Abstract
The mammary gland acts as a bio-factory to produce in large amount few proteins by transcribing temporally and spatially regulated genes and translating their mRNA. The aim of this chapter is to summarize briefly our knowledge on the structure of the milk protein genes and to put into context the rapid growth of information on the regulatory elements involved in controlling the expression of these genes. It also describes the amino acid supply to the mammary gland and the intracellular transport and sorting of milk proteins in the secretory pathway of mammary cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
14.1 Introduction
During lactation, mammary epithelial cells secrete large quantities of milk proteins. More than 90 % of these proteins are derived from the transcription of a few tissue-specific genes, expression of which is under a complex multi-hormonal regulation that involves both transcriptional and post-transcriptional mechanisms. Furthermore, to fulfil its bioreactor activity, the mammary gland needs an optimal supply of amino acids as well as efficient translation and transport machineries during lactation.
The previous edition of this chapter (Vilotte et al., 2002) described in detail the hormonal regulation of milk protein gene expression, their mRNA and gene structures, their co- and post-translational modifications and the transport and the secretion of milk proteins. The aim of this revision is to summarize briefly our knowledge on the structure of the milk protein genes and to put into context the rapid growth of information on the regulatory elements involved in controlling the expression of these genes. We will also focus on the amino acid supply to the mammary gland and on the intracellular routing and sorting of milk proteins in mammary cells. However, other important topics will not be discussed. The widespread presence of caseins variants will be covered in Chap. 15, while the practical applications of these studies for the dairy field will be described in Chap. 16. Similarly, global analysis of genome evolution with regard to the mammary gland, as described in Lemay et al. (2009), will be discussed in other chapters of this book.
14.2 Structure of Milk Protein Genes
The major milk protein genes have been sequenced in several species. Overall, the mosaic structure of these genes has been well conserved during evolution, and observed species differences in the length of their transcription unit can often be attributed to the occurrence of repetitive DNA within some introns, mainly artiodactyl retroposons. Similarly, deletions or insertions of amino acids between caseins from different species or variants appear to occur mostly by exon skipping. These genes share the canonical structure of tissue-specific eukaryotic genes. Beyond these basic similarities, these genes differ substantially in their genomic organization.
14.2.1 The Casein-Encoding Genes
14.2.1.1 General Organization of the Gene Cluster
Classical genetic studies have demonstrated that the four casein genes are closely linked, and a general organization of the gene cluster was deduced (reviewed by Grosclaude, 1979). Since then, structural analysis of the gene cluster at the DNA level in various species has confirmed and refined the protein data (Fig. 14.1; Tomlinson et al., 1996; George et al., 1997; Fujiwara et al., 1997; Rijnkels et al., 1997a,b,c; 2002; Lefèvre et al., 2009). The overall size of the casein locus varies from around 50 kb in opossum to 370 kb in humans, but the order and the orientation of the genes within the cluster are conserved (Fig. 14.1). In mice, the γ- and δ-casein genes, which are linked within 60 kb, encode an αs2-like protein, and sequence analysis suggests that the ancestral αs2-casein gene duplicated at the time of radiation between rodents and Artiodactyla (George et al., 1997; Rijnkels et al., 1997a). A similar duplication of the αs2-casein gene is also suspected to have occurred in rabbits (Dawson et al., 1993). A recent duplication of β-casein was evidenced in the monotreme lineage, and the duplication of the α-casein that occurred in the eutherian lineage is absent in marsupials (Lefèvre et al., 2009). Interestingly, the casein locus encompasses several other genes, expression profile of which may not be restricted to the mammary gland. Inactivation of the β-casein gene (Kumar et al., 1994) did not prevent expression of the remaining caseins within the locus, suggesting that they are expressed independently of each other.
The casein locus has been localized to chromosome 6 in the cow, sheep and goat (Threadgill and Womack, 1990; Hayes et al., 1992; Hayes et al., 1993a; Gallagher et al., 1994); 5 in mice (Geissler et al., 1988); 12 in rabbits (Gellin et al., 1985); 4 in humans; 3 in chimpanzees (McConkey et al., 1996); and 5 in opossum (Lefèvre et al., 2009).
14.2.1.2 Individual Gene Structures
Internal homologies within αs1- and αs2-casein proteins suggested that the cognate genes evolved through intragenic duplications, a result confirmed at the DNA level by the observed duplication of intron-exon-intron stretches. The ubiquity of the major phosphorylation site and the striking homology of signal peptides of αs1-, αs2- and β-casein proteins indicated a possible common origin of these calcium-sensitive casein-encoding genes. This hypothesis was further substantiated by the identification of common sequence motifs in the proximal 5′-flanking region and the similar structural organization of the first four exons. Despite its location within the casein genes cluster, the κ-casein gene appears to have no evolutionary relationship with the calcium-sensitive casein-encoding genes. It was recently hypothesized that the milk casein genes have evolved from genes involved in tooth development before the origin of mammals (Kawasaki et al., 2011).
The structure of the αs1-casein gene has been partially analysed in rat (Yu-Lee et al., 1986) and fully determined in cow (Koczan et al., 1991), goat (Leroux et al., 1992) and rabbit (Jolivet et al., 1992), or could be deduced from several other sequenced genomes, the same being true for the other individual milk protein genes. The transcription unit of the gene is split into 19 exons and spans around 17.5 kb in ruminants (Fig. 14.2).
Organization of the bovine casein-encoding genes. Exons are represented by boxes. White boxes: untranslated regions, black boxes: coding frame. Exons are not to scale and their sizes are indicated as base pairs. Two numbers are indicated below exons that comprise both untranslated and coding sequences. Exon I′ from the αs2-casein gene corresponds to a partially skipped exon in the sheep species. Origins of the data are mentioned in the text
The structure of the αs2-casein gene has been described in cow (Groenen et al., 1993). The gene transcription unit is split into 18 exons and spans 18.5 kb (Fig. 14.2). The first intron contains a non-coding exon (exon I′ in Fig. 14.2) that is known to be retained in 4 % of the ovine mRNA (Boisnard et al., 1991). In this species, exon VI is also partially skipped. Sequence comparisons suggest that this gene is more closely related to the β-casein gene than it is to the αs1-casein gene (Groenen et al., 1993).
The structure of the β-casein gene is known in mouse (Yoshimura and Oka, 1989), cow (Bonsing et al., 1988), rat (Jones et al., 1986), rabbit (Thépot et al., 1991), goat (Roberts et al., 1992), human (Hansson et al., 1994) and sheep (Provot et al., 1995). Its transcription unit is composed of 9 exons and spans between 8 and 10 kb according to differences between species in the length of intronic sequences (Fig. 14.2). Exon III is skipped in humans, leading to the deletion of 9 amino acids in the mature protein.
Characterisation of the κ-casein gene has been reported in cow (Alexander et al., 1988; Kapelinskaia et al., 1989), human (Edlund et al., 1996) and rabbit (Baranyi et al., 1996). The transcription unit of this gene comprises 5 exons, and its length varies from 7.5 kb in rabbit to 12.5 kb in cow (Fig. 14.2). This gene is evolutionarily related to fibrinogens (Jollès et al., 1974). Indeed, a 24 bp sequence located at the 5′ end of exon IV of the gene was found to be similar with the end of exon II of the γ-fibrinogen gene, suggesting that it represents an exon from the ancestral gene (Alexander et al., 1988).
14.2.2 The Major Whey Protein-Encoding Genes
14.2.2.1 The β-Lactoglobulin-Encoding Gene and Pseudogenes
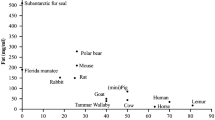
The structure of the β-lactoglobulin-encoding gene has been reported in sheep (Ali and Clark, 1988; Harris et al., 1988), cow (Alexander et al., 1993), goat (Folch et al., 1994), tammar wallaby (Collet and Joseph, 1995) and horse (Lear et al., 1998). The structure of this gene, with seven exons and a transcription unit length of around 4.8 kb, was conserved during evolution (Fig. 14.3). In dogs and horses, two functional and closely linked genes are present in the genome (Halliday et al., 1990; Lear et al., 1998). In cats, a third functional gene is present (Halliday et al., 1990; Pena et al., 1999).
Organization of the major whey protein-encoding genes. Exons are represented by boxes. White boxes : untranslated regions, black boxes: coding frame, grey box: exon 2 from marsupials and monotremes whey acidic protein-encoding gene. Exons are not to scale and their sizes are indicated as base pairs. Two numbers are indicated below exons that comprise both untranslated and coding sequences. Origins of the data are mentioned in the text
In ruminants, a β-lactoglobulin pseudogene has been identified (Passey and MacKinlay, 1995; Folch et al., 1996), while in cow and goat a gene conversion event has occurred. The bovine and caprine pseudogenes contain seven exons, and the ancestral protein that they encode is related to the monomeric β-lactoglobulin II protein. The bovine pseudogene is located 14 kb 5′ from the functional gene, in a similar orientation (Passey and MacKinley, 1995).
β-Lactoglobulin belongs to the lipocalin protein family (Flower, 1996, for review). Comparison of the structure of various lipocalin genes with that of the β-lactoglobulin gene has revealed striking similarities, confirming further their evolutionary relationship (Ali and Clark, 1988). The β-lactoglobulin gene(s) and pseudogene were assigned to chromosome 11 in cow and goat, 3 in sheep (Hayes and Petit, 1993c; Folch et al., 1996) and 28 in horse (Lear et al., 1998).
14.2.2.2 The α-Lactalbumin Gene and Pseudogenes
The α-lactalbumin gene has been sequenced in rat (Quasba and Safaya, 1984), cow (Vilotte et al., 1987), human (Hall et al., 1987), guinea pig (Laird et al., 1988), goat (Vilotte et al., 1991), mouse (Vilotte and Soulier, 1992), tammar wallaby (Collet and Joseph, 1995) and otariid and phocid seals (Sharp et al., 2008). In eutherians, the gene is composed of four exons and its transcription unit is about 2 kb in length (Fig. 14.3). It shares the same structural organization with the lysozyme gene, corroborating the hypothesis of a common ancestor (Quasba and Safaya, 1984). The structure of the tammar wallaby α-lactalbumin gene appears different with the occurrence of a putative 5′-untranslated first exon (Collet and Joseph, 1995). In ruminants, the occurrence of related sequences, probably pseudogenes, has been reported (Soulier et al., 1989; Vilotte et al., 1991; 1993). All α-lactalbumin-related sequences are closely linked (Hayes et al., 1993b; Gallagher et al., 1993) and located 3′ to the functional gene (quoted in Stinnakre et al., 1999). The α-lactalbumin-encoding gene (and related sequences) has been assigned to human chromosome 12 (Davies et al., 1987), sheep chromosome 3, bovine and goat chromosomes 5 (Hayes et al., 1993b) and pig chromosome 5 (Rohrer et al., 1997). In Cape fur seals, the gene appears to be transcriptionally silenced and, although comparative analysis of proximal promoter sequence revealed some differences, for which none appears to be responsible (Sharp et al., 2008).
14.2.2.3 The Whey Acidic Protein-Encoding Gene
The whey acidic protein (WAP) gene was originally thought to be present only in rodents (Campbell et al., 1984) but has been sequenced in various eutherians (Thépot et al., 1990; Rival-Gervier et al., 2003), marsupials and monotremes (Topcic et al., 2009, for review). The 2 kb transcription unit is composed of four exons in eutherians and of five exons in marsupials and monotremes (Fig. 14.3). In ruminants, the WAP gene is characterized by a nucleotide deletion at the end of exon one and a lack of detectable transcription. It thus appeared to be a pseudogene (Hajjoubi et al., 2006). The functional role for WAP in milk is unknown, although it bears a similarity to a family of protease inhibitors; however, its absence in deficient mice leads to nutritional deficiencies in the offspring which appear to be unrelated to its activity as a protease inhibitor (Triplett et al. 2005). Both WAP and protease inhibitors of the Kunitz family are characterized by highly conserved cysteine residues located in two proteic domains (Hennighausen and Sippel, 1982). The murine WAP gene has been assigned to chromosome 11 (Gupta et al., 1982).
14.3 Milk Protein-Encoding Gene Expression and Regulation
14.3.1 Tissue Specificity and Developmental Regulation
The major milk protein genes are defined as mammary-specific and developmentally regulated expressed genes. As such, they represent markers of mammary differentiation. The amount of milk protein mRNA in mammary epithelial cells increases steadily from mid-pregnancy to lactation, although at different rates according to the different genes (Harris et al., 1990; Robinson et al., 1995). This is due to an increase in the transcription rate of these genes as well as a stabilization of the transcripts (Guyette et al., 1979). An observed asynchrony of mammary epithelial cell maturation during pregnancy (Robinson et al., 1995) is paralleled by heterogeneous expression during lactation of the major milk protein genes in sheep and cattle (Molenaar et al., 1992). This heterogeneous pattern of expression was not observed in lactating mouse mammary glands (Dobie et al. 1996). Nevertheless, a short closure of lactating murine mammary gland resulted in local perturbation of milk protein gene expression, leading to the appearance of a mosaic pattern (Faerman et al., 1995). These results strongly suggest that the regulation of the expression of the major milk protein genes in mammary epithelial cell is under a complex regulation that could involve both a graded and a binary mechanism.
Over the last few years, the concept that the expression of the milk protein genes is restricted to the mammary gland has been questioned. Expression of α-lactalbumin in the rat epididymis was reported (Qasba et al., 1983) and denied (Moore et al., 1990; Tang, 1993). The murine α-lactalbumin gene was also reported to be expressed, alongside the β-casein gene, in the sebaceous glands during lactation (Maschio et al., 1991), but this observation has not been confirmed (Persuy et al., 1992; Vilotte and Soulier, 1992). RT-PCR experiments have suggested that the rat α-lactalbumin gene is also expressed at low levels in the brain of some lactating animals (Fujiwara et al., 1999), and casein mRNA expression has been observed in cytotoxic T-lymphocyte-derived cell lines (Grusby et al., 1990). Occurrence of casein-like immunoreactive substances in diverse organs, including the thymus, was reported in the rat (Onoda and Inano, 1997). More recently, promiscuous milk protein gene expression was observed in medullary thymic epithelial cells, probably in relation with the development of central T cell tolerance (Derbinski et al., 2008). It is of interest to note that many of the tissues where ectopic expression is observed contain some of the transcription factors required for mammary expression of the milk protein genes, for example, in T cells, interleukin-2 activates STAT5 (Gilmour et al., 1995).
14.3.2 Hormonal Regulation and Identification of cis-Regulatory Elements
Expression of the major milk protein-encoding genes is under a complex multi-hormonal regulation, resulting from the interplay of steroid and polypeptide hormones. In addition, local growth factors and cell-cell and cell-substratum interactions are also involved. Since many reviews have already focused on this topic, including the previous edition of this chapter (Vilotte et al., 2002), we will only give a brief survey here.
Schematically, lactogenic hormones, such as insulin, prolactin and glucocorticoids, activate the transcription of the major milk protein genes whereas other hormones, such as progesterone, inhibit this activation in the early stages of pregnancy to favour cell proliferation over cell differentiation. It should be noted that in many systems it is difficult to separate direct induction of milk protein transcription from indirect differentiation-related events. As already mentioned, transcription activation of the major milk protein genes is not concomitant during pregnancy, perhaps due to the presence of various hormonal micro-environments and/or different responses to this environment displayed by different milk protein genes. For example, expression of calcium-sensitive casein genes that are activated at mid-pregnancy relies on prolactin and is increased by the synergetic action of glucocorticoids (Kabotyanski et al., 2006, 2009), while the WAP promoter, a gene expressed late in pregnancy, is reciprocally regulated by these two hormones. In addition, whereas the glucocorticoid induction of the WAP gene expression is rapid, its action on the β-casein gene occurs only with a significant time-lag and requires de novo protein synthesis. Expression of the β-lactoglobulin gene, although displaying a similar temporal expression profile to the caseins, appears to be less dependent on lactogenic hormones. Finally, the α-lactalbumin gene, although displaying a temporal expression profile similar to WAP, is induced by prolactin in the presence of low concentrations of glucocorticoids whereas high concentrations of glucocorticoids inhibit its expression, at least in eutherians (Funder, 1989). Induction of the α-lactalbumin gene in the absence of prolactin could be observed in the pregnant murine mammary explants in the presence of insulin and cortisol (Warner et al., 1993), while in marsupials, α-lactalbumin gene expression depends only on prolactin (Collet et al., 1990). The mechanistic rationale for these intriguing differences has not been identified. Finally, it was recently shown that beside transcriptional regulation and transcript stabilization, lactogenic hormones are also involved in the translational regulation of milk protein synthesis (Rhoads and Grudzien-Nogalska, 2007, for review).
Regulation of mammary gene expression is also controlled by the epithelial cell basement membrane. For example, laminin can induce expression of α-lactalbumin, αS1-casein and β-casein by 160-fold (Aggeler et al., 1988; Blum et al., 1989). The differences observed between milk protein genes with regard to hormonal induction are also evident in their varying requirement for a basement membrane. Transcriptional control of the β-casein promoter appears less dependent on the three-dimensional structure of the mammary epithelial cells than does the WAP promoter, although both of them are sensitive to extracellular-matrix (ECM) components (Lin et al., 1995). At least for the β-lactoglobulin gene, regulation of expression by the ECM occurs through activation of STAT5 (Streuli et al., 1995), and this may occur through an ECM-dependent modulation of protein-tyrosine phosphatase activity (Edwards et al., 1998). Cell-substratum components as well as glucocorticoids can, at least for the casein genes, also act at the post-transcriptional level (Eisenstein and Rosen, 1988).
Beside these differences, milk protein genes share specific hormonal responses. For example, in most species, progesterone inhibits milk protein gene expression. Although the exact mechanism is still unclear, it has been reported that progesterone might repress expression of the long form of the prolactin receptor mRNA in the mammary gland (Mizoguchi et al., 1997) and/or directly repress the prolactin/STAT5-mediated transcription at the milk protein gene promoter level (Buser et al., 2007).
Transfection and transgenic studies have revealed that the promoters of most of the major milk protein-encoding genes are responsive to lactogenic hormones sufficiently to target mammary-specific expression of reporter genes in transgenic animals. However, these promoters cannot sustain full expression on their own (compare Webster et al., 1995 with Whitelaw et al., 1992). Indeed, intragenic sequences of some of the major milk protein-encoding genes were shown to be able to contribute to their hormonal regulation (Lee et al., 1989), and important regulatory elements have been identified within the introns (Kang et al., 1998, Kolb, 2003) and/or in the 3′ untranslated region (UTR) and flanking regions (Dale et al., 1992). Furthermore, and as already mentioned, milk protein genes are also regulated at a post-transcriptional level.
Sequence comparison of a particular gene between several species or from different milk protein genes has led to the identification of conserved DNA motifs that were suspected to be involved in the control of the gene transcription. A classical example is the high conservation of sequence elements in the proximal 5′-flanking region (−200/+1) of the calcium-sensitive casein genes (Rosen, 1987; Kolb, 2002). These early identified elements were subsequently found to be recognized by regulatory nuclear factors. Consensus sequences recognized by effectors known to be involved in lactogenesis were also identified within the major milk protein gene promoter sequences by computer searches. However, evidence for the presence of a cis-regulatory element within a DNA fragment came from transfection experiments in cell cultures, transgenic studies, DNAse I protection, footprinting and/or gel shift assays. Site-directed mutagenesis of the identified binding sites and observations, either in cell culture or in transgenics, of the consequences of such mutations on the promoter transcriptional regulation was sometimes performed to further define the functional role of these elements.
14.3.3 Transcriptional Control of Milk Protein Genes
Binding sites for several transcription factors have been identified within the promoters of most of the major milk protein-encoding genes, such as binding sites for OCT-1, NF-1, C/EBP, STAT5, GR, Ets-1 and YY1 (see Rosen et al., 1996, for review). Other DNA elements have been shown to interact with yet unidentified effectors, such as the negative regulatory elements of the WAP promoter (Kolb et al., 1994) and of the β-casein promoter (Lee and Oka, 1992; Altiok and Groner, 1993, 1994). Most of these sequences appear to be clustered within short DNA fragments of several hundred base pairs in length that encompassed both positive and negative regulatory elements. Such composite response elements have been identified in the proximal 5′-flanking regions of the β-lactoglobulin gene (region −406/+1; Watson et al., 1991), of the calcium-sensitive casein genes (region −200/+1; Rosen et al., 1986; 1998 for review), in more distal regions of the bovine (BCE-1 element: region −1613/−1562; Schmidhausser et al., 1992, Myers et al., 1998) and human (region −4700/−4550; Winklehner-Jennewein et al., 1998) β-casein genes, of the rabbit αs1-casein gene (region −3442/−3118; Pierre et al., 1994) and of the rat WAP gene (region −949/−720; Li and Rosen 1994a, 1995; Raught et al., 1995). A cooperation of distal and proximal promoter elements is required to achieve both maximum expression and maximum hormone responsiveness of the murine β-casein gene in mouse HC11 cells (Robinson and Kolbs, 2009). Thus, it appears that the transcriptional regulation of the major milk protein genes is under a combinatorial control with the binding of multiprotein complexes that can either repress or activate gene expression (see Wolberger, 1998, for review).
Differences in the composition of these composite regulatory elements may explain the observed hormonal differences in the transcriptional developmental regulation between the various major milk protein genes. In the WAP gene, for example, an Ets-1 binding site located at −110 appears to be important for the stage-specific transcriptional activation of the gene but not for its stable expression during lactation (McKnight et al., 1995). Activation of promoters by transcription factors can be mediated by the relief of the binding of transcription repressors through both the competitive binding of these activators and the hormonal regulation of the expression or activation of these factors (Schmitt-Ney et al., 1991). Thus, some negative binding factors appear to be present in the mammary gland only during pregnancy but not during lactation (Lee and Oka, 1992). Some of them mediate the inhibitory action of progesterone. Similarly, expression of C/EBPβ protein isoforms that are essential both for mammogenesis and lactogenesis (Robinson et al., 1998; Seagroves et al., 1998) is regulated during pregnancy and lactation. The ratio between LIP, a dominant-negative transcriptional repressor, and LAP, which is an activator of transcription, is high during the pregnancy stage and decreases during lactation due, in part, to the inhibition of LIP expression by glucocorticoids (Raught et al., 1995). Expression of C/EBPα is also increased during lactation, possibly following stimulation by some ECM components (Raught et al., 1995). The action of another factor, the transcription factor YY1, that represses expression of the β-casein promoter by binding to it at position −120/−110 is counteracted by its replacement from its binding site by the prolactin-activated STAT5 protein (see below), which in turn positively regulates the promoter activity (Meier and Groner, 1994). A more surprising example of regulation via binding of negative regulatory factors is given by the two proteins that bind the upper strand of the β-casein promoter at position −221/−170 during pregnancy and involution (Altiok and Groner, 1994). During lactation, a molecule inhibits the binding of these factors to the gene promoter, and it is suspected that this molecule could be the β-casein mRNA itself that possesses high-affinity binding sites for the two proteins in its 5′ UTR (Altiok and Groner, 1994).
Much has been discovered about how milk protein genes are regulated, and the involvement of many transcription factors has been described. Notwithstanding all this information, the identification of STAT5 as the end point of prolactin signalling in the mammary gland heralded a new era in our understanding of mammary gene regulation. All the more so, since the STAT proteins are central to all cytokine responses (Heim, 1999). Much of the work on STAT proteins has been pioneered by studies involving mammary genes (Schmitt-Ney et al., 1991; Watson et al., 1991).
14.3.4 Prolactin Signal Transduction
In the mammary gland, prolactin induction results in the expression of the milk protein genes. This occurs through a rapid but transient signalling transduction pathway (Heim, 1999). First, prolactin-induced dimerisation of its receptor causes trans-phosphorylation of the kinase which is constitutively associated with the cytoplasmic domain of the receptor. The kinase is called JAK2 (for janus kinase 2). The activated JAK2 phosphorylates the receptor creating a docking site for STAT5 through its SH2 domain. Subsequent phosphorylation and dimerisation of STAT5 result in its translocation to the nucleus where it binds to GAS (γ-interferon activation sequences) elements in target genes, for example, β-lactoglobulin. Many other proteins are associated with this pathway, for example, MAPK and SOCS, resulting in a logistical problem for the cell to manage if it is to maintain tight regulation of the signal. It does this by balancing the activation signal with the generation of factors that inhibit the signal (Starr and Hilton, 1999; Tomic et al., 1999).
STAT5 is an important positive transcription factor in the transcriptional regulation of milk protein genes (see Barash, 2006, for review). It is involved in the transduction of the prolactin signal. Functional STAT5 binding sites have been identified in the promoter region of almost all major milk protein-encoding genes, with the potential exception of the κ-casein-encoding gene (Adachi et al., 1996). Within calcium-sensitive casein and β-lactoglobulin composite elements, the occurrence of multiple STAT5 binding sites is observed. These STAT5 binding sites were shown to be essential to confer prolactin transcriptional stimulation to the linked promoter (Schmitt-Ney et al., 1991; Demmer et al., 1995; Jolivet et al., 1996; Soulier et al., 1999). Furthermore, several experiments suggest that STAT5 effects are limited to the modulation of expression level, but are not involved in determining the tissue specificity of expression. For example, mutation of one or several of the STAT5 binding sites within the β-lactoglobulin promoter did not affect the tissue-specific expression of this gene in transgenic mice (Burdon et al., 1994).
The STAT5 transcription factor actually consists of two proteins, STAT5a and STAT5b. These highly similar proteins are encoded by two genes, with differences in their binding affinities due to a single amino acid substitution (Boucheron et al., 1998). The role of these proteins has been studied using gene knockout approaches, with STAT5a emerging as the major factor required for milk protein gene expression. STAT5a-deficient mice exhibit defective mammary gland development (Liu et al., 1997). As one might expect, given the different response of the various milk protein genes to hormones, STAT5a knockout mice express the milk protein genes to different levels. Essentially normal levels of β-casein and α-lactalbumin mRNA levels are detected, with only WAP mRNA levels showing a reduction (Liu et al., 1997). Indeed, there is no correlation between the expression of the STAT5a and the β-casein genes (Kazansky et al., 1995). Studies with STAT5b knockout mice indicate that this protein is associated with growth hormone effects (Udy et al., 1997).
Altogether, these observations suggested that STAT5 may facilitate the interaction of other transcription factors to allow the transcriptional activation of the promoter. Indeed, functional interaction between STAT5 and the glucocorticoid receptor was reported, and this molecular complex was demonstrated to cooperate in the induction of the β-casein promoter, independently of the DNA-binding function of the glucocorticoid receptor (Stöcklin et al., 1996 , 1997; Lechner et al., 1997).
Better understanding of the nature and structural arrangement of the cis-regulatory elements involved in the control of the transcription of the major milk protein genes has given an insight into the differential developmental regulation of their expression. However, many questions remain. Why is expression of WAP in preference to β-casein affected in the STAT5a-null mice given that the reciprocal requirement for prolactin is observed? If it is not STAT5, and it appears not to be, what confers mammary specificity to milk protein gene expression? How do the various transcription factors involved interact with each other to generate a production transcription complex? How does this transcription complex interact with the underlying nucleosomes?
14.3.5 Milk Protein Gene Chromatin Domains
It is now generally accepted that chromatin, or its epigenetic regulation, plays a central role in the regulation of gene expression (Rijnkels et al., 2010). Studies on the ovine β-lactoglobulin gene brought indirect, although compelling, evidence that chromatin reorganization is important for milk protein gene expression. Concomitant to the increase in β-lactoglobulin gene expression during pregnancy, due to activation of STAT5, changes in DNase I hypersensitivity in the β-lactoglobulin gene were reported to occur (Whitelaw and Webster, 1998). This implies a reorganization of the chromatin in response to the interaction of transcription factors. Furthermore, this assay has detected species-specific differences in this reorganization of the chromatin (Pena et al., 1998). These differences are important clues to understand the various levels of expression of the same gene between species or between alleles (Whitelaw, 2000). Similar studies have shown that chromatin reorganization events also occur during the induction of the expression of the rat WAP gene (Li and Rosen, 1994b). However, the clearest evidence yet describing a role for chromatin structure in the regulation of milk protein gene expression comes from the analysis of the β-casein BCE-1 element. This element is not activated in transient transfections, in which true chromatin is not formed, and is responsive to the state of acetylation of the histones (Myers et al., 1998).
The transcription domains of the major milk protein genes are poorly defined. As yet, they have generally been defined through functional analyses only. Transgenic studies have revealed that while the αs1- and the β-casein genes from various species can be expressed at relatively high levels in mice, only weak expression of the αs2- and the κ-casein transgenes was observed. It is thus hypothesized that a mammary-specific locus control region (LCR) might control the expression of the casein locus and that this putative element is located close to the αs1- and the β-casein loci. So far, however, no direct evidence for the existence of such an element has been provided. Furthermore, as the casein gene clusters of some species contain several genes which are not expressed tissue specifically in the mammary gland (Fig. 14.1), the influence of a putative LCR would have to be selective for the casein genes.
A consequence of chromatin gene domains is that boundaries to these domains must exist. There are several documented examples (see Geyer, 1997, for review), but again, as yet, none has been conclusively identified for a milk protein gene. However, some predictions are possible using genome comparison. The boundaries of the α-lactalbumin gene are not known, but those of the related lysozyme gene are well defined (Phi-Van and Strätling, 1988). It is likely that the α-lactalbumin gene contains similar elements, probably in a similar location. This hypothesis is supported by the observed position-independent and copy number-related expression of a human 250 kb YAC and of a 160 kb goat BAC-α-lactalbumin transgene (Fujiwara et al., 1997; Stinnakre et al., 1999).
At present, the transcription domain of the β-lactoglobulin gene might be one of the best studied within milk protein genes. Several DNase I-hypersensitive sites that reflect its expression status have been located within the promoter, intronic and 3′-flanking regions (Whitelaw and Webster, 1998). These sites spatially reflect the limit of the chromatin domain, as defined by nuclease sensitivity. The 5′ limit of this domain resides very close to the promoter. In addition, the proximal 3′-flanking β-lactoglobulin sequences can interact with the nuclear matrix in vitro. This suggests that the β-lactoglobulin gene resides in a very small chromatin domain (Whitelaw, unpublished results). Sequences that interact with the nuclear matrix, usually AT-rich in nature, may be involved in regulating chromatin structure thereby facilitating gene expression. Although several studies have addressed the ability of such sequences to enhance milk protein gene expression, a clear picture has not yet emerged (e.g., Attal et al., 1995; McKnight et al., 1996). Another well-studied locus is that encompassing the WAP gene, the DNA of which adopts different chromatin loop structures according to the studied tissue and developmental stage that allow the differential expression of the genes it contains (Montazer-Torbati et al., 2008) and regulation from distant enhancers and/or repressors (Saidi et al., 2007).
14.3.6 Milk Protein mRNAs
The structure of milk protein mRNAs has been investigated in numerous species (see Mercier and Vilotte, 1993, for review). In lactation, these RNAs account for up to 60 %–80 % of the total RNA present in mammary epithelial cells. The calcium-sensitive casein mRNAs are characterized by a better interspecies conservation of the UTRs and of the sequence encoding the signal peptide compared to the mature protein-coding frame. Since part of the regulation of milk protein gene regulation is exerted at a post-transcriptional level (see Aggeler et al., 1988; Blum et al., 1989; Golden and Rillema, 1995, for examples), conservation of the UTR might reflect their importance for the mRNA processing. In many cases, the rate of translation is influenced by the 5′ UTR sequence and its secondary structure. Sequences located in the 3′ UTR are also known to interact with proteins to either stabilize or degrade the RNA, and/or alter mRNA translation efficiency, and to be potential targets for miRNA. Involvement of miRNA in the biology of the mammary gland is still poorly documented. However, recent studies suggested their implication during normal mammary gland development and differentiation (Gu et al., 2007; Wang and Li, 2007; Avril-Sassen et al., 2009; Sdassi et al., 2009; Tanaka et al., 2009; Ucar et al., 2010). A direct role for miRNA in the synthesis of a milk protein was recently evidenced with the identification of a conserved region in the 3′ UTR of the lactoferrin gene targeted by miR-214 (Liao et al., 2010).
As already mentioned, total or partial exon skipping during the splicing of the pre-mRNA is responsible for the differences observed between casein variants and between homologous proteins from different species. In this section, we will just describe variations that affect the overall level of gene expression. Deletion or transition of a single nucleotide within coding exons of a caprine αs1-casein allele and of two β-casein alleles creates nonsense codons (Leroux et al., 1992; Persuy et al., 1996; Rando et al., 1996). These alleles are characterized by a much lower level of mRNAs compared to other alleles and, for some of them, with multiple exon-skipping events. This phenomenon has been observed for several other genes where nonsense codons have been found to be associated with mRNA decay and/or exon skipping (see Valentine, 1998; Hentze and Kulozik, 1999, for reviews). Goat and bovine αs1-casein alleles, also associated with reduced amount of mRNA and milk protein, were found to contain a truncated inserted LINE element in the 3′UTR (Perez et al., 1994; Rando et al., 1998). Insertion of these elements is supposed to reduce mRNA stability.
14.4 Milk Protein Synthesis and Secretion
Secretion of milk protein is obviously of interest due to its physiological and economic importance but also from the point of view of the study of high-efficiency secretory pathways. The trafficking and processing events leading to the secretion of milk proteins are known in general outline, but relatively little is established of the molecular cell biology of milk protein transport in the secretory pathway of mammary epithelial cells.
14.4.1 Morphological Organization of the Mammary Secretory Epithelium
Functional differentiation of the mammary gland is linked to the development of its epithelial tissues. From the onset of pregnancy, the duct cells enter a proliferation and differentiation period leading to the development of a highly branched ductal tree which fills the entire mammary fat pad. Alveolar structures, or acini, develop at the ends of the side branches, and terminal differentiation of the alveolar mammary epithelial cells is completed at the end of gestation with the start of milk secretion at parturition. Functional acini are embedded in a stroma composed of connective and adipose tissues, fibroblasts, plasma cells, blood vessels and nerve terminals. These morphological aspects are well documented at http://mammary.nih.gov/
The acini consist of a single layer of cuboidal mammary epithelial secretory cells sealed together at the luminal border by tight junctions so that passage of molecules from the interstitial space to the lumen of the acini by a paracellular route is restricted during lactation, confining such molecular movements to the cellular trans-cytotic pathway. Progenitor cells are believed to reside below the monolayer of luminal cells. Contractile myoepithelial cells that have long spidery processes embrace the alveoli secretory cells. They participate in milk ejection by squirting milk out of the acini lumen into the ducts. Finally, these cells that constitute the acini are separated from the interstitial space by a basement membrane like in any epithelial tissue.
14.4.2 The Functional Compartmentalisation of the Biosynthetic-Secretory Pathway of Mammary Epithelial Cells
During lactation, mammary epithelial cells are highly polarized and display features typical of cells specialized for secretion (Bargmann and Knoop, 1959; Wooding, 1977; Pitelka and Hamamoto, 1983; illustrated in Figs. 14.4 and 14.5). Their cytoplasm contains an extended network of numerous parallel lamellar cisternae and branching tubules decorated with electron-dense ribosomal particles: the rough endoplasmic reticulum (ER). As described in other polarized epithelial cells, the Golgi apparatus is typically located in the peri- and supranuclear region of the cell, close to the centrosome. The Golgi cisternae are well developed, more or less distended, often containing electron-dense particles and filamentous materials. Of note, unusually large vesicles are observed on the trans side of the Golgi apparatus. It is not clear whether these dilated structures constitute the trans-Golgi network (TGN) or represent newly formed secretory vesicles en route to the apical plasma membrane (APM). In addition, numerous smaller vesicles, some of them coated with the typical spike structure characteristic of clathrin, are associated with the trans side of the Golgi. Secretory vesicles that have pinched off from the TGN contain filamentous structures and casein micelles similar to those found in milk, in an electron-lucent fluid.
The intracellular compartments of the mammary epithelial cell involved in the biosynthesis and secretion of milk proteins and lipids. Milk proteins undergo various co- and post-translational modifications during their translocation into the lumen of the rough endoplasmic reticulum (RER) and further transport to the apical cell surface, via the Golgi apparatus. Casein self-association starts in the lumen of the RER and proceeds into long loose linear aggregates in the trans-Golgi compartments and early secretory vesicles (SV). They further self-associate before release, and structures with the characteristic morphological aspect of casein micelles are found in distal SV (see Fig 14.5). Casein-containing SV reach and fuse with the apical plasma membrane (APM) by exocytosis (pathway A). Microlipid droplets (MLDs) emerge from the RER and may fuse with each other and with larger cytoplasmic lipid droplets (CLDs) as they are transported to the apex of the mammary epithelial cells. CLDs and caseins secretion may be partly (pathway B) or significantly (pathway C) coupled (dotted lines indicate that membrane of the casein vesicles has fused together) since the budding of the CLDs requires considerable amounts of APM. Pathway B may only involve heterotypic fusion between the casein vesicles and the APM while pathway C may imply both homotypic between SVs and heterotypic fusion of SVs with the APM. Alternatively, CLDs may be secreted independently of casein-containing SV (pathway D), and some MLDs may also be directly secreted at the apical side of the MECs (not shown). Basal plasma membrane (BPM), cis-Golgi network (CGN), ER-Golgi intermediate compartment (ERGIC), lipid droplet (LD), milk fat globule (MFG), tight junction (TJ), trans-Golgi network (TGN). Redrawn from Chat et al. (2011) (Courtesy of S. Truchet)
Transmission electron micrograph of part of a mouse mammary epithelial cell during lactation. The major compartments of the secretory pathway are illustrated. Filamentous material is found in dilated Golgi cisternae and in secretory vesicles (SV) close to the trans-Golgi, whereas more condensated protein aggregates and typical casein micelles are present in more distal SV and in the lumen of the acini. ER, endoplasmic reticulum (Courtesy of S. Chat and C. Longin)
14.4.3 Intracellular Transport and Co- and Post-translational Modifications of Milk Proteins
In mammary epithelial cells, transport of newly synthesized proteins destined for secretion proceeds according to the general biosynthetic-secretory pathway (Palade, 1975). Following a 3-min pulse labelling with a radioactive amino acid, newly synthesized proteins are detectable in the ER 5 min after the beginning of the pulse, concentrated in the Golgi region after 15 min, accumulated in apical secretory vesicles after 45 min and are predominantly located in the lumen of the acini after 60 min (Seddiki and Ollivier-Bousquet, 1991).
14.4.3.1 Translocation into and Transport from the Endoplasmic Reticulum
Milk proteins are of two types: transmembrane proteins and water-soluble proteins. An example of milk transmembrane protein is butyrophilin which is first targeted to the plasma membrane from which it is released into milk as part of the milk fat globule (MFG) membrane. The vast majority of milk proteins, however, are water-soluble proteins, for example, the caseins. Entry into the ER requires a signal sequence (Blobel and Dobberstein, 1975). Translocation of milk protein polypeptide chains into the ER lumen and subsequent cleavage of their signal sequence involve classical mechanisms (Schatz and Dobberstein, 1996).
Once translocated into the ER lumen, proteins are in an oxidizing environment which promotes the formation of disulfide bonds between cysteine residues. Most proteins synthesized in the rough ER are glycoproteins. They are modified by a common oligosaccharide on target asparagines residues (N-glycosylation). N-linked oligosaccharides are added co-translationally and serve as tags to monitor the state of protein folding. The proper folding of proteins that are made in the ER is assisted by proteins with chaperone activities, including BiP/GRP78, Erp77, calnexine and calreticulin (Pelham, 1989). These ER-resident proteins bind to misfolded newly synthesized proteins and retain them in the ER until they have achieved their properly folded or oligomeric state. Correct protein folding or oligomerisation is prerequisite for protein export from the ER and transport to the Golgi complex. This process has been named “quality control” (Hammond and Hellenius, 1995). Accumulation of improperly folded proteins in the ER causes a stress which triggers a coordinated adaptive programme called the unfolded protein response (UPR).
Elucidation of the secondary structure of α-lactalbumin and β-lactoglobulin has revealed the presence of four and two disulfide bridges, respectively (Brew et al., 1970; Papiz et al., 1986). As to WAP, it contains numerous cysteine residues which form multiple intramolecular disulfide bonds (Hennighausen and Sippel, 1982, Devinoy et al., 1988). Moreover, native WAP is dimeric (Baranyi et al., 1995). Analysis of the primary structure of κ-casein reveals the very high degree of conservation of at least one cysteine residue in the N-terminal domain of the protein (Bouguyon et al., 2006). Dimers of κ-casein were found in all milk studied, and interchain disulfide bridges were also found for any casein possessing a cysteine residue (Bouguyon et al., 2006), for example, in mouse and rat which express four or five cysteine-containing caseins, respectively. As expected, disulphide bond formation between casein molecules was demonstrated to occur within the ER lumen (Le Parc et al., 2010). Finally, N-glycosylation of rat α-lactalbumin has been detected in mammary microsomal membranes (Lingappa et al., 1978). Although N-glycosylated forms of α-lactalbumin have been observed in several species, only rat α-lactalbumin is efficiently glycosylated (Prasad et al., 1979; Chanat, 2006).
Caseins are not N-glycosylated and were shown to lack appreciable amount of regular secondary structure. β- and κ-caseins might possess premolten or molten globule conformations whereas αS1- and αS2-caseins are intrinsically unstructured proteins (Farrell et al., 2006; see Chap. 5) or natively unfolded proteins (Holt and Sawyer, 1993). The characteristic structural feature of natively unfolded proteins is a combination of low mean hydrophobicity and relatively high proportion of charged residues at physiological pH (Uversky et al., 2000). Proteins with such an open structure possess a peculiar aggregative behaviour and are prone to interact with their specific ligand in vivo. The caseins, however, do not fulfil these two criteria since they present relatively high hydrophobicities.
To date, the question whether caseins are subjected to the ER quality control machinery has not been directly addressed. Moreover, whether or not soluble luminal cargo proteins need to be concentrated and/or require intrinsic sorting information for loading into COPII-coated transport carriers at the ER exit sites to move to the Golgi apparatus is still controversial (for review see Lee et al., 2004). Some secretory proteins seem to enter a transport carrier without positive selection by a signal-independent mechanism known as “bulk flow” (Rothman and Wieland, 1996). Finally, as stated above, subunit oligomerisation is required for export of multimeric proteins from the ER (Copeland et al., 1986; Kim and Arvan, 1991). Whether interaction between at least some of the caseins in the ER lumen is a prerequisite for their forward transport to the Golgi apparatus is not clear. However, investigation of the impact of the polymorphism at the αs1-casein locus on goat milk secretion has shown that, in the absence of αS1-casein, other caseins accumulate in the ER (Chanat et al., 1999). The efficiency of casein transport from the ER to the Golgi apparatus was strongly affected in this context. Data suggested that interaction of caseins in a yet-to-be-identified structure including αS1-casein is required for efficient transport of these proteins to the Golgi apparatus. More recently, the existence of a membrane-associated form of αS1-casein in the ER and more distal compartments of the secretory pathway of mammary epithelial cells has been reported (Le Parc et al., 2010), further suggesting a key role of αS1-casein in casein transport in the biosynthetic pathway.
From the above considerations, it is now obvious that first interactions between the caseins take place in the ER and that the exit of casein from this compartment is a key step in casein micelle biogenesis and casein transport in the secretory pathway.
14.4.3.2 Transport Through the Golgi Apparatus
Protein transport through the Golgi apparatus may occur according to the vesicular transport model (stable Golgi cisternae) or the cisternal maturation model (Glick and Malhotra, 1998). Many lines of evidence now support this later model in which the Golgi cisternae themselves move through the Golgi stack. The observation that electron-dense structures, most likely casein aggregates, are detectable in Golgi cisternae of lactating mammary epithelial cells but are excluded from Golgi-associated vesicles is in agreement with this cisternal maturation model (Clermont et al., 1993).
Golgi enzymes carry out protein modifications that include glycosylation, phosphorylation, sulphation and proteolytic processing (Fig. 14.4). In mammary epithelial cells, glycosyltransferases are notably involved in the O-glycosylation of κ-casein. Moreover, galactosyltransferase, with the help of α-lactalbumin, is also responsible for the synthesis of lactose (Ebner and Brodbeck, 1968). Galactosyltransferase activity has been detected within the Golgi apparatus but also in secretory vesicles and milk (Witsell et al., 1990; Boisgard and Chanat, 2000). The synthesis of lactose in the trans-most cisternae of the Golgi apparatus, and most likely in secretory vesicles, surely explains the swollen aspect of these organelles in mammary epithelial cells. The striking reduction of the volume of casein-containing secretory vesicles in α-lactalbumin-deficient mice is in agreement with this hypothesis (Stinnakre et al., 1994). Phosphorylation of the calcium-sensitive caseins on serine clusters allows calcium phosphate binding and further interactions between caseins. Like in other cell systems, the kinases that phosphorylate the caseins are located within the Golgi apparatus (Bingham and Farrell, 1974; West and Clegg, 1984). Notably, the phosphorylation of β-casein seems delayed compared to that of αS1-casein (Turner et al., 1993; Boisgard and Chanat, 2000; Péchoux et al., 2005). This suggests that strong interaction of β-casein with casein polymers might be postponed until it is trafficked to trans-Golgi cisternae (see below). Protein sulphation is a ubiquitous TGN-specific post-translational modification. Beside sulphated proteoglycans (Boisgard et al., 1999), the sulphation of both α-lactalbumin and κ-casein from rat has been described (Chanat, 2006). One the other hand, although no direct evidence for the cleavage of milk proteins by endoproteases has been reported, furin has been shown to be relatively abundant in Golgi-derived clathrin-coated vesicles from lactating rabbit mammary epithelial cells (Pauloin et al., 1999).
14.4.3.3 Transport from the trans-Golgi Network and Secretory Vesicle Exocytosis
Secretory proteins are segregated, highly concentrated and packaged into appropriate transport vesicles in the TGN. Sorting and concentration are believed to involve the selective aggregation of the secretory proteins in the ionic environment of the TGN (Chanat and Huttner, 1991), as well as retrieval of excess membrane and luminal content present in newly formed secretory vesicles in clathrin-coated vesicles (Tooze, 1998).
Pre-micellar aggregates have been observed by electron microscopy in the lumen of the Golgi cisternae (Clermont et al., 1993). Consistent with the presence of a high calcium concentration in the trans-most Golgi cisternae (Neville and Watters, 1983), there is a drastic rearrangement of the micellar structure during the formation of secretory vesicles at the TGN and their transport to the APM for exocytosis. In newly formed transport vesicles, caseins are concentrated in the form of long loose linear aggregates (Mather and Keenan, 1983; Clermont et al., 1993). These progressively self-associate and become bigger and denser, and structures with the characteristic honeycomb texture of casein micelles from milk are found in distal secretory vesicles (see Fig. 14.5). The mean size of casein micelles varies widely across species, and the relative proportion of κ-casein was demonstrated to be a modulator of micelle size (Gutierrez-Adan et al., 1996). Noteworthy, casein micelles, or at least big aggregates, still form in mammary epithelial cells from of αS1-, β- or κ-casein-deficient animals (Kumar et al., 1994; Chanat et al., 1999; Shekar et al., 2006). Interactions between the various caseins and minerals during micelle biogenesis in the secretory pathway might therefore involve rather general physico-chemical and biochemical characteristics of these components. However, these characteristics are specific enough to avoid incorporation of whey proteins in the micelles. On the other hand, casein-containing secretory vesicles might undergo maturation before exocytosis (Pauloin et al., 1999). Finally, reports support the notion that secretory vesicles destined to fuse with the apical cell surface transport both caseins and whey proteins (Devinoy et al., 1995; Neville et al., 1998; Ollivier-Bousquet, unpublished observation).
A large body of evidence supports the concept that local modifications of the lipid composition of membranous sub-domains by lipid-modifying enzymes also contribute to vesicular traffic. In line with this, phospholipase D and calcium-independent phospholipase A2 were reported to be involved in both the transport of milk proteins from the ER to the Golgi and in the formation of secretory vesicles from the TGN (Boisgard and Chanat, 2000; Péchoux et al., 2005), as was observed in other cell systems (Riebeling et al., 2009; Schmidt et al., 2010).
Trafficking steps within the secretory pathway of mammary epithelial cells and exocytosis of casein-containing vesicles with the plasma membrane might involve SNARE (soluble N-ethylmaleimide-sensitive fusion (NSF) attachment protein (SNAP) receptor) proteins, as already described in other cell types (Sollner et al., 1993; Jahn and Scheller, 2006). To date, however, only a few studies have directly addressed the functions of SNARE proteins in mammary epithelial cells. One of these suggests that VAMP-8 (vesicle-associated membrane protein 8) may be involved in casein secretion (Wang et al., 2007). On the other hand, SNAP-23 (synaptosomal-associated protein 23), syntaxin-3 and syntaxin-5, and Ykt6 have been described as being associated with lipid droplets (Boström et al., 2007; Reinhardt and Lippolis, 2008). SNAP-23 is believed to play a role in homotypic fusion of intracellular lipid droplets. Moreover, the large amount of membrane necessary for MFG secretion by budding of the APM could be partly provided by exocytosis of casein-containing vesicles (Mather and Keenan, 1998; see Fig. 14.4). Recently, the endogenous expression levels of a large set of SNAREs were investigated in mouse mammary gland (Chat et al., 2011). This study points to SNAP-23 as a potential central player for the coupling of casein and MFG secretion during lactation.
14.4.4 Hormonal Regulation of Milk Protein Secretion
Proteins destined for the cell exterior are secreted by either the constitutive or the regulated secretory pathway (Glombik and Gerdes, 2000; Morvan and Tooze, 2008). The fact that there is no substantial storage of newly synthesized milk proteins in mammary epithelial cells does not support the later hypothesis (Devinoy et al. 1995; Pauloin et al. 1997). On the other hand, hormones including oxytocin and prolactin are able, at least in vitro, to increase casein secretion (secretagogue effect) in mammary epithelial cells from rabbits and rodents (see Ollivier-Bousquet, 1993, 1997). Prolactin seems to act on a late step of casein trafficking, possibly exocytosis. In contrast, oxytocin was reported to also accelerate the transport of newly synthesized proteins from the ER to the Golgi apparatus and to secretory vesicles (Lollivier et al., 2006).
14.5 Amino Acid Transport by the Mammary Gland
Amino acids, extracted from interstitial fluid, are the major source of amino nitrogen for milk protein synthesis; therefore, a knowledge of mammary tissue amino acid transport mechanisms and their regulation is important if we are to understand fully the process of milk protein secretion. Such knowledge will help those wishing to manipulate milk protein content via dietary means. The uptake of amino acids across the basolateral membranes of mammary secretory cells, the major point of entry, is accomplished by an array of distinct transport mechanisms. The amino acid transporters differ from one another with respect to kinetics, substrate specificity and ion dependency; however, it is evident that they operate in a coordinated fashion to supply amino nitrogen to support milk protein synthesis.
The study of mammary epithelial amino acid transport is hampered by the relatively complex anatomy of the mammary gland. Nevertheless, the use of mammary tissue explants and the perfused mammary gland has enabled the transport of amino acids (using radiotracers) by mammary tissue to be characterized. Mammary explants are easy to prepare and have the advantage that the cellular architecture remains intact. Furthermore, the preparation of mammary explants does not require digestive agents: enzymes such as collagenase could ultimately alter the properties of membrane transport proteins. Although mammary tissue explants isolated from lactating animals are comprised of more than one cell type, it can be assumed that the vast majority of the surface area of explants is that of the basolateral membrane of the secretory cells. Therefore, it is reasonable to assume that mammary tissue explants can be used to give a measure of amino acid transport across the blood-facing side of the mammary epithelium. The large tissue extracellular space associated with mammary explants does, however, place limitations on the design of experiments. The perfused mammary gland allows the transport of amino acids across the blood-facing aspect of the mammary epithelium to be measured under near physiological conditions: perfusates can be delivered to the gland with a flow and pressure profile similar to that found in vivo. The perfused mammary gland used in combination with a rapid, paired-tracer dilution technique allows the transport of amino acids to be measured over very short time courses (Mepham et al., 1985; Calvert and Shennan 1996).
14.5.1 Mammary Tissue Amino Acid Transport Systems
The identification of individual amino acid transport systems is difficult on account of the fact that a single amino acid may be able to utilize several transport systems (see Barker and Ellory 1990). Furthermore, the difficulty in characterizing amino acid transport is compounded by the lack of specific inhibitors of amino acid transporters. In spite of these drawbacks, significant progress has been made towards identifying mammary tissue amino acid transport systems together with their putative molecular correlates. Mammary tissue amino acid transport mechanisms fall into two categories: Na+-dependent and Na+-independent systems.
14.5.1.1 Na+-Dependent Transport Mechanisms
Lactating mammary cells are able to concentrate free amino acids (particularly the non-essential ones) with respect to plasma, suggesting that there must be an input of free energy (Shennan et al., 1997). It is apparent that several amino acid transport systems utilize the electrochemical Na+ gradient to drive the movement of amino acids into mammary cells. The Na+-dependent mechanisms which have been identified in mammary tissue include systems A, ASC, X -AG and β.
System A prefers short-chain neutral amino acids as substrates and is characterized by its tolerance of N-methylated amino acids such as N-methylaminoisobutyrate (MeAIB). Indeed, Na+-dependent amino acid uptake inhibited by MeAIB is usually taken as a measure of transport via system A. It appears that mouse, rat and bovine mammary tissue possesses system A activity (Neville et al., 1980; Baumrucker 1984; Verma and Kansal 1993; Shennan and McNeillie 1994a). Lopez et al. (2006) provided convincing evidence that SNAT2 may be the molecular correlate of system A in rat mammary tissue. In contrast, no evidence for the presence of system A at the blood-facing aspect of the guinea pig mammary gland could be found (Mepham et al., 1985). The limited kinetic data available suggest that system A in lactating mammary tissue operates with relatively low affinity: the KM of methionine and α-aminoisobutyric acid uptake via system A in mouse mammary tissue is 0.47 mM and 2.0 mM, respectively (Neville et al., 1980; Verma and Kansal, 1993). Several lines of evidence suggest that system A is regulated by milk stasis, by starvation and by the stage of lactation (Neville et al., 1980; Shennan and McNeillie 1994c; Verma and Kansal 1995). In accordance with these findings, SNAT2 mRNA expression, respectively, increases and decreases during lactation and weaning. Moreover, the abundance of SNAT2 mRNA is increased by oestrogen which may explain the high levels observed during pregnancy (Lopez et al., 2006). System A activity is regulated by prolactin: treating animals with bromocriptine, a drug which inhibits prolactin secretion from the pituitary gland, markedly reduces the arteriovenous concentration differences of amino acids which are potential substrates of the A system (Vina et al., 1981).
Mammary tissue from several species (e.g., bovine, mouse, guinea pig) has been shown to posses system ASC (Baumrucker 1985; Mepham et al., 1985; Verma and Kansal 1993). It has been established in other tissues that this mechanism cotransports neutral amino acids such as alanine, threonine and cysteine with Na+. System ASC in mammary tissue, like system A, operates with relatively low affinity: the K M of methionine uptake by lactating mouse mammary tissue via system ASC is 0.46 mM (Verma and Kansal, 1993). At present, there appears to be a paucity of information on the regulation of mammary tissue amino acid transport via system ASC except for the finding that starvation gives rise to a large increase in system ASC activity in mouse mammary tissue (Verma and Kansal, 1995). There are at least two molecular isoforms of system ASC, both of which have been detected in mammary tissue. Thus, the expression of ASCT1 and ASCT2 mRNA has respectively been described in lactating rat and porcine mammary tissue (Aleman et al., 2009; Laspiur et al., 2009). The expression of both transcripts increases with the onset of lactation.
The transport of anionic amino acids by lactating mammary tissue has been extensively studied (Millar et al., 1996, 1997a, b). The predominant, if not the only, pathway for L-glutamate and L-aspartate transport is a high-affinity (K M = 18 μM for L-glutamate), Na+-dependent system analogous to system X -AG (Kanai et al., 1994). The Na+-dependent anionic amino acid carrier is very selective for anionic amino acids: it does not interact with neutral or cationic amino acids (Millar et al., 1996, 1997b). An unusual feature of system X -AG is the ability to discriminate between the opical isomers of glutamate but not those of aspartate. The high-affinity anionic amino acid carrier is able to act as an exchange system as well as a cotransport mechanism suggesting that the transport of L-glutamate will affect the intracellular concentration of L-aspartate (and vice versa) (Millar et al., 1997b). Several high-affinity anionic amino acid carriers, which have identity with system X-AG, have been cloned and characterized (e.g., see Kanai and Hediger, 1992; Pines et al., 1992; Storck et al., 1992). Three clones, EAAC1, GLAST and GLT-1, have been identified in rat mammary tissue (Martinez-Lopez et al., 1999; Aleman et al., 2009). However, the exact contribution of these isoforms to anionic amino acid transport across the basolateral membranes of mammary secretory cells is unknown.
Taurine, a nonprotein amino acid, is taken up by lactating rat and porcine mammary tissue via a high-affinity, Na+-dependent transport system analogous to system β (Shennan and McNeillie, 1994b; Bryson et al., 2001). The mammary (Na++taurine) cotransport system also requires Cl- for maximal activity. System β has narrow substrate specificity: only β-amino acids such as taurine and β-alanine interact with the transporter. The activity of the rat mammary (Na+-Cl--taurine) cotransporter decreases as lactation progresses (Millar and Shennan 1999). In accordance with this, Aleman et al. (2009) have reported that the expression of rB16 (a cloned taurine transporter) mRNA decreases between early and peak lactation. The lactating gerbil mammary gland expresses a high-affinity (Na+-taurine) cotransport system which, unlike the rat mammary taurine transporter, is not dependent upon Cl- (Shennan, 1995).
14.5.1.2 Na+-Independent Transport Mechanisms
System L has been identified in mouse, rat, guinea pig and bovine mammary tissue (Neville et al., 1980; Mepham et al., 1985; Verma and Kansal, 1993; Shennan and McNeillie, 1994c). System L is a Na+-independent transport mechanism that has wide substrate specificity. Indeed, system L may be the most important transport system for the uptake of essential neutral amino acids by the lactating mammary gland. Na+-independent amino acid transport sensitive to 2-aminobicyclo-heptane-2-carboxylic acid (BCH) is taken as a measure of system L activity. It is generally accepted that system L can act as an amino acid exchange mechanism. Accordingly, methionine uptake by mouse mammary gland via system L can be trans-accelerated by intracellular amino acids. However, amino acid efflux from rat mammary tissue, via system L, is not trans-stimulated by extracellular amino acids, suggesting that the transporter operates with asymmetric kinetics which could favour the retention of substrates within the gland. System L has been localized to the basolateral aspect of the lactating rat mammary gland (Shennan et al., 2002). There are at least four molecular isoforms of system L (LAT 1–4), two of which have been described in the mammary gland. Thus, LAT1 and LAT2 mRNA are expressed in the rat mammary gland (Shennan et al., 2002; Aleman et al., 2009). Interestingly, Aleman et al. (2009) have shown that LAT1 mRNA expression in rat mammary tissue markedly increases during lactation. LAT mRNA is also expressed in the mouse and bovine mammary gland (Rudolph et al., 2007; Finucane et al., 2008; Connor et al., 2008). The expression of LAT1 mRNA increases during the transition from pregnancy to lactation and is also up-regulated by milking frequency.
The transport of cationic amino acids by lactating mammary tissue is a Na+-independent process (Baumrucker 1984; Shennan et al., 1994b; Hurley et al., 2000). The pathway for lysine and arginine uptake by bovine mammary tissue is not affected by replacing extracellular Na+ and appears to be relatively specific for cationic amino acids. On this basis Baumrucker (1984) concluded that cationic amino acids are transported via system y+. There is evidence suggesting that CAT-1 may be responsible for system y+ activity in rat mammary tissue (Aleman et al., 2009). CAT-1 mRNA expression is low in mammary tissue isolated from pregnant rats and increases with the onset of lactation (Aleman et al., 2009). Transcripts for two cloned Na+-independent cationic amino acid transporters, CAT-1 and CAT-2B, have been detected in lactating porcine tissue (Laspiur et al., 2009), However, it appears that the expression of both transcripts is relatively unaffected by the stage of lactation. Cationic amino acid uptake by lactating rat mammary tissue is also facilitated by a transporter which interacts with neutral amino acids such as glutamine and leucine (Shennan et al., 1994a; Calvert and Shennan 1996). Thus, certain neutral amino acids respectively inhibit and stimulate lysine uptake by and efflux from rat mammary tissue. It is likely that this pathway is y+L: y + LAT1 mRNA has been detected in lactating rat mammary tissue (Boyd, Kudo and Shennan, unpublished).
System T, a mechanism specific for aromatic amino acids, has been described in lactating mouse mammary tissue (Kansal and Kansal, 1996). Thus, the moiety of tyrosine uptake by mouse mammary tissue which is not dependent upon extracellular Na+ and is not sensitive to BCH has been ascribed to system T. This mechanism operates with low affinity; the K M for tyrosine transport is approximately 15 mM.
14.5.1.3 Volume-Activated Amino Acid Transport
To survive, cells have to regulate their volume within relatively narrow limits (see Hoffmann and Simonsen, 1989, for a review). Cell membranes are very permeable to water which means that cell volume, otherwise termed the cellular hydration state, will be determined by the osmolarity of the extracellular fluid and by the intracellular content of osmotically active solutes. The cellular hydration state can be changed as a consequence of anisosmotic conditions, cellular accumulation of solutes or oxidative metabolism. Cells are able to regulate their volume following swelling or shrinking. Cell volume regulation involves the transmembrane movement of solutes together with osmotically obliged water (Hoffmann and Simonsen, 1989).
A knowledge of volume-regulatory processes in mammary tissue is of particular importance given that cell volume changes markedly affect the rate of milk protein synthesis (Millar et al., 1997a). It has been established that cell swelling activates the transport of amino acids in mammary tissue (Shennan et al., 1994; 1996). Thus, cell swelling, induced by a hypoosmotic shock, increases the efflux of amino acids such as taurine and glycine via a pathway which has the characteristics of a channel rather than a carrier. The swelling-induced amino acid efflux pathway appears to be situated in the blood-facing aspect of the mammary epithelium (Calvert and Shennan, 1998). The volume-activated efflux of taurine is dependent upon the extent of cell swelling and can be inhibited by a number of pharmacological agents such as niflumic acid (Shennan et al., 1996). There is the strong possibility that volume-activated amino acid efflux may participate in mammary cell volume regulation given that amino acids are concentrated within mammary cells with respect to plasma.
14.5.2 Transport and Metabolism of Peptides
Although free amino acids are the major source of amino nitrogen for milk protein synthesis, it is evident that the uptake of several essential amino acids is less than their output in milk, suggesting that other circulating forms of amino acids, such as peptides, may be available for casein production (e.g., see Backwell et al., 1996). In this connection, it has been demonstrated that the goat mammary gland is able to use intravenously administered peptides for milk protein synthesis (Backwell et al., 1994 , 1996). The in vivo studies were unable to show whether the mammary gland transported peptides intact or whether the peptides were hydrolysed extracellularly followed by uptake of the liberated amino acids. It has been shown, albeit indirectly, that the mouse mammary tissue does not significantly hydrolyse peptides extracellularly but is able to transport peptides intact (Wang et al., 1996). On the other hand, studies using the rat mammary gland as a model suggest that mammary tissue is able to both transport and hydrolyse dipeptides (Shennan et al., 1998 , 1999). It is evident that the perfused lactating rat mammary is able to transport dipeptides which are resistant to hydrolysis (i.e., D-Phe-L-Gln; D-Phe-L-Glu); the nature of the pathway remains to be identified precisely. However, it is apparent that the capacity of the rat mammary gland to transport peptides intact across the basolateral pole of the epithelium is relatively limited (Shennan et al., 1998). Transcripts for two proton-dependent peptide transporters, namely, PepT1 and PepT2, have been identified in lactating rat and human mammary epithelial cells (Alcorn et al., 2002; Groneberg et al., 2002; Gilchrist and Alcorn, 2010). PepT2 protein is localized in the apical membrane of rat ductal epithelial cells and is therefore not in a position to facilitate the uptake of peptides from the circulation. Rather, it is likely that PepT2 provides a route for the reuptake of peptides from milk as postulated by Shennan and Peaker (2000).
Rat mammary tissue is able to extensively hydrolyse peptides extracellularly: this has been demonstrated using both direct and indirect experimental approaches (Shennan et al., 1998; 1999). Anionic dipeptides presented to rat mammary tissue can trans-stimulate D-aspartate efflux via the high-affinity anionic amino acid carrier. If it is accepted that dipeptides do not interact directly with the amino acid carrier, then it can be assumed that anionic amino acids, produced as a consequence of extracellular hydrolysis, act to stimulate D-aspartate efflux. In this connection, it has been shown that mammary tissue is capable of hydrolysing a variety of aminoacyl-p-nitroanilides (peptide analogues). Quantitatively, it appears, at least in the rat, that hydrolysis of peptides followed by uptake of the free amino acids may be more important than the transport of peptides (Shennan et al., 1998).
The identity of the peptidases involved in the extracellular hydrolysis of peptides by mammary tissue has not been established. However, γ-glutamyltranspeptidase appears to be involved given that glutathione can stimulate D-aspartate efflux from rat mammary tissue in a fashion sensitive to acivicin (Shennan et al., 1998). The hydrolysis of glutathione may be an important route for providing the mammary gland with cysteine. There is evidence to suggest that aminopeptidase N plays a role in providing free amino acids for protein synthesis in the goat mammary gland (Liu et al., 2010).
References
Adachi, T., Ahn, J.Y., Yamamoto, K., Aoki, N., Nakamura, R. and Matsuda, T. (1996). Characterization of the bovine kappa-casein gene promoter. Biosci. Biotech. Biochem. 60, 1937–1940.
Aggeler, J., Park, C.S. and Bissell, M.J. (1988). Regulation of milk protein and basement membrane gene expression: the influence of the extracellular matrix. J. Dairy Sci. 71, 2830–2842.
Alcorn, J., Moscow, J.A. and McNamara, P.J. (2002). Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J. Pharmacol. Ther. 303, 487–496.
Aleman, G., Lopez, A., Ordaz, G., Torres, N. and Tovar, A.R. (2009). Changes in messenger RNA abundance of amino acid transporters in rat mammary gland during pregnancy, lactation and weaning. Metabolism 58, 594–601.
Alexander, L.J., Hayes, G., Bawden, W., Stewart, A.F. and MacKinlay, A.G. (1993). Complete nucleotide sequence of the bovine beta-lactoglobulin gene. Anim. Biotechol. 4, 1–10.
Alexander, L.J., Stewart, A.F., MacKinlay, A.G., Kapelinskaya, T.V., Tkach, T.M. and Gorodetsky, S.I. (1988). Isolation and characterization of the bovine kappa-casein gene. Eur. J. Biochem. 178, 395–401
Ali, S. and Clark, J. (1988). Characterization of the gene encoding ovine beta-lactoglobulin. Similarity to the genes for retinol binding protein and other secretory proteins. J. Mol. Biol. 199, 415–426
Altiok, S. and Groner, B. (1993). Interaction of two sequence-specific single-stranded DNA-binding proteins with an essential region of the beta-casein promoter is regulated by lactogenic hormones. Mol. Cell. Biol. 13, 7303–7310.
Altiok, S. and Groner, B. (1994). Beta-casein mRNA sequesters a single stranded nucleic acid-binding protein which negatively regulates the beta-casein gene promoter. Mol. Cell. Biol. 14, 6004–6012.
Attal, J., Cajero-Juarez, M., Petitclerc, D., Theron, M.C., Stinnakre, M.G., Bearzotti, M., Kann, G. and Houdebine, L.M. (1995). The effect of matrix attached regions (MAR) and specialized chromatin structure (SCS) on the expression of gene constructs in cultured cells and transgenic mice. Mol. Biol. Rep. 22, 37–46.
Avril-Sassen, S., Goldstein, L.D., Stingl, J., Blenkiron, C., Le Quesne, J., Spiteri, I., Karagavriilidou, K., Watson, C.J., Tavaré, S., Miska, E.A. and Caldas, C. (2009). Characterisation of miRNA expression in post-natal mouse mammary gland development. BMC Genomics 10, 548
Backwell, F.R.C., Bequette, B.J., Wilson, D., Calder, A.G., Metcalf, J.A., Wray Cahen, D., MacRae, J.C., Beever, D.E. and Lobley, G.E. (1994). Utilization of dipeptides by the caprine mammary-gland for protein-synthesis. Am. J. Physiol. 267, R1–R6.
Backwell, F.R.C., Bequette, B.J., Wilson, D., Metcalf, J.A., Franklin, M.F., Beever, D.E., Lobley, G.E. and MacRae, J.C. (1996). Evidence for the utilization of peptides for milk protein synthesis in the lactating dairy goat in vivo. Am. J. Physiol. 271, R955–R960.
Baranyi, M., Aszodi, A., Devinoy, E., Fontaine, M.L., Houdebine, L.M. and Bosze, Z. (1996). Structure of the rabbit kappa-casein encoding gene: expression of the cloned gene in the mammary gland of transgenic mice. Gene 174, 27–34.
Baranyi, M., Brignon, G., Anglade, P. and Ribadeau-Dumas, B. (1995). New data on the proteins of rabbit (Oryctolagus cuniculus) milk. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 111, 407–415.
Barash, I. (2006). Stat5 in the mammary gland: controlling normal development and cancer. J. Cell Physiol. 209, 305–313
Bargmann, W. and Knoop, A. (1959). Morphology of lactation; light and electro-microscopic studies on the mammary glands of rats. Z. Zellforsch. Mikrosk. Anat. 49, 344–388.
Barker, G.A. and Ellory, J.C. (1990). The identification of neutral amino acid transport systems. Exp. Physiol. 75, 3–26.
Baumrucker, C.R. (1984). Cationic amino acid transport by bovine mammary tissue. J. Dairy Sci. 67, 2500–2506.
Baumrucker CR. (1985). Amino acid transport systems in bovine mammary tissue. J. Dairy Sci. 68(9), 2436–2451.
Bingham, E.W. and Farrell, H.M. Jr (1974). Casein kinase from the Golgi apparatus of lactating mammary gland. J. Biol. Chem. 249, 3647–3651.
Blobel, G. and Dobberstein, B. (1975). Transfer of proteins across membranes 1. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 67, 835–851.
Blum, J.L., Zeigler, M.E. and Wicha, M.S. (1989). Regulation of mammary differentiation by the extracellular matrix. Environ. Health Perspect. 80, 71–83.
Boisgard, R. and Chanat, E. (2000). Phospholipase D-dependent and -independent mechanisms are involved in milk protein secretion in rabbit mammary epithelial cells. Biochim Biophys Acta 1495, 281–296.
Boisgard, R., Charpigny, G. and Chanat, E. (1999). Polymeric IgA are sulfated proteins. FEBS Lett 463, 250–254.
Boisnard, M., Hue, D., Bouniol, C., Mercier, J.C. and Gaye, P. (1991). Multiple mRNA species code for two non-allelic forms of ovine alpha-s2-casein. Eur. J. Biochem. 201, 633–641
Bonsing, J., Ring, J.M., Stewart, A.F. and MacKinlay, A.G. (1988). Complete nucleotide sequence of the bovine beta-casein gene. Aust. J. Biol. Sci. 41, 527–537.
Boström, P., Andersson, L., Rutberg, M., Perman, J., Lidberg, U., Johansson, B.R., Fernandez-Rodriguez, J., Ericson, J., Nilsson, T., Borén, J. and Olofsson, S.-O. (2007). SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 9, 1286–1293.
Boucheron, C., Dumon, S., Santos, S.C., Moriggl, R., Hennighausen, L., Gisselbrecht, S. and Gouilleux, F. (1998). A single amino acid in the DNA binding regions of Stat5A and Stat5B confers distinct binding specificities. J. Biol. Chem. 273, 33936–33941.
Bouguyon, E., Beauvallet, C., Huet, J.C. and Chanat, E. (2006). Disulphide bonds in casein micelle from milk. Biochem. Biophys. Res. Commun. 343, 450–458.
Brew, K., Castellino, F.J., Vanaman, T.C., Hill, R.L. (1970). The complete amino acid sequence of bovine alpha-lactalbumin. J. Biol. Chem. 245, 4570–4582.
Bryson, J.M., Jackson, S.C., Wang, H. and Hurley, W.L. (2001). Cellular uptake of taurine by lactating porcine mammary gland. Comp. Biochem. Physiol. B . 128, 667–673.
Burdon, T.G., Maitland, K.A., Clark, A.J., Wallace, R. and Watson, C.J. (1994). Regulation of the sheep beta-lactoglobulin gene by lactogenic hormones is mediated by a transcription factor that binds an interferon gamma activation site-related element. Mol. Endocrinol. 8, 1528–1536.
Buser, A.C., Gass-Handel, E.K.., Wyszomierski, S.L., Doppler, W., Leonhardt, S.A., Schaack, J., Rosen, J.M., Watkin, H., Anderson, S.M. and Edwards, D.P. (2007). Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the beta-casein gene in mammary epithelial cells. Mol Endocrinol.1, 106–25.
Calvert, D.T. and Shennan, D.B. (1996). Evidence for an interaction between cationic and neutral amino acids at the blood-facing aspect of the lactating rat mammary epithelium. J. Dairy Res. 63, 25–33.
Calvert, D.T. and Shennan, D.B. (1998). Volume-activated taurine efflux from the in situ perfused lactating rat mammary gland. Acta Physiol. Scand. 162, 97–105.
Campbell, S.M., Rosen, J.M., Hennighausen, L.G., Strech-Jurk, U. and Sippel, A.E. (1984). Comparison of the whey acidic protein genes of the rat and mouse. Nucleic Acids Res. 12, 8685–8697
Chanat, E. (2006). Sulphated proteins secreted by rat mammary epithelial cells. Reprod Nutr Dev 46, 557–566.
Chanat, E. and Huttner, W.B. (1991). Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J. Cell. Biol. 115, 1505–1519.
Chanat, E., Martin, P. and Ollivier-Bousquet, M. (1999). αs1-Casein is required for the efficient transport of b- and k-casein from the endoplasmic reticulum to the Golgi apparatus of mammary epithelial cells. J. Cell Sci. 112 (Pt 19), 3399–3412.
Chat, S., Layani, S., Mahaut, C., Henry, C., Chanat, E. and Truchet, S. (2011). Characterisation of the potential SNARE proteins relevant to milk product release by mouse mammary epithelial cells. Eur. J. Cell. Biol. 90 , 401–413.
Clermont, Y., Xia, L., Rambourg, A., Turner, J.D. and Hermo, L. (1993). Transport of casein submicelles and formation of secretion granules in the Golgi apparatus of epithelial cells of the lactating mammary gland of the rat. Anat. Rec. 235, 363–373.
Collet, C. and Joseph, R. (1995). Exon organization and sequence of the genes encoding alpha-lactalbumin and beta-lactoglobulin from the Tammar Wallaby (Macropodidae, Marsupialia). Biochem. Genet. 33, 61–72.
Collet, C., Joseph, R. and Nicholas, K. (1990). Cloning, cDNA analysis and prolactin-dependent expression of a marsupial alpha-lactalbumin. Reprod. Fertil. Dev. 2, 693–701.
Connor, E.E., Siferd, S., Elsasser, T.H., Evock-Clover, C.M., Van Tassel, C.P., Sonstegard, T.S., Fernandes, V.M. and Capuco, A.V. (2008). Effects of increased milking frequency on gene expression in the bovine mammary gland. BMC Genomics 9, 362
Copeland, C.S., Doms, R.W., Bolzau, E.M., Webster, R.G. and Helenius, A. (1986). Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell. Biol. 103, 1179–1191.
Dale, T.C., Krnacik, M.J., Schmidhauser, C., Yang, C.L.Q., Bissell, M.J. and Rosen, J.M. (1992). High-level expression of the rat whey acidic protein gene is mediated by elements in the promoter and 3′ untranslated region. Mol. Cell. Biol. 12, 905–914.
Davies, M.S., West, L.F., Davis, M.B., Povey, S. and Craig, R.K. (1987). The gene for human alpha-lactalbumin is assigned to chromosome 12q13. Ann. Hum. Genet. 51, 183–188
Dawson, S.P., Wilde, C.J., Tighe, P.J. and Mayer, R.J. (1993). Characterization of two novel casein transcripts in rabbit mammary gland. Biochem. J. 15, 777–784.
Demmer, J., Burdon, T.G., Djiane, J., Watson, C.J. and Clark, A.J. (1995). The proximal milk protein binding factor binding site is required for the prolactin responsiveness of the sheep beta-lactoglobulin promoter in Chinese hamster ovary cells. Mol. Cell. Endocrinol. 107, 113–121.
Derbinski, J., Pinto, S., Rösch, S., Hexel, K. and Kyewski, B. (2008). Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proc. Natl. Acad. Sci. U.S.A. 105, 657–662
Devinoy, E., Hubert, C., Schaerer, E., Houdebine, L.M. and Kraehenbuhl, J.P. (1988). Sequence of the rabbit whey acidic protein cDNA. Nucleic Acids Res. 16, 8180.
Devinoy, E., Stinnakre, M.G., Lavialle, F., Thepot, D. and Ollivier-Bousquet, M. (1995). Intracellular routing and release of caseins and growth hormone produced into milk from transgenic mice. Exp. Cell Res. 221, 272–280.
Dobie, K.W., Lee, M., Fantes, J.A., Graham, E., Clark, A.J., Springbett, A., Lathe, R. and McClenaghan, M. (1996). Variegated transgene expression in mouse mammary gland is determined by the transgene integration locus. Proc. Natl. Acad. Sci. U.S.A. 93, 6659–6664.
Ebner, K. and Brodbeck, U. (1968). Biological role of alpha-lactalbumin: a review. J Dairy Sci 51, 317–322.
Edlund, A., Johansson, T., Leidvik, B. and Hansson, L. (1996). Structure of the human kappa-casein gene. Gene 174, 65–69.
Edwards, G.M., Wilford, F.H., Liu, X., Hennighausen, L., Djiane, J. and Streuli, C.H. (1998). Regulation of mammary differentiation by extracellular matrix involves protein-tyrosine phosphatases. J. Biol. Chem. 17, 9495–9500.
Eisenstein, R.S. and Rosen, J.M. (1988). Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol. Cell. Biol. 8, 3183–3190.
Faerman, A., Barash, I., Puzis, R., Nathan, M., Hurwitz, D.R. and Shani, M. (1995). Dramatic heterogeneity of transgene expression in the mammary gland of lactating mice: a model system to study the synthetic activity of mammary epithelial cells. J. Histochem. Cytochem. 43, 461–470.
Finucane, K.A., McFadden, T.B., Bond, J.P., Kenelly, J.J. and Zhao, F.-Qi. (2008). Onset of lactation in the bovine mammary gland: gene expression profiling indicates a strong inhibition of gene expression in cell proliferation. Funct. Integr. Genomics 8, 251–264.
Farrell, H. M. Jr., Malin, E.L., Brown, E.M. and Qi, P.X. (2006). Casein micelle structure: what can be learned from milk synthesis and structural biology? Curr. Opin. Coll. Interf. Sci. 11, 135–147.
Flower, D.R. (1996). The lipocalin protein family: structure and function. Biochem. J. 318, 1–14.
Folch, J.M., Coll, A. and Sanchez, A. (1994). Complete sequence of the caprine beta-lactoglobulin gene. J. Dairy Sci. 77, 3493–3497.
Folch, J.M., Coll, A., Hayes, H.C. and Sanchez, A. (1996). Characterization of a caprine beta-lactoglobulin pseudogene, identification and localization by in situ hybridization in goat, sheep and cow. Gene 24, 87–91.
Fujiwara, Y., Miwa, M., Nogami, M., Okumura, K., Nobori, T., Suzuki, T., Ueda, M. (1997). Genomic organization and chromosomal localization of the human casein gene family. Hum. Genet. 99, 368–373.
Fujiwara, Y., Miwa, M., Takahashi, R., Kodaira, K., Hirabayashi, M., Suzuki, T. and Ueda, M. (1999). High-level expressing YAC vector for transgenic animal bioreactors. Mol. Reprod. Dev. 52, 14–20.
Funder, J.W. (1989). Hormonal regulation of gene expression. Biochem. Soc. Symp. 55, 105–114.
Gallagher, D.S., Schelling, C.P., Groenen, M.M.A. and Womack, J.E. (1994). Confirmation that the casein gene cluster resides on cattle chromosome 6. Mamm. Genome 5, 524.
Gallagher, D.S., Treadgill, D.W., Ryan, A.M., Womack, J.E. and Irwin, D.M. (1993). Physical mapping of the lysozyme gene family in cattle. Mamm. Genome 4, 368–373.
Geissler, E.N., Cheng, S.V., Gusella, J.F. and Housman, D.E. (1988). Genetic analysis of the dominant white-spotting (W) region on mouse chromosome 5: identification of clones DNA markers near W. Proc. Natl. Acad. Sci. U.S.A. 85, 9635–9639
Gellin, J., Echard, G., Yerle, M., Dalens, M., Chevalet, C. and Gillois, M. (1985). Localization of the alpha and beta casein genes to the q24 region of chromosome 12 in the rabbit (Oryctolagus cuniculus L.) by in situ hybridization. Cytogenet. Cell Genet. 39, 220–223.
George, S., Clark, A.J. and Archibald, A.L. (1997). Physical mapping of the murine casein locus reveals the gene order as alpha-beta-gamma-epsilon-kappa. DNA Cell. Biol. 16, 477–484.
Geyer, P.K. (1997). The role of insulator elements in defining domains of gene expression. Curr. Opin. Genet. Dev. 7, 242–248.
Gilchrist, S.E. and Alcorn J (2010). Lactation stage-dependent expression of transporters in rat whole mammary gland and primary epithelial organoids. Fundam. Clin. Pharmacol. 24, 205–214.
Gilmour, K.C., Pine, R. and Reich, N. (1995). Interleukin 2 activates STAT5 transcription factor (mammary gland factor) and specific gene expression in T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 92, 10772–10776.
Glick, B.S., Malhotra, Y. (1998). The curious status of the Golgi apparatus. Cell 95, 883–889.
Glombik, M.M. and Gerdes, H.H. (2000). Signal-mediated sorting of neuropeptides and prohormones: secretory granule biogenesis revisited. Biochimie 82, 315–326.
Golden, K.L. and Rillema, J.A. (1995). Effects of prolactin on galactosyl transferase and alpha-lactalbumin mRNA accumulation in mouse mammary gland explants. Proc. Soc. Exp. Biol. Med. 209, 392–396.
Groenen, M.A.M., Dijkhof, R.J.M., Verstege, A.J.M. and van der Poel, J.J. (1993). The complete sequence of the gene encoding bovine alpha-s2-casein. Gene 123, 187–193.
Groneberg, D.A., Doring, F., Theis, S., Nickolaus, M., Fischer, A. and Daniel, H. (2002). Peptide transport in the mammary gland: expression and distribution of PEPT2 mRNA and protein. Am. J. Physiol. Endocrinol. Metab. 282, E1172–E1179.
Grosclaude, F. (1979). Polymorphism of milk proteins: some biochemical and genetical aspects, in, Proc.16th International Conference Animal Blood Groups Biochemical Polymorphisms, International Society for Animal Blood Group Research, ed., International Society for Animal Blood Group Research, Leningrad. pp. 54–92.
Grusby, M.J., Mitchell, S.C., Nabavi, N. and Glimcher, L.H. (1990). Casein expression in cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U.S.A 87, 6897–6901.
Gu, Z., Eleswarapu, S. and Jiang, H. (2007). Identification and characterization of microRNAs from the bovine adipose tissue and mammary gland. FEBS Lett. 581, 981–988.
Gupta, P., Rosen, J.M., D’Eustachio, P. and Ruddle, F.H. (1982). Localization of the casein gene family to a single mouse chromosome. J. Cell Biol. 93, 199–204
Gutierrez-Adan, A., Maga, E.A., Meade, H., Shoemaker C.F., Medrano J.F., Anderson G.B. and Murray J.D. (1996). Alterations of the physical characteristics of milk from transgenic mice producing bovine kappa-casein. J. Dairy Sci. 79, 791–799.
Guyette, W.A., Matusik, R.J. and Rosen, J.M. (1979). Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell 17, 1013–1023
Hajjoubi, S., Rival-Gerbier, S., Hayes, H., Floriot, S., Eggen, A., Piumi, F., Chardon, P., Houdebine, L.M. and Thepot, D (2006). Ruminants genome no longer contains whey acidic protein gene but only a pseudogene. Gene 370, 104–112
Hall, L., Emery, D.C., Davies, M.S., Parker, D. and Craig, R.K. (1987). Organization and sequence of the human alpha-lactalbumin gene. Biochem. J. 242, 735–742
Halliday, J.A., Bell, K., McKenzie, H.A. and Shaw, D.C. (1990). Feline whey proteins: identification, isolation and initial characterization of alpha-lactalbumin, beta-lactoglobulin and lysozyme. Comp. Biochem. Physiol. 95, 773–779.
Hammond, C. and Helenius, A. (1995). Quality control in the secretory pathway. Curr. Opin. Cell. Biol. 7, 523–529.
Hansson, L., Edlund, A., Johansson, T., Hernell, O., Strömqvist, M., Lindquist, S., Lönnerdal, B. and Bergström, S. (1994). Structure of the human beta-casein encoding gene. Gene 139, 193–199.
Harris, S., Ali, S., Anderson, S., Archibald, A.L. and Clark, A.J. (1988). Complete nucleotide sequence of the genomic ovine beta-lactoglobulin gene. Nucleic Acids Res. 16, 10379–10380
Harris, S., McClenaghan, M., Simons, J.P., Ali, S. and Clark, A.J. (1990). Gene expression in the mammary gland. J. Reprod. Fertil. 88, 707–715
Hayes, H., Petit, E., Bouniol, C. and Popescu, P. (1993a). Localization of the alpha-S2-casein gene (CASAS2) to the homeologous cattle, sheep and goat chromosomes 4 by in situ hybridization. Cytogenet. Cell. Genet. 64, 282–285.
Hayes, H., Petit, E., Lemieux, N. and Dutrillaux, B. (1992). Chromosomal localization of the ovine beta-casein gene by non-isotopic in situ hybridization and R-banding. Cytogenet. Cell. Genet. 61, 286–288.
Hayes, H., Popescu, P. and Dutrillaux, B. (1993b). Comparative gene mapping of lactoperoxidase, retinoblastoma and alpha-lactalbumin genes in cattle, sheep and goats. Mamm. Genome 4, 593–597.
Hayes, H.C. and Petit, E.J. (1993c). Mapping of the beta-lactoglobulin gene and of an immunoglobulin M heavy chain-like sequence to homoeologous cattle sheep, and goat chromosomes. Mamm. Genome 4, 207–210.
Heim, M.H. (1999). The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. J. Recept. Signal Transduct. Res. 19, 75–120.
Hennighausen, L.G. and Sippel, A.E. (1982). Mouse whey acidic protein is a novel member of the family of four-disulfide core proteins. Nucleic Acids Res. 10, 2677–2684.
Hentze, M. and Kulozik, A.E. (1999). A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96, 307–310.
Hoffmann, E.K. and Simonsen, L.O. (1989). Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol. Rev. 69, 315–382.
Holt, C. and Sawyer, L. (1993). Caseins as rheomorphic proteins: interpretation of the primary and secondary structures of the ασ1-, β- and κ-caseins. J. Chem. Soc. Farad. Trans. 89, 2683–2692.
Hurley, W.L., Wang, H., Bryson, J.M. and Shennan D.B. (2000). Lysine uptake by mammary gland tissue from lactating sows. J. Anim. Sci. 78, 391–395.
Jahn, R. and Scheller, R.H. (2006). SNAREs–engines for membrane fusion. Nat. Rev. Mol. Cell. Biol. 7, 631–643.
Jolivet, G., Devinoy, E., Fontaine, M.L. and Houdebine, L.M. (1992). Structure of the gene encoding rabbit alpha s1-casein. Gene 113, 257–262.
Jolivet, G., L’Hotte, C., Pierre, S., Tourkine, N. and Houdebine, L.M. (1996). The MGF/STAT5 binding site is necessary in the distal enhancer for high prolactin induction of transfected rabbit alpha-s1-casein-CAT gene transcription. FEBS Lett. 8, 257–262.
Jollès, J., Fiat, A.M., Schoentgen, F., Alais, C. and Jollès, P. (1974). The amino acid sequence of sheep kappa-A-casein. II. Sequence studies concerning the kappa-A-caseinoglycopeptide and establishment of the complete primary structure of the protein. Biochim. Biophys. Acta 365, 335–342.
Jones, W.K., Yu-Lee, L.Y., Clift, S.M., Brown, T.L. and Rosen, J.M. (1986). The rat casein multigene family. Fine structure and evolution of the beta-casein gene. J. Biol. Chem. 260, 7042–7050
Kabotyanski, E.B., Huetter, M., Xian, W., Rijnkels, M. and Rosen, J.M. (2006). Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol. Endocrinol. 10, 2355–2368.
Kabotyanski, E.B., Rijnkels, M., Freeman-Zadrowski, C., Buser, A.C., Edwards, D.P. and Rosen, J.M. (2009). Lactogenic hormonal induction of long distance interactions between beta-casein gene regulatory elements. J. Biol. Chem. 284, 22815–22824.
Kanai, Y. and Hediger, M.A. (1992). Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 360, 467–471.
Kanai, Y., Smith, C.R. and Hediger, M.A. (1994). A new family of neurotransmitter transporters: the high affinity glutamate transporters. FASEB J. 8, 1450–1459.
Kang, Y.K., Lee, C.S., Chung, A.S. and Lee, K.K. (1998). Prolactin-inducible enhancer activity of the first intron of the bovine beta-casein gene. Mol. Cells 30, 259–265.
Kansal, R. and Kansal, V.K. (1996). Discrimination of transport systems of L-tyrosine in mouse mammary gland: characterisation of system T. Indian J. Exp. Biol. 34, 750–757.
Kapelinskaia, T.V., Tkach, T.M., Smirnov, I.K. and Gorodetskii, S.I. (1989). The Bos taurus casein genes. Isolation and characterization of the kappa-casein gene. Genetika 25, 15–23.
Kawasaki, K., Lafont, A.G. and Sire, J.Y. (2011). The evolution of milk casein genes from tooth genes before the origin of mammals. Mol Biol Evol. 28, 2053–2061.
Kazansky, A.V., Raught, B., Lindsey, S.M., Wang, Y.F. and Rosen, J.M. (1995). Regulation of mammary gland factor/Stat5 during mammary gland development. Mol. Endocrinol. 9, 1598–1609.
Kim, P.S. and Arvan, P. (1991). Folding and assembly of newly synthesized thyroglobulin occurs in a pre-Golgi compartment. J. Biol. Chem. 266, 12412–12418.
Koczan, D., Hobom, G. and Seyfert, H.M. (1991). Genomic organization of the bovine αs1-casein gene. Nucleic Acids Res. 19, 5591–5596.
Kolb, A.F., Günzburg, W.H., Albang, R., Brem, G., Erfle, V. and Salmons, B. (1994). Negative regulatory element in the mammary specific whey acidic protein promoter. J. Cell. Biochem. 56, 245–261.
Kolb, A. F. (2002). Structure and regulation of the murine γ-casein gene. Biochem. Biophys. Acta 1579, 101–116.
Kolb, A. F. (2003). The first intron of the murine β-casein gene contains a functional promoter. Biochem. Biophys. Res. Commun. 306, 1099–1105.
Kumar, S., Clarke, A.R., Hooper, M.L., Horne, D.S., Law, A.J., Leaver, J., Springbett, A., Stevenson, E. and Simons, J.P. (1994). Milk composition and lactation of beta-casein deficient mice. Proc. Natl. Acad. Sci. U.S.A 91, 6138–6142.
Laird, J.E., Jack, L., Hall, L., Boulton, A., Parker, D. and Craig, R.K. (1988). Structure and expression of the guinea-pig alpha-lactalbumin gene. Biochem. J. 254, 85–94
Laspiur, J.P., Burton, J.L., Weber, P.S.D., Moore, J., Kirkwood, R.N. and Trottier, N.L. (2009). Dietary protein intake and stage of lactation differentially modulate amino acid transporter mRNA abundance in porcine mammary tissue. J. Nutr. 139, 1677–1684.
Lear, T.L., Brandon, R., Masel, A., Bell, K. and Bailey, E. (1998). Molecular cloning and chromosomal localization of horse alpha-1-antitrypsin (AAT), beta-lactoglobulin 1 and 2 (BLG, BLG2), lactotransferrin (LTF) and transferrin (TF). Anim. Genet. 29, 43–49.
Lechner, J., Welte, T., Tomasi, J.K., Bruno, P., Cairns, C., Gustafsson, J.A. and Doppler, W. (1997). Promoter-dependent synergy between glucocorticoid receptor and Stat5 in the activation of beta-casein gene transcription. J. Biol. Chem. 272, 20954–20960.
Lee, C.S. and Oka, T. (1992). A pregnancy-specific mammary nuclear factor involved in the repression of the mouse beta-casein gene transcription by progesterone. J. Biol. Chem. 267, 5797–5801.
Lee, K.F., Atiee, S.H., Henning, S.J. and Rosen, J.M. (1989). Relative contribution of promoter and intragenic sequences in the hormonal regulation of rat beta-casein transgenes. Endocrinology 3, 447–453.
Lee, M.C., Miller, E.A., Goldberg, J., Orci, L. and Schekman, R. (2004). Bi-directional protein transport between the ER and Golgi. Ann. Rev. Cell Dev. Biol. 20, 87–123.
Lefèvre, C.M., Sharp, J.A. and Nicholas, K.R. (2009). Characterisation of monotreme caseins reveals lineage-specific expansion of an ancestral casein locus in mammals. Reprod. Fertil. Dev. 21, 1015–1027.
Lemay D.G., Lynn D.J., Martin W.F., Neville M.C., Casey T.M., Rincon G., Kriventseva E.V., Barris W.C., Hinrichs A.S., Molenaar A.J., Pollard K.S., Maqbool N.J., Singh K., Murney R., Zdobnov E.M., Tellam R.L., Medrano J.F., German J.B. and Rijnkels M. (2009). The bovine lactation genome: insights into the evolution of mammalian milk. Genome Biol. 10, R43
Le Parc, A., Leonil, J. and Chanat, E. (2010). αs1-Casein, which is essential for efficient ER-to-Golgi casein transport, is also present in a tightly membrane-associated form. BMC Cell Biol. 11, 65–76.
Leroux, C., Mazure, N. and Martin, P. (1992). Mutations away from splice site recognition sequences might cis-modulate alternative splicing of goat alpha-S1-casein transcripts. Structural organization of the relevant gene. J. Biol. Chem. 267, 6147–6157.
Li, S. and Rosen, J.M. (1994a). Distal regulatory elements required for rat whey acidic protein gene expression in transgenic mice. J. Biol. Chem. 269, 14235–14243.
Li, S. and Rosen, J.M. (1994b). Glucocorticoid regulation of rat whey acidic protein gene expression involves hormone-induced alterations of chromatin structure in the distal promoter region. Mol. Endocrinol. 8, 1328–1335.
Li, S. and Rosen, J.M. (1995). CTF/NF1 and mammary gland factor (Stat5) play a critical role in regulating rat whey acidic protein gene expression in transgenic mice. Mol. Cell. Biol. 15, 2063–2070.
Liao, Y., Du, X. and Lönnerdal, B. (2010). miR-214 regulates lactoferrin expression and pro-apoptotic function in mammary epithelial cells. J. Nutr. 140, 1552–1556.
Lin, C.Q., Dempsey, P.J., Coffey, R.J. and Bissell, M.J. (1995). Extracellular matrix regulates whey acidic protein gene expression by suppression of TGF-alpha in mouse mammary epithelial cells: studies in culture and in transgenic mice. J. Cell Biol. 129, 1115–1126.
Lingappa, V.R., Lingappa, J.R., Prasad, R., Ebner, K.E. and Blobel, G. (1978). Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc. Natl. Acad. Sci. U. S. A. 75, 2338–2342.
Liu, H., Wang, L., Wang, L.-B., Li, S.-L. and Cao, Z.-J. (2010). Responses of mammary amino acid metabolism and aminopeptidase N gene expression to duodenal soyabean small peptides and infusion of free amino acids in lactating goats. J. Anim. Feed Sci. 19, 24–36.
Liu, X., Robinson, G.R., Wagner, K.U., Garrett, L., Wynshaw-Boris, A. and Hennighausen, L. (1997). Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186.
Lopez, A., Torres, N., Ortiz, V., Aleman, G., Hernandez-Pando, R. and Tovar, A.R. (2006). Characterization and regulation of the gene expression of amino acid transport system A (SNAT2) in rat mammary gland. Am. J. Physiol. Endocrinol. Metab. 291, E1059–E1066.
Lollivier, V., Marnet, P.G., Delpal, S., Rainteau, D., Achard, C., Rabot, A. and Ollivier-Bousquet, M. (2006). Oxytocin stimulates secretory processes in lactating rabbit mammary epithelial cells. J. Physiol. 570, 125–140.
Martinez-Lopez, I., Garcia, C., Barber, T., Vina, J.R. and Miralles, V.J. (1999). The L-glutamate transporters GLAST (EAAT1) and GLT-1(EAAT2): expression and regulation in the rat lactating mammary gland. Mol. Membr. Biol. 15, 237–242.
Maschio, A., Brickell, P.M., Kioussis, D., Mellor, A.L., Katz, D. and Craig, R.K. (1991). Transgenic mice carrying the guinea-pig alpha-lactalbumin gene transcribe milk protein genes in their sebaceous glands during lactation. Biochem. J. 275, 459–467.
Mather, I.H. and Keenan, T.W. (1983). Function of endomembranes and the cell surface in the secretion of organic milk constituents, in, Biochemistry of Lactation. T.B. Mepham, ed. Amsterdam, Elsevier IP. pp. 231–283.
Mather, I.H. and Keenan, T.W. (1998). Origin and secretion of milk lipids. J. Mammary Gland Biol. Neoplasia 3, 259–273.
McConkey, E.H., Menon, R., Williams, G., Baker, E. and Sutherland, G.R. (1996). Assignment of the gene for beta-casein (CSN2) to 4q13 - > q21 in humans and 3p13 - > p12 in chimpanzees. Cytogenet. Cell Genet. 72, 60–62.
McKnight, R.A., Spencer, M., Dittmer, J., Brady, J.N., Wall, R.J. and Hennighausen, L. (1995). An Ets site in the whey acidic protein gene promoter mediates transcriptional activation in the mammary gland of pregnant mice but is dispensable during lactation. Mol. Endocrinol. 9, 717–724.
McKnight, R.A., Spencer, M., Wall, R.J. and Hennighausen, L. (1996). Severe position effects imposed on a 1 kb mouse whey acidic protein gene promoter are overcome by heterogeneous matrix attachment regions. Mol. Reprod. Dev. 44, 179–184.
Meier, V.S. and Groner, B. (1994). The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction. Mol. Cell. Biol. 14, 128–137.
Mepham, T.B., Overthrow, J.I. and Short A.H. (1985). Epithelial cell entry and exit competition amongst amino acids in the perfused isolated lactating mammary gland of guinea pig, in, Carrier Mediated Transport of Solutes from Blood to Tissue, D.L. Yudilevich and G.E. Mann, eds., Longman, London. pp 369–372.
Mercier, J.C. and Vilotte, J.L. (1993). Structure and function of milk protein genes. J. Dairy Sci. 76, 3079–3098.
Millar, I.D., Barber, M.C., Lomax, M.A., Travers, M.T. and Shennan, D.B. (1997a). Mammary protein synthesis is acutely regulated by the cellular hydration state. Biochem. Biophys. Res. Commun. 230, 351–355.
Millar, I.D., Calvert, D.T., Lomax, M.A. and Shennan, D.B. (1996). The mechanism of L-glutamate transport by lactating rat mammary tissue. Biochim. Biophys. Acta 1282, 200–206.
Millar, I.D., Calvert, D.T., Lomax, M.A. and Shennan, D.B. (1997b). Substrate specificity of the mammary tissue anionic amino acid carrier operating in the cotransport and exchange modes. Biochim. Biophys. Acta 1326, 92–102.
Millar, I.D. and Shennan, D.B. (1999). The regulation of Na+-dependent anionic amino acid transport by the rat mammary gland. Biochim. Biophys. Acta 1421, 340–346.
Mizoguchi, Y., Kim, J.Y., Enami, J. and Sakai, S. (1997). The regulation of the prolactin receptor gene expression in the mammary gland of early pregnant mouse. Endocr. J. 44, 53–58.
Molenaar, A.J., Davis, S.R. and Wilkins, R.J. (1992). Expression of alpha-lactalbumin, alpha-s1-casein and lactoferrin genes is heterogeneous in sheep and cattle mammary tissue. J. Histochem. Cytochem. 40, 611–618.
Montazer-Torbati, M.B., Hue-Beauvais, C., Droineau, S., Ballester, M., Coant, N., Aujean, E., Petitbarat, M., Rijnkels, M. and Devinoy, E. (2008). Epigenetic modifications and chromatin loop organization explain the different expression profiles of the Tbrg4, WAP and Ramp3 genes. Exp. Cell Res. 314, 975–987.
Moore, A., Hall, L. and Hamilton, D.W. (1990). An 18-kDa androgen-regulated protein that modifies galactosyltransferase activity is synthesized by the rat caput epididymidis, but has no structural similarity to rat milk alpha-lactalbumin. Biol. Reprod. 43, 497–506.
Morvan, J. and Tooze, S.A. (2008). Discovery and progress in our understanding of the regulated secretory pathway in neuroendocrine cells. Histochem. Cell Biol. 129, 243–252.
Myers, C.A., Schmidhauser, C., Mellentin-Michelotti, J., Fragoso, G., Roskelley, C.D., Casperson, G., Mossi, R., Pujuguet, P., Hager, G. and Bissell, M.J. (1998). Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol. Cell. Biol. 18, 2184–2195.
Neville, M.C., Chatfield, K., Hansen, L., Lewis, A., Monks, J., Nuijens, J., Ollivier-Bousquet, M., Schanbacher, F., Sawicki, V. and Zhang, P. (1998). Lactoferrin secretion into mouse milk. Development of secretory activity, the localization of lactoferrin in the secretory pathway, and interactions of lactoferrin with milk iron. Adv. Exp. Med. Biol. 443, 141–153.
Neville, M.C., Lobitz, C.J., Ripoll, E.A. and Tinney, C. (1980). The sites of α-aminoisobutyric acid uptake in normal mammary gland and ascites tumor cells. J. Biol. Chem. 255, 7311–7316.
Neville, M.C. and Watters, C.D. (1983). Secretion of calcium into milk: review. J. Dairy Sci. 66, 371–380.
Ollivier-Bousquet, M. (1993). Secrétion des caséines: régulation hormonale, in, Biologie de la Lactation, Martinet, J. and Houdebine, L.M. eds., pp. 367–387. NRA-INSERM, France.
Ollivier-Bousquet, M. (1997). Milk protein transfer in the mammary cell. Flem. Vet. J. 66 (Suppl.), 125–142.
Onoda, M. and Inano, H. (1997). Distribution of casein-like proteins in various organs of rat. J. Histochem. Cytochem. 45, 663–674.
Palade, G. (1975). Intracellular aspects of the process of protein synthesis. Science 189, 347–358.
Papiz, M.Z., Sawyer, L., Eliopoulos, E.E., North, A.C.T., Findlay, J.B.C., Sivaprasadavao, R., Jones, T.A., Newcomer, M.E. and Kraulis, P.J. (1986). The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature 324, 383–385.
Passey, R.J. and McKinlay, A.G. (1995). Characterization of a second, apparently inactive, copy of the bovine beta-lactoglobulin gene. Eur. J. Biochem. 233, 736–743.
Pauloin, A., Delpal, S., Chanat, E., Lavialle, F., Aubourg, A. and Ollivier-Bousquet M. (1997). Brefeldin A differently affects basal and prolactin-stimulated milk protein secretion in lactating rabbit mammary epithelial cells. Eur. J. Cell Biol. 72, 324–336.
Pauloin, A., Tooze, S.A., Michelutti, I., Delpal, S. and Ollivier-Bousquet, M. (1999). The majority of clathrin coated vesicles from lactating rabbit mammary gland arises from the secretory pathway. J. Cell Sci. 112(Pt 22), 4089–4100.
Pechoux, C., Boisgard, R., Chanat, E. and Lavialle, F. (2005). Ca(2+)-independent phospholipase A2 participates in the vesicular transport of milk proteins. Biochim. Biophys. Acta 1743, 317–329.
Pelham, H.R. (1989). Control of protein exit from the endoplasmic reticulum. Ann. Rev. Cell Biol. 5, 1–23.
Pena, R.N., Folch, J.M., Sanchez, A. and Whitelaw, C.B.A. (1998). Chromatin structures of goat and sheep beta-lactoglobulin gene differ. Biochem. Biophys. Res. Commun. 252, 649–653.
Pena, R.N., Sanchez, A., Coll, A. and Folch, J.M. (1999). Isolation, sequencing and relative quantification by fluorescent-ratio PCR of feline beta-lactoglobulin I, II and III cDNAs. Mamm. Genome 10, 560–564.
Perez, M.J., Leroux, C., Bonastre, A.S. and Martin, P. (1994). Occurrence of a LINE sequence in the 3′ UTR of the goat alpha-s1-casein E-encoding allele associated with reduced protein synthesis level. Gene 147, 179–187.
Persuy, M.A., Printz, C., Medrano, J.F. and Mercier, J.C. (1996). One mutation might be responsible for the absence of beta-casein in two breeds of goats. Anim. Genet. 27, 96–102.
Persuy, M.A., Stinnakre, M.G., Printz, C., Mahé, M.F. and Mercier, J.C. (1992). High expression of the caprine beta-casein gene in transgenic mice. Eur. J. Biochem. 205, 887–891
Phi-Van, L. and Strätling, W.H. (1988). The matrix attachment regions of the chicken lysozyme gene co-map with the boundaries of the chromatin domain. EMBO J. 7, 655–664.
Pierre, S., Jolivet, G., Devinoy, E. and Houdebine, L.M. (1994). A combination of distal and proximal regions is required for efficient prolactin regulation of transfected rabbit alpha-s1-casein chloramphenicol acetyltransferase constructs. Mol. Endocrinol. 8, 1720–1730.
Pines, G., Danbolt, N.C., Bjoras, M., Zhang, Y., Bendahan, A., Eide, L., Koepsell, H., Storm-Mathisen, J., Seeberg, E. and Kanner, B.L. (1992). Cloning and expression of a rat brain L-glutamate transporter. Nature 360, 464–467.
Pitelka, D.R. and Hamamoto, S.T. (1983). Ultrastructure of the mammary secretory cell, in, Biochemistry of Lactation, T.B. Mepham, ed., Elsevier Science Publishers B.V., pp. 29–70. Amsterdam, New York.
Prasad, R., Hudson, B.G., Butkowski, R., Hamilton, J.W. and Ebner, K.E. (1979). Resolution of the charge forms and amino acid sequence and location of a tryptic glycopeptide in rat alpha-lactalbumin. J. Biol. Chem. 254, 10607–10614.
Provot, C., Persuy, M.A. and Mercier, J.C. (1995). Complete sequence of the ovine beta-casein-encoding gene and interspecies comparison. Gene 154, 259–263.
Qasba, P.K. and Safaya, S.K. (1984). Similarity of the nucleotide sequences of rat alpha-lactalbumin and chicken lysozyme genes. Nature 608, 377-380
Qasba, P.K., Hewlett, I.K. and Byers, S. (1983). The presence of the milk protein alpha-lactalbumin and its mRNA in the rat epididymis. Biochem. Biophys. Res. Commun. 30, 306–312.
Rando, A., Di Gregorio, P., Ramunno, L., Mariani, P., Fiorella, A., Senese, C., Marletta, D. and Masina, P. (1998). Characterization of the CSNAG allele of the bovine alpha-s1-casein locus by the insertion of a relict of a long interspersed element. J. Dairy Sci. 81, 1735–1742.
Rando, A., Pappalardo, M., Capuano, M., Di Gregorio, P. and Ramunno, L. (1996). Two mutations might be responsible for the absence of beta-casein in goat milk. Anim. Genet. 27, 31.
Raught, B., Liao, W.S.L. and Rosen, J.M. (1995). Developmentally and hormonally regulated CCAAT/enhancer-binding protein isoforms influence beta-casein gene expression. Mol. Endocrinol. 9, 1223–1232.
Reinhardt, T.A. and Lippolis, J.D. (2008). Developmental changes in the milk fat globule membrane proteome during the transition from colostrum to milk. J. Dairy Sci. 91, 2307–2318.
Rhoads, R.E. and Grudzien-Nogalska, E. (2007). Translational regulation of milk protein synthesis at secretory activation. J. Mammary Gland Biol. Neoplasia 12, 283–292.
Riebeling, C., Morris, A.J. and Shields, D. (2009). Phospholipase D in the Golgi apparatus. Biochim. Biophys. Acta 1791, 876–880.
Rijnkels, M. (2002). Multispecies comparison of the casein gene loci and evolution of casein gene family. J. Mammary Gland Biol. Neoplasia 7, 327–345.
Rijnkels, M., Kabotyanski, E., Montazer-Torbati, M.B., Beauvais, C.H., Vassetzky, Y., Rosen, J.M. and Devinoy, E. (2010). The epigenetic landscape of mammary gland development and functional differentiation. J. Mammary Gland Biol. Neoplasia 15, 85–100.
Rijnkels, M., Kooiman, P.M., de Boer, H.A. and Pieper, F.R. (1997b). Organization of the bovine casein gene locus. Mamm. Genome 8, 148–152.
Rijnkels, M., Meershoek, E., de Boer, H.A. and Pieper, F.R. (1997c). Physical map and localization of the human casein gene locus. Mamm. Genome 8, 285–286.
Rijnkels, M., Wheeler, D.A., de Boer, H.A. and Pieper, F.R. (1997a). Structure and expression of the mouse casein gene locus. Mamm. Genome 8, 9–15.
Rival-Gervier, S., Thepot, D., Jolivet, G. and Houdebine, L.M. (2003). Pig whey acidic protein gene is surrounded by two ubiquitously expressed genes. Biochim. Biophys. Acta 1627, 7–14.
Roberts, B., DiTullio, P., Vitale, J., Hehir, K. and Gordon, K. (1992). Cloning of the goat beta-casein-encoding gene and expression in transgenic mice. Gene 121, 255–262.
Robinson, C. and Kolb, A. F. (2009). Analysis of mammary specific gene locus regulation in differentiated cells derived by somatic cell fusion. Exp. Cell Res. 315, 508–22.
Robinson, G.W., Johnson, P.F., Hennighausen, L. and Sterneck, E. (1998). The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 12, 1907–1916.
Robinson, G.W., McKnight, R.A., Smith, G.H. and Hennighausen, L. (1995). Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121, 2079–2090.
Rohrer, G.A., Alexander, L.J. and Beattie, C.W. (1997). Mapping genes located on human chromosomes 2 and 12 to porcine chromosomes 15 and 5. Anim. Genet. 28, 448–450.
Rosen, J.M. (1987). Milk protein gene structure and expression, in, The Mammary Gland, Neville, M.C. and Daniel, C.W., eds., Plenum Publishing Corporation, New York. pp. 301–322
Rosen, J.M., Li, S., Raught, B. and Hadsell, D. (1996). The mammary gland as a bioreactor: factors regulating the efficient expression of milk protein-based transgenes. Am. J. Clin. Nutr. 63, 627–632.
Rosen, J.M., Mediona, D., Schlein, A.R., Eisenstein, R.S. and Yu-Lee, L.Y. (1988). Hormonal and cell-substratum regulation of casein gene expression at the posttranscriptional level, in, Steroid Hormone Action, G. Ringold (ed.) Alan R. Liss. Inc. New York. pp 269–278
Rosen, J.M., Rodgers, J.R., Couch, C.H., Bisbee, C.A., David-Inouye, Y., Campbell, S.M. and Yu-Lee, L.Y. (1986). Multihormonal regulation of milk protein gene expression. Ann. N. Y. Acad. Sci. 478, 63–76
Rosen, J.M., Zahnow, C., Kazansky, A. and Raught, B. (1998). Composite response elements mediate hormonal and developmental regulation of milk protein gene expression. Biochem. Soc. Symp. 63, 101–113.
Rudolph, M.C., McManaman, J. L., Phang, T., Russell, T., Kominsky, D.J., Serkova, N.J., Stein, T., Anderson, S.M. and Neville, M.C. (2007). Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol. Genomics 28, 323–336.
Rothman, J.E. and Wieland, F.T. (1996). Protein sorting by transport vesicles. Science 272, 227–234.
Saidi, S., Rival-Gervier, S., Daniel-Carlier, N., Thepot, D., Morgenthaler, C., Viglietta, C., Prince, S., Passet, B., Houdebine, L.M. and Jolivet, G. (2007). Distal control of the pig whey acidic protein (WAP) locus in transgenic mice. Gene 401, 97–107.
Schatz, G., Dobberstein, B. (1996). Common principles of protein translocation across membranes. Science 271, 1519–1526.
Schmidhausser, C. Casperson, G.F., Myers, C.A., Sanzo, K.T., Bolten, S. and Bissell, M.J. (1992). A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol. Biol. Cell 3, 699–709.
Schmidt, J.A., Kalkofen, D.N., Donovan, K.W. and Brown, W.J. (2010). A role for phospholipase A2 activity in membrane tubule formation and TGN trafficking. Traffic 11, 1530–1536.
Schmitt-Ney, M., Doppler, W., Ball, R.K. and Groner, B. (1991). Beta-casein gene promoter activity is regulated by the hormone-mediated relief of transcriptional repression and a mammary-gland-specific nuclear factor. Mol. Cell. Biol. 11, 3745–3755.
Sdassi, N., Silveri, L., Laubier, J., Tilly, G., Costa, J., Layani, S., Vilotte, J.L. and Le Provost, F. (2009). Identification and characterization of new miRNA cloned from normal mouse mammary gland. BMC Genomics 10, 149.
Seagroves, T.N., Krnacik, S., Raught, B., Gay, J., Burgess-Beusse, B., Darlington, G.J. and Rosen, J.M. (1998). C/EBPbeta, but not C/EBPalpha is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Gene Devp. 12, 1917–1928
Seddiki, T. and Ollivier-Bousquet, M. (1991). Temperature dependence of prolactin endocytosis and casein exocytosis in epithelial mammary cells. Eur. J. Cell Biol. 55, 60–70.
Sharp, J.A., Lefèvre, C. and Nicholas, K.R. (2008). Lack of functional alpha-lactalbumin prevents involution in Cape fur seals and identifies the protein as an apoptotic milk factor in mammary gland involution. BMC Biol. 6, 48–57.
Shekar, P.C., Goel, S., Rani, S.D.S., Sarathi, D.P., Alex, J.L., Singh, S. and Kumar, S. (2006). kappa-Casein-deficient mice fail to lactate. Proc. Natl. Acad. Sci. U.S.A 103, 8000–8005.
Shennan, D.B. (1995). Identification of a high affinity taurine transporter which is not dependent on chloride. Biosci. Rep. 15, 231–239.
Shennan, D.B. and McNeillie, S.A. (1994a). Characteristics of α-aminoisobutyric acid transport by the lactating rat mammary gland. J. Dairy Res. 61, 9–19.
Shennan, D.B. and McNeillie, S.A. (1994b). High affinity (Na+ + Cl-)-dependent transport by lactating mammary tissue. J. Dairy Res. 61, 335–343.
Shennan, D.B. and McNeillie, S.A. (1994c). Milk accumulation down regulates amino acid uptake via systems A and L by lactating mammary tissue. Horm. Metab. Res. 26, 611.
Shennan, D.B. and Peaker, M. (2000).Transport of milk constituents by the mammary gland. Physiol. Rev. 80, 925–951.
Shennan, D.B., Backwell, F.R.C. and Calvert, D.T. (1999). Metabolism of aminoacyl-p-nitroanilides by rat mammary tissue. Biochim. Biophys. Acta 1427, 227–235.
Shennan, D.B., Calvert, D.T., Backwell, F.R.C. and Boyd, C.A.R. (1998). Peptide aminonitrogen transport by the lactating rat mammary gland. Biochim. Biophys. Acta 1373, 252–260.
Shennan, D.B., Calvert, D.T., Travers, M.T., Kudo, Y. and Boyd, C.A.R. (2002). A study of L-leucine, L-phenylalanine and L-alanine transport in the perfused rat mammary gland: possible involvement of LAT1 and LAT2. Biochim. Biophys. Acta 1564, 133–139.
Shennan, D.B., Cliff, M.J. and Hawkins, P. (1996). Volume-sensitive taurine efflux from mammary tissue is not obliged to utilize volume-activated anion channels. Biosci. Rep. 16, 459–465.
Shennan, D.B., McNeillie, S.A. and Curran, D.E. (1994). The effect of a hyposmotic shock on amino acid efflux from lactating rat mammary tissue : stimulation of taurine and glycine efflux via a pathway distinct from anion exchange and volume-activated anion channels. Exp. Physiol. 79, 797–808.
Shennan, D.B., McNeillie, S.A., Jamieson, E.A. and Calvert, D.T. (1994). Lysine transport in lactating rat mammary tissue : evidence for an interaction between cationic and neutral amino acids. Acta Physiol. Scand. 151, 461–466.
Shennan, D.B., Millar, I.D. and Calvert, D.T. (1997). Mammary-tissue amino acid transport systems. Proc. Nutr. Soc. 56, 177–191.
Sollner, T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P. and Rothman, J.E. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324.
Soulier, S., Lepourry, L., Stinnakre, M.G., Langley, B., L’Huillier, P.J., Paly, J., Djiane, J., Mercier, J.C. and Vilotte, J.L. (1999). Introduction of a proximal Stat5 site in the murine alpha-lactalbumin promoter induces prolactin dependency in vitro and improves expression frequency in vivo. Transgenic Res. 8, 23–31.
Soulier, S., Mercier, J.C., Vilotte, J.L., Anderson, J., Clark, J. and Provot, C. (1989). Characterization of genomic clones homologous to the alpha-lactalbumin gene in the bovine and ovine species. Gene 83, 331–338.
Starr, R. and Hilton, D.J. (1999). Negative regulation of the JAK/STAT pathway. Bioessays 21, 47–52.
Stinnakre, M.G., Soulier, S., Schibler, L., Lepourry, L., Mercier, J.C. and Vilotte, J.L. (1999). Position-independent and copy-number-related expression of a goat bacterial artificial chromosome alpha-lactalbumin gene in transgenic mice. Biochem. J. 339, 33–36.
Stinnakre, M.G., Vilotte, J.L., Soulier, S. and Mercier, J.C. (1994). Creation and phenotypic analysis of alpha-lactalbumin-deficient mice. Proc. Natl. Acad. Sci. U.S.A 91, 6544–6548.
Stöcklin, E., Wissler, M., Gouilleux, F. and Groner, B. (1996). Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383, 726–728.
Stoecklin, E., Wissler, M., Morriggl, R. and Groner, B. (1997). Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional cooperation in the regulation of gene transcription. Mol. Cell. Biol. 17, 6708–6716.
Storck, T., Schulte, S., Hofmann, K. and Stoffel, W. (1992). Structure, expression and functional analysis of a Na+-dependent glutamate aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U.S.A. 89, 10959–10965.
Streuli, C.H., Edwards, G.M., Delcommenne, M., Whitelaw, C.B.A., Burdon, T.G., Schindler, C. and Watson, C.J. (1995). Stat5 as a target for regulation by extracellular matrix. J. Biol. Chem. 270, 21639–21644.
Tanaka, T., Haneda, S., Imakawa, K., Sakai., S. and Nagaoka., K. (2009). A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 77, 181–187.
Tang, Y. (1993). No alpha-lactalbumin-like activity detected in a low molecular mass protein fraction of rat epididymal extract. Reprod. Fertil. Dev. 5, 229–237.
Thépot, D., Fontaine, M.L., Houdebine, L.M. and Devinoy, E. (1990). Complete sequence of the rabbit WAP gene. Nucleic Acids Res. 18, 3641.
Thépot, D., Fontaine, M.L., Houdebine, L.M. and Devinoy, E. (1991). Structure of the gene encoding rabbit beta-casein. Gene 97, 301–306
Threadgill, D.W. and Womack, J.E. (1990). Genomic analysis of the major bovine casein genes. Nucleic Acids Res. 18, 6935–6942
Tomic S, Chughtai N. and Ali S. (1999). SOCS-1, -2, -3: selective targets and functions downstream of the prolactin receptor. Mol. Cell. Endocrinol. 158, 45–54.
Tomlinson, A.M., Cox, R.D., Lehrach, H.R. and Dalrymple, M.A. (1996). Restriction map of two yeast artificial chromosomes spanning the murine casein locus. Mamm. Genome 7, 342–344.
Tooze, S.A. (1998). Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim. Biophys. Acta 1404, 231–244.
Topcic, D., Auguste A., De Leo, A.A., Lefevre, C., Digby, M.R. and Nicholas, K.R. (2009). Characterization of the tammar wallaby (Macropus eugenii) whey acidic protein gene; new insights into the function of the protein. Evol. Dev. 11, 363–375.
Triplett, A. A., Sakamoto, K., Matulka, L. A., Shen, L., Smith, G. H. and Wagner, K. U. (2005). Expression of the whey acidic protein (WAP) is necessary for adequate nourishment of the offspring but not functional differentiation of mammary epithelial cells. Genesis 43, 1–11.
Turner, M.D., Handel, S.E., Wilde, C.J. and Burgoyne, R.D. (1993). Differential effect of brefeldin A on phosphorylation of the caseins in lactating mouse mammary epithelial cells. J. Cell Sci. 106, 1221–1226.
Ucar, A., Vafaizadeh, V., Jarry, H., Fiedler, J., Klemmt, P.A., Thum, T., Groner, B. and Chowdhury, K. (2010). miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat. Genet. 42, 1101–1108.
Udy, G.B., Towers, R.P., Snell, R.G., Wilkins, R.J., Park, S.H., Ram, P.A., Waxman, D.J. and Davey, H.W. (1997). Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. U.S.A. 94, 7239–7244.
Uversky, V.N., Gillespie, J.R. and Fink, A.L. (2000). Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 41, 415–427.
Valentine, C.R. (1998). The association of nonsense codons with exon skipping. Mutat. Res. 411, 87–117.
Verma, N. and Kansal V.K. (1995). Characterisation and starvation induced regulation of methionine uptake sites in mouse mammary gland. Indian J. Exp. Biol. 33, 516–530.
Verma, N. and Kansal, V.K. (1993). Characterisation of the routes of methionine transport in mouse mammary glands. Indian J. Med. Res. [B] 98, 297–304.
Vilotte J.L., Whitelaw, C.B.A., Ollivier-Bousquet, M. and Shennan, D.B. (2002). Biosynthesis of milk proteins, in, Advanced Dairy Chemistry 1, Proteins, 3rd edn., P.F. Fox and P.L.H. McSweeney, eds., Kluwer Academic/Plenum Publishers. New York. pp.699–738.
Vilotte, J.L. and Soulier, S. (1992). Isolation and characterization of the mouse alpha-lactalbumin-encoding gene: interspecies comparison, tissue- and stage-specific expression. Gene 119, 287–292.
Vilotte, J.L., Soulier, S. and Mercier, J.C. (1993). Complete sequence of a bovine alpha-lactalbumin pseudogene: the region homologous to the gene is flanked by two directly repeated LINE sequences. Genomics 16, 529–532.
Vilotte, J.L., Soulier, S., Mercier, J.C., Gaye, P., Hue-Delahaie, D. and Furet, J.P. (1987). Complete nucleotide sequence of bovine alpha-lactalbumin gene. Comparison with its rat counterpart. Biochimie 69, 609–620
Vilotte, J.L., Soulier, S., Printz, C. and Mercier, J.C. (1991). Sequence of the goat alpha-lactalbumin-encoding gene: comparison with the bovine gene and evidence of related sequences in the goat genome. Gene 98, 271–276
Vina, J., Puertes, I.R., Saez, G.T. and Vina, J.R. (1981). Role of prolactin in amino acid uptake by the lactating mammary gland of the rats. FEBS Lett. 126, 250–252.
Wang, C. and Li, Q. (2007). Identification of differentially expressed miRNA during the development of Chinese murine mammary gland. J. Genet. Genomics 34, 966–973.
Wang, C.C., Shi, H., Guo, K., Ng, C.P., Li, J., Gan, B.Q., Chien Liew, H., Leinonen, J., Rajaniemi, H., Zhou, Z.H., Zeng, Q. and Hong, W. (2007). VAMP8/endobrevin as a general vesicular SNARE for regulated exocytosis of the exocrine system. Mol. Biol. Cell 18, 1056–1063
Wang S, Webb KE Jr, Akers MR. (1996) Peptide-bound methionine can be a source of methionine for the synthesis of secreted proteins by mammary tissue explants from lactating mice. J. Nutr. 126, 1662–1672.
Warner, B., Janssens, P. and Nicholas, K. (1993). Prolactin-independent induction of alpha-lactalbumin gene expression in mammary gland explants from pregnant Balb/C mice. Biophys. Biochem. Res. Commun. 194, 987–991.
Watson, C.J., Gordon, K.E., Robertson, M. and Clark, A.J. (1991). Interaction of DNA-binding proteins with a milk protein gene promoter in vitro: identification of a mammary gland-specific factor. Nucleic Acids Res. 19, 6603–6610.
Webster, J., Wallace, R.M., Clark, A.J. and Whitelaw, C.B.A. (1995). Tissue-specific, temporally regulated expression mediated by the proximal ovine beta-lactoglobulin promoter in transgenic mice. Cell. Mol. Biol. Res. 41, 11–15.
West, D.W. and Clegg, R.A. (1984). Casein kinase activity in rat mammary gland Golgi vesicles. Demonstration of latency and requirement for a transmembrane ATP carrier. Biochem. J. 219, 181–187.
Whitelaw C.B.A. (2000). Nucleosome organization of the beta-lactoglobulin gene. Transcription complex formation. Adv. Exp. Med. Biol. 480, 147–153
Whitelaw, C.B.A. and Webster, J. (1998). Temporal profiles of appearance of DNAse I hypersensitive sites associated with the ovine beta-lactoglobulin gene differ in sheep and transgenic mice. Mol. Gen. Genet. 257, 649–654.
Whitelaw, C.B.A., Harris, S., McClenaghan, M., Simons, J.P. and Clark, A.J. (1992). Position-independent expression of ovine beta-lactoglobulin in transgenic mice. Biochem. J. 286, 31–39.
Winklehner-Jennewein, P., Geymayer, S., Lechner, J., Welte, T., Hansson, L., Geley, S. and Doppler, W. (1998). A distal enhancer region in the human beta-casein gene mediates the response to prolactin and glucocortocoid hormones. Gene 217, 127–139.
Witsell, D.L., Casey, C.E. and Neville, M.C. (1990). Divalent cation activation of galactosyl transferase in native mammary Golgi vesicles. J. Biol. Chem. 265, 15731–15737.
Wolberger, C. (1998). Combinatorial transcription factors. Curr. Opin. Genet. Dev. 8, 552–559.
Wooding, F.B.P. (1977). Comparative mammary fine structure. Symp. Zool. Soc. Lond. 41, 1–41.
Yoshimura, M. and Oka, T. (1989). Isolation and structural analysis of the mouse beta-casein gene. Gene 78, 267–275
Yu-Lee, L.Y., Richter-Mann, L., Couch, C.H., Stewart, A.F., MacKinlay, R.G. and Rosen, J.M. (1986). Evolution of the casein multigene family conserved sequences in the 5′ flanking and exon regions. Nucleic Acids Res. 14, 1883–1902
Martin, P. and Leroux, C. (1992) Exon-skipping is responsible for the 9 amino acid residue deletion occurring near the N-terminal of human β-casein. Biochem. Biophys. Res. Commun. 183, 750–757.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Vilotte, JL., Chanat, E., Le Provost, F., Whitelaw, C.B.A., Kolb, A., Shennan, D.B. (2013). Genetics and Biosynthesis of Milk Proteins. In: McSweeney, P., Fox, P. (eds) Advanced Dairy Chemistry. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-4714-6_14
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4714-6_14
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-4713-9
Online ISBN: 978-1-4614-4714-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)