Chapter Overview
The treatment of osteosarcoma forms the basis of therapy for most other sarcomas. The concepts that were developed originally for the staging, pathologic analysis, chemotherapy, and surgical management of osteosarcoma have now been applied to many other diseases. Most cases of osteosarcoma are classified as conventional osteosarcoma, which is a high-grade tumor arising typically in an adolescent patient or young adult. For these patients, the standard treatment consists of preoperative chemotherapy, wide surgical excision, careful pathologic mapping of the resected tumor, and postoperative chemotherapy based upon the percentage of necrosis of the tumor. There are many uncommon variants of osteosarcoma that behave differently than conventional osteosarcoma. Osteosarcoma of the craniofacial bones resembles conventional osteosarcoma histologically, but its prognosis is different since metastasis is uncommon. Other variants discussed in this chapter have distinctive radiographic, histologic, or demographic characteristics. Secondary osteosarcoma, which arises in a preexisting bone lesion, has a markedly worse prognosis than other forms of osteosarcoma.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- National Comprehensive Cancer Network
- Postoperative Chemotherapy
- Malignant Fibrous Histiocytoma
- Craniofacial Bone
- Parosteal Osteosarcoma
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Osteosarcoma is the most common primary sarcoma of bone. Nevertheless, it is, like all sarcomas, a rare disease. Approximately 1,000 new cases arise in the United States each year. Most of these occur in young patients, with a peak age of incidence in the second decade. Cases of primary conventional osteosarcoma may arise in older patients, but as age increases, secondary osteosarcoma is more likely. Such tumors develop in patients who have had a preexisting lesion or disease in the bone, such as Paget disease. The diagnosis of secondary osteosarcoma carries significance in terms of prognosis and expected response to treatment; secondary osteosarcoma does not respond well to chemotherapy and has a worse outcome than that of primary conventional osteosarcoma.
The term osteosarcoma usually carries the connotation of a high-grade, bone-forming sarcoma that has occurred in a young person. In essence, this description characterizes conventional osteosarcoma, which is the proper name for such disease. Conventional, or classic, osteosarcoma accounts for most cases of osteosarcoma, but there also exist many other, rarer variants of osteosarcoma that have different clinical characteristics, prognoses, and treatment approaches. Thus, a clear distinction should be made as to which type of osteosarcoma is meant when the disease is discussed.
The management of conventional osteosarcoma represents the model of multidisciplinary treatment that is the foundation of therapy for other sarcomas. The management of certain rare sarcomas, such as dedifferentiated chondrosarcoma, is based on the protocols used for conventional osteosarcoma. The hope is that sarcomas that are currently considered resistant to therapy might one day be treated successfully with a similar strategy, if newer, more effective agents can be identified in the future.
It should be recognized that certain other tumors are now believed to be closely related to osteosarcoma. In particular, malignant fibrous histiocytoma (MFH) of bone, which is discussed in Chap. 8 (“Rare Bone Sarcomas”), may be a variant of osteosarcoma. Although MFH of bone may be nearly identical in histologic appearance to MFH of soft tissue, the behavior of MFH of bone and its response to treatment are more akin to those of conventional osteosarcoma.
This chapter discusses the staging and diagnostic workup of suspected cases of osteosarcoma, as well as the management of conventional osteosarcoma and its variants. The classification of osteosarcoma is shown in Table 5.1, which is based on the World Health Organization (WHO) classification of bone-forming tumors (Schajowicz 1993), with a few modifications. In particular, several clinical variants that are recognized in Table 5.1 are not part of the original WHO classification. Osteosarcoma of the craniofacial bones and secondary osteosarcoma are two important entities with characteristics that clearly set them apart from conventional osteosarcoma. These diseases are discussed in separate sections below. In addition, several rare histologic variants are noted. These include telangiectatic, small cell, epithelioid, and giant cell-rich osteosarcoma. Emphasis in this chapter will be placed upon variants that are unique in their clinical presentation, prognosis, or treatment. Other variants may be distinctive in terms of their histologic appearance but have the same treatment and prognosis as those of conventional osteosarcoma and will therefore be mentioned only briefly.
Diagnostic Workup and Staging
The workup of osteosarcoma includes a detailed history taking and physical examination. The presenting symptoms typically include deep-seated, constant, gnawing pain and swelling at the affected site. Pain in multiple areas may portend skeletal metastasis and should be investigated appropriately. The family history is important, as there may be clues to inherited familial disorders such as retinoblastoma and Li–Fraumeni syndrome (TP53 gene mutation), both of which give rise to osteosarcoma. Beyond the history and examination, the standard studies for evaluation of potential osteosarcoma are laboratory tests, an X-ray of the entire affected bone, a magnetic resonance imaging (MRI) scan of the entire affected bone, a chest X-ray, a chest computed tomography (CT) scan, a whole-body technetium bone scan, and a percutaneous image-guided biopsy.
Laboratory tests should include a complete blood count; measurement of serum electrolytes, blood urea nitrogen, creatinine, calcium, phosphate, and magnesium; and liver function tests. High levels of the enzymes alkaline phosphatase and lactic dehydrogenase, which offer a rough measure of overall tumor burden, have been correlated with worse prognosis by some authors (Meyers et al. 1992).
An X-ray of the affected bone is an important test since it provides diagnostic information and offers a measure by which response to treatment can be judged qualitatively. For diagnostic purposes, the entire bone must be included to evaluate for skip metastases, which are discontinuous tumors in the primary bone site. The presence of fluffy, cloudlike ossification in the soft tissues combined with a permeative, destructive lesion of bone is the classic presentation of osteosarcoma (Fig. 5.1). The amount of ossification can be quite variable, and initially there may not be much ossification, particularly with telangiectatic osteosarcomas, which are almost purely osteolytic lesions. With a positive response to treatment, the extraosseous tumor ossifies and becomes more radiodense. Development of a very clear, mature edge of ossification delineating the border of the tumor portends an excellent response to systemic therapy.
Radiographic and histologic hallmarks of osteosarcoma. (a) Osteosarcoma classically presents in the distal femur of a skeletally immature patient or young adult. Fluffy ossification is present in the soft tissues, while the bone shows mixed sclerotic and lytic areas. (b) Histologically, areas of osteoblastic (single arrowhead), chondroblastic (double arrowhead), and fibroblastic (single arrow) differentiation may be visible in the same tumor to varying degrees. The presence of osteoid formation by malignant spindle cells is the essential diagnostic criterion for osteosarcoma. Image © 2013, A. Kevin Raymond, used with permission
Cross-sectional and multiplanar imaging is obtained with MRI, which provides detailed anatomic information about the extent of disease in the extraosseous soft tissues and the bone marrow. Again, the entire bone should be included. A chest X-ray and chest CT scan are important to screen for pulmonary metastases. A whole-body technetium Tc 99m bone scan is obtained to evaluate possible skeletal metastasis. Occasionally, a bone scan shows activity in pulmonary lesions, which may help to determine whether the lesions represent metastatic disease.
Beyond these standard studies, another imaging study, 18F-labeled fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) with CT, is often performed at presentation. Although it is still not widely accepted as the standard of care, the test is attractive to clinicians because of its potential use for screening for occult metastases and, more importantly, for monitoring response to treatment. Improvements in the specificity and sensitivity of the scan may enable the test to be used as a surrogate measure of chemotherapy response before resection of the tumor. Such use may have important therapeutic implications for both medical oncologists and orthopedic surgeons.
Like the PET/CT scan, an arteriogram of the tumor is not a standard test, but it is vital to intra-arterial therapy. The arteriogram offers an excellent means of monitoring the effectiveness of treatment. A marked decrease in the hypervascular blush associated with radiocontrast dye uptake indicates a positive response to chemotherapy.
The diagnosis of osteosarcoma still must be verified by histopathologic examination of biopsy tissue. At MD Anderson Cancer Center, needle biopsy has traditionally been favored over open biopsy. Biopsy techniques are discussed in detail in Chap. 3, “Percutaneous Image-Guided Biopsy for Diagnosis of Bone Sarcomas.” In the rare instance in which diagnostic tissue is not obtained by needle biopsy, a small open biopsy should be performed by an experienced orthopedic oncologist. Placement of the biopsy incision in line with the future incision for tumor resection is important since the biopsy track must be subsequently resected.
The Musculoskeletal Tumor Society (MSTS) staging system (Table 5.2) has traditionally been used for staging purposes. Using this system, nonmetastatic conventional osteosarcoma is typically staged as IIB. Stage IIA applies in the rare instance in which the tumor is purely intramedullary without extraosseous growth. Stage III applies if metastasis is present, regardless of where it occurs. Most commonly, metastasis occurs in the lungs through hematogenous spread. The second most common site is the skeleton. Lymph node involvement is rare and generally occurs late in the course of disease.
The MSTS-based system is still widely used and is currently favored at MD Anderson. However, one of the criticisms of this staging system is that it does not stratify patients with conventional osteosarcoma except on the basis of metastasis. Conventional osteosarcoma, by definition, is a high-grade tumor and therefore is considered a minimum of stage II, not stage I. The distinction between intra- and extraosseous tumor (stage IIA vs IIB) does not separate patients in a manner that carries much prognostic significance.
The more recent version (seventh edition) of the American Joint Committee on Cancer (AJCC) staging system (Edge et al. 2010) incorporates into the system the size of the tumor and the presence of skip metastases (Table 5.3). Once again, stage I disease represents low-grade tumor, while stage II disease is characterized by high-grade tumor. The designations A and B are determined by tumor size, with 8 cm being the cutoff point. Stage III represents cases with skip metastases, and the new stage IV is characterized by metastatic disease. At the time of this writing, this staging system has not yet gained widespread acceptance or usage. The 8-cm cutoff is not universally accepted as the most appropriate way of distinguishing large versus small tumors. It is unknown whether the staging system will be modified further in future editions or retained in its present form as the standard. Given the ongoing advances in the understanding of the basic biology of the disease, it is likely that the staging of the future will incorporate more molecular-based parameters.
Conventional Osteosarcoma
Clinical Features
Conventional osteosarcoma is primarily a disease of young people; the peak age of incidence is in the second to third decade. This disease is rare in the elderly and, curiously, the very young. It has a slight predominance in males. The most common site of occurrence is around the knee: foremost the distal femoral metaphysis, followed by the proximal tibial metaphysis. Together, these sites account for nearly half of all cases. The proximal femoral and proximal humeral metaphyses are the next most common sites. Occurrence in the pelvis and spine is rarer, which is fortunate because in these areas the surgical difficulties are greater and the rates of recurrence correspondingly higher.
Conventional osteosarcoma has three histologic subtypes: fibroblastic, chondroblastic, and osteoblastic osteosarcoma. In all three subtypes, there is production of osteoid (immature bone matrix) by malignant spindle-shaped sarcoma cells. The presence of osteoid, in essence, defines osteosarcoma. Typically, in any given tumor, all three subtypes may be found to varying degrees; a tumor’s designation as a particular subtype simply refers to the predominant subtype of the tumor. Fibroblastic osteosarcoma has a greater proportion of fibroblastic spindle cells relative to the amount of osteoid. Chondroblastic osteosarcoma has a greater proportion of chondrocytes and cartilaginous matrix relative to the amount of osteoid. Osteoblastic osteosarcoma is composed chiefly of osteoblasts and dense, abundant osteoid.
Primary Treatment
Approach and Rationale
The treatment regimen for conventional osteosarcoma at MD Anderson is unique. Although similar to that of other centers in that it involves systemic chemotherapy and surgery, it is distinguished by the tailoring of postoperative chemotherapy and the use of intra-arterial preoperative chemotherapy. As we describe below, the rationale for this approach is based on both theoretical grounds and experience at MD Anderson.
Many articles have been published on different chemotherapeutic regimens for conventional osteosarcoma. Comparison across different trials is difficult, and it is hazardous to ascribe superiority to one therapeutic strategy over another. Although various protocols employ similar chemotherapeutic agents, differences in dosing and scheduling can affect the comparability of the results.
A number of principles emerge from the published experience with the disease. First, successful treatment requires both systemic chemotherapy and surgical resection of disease. The chances of cure with either alone are quite low, although not zero. Before the era of effective chemotherapy, the rate of cure for patients who underwent immediate amputation was approximately 15–20%. That low rate indicates that in most patients, microscopic metastatic disease is present at the time of presentation. As with surgery alone, the chances of long-term disease control with chemotherapy alone are poor. The experience at MD Anderson has shown that patients who were treated aggressively with chemotherapy alone and had durable remissions eventually developed relapses (Jaffe et al. 2002).
Another principle that has emerged from the published experience is that a combination of active agents is necessary to achieve optimal results. The most active agents currently include doxorubicin, cisplatin, ifosfamide, and methotrexate. Historically, methotrexate was one of the first agents identified as being effective for osteosarcoma, but as a single agent, it results in a somewhat lower rate of response compared with the other drugs. Doxorubicin and cisplatin have higher rates of response and are therefore now the frontline agents. Ifosfamide and methotrexate are the next most active agents. The combination of gemcitabine and docetaxel (Taxotere) has been shown recently to have some activity against the disease and may be considered a reasonable alternative second-line regimen. Older drugs with weaker activity include dactinomycin, bleomycin, and cyclophosphamide.
Several key questions regarding chemotherapy remain unanswered at this time. There continues to be debate over what constitutes the optimal combination of chemotherapeutic agents. Should two, three, four, or more agents be employed? Addition of more agents is not wholly beneficial because it may compromise the dose intensity of the most effective agents. There is also debate over whether the strategy of “tailoring” chemotherapy has merit. This concept involves changing the postoperative chemotherapeutic agents for patients who have not responded well to the initial agents. Finally, there is controversy over the utility of intra-arterial versus intravenous therapy.
To make some sense of the controversy over these issues, it is useful to bear in mind that there are inherent differences among tumors, and all tumors are not created equal. Some respond well to chemotherapy, and some do not. More importantly, some tumors respond well to certain agents but not others. These differences are simply a manifestation of the genetic heterogeneity of tumors.
When viewed in this context, the rationale of the MD Anderson approach to chemotherapy becomes clearer. Rather than relying on a uniform combination of agents to treat all tumors, we build flexibility into the treatment scheme. The two drugs that are given preoperatively, doxorubicin and cisplatin, are the ones that have induced greatest responsiveness in previous trials. They are ideal candidates for combination in full doses because of their nonoverlapping mechanisms of action and toxicity profiles. This combination gives patients the highest probability of achieving a response to the initial therapy. After four cycles of preoperative treatment, patients undergo surgical resection of the primary tumor. If the percentage of tumor necrosis is not outstanding (i.e., if it is less than 95%), then postoperatively patients are switched to the next two most active agents, ifosfamide and methotrexate. These drugs are given at high doses to maximize their effectiveness.
The concept of tailoring chemotherapy based on patients’ therapeutic response has existed for some time. In a study by Meyers et al. (1992) from Memorial Sloan-Kettering Cancer Center, tailoring chemotherapy was attempted, but no significant improvement in survival was found. It should be noted, however, that in this and other studies, many agents were included in the preoperative regimen, and it was impossible for the clinician to know which of the agents were active or inactive. The T12 protocol at Memorial Sloan-Kettering included methotrexate, doxorubicin, cisplatin, bleomycin, cyclophosphamide, and dactinomycin before surgery.
MD Anderson’s approach to tailoring chemotherapy represents a clear departure from previous attempts. Only two agents, doxorubicin and cisplatin, are given before surgery. If the tumor response is inadequate, it seems logical to switch to alternative agents rather than to persevere with inactive agents. Indeed, review of data at MD Anderson has shown that the survival rate of patients who have poor responses to the frontline regimen can be significantly improved by switching to high-dose ifosfamide and methotrexate. Between 1980 and 1992, 123 patients age 16 years and over were treated for conventional osteosarcoma of the extremities. Throughout this period, patients received doxorubicin and cisplatin as the primary agents, and these were the exclusive agents in the early years. During the latter part of the study period, between 1989 and 1992, high-dose ifosfamide and methotrexate were given to patients who had had poor responses (less than 90% tumor necrosis) after the frontline regimen. The 5-year continuous disease-free survival rate was 67% for these patients, a significant improvement over the 24% rate for poor-responding patients who did not receive ifosfamide and methotrexate after surgery (P = 0.015, Benjamin et al. 1995). Of note, patients who received methotrexate alone did not fare as well as did patients who received both ifosfamide and methotrexate. Nevertheless, even with the use of postoperative tailoring with ifosfamide and methotrexate, the survival rates were significantly worse than those for patients who had had a good response (at least 90% necrosis) after preoperative therapy; at 10 years, the latter group’s continuous disease-free survival and overall survival rates were 74% and 76%, respectively.
It is likely that the tailoring of chemotherapy is still in its infancy. At present, our ability to characterize tumors on a molecular level is still at a primitive state. There may one day be a means to determine at the time of diagnosis whether a patient’s tumor carries the biological targets for specific agents. As our ability to characterize tumors in molecular terms improves, it is conceivable that the choice of postoperative chemotherapy will be guided by biological targets within the resistant clones of tumor cells.
One of the benefits of tailoring chemotherapy is that patients are not uniformly subjected to the harsh toxic effects of chemotherapy. All of the agents mentioned above can have serious long-term deleterious effects. Maximizing dose intensity of chemotherapy for all patients indiscriminately subjects many patients to complications that seriously detract from their overall quality of life and functional outcomes. Identification of patients who need only a moderate course of postoperative chemotherapy should be an important aspect of future efforts to improve treatment.
Apart from tailoring chemotherapy, the other unique aspect of the chemotherapy approach at MD Anderson is the intra-arterial administration of cisplatin, when feasible, before surgery. The conceptual basis for this strategy is that it delivers a higher dose of chemotherapy to the primary site compared with other means of administration. Data from the Rizzoli Institute have shown that the percentage of patients who have a good response to initial chemotherapy is higher for patients who receive intra-arterial, as opposed to intravenous, cisplatin (Bacci et al. 1992). Furthermore, data at MD Anderson clearly indicate that the rate of local recurrence is directly related to patients’ responses to chemotherapy. Patients who have excellent responses to induction chemotherapy have significantly lower rates of local recurrence. By enhancing the chemotherapeutic effect at the primary site, quick palliation of symptoms is achieved. Furthermore, limb-salvage surgery is facilitated, and a greater percentage of patients have successful preservation of the extremity.
Chemotherapy Schedule
Our standard treatment protocol is shown in Fig. 5.2. To summarize, patients receive four cycles of intravenous doxorubicin (90 mg/m2) and intra-arterial cisplatin (120 mg/m2) before surgery. The percentage of tumor necrosis is determined by careful pathologic mapping of the resection specimen. Our current practice is to separate optimal from suboptimal necrosis based on a cutoff of 95%, instead of the 90% cutoff used in MD Anderson’s published data (described above). This practice is based on a separate analysis of our data set evaluating the effect of necrosis in increments of 5% instead of 10%, which suggested that the continuous disease-free survival rate of patients with 85–90% tumor necrosis was superior to that of patients with 91–95% necrosis. One possible explanation for this finding could be that the group of patients with 85–90% necrosis was switched to ifosfamide and methotrexate, while the 91–95% group did not receive this intensified postoperative therapy. So, currently, patients who have excellent responses (at least 95% tumor necrosis) receive an abbreviated postoperative regimen: four cycles of doxorubicin (75 mg/m2) and ifosfamide (10 g/m2). Although cisplatin has also been used instead of ifosfamide for good responders, the preference at MD Anderson is for ifosfamide in the postoperative setting. Patients who have less-than-excellent responses (less than 95% tumor necrosis) are switched to a regimen of ifosfamide (14 g/m2) and methotrexate (10–12 g/m2). Depending on tolerance, patients are given up to six cycles of high-dose ifosfamide at 3- to 4-week intervals and six cycles of high-dose methotrexate at 2-week intervals, alternating the drugs every three cycles.
A schematic diagram of the schedule for standard chemotherapy for conventional osteosarcoma at MD Anderson. All patients receive the same preoperative chemotherapy. Postoperative chemotherapy is based on the response to preoperative chemotherapy, as determined by the percentage of tumor necrosis in the resected specimen. Historically, patients were considered “good responders” if at least 90% necrosis was observed. Currently, the preferred cutoff for “excellent responders” at MD Anderson is 95% necrosis. Patients achieving this tumor response are treated with a shorter course of postoperative chemotherapy. Conversely, patients who do not have an excellent response are treated postoperatively with ifosfamide and methotrexate (six cycles each, depending on tolerance). Dox doxorubicin (90 or 75 mg/m2), IA-Cis intra-arterial cisplatin, Ifx ifosfamide, HD-Ifx high-dose ifosfamide, Mtx methotrexate
Several points regarding the delivery of drugs are worth noting. The cardiotoxicity of doxorubicin is lessened by administering it using a continuous infusion, which is given over 72–96 h. Intra-arterial cisplatin (120 mg/m2) may be given concurrently with doxorubicin intravenously via central venous access. Pretreatment hydration with 5% dextrose (D5) half-normal saline and posttreatment hydration with intravenous mannitol solution are important to decrease the nephrotoxic effects of cisplatin.
Surgery
Definitive management of osteosarcoma requires surgical resection of the primary site of disease with wide, negative margins. In the past, various authors recommended a bone margin of 3–7 cm, but most of these recommendations were made before the advent of MRI scans. Smaller margins are acceptable now that MRI enables visualization of the tumor border. Whenever feasible, a 1- to 3-cm margin is advisable, particularly for diaphyseal osteotomies, in which the removal of an extra centimeter of bone usually carries no functional consequence. However, for epiphyseal-sparing resections and other difficult situations, a 1-cm (or less) margin may be acceptable if the preoperative MRI shows clear delineation of the tumor border. Careful intraoperative inspection of a close margin with the pathologist is important to minimize the chance of recurrence at that location.
An adequate soft tissue margin is more difficult to define. Data from the surgical experience at MD Anderson indicate that the risk of local recurrence largely depends on the response to chemotherapy. Patients with 99–100% tumor necrosis have a 1% local recurrence rate. This finding has relevance for the debate regarding surgical margins. Assignment of an arbitrary numeric distance as being an “adequate” margin for all cases is simplistic and not especially helpful. The current challenge is to determine before surgery how well patients have responded to chemotherapy. This information can modulate how much of a margin the surgeon feels is necessary in different areas.
At present, there are no tests that will predict with high correlation the percentage of tumor necrosis. Careful assessment with all imaging modalities is important to determine how effective the preoperative chemotherapy has been. Of note, the Response Evaluation Criteria in Solid Tumors (RECIST) rules are not applicable to osteosarcoma and should not be used. Reduction in tumor size, which can be appreciated on X-rays, CT scans, and MRI scans, is definitely a favorable sign, but it occurs variably because the tumor mass may ossify without shrinkage. In addition to tumor size, the vascularity of the tumor is also an indicator of response. Serial arteriograms are important to monitor in this regard. Disappearance of all hypervascular areas is considered a good prognostic sign.
Ossification with sharp delineation of the borders of the tumor is a positive finding. This development usually can be viewed on plain X-rays. CT scans can be helpful in equivocal cases, particularly if the tumor is adjacent to critical neurovascular and soft tissue structures. A smooth, contiguous zone of calcification at the periphery of the tumor is indicative of a good response to chemotherapy. Tissue outside this zone of calcification is not likely to be involved with tumor and can be safely preserved. This assessment is often most critical in determining whether it is safe to preserve nerves and blood vessels in the popliteal fossa for distal femoral and proximal tibial lesions. In areas in which the tumor does not appear to have calcified, it may be necessary to dissect more widely around the tumor and obtain wider margins.
In most cases of conventional osteosarcoma, limb-sparing surgery can be performed. If preoperative MRI and CT scans do not show neurovascular or massive soft tissue involvement, the tumor can be safely resected and the limb reconstructed. At the time of resection, to ensure the bone margins are negative, the marrow margin is evaluated by frozen section and the tumor is grossly sectioned by the pathologist.
At the common distal femoral and proximal tibial locations, an intra-articular resection can usually be performed. However, first the preoperative MRI scan must be closely studied to ensure the tumor does not extend into the knee joint along the cruciate or collateral ligaments. At MD Anderson, we prefer to reconstruct the distal femur with an endoprosthesis and the proximal tibia with an allograft-prosthesis composite, as discussed in Chap. 9, “Skeletal Reconstruction after Bone Sarcoma Resection.” In the proximal humerus, there is an increased possibility of extension into the glenohumeral joint, in which case an extra-articular resection outside the joint capsule must be performed. Also, the deltoid can be directly invaded by the tumor, necessitating resection of that muscle. However, a review of our surgical experience has shown that in most cases, an intra-articular, deltoid-sparing surgery can be performed. If the rotator cuff can be preserved, an allograft-prosthesis composite can provide excellent function. If the cuff cannot be preserved, but the deltoid and axillary nerve remain intact, then a reverse-shoulder prosthesis provides good function.
In a few cases, extensive involvement of the adjacent neurovascular structures or soft tissues precludes obtaining negative margins and mandates an amputation. For osteosarcomas of the distal femur and proximal tibia, an above-knee amputation is performed. An amputation, though traditionally thought to be more difficult for the patient to accept psychologically, does enable quicker mobilization and eliminates the potential prosthesis- and allograft-related complications seen with limb-sparing surgery. The functional level obtained with modern prostheses can be quite high.
Follow-Up
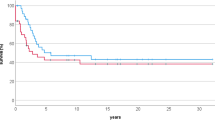
The prognosis for patients with nonmetastatic conventional osteosarcoma is reasonably good. The overall survival rate at 5 years is roughly 65–75% (Fig. 5.3; Meyers et al. 1992; Bacci et al. 1998; Meyers et al. 1998; Le Deley et al. 2007; Meyers et al. 2008; Bielack et al. 2009). As described above, the most important factor that affects survival rate is the response to chemotherapy. Other prognostic variables that affect survival duration include the size of the tumor, the location of the tumor, the age of the patient, and inherited genetic mutations.
Long-term follow-up on a strict schedule is vital to the success of treatment. The general timing for follow-up of high-grade sarcomas is described in Chap. 14, “Follow-up Evaluation and Surveillance after Treatment of Bone Sarcomas”; modified National Comprehensive Cancer Network (NCCN) guidelines are employed. Patients are seen every 3 months during the first 2 years and then gradually less often thereafter. Between years 5 and 10, patients are seen on an annual basis. Our experience suggests that after 10 years the risk of recurrence or secondary malignancy is extremely low, but follow-up may still be worthwhile for other reasons, including evaluation of orthopedic implants, cardiac function, and hearing.
A few points relevant to osteosarcoma in particular are worth emphasizing here. The reason that patients are evaluated for a minimum of 10 years is that the use of effective chemotherapy has changed the natural history of the disease. Recurrences, which used to occur well within 5 years, are delayed by chemotherapy. Furthermore, treatment-related complications may arise after 5 years. Specific chemotherapy-related complications are important to address. Doxorubicin-induced cardiomyopathy can arise as a function of the cumulative dose and duration of infusion of the drug. Affected patients should undergo echocardiograms and stress tests as clinically indicated. Nephrotoxicity can result from treatment with ifosfamide and cisplatin. The renal effects of ifosfamide may not become evident until well after cessation of treatment, and they are sometimes potentiated by nonsteroidal anti-inflammatory drugs and other nephrotoxic medications. Ototoxicity and sensory neuropathy are prominent side effects of cisplatin. They are consistently present, though to variable degrees, in patients who receive a cumulative dose greater than 300 mg/m2. High-dose ifosfamide can exacerbate these effects. As clinically indicated, patients should undergo otologic examinations to assess hearing impairment and the need for hearing-assistive devices.
Surgical complications usually vary according to the type of reconstruction employed. After surgery, all patients with some form of endoprosthesis will need lifelong monitoring for late complications, such as aseptic loosening and hardware failure. Patients with allografts, particularly osteoarticular allografts, may eventually need joint replacement for subsequent arthritis. After amputations, patients also need regular follow-up with a prosthetist for maintenance of their prostheses.
Relapse and Spread of Disease
Local Recurrence
Local recurrence is a significant problem and occurs in approximately 10% of patients (Fig. 5.4; Grimer et al. 2005a; b). To maximize the likelihood of long-term survival, locally recurrent tumors need to be completely resected. If the tumor’s size and location are favorable to allowing a wide local excision with negative margins, a limb-sparing surgery should be performed. However, this scenario tends to be more the exception than the rule. Recurrent disease tends to be large and diffuse. The soft tissue involvement can be extensive and difficult to delineate on MRI or other scans. In recurrences in the distal femur and proximal tibia, the popliteal neurovascular structures are often involved. Similarly, proximal humerus recurrences can involve the brachial plexus and artery. Chemotherapeutic options may be limited, thereby decreasing the potential beneficial effect of chemotherapy on the ability to achieve local disease control. Thus, in most cases, local recurrences cannot be treated adequately by a wide excision and warrant an amputation. In two recent series, more than 50% of local recurrences that were managed surgically required an amputation (Grimer et al. 2005a; b; Nathan et al. 2006).
A local recurrence often occurs together with distant metastasis. In such cases, the extent of disease seems to reflect an inherent aggressiveness of the tumor, and the effect of chemotherapy is often noted to be poor. In these cases, the treatment of the locally recurrent tumor may need to be more palliative than curative. Although radiation may be tried, historically it has not been found to be especially effective in controlling disease. Radioactive bone-seeking agents such as samarium might also be considered, but this modality’s bone marrow toxicity may limit its usefulness. Although amputation is not necessarily the ideal procedure for a patient with incurable disease, it may offer better control of disease and relief of pain than can be obtained with other measures.
Metastasis
Patients who present with overtly metastatic disease have a significantly worse prognosis, with 5-year survival rates of 30–50% (Bielack et al. 2009; Chou et al. 2009). The definition of metastasis at presentation, however, may need to be reexamined in the future because it is known that most patients have microscopic metastatic disease at the time of presentation. The improvements over the years in CT scanning of the lungs have yielded increasingly higher image resolutions, with the result that tiny pulmonary nodules previously invisible on plain chest X-rays are now noticeable on CT scans. It has yet to be determined how these findings will affect staging and treatment.
There are important therapeutic implications for patients who present with metastases initially in comparison with patients who have metastatic relapses of disease. Patients who present with metastases are chemotherapy naïve and therefore have all treatment options available. The initial preoperative treatment approach for these patients is the same as that for patients with nonmetastatic disease. Surgical resection of the primary tumor and the pulmonary (or other) metastases may be performed if all of the gross disease is considered resectable. Aggressive postoperative chemotherapy is employed until maximal tolerance is reached.
Patients who present with multiple osseous metastases or such extensive pulmonary disease that surgical eradication is not feasible are likely to be incurable. For such patients, enrollment in experimental protocols may be considered. In most cases, however, treatment is directed at palliation and improving quality of life.
Patients who have relapses with metachronous metastases after having been rendered disease-free for a period of time are more difficult to treat than are patients who present with metastasis at initial diagnosis. The disease-free interval and the number of metastases both affect the duration of survival. Approximately 15–20% of patients with metastatic relapses achieve long-term survival beyond 5 years (Harting et al. 2006). Chemotherapeutic options may be limited, particularly for patients who had poor responses previously and received higher cumulative doses of drugs. Nevertheless, retreatment with the standard agents doxorubicin, cisplatin, ifosfamide, and methotrexate can be considered. The combination of gemcitabine and docetaxel can also be given to patients who cannot tolerate further chemotherapy with prior agents. Alternatives include participation in experimental trials of new agents.
Patients who have relapses in the form of a single pulmonary nodule have a relatively favorable prognosis and are sometimes curable by thoracotomy alone. In such patients, it may be worth performing surgery first and giving additional chemotherapy in an adjuvant setting. In most instances, however, there are multiple metastases, and it is more logical to treat with systemic agents before considering surgical resection. In nearly all cases, cure is not possible without surgical excision of the recurrent disease.
Rare Variants of High-Grade Osteosarcoma
Rare histologic variants of high-grade osteosarcoma are telangiectatic, small cell (round cell), epithelioid, and giant cell–rich osteosarcoma. Like conventional osteosarcoma, these tumors arise from the interior of the bone in the intramedullary cavity, as opposed to the surface of the bone, which characterizes other subtypes such as parosteal osteosarcoma (see below).
Radiographically, these variants produce lytic lesions in bone without the abundant fluffy ossification that is commonly associated with conventional osteosarcoma. As a result, many cases are misdiagnosed as other tumors, such as benign giant cell tumor of bone. The reason for the lack of ossification may be that the tumors tend to produce only a scant amount of osteoid, thus leading to faint calcification on X-rays. Histologically, the tumors are distinguished by features suggested by their names. Telangiectatic osteosarcoma is notable for large vascular-like spaces; small cell osteosarcoma is composed of sheets of small, round, blue cells; epithelioid osteosarcoma is characterized by epithelioid cells; and giant cell–rich osteosarcoma is marked by multinucleated giant cells.
Telangiectatic osteosarcoma, the more common of the rare variants, responds well to the standard chemotherapeutic agents used for conventional osteosarcoma. The other variants do not respond reliably well. Small cell osteosarcoma in particular tends not to respond to standard osteosarcoma protocols, so a different therapeutic approach to this variant may be warranted. Agents that are effective against Ewing sarcoma, rhabdomyosarcoma, and other primitive sarcomas may be worth considering for the small cell variant.
Osteosarcoma of the Craniofacial Bones
Osteosarcoma of the craniofacial bones resembles conventional osteosarcoma histologically but not in its clinical behavior. The reason for this puzzling difference in biological behavior is unknown, but it is believed to relate in part to the fact that the craniofacial bones are formed by membranous ossification, whereas the long bones are formed by enchondral ossification. Of the different craniofacial bones, the mandible is affected most often, followed closely by the maxilla. Compared with conventional osteosarcoma, craniofacial osteosarcomas occur in an older population, with a peak in the third to fourth decade. The craniofacial tumors are more indolent and much less likely to metastasize (Clark et al. 1983). The primary treatment is wide surgical excision, and the strongest predictor for survival is the adequacy of the surgical margins. Craniofacial osteosarcomas tend not to respond to chemotherapy as well as conventional osteosarcomas of the limbs do, but there is a growing recognition that chemotherapy might improve survival for some patients (Smeele et al. 1997). At MD Anderson, if margin-negative surgical resection is not technically feasible, we employ systemic chemotherapy similar to that used for conventional osteosarcoma.
Well-Differentiated Intramedullary and Parosteal Osteosarcoma
There are two types of well-differentiated osteosarcoma (sometimes referred to as low-grade osteosarcoma). Well-differentiated intramedullary osteosarcoma, as the name suggests, occurs within the cortical confines of bone. In contrast, parosteal osteosarcoma develops on the surface of bone, often with just a small area of contact between the cortical bone and the extraosseous mass; in approximately 25% of cases, there is some penetration of the tumor into the medullary cavity (Okada et al. 1994). Most cases of parosteal osteosarcoma occur in the posterior aspect of the distal femur. While both of these forms of osteosarcoma are rare, parosteal osteosarcoma is seen more frequently than is well-differentiated intramedullary osteosarcoma.
The diagnosis of these tumors may not be straightforward or simple. Both tumor types can present radiographically as densely ossified masses, but areas of fibrous tissue and poor ossification can be present in either lesion (Bertoni et al. 1985). Another difficulty is distinguishing these tumors from other ossified lesions, both radiographically and histologically. The differential diagnosis includes trauma, reactive periosteal bone formation, osteomyelitis, and other tumors, such as osteoid osteoma, which can produce florid cortical bone formation. Parosteal osteosarcoma must be distinguished from heterotopic ossification (also known as myositis ossificans), which arises acutely from direct trauma to muscle.
At MD Anderson, the workup of a suspected case of well-differentiated osteosarcoma is similar to that for conventional osteosarcoma, with one notable exception: an arteriogram is often obtained to assist with the choice of biopsy site. If a region of hypervascularity is encountered, particularly if associated with a less radiodense region, it is given preference as the biopsy site; it may represent an area of dedifferentiation. One of the pitfalls in diagnosing parosteal osteosarcoma is missing such an area of dedifferentiation, which can have grave consequences for the patient because the prognoses and treatments of parosteal osteosarcoma and dedifferentiated parosteal osteosarcoma, which is discussed below, are vastly different.
Well-differentiated osteosarcomas are typically treated with surgery alone. Even though they are low in grade, they still should be completely excised with wide, negative margins. Because parosteal osteosarcomas commonly occur on the surfaces of bones without frank intraosseous invasion, they are amenable to treatment with a hemicortical resection and reconstruction with a hemicortical allograft. In this procedure, the posterior femur is exposed through either a posterior incision or dual medial and lateral incisions. The posterior portion of the distal femur is resected through normal tissue. Reconstruction is then performed using a similarly sized allograft. The advantage of a hemicortical allograft over a circumferential allograft is that with the former, healing is much more prompt and reliable. Normal knee function is typically restored.
A hemicortical allograft may not be appropriate if the parosteal osteosarcoma partially extends into the intramedullary space. If doubt exists as to whether a hemicortical resection can be performed with safe margins, it is preferable to err on the side of resecting the entire circumference of the bone rather than to risk subsequent recurrence. Similarly, well-differentiated intramedullary osteosarcoma is usually not amenable to treatment with a hemicortical resection. In these cases, a conventional resection of a segment of bone is required, and reconstruction with an endoprosthesis or allograft is performed.
The prognosis is excellent for well-differentiated osteosarcomas, and the overall survival rate at 5 years is approximately 95% (Okada et al. 1994). Poor outcomes are generally associated with local recurrence of disease, thus underscoring the importance of wide surgical margins around the primary tumor. When tumors recur, they often become high-grade osteosarcomas. This change in status suggests that either transformation of the low-grade tumor has occurred or that a small, dedifferentiated component of the original tumor was not appreciated.
Periosteal Osteosarcoma
Periosteal osteosarcoma is similar to parosteal osteosarcoma in that the tumor arises on the surface of a long bone, in contrast with conventional osteosarcoma, which arises from the intramedullary cavity of the bone. Despite the similarity in names, periosteal osteosarcoma is distinguished from parosteal osteosarcoma by several features. Periosteal osteosarcoma occurs primarily on the diaphysis, as opposed to the metaphysis, of long bones. Periosteal osteosarcoma tends to be chondroblastic histologically and has less pronounced osteoblastic bone formation. Finally, and most importantly, periosteal osteosarcoma is histologically an intermediate-grade tumor and exhibits a greater degree of atypia and pleomorphism than is seen in parosteal osteosarcoma.
Although it is not disputed that a wide surgical resection is essential to the treatment of periosteal osteosarcoma, the role of chemotherapy is somewhat controversial. The published data are conflicting; some studies purport a benefit, whereas others do not. Some of the discrepancy may arise from diagnostic criteria for periosteal osteosarcoma, which may differ from series to series. It can be challenging to distinguish periosteal osteosarcoma from conventional chondroblastic osteosarcoma of the diaphysis and from periosteal chondrosarcoma.
In the experience of the European Musculoskeletal Oncology Society, the overall prognosis for patients with periosteal osteosarcoma was good. Most patients received doxorubicin-based chemotherapy, and a 5-year overall survival rate of 89% was reported (Grimer et al. 2005a; b). The data for the control arms of this and other retrospective studies, however, are somewhat compromised by potential selection bias. The data at MD Anderson also favor the use of chemotherapy for periosteal osteosarcoma. The same agents used for conventional osteosarcoma (doxorubicin and cisplatin) are employed preoperatively. If a response to the chemotherapy is noted, patients may continue with postoperative chemotherapy. However, if the percentage of necrosis after preoperative chemotherapy is poor (well below 90%), it may not be beneficial to continue with postoperative chemotherapy.
Dedifferentiated Parosteal Osteosarcoma
The diagnosis of dedifferentiated parosteal osteosarcoma requires the presence of a high-grade sarcoma arising from a portion of a low-grade parosteal osteosarcoma. The implication is that the tumor arose as a well-differentiated parosteal osteosarcoma, but a portion of the tumor transformed into a dedifferentiated, high-grade sarcoma. The high-grade portion may be appreciated radiographically as a more lytic, less ossified tumor with permeative, poorly defined borders. On an arteriogram, this portion of the tumor corresponds to the hypervascular region.
Although the dedifferentiated portion of the tumor may represent only a small part of the tumor, it dictates the biological behavior of the tumor. When a metastasis occurs in the lung, it usually recapitulates the morphologic character of the high-grade portion of the tumor. Thus, even if there is only a very small dedifferentiated component, strong consideration must be given to systemic chemotherapy. This approach is controversial, and patients have apparently been cured with wide excision only.
The chemotherapeutic strategy for dedifferentiated parosteal osteosarcoma is similar to that for conventional osteosarcoma, and its response rate is nearly as good. When recognized early, dedifferentiated parosteal osteosarcoma tends to have a favorable outcome. Although the numbers of patients in reported series have been small, published experience suggests that the prognosis is similar to that of conventional osteosarcoma. The long-term overall survival rate in different series has been approximately 50%. The rarity of the disease precludes definitive statements regarding the efficacy of chemotherapy.
Secondary Osteosarcoma
Secondary osteosarcoma arises in the setting of a preexisting lesion (most commonly Paget disease), radiation exposure to bone, fibrous dysplasia, or a bone infarct. Secondary osteosarcomas occur in an older age group, with a peak around the sixth decade. The prognosis is distinctly worse than that of conventional osteosarcoma; the 5-year overall survival rate is 10–20% (Frassica et al. 1991; Shaylor et al. 1999; Longhi et al. 2008). Patients generally respond poorly to chemotherapy. Furthermore, this older patient group does not tolerate chemotherapy as well as younger patients do, and dose reductions may be needed. In a relatively healthy individual with few comorbidities, treatment with standard preoperative chemotherapy, limb-salvage surgery, and postoperative chemotherapy can be tried.
Key Practice Points
-
Osteosarcoma encompasses many variants, including low-, intermediate-, and high-grade tumors.
-
Conventional osteosarcoma refers to a primary, high-grade sarcoma arising within bone, typically in a patient younger than 30 years.
-
The workup of osteosarcoma includes a history and physical examination, laboratory studies, an X-ray of the entire affected bone, an MRI scan of the entire affected bone, a chest X-ray, a chest CT scan, a whole-body technetium bone scan, and a needle biopsy.
-
The treatment of conventional osteosarcoma forms the basis for treatment of a number of other high-grade sarcomas of bone, including MFH of bone, dedifferentiated parosteal osteosarcoma, and dedifferentiated chondrosarcoma.
-
The treatment of nonmetastatic conventional osteosarcoma includes preoperative induction chemotherapy, wide surgical resection of the primary tumor, quantitative histopathologic assessment of the chemotherapy response, and postoperative chemotherapy.
-
The frontline active agents in standard treatment regimens are doxorubicin, cisplatin, ifosfamide, and methotrexate. Second-line agents with activity include gemcitabine and docetaxel.
-
Preoperative chemotherapy for conventional osteosarcoma consists of four cycles of intravenous doxorubicin (90 mg/m2), given as a continuous 72–96 h infusion (to decrease cardiotoxicity), and intra-arterial cisplatin (120 mg/m2).
-
Postoperative chemotherapy for conventional osteosarcoma is based upon the percentage of tumor necrosis in the resected tumor. Patients who do not have an excellent response (at least 95% necrosis) to preoperative chemotherapy are given high-dose ifosfamide (14 g/m2 for up to six cycles) and high-dose methotrexate (10–12 g/m2 for six cycles). Patients who have a favorable response are given a shorter course of less intensive chemotherapy, usually consisting of three cycles of doxorubicin (75 mg/m2) and ifosfamide (10 mg/m2).
-
Resection of pulmonary metastases, whenever feasible, is performed to render the patient free of gross disease.
-
Treatment of well-differentiated parosteal osteosarcoma consists of wide surgical excision only, which is associated with greater than 90% survival and excellent prognosis, whereas treatment of dedifferentiated parosteal osteosarcoma includes preoperative and postoperative chemotherapy.
-
The prognosis for secondary osteosarcoma is significantly worse than that for conventional osteosarcoma, and these patients do not often have favorable responses to chemotherapy.
Suggested Readings
Bacci G, Picci P, Avella M, et al. Effect of intra-arterial versus intravenous cisplatin in addition to systemic adriamycin and high-dose methotrexate on histologic tumor response of osteosarcoma of the extremities. J Chemother. 1992;4:189–95.
Bacci G, Ferrari S, Mercuri M, et al. Neoadjuvant chemotherapy for extremity osteosarcoma–preliminary results of the Rizzoli’s 4th study. Acta Oncol. 1998;37:41–8.
Benjamin RS, Chawla SP, Carrasco CH, et al. Preoperative chemotherapy for osteosarcoma with intravenous adriamycin and intra-arterial cis-platinum. Ann Oncol. 1992;3(Suppl 2):S3–6.
Benjamin RS, Patel SR, Armen T, et al. The value of ifosfamide in postoperative neoadjuvant chemotherapy of osteosarcoma [meeting abstract]. Proc Annu Meet Am Soc Clin Oncol. 1995;14:A1690.
Bertoni F, Present D, Hudson T, Enneking WF. The meaning of radiolucencies in parosteal osteosarcoma. J Bone Joint Surg Am. 1985;67:901–10.
Bielack S, Jurgens H, Jundt G, et al. Osteosarcoma: the COSS experience. Cancer Treat Res 2009;152:289–308.
Chou AJ, Kleinerman ES, Krailo MD, et al. Addition of muramyl tripeptide to chemotherapy for patients with newly diagnosed metastatic osteosarcoma: a report from the Children’s Oncology Group. Cancer. 2009;115:5339–48.
Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer. 1983;51:2311–6.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III (eds). AJCC cancer staging manual, 7th edn. Springer, New York: 2010.
Edmonson JH, Green SJ, Ivins JC, et al. A controlled pilot study of high-dose methotrexate as postsurgical adjuvant treatment for primary osteosarcoma. J Clin Oncol. 1984;2:152–6.
Eilber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5:21–6.
Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20.
Fleming ID, Cooper JS, Henson DE, et al (eds). AJCC cancer staging manual, 5th edn. Lippincott-Raven, Philadelphia: 1997.
Frassica FJ, Sim FH, Frassica DA, Wold LE. Survival and management considerations in postirradiation osteosarcoma and Paget’s osteosarcoma. Clin Orthop Relat Res. 1991;270:120–7.
Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9:5442–53.
Grimer RJ, Bielack S, Flege S, et al. Periosteal osteosarcoma—a European review of outcome. Eur J Cancer. 2005a;41:2806–11.
Grimer RJ, Sommerville S, Warnock D, et al. Management and outcome after local recurrence of osteosarcoma. Eur J Cancer. 2005b;41:578–83.
Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2005b;41:194–9.
Jaffe N, Knapp J, Chuang VP, et al. Osteosarcoma: intra-arterial treatment of the primary tumor with cis-diamminedichloroplatinum II (CDP). Angiographic, pathologic, and pharmacologic studies. Cancer. 1983;51:402–7.
Jaffe N, Raymond AK, Ayala A, et al. Effect of cumulative courses of intraarterial cis-diamminedichloroplatinum-II on the primary tumor in osteosarcoma. Cancer. 1989;63:63–7.
Jaffe N, Patel SR, Benjamin RS. Chemotherapy in osteosarcoma. Basis for application and antagonism to implementation; early controversies surrounding its implementation. Hematol Oncol Clin North Am. 1995;9:825–40.
Jaffe N, Carrasco H, Raymond K, Ayala A, Eftekhari F. Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer. 2002;95:2202–10.
Kleinerman ES, Jia SF, Griffin J, et al. Phase II study of liposomal muramyl tripeptide in osteosarcoma: the cytokine cascade and monocyte activation following administration. J Clin Oncol. 1992;10:1310–6.
Le Deley MC, Guinebretiere JM, Gentet JC, et al. SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007;43:752–61.
Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–6.
Longhi A, Errani C, Gonzales-Arabio D, et al. Osteosarcoma in patients older than 65 years. J Clin Oncol. 2008;26:5368–73.
Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10:5–15.
Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998;16:2452–8.
Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children’s Oncology Group. J Clin Oncol. 2008;26:633–8.
Nathan SS, Gorlick R, Bukata S, et al. Treatment algorithm for locally recurrent osteosarcoma based on local disease-free interval and the presence of lung metastasis. Cancer. 2006;107:1607–16.
Okada K, Frassica FJ, Sim FH, et al. Parosteal osteosarcoma. A clinicopathological study. J Bone Joint Surg Am. 1994;76:366–78.
Patel SR. Radiation-induced sarcoma. Curr Treat Options Oncol. 2000;1:258–61.
Patel SR, Papadopolous N, Raymond AK, et al. A phase II study of cisplatin, doxorubicin, and ifosfamide with peripheral blood stem cell support in patients with skeletal osteosarcoma and variant bone tumors with a poor prognosis. Cancer. 2004;101:156–63.
Schajowicz F. Histological typing of bone tumors. World Health Organization international histological classification of tumors. Springer, Berlin: 1993.
Shaylor PJ, Peake D, Grimer RJ, et al. Paget’s osteosarcoma: no cure in sight. Sarcoma. 1999;3:191–2.
Smeele LE, Kostense PJ, van der Waal I, Snow GB. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol. 1997;15:363–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lin, P.P., Patel, S. (2013). Osteosarcoma. In: Lin, P., Patel, S. (eds) Bone Sarcoma. MD Anderson Cancer Care Series. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-5194-5_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-5194-5_5
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-5193-8
Online ISBN: 978-1-4614-5194-5
eBook Packages: MedicineMedicine (R0)