1999; Hill et al. 1997) introduced an alternative classification of the PrPSc types and their pairing with CJD phenotypes that differed from the previous one in two aspects (a) it recognized three different electrophoretic mobilities of PrPSc and (b) differentiated distinct glycoform ratios in PrPSc (Collinge and Clarke 2007).
Because the disease duration and phenotypes associated with 21-kDa fragments of unglycosylated PrPSc (type 1) frequently differ from the 19-kDa fragments of PrPSc (type 2) (Fig. 12.3) (Gambetti et al. 2003; Telling et al. 1996; Parchi et al. 1997; Monari et al. 1994), these findings argue that the PrPSc type may represent another modifier of the phenotype in human prion diseases. Consequently, WB-based clinicopathologic classifications became useful tool in studies of prion pathogenesis in transgenic mice models of human prion diseases and in human brains (Telling et al. 1996; Collinge and Clarke 2007). Because two distinct PK cleavage sites in PrPSc types 1 and 2 most likely originate from different conformations, some investigators contend that PrPSc types 1 and 2 code distinct prion strains (Parchi et al. 1996; Telling et al. 1996; Collinge et al. 1996; Monari et al. 1994). However, the findings of the co-occurrence of PrPSc types 1 and 2 in 40% or more of sCJD cases suggested that the originally observed differences were quantitative rather than qualitative (Puoti et al. 1999; Kovacs et al. 2002; Head et al. 2004; Lewis et al. 2005; Schoch et al. 2006; Cali et al. 2009). Additionally, the extensive phenotypic heterogeneity of sCJD, along with a growing number of studies including bioassays, all suggests that the range of prions causing sCJD exceeds the number of categories recognized within the original WB-based clinicopathologic schemes (Safar et al. 2005b; Uro-Coste et al. 2008; Polymenidou et al. 2005). Finally, up to 90% of PrPSc is protease sensitive (s), and the conformation and the role of this fraction in the pathogenesis of the disease are unknown and remain a subject of speculation (Safar et al. 2005b, c; Cronier et al. 2008) because it is destroyed by proteinase K treatment, which is necessary to eliminate PrPC (Safar et al. 2005b). Cumulatively, no direct structural data are available for sCJD brain PrPSc beyond the evidence that it is variably resistant to proteolytic digestion.
To determine the conformational range and strain-dependent structural characteristics of sCJD PrPSc in patients who were homozygous for codon 129 of the PRNP gene and thus advance our understanding of the molecular pathogenesis of human prion diseases, we introduced the conformation-dependent immunoassay (CDI) (Safar et al. 1998, 2002, 2005b). The conformational stability of the protein in a denaturant such as Gdn HCl (Shirley 1995) is reflecting the original conformation of the protein. If the protein has the same amino acid sequence, the difference in stability indicates the difference in conformation. Thus, even relatively minute variations in a protein structure can be determined. Using this concept, we developed conformational stability assay in which PrPSc is first exposed to denaturant Gdn HCl and then to europium-labeled mAb against the epitopes hidden in the native conformation (Safar et al. 1998). With sequentially increasing concentration of Gdn HCl, PrPSc dissociates and unfolds from native β-sheet-structured aggregates and more epitopes become available to antibody binding. Because PrPSc is insoluble oligomer and denaturation of this protein is irreversible in vitro, the Gibbs free energy change (ΔG) of PrPSc cannot be calculated (Safar et al. 1994). Therefore, we introduced instead the Gdn HCl value found at the half-maximal denaturation ([GdnHCl]1/2) as a measure of the relative conformational stability of PrPSc. The differences in [GdnHCl]1/2 reveal evidence of distinct conformations of PrPSc (Safar et al. 1994, 1998; Shirley 1995).
The process of disaggregation and unfolding of PrPSc in the presence of increasing concentration of Gdn HCl has been described as follows:
where [PrPSc]n are native aggregates of PrPSc, [sPrPSc]n are soluble protease-sensitive oligomers of PrPSc, iPrP is an intermediate, and uPrP is completely unfolded (denatured) PrP (Safar et al. 1993, 1994, 2011; Tzaban et al. 2002; Safar 2012). Since CDI is not dependent on protease treatment, it allowed us to address fundamental questions concerning the concentration and conformation of different isoforms of sCJD PrPSc, including protease-sensitive (s) and protease-resistant (r) PrPSc (Kim et al. 2011; Safar 2012). Consequently, the CDI monitors the global transition from native aggregates to fully denatured monomers of PrPSc. In contrast, the WB-based techniques monitor either the partial solubilization of PrPSc (Pirisinu et al. 2011) or conversion of rPrPSc to protease-sensitive conformers (Peretz et al. 2001) after exposure to denaturant. Therefore, stability data on protease-sensitive oligomers and intermediates of PrPSc cannot be obtained with WB and may lead to some markedly different values (Choi et al. 2011).
We found with CDI a remarkable heterogeneity of PrPSc conformations within sCJD patients homozygous for codon 129 polymorphism of the PRNP gene and a range corresponded to that of stabilities found in ∼30 distinct strains of natural and de novo laboratory rodent prions that were examined so far (Safar et al. 1998; Peretz et al. 2001; Kim et al. 2011; Colby et al. 2010). The unexpected differential effect of PK treatment with increasing stability of type 1 and decreasing stability of type 2 PrPSc(129M) suggests that in contrast to type 1, the protease-resistant core of type 2 is less stable (Fig. 12.4). The increased frequency of exposed epitopes and decreased stability in type 2 PrPSc after PK treatment (Kim et al. 2011) are counterintuitive and may indicate one of three possibilities: that the PK sensitivity is not an obligatory measure of protein stability and rPrPSc may be in some prion strains less stable than sPrPSc, that removal of the N-terminus from PrPSc resulted in less stable conformation with more exposed 108–112 epitopes, or that the ligand protecting the 108–112 epitopes and stabilizing the PrPSc was removed by PK. Whether the epitopes’ hindrance in undigested PrPSc is the result of lipid, glycosaminoglycan, nucleic acid, or protein binding to the conformers unique to the MM2 sCJD PrPSc remains to be established. Since sCJD cases with type 2 PrPSc(129M) have generally extended disease durations, the molecular mechanism underlying this effect calls for detailed investigation. Cumulatively, our findings indicate that sCJD PrPSc exhibits extensive conformational heterogeneity and suggest that a wide spectrum of sCJD prions cause the disease (Safar 2012). Whether this heterogeneity originates in a stochastic misfolding process that generates many distinct self-replicating conformations (Collinge and Clarke 2007; Prusiner 2001) or in a complex process of evolutionary selection during development of the disease (Li et al. 2010) remains to be established (Kim et al. 2011; Safar 2012).
Despite the inevitable influence of the potential difficulties in evaluating initial symptoms and variable genetic background, our recent data indicate that the levels as well as stability of sPrPSc are a good predictor of the progression rate in sCJD (Kim et al. 2011). The disease progression rate and incubation time jointly represent replication rate, propagation, and clearance of prions from the brain (Prusiner et al. 2004a; Safar et al. 2005c). Therefore, the correlations among the levels of sPrPSc, the stability of sPrPSc, and the duration of the disease all indicate that sPrPSc conformers play an important role in the pathogenesis. When sPrPSc is less stable than rPrPSc, the difference in stability correlates with less accumulated sPrPSc and shorter duration of the disease. An opposite effect is observed when sPrP conformers are more stable than rPrPSc—more accumulated sPrPSc and extended disease duration (Fig. 12.4) (Kim et al. 2011). These observations parallel the experiments on yeast prions and suggest that the stability of misfolded protein is inversely related to the replication rate (Kim et al. 2011; Tanaka et al. 2006). Thus, the data from both yeast and human prions lead to the hypothesis that the less stable prions replicate faster by exposing more available sites for growth of the aggregates. Although the modulating effect of prion clearance in the mammalian brains is likely (Safar et al. 2005c), faster prion replication leads to shorter incubation time and faster progression of the disease.
12.7 Outlook
The continuing mystery surrounding replication of the PrPSc conformer poses a fundamental challenge in modern biology, and important questions regarding prion strains remain to be answered. For example, is each strain composed of a unique conformer or of a spectrum of conformations, which may shift by selection or conformational evolution? Additionally, the conformational concept of prion strain replication raises the question of which conformational features of PrPSc are important for replication and which determine clearance. Although there is now convincing evidence that the PrPSc conformation of distinct strains is different, it is not known to what extent the conformation or replication rate of different conformers might depend on factors other than conformation of the PrP, for example, the nature of the glycans or additional cell-derived ligands (cofactors). An attractive experiment would be to obtain large quantities of highly purified PrPSc from a single cell line, infected separately with several different prion strains; determine the glycans carried by each strain-associated PrPSc; and search for associated molecules, such as small RNAs or other cell components. Finally, the deepest insight will be gained once the three-dimensional structure of PrPSc can be determined at high resolution, currently a still formidable task.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- CDI:
-
Conformation-dependent immunoassay
- CHO:
-
N-linked complex glycosylation chains
- CJD:
-
Creutzfeldt–Jakob disease
- CPA:
-
Cell panel assay
- ER:
-
Endoplasmic reticulum
- FFI:
-
Fatal familial insomnia
- GSS:
-
Gerstmann–Sträussler–Scheinker syndrome
- PMCA:
-
Protein misfolding cyclic amplification
- PRNP:
-
Prion protein gene
- PrP:
-
Prion protein
- PrPC :
-
Normal or cellular prion protein
- PrPSc :
-
Pathogenic prion protein
- rPrPSc :
-
Protease-resistant conformers of pathogenic prion protein (PrP 27-30)
- sCJD:
-
Sporadic Creutzfeldt–Jakob disease
- SFI:
-
Sporadic fatal insomnia
- sPrPSc :
-
Protease-sensitive conformers of pathogenic prion protein
- SSCA:
-
Standard scrapie cell assay
- TSE:
-
Transmissible spongiform encephalopathy
- VPSPr:
-
Variable protease-sensitive prionopathy
- WB:
-
Western blot
References
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 181:223–230
Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C (2009) De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog 5:e1000421
Bennett MJ, Schlunegger MP, Eisenberg D (1995) 3D domain swapping: a mechanism for oligomer assembly. Protein Sci 4:2455–2468
Bergstrom AL, Jensen TK, Heegaard PM, Cordes H, Hansen VB, Laursen H et al (2006) Short-term study of the uptake of PrP(Sc) by the Peyer’s patches in hamsters after oral exposure to scrapie. J Comp Pathol 134:126–133
Bessen RA, Marsh RF (1992) Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66:2096–2101
Bessen RA, Marsh RF (1994) Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68:7859–7868
Bishop MT, Will RG, Manson JC (2010) Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc Natl Acad Sci USA 107:12005–12010
Borchelt DR, Scott M, Taraboulos A, Stahl N, Prusiner SB (1990) Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol 110:743–752
Brown P, Gibbs CJ Jr, Rodgers-Johnson P, Asher DM, Sulima MP, Bacote A et al (1994) Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann Neurol 35:513–529
Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C et al (2004) Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78:13345–13350
Bruce ME, Dickinson AG (1979) Biological stability of different classes of scrapie agent. In: Prusiner SB, Hadlow WJ (eds) Slow transmissible diseases of the nervous system, vol 2. Academic, New York, pp 71–86
Bruce ME, Dickinson AG (1987) Biological evidence that the scrapie agent has an independent genome. J Gen Virol 68:79–89
Büeler H, Aguzzi A, Sailer A, Greiner R-A, Autenried P, Aguet M et al (1993) Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347
Cali I, Castellani R, Alshekhlee A, Cohen Y, Blevins J, Yuan J et al (2009) Co-existence of scrapie prion protein types 1 and 2 in sporadic Creutzfeldt-Jakob disease: its effect on the phenotype and prion-type characteristics. Brain 132:2643–2658
Carlson GA, Ebeling C, Yang S-L, Telling G, Torchia M, Groth D et al (1994a) Prion isolate specified allotypic interactions between the cellular and scrapie prion proteins in congenic and transgenic mice. Proc Natl Acad Sci USA 91:5690–5694
Carlson GA, DeArmond SJ, Torchia M, Westaway D, Prusiner SB (1994b) Genetics of prion diseases and prion diversity in mice. Philos Trans R Soc Lond B Biol Sci 343:363–369
Castilla J, Saa P, Hetz C, Soto C (2005) In vitro generation of infectious scrapie prions. Cell 121:195–206
Caughey B, Raymond GJ (1991) The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem 266:18217–18223
Caughey B, Raymond GJ, Bessen RA (1998) Strain-dependent differences in b-sheet conformations of abnormal prion protein. J Biol Chem 273:32230–32235
Caughey B, Baron GS, Chesebro B, Jeffrey M (2009) Getting a grip on prions: oligomers, amyloids, and pathological membrane interactions. Annu Rev Biochem 78:177–204
Chandler RL (1961) Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet 277:1378–1379
Choi YP, Peden AH, Groner A, Ironside JW, Head MW (2011) Distinct stability states of disease-associated human prion protein identified by conformation-dependent immunoassay. J Virol 84:12030–12038
Cobb NJ, Surewicz WK (2009) Prion diseases and their biochemical mechanisms. Biochemistry 48:2574–2585
Cohen FE, Prusiner SB (1998) Pathologic conformations of prion proteins. Annu Rev Biochem 67:793–819
Colby DW, Wain R, Baskakov IV, Legname G, Palmer CG, Nguyen HO et al (2010) Protease-sensitive synthetic prions. PLoS Pathog 6:e1000736
Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318:930–936
Collinge J, Sidle KCL, Meads J, Ironside J, Hill AF (1996) Molecular analysis of prion strain variation and the aetiology of “new variant” CJD. Nature 383:685–690
Collinge J, Gorham M, Hudson F, Kennedy A, Keogh G, Pal S et al (2009) Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol 8:334–344
Cronier S, Gros N, Tattum MH, Jackson GS, Clarke AR, Collinge J et al (2008) Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J 416:297–305
DeArmond SJ, Sánchez H, Yehiely F, Qiu Y, Ninchak-Casey A, Daggett V et al (1997) Selective neuronal targeting in prion disease. Neuron 19:1337–1348
Deleault NR, Geoghegan JC, Nishina K, Kascsak R, Williamson RA, Supattapone S (2005) Protease-resistant prion protein amplification reconstituted with partially purified substrates and synthetic polyanions. J Biol Chem 280:26873–26879
Deleault NR, Harris BT, Rees JR, Supattapone S (2007) Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA 104:9741–9746
Deleault NR, Kascsak R, Geoghegan JC, Supattapone S (2010) Species-dependent differences in cofactor utilization for formation of the protease-resistant prion protein in vitro. Biochemistry 49(18):3928–3934
Deleault NR, Piro JR, Walsh DJ, Wang F, Ma J, Geoghegan JC et al (2012) Isolation of phosphatidylethanolamine as a solitary cofactor for prion formation in the absence of nucleic acids. Proc Natl Acad Sci USA 109:8546–8551
Dickinson AG, Fraser HG (1977) Scrapie: pathogenesis in inbred mice: an assessment of host control and response involving many strains of agent. In: ter Meulen V, Katz M (eds) Slow virus infections of the central nervous system. Springer, New York, pp 3–14
Dickinson AG, Outram GW (1988) Genetic aspects of unconventional virus infections: the basis of the virino hypothesis. In: Bock G, Marsh J (eds) Novel infectious agents and the central nervous system. CIBA Foundation Symposium 135. Wiley, Chichester, pp 63–83
Dickinson AG, Fraser H, Meikle VMH, Outram GW (1972) Competition between different scrapie agents in mice. Nat New Biol 237:244–245
Endo T, Groth D, Prusiner SB, Kobata A (1989) Diversity of oligosaccharide structures linked to asparagines of the scrapie prion protein. Biochemistry 28:8380–8388
Fraser H, Dickinson AG (1973) Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J Comp Pathol 83:29–40
Gambetti P, Kong Q, Zou W, Parchi P, Chen SG (2003) Sporadic and familial CJD: classification and characterisation. Br Med Bull 66:213–239
Geoghegan JC, Valdes PA, Orem NR, Deleault NR, Williamson RA, Harris BT et al (2007) Selective incorporation of polyanionic molecules into hamster prions. J Biol Chem 282:36341–36353
Geoghegan JC, Miller MB, Kwak AH, Harris BT, Supattapone S (2009) Trans-dominant inhibition of prion propagation in vitro is not mediated by an accessory cofactor. PLoS Pathog 5:e1000535
Ghaemmaghami S, Phuan PW, Perkins B, Ullman J, May BC, Cohen FE et al (2007) Cell division modulates prion accumulation in cultured cells. Proc Natl Acad Sci USA 104:17971–17976
Gibbs CJ Jr, Gajdusek DC, Asher DM, Alpers MP, Beck E, Daniel PM et al (1968) Creutzfeldt-Jakob disease (spongiform encephalopathy): transmission to the chimpanzee. Science 161:388–389
Giles K, Glidden DV, Patel S, Korth C, Groth D, Lemus A et al (2010) Human prion strain selection in transgenic mice. Ann Neurol 68:151–161
Head MW, Bunn TJ, Bishop MT, McLoughlin V, Lowrie S, McKimmie CS et al (2004) Prion protein heterogeneity in sporadic but not variant Creutzfeldt-Jakob disease: UK cases 1991–2002. Ann Neurol 55:851–859
Hill AF, Desbruslais M, Joiner S, Sidle KCL, Gowland I, Collinge J et al (1997) The same prion strain causes vCJD and BSE. Nature 389:448–450
Jones EM, Surewicz WK (2005) Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell 121:63–72
Kaneko K, Zulianello L, Scott M, Cooper CM, Wallace AC, James TL et al (1997) Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc Natl Acad Sci USA 94:10069–10074
Karapetyan YE, Saa P, Mahal SP, Sferrazza GF, Sherman A, Sales N et al (2009) Prion strain discrimination based on rapid in vivo amplification and analysis by the cell panel assay. PLoS One 4:e5730
Kellings K, Meyer N, Mirenda C, Prusiner SB, Riesner D (1992) Further analysis of nucleic acids in purified scrapie prion preparations by improved return refocussing gel electrophoresis (RRGE). J Gen Virol 73:1025–1029
Kellings K, Prusiner SB, Riesner D (1994) Nucleic acids in prion preparations: unspecific background or essential component? Philos Trans R Soc Lond B Biol Sci 343:425–430
Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R et al (2010) Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285:14083–14087
Kim C, Haldiman T, Cohen Y, Chen W, Blevins J, Sy MS et al (2011) Protease-sensitive conformers in broad spectrum of distinct PrP structures in sporadic Creutzfeldt-Jakob disease are indicator of progression rate. PLoS Pathog 7:e1002242
Kimberlin RH, Walker CA (1978) Pathogenesis of mouse scrapie: effect of route of inoculation on infectivity titres and dose–response curves. J Comp Pathol 88:39–47
Kimberlin RH, Cole S, Walker CA (1987) Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. J Gen Virol 68:1875–1881
King DJ, Safar JG, Legname G, Prusiner SB (2007) Thioaptamer interactions with prion proteins: sequence-specific and non-specific binding sites. J Mol Biol 369:1001–1014
Kocisko DA, Come JH, Priola SA, Chesebro B, Raymond GJ, Lansbury PT Jr et al (1994) Cell-free formation of protease-resistant prion protein. Nature 370:471–474
Kovacs GG, Head MW, Hegyi I, Bunn TJ, Flicker H, Hainfellner JA et al (2002) Immunohistochemistry for the prion protein: comparison of different monoclonal antibodies in human prion disease subtypes. Brain Pathol 12:1–11
Legname G, Baskakov IV, Nguyen H-OB, Riesner D, Cohen FE, DeArmond SJ et al (2004) Synthetic mammalian prions. Science 305:673–676
Legname G, Nguyen H-OB, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB (2006) Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA 103:19105–19110
Lewis V, Hill AF, Klug GM, Boyd A, Masters CL, Collins SJ (2005) Australian sporadic CJD analysis supports endogenous determinants of molecular-clinical profiles. Neurology 65:113–118
Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C (2010) Darwinian evolution of prions in cell culture. Science 327:869–872
Mahal SP, Baker CA, Demczyk CA, Smith EW, Julius C, Weissmann C (2007) Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci USA 104:20908–20913
Meyer N, Rosenbaum V, Schmidt B, Gilles K, Mirenda C, Groth D et al (1991) Search for a putative scrapie genome in purified prion fractions reveals a paucity of nucleic acids. J Gen Virol 72:37–49
Mishra RS, Basu S, Gu Y, Luo X, Zou WQ, Mishra R et al (2004) Protease-resistant human prion protein and ferritin are cotransported across Caco-2 epithelial cells: implications for species barrier in prion uptake from the intestine. J Neurosci 24:11280–11290
Monari L, Chen SG, Brown P, Parchi P, Petersen RB, Mikol J et al (1994) Fatal familial insomnia and familial Creutzfeldt-Jakob disease: different prion proteins determined by a DNA polymorphism. Proc Natl Acad Sci USA 91:2839–2842
Morales R, Abid K, Soto C (2007) The prion strain phenomenon: molecular basis and unprecedented features. Biochim Biophys Acta 1772:681–691
Paravastu AK, Leapman RD, Yau WM, Tycko R (2008) Molecular structural basis for polymorphism in Alzheimer’s beta-amyloid fibrils. Proc Natl Acad Sci USA 105:18349–18354
Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG et al (1996) Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol 39:767–778
Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, Kopp P et al (1997) Typing prion isoforms. Nature 386:232–233
Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O et al (1999) Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46:224–233
Pattison IH, Millson GC (1961) Scrapie produced experimentally in goats with special reference to the clinical syndrome. J Comp Pathol 71:101–108
Peretz D, Scott M, Groth D, Williamson A, Burton D, Cohen FE et al (2001) Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10:854–863
Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F et al (2002) A structural model for Alzheimer’s beta-amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci USA 99:16742–16747
Pirisinu L, Di Bari M, Marcon S, Vaccari G, D’Agostino C, Fazzi P et al (2011) A new method for the characterization of strain-specific conformational stability of protease-sensitive and protease-resistant PrP. PLoS One 5:e12723
Piro JR, Supattapone S (2011) Photodegradation illuminates the role of polyanions in prion infectivity. Prion 5:49–51
Piro JR, Harris BT, Nishina K, Soto C, Morales R, Rees JR et al (2009) Prion protein glycosylation is not required for strain-specific neurotropism. J Virol 83:5321–5328
Polymenidou M, Stoeck K, Glatzel M, Vey M, Bellon A, Aguzzi A (2005) Coexistence of multiple PrPSc types in individuals with Creutzfeldt-Jakob disease. Lancet Neurol 4:805–814
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Prusiner SB (1997) Prion diseases and the BSE crisis. Science 278:245–251
Prusiner SB (1998a) Prions (Les Prix Nobel Lecture). In: Frängsmyr T (ed) Les Prix Nobel. Almqvist & Wiksell International, Stockholm, pp 268–323
Prusiner SB (1998b) Prions. Proc Natl Acad Sci USA 95:13363–13383
Prusiner SB (2001) Shattuck lecture—neurodegenerative diseases and prions. N Engl J Med 344:1516–1526
Prusiner SB (ed) (2004) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Prusiner SB, Cochran SP, Groth DF, Downey DE, Bowman KA, Martinez HM (1982) Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol 11:353–358
Prusiner SB, Scott M, Foster D, Pan K-M, Groth D, Mirenda C et al (1990) Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63:673–686
Prusiner SB, Scott MR, DeArmond SJ, Cohen FE (1998) Prion protein biology. Cell 93:337–348
Prusiner SB, Tremblay P, Safar J, Torchia M, DeArmond SJ (1999a) Bioassays of prions. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 113–145
Prusiner SB, Scott MR, DeArmond SJ, Carlson G (1999b) Transmission and replication of prions. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 147–190
Prusiner SB, Scott MR, DeArmond SJ, Carlson G (2004a) Transmission and replication of prions. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 187–242
Prusiner SB, Legname G, DeArmond SJ, Cohen FE, Safar J, Riesner D et al (2004b) Some strategies and methods for the study of prions. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 857–920
Puoti G, Giaccone G, Rossi G, Canciani B, Bugiani O, Tagliavini F (1999) Sporadic Creutzfeldt-Jakob disease: co-occurrence of different types of PrP(Sc) in the same brain. Neurology 53:2173–2176
Safar JG (2012) Molecular pathogenesis of sporadic prion diseases in man. Prion 6:108–115
Safar J, Roller PP, Gajdusek DC, Gibbs CJ Jr (1993) Conformational transitions, dissociation, and unfolding of scrapie amyloid (prion) protein. J Biol Chem 268:20276–20284
Safar J, Roller PP, Gajdusek DC, Gibbs CJ Jr (1994) Scrapie amyloid (prion) protein has the conformational characteristics of an aggregated molten globule folding intermediate. Biochemistry 33:8375–8383
Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M et al (1998) Eight prion strains have PrPSc molecules with different conformations. Nat Med 4:1157–1165
Safar J, Cohen FE, Prusiner SB (2000) Quantitative traits of prion strains are enciphered in the conformation of the prion protein. Arch Virol Suppl 2000:227–235
Safar JG, Scott M, Monaghan J, Deering C, Didorenko S, Vergara J et al (2002) Measuring prions causing bovine spongiform encephalopathy or chronic wasting disease by immunoassays and transgenic mice. Nat Biotechnol 20:1147–1150
Safar JG, Kellings K, Serban A, Groth D, Cleaver JE, Prusiner SB et al (2005a) Search for a prion-specific nucleic acid. J Virol 79:10796–10806
Safar JG, Geschwind MD, Deering C, Didorenko S, Sattavat M, Sanchez H et al (2005b) Diagnosis of human prion disease. Proc Natl Acad Sci USA 102:3501–3506
Safar JG, DeArmond SJ, Kociuba K, Deering C, Didorenko S, Bouzamondo-Bernstein E et al (2005c) Prion clearance in bigenic mice. J Gen Virol 86:2913–2923
Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F et al (2008) Transmission and detection of prions in feces. J Infect Dis 198:81–89
Safar JG, Giles K, Lessard P, Letessier F, Patel S, Serban A (2011) et al. Conserved properties of human and bovine prion strains on transmission to guinea pigs, Lab Invest
Schoch G, Seeger H, Bogousslavsky J, Tolnay M, Janzer RC, Aguzzi A et al (2006) Analysis of prion strains by PrPSc profiling in sporadic Creutzfeldt-Jakob disease. PLoS Med 3:e14
Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M et al (1989) Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59:847–857
Scott MR, Groth D, Tatzelt J, Torchia M, Tremblay P, DeArmond SJ et al (1997) Propagation of prion strains through specific conformers of the prion protein. J Virol 71:9032–9044
Scott MR, Will R, Ironside J, Nguyen H-OB, Tremblay P, DeArmond SJ et al (1999) Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA 96:15137–15142
Scott M, Peretz D, Ridley RM, Baker HF, DeArmond SJ, Prusiner SB (2004) Transgenetic investigations of the species barrier and prion strains. In: Prusiner SB (ed) Prion biology and diseases. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 435–482
Scott MR, Peretz D, Nguyen H-OB, DeArmond SJ, Prusiner SB (2005) Transmission barriers for bovine, ovine, and human prions in transgenic mice. J Virol 79:5259–5271
Shirley BA (ed) (1995) Protein stability and folding: theory and practice. Humana, Totowa, NJ
Stephenson DA, Chiotti K, Ebeling C, Groth D, DeArmond SJ, Prusiner SB et al (2000) Quantitative trait loci affecting prion incubation time in mice. Genomics 69:47–53
Tamguney G, Giles K, Glidden DV, Lessard P, Wille H, Tremblay P et al (2008) Genes contributing to prion pathogenesis. J Gen Virol 89:1777–1788
Tanaka M, Collins SR, Toyama BH, Weissman JS (2006) The physical basis of how prion conformations determine strain phenotypes. Nature 442:585–589
Taraboulos A, Rogers M, Borchelt DR, McKinley MP, Scott M, Serban D et al (1990) Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc Natl Acad Sci USA 87:8262–8266
Taraboulos A, Jendroska K, Serban D, Yang S-L, DeArmond SJ, Prusiner SB (1992) Regional mapping of prion proteins in brains. Proc Natl Acad Sci USA 89:7620–7624
Telling GC (2008) Transgenic mouse models of prion diseases. Methods Mol Biol 459:249–263
Telling GC, Scott M, Hsiao KK, Foster D, Yang S-L, Torchia M et al (1994) Transmission of Creutzfeldt-Jakob disease from humans to transgenic mice expressing chimeric human-mouse prion protein. Proc Natl Acad Sci USA 91:9936–9940
Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R et al (1996) Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274:2079–2082
Tremblay P, Ball HL, Kaneko K, Groth D, Hegde RS, Cohen FE et al (2004) Mutant PrPSc conformers induced by a synthetic peptide and several prion strains. J Virol 78:2088–2099
Trevitt CR, Collinge J (2006) A systematic review of prion therapeutics in experimental models. Brain 129:2241–2265
Tuzi NL, Cancellotti E, Baybutt H, Blackford L, Bradford B, Plinston C et al (2008) Host PrP glycosylation: a major factor determining the outcome of prion infection. PLoS Biol 6:e100
Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G et al (2002) Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41:12868–12875
Uro-Coste E, Cassard H, Simon S, Lugan S, Bilheude JM, Perret-Liaudet A et al (2008) Beyond PrP9res) type 1/type 2 dichotomy in Creutzfeldt-Jakob disease. PLoS Pathog 4:e1000029
Wadsworth JDF, Hill AF, Joiner S, Jackson GS, Clarke AR, Collinge J (1999) Strain-specific prion-protein conformation determined by metal ions. Nat Cell Biol 1:55–59
Wang F, Wang X, Yuan CG, Ma J (2010) Generating a prion with bacterially expressed recombinant prion protein. Science 327:1132–1135
Watts JC, Westaway D (2007) The prion protein family: diversity, rivalry, and dysfunction. Biochim Biophys Acta 1772:654–672
Weissmann C (2004) The state of the prion. Nat Rev Microbiol 2:861–871
Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, Chen SG (2003) Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem 278:40429–40436
Acknowledgments
This work was supported by grants from NIA (AG-14359), NINDS (NS074317), CDC (UR8/CCU515004), and the Charles S. Britton Fund.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Safar, J.G. (2013). Molecular Mechanisms Encoding Quantitative and Qualitative Traits of Prion Strains. In: Zou, WQ., Gambetti, P. (eds) Prions and Diseases. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5305-5_12
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5305-5_12
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-5304-8
Online ISBN: 978-1-4614-5305-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)