Abstract
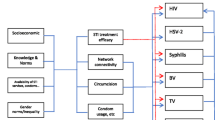
Social networks are natural social units of people (nodes, actors) linked directly or indirectly to others by interaction, affections, associations or relationships and, for the purposes of this chapter, sexual intercourse. Sexual networks through which sexually transmitted pathogens are transmitted form the centre of this review of sexual networks. The fundamental concept of a social network is that the collection of links (paths or edges) and nodes forms an entity far greater than the sum of its parts [1], including interdependent norms, members, organisation and culture.
With apologies to Dr. Mark Granovetter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Social networks are natural social units of people (nodes, actors) linked directly or indirectly to others by interaction, affections, associations or relationships and, for the purposes of this chapter, sexual intercourse. Sexual networks through which sexually transmitted pathogens are transmitted form the centre of this review of sexual networks. The fundamental concept of a social network is that the collection of links (paths or edges) and nodes forms an entity far greater than the sum of its parts [1], including interdependent norms, members, organisation and culture.
Considering a sexual network in which Neisseria gonorrhoeae, Treponema pallidum, Chlamydia trachomatis or HIV circulate exclusively and independently of one another, may be somewhat artificial. Technically, these and other pathogens can spread from any infected individual to any susceptible people with whom he or she has unprotected sex and thence to another generation of sex partners within a sexual network. Therefore, sexually transmitted infections (STIs) should spread through networks of varying structures, as long as they contain infectious people and unprotected sexual intercourse takes place. However, empirical work on STI networks [2, 3], and basic epidemiological data [4, 5], has provided evidence that networks harbouring gonorrhoea differ from those which harbour chlamydia, which again differ from those harbouring HIV, suggesting that different networks have specific innate properties suited to specific organisms. Specific structures of different networks with identical number of contacts, links between them, the same type and number of sexual relationships, and the same variation in sex partners between dyads (sexual activity classes) were elucidated by Klovdahl et al. in a hypothetical graph (Fig. 5.1) [6]. Not only are the number of people who had three sex partners the same in each graph, but so are the number of links also, such that people with three partners had contact with people with only one partner in both graphs. However, a pathogen may pass from one end of the graph to the other in one component by only one route, whereas in the other transmission it is made possible simultaneously by three routes. Thus the authors show that the structure of a network has implications for pathogen transmission through different routes of transmission over and above those described by its basic numeric properties, such as the number of links or the number of nodes, and even the pairing of degree combinations.
Two graphs, each with an equal number of people represented by dots, and relationships represented by lines, where transmission of infection through relationships is facilitated in the second graph because the structure of relationships makes passage easier, rather than in the first where transmission through the network relies on individuals 5 and 6. Adapted from Klovdahl AS, Potterat JJ, Woodhouse D, Muth J, Muth S, Darrow WW. HIV infection in an urban social network: a progress report. Bulletine Methodologie Sociologique 1992;36:24–33
In a seminal paper in 1996 [7], Wasserheit and Aral elucidated a theory that STI epidemiology of different sexually transmitted pathogens changes over time in response to control strategies, and to changes in behaviours of the hosts. They hypothesise that due to increased STI prevention efforts, pathogens can survive only in members of social and sexual networks who have unprotected sex with higher number of sex partners, and less access to effective sexual health care than other populations. The current review builds on that evolutionary approach, exploring the common characteristics of sexual networks using network theories of homophily, heterogeneity, and social aggregation. Then we will review specific differences in the networks which seem essential for the survival of gonorrhoea, chlamydia, syphilis, viral STI, and HIV in sociometric networks where data for more than one generation of sex partners are available. There will be less emphasis on egocentric data, usually collected from one individual with proxy data on his or her sex partners and, in some instances, from first-generation sex partners themselves. Findings from molecular analysis of pathogens within couples or networks will be integrated into pathogen-specific network reviews. Finally, as many social network-inspired prevention strategies are similar for different pathogens, these are reviewed in the final section of the paper, in a more general context.
Sexual Networks, Transmission Networks and Disease Networks
It is important to differentiate here between (1) a sexual network where members have intercourse with each other, some of whom may or may not have an STI; (2) a network which definitely contains members infected and transmitting an STI (transmission and infection network); and (3) a network in which members have symptoms, diagnoses and possibly long-term symptomatic infections (disease network). In the first case, the network can be viewed as a complete network containing all sexual intercourse between all couples in a network, linking them all directly or indirectly through a third person to each other. Of course in reality this is subject to incomplete reporting, omitting and forgetting, but basically the network depicts a subset of all recalled coital links. A transmission network is a subset of the sexual network, containing only those named as sex partners, and likely exposed, by infected people, some of whom may actually be uninfected, but who nevertheless were potentially infected and who should be tested. Ideally, a transmission network is directed, with the arrows denoting who infected whom. However, due to the asymptomatic nature of many STIs, it is challenging in many cases to determine the direction of transmission. For this reason, unless one has specific data to the contrary, transmission networks have been graphed as undirected. It should be noted here that the process of contact tracing or respondent nomination is not a proxy for transmission; it may indeed be synchronised with the onset of symptoms, but by no means should it be taken for granted that because person A named person B, C, and D that person A is the transmitter, and persons B, C, and D are the susceptibles who were infected subsequently.
Defining a transmission network implies identification of actual transmission events within a sexual network. A number of techniques are useful in determining transmission, such as careful documentation of dates of first and last intercourse; use of condoms; type of sex, vaginal/penile, anal/penile and/or oral/penile; recording and listing of symptoms for those unfamiliar with them, dates of onset, specimen collection, test results, accuracy of test method; and finally confirming strain concordance [8, 9]. For diseases such as syphilis, transmission direction may be easier to ascertain than HIV or chlamydia for example, as the disease in individuals can be staged by symptoms appearing at different times, although the presence of HIV may cause acceleration or atypical disease and should be taken into account [10].
Last, a disease network is again a subset of the transmission network, containing only those individuals who have symptoms. For many STIs this is an impractical approach to graphing and investigation, as many are asymptomatic for the whole course (chlamydia) or a part of the course (HSV) in a high proportion of people, or the infection may have a long, mostly asymptomatic incubation period (HIV). Nevertheless, the first sexual network which provided definite proof of an infectious source of AIDS contained only those men who had symptoms, so disease networks cannot be discounted [1, 11].
Methods of Collecting Sexual Network Data
Contact Tracing
Two most commonly used methods of collecting sexual network data from individuals have been (a) contact tracing or partner notification and (b) variations of snowball sampling, such as chain link sampling or respondent-driven sampling. The first and oldest of these is contact tracing or partner notification in which index individual has a positive laboratory test for a notifiable sexually transmitted pathogen which is recognised in the public health legislation of the region as being amenable to intervention. The person’s name, birth date, gender and other relevant identifying and locating information are recorded such that he or she can be contacted by public health staff who then interviews the person with the goals of education on risk reduction, confirming adequate treatment, and management of sex partners to or from whom the STI may have been transmitted [12]. If a sex partner is found to be positive, he or she becomes an “index” case and the process is reiterated, until no new positive partners are found. In this way, a sexual network is sampled with an intentional bias towards interviewing only those who are infected.
There are three ways in which partner notification is conducted: provider referral, contracting and self-referral. First, the public health nurse can elicit the names, identifying and locating information of sex partners of the client for the infectious period, since just before symptom onset, or in the absence of symptoms, usually 3 months before the current diagnosis date [13, 14]. Then the nurse or disease investigation specialist (DIS) will notify the partner, without divulging the name of the index case, that he or she has been exposed to an STI and encourage him or her to present for testing and examination. The client may wish to notify his or her own partners, in which case the nurse or the DIS may still note the partner information, with the proviso that if the partner(s) has not presented for care within a certain period of time, the public health officer will do so (contracting). Last, in many jurisdictions, the names and locating information of sex partners are not recorded by public health staff and the patient is solely responsible for notifying his or her own partners (self-referral). This last method is considered to be the least effective with the fewest number of clients examined [15], as is partner notification attempted by untrained physicians [16]. It is important to note that partner notification practices and effectiveness [17] differ depending on infecting pathogen [15] such that median numbers of clients newly brought to treatment and those newly diagnosed of all those elicited are highest for gonorrhoea and chlamydia, a little lower for syphilis and lowest of all for HIV.
Evaluating the completeness of network data generated by contact tracing has been done mostly by Brewer, who found that people forget 14–25% of sex partners from the past 2 years, elicited during two interviews spaced 1 week and 3 months apart [18, 19]. About 40% were forgotten if one imputed the number of partners over 2 years from a period of recent partner recruitment. However the number of partners forgotten was moderately correlated with the number reported (r = 0.67), even in people who had many or very few number of recent partners, which is of interest when examining the number of partners and network structure required for different pathogens. Reassuringly, sex partners remembered did not differ significantly from those forgotten, by demographic characteristics, such as sexual orientation; type of partnership; positive or negative feelings towards partners and network characteristics, such as frequency of contact. Forgetting partners leads, of course, to an under-reporting of partners; lower number of links between partners (density), and smaller network size, which in turn results in fewer nominations; examinations and lower number of infected clients brought to treatment.
Re-interviewing patients, reading back lists of already nominated partners, prompting the client to recall more partners, using memory cues such as identifying important events and relating those to partners can significantly improve the number of patients nominated and thus the number of those brought to treatment [19]. Ethnographic enquiries, participant observation and other methods commonly used by sociologists have been found to be useful in STI investigations [9, 20], not only for eliciting more partner data but also for refining a general understanding of the social context in which STI occurs. Early evaluations of social network-enhanced contact tracing have revealed between 30% and 100% additional cases diagnosed compared with traditional contact tracing only for syphilis and HIV infection, justifying the adoption and evaluation of these additional methods, which are being incorporated into guidelines on source investigations [13, 14, 21].
Mathematical models of simulated populations have also been used to evaluate the completeness and biases in contact investigations within transmission networks [22]. Three methods of estimating the frequency of number of partners in a network were tested: (1) asking participants to estimate the number of partners which their partners may have; (2) snowball sample participants by selecting certain people initially and sampling a proportion of progressive generations of sex partners and (3) contact tracing, where initial index cases who have confirmed infection are selected, and they are encouraged to notify their partners, or have public health do so. While all three methods resulted in an underestimation of both sex partners and the links between them, contact tracing resulted in the least bias, especially when applied to a high proportion of cases and provided the least underestimation of component sizes.
Respondent-Driven Sampling
This method is more frequently used in research rather than for routine disease investigation, where a small number of “seeds” (initial participants) are selected to begin the study. In principle, it is not necessary to choose a representative group of seeds (e.g., covering all ethnic groups), as the nature of RDS ensures that any bias in seed selection is overcome by the sampling design. However, in practice using seeds that differ in their ethnicity, gender and geographic location within a city minimises the number of waves of recruitment required [23]. Initial participants (seeds) are selected from a population who are then provided with cards or tokens on which the unique number of the seed is recorded along with the study recruitment phone number, to give to friends who may fall into the study target group, and who may be interested in participating. When the friends present with the card, they are entitled to participate in the study and are compensated for their time and effort. In many situations the initial respondent is compensated for each new person he or she recruits. Ideally, in sampling a sexual network, the respondents would refer all sexual partners or friends who may benefit from an STI test within a given time period, who again would refer all of their sex partners in turn such that sequential waves of enrolment occur and anonymous linked chains of study participants are created [24–26]. RDS is a relatively new form of chain-referral network sampling designed to overcome many of the problems typically associated with chain-referral methods, including the generation of a non-probability sample. Mathematical evaluations of RDS showed that probability samples can be generated using this method, facilitating the use of conventional statistics to analyse the results [27] http://www.respondentdrivensampling.org/, thus correcting for biases in sampling to reflect the target population.
Sexual Network Data
One of the first detailed descriptions of sexual networks containing gonorrhoea was published in 1985, a product of the traditional work of DIS combined with innovative analysis. The Colorado Springs study by Potterat and Rothenberg et al. was unique in that it contained most, if not all, of the main themes of STI epidemiology for the foreseeable future and will form the basis for our discussions in this review [28]. In that study, from 6 months of data, there were six “lots” or components which contained 20% of all cases. Components include all people connected directly by one link or indirectly by many links, denoting sexual intercourse. In this study, the small minority of six components contained 20% of all the cases. The fact that they contained a disproportionate number of cases presages later network analysis of similar groups, as did the small number of social venues where sex partners met (pick up joints). Of 300 venues available only six were frequented by 51% of the cases. This study was ground-breaking not only as an initial description of a sexual network containing gonorrhoea constructed without the benefit of modern network software, but also because the investigators identified (1) the fact that many in fact most of the people in the study chose sexual partners who were similar to themselves—homophily; (2) that a relatively small group generated a disproportionately high number of infections—heterogeneity and (3) the small number of common “pick up” joints through which a majority of the at-risk population could be reached—social aggregation.
Homophily
Homophily in social networks is a well-known phenomenon whereby people with similar demographic, behavioural and personal characteristics are more likely to form bonds with people similar to them, summarised in the adage, “birds of a feather flock together”. The mechanism for this is that a close friendship between A and B will limit the time spent between A and another friend C, unless it is spent with both C and B simultaneously. This enhances all three friendships, or if a link between C and B does not yet exist, the common relationship with A is likely to generate an acquaintance if not a friendship [29]. Such ties not only reinforce relationships but also limit them, as documented by many sociological studies, controlling the information people receive, the experiences they undergo, the attitudes they form and the behaviours they adopt [30].
Geographic Homophily
The initial description of how “like mixes with like” was in the form of contiguous census tract areas in which cases of gonorrhoea were clustered [28]. Fifty-one percent of cases were from four census tracts in the core downtown area of Colorado Springs; 5 other adjacent tracts accounted for 21% more of the cases, and the remaining 10 cases were in peripheral census tracts. Sixty-five percent of all relationships containing one core person were with another core person. Fifty percent of people who came from periphery had a partner also from the periphery, while the minority partnered with people from the core or adjacent census tracts. Although a residence in an area defined post hoc by the proportion of gonorrhoea cases is a proxy for sociodemographic and other behavioural factors, this early analysis presaged later work.
As STI incidence decreased, targeting of whole neighbourhoods [31] was not as efficient as it had been in previous years [32]. Wylie and Jolly showed that central member of the largest component containing people infected with gonorrhoea and chlamydia lived in one instance outside the city of Winnipeg, and in another, outside the high-incidence or “core” area of Winnipeg but both were essential in transmitting infection to persons inside and outside the area [2]. A certain amount of geographic heterogeneity (addressed below), like homogeneity, is an important element of sustained transmission, as demonstrated in a study of distances between sex partners [33].
Ethnic Homophily
Correlated with geographic clustering is ethnic homophily, due to the fact that ethnic groups tend to cluster in certain neighbourhoods, although some people choose to have sex with someone outside their own ethnic group. The most conclusive research was a study measuring how likely it is that a person with two partners a year chooses a partner demographically and behaviourally similar to him- or herself [34]. Also measured was homophily in sex partners’ ethnic backgrounds, ages and education levels, known to be important sociologically. Homophily was highest amongst African-American women who reported that 91.8% of their partners were African-American men, while 52% of partners of African-American men were of the same ethnic background. Consistent with this, 56% of White women reported White partners, and 84% of White men reported White female partners. Eighty-three percent of men who were 19 years or younger reported partners in the same age range, 64% in the 20–29-year age group had partners within the same age group and 53% of people had partners above the age of 30. While people tended to pick partners with similar number of partners as themselves, the concordance was not as striking as more observable social groupings. These authors also showed that mixing within a population defined by low prevalence within all segments of subpopulations such as those defined by age, education, ethnic backgrounds and number of partners is an essential component of STI transmission in the population.
Thorough studies of geographic epidemiology of STI [4, 5, 31–33, 35, 36] have shown high STI rates in inner-city, low-income, minority populations, without good access to health care. These papers emphasise the need to focus prevention efforts on relatively small, geographically well-identified populations which contain a large proportion of STI cases. While precise descriptions of person, place and time with a view to intervention are cornerstones of epidemiology and good public health practice, they may be only proxy measures of the ultimate causes of concentrations of STI. Social networks have been cited in some of these papers as being the root of the concentration of STI, as people recruit sex partners in some cases from social networks [37], determined by cost of housing and confined by geographic space [38], particularly in less affluent communities where transport is relatively costly.
Age Homophily
An early indication of homophily in age-based sexual networks was contained in a study which showed that syphilis in adult males was associated with crack cocaine, while diagnoses with gonorrhoea were in much younger men who had similar number of sex partners, but were less likely to use crack and were also much less likely to have syphilis. Further analysis of young men who did use crack showed that they were still far more likely to have gonorrhoea and far less likely to have syphilis than older men, which reinforced the fact that they and the young women they had sex with were in different sexual networks or at least distant from each other [39]. This early work was confirmed later by Jennings et al. [40] who showed that approximately 75% of men have sex with women who are less than 2 years older or younger than themselves. More recent studies in specific populations such as those with chlamydia and gonorrhoea in an STD clinic found that the majority of men and women select partners within their own age categories, <19; 20–29 and >29, indicating that sexual partnerships were formed within the context of social relationships where aggregation by age is common [34]. Other research into sex partners of people using dyadic data shows similar patterns [2, 41].
Heterogeneity
The second interesting phenomenon in the 1985 Colorado Springs paper was that although the people who originally tested positive for gonorrhoea had similar number of partners as others in different components, a small group of them and their partners linked in one component had the highest number of infectious days or “force of infectivity”, allowing exposure to many more partners over a longer period of time. The finding that a small number of people affect gonorrhoea epidemiology on a large scale had been theorised earlier by Yorke and Hethcote who discovered that when screening for gonorrhoea was introduced in the United States, the detection of an additional 10% of gonococcal infections in women resulted in a 20% decrease of infections in the years immediately following [42]. This gave rise to the theory that a subpopulation must exist to maintain an epidemic of gonorrhoea, in which the incidence is so high that 20% of all new infections are pre-empted by the susceptible already being infected. Reinfection with gonorrhoea was estimated to occur twice annually in this population. In 1991 Brunham unified empirical data with mathematical theory when he posited that for an infection to be sustained in such a small subpopulation (given sufficient number of partners to whom infection could be transmitted), productive chains of transmission must exist, therefore linking members together directly or indirectly through a network of sexual relationships [43].
The importance of this heterogeneity in numbers of sex partners in addition to duration of infectiousness was emphasised in a landmark study in 2001 by Liljeros and Stanley. They noticed the peculiar shape of the number of reported lifetime sex partners reported in population-based samples of men and women from Sweden, to which they fit a power law [44]. A true power law is analogous to by far the majority of people being between 4 and 7 ft tall, while the minority are spread out over the next two or three orders of magnitude with a minute proportion being over 4,000 ft tall, which clearly does not represent reality. However, the number of flights leaving major cities across the globe may well satisfy this distribution, with many cities such as Cape Town, South Africa, having lower number of flights, and places such as London, England, having greater number. Although the fit of their model and precise definitions and generating functions of scale-free distributions have been hotly debated [45, 46], the fact remained that a small proportion of highly active people with number of partners in the thousands or tens of thousands are likely to be efficient transmitters of gonorrhoea and other sexually transmitted pathogens, who affect the transmission and the epidemiology of STIs disproportionately through their networks. Following closely on the first study, Schneeberger published a similar analysis of empirical data [45, 46], which stimulated a focus on new methods by which to describe the non-random process by which sex partners are recruited [46–48], and the resulting skewed frequency distribution of numbers of partners [49]. Assumptions in the traditional compartmental model (a) of homogeneity, even when populations are divided into classes denoting the number of sex partners, and (b) of randomness in which an individual has the same probability of coming into contact with any other individual in the population regardless of demographic, physical proximity or disease status are insufficient in allowing for heterogeneity, and the interdependence of one individual’s disease status on that of his or her neighbours remains problematic. Last, the fact that people generally have a finite number of sex partners whom they can infect and be infected by, means that random mixing of sexual partners within compartments is invalid, as when the number of sex partners infected rises, the number of potential susceptibles decreases.
The range in numbers of sex partners which people from samples of the general population report may not resemble that of populations with confirmed STI, or of contacts of people with STI, simply because the latter generally have more sex partners, at least enough to sustain transmission than those populations in which STI are not transmitted. Second, the number of sex partners may not be recorded—only those who have enough locating data to allow contact tracing to take place, therefore underestimating the total number of partners. Allowing for various biases, it is clear that York and Hethcote’s 1978 concept of the population incidence of gonorrhoea being made up of an average of two rates, one in the core where the prevalence is about 20% and where a significant proportion of infections are transmitted to those who were previously infected [42] and the rate in the remainder of the STI-susceptible population with fewer number of partners in which new infections were seldom pre-empted, presages both the current empirical and theoretical data.
Ethnic Heterogeneity
Discussions of initial investigations of ethnic differences and partnerships between people of two different backgrounds have also evolved. People who have sex with people from a different ethnic group can potentially serve as conduits for pathogen transmission where the disease incidence between the two groups differs substantially. Inter-ethnic partnerships are associated with higher risk of disease [34, 50, 51] and exposure to higher number of partners [50]. In a study of women attending an STD clinic in Tennessee [52], sexual behaviour for Black and White women was similar. However, the prevalence of infection in Black women was 36.7% when compared with 27.1% in White women. The likely reason for higher rates in Black women was that their chance of having sex with an infected man was higher due to his having high number of partners (see below for why this pairing pattern may occur). This illustrates the point that even in the absence of sexual network data, using only the number of partners of each case can lead to incorrect assumptions of disease risk, and that mixing matrices based on the number of partners may also be inadequate to describe a pattern which relies on race and not just sexual activity class. Also, the fact that Blacks have, and have had, historically higher rates of STIs, and that they form a marginalised group in American society [52–54], is important to one’s understanding of the risk of gonorrhoea in Black women attending an STD clinic in Tennessee.
A comparison of networks containing gonorrhoea and chlamydia revealed disproportionately large number of cases in African Americans in Colorado Springs, USA, and First Nations (North American Indians) in Winnipeg, Canada [55]. Both populations formed only 7% and 3% of the population but bore 34% and 18% of the number of cases, respectively, which raises interesting questions concerning the structure of these networks and whether similar structures may exist in all minority populations which bear disproportionately higher burdens of illness. In the absence of empirical network data, Laumann and Youm [56] have provided a network explanation for consistently higher rates of STI in African Americans. They demonstrated that, like in the earlier study by Quinn, while many African Americans have comparable number of partners to their White counterparts, more African Americans have partners who have many more partners. This was confirmed empirically in a household sample of respondents living in a high-incidence, minority neighbourhood who were asked to nominate up to six people with whom they had had sex in the last 3 months [50, 57]. The partners of African-American women were more likely to have many partners than those of Caucasian women. In addition many more partnerships take place between African Americans only, whereas a higher proportion of Whites and Hispanics have sex with African Americans, thus concentrating the infections within minority communities. In a notable example, 92% of African-American women reported having sex with African-American men, but only half of the African-American men reported sex with partners from their own ethnic background [34].
Geographic Heterogeneity
While a certain amount of homophily within sexual networks defined by geography and socioeconomic characteristics is ideal for targeting certain populations, intervention with only the residents of that area only would ignore important routes of infection into the community. The existence of people who have sex with others from another region—spatial bridgers—in itself may be an adaptation to a geographic, ethnic, language-specific or age group-targeted approach, in that relationships which elude the intervention are best “fitted” for survival as they are less likely to be detected and thus chains of transmission less likely to be interrupted. Sexual partnerships have been documented in a Canadian province between people living in communities 500 or more km apart with only water and ice road access [58] (Fig. 5.2); across the United States [59] (Fig. 5.2) and internationally [1] (Fig. 5.3), [60] (Fig. 5.4), where people from inside the community have sex with those from far away [50, 61–63], forming a conduit through which infection can pass.
Overview of potential transmission routes in the province of Manitoba, Canada. Individual points represent cities, towns or aboriginal reserves. The largest point in southern Manitoba represents Winnipeg. Lines represent sexual contacts between individuals in different geographic locations. The number of lines between points represents the number of sexual contacts identified. The exception is the thick line between Winnipeg and northern Manitoba. This line represents approximately 25 sexual contacts. Numbers at each of the points represent components of the graphs and provide a means to trace the extent to which geographic bridges occur within that component. Shaded numbers indicate components containing both gonorrhoea and chlamydia. Clear numbers are chlamydia-only components, BC, SK, ON and ND represent network connections to British Columbia, Saskatchewan, Ontario and North Dakota, respectively. Some community locations have been altered slightly for visual clarity. The locations of sexual northern communities with small populations have also been altered. Reprinted with the permission of the Editor, Sexually Transmitted Diseases from Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001;28(1):14–24
Geographic graph of the first 40 cases of AIDS, by city residence. Where only state information was available, nodes were placed at the capitals of that state. Adapted from Auerbach DM, Darrow WW, Jaffe HW, Curran JW. Cluster of cases of the Acquired Immune Deficiency Syndrome; patients linked by sexual contact. Am J Med. 1984;76:487–492
Residence of out-of-city sex contacts named by syphilis patients in cities of 1,000,000 or more population March 1962 (for distances of more than 50 miles). From Donohue JF. Problems posed by population mobility in control of syphilis. In: Proceedings of the world forum on syphilis and other trepanematoses. Atlanta, GA: U.S. Department of Health, Education and Welfare; 1964:38
Recently, a graph of places where male sex workers who also used drugs, had previously worked prior to coming to Houston had many of the cities in common with the above two maps [59]. The importance of people who have sex partners from their own geographic area and outside it, though small in number (5–8%) [61, 63], is not to be underestimated. “Bridging” is an essentially social/sexual network mechanism by which well-defined communities revealed in epidemiological investigations with disproportionately large burdens of STI transmit to the remainder of the lower risk more general population.
In addition to the challenges of effectively managing patients and their partners across jurisdictional boundaries, spatial bridgers have been found to have more sex partners compared with residents who have sex with people from within their communities in Ontario, Canada [64], as well as higher HIV rates and increased amounts of drug injecting in Houston, Texas [59]. Although data analysed from Sweden revealed no demographic, laboratory test or socio-economic differences between individuals with long-distance relationships and those without, concurrency and numbers of sex partners were not measured [61]. The higher risk nature of people who have sex with people both within and outside of their own communities is supported to some extent by descriptions of exogenous higher income, highly educated males, who have higher number of sex partners than men from King County, Washington, consistent with a profile of sex work clients [33, 63].
Spatial bridgers seem to have higher risk behaviours and infection levels than do people who have sex with members of their own geographic region and form effective conduits for transmission. Further investigation is needed into networks containing extra regional relationships in order to establish causes such as individual personality traits such as extroversion, adventurer and leadership roles within networks, and most important, the existence of economic networks where sex is exchanged for money, goods or shelter.
Heterogeneity in Age
Disparities in age have also been associated with higher risk behaviours. Jennings et al. [40] showed that approximately 25% of men have sex with women who are more than 2 years older or younger than themselves. These men were more likely to have had more sex partners in the past 2 months; engaged in commercial sex, been drunk at least once a week in the last 3 months, used drugs including alcohol and marijuana, or sex with someone who used drugs or alcohol at last intercourse. On multivariate analysis only the partners’ taking drugs or alcohol at last sex was associated with age bridging. A more recent and noteworthy account of the effects of disparate ages within sexual networks has been in HIV networks in Africa, where the tendency of much older men to partner with younger women has accelerated the spread of HIV into younger, lower prevalence sexual networks, and increased the epidemic size by continually infecting new generations of susceptibles [65–67]. Sociometric network data have revealed wide age ranges within components due to the presence of some members who are 10 or more years older than the mean age. These older members are the equivalent of “spatial bridgers” who form a conduit between populations with different rates, adversely affecting the epidemiology of HIV, and HSV2. In another empirical study young African Americans who had sex with people 2 or more years older or younger than themselves were more likely also to have sex with people outside of their social network [68–70].
Social Aggregation
The third major finding in the 1985 Colorado Springs paper on gonorrhoea was that although 529 people named 1,009 places which they frequented to “pick up” sex partners, only six places accounted for common establishments frequented by 51% of all cases [28]. The importance of a large number of people clustering around a relatively small number of common social venues was re-emphasised in the sexual network analysis of a gonorrhoea outbreak involving 182 people in Alberta, Canada, carried out simultaneously with a traditional epidemiological case–control study [71]. The case–control study, in which controls were drawn from those with medical visits to the local health centres who did not have a laboratory diagnosis for gonorrhoea nor had been named as contacts, demonstrated that risk behaviours, such as numbers of partners, and risk markers such as age, were not associated with infection. However, those who had frequented a certain bar which was a known “pick up joint” were more likely to be infected than those who had attended different bars or none at all. Construction of the sexual networks of all cases and contacts revealed a giant component of 39 individuals (21% of the cases and contacts). All clients with gonorrhoea were linked to the bar, and then by sexual intercourse to their corresponding network members, resulting in a bipartite network of one place (the bar) and 89 people representing 49% of the entire population of cases and contacts. Identifying central locations such as this can be immensely helpful in targeting prevention efforts such as urine or point-of-care testing, condom distribution and educational messages (Figs. 5.5 and 5.6).
Network members (n = 89) viewed by their connection to a bar associated with gonorrhoea acquisition. A prefix to the identifier of “m” denotes a male, while “f” denotes a female sex partner. Bar patrons possessed significantly higher centrality measures compared to non-patrons. Adapted from Sexually Transmitted Infections; De P, Singh AE, Yacoub W, Jolly AM. Sexual network analysis of a gonorrhoea outbreak. Sexually Transmitted Infections 2004;80:280–285
In conclusion, assortative sexual mixing patterns between age groups, ethnic groups and geographic areas are proxy indicators of social aggregation—the primary driving factor in our choices of both social and sexual associates, and an essential part of an ecological niche for STIs. Another essential part are the disassortative or heterogeneous associations inherent in the structure of sexual networks, which transmit pathogens from higher to lower incidence regions of the network and enhance the resilience of both against local change. We have attempted to show here that social and sexual network affiliations are reasonable explanations for geographic, ethnic and age mixing patterns. Mixing between people with different numbers of partners is an inherent structural characteristic within a social or a sexual network, the implications of which cannot be totally accounted for by dyadic data or partner mixing matrices alone. Measuring only the number of partners each person has cannot fully explain two graphs with differing capacities for spread despite identical number of nodes and links [6]. Likewise, epidemiologists [71] have demonstrated that information centrality and not numbers of partners is associated with gonorrhoea exposure in an outbreak. Last, physicists [47, 72, 73] have elucidated naturally occurring graphs which are highly connected, yet extraordinarily resistant, to random disturbances of links or nodes. Thus, scientists from three different disciplines have shown that the entire network and its structure cannot be reduced to the number of sex partners of each member without considerable loss of meaning.
While the first part of the paper focussed on general themes and common results found in most sexual networks, the remainder of the paper is dedicated to aspects of pathogen-specific networks.
Pathogen-Specific Networks
In this section we review the distinctive characteristics of the networks in which specific pathogens circulate as these may be key to the pathogen’s survival. At the start of each section we give a brief review of the molecular epidemiology of pathogens with respect to transmission within networks. This molecular focus is on how variation in the genetic characteristics within a species can be used to identify different “types” or strains within that species. In turn, analysing the transmission dynamics of these individual strains can provide a richer understanding of transmission dynamics within a network. In general, pathogen strains in people who have had direct sexual contact have shown concordance. This is particularly true of bacterial STI where strain types are more stable over time, but even with HIV infection, couples have closely related types of virus. It should be noted that particularly in viral transmission, the time since HIV infection and strain typing is crucial, in that current partners may not have similar strain types to those partners with whom the index case was having sex in previous years. Another primary consideration is that of selecting an appropriate gene from the organism. There is a balance between selecting a gene or genes with sufficient variation such that two infected people who have not had sex will have a greater probability of being discordant than those who are infected and have had sex. Likewise, a gene which is highly variable and changes randomly as an adaptation to the human immune system may not be concordant in two infected people who have had sex, even though they were tested within a few days of each other. Last, it must be noted that just because people share a common strain of a bacterium, this does not always indicate a direct sexual relationship. However, if one assumes that one individual infected another and the strains are discordant, this is a very specific indication that even though they may have had sex, the index case could not have infected that particular sex partner.
Gonorrhoea and Chlamydia
There are only a small number of articles which describe sexual networks containing gonorrhoea, reflecting not only the small number of scientists working with social networks and infectious diseases but also the number who have access to experienced, knowledgeable and meticulous and public health or research staff. The advantages and validity of contact tracing as a method for collecting sexual network data of people with gonorrhoea have been confirmed consistently with a variety of old and new typing systems. Wylie et al. demonstrated that reported partnerships of people infected with N. gonorrhoea were consistent with serotypes of gonorrhoea, and by pulsed field gel electrophoresis (PFGE), but not by typing of the opa gene [74]. The opa gene encodes an outer membrane protein of the gonococcus and as such the protein is exposed to the human immune system. The variability observed in this protein is believed to be one of the means by which a gonococcal cell can evade the host immune system. This variability arises from genetic variability within the opa gene itself and it is this variability that is the basis for designating different strains based on opa gene type. Since the gene is responding to the human immune system, typing methods based on this gene may therefore be subject to more variability than the other two methods. However, it was used successfully by Ward, Day and Ison in an analysis of gonorrhoea-infected sexual network members in Sheffield and London [75]. Later on, validation of contact tracing data in early work was confirmed by sequencing the porB gene [76], and by NG-Mast which includes porB and TbpB [77, 78]. While gene sequencing is not a sensitive discriminating method by which to detect unreported relationships between people, it is very specific. Concordant strains between the majority of partners have been validated, with only a small minority of strain discordant partners. This small minority were usually a result of multiple partnerships of both individuals in a short space of time.
Microbiological analysis of sequences of chlamydia and contact tracing show good convergence [79, 80]. The most common recently used method to distinguish chlamydia types is to sequence omp1 which encodes the major outer membrane protein (MOMP). Due to stability of omp1 one can use the sequencing results to confirm that contact tracing actually follows transmission routes with high specificity but not sensitivity. Where it may be of most help is assessing infection source in the case of discordant gene types by ruling out transmission if the sequences are discordant, and searching for others.
To form a viable eco-niche for gonorrhoea the number of susceptible people infected by each infectious person must be greater than one, on average. Mathematical ecologists have contributed a valuable heuristic device by which some minimum requirements may be defined [42, 81, 82]. There needs to be sufficient number of sexual partners in at least some of the networks to transmit infection with a high enough probability during the duration of the infectious period of gonorrhoea. Given the effort and human error in defining most of these estimates, few empirical data are available. As gonorrhoea is one infection for which more data exist than for many others it is a useful template for analysis, which is extendible to other STIs for which less data are available. Table 5.1 gives some basic parameters [83].
The infectious period for gonorrhoea starts at the earliest 1 day before the onset of symptoms, during the last day of the incubation period, and ends when the person is treated (which could be incidental) or when the infection spontaneously resolves (about 6 months). As many studies have reported substantial number of patients who continue to have sex despite frank symptoms [3], it is wise to assume that not all those who have symptoms will seek treatment immediately, or ever. The transmission probability per intercourse has been reported as 0.25 using positive cultures from infected cases exposed to the contact population in which the rate was estimated [84] and 0.32, using strain-specific methods where each person in the couple had confirmed gonorrhoea (Apedaile, unpublished). This relatively high transmission probability compared with that of chlamydia at 0.10 may make up for the shorter duration of infection which may be truncated by symptoms appearing within 5–7 days in about 65% of males, leading the client to seek treatment. Also it is much more likely to be transmitted in a short-term sexual relationship than some other pathogens, with lower transmission probabilities but long duration times, thereby “favouring” transmission by casual, anonymous and commercial sex. It is likely also that those people who prefer short-term casual sexual relationships have time to recruit more sex partners.
In 2001, Wylie and Jolly used laboratory and sexually transmitted disease notifiable disease registry data to clearly define specific networks in which gonorrhoea and/or chlamydia were present, in Manitoba, Canada [2]. They examined the 23 largest networks of 10 or more people. Two basic types of networks were identified: radial, in which a few members had five to 13 partners linked together by the majority who had only between one and three partners, and linear, in which degree centrality was not as varied, and people were linked through only one to four partners (Figs. 5.7 and 5.8). Comparison of the two types of networks revealed that the 16 linear networks contained individuals with gonorrhoea, and 10 of those contained individuals infected with both gonorrhoea and chlamydia. Members of linear components were more likely to be North American Indians (First Nations), have positive test results and come from different geographic locations. The higher proportion of First Nations people diagnosed with gonorrhoea, and correspondingly with chlamydia and gonorrhoea coinfection, was quantified in a later publication [58], and definitively in 2007 [3], where a cluster analysis of STI in the Manitoba population demonstrated three distinct types of networks, two of which had lower degree centralisation (variation in degree) denoting linear networks. They were composed of predominantly First Nations people from rural Manitoba. Members of these networks were also more likely to have gonorrhoea, and coinfection with chlamydia, which was more likely to have been laboratory confirmed when compared with the largest cluster of primarily urban, Caucasian, chlamydia-infected networks. Higher proportions of ethnic minorities have also been reported within gonorrhoea networks in the United States [63] as well as higher proportions of bridgers when compared with chlamydia networks.
Examples of radial (a) and linear components (b) identified in Manitoba by network analysis. Each square and circle represents an individual, with squares denoting gonorrhoea, circles chlamydia and both together coinfection. “M” within the square or the circle denotes males and “F”, females. Grey rectangles represent unnamed contacts, and solid black shapes represent confirmed cases of infection. Reprinted with permission of the Editor, Sexually Transmitted Diseases, from Wylie JL, Jolly AM. Sexually Transmitted Diseases 2001; 14–24
A network from a sampling study in Winnipeg, Manitoba, of all reported cases of gonorrhoea and chlamydia and their contact. The circles represent individuals who were not tested or who tested negative. The triangles show infection with chlamydia and the squares show people with gonorrhoea. The person represented by the solid triangle was selected by random sampling, and the other two solid shapes represent individuals with chlamydia and gonorrhoea coinfection who also had repeated infections over a 7-month period. Adapted from Wylie JL, Jolly AM. Sexually Transmitted Diseases 2001; 14–24
The lower median number of partners in the gonorrhoea components in Manitoba compared with chlamydia networks seems counter-intuitive, as many earlier studies characterised individuals with chlamydia as having higher education and income levels, and lower risk profiles [4, 5, 32, 92]. However, a study comparing sociometric gonorrhoea and chlamydia networks in which partner elicitation was standardised at 3 months prior to diagnosis showed that people with gonorrhoea did have more sex partners both in the past year and past 3 months. In network terms, this would result in more star-shaped or radial structures, and is consistent with the short duration time for gonorrhoea, and the relatively high transmission probability, required for successful diffusion of gonorrhoea in a network [51]. Also associated with higher number of partners is concurrency [93], as it allows for more partners to be recruited in a short time frame. In the Manitoba study, the lower number of named partners of people with gonorrhoea may have been due to the shorter time over which people are infectious due to higher likelihood of the onset of symptoms, prompting many to seek care. Chlamydia infections are more likely to be asymptomatic and when diagnosed are routinely interviewed for partners within the last 3 months [94]. The differences in findings at 3 months and a whole year also suggest that gonorrhoea transmission within a network has a significantly shorter “lifespan” than does chlamydia.
The fact that the majority of gonorrhoea networks, if not all of them, harbour chlamydia also suggests that the network structure for gonorrhoea forms a viable ecological niche for both pathogens. This is supported by the fact that the giant components of bacterial STI networks often contain both pathogens [2, 3, 17, 55, 71, 95–97]. While it is likely that giant components result from expending more effort in contact tracing for clients with both notifiable infections compared to only one [17], and with correspondingly higher number of partner nominations, locations, tests and treatment, it is also clear that the giant components are a minimum estimate of network completeness and connectivity, and in reality the giant component is much larger and more densely linked. Therefore, it is possible that smaller components containing individuals with only gonorrhoea are fragments of de facto giant components, an artificial result of incomplete partner reporting. This is supported by the morphology of networks containing only gonorrhoea [2], which appear similar to that of the periphery of the giant components, with linear branches of one to four partners per client (Fig. 5.7). In one study, a giant component contained higher concentrations of coinfected people with the highest reproductive rates of 0.97 in coinfected non-repeaters, and 1.41 in repeaters [98] compared with people who had either gonorrhoea or chlamydia only. It is logical to hypothesise that the smaller, seemingly disconnected gonorrhoea components are “fed” by similar regions in all STI networks.
The absence of chlamydia in some components, or regions of them, is likely due to STI management practice guidelines which recommend treatment be given for chlamydia on the diagnosis of gonorrhoea given the high rates of coinfection [13, 99]. This is supported by the epidemiological studies which show higher socio-economic status of individuals infected with chlamydia, and more diverse geographical range, associated with screening incidence rather than true occurrence of infection. The reality is that these demographic characteristics reflect (a) those who are screened and tested positive, which in turn indicates good access to preventive care, and/or (b) those who are well educated and able to attend clinics for annual or biannual physical examinations at which STI screening is recommended.
Age differences in people with chlamydia and gonorrhoea reflect the propensity of C. trachomatis for columnar epithelial cells, which are more exposed in the cervices of younger women due to ectopy, lack of immunity [100] and, of course, their choice of partners who are of similar ages. The average age of people infected with gonorrhoea is higher and due to homophily, network members also are older. Many of the differences between networks affected by the two pathogens can be explained by homophily, whether geographic, demographic and behavioural.
Syphilis and HIV
Contact tracing for syphilis is the most long-standing intervention to prevent infection [101]. Testing for syphilis usually involves indirect methods detecting non treponemal antibodies, and those which detect treponemal antibodies in sera. Microscopy of swabs of the chancres in primary syphilis reveals treponemae, but this test is restricted to those sites in the early stage of the disease. Recent advances in amplification technology have prompted scientists to amplify three genes for detection, diagnosis and typing with limited results, especially in sera samples. Only half the samples from ulcerative swabs in one study were appropriate for sequencing, although no between-couple comparisons were done [102]. Due to the difficulty in obtaining good amplified DNA from sera during the secondary and latent periods, the short period and limited number of chancre sites for swabs during the primary period, and the early stage of syphilis sequencing science, contact tracing has not yet been confirmed or refuted by treponemal sequencing.
In contrast, much work has been done with HIV, although the results and their implications are complex due to the clonal nature of the infection, and the long asymptomatic period which delays testing. However, a study of a network of people transmitting and contracting HIV was correlated with phylogenetic data spanning a decade [103]. Two regions of viral DNA were used in the analysis, p17 gag and env V3, and common methods of constructing the trees, neighbour joining (used in the gonorrhoea sequencing above), Fitch–Margoliash and maximum likelihood methods, gave the most accurate trees, while the use of data from both regions resulted in greater accuracy than either one alone or different methods of phylogenetic tree construction [104]. Due to the rapid changes in this virus over time and within and between people, additional appropriate regions can be selected and analysed to reflect transmission networks accurately.
The first sexual network of men with AIDS linked by sexual intercourse [11] (Fig. 5.8) resembled some of the early networks of syphilis, which spanned the whole of the United States and parts of Europe (Fig. 5.4) [60]. After a long decrease, syphilis has been on the increase in the developed world since the late 1990s [105], due in part, to the reduction in funding devoted to it [63, 106]. Major outbreaks have usually involved men who have sex with men (MSM) [107–111], and sex workers who exchange injection drugs or money for sex [112–114]. Occasionally, recent outbreaks have been in diverse populations not usually associated with syphilis transmission, such as a group of middle class young women from suburban Atlanta who hosted sex parties with African-American and White men on alternate weekends (Fig. 5.9) [114], and another group of predominantly First Nations heterosexual adults, many of whom used alcohol (http://www.wrha.mb.ca/healthinfo/preventill/files/Syphilis_080604.pdf). Sexual networks of MSM and those in which sex is exchanged directly for money or drugs seem to provide the most common environment for syphilis transmission, evinced by a great variation in the number of partners, some with very high number of partners and concurrent sex partners. Less traditional sexual networks such as those in Atlanta are remarkable in that they also had unusually high number of partners and concurrency and one other essential feature—they contained some network members from other socially distant networks.
As syphilis was a relatively rare infection in 1996, transmission outside of traditional MSM, sex work and IDU populations was enabled only by bridging, achieved only if networks contained members from diverse groups in which syphilis is endemic. This importance of sex partners who travel long distances was recognised in 1962 (Fig. 5.3, above). This is consistent with recent findings from a cluster analysis of sexual networks in which all of the syphilis cases were found in one cluster, characterised by network members bridging all geographic regions of Manitoba [3]. The existence of social or geographic “long distance” links may be indicative of prevention programs successfully eradicating infection from certain populations such that incidence in other populations in which control measures are relaxed becomes more critical. As above, these long distance or heterogeneous links are all the more effective for transmission due to associations with other high-risk behaviours [59, 64].
A second feature of the syphilis networks is their density; that is, a high proportion of connections of all that are possible exist—higher than those in gonorrhoea or chlamydia graphs. The graphs of the young women who initiated sex parties in Atlanta and the other from Fulton County, Atlanta, demonstrate a plethora of cliques and cores, areas in the graph where there are more connections between people than in other areas. Interestingly, a graph of penicillinase-producing N. gonorrhoeae outbreak in a gang showed similar features, and did contain two cases of early syphilis (Fig. 5.10) [95]. Although more sparsely connected, networks of clients with syphilis in Vancouver, British Columbia, show similar multiple connections between people [20], rather than the linear graphs of chlamydia and gonorrhoea. The sparseness may be a result of unreported or anonymous partnerships, as a later paper on this outbreak revealed 88 or 6% repeated infections [115]. It is legitimate to argue that these graphs are highly connected given the fact initial rises in the number of cases stimulated deeper investigations, resulting in higher number of connections being found. However, it is equally valid to hypothesise that the networks with connections marked currently by syphilis transmission were as dense as previously, and required only occasional long distance or heterogeneous bridges to funnel the infection into a viable network. In conclusion, it appears that in order for syphilis to be endemic, higher number of sexual connections are required than those for gonorrhoea or chlamydia maintenance alone.
Graph of the largest component in gang-associated STD outbreak, Colorado Springs 1989–1991, n = 410. Reprinted with permission from the Editor, Sexually Transmitted Infections from Potterat JJ, Phillips-Plummer L, Muth SQ, et al. Risk network structure in the early epidemic phase of HIV transmission in Colorado Springs. Sexually Transmitted Infections. 2002;78(Suppl 1):I159–I163
Two of the earliest graphs of sexual networks of people with HIV are similar in one respect; they contain people surrounded by many sexual contacts like star bursts. They also contain loops or enclosed circles known as bicomponents in which each person within the subgroup is connected to at least two others, and if one of the members is removed, the linked component does not break apart. In other words, superfluous members within a bicomponent supply each member with at least one extra source of infection, rendering the transmission path robust to intervention. It is remarkable that even in 1982, early on in the AIDS epidemic, a path of six links enclosing six men from New York, San Francisco and Los Angeles who had direct and indirect sexual contact with each other and displayed symptoms of HIV infection was able to be identified (Fig. 5.11) [11]. At the time it was estimated that the population at risk comprised 2,500,000 male Americans between the ages of 15 and 54 who were homosexual [1]. These loops were all the more evident in a small group of MSM from Iceland [116], due to the isolated nature of the island and low population (Fig. 5.12), but are still present in later graphs.
Graph of the sexual links of the first 40 cases of AIDS showing an enclosed loop of NY11, NY17, NY9, 0, LA6 and SF1 reprinted with permission of the Editor, Social Science and Medicine, from Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Social Science and Medicine 1985;21(11):1203–1216
Sexual network of a male homosexual network in Iceland, showing enclosed loops. Reprinted with permission of the Editor, Journal of Acquired Immune Deficiency Syndrome from Haraldsdottir S, Gupta S, Anderson RM. Preliminary studies of sexual networks in a male homosexual community in Iceland. Journal of Acquired Immune Deficiency Syndromes 1992;5(4):374–81
The presence of the radial structures of high-degree males is explained by the finding that in the first group of men from North America, the highest number of sex partners reported was 1,560 per year (range 10–1,560), with the average being 227 [1]. This was an early indication of the heterogeneity in partner choice needed to first introduce HIV into a population, which then could be sustained by the densely linked networks containing a number of circular structures. Population-based data collected much later and analysed using methods from physics and mathematics confirmed the important role of such people with very high number of partners in transmission.
Similar to the relatively rare syphilis infections, HIV is uncommon in many populations infected with chlamydia and gonorrhoea in the developed world. Therefore, new HIV infections within sexual networks are mostly exogenous, whether by virtue of geography, demography, culture or behaviour. This was demonstrated in Figs. 5.3 and 5.4 above, and elucidated in a recent paper showing cities in which male sex workers had worked prior to working in Houston, Texas, Fig. 5.13 [59]. The male sex workers who had worked in multiple cities were also more likely to be HIV positive, three times more likely to inject drugs and had double the number of sex partners than male sex workers who worked in Houston only. Higher number of partners and rates of anal intercourse were also reported in people who bridged small, remote aboriginal Canadian communities [64], and in men who arrange to have unprotected sex with other men through Internet Websites [117].
Cities where male sex workers (MSWs) had traded sex for money before coming to Houston. A line indicates a bridge between cities. At least one MSW traded sex for money in each of the two cities connected by the lines. Reprinted with permission of the Editor, Journal of Urban Health from Williams ML, Atkinson J, Klovdahl A, Ross MW, Timpson S. Spatial bridging in a network of drug-using male sex workers. Journal of Urban Health: Bulletin of the New York Academy of Medicine 2005;82:i35–i41
Syphilis networks and HIV networks are similar in that they contain people with large number of sex partners, they contain circular bicomponents with more than one independent transmission route between people [118, 119] and they contain sex partners from distant locations. These characteristics converge to form viable ecological niches for both pathogens, as both are relatively rare, and both have low transmission probabilities. Of course, once both are extant in a network, the ulcerative nature of syphilis potentiates HIV infection, while the assault of HIV on the immune system exacerbates syphilis infection and may prolong syphilis infectiousness [120].
Prevention and Networks
The traditional method of contact tracing to interrupt the chain of STI transmission has been in use in various jurisdictions at least since the 1940s and longer in others [101]. In essence it is a form of network sampling in which the infection or transmission network is sampled such that only those contacts or network members who have positive laboratory results are interviewed for their contacts. The emphasis, therefore, is on people with infection and on locating and eliciting their contacts so that they can be examined, tested and treated. Interestingly, the concept of a sexual network was referenced in early work by mathematical modellers who pointed out that greater emphasis should be placed on the identification of contacts with whom the case had had sex just prior to the onset of symptoms (upstream contacts), rather on those who are infected by the index case (downstream contacts) [42]. This concept is important, as it is more likely that the upstream contact is an effective transmitter as he or she has infected one person already—the current case—whereas the ability of the subsequent contacts to spread infection is unknown.
Enhancement of traditional contact tracing using social network methods provides public health staff with various advantages. The first of these is a more client-friendly approach such as that used by sociologists and anthropologists which is fundamentally more interactive than that employed by medical staff which is more clinical, objective and more socially alien. Social scientists have traditionally collected information from people by means of observation, ethnographies and open-ended questionnaires, all of which are more varied and scientifically rigorous than the questionnaires used in contact tracing [121, 122]. Social network methods are focussed on the entire component rather than on each individual, and on the social context in which the sex is taking place. There is more emphasis placed on interviewing, testing or treating the contacts epidemiologically as they may be equally likely, if not more likely, to transmit infection as the cases [123], and last, a focus on the whole network has the added benefit of being more aware of the presence of coinfections circulating. Less obvious advantages of social network-enhanced interviewing include more careful designation of source and spread cases; characterisation and relationship building with groups at risk of STI; continual evaluation of network membership, risk behaviours, infections and relationships over time and increased opportunities for community-based involvement in interventions [124]. Evaluations of network-enhanced partner notification compared with traditional partner notification have revealed additional people with infections which traditional methods would not have identified, as under the methods section, above.
Venue-Specific Interventions
The early work identifying “lots” of people infected with gonorrhoea and their contacts and the common “pick up joints” patronised by the majority has been confirmed by more recent data, and valuable opportunities for education, testing and treating at those locations have also been identified. Despite the apparent ease of launching interventions in this way, studies evaluating such prevention strategies are rare if not non-existent. The most significant barrier to these initiatives may be establishing trust and gaining permission from the owner of the establishment, though in some cases this has been surprisingly forthcoming [125]. It is important for some establishments, including bathhouses, commercial sex establishments, bars and Internet sites, that the owner’s business depends on returning clients and their well-being is therefore of concern.
Network Interventions
Interventions with networks of individuals as a group have been evaluated in some settings. In an outbreak of syphilis in Vancouver’s population of injection drug users and sex workers an attempt to pre-empt further infections was made by distributing azithromycin (1.8–1.2 g according to weight) to people in the affected area of the city, with additional doses distributed to associates and sex partners [126]. This strategy was intended to reach otherwise unaccessible people at risk in the network, by increasing trust in having people in the affected community deliver the medication, and by its anonymity, which due to the taboo and often illegal nature of exchanges in the area may be welcome. Although reported safe sex practices and knowledge of syphilis had increased in participants when they responded to an evaluation a year later, compared with eligible non-participants, the rebound in incidence of syphilis was greater than expected as infection filtered back into the population. This is consistent with known geographic links with people in northern British Columbia and in the Yukon, the adjacent northern territory, and undisclosed links with infected people, which resulted in a 6% repeat infection rate [115].
More commonly used network interventions involve including leaders or members of a peer group who receive training in intervention and then deliver it, in some manner, within their own networks. Work with peer networks of injection drug users in reducing the risk of infection from injection and sex is likely more efficient and possibly more effective than traditional peer interventions [127]. This approach was tried in Romany people in Bulgaria and in MSM in Russia and Bulgaria and evaluated [128, 129]. Both interventions used sociological methods to identify leaders, who then were trained and conducted HIV prevention by counselling, discussing and offering advice on HIV prevention. After the intervention, participants were more willing to discuss risk reduction, perceptions of network norms of safer sex became more positive and reports of condom use increased.
Future Directions
Network methods, whether used in case contact investigations, outbreak investigations and in analytic approaches to both routine surveillance and research, are intuitively appealing because of a common immediate grasp of the network graphs. The increasing shortage of funding devoted to public health, the globalisation of STIs and their resurgence may be addressed well by network methods. For example, the traditional case–control study in an STI outbreak is unintuitive in that the selection of controls with equal opportunity for exposure is problematic, as is the possibility that they may also become infected. These two challenges render the case–control study labour intensive and costly, while the network approach is strategic in that it focuses attention on both cases and contacts, and the routes by which people become infected. Also, the international spread of LGV has demonstrated the transmission of STI over a vast area such that localised approaches which disregard transmission routes may be less successful than in the past. Last, the changing eco niches which harbour STI have grown in response to screening and treatment in recommended groups, as recognised by Wasserheit and Aral. [7]. The discovery of STI in groups not usually affected again calls for a network approach focussed on specific people and the routes of transmission into and out of those non-traditional groups and reaffirms findings of those ideas.
What remains a challenge is to define more specifically the extent to which network analysis application results in an improvement over previous methods [9]. One of the most important of these is the evaluations of network enhanced contact tracing effectiveness and efficiency, both routinely and during an outbreak. It is to be noted that in some jurisdictions contact tracing interviews have attenuated over time, and many for the bacterial STI are interviews conducted over the phone, which may not give a true reflection of a good baseline for contact tracing. In such settings it is possible that the value network-enhanced contact tracing may be overestimated. However, contact investigations can be amended to include the network approach, both in the interview process and in content, with little additional work over and above traditional methods.
Evaluations of the network methods against “routine practice” can therefore be biased, as much of the improvement may be ascribed to thorough and more complete organisation and delivery of contact interviews, resulting in a higher percentage of contacts interviewed and better and more accurate data from that. A second challenge is the complicated nature of study design and replicability. As contact tracing practices differ so much in jurisdictions and also for different pathogens, what added advantage network investigation brings also varies, though consistent improvements in the number of contacts interviewed, tested and brought to treatment should satisfy most that it is effective. This observational approach is not counted amongst the highest standards of evidence, though it is impossible and may be unethical to perform a randomised clinical trial. First, this kind of intervention is susceptible to changes in social context, which goes against the sterile objectivity of the clinical trial. Second, it is serving largely a vulnerable, marginalised population which by its nature has never typically been well accepted in mainstream institutions such as hospitals. Third, an adequate sampling frame from which to draw a representative random sample of marginalised people seldom exists, and fourth, if a sample is recruited, the chances that the individuals are acquainted with each other and may even have encouraged each other to participate are high, thus compromising the requirement for independent observations.
In order to minimise bias and adhere to high standards of proof, outcome evaluations based on the number of patients nominated, tested and brought to treatment should be conducted alongside process evaluations of the amounts of time spent on interviews: timeliness of interviews after receipt of positive laboratory tests, completeness of data and interviewee opinion of the process. As drug use, sex work and culture of vulnerable people differ between jurisdictions, comparing network-enhanced contact tracing with traditional methods in two different areas may not yield accurate results, so before and after studies may be more suitable. Yet another complication involves the efficiency of public health workers initially spending large amount of time within disadvantaged neighbourhoods. Once staff have identified key informants, the places where people assemble and the general rhythm of life, these may aid immensely in future interventions and inquiries as to changes in drug choices, sex work, availability of injecting equipment and outbreaks. This last is not easily quantifiable, but no less important.
Research into networks has already come far, and future research is bound to reveal new and relevant information. One of the most immediate of the network questions, which has already been partly addressed, is how STI networks differ from those of people without STI. The sexual network of high school students (Fig. 5.14) and another of adults on Likoma Island, Malawi (Fig. 5.15), are the sole examples of a sexual network within a general, relevant population, collected without STI as the motivation. From the high school example it does seem possible that sexual networks of those who are uninfected may resemble those who are, although only for some pathogens such as chlamydia. The implication for prevention is that should chlamydia be able to survive in typical adolescent networks, careful thought may have to be given in intervening the structure of the network, but routine screening, peer education and treatment of key people may be more effective than mass interventions. The second network from Africa demonstrates that the structure and density associated with HIV, syphilis, chlamydia and gonorrhoea can exist in the absence of high rates of these infections. In this example, bicomponents contained people with lower rates of infection than smaller components or other regions of the giant components, indicating a possible protective structure. Higher infection rates in smaller components separate from the main population was also observed in Colorado Springs [130] (Fig. 5.16). However, far more examples of sexual networks in far more diverse contexts are needed before definitive, generalisable statements can be made.
Romantic and sexual network of high school students of a school in a midsize town in the United States Midwest. Reprinted with permission of the editor American Journal of Sociology, from Bearman, PS, Moody J, Stovel K. Chains of affection: the structure of adolescent romantic and sexual networks. American Journal of Sociology 2004;110:44–91
Components of the Likoma sexual networks of size six and larger. Circles represent individuals. Lines represent sexual partnerships between individuals. Black circles: male survey respondents; grey circles: female survey respondents. Larger circles represent network members who were interviewed during the sexual network survey and who were sexually active during the recall period (n = 896). Smaller circles represent network members who were found within the village rosters but were not interviewed because they were outside the sampling frame of this study (all young adults aged 18–35 years and their spouses living in the seven sample villages). The subset of lines not connecting two circles represent partnerships with individuals we were not able to identify in rosters of potential partners. The subset of thicker lines represent partnerships within bicomponents that are between network member who are connected by more than one independent pathway within the sexual network. This graph represents the full network of individuals identified during this study, over a 3-year recall period. Reprinted with permission of the Editor of AIDS, from Helleringer, S, Kohler H-P. Sexual network structure and the spread of HIV in Africa: evidence from Likoma Island, Malawi. AIDS 2007;21:2323–2332
Social network of close personal friends; sexual and drug sharing partners with whom participants shared lodging or food. This diagram shows by the arrows those respondents who were HIV positive located for the most part on the periphery of the network. Reprinted with the permission of the Editor, Sociological Focus, from Darrow WW, Potterat JJ, Rothenberg RB, Woodhouse DE, Muth SQ. Using knowledge of social networks to prevent human immunodeficiency virus infections: The Colorado Springs study. Sociological Focus 1999;32(2):143–158
Research concerning the diffusion processes of different STI may also help in our understanding of STI epidemics. Specifically, the properties of the agent itself may affect diffusion, as does the network itself. As time passes, the interaction between the agent, network and prevention strategies evolves which again affects each of the above. Some work has been done in networks evolving over time [114], though much more needs to be done to explain the roles of individuals with repeat STI, those who do not seek treatment, asymptomatic carriers, members of circular, ring-shaped bicomponents and those with many and possibly concurrent partners who facilitate pathogen spread within a network. Facilitating this work is the advancement in strain typing methods which can be used to more accurately mark the route of a pathogen through a transmission network.
Geographic analyses of networks have also more to divulge, especially as they influence network formation. Concentrations of inner-city minority populations with historically high STI rates influence the dynamics of network formation so that there is a continuing relationship between likelihood of people meeting their neighbours and formation of an STI network. After introduction into a network, shared practices, beliefs and norms may serve to perpetuate STIs over and above individuals’ choices. Using a social network approach may facilitate analyses of social cohesion and social capital which positively influence network norms and lower STI rates.
Last, a deeper understanding of personalities, roles, risk perception and norms of networks may aid in the understanding of people who bridge different ethnic, geographic and age groups, with those who have many more sex partners than their associates. While these encounters may mark higher risks for STI transmission, these same people may be ideal leaders in reaching others to disseminate interventions.
In conclusion, successful sexually transmitted pathogens are those which not only adapt and infect the host but also those that adapt to the whole reservoir—a changing network of humans and control measures. Incidental infection of a single host is simply a “polling” event which is only truly effective if the pathogen can pass further into a network where it can remain endemic. It follows then, that in the changing networks, where we have reached the point of diminishing returns through mass screening, we should focus more precisely on those network structures which complement pathogen characteristics, facilitating their spread. For syphilis and HIV, network density seems to be one of the keys to transmission, as are lower number of partners (degree centrality) and bicomponents in chlamydia and gonorrhoea networks. When these structures are observed in the routine follow-up of STIs, proactive screening for existing and other STIs, outreach and more specific investigation of the context in which the sex occurs will be helpful. In addition, people central to the network can be re-interviewed and offered testing either at the first sign of reintroduction of a pathogen or routinely. Consistent, regular contact with health care providers, whether in clinics or in an outreach setting, can only help build good interactive relationships between clients and health care staff. The advantage of these more precise, locally based approaches is that some of the treatment and testing pressure on pathogens is removed, slowing the evolution of resistance and loss of genes required for testing.
References
Klovdahl AS. Social networks and the spread of infectious diseases: the AIDS example. Soc Sci Med. 1985;21(11):1203–16.
Wylie JL, Jolly A. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex Transm Dis. 2001;28(1):14–24.
De Rubeis E, Wylie JL, Cameron DW, Nair RC, Jolly AM. Combining social network analysis and cluster analysis to identify sexual network types. Int J STD AIDS. 2007; 18(11):754–59. http://ijsa.rsmjournals.com/cgi/reprint/18/11/754; http://dx.doi.org/10.1258/095646207782212234.
Zimmerman HL, Potterat JJ, Dukes RL, et al. Epidemiologic differences between chlamydia and gonorrhea. Am J Public Health. 1990;80(11):1338–42.
Rice RJ, Roberts PL, Handsfield HH, Holmes KK. Sociodemographic distribution of gonorrhea incidence: implications for prevention and behavioral research. Am J Public Health. 1991;81:1252–8.
Klovdahl AS, Potterat J, Woodhouse D, Muth J, Muth S, Darrow WW. HIV infection in an Urban Social Network: a progress report. Bull Methodol Sociol. 1992;36:24–33.
Wasserheit JN, Aral SO. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. J Infect Dis. 1996;174(2):S201–13.
Potterat JJ, Meheus A, Gallwey J. Partner notification: operational considerations. Int J STD AIDS. 1991;2(6):411–5.
Rothenberg R. The transformation of partner notification. Clin Infect Dis. 2002;35(2):S138–45. http://dx.doi.org/10.1086/342101.
Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Intern Med. 2009;20(1):9–13. doi:10.1016/j.ejim.2008.04.002.
Auerbach DM, Darrow WW, Jaffe HW, Curran JW. Cluster of cases of the acquired immune deficiency syndrome; patients linked by sexual contact. Am J Med. 1984;76:487–92.
Rothenberg RB, Potterat JJ, Holmes KK, Mardh P, Sparling PF, et al., editors. Strategies for management of sex partners; sexually transmitted diseases. Vol. 2. New York: McGraw-Hill; 1990. p. 1081–86.
Canadian guidelines on sexually transmitted infections. Ottawa, ON: Public Health Agency of Canada; 2006.
Sexually transmitted diseases treatment guidelines, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(RR-11):1–100.
Brewer DD. Case-finding effectiveness of partner notification and cluster investigation for sexually transmitted diseases/HIV. Sex Transm Dis. 2005;32(2):78–83.
Langille DB, Shoveller J. Partner notification and patient education for cases of Chlamydia trachomatis infection in a rural Nova Scotia health unit. Can J Public Health. 1992;83(5):358–61.
Rasooly I, Millson ME, Frank JW, et al. A survey of public health partner notification for sexually transmitted diseases in Canada. Can J Public Health. 1994;85 Suppl 1:S48–52.
Brewer DD, Webster CM. Forgetting of friends and its effect on measuring friendship networks. Soc Net. 1999;21:361–73.
Brewer DD, Garrett SB, Kulasingam S. Forgetting as a cause of incomplete reporting of sexual and drug injection partners. Sex Transm Dis. 1999;26(3):166–76.
Ogilvie G, Knowles L, Wong E, et al. Incorporating a social networking approach to enhance contact tracing in a heterosexual outbreak of syphilis. Sex Transm Infect. 2005;81(2):124–7.
Annan T, Evans B, Hughes G. Guidance for managing STI outbreaks and incidents. London, UK: Health Protection Agency; 2008:12.
Ghani AC, Donnelly CA, Garnett GP. Sampling biases and missing data in explorations of sexual partner networks for the spread of sexually transmitted diseases. Stat Med. 1998;17(18):2079–97.
Ramirez-Valles J, Heckathorn DD, Vázquez R, Diaz RM, Campbell RT. From networks to populations: the development and application of respondent-driven sampling among IDUs and Latino gay men. AIDS Behav. 2005;9(4):387–402. http://dx.doi.org/10.1007/s10461-005-9012-3.
Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. http://dx.doi.org/10.1525/sp.1997.44.2.03x0221m.
Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc Probl. 2002;49(1):11–34. http://dx.doi.org/10.1525/sp.2002.49.1.11.
Abdul-Quader AS, Heckathorn DD, Sabin K, Saidel T. Implementation and analysis of respondent driven sampling: lessons learned from the field. J Urban Health. 2006;83(1 Suppl):1–5. http://dx.doi.org/10.1007/s11524-006-9108-8.
Salganik MJ, Heckathorn DD. Making unbiased estimates from hidden populations using respondent-driven sampling. Sunbelt Social Network Conference, Cancun, Mexico; 2003.
Potterat JJ, Rothenberg RB, Woodhouse DE, Muth JB, Pratts CI, Fogle JS. Gonorrhea as a social disease. Sex Transm Dis. 1985;12(1):25–32.
Granovetter M. The strength of weak ties. Am J Sociol. 1973;78(6):1360–80.
McPherson M, Smith-Lovin L, Cook JM. Birds of a feather: homophily in social networks. Annu Rev Sociol. 2001;27(1):415–44. http://dx.doi.org/10.1146/annurev.soc.27.1.415.
Blanchard JF, Moses S, Greenaway C, Orr P, Hammond GW, Brunham RC. The evolving epidemiology of chlamydial and gonococcal infections in response to control programs in Winnipeg, Canada. Am J Public Health. 1998;88(10):1496–502.
Rothenberg RB. The geography of gonorrhea. Am J Epidemiol. 1983;117:688–94.
Rothenberg R, Muth SQ, Malone S, Potterat JJ, Woodhouse DE. Social and geographic distance in HIV risk. Sex Transm Dis. 2005;32(8):506–12. http://dx.doi.org/10.1097/01.olq.0000161191. 12026.ca.
Aral SO, Hughes JP, Stoner B, et al. Sexual mixing patterns in the spread of gonococcal and chlamydial infections. Am J Public Health. 1999;89(6):825–33.
Rothenberg R. The relevance of social epidemiology in HIV/AIDS and drug abuse research. Am J Prev Med. 2007;32(6 Suppl 1):S147–53. http://dx.doi.org/10.1016/j.amepre.2007.02.007.
Bush KR, Henderson EA, Dunn J, Read RR, Singh A. Mapping the core: chlamydia and gonorrhea infections in Calgary, Alberta. Sex Transm Dis. 2008;35(3):291–97. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=N&PAGE=fulltext&AN= 00007435-200803000-00015&D=ovftj. 10.1097/OLQ.0b013e31815c1edb.
Laumann EO, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: sexual practices in the United States. Chicago, IL: University of Chicago Press; 1994. p. 750.
Wallace R, Fullilove MT, Flisher AJ. AIDS, violence and behavioral coding: information theory, risk behavior and dynamic process on core-group sociogeographic networks. Soc Sci Med. 1996;43(3):339–52.
Ellen JM, Langer LM, Zimmerman RS, Cabral RJ, Fichtner R. The link between the use of crack cocaine and the sexually transmitted diseases of a clinic population. A comparison of adolescents with adults. Sex Transm Dis. 1996;23(6):511–6.
Jennings JM, Luo RF, Lloyd LV, Gaydos C, Ellen JM, Rietmeijer CA. Age-bridging among young, urban, heterosexual males with asymptomatic Chlamydia trachomatis. Sex Transm Infect. 2007;83(2):136–41. http://dx.doi.org/10.1136/sti.2006.023556; http://sti.bmj.com/cgi/content/abstract/83/2/136.
Foulkes HB, Pettigrew MM, Livingston KA, Niccolai LM. Comparison of sexual partnership characteristics and associations with inconsistent condom use among a sample of adolescents and adult women diagnosed with Chlamydia trachomatis. J Womens Health (Larchmt). 2009;18(3):393–99. http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2010222653&site=ehost-live.
Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis. 1978;5(2):51–6.
Brunham RC. The concept of core and its relevance to the epidemiology and control of sexually transmitted diseases [editorial]. Sex Transm Dis. 1991;18(2):67–8.
Liljeros F, Edling CR, Amaral LAN, Stanley HE, Aberg Y. The web of human sexual contacts. Nature. 2001;411(6840):907–8. http://dx.doi.org/10.1038/35082140.
Schneeberger A, Nat R, Mercer CH, et al. Scale-free networks and sexually transmitted diseases: a description of observed patterns of sexual contacts in Britain and Zimbabwe. Sex Transm Dis. 2004;31(6):380–7.
Eames KTD, Keeling MJ. Monogamous networks and the spread of sexually transmitted diseases. Math Biosci. 2004;189(2):115–30.
Newman M. Power laws, Pareto distributions and Zipf’s law. Contemp Phys. 2005;46(5):323–51. http://www.informaworld.com/10.1080/0010751052444.
Chan DYC, Hughes BD, Leong AS, Reed WJ. Stochastically evolving networks. Phys Rev E. 2003;68(066124):066124-4–24.
Britton T, Nordvik MK, Liljeros F. Modelling sexually transmitted infections: the effect of partnership activity and number of partners on R0. Theor Popul Biol. 2007;72(3):389–99.
Gorbach PM, Drumright LN, Holmes KK. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted infections. Sex Transm Dis. 2005;32(1):7–12.
Stoner BP, Whittington WL, Hughes JP, Aral SO, Holmes KK. Comparative epidemiology of heterosexual gonococcal and chlamydial networks: implications for transmission patterns. Sex Transm Dis. 2000;27(4):215–23.
Quinn RW, O’Reilly KR, Khaw M. Gonococcal infections in women attending the venereal disease clinic of the Nashville Davidson County Metropolitan Health Department, 1984. South Med J. 1988;81:851–4.
Moran JS, Aral SO, Jenkins WC, Peterman TA, Alexander ER. The impact of sexually transmitted diseases on minority populations. Public Health Rep. 1989;104(6):560–5.
Zenilman JM. Gonorrhea, chlamydia and the sexual network: pushing the envelope. Sex Transm Dis. 2000;27(4):224–5.
Jolly AM, Muth SQ, Wylie JL, Potterat JJ. Sexual networks and sexually transmitted infections: a tale of two cities. J Urban Health. 2001;78(3):433–45.
Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation [see comments]. Sex Transm Dis. 1999;26(5):250–61.
Fichtenberg CM, Muth SQ, Brown B, Padian NS, Glass TA, Ellen JM. Sexual network structure among a household sample of urban African American adolescents in an endemic sexually transmitted infection setting. Sex Transm Dis. 2009;36(1):41–8.
Jolly AM, Moffatt MEK, Fast MV, Brunham RC. Sexually transmitted disease thresholds in Manitoba, Canada. Ann Epidemiol. 2005;15(10):781–8. http://dx.doi.org/10.1016/j.annepidem.2005.05.001.
Williams ML, Atkinson J, Klovdahl A, Ross MW, Timpson S. Spatial bridging in a network of drug-using male sex workers. J Urban Health. 2005;82:i35–42. http://jurban.oupjournals.org/.
Donohue JF. Problems posed by population mobility in control of syphilis. In: Proceedings of the world forum on syphilis and other trepanematoses. Atlanta, GA: U.S. Department of Health, Education and Welfare; 1964:38.
Nordvik MK, Liljeros F, Osterlund A, Herrmann B. Spatial bridges and the spread of chlamydia: the case of a County in Sweden. Sex Transm Dis. 2007;34(1):47–53. http://dx.doi.org/10.1097/01.olq.0000222722.79996.4b.
Wylie JL, Cabral T, Jolly AM. Identification of networks of sexually transmitted infection: a molecular, geographic, and social network analysis. J Infect Dis. 2005;191(6):899–906. http://dx.doi.org/10.1086/427661.
Kerani RP, Golden MR, Whittington WLH, Handsfield HH, Hogben M, Holmes KK. Spatial bridges for the importation of gonorrhea and chlamydial infection. Sex Transm Dis. 2003;30(10):742–9.
Calzavara LM, Bullock SL, Myers T, Marshall VW, Cockerill R. Sexual partnering and risk of HIV/STD among Aboriginals. Can J Public Health. 1999;90(3):186–91.
Brunham RC, Cheang M, McMaster J, Garnett G, Anderson R. Chlamydia trachomatis, infertility, and population growth in sub-Saharan Africa [see comments]. Sex Transm Dis. 1993;20(3):168–73.
Boily MC, Brunham RC. The impact of HIV and other STDs on human populations. Are predictions possible? [published erratum appears in Infect Dis Clin North Am 1994 Jun;8(2):xi-xii]. Infect Dis Clin North Am. 1993;7(4):771–92.
Anderson RM, Ng TW, Boily MC, May RM. The influence of different sexual-contact patterns between age classes on the predicted demographic impact of AIDS in developing countries. Ann N Y Acad Sci. 1989;569:240–74.
Ellen JM, Brown BA, Chung S, et al. Impact of sexual networks on risk for gonorrhea and chlamydia among low-income urban African American adolescents. J Pediatr. 2005;146(4):518–22. http://dx.doi.org/10.1016/j.jpeds.2004.11.023.
Morris M, Zavisca J, Dean L. Social and sexual networks: their role in the spread of HIV/AIDS among young gay men. AIDS Educ Prev. 1995;7(5 Suppl):24–35.
Service SK, Blower SM. HIV transmission in sexual networks: an empirical analysis. Proc R Soc Lond B Biol Sci. 1995;260(1359):237–44.
De P, Singh AE, Wong T, Yacoub W, Jolly AM. Sexual network analysis of a gonorrhoea outbreak. Sex Transm Infect. 2004;80(4):280–5.
Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393(6684):440–2.
Barabasi AL, Bonabeau E. Scale-free networks. Sci Am. 2003;288(5):60–9.
Wylie JL, Maclean I, Brunham R, Craig C, Jolly AM. Gonorrhea types and sexual networks in Manitoba, Canada. Sunbelt XIX International Sunbelt Social Network Conference. 1999.
Ward H, Ison CA, Day SE, et al. A prospective social and molecular investigation of gonococcal transmission. Lancet. 2000;356(9244):1812–7.
Liao M, Bell K, Gu WM, et al. Clusters of circulating Neisseria gonorrhoeae strains and association with antimicrobial resistance in Shanghai. J Antimicrob Chemother. 2008;61(3):478–87.
Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189(8):1497–505.
Fredlund H, Falk L, Jurstrand M, Unemo M. Molecular genetic methods for diagnosis and characterisation of Chlamydia trachomatis and Neisseria gonorrhoeae: impact on epidemiological surveillance and interventions. APMIS. 2004;112(11–12):117–84.
Falk L, Lindberg M, Jurstrand M, Backman A, Olcen P, Fredlund H. Genotyping of Chlamydia trachomatis would improve contact tracing. Sex Transm Dis. 2003;30(3):205–10.
Cabral T, Jolly AM, Wylie JL. Chlamydia trachomatis omp1 genotypic diversity and concordance with sexual network data. J Infect Dis. 2003;187(2):279–86. http://dx.doi.org/10.1086/346048.
Anderson RM. Wasserheit JN, Aral SO, Holmes KK, Hitchcock PJ, editors. The transmission dynamics of sexually transmitted diseases: the behavioral component; Research issues in human behavior and sexually transmitted diseases in the AIDS era. Vol Ist. Washington, DC: American Society for Microbiology; 1991:38–60.
Anderson RM, May RM. Infectious diseases of humans; dynamics and control. Vol 1st. Oxford, England: Oxford University Press; 1992:234.
Brunham RC, Plummer FA. A general model of sexually transmitted disease epidemiology and its implications for control. Med Clin North Am. 1990;74(6):1339–52.
Holmes KK, Johnson DW, Trostle HJ. An estimate of the risk of men acquiring gonorrhea by sexual contact with infected females. Am J Epidemiol. 1970;91(2):170–4.
Hook EW, Handsfield HH, Holmes KK, Mardh P, Sparling PF, et al. editors. Gonococcal infections in the adult; sexually transmitted diseases. Vol 2nd. Toronto: McGraw-Hill Information Services Co; 1992:149–65.
Stigum H, Magnus P, Bakketeig LS. Effect of changing partnership formation rates on the spread of sexually transmitted diseases and human immunodeficiency virus. Am J Epidemiol. 1997;145(7):644–52.
McCormack WM, Alpert S, McComb DE, Nichols RL, Semine DZ, Zinner SH. Fifteen month follow-up study of women infected with Chlamydia trachomatis. N Engl J Med. 1979;300(5):123–5.
Garnett GP, Aral SO, Hoyle DV, Cates W, Anderson Jr RM. The natural history of syphilis. Implications for the transmission dynamics and control of infection [see comments]. Sex Transm Dis. 1997;24(4):185–200.
Schroeter et al. Therapy for incubating syphilis: effectiveness of gonorrhea treatment. JAMA. 1971;218(5):711. http://jama.ama-assn.org/cgi/reprint/218/5/711. Accessed 6 May 2010.
Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53.
Renton AM, Whitaker L, Riddlesdell M. Heterosexual HIV transmission and STD prevalence: predictions of a theoretical model. Sex Transm Infect. 1998;74(5):339–44.
Rothenberg R. Change your friends. Addiction. 2006;101(7):913–4, discussion 914.
Potterat JJ, ZimmermanRogers H, Muth SQ, et al. Chlamydia transmission: concurrency, reproduction number, and the epidemic trajectory. Am J Epidemiol. 1999;150(12):1331–9.
Communicable Disease Control. Manitoba health. Sexually transmitted disease control. Winnipeg, Manitoba; 1997.
Potterat JJ, Muth SQ, Rothenberg RB, et al. Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78 Suppl 1:I152–8.
Jolly AM, Wylie JL. Sampling individuals with large sexual networks – an evaluation of four approaches. Sex Transm Dis. 2001;28(4):200–7.
Potterat JJ, Muth SQ, Rothenberg RB. Sexual network structure as an indicator of epidemic phase. Sex Transm Infect. 2002;78 Suppl 1:I152–8.
Jolly AM, Wylie JL. Gonorrhoea and chlamydia core groups and sexual networks in Manitoba. Sex Transm Infect. 2002;78 Suppl 1:I145–51.
CDC. Sexually transmitted diseases treatment guidelines – 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1–80.
Schachter J, Stephens RS. Biology of Chlamydia trachomatis. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sexually transmitted diseases. 4th ed. New York, NY: McGraw Hill; 2008. p. 555–74.
Iskrant AP, Kahn HA. Statistical indices used in the evaluation of syphilis contact investigation. J Vener Dis Inf. 1948;29(1):1–6.
Martin I, Gu W, Yang Y, Tsang R. Macrolide resistance and molecular types of Treponema pallidum causing primary syphilis in Shanghai, China. Clin Infect Dis. 2009;49(4):515–21. http://dx.doi.org/10.1086/600878.
Leitner T, Escanilla D, Franzen C, Uhlen M, Albert J. Accurate reconstruction of a known HIV-1 transmission history by phylogenetic tree analysis. Proc Natl Acad Sci USA. 1996;93(20):10864–9.
Paraskevis D, Magiorkinis E, Magiorkinis G, et al. Phylogenetic reconstruction of a known HIV-1 CRF04_cpx transmission network using maximum likelihood and Bayesian methods. J Mol Evol. 2004;59(5):709–17. doi:10.1007/s00239-004-2651-6.
European Centre for Disease Prevention and Control. Annual epidemiological report on communicable diseases in Europe. 2009:69–71.
Brown WJ, U.S. Department of Health, Education and Welfare, Public Health Sevice, Communicable Disease Center, editors. The first step toward eradication. In: Proceedings of the World Forum on Syphilis and other Trepanematoses. Atlanta: U.S. Department of Health, Education and Welfare; 1964:21–5.
Primary and secondary syphilis – United States 2002. MMWR Morb Mortal Wkly Rep. 2003;52(46):1117–20.
Knapper CM, Roderick J, Smith J, Temple M, Birley HDL. Investigation of an HIV transmission cluster centred in South Wales. Sex Transm Infect. 2008;84(5):377–380. http://dx.doi.org/10.1136/sti.2008.030536.
Northern Ireland, Department of Health and Social Security. Syphilis outbreak. Communicable Diseases Monthly Report (Northern Ireland). 2001;10(9):1–3.
Leber A, MacPherson P, Lee BC. Epidemiology of infectious syphilis in Ottawa: recurring themes revisited. Can J Public Health. 2008;99(5):401–5. http://www.cpha.ca.
Jayaraman GC, Read RR, Singh AE. Characteristics of individuals with male-to-male and heterosexually acquired infectious syphilis during an outbreak in Calgary, Alberta, Canada. Sex Transm Dis. 2003;30(4):315–9.
Patrick DM, Rekart ML, Jolly A, et al. Heterosexual outbreak of infectious syphilis: epidemiological and ethnographic analysis and implications for control. Sex Transm Infect. 2002;78(1):164–9.
2004 Canadian sexually transmitted infections surveillance report. Can Commun Dis Rep. 2007;33S1:1–69.
Rothenberg RB, Sterk C, Toomey KE, et al. Using social network and ethnographic tools to evaluate syphilis transmission. Sex Transm Dis. 1998;25(3):154–60.
Ogilvie G, Taylor D, Moniruzzaman A, et al. A population based study of infectious syphilis rediagnosis in British Columbia, 1995–2005. Clin Infect Dis. 2009;48(11):1554–58. http://dx.doi.org/10.1086/598997.
Haraldsdottir S, Gupta S, Anderson RM. Preliminary studies of sexual networks in a male homosexual community in Iceland. J Acquir Immune Defic Syndr. 1992;5(4):374–81.
Adam BD, Husbands W, Murray J, Maxwell J. Circuits, networks, and HIV risk management. AIDS Educ Prev. 2008;20(5):420–34.
Rothenberg R. Commentary – how a net works – implications of network structure for the persistence and control of sexually transmitted diseases and HIV. Sex Transm Dis. 2001;28(2):63–8.
Potterat JJ, Phillips-Plummer L, Muth SQ, et al. Risk network structure in the early epidemic phase of HIV transmission in Colorado Springs. Sex Transm Infect. 2002;78 Suppl 1:I159–63.
Walter T, Lebouche B, Miailhes P, et al. Symptomatic relapse of neurologic syphilis after benzathine penicillin G therapy for primary or secondary syphilis in HIV-infected patients. Clin Infect Dis. 2006;43(6):787–90.
Brewer DD. Supplementary interviewing techniques to maximize output in free listing tasks. Field Methods. 2002;14(1):108–18. doi:10.1177/1525822X02014001007.
Brewer DD, Garrett SB, Rinaldi G. Free-listed items are effective cues for eliciting addition items in semantic domains. Appl Cognitive Psychol. 2002:343–58.
Zimmerman-Rogers H, Potterat JJ, Muth SQ, et al. Establishing efficient partner notification periods for patients with chlamydia. Sex Transm Dis. 1999;26(1):49–54.
Rothenberg R, Narramore J. The relevance of social network concepts to sexually transmitted disease control. Sex Transm Dis. 1996;23(1):24–9.
Remple VP, Patrick DM, Johnston C, Tyndall MW, Jolly AM. Clients of indoor commercial sex workers: heterogeneity in patronage patterns and implications for HIV and STI propagation through sexual networks. Sex Transm Dis. 2007;34(10):754–60.
Rekart ML, Patrick DM, Chakraborty B, et al. Targeted mass treatment for syphilis with oral azithromycin. Lancet. 2003;361(9354):313–4. doi:10.1016/S0140-6736(03)12335-5.
Broadhead RS, Heckathorn DD, Weakliem DL, et al. Harnessing peer networks as an instrument for AIDS prevention: results from a peer-driven intervention. Public Health Rep. 1998;113 Suppl 1:42–57.
Amirkhanian YA, Kelly JA, McAuliffe TL. Identifying, recruiting, and assessing social networks at high risk for HIV/AIDS: methodology, practice, and a case study in St Petersburg, Russia. AIDS Care. 2005;17(1):58–75. http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2009401955&site=ehost-live.
Kelly JA, Amirkhanian YA, Kabakchieva E, et al. Prevention of HIV and sexually transmitted diseases in high risk social networks of young Roma (Gypsy) men in Bulgaria: randomised controlled trial. BMJ. 2006;333(7578):1098–1101. http://search.ebscohost.com/login.aspx?direct=true&db=cin20&AN=2009350637&site=ehost-live.
Darrow WW, Potterat JJ, Rothenberg RB, Woodhouse DE, Muth SQ. Using knowledge of social networks to prevent human immunodeficiency virus infections: the Colorado Springs Study. Sociol Focus. 1999;32(2):143–58.
Acknowledgement
The authors thank Ms. Aristea Kokkinos for her precise and painstaking help with the references.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jolly, A.M., Wylie, J.L. (2013). Sexual Networks and Sexually Transmitted Infections; “The Strength of Weak (Long Distance) Ties”. In: Aral, S., Fenton, K., Lipshutz, J. (eds) The New Public Health and STD/HIV Prevention. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-4526-5_5
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4526-5_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-4525-8
Online ISBN: 978-1-4614-4526-5
eBook Packages: MedicineMedicine (R0)