Abstract
Pineal and related organs are major extraocular photoreceptors in non-mammalian vertebrates. The pineal organ contains several types of photoreceptor cells, which contribute to regulating light-dependent melatonin secretion and the neural light response, including irradiance detection and wavelength discrimination. Visual opsins and pineal-specific opsins have been identified from the pineal and related organs in a wide variety of non-mammalian vertebrates. Pinopsin and parapinopsin are key opsins for understanding melatonin secretion in the chicken pineal organ and wavelength discrimination in the lamprey pineal organ, respectively. Interestingly, parapinopsin has the molecular characteristics of both vertebrate and invertebrate opsin-based pigments, making it an important photopigment for understanding the molecular evolution of vertebrate visual opsins. In this chapter, we discuss the opsin-based pigments functioning in the pineal and related organs with a focus on parapinopsin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Pineal and Related Organs
The pineal organ is common among vertebrates and is known to be involved in synthesizing and secreting the pineal hormone melatonin, a regulator of circadian rhythms (Underwood 1985; Gern and Greenhouse 1988; Falcon et al. 1989; Samejima et al. 1997, 2000). In mammals, melatonin synthesis is controlled by light information from the retina via the suprachiasmatic nuclei (SCN); however, most non-mammalian vertebrates possess an intrinsically photosensitive pineal organ that regulates melatonin synthesis (Oksche 1971; Collin et al. 1986; Falcon 1999; Okano and Fukada 2001; Bell-Pedersen et al. 2005). In addition to melatonin secretion, pineal photoreceptor cells of lower vertebrates transduce a light signal to an electrical response, which is transmitted to the brain through pineal ganglion cells (Dodt and Heerd 1962; Morita 1969; Dodt 1973). Interestingly, pineal and related organs of lower vertebrates have the ability of not only irradiance detection but also wavelength discrimination, namely “color discrimination” (Dodt and Heerd 1962; Morita 1966; Dodt 1973; Morita and Dodt 1973). Recent studies have revealed that pineal photoreception involves various types of opsin-based pigments including those that are pineal-specific. The photoreceptive mechanism in the pineal and related organs will be discussed in more detail, with a focus on opsin-based pigments in lower vertebrates.
1.1.1 Anatomical and structural Observation of the Pineal and Related Organs
The pineal organ is an outgrowth of the dorsal diencephalon and mostly located just below the skull (Fig. 1.1) (Oksche 1965; Collin et al. 1989; Falcon 1999). Most pineal and related organs in non-mammalian vertebrates are directly photosensitive. It is generally accepted that the pineal organ receives sufficient light to activate pineal photoreceptor cells through the overlaying tissues, which absorb, scatter, and reflect light (Dodt and Meissl 1982). The pineal organ is mainly composed of photoreceptor cells (often called pineal cells) as well as neural cells and glial cells. The pineal photoreceptor cells are broadly classified into two morphological types, the typical and the modified photoreceptor cells (Meiniel 1980). The typical photoreceptor cells in the lamprey, fish, and amphibian pineal organs possess relatively developed morphological features similar to those of the cone photoreceptor cell of the retina, such as outer segments and synaptic ribbons. Furthermore, the typical photoreceptor cells connect synaptically with the second-order neurons, which relay the neural light response (Vigh et al. 2002). The axons of the second-order neurons converge on the pineal stalk to form the pineal nerve that projects to the brain. In contrast, the modified photoreceptor cells have regressed outer segments and are involved in melatonin synthesis and secretion (Meiniel 1979, 1980; Collin et al. 1989). The modified photoreceptor cells are present in pineal organs of a wide variety of non-mammalian vertebrates and are predominant in the reptile and bird pineal organs. It is speculated that during the course of vertebrate evolution, the typical photoreceptor cells were gradually replaced by the modified photoreceptor cells and in mammals by the pinealocytes that do not have the membrane stacks or the capacity for photoreception. This hypothesis is supported by the presence of phototransduction-related molecules, such as opsin and arrestin, in mammalian pinealocytes (Korf et al. 1985a, b).
Schematic drawings of the lamprey brain. (a) Lateral view of the lamprey brain. The pineal organ is situated at the dorsal diencephalon. D diencephalon, M mesencephalon, ON optic nerve, R rhombencephalon, T telencephalon. (b) The lamprey pineal and parapineal organ. The pineal and parapineal organ are located just below the skull, and are connected to the brain by the pineal stalk. The parapineal organ has a neuronal area called the parapineal ganglion. L lumen, P pineal organ, PP parapineal organ, PPG parapineal ganglion, ST pineal stalk
Lower vertebrates have pineal-related organs, such as the parapineal organ (lampreys and fishes), the frontal organ (anuran amphibians), and the parietal eye (lizards), in addition to the pineal organ. The parapineal organ is located in the intracranial region below the pineal organ. In the lamprey, the parapineal organ has a well-developed structure similar to the pineal organ, and a characteristic neuronal area called the parapineal ganglion, containing many ganglion cells (Fig. 1.1b). In teleosts, the parapineal is very small and has a vestigial morphology. The frontal organ of anuran amphibians is found in the extracranial region and is connected to the pineal organ by the nerve fiber. The lizard parietal eye, situated on the top of the head, has a lens and a retina, similar to the lateral eyes. It is speculated that the frontal organs and parietal eyes developed from a frontal part of the pineal organ and parapineal organ at an ancestral stage, respectively (Kappers 1965; Oksche 1965). The frontal organ and parietal eye exhibit a neural light response, which will be discussed later.
1.1.2 Functional Properties of Pineal and Pineal-Related Organs and Functional Opsins
As described above, pineal and related organs are involved in both light-dependent melatonin secretion and neural light-sensing. Opsins involved in melatonin secretion will be discussed first followed by neural light-sensing opsins, with a particular focus on wavelength discrimination.
1.1.2.1 Opsins Important for Melatonin Secretion in the Pineal Organ
The pineal photoreceptor cells that secrete melatonin, which correspond to the modified photoreceptor cells through morphological classification, have been identified immunohistochemically (Meiniel 1979, 1980). In the lamprey pineal organ, the melatonin-secretory photoreceptor cells were identified by both antibodies against serotonin, a precursor of melatonin, and against opsins (Tamotsu et al. 1990, 1994). These studies revealed that a red-sensitive cone opsin was present in most of the melatonin-secretory photoreceptor cells in the lamprey. A red-sensitive cone opsin was also detected in the lizard and frog pineal organs by immunohistochemistry (Masuda et al. 1994), suggesting that melatonin secretion may be controlled by long wavelength light in lower vertebrates.
In the chicken pineal organ, the red-sensitive cone opsin (iodopsin) is also present and considered to be involved in melatonin secretion (Okano et al. 1994, 1997). However, the involvement of other opsins in controlling melatonin secretion was investigated because the spectral sensitivity of red-sensitive opsin-based pigments could not completely account for the action spectrum of the inhibitory effect of light on serotonin N-acetyltransferase (NAT) activity, a key enzyme in the synthetic pathway of melatonin in the chicken pineal organ (Deguchi 1981). “Pinopsin” was identified as the first extraocular photopigment in the chicken pineal organ (Okano et al. 1994; Max et al. 1995). Pinopsin was successfully expressed in HEK293 cells and a recombinant pinopsin pigment reconstituted with 11-cis retinal exhibited an absorption maximum at 468 nm, identifying it as a blue sensitive pigment (Okano et al. 1994). The photoproduct of the recombinant pinopsin activates a visual G protein transducin in vitro in a light-dependent manner (Nakamura et al. 1999). The absorption spectrum of pinopsin together with that of the red-sensitive opsin-based pigment accounts for the action spectrum of inhibition of pineal NAT activity in the chicken pineal organ (Okano et al. 1994; Okano and Fukada 1997).
It has been suggested that pinopsin may play a role in synchronizing the phase of the endogenous circadian oscillator with an environmental dark–light cycle, such as the photic entrainment of the melatonin production rhythms (Okano and Fukada 1997, 2001). In chicken pineal cells, the rhythmic production of melatonin is regulated by two pathways, the acute suppression pathway and a photic-input pathway to the oscillator to reset the circadian clock (the phase-shifting effect of the circadian clock). Two types of G protein, Gt1 and Gq/11, colocalize with pinopsin in the chicken pineal photoreceptor cells (Matsushita et al. 2000). It has been hypothesized that pinopsin triggers two types of phototransduction cascades involving Gt1 and Gq/G11. On the other hand, recent studies have shown that melanopsin, which functions as a circadian photoreceptor in mammals (Provencio et al. 1998; Hattar et al. 2002; Panda et al. 2002), is also expressed in the chicken pineal organ (Bailey and Cassone 2005). It is of interest to see how melanopsin, which is suggested to drive a Gq/G11-type G protein signaling (Panda et al. 2002; Koyanagi and Terakita 2008; Bailes and Lucas 2013), contributes to the regulatory mechanisms cooperatively with pinopsin.
Reptiles (Kawamura and Yokoyama 1997) and amphibians (Yoshikawa et al. 1998) also possess the pinopsin gene, but fish and mammals do not. In the toad (Bufo japonicus), pinopsin is localized to the brain, specifically the anterior preoptic nucleus of the hypothalamus (Yoshikawa et al. 1998). Interestingly, pinopsin is localized in both the retina and pineal of a diurnal gecko (Phelsuma madagascariensis longinsulae) (Taniguchi et al. 2001), whereas in lizards, pinopsin is expressed only in the pineal [ruin lizard (Podarcis sicula), iguana (Iguana iguana)] and parietal eye [ruin lizard and side-blotched lizard (Uta stansburiana)] (Frigato et al. 2006; Su et al. 2006; Wada et al. 2012).
1.1.2.2 Opsins Involved in the Neural Light Response in the Pineal and Related Organs
In addition to the photoreceptor cells secreting melatonin, pineal organs in most lower vertebrates, such as lamprey, fishes, amphibians, and reptiles, contain typical photoreceptor cells (based on morphology) that transduce a captured light signal to an electrical response, which is then transmitted to the brain via the pineal ganglion cells (Dodt and Heerd 1962; Morita 1969; Dodt 1973). The frequency of neural discharge in the pineal ganglion cells is modulated by light, demonstrating that the pineal organ provides light information to the brain (Dodt 1973). In addition, several electrophysiological studies have established that the pineal organs of lampreys, fishes, and frogs have two types of ganglion cells, which show chromatic and achromatic responses. In the lamprey, the neural activity of achromatic-type ganglion cells is inhibited by visible light that can be detected by green-sensitive photoreceptor cells (Fig. 1.2a). On the other hand, chromatic-type ganglion cells receive and integrate light signals from two types of the photoreceptor cells, UV- and green-sensitive, and the neural activity of chromatic-type ganglion cells is inhibited and excited by UV and visible light, respectively (Fig. 1.2b).
Two types of the neural light responses in the lamprey pineal ganglion cells. (a) The neural activity of achromatic-type ganglion cells is inhibited by visible light that is received by green-sensitive photoreceptor cells. (b) The neural activity of chromatic-type ganglion cells is inhibited by UV light and excited by visible light. The chromatic-type ganglion cells receive and integrate light signals from two types of the photoreceptor cells, UV- and green-sensitive photoreceptor cells
The functional relation of the pineal photoreceptor cells to the ganglion cells has been well investigated for the pineal organ of the river lamprey, Lethenteron japonicum (Morita et al. 1989). The achromatic-type ganglion cells of the lamprey pineal show a maximum sensitivity at 525 nm, which correlates with the wavelength of maximal sensitivity of the green-sensitive pineal photoreceptor cells (Uchida et al. 1992). In addition, recombinant lamprey rhodopsin bound to the native chromophore 3,4-dehydroretinal (retinal A2) exhibits an absorption maximum at ~525 nm (Fig. 1.3a). The absorption spectrum of lamprey rhodopsin corresponds to the spectral sensitivity of the green-sensitive photoreceptor cells (Fig. 1.3a). Immunohistochemical analysis clearly showed that lamprey rhodopsin was distributed to the outer segments of the pineal green-sensitive cells, which were identified by electrical recordings (Fig. 1.3c). These results suggest that the achromatic-type ganglion cells receive and integrate light signals from the green-sensitive photoreceptor cells containing rhodopsin in the lamprey pineal organ (Fig. 1.2a). On the other hand, in the pineal organ of the river lamprey, the maximum sensitivities of the inhibitory and excitatory responses of the chromatic-type ganglion cell were recorded at approximately 380 nm and 540 nm, respectively (Uchida and Morita 1994). Comparison of the spectral sensitivity of the achromatic and the antagonistic chromatic responses indicates that the pineal UV sensitivity is involved in the antagonistic chromatic response, which underlies the pineal wavelength discrimination (Uchida and Morita 1994), but not the achromatic response (Uchida et al. 1992), even though the green-sensitivity relates to both the chromatic and achromatic responses in the river lamprey.
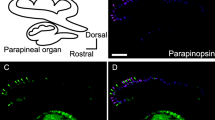
Comparison of spectral sensitivities of UV- and green-sensitive photoreceptor cells and absorption spectra of opsin-based pigments in the lamprey pineal organ. (a) Relative spectral sensitivities of UV-sensitive cells (magenta squares) and green-sensitive cells (green circles). The response amplitude to each wavelength light stimulus was normalized by the maximum amplitude. The individual relative response curves were averaged (n = 3). Vertical bars indicate standard deviations. The absorption spectra of the recombinant parapinopsin (magenta broken curve) and green-sensitive porphyropsin (retinal A2-based rod opsin pigment) (green broken curve), which bind to the native chromophore, 3,4-dehydroretinal (retinal A2) and exhibit absorption maxima at ~370 nm and ~525 nm, respectively, are superimposed with the relative spectral sensitivities. (b) Immunohistochemical localization of parapinopsin (magenta) at the dorsal region in the lamprey pineal organ. Scale bar, 100 μm. L lumen, P pineal organ, PP parapineal organ. (Inset in b) UV-sensitive cell is labeled with intracellularly injected neurobiotin (red) after recording spectral sensitivities as shown in a. The parapinopsin immunoreactivity (green) is observed in the outer segment of neurobiotin-labeled cell (arrow). (c) Immunohistochemical localization of rod opsin (porphyropsin, green) at the ventral region in the lamprey pineal organ. (Inset in c) Green-sensitive cell is labeled with intracellularly injected neurobiotin (red) after recording spectral sensitivities as shown in a. The rod opsin immunoreactivity (green) is observed in the outer segment of neurobiotin-labeled cell (arrow)
1.1.2.3 UV Photopigment Underlying Wavelength Discrimination in the Pineal Organ
Tamotsu and Morita (1990) showed that a UV-sensitive opsin-based pigment with molecular properties different from those of vertebrate visual pigments may be present in the pineal organ of the lamprey (Tamotsu and Morita 1990), namely reversible photoisomerization of the chromophore retinal with UV and visible light, thereby suggesting the existence of a novel UV-sensitive opsin-based pigment. In fact, an opsin underlying UV sensitivity, including a member of the UV/violet group, has not been isolated from the pineal organ or eye of the river lamprey, although rod opsin (rhodopsin in rod photoreceptors) and red-sensitive cone opsins were identified immunohistochemically (Tamotsu et al. 1990, 1994). This stimulated the search for a novel UV-sensitive pigment in the pineal organ.
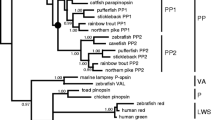
Parapinopsin, which was first identified from the catfish pineal and parapineal organs (Blackshaw and Snyder 1997), is such a UV-sensitive pigment (Koyanagi et al. 2004); spectroscopic analysis of recombinant lamprey parapinopsin containing native chromophore 3,4-dehydroretinal (retinal A2) showed that parapinopsin exhibits an absorption maximum at 370 nm in the UV region, clearly indicating that parapinopsin is a UV-sensitive opsin (Fig. 1.3a). Interestingly, the phylogenetic tree of opsins, including parapinopsin, indicates that two lines of UV pigments, UV cone opsin (UV/violet group) and parapinopsin (PP group), have evolved independently in the vertebrate lineage, providing a striking example of convergent evolution (Fig. 1.4b). In addition, we also revealed that parapinopsin has molecular properties different from that of vertebrate visual pigments, yet is similar to those of invertebrate visual pigments, in agreement with the finding of reversible photoisomerization of chromophore retinal in the lamprey pineal organ by Tamotsu et al. (1990).
Phylogenetic positions of opsins specific to the pineal and related organs. (a) Opsins have been classified into eight groups based on amino acid sequence similarity. (b) Phylogenetic relationships of vertebrate visual and non-visual pigments. Opsins specific to pineal and related organs, such as pinopsin, parapinopsin, parietopsin, and exo-rhodopsin, belong to the vertebrate visual pigment/non-visual pigment group. PT parietopsin, PP parapinopsin, VA VA-opsin, P pinopsin, LWS long wavelength-sensitive pigment, SWS1 short wavelength-sensitive pigment 1, SWS2 short wavelength-sensitive pigment 2, MWS middle wavelength-sensitive pigment, RH rhodopsin (rod opsin). The scale bar indicates 0.1 substitutions per site
The possible expression of parapinopsin in the UV-sensitive photoreceptor cells of the pineal organ was investigated using electrophysiological and immunohistochemical techniques (Koyanagi et al. 2004). Intracellular hyperpolarizing responses demonstrate the highest sensitivity at ~380 nm from the lamprey pineal cells. The spectral sensitivity closely resembles the absorption spectrum of recombinant parapinopsin (Fig. 1.3a). The immunohistochemical analysis clearly shows that parapinopsin is distributed in the outer segments of the UV-sensitive cells that were identified by electrophysiological recordings (Fig. 1.3b). These results demonstrate that parapinopsin is responsible for UV reception in the lamprey pineal organ. In addition, parapinopsin expression is found only in the pineal (Kawano-Yamashita et al. 2007), suggesting that parapinopsin involvement may be limited to pineal UV reception.
Parapinopsin homologs have also been isolated from rainbow trout and the clawed frog pineal and related organs, where the UV sensitivity has been electrophysiologically detected (Morita 1966; Korf et al. 1981; Koyanagi et al. 2004). Recently, parapinopsin was found in the parietal eye of the green iguana, Iguana iguana, which responds to UV light (Wada et al. 2012). Therefore, parapinopsin may be a common molecular basis of pineal UV reception for wavelength discrimination.
1.1.2.4 Characterization of the Parapinopsin-Containing UV Photoreceptor Cells in the Lamprey Pineal Organ
In order to understand how UV light signals captured by parapinopsin are transmitted to ganglion cells, which generate the antagonistic chromatic response, it is important to investigate the distribution of parapinopsin-containing cells and their neural projections histologically. Parapinopsin is localized predominantly in the dorsal layer of the lamprey pineal organ (Fig. 1.3b) (Koyanagi et al. 2004). In contrast, rhodopsin is distributed predominantly in the ventral layer (Fig. 1.3c) (Koyanagi et al. 2004). Remarkably, both parapinopsin and rhodopsin are expressed in the peripheral region, which is the dorsoventral border region of the lamprey pineal organ; however, the two pigments are never colocalized in the same photoreceptor cell (Kawano-Yamashita et al. 2007).
Parapinopsin-containing cells possess important histological characteristics that allow the assignment of the properties of pineal UV reception and neural projection to chromatic-type ganglion cells. Dye coupling, which indicates cell connection through gap junctions, was characterized in the basal processes of UV photoreceptor cells by intracellular injections (Koyanagi et al. 2004; Kawano-Yamashita et al. 2007). The UV photoreceptor cells are connected to each other, making a large photoreceptive field of at least 250 μm × 100 μm in area. Coupling in a large photoreceptive field may enable an averaging of the UV light information reaching the pineal organ and to help cancel the effect of shade.
In the lamprey pineal organ, most of the ganglion cells are localized in the ventral and peripheral regions, whereas only a few are localized in the dorsal region; the localization pattern of the ganglion cells is therefore different from that of the parapinopsin-containing cells, implying that they do not interact directly. However, parapinopsin-containing cells form a wide neural network, and almost all the basal processes from these cells are in direct contact with the ganglion cells in the peripheral region (Kawano-Yamashita et al. 2007), where many responses of chromatic-type ganglion cells have been recorded (Uchida and Morita 1994). These results suggest that in the lamprey pineal organ, the ganglion cells of the peripheral portion receive UV light information from the UV photoreceptor cells and that this drives the antagonistic chromatic response.
1.1.2.5 Visible Light Absorption in Wavelength Discrimination in the Lamprey Pineal
How visible light is captured and transduced in wavelength discrimination of lamprey pineal organ is of high interest. In chromatic-type ganglion cells, neural firing is inhibited and excited by UV and visible light, respectively (Uchida and Morita 1994). UV photoreceptor cells show hyperpolarizing responses to all wavelengths of measuring light (Uchida and Morita 1990; Koyanagi et al. 2004). Taken together with the finding that chromatic-type ganglion cells directly receive UV light information from the parapinopsin-containing cells (Kawano-Yamashita et al. 2007), it has been suggested that hyperpolarization of UV photoreceptor cells may cause suppression of the release of excitatory transmitters, such as glutamate, and produce the subsequent inhibitory responses of chromatic-type ganglion cells, analogous to light response and phototransduction of retinal photoreceptor cells. However, it remains unclear whether the pineal photoreceptor cells involved in the excitatory response to visible light are depolarized or hyperpolarized by light stimulation and whether they connect indirectly with chromatic-type ganglion cells through an interneuron. The maximum sensitivities of the excitatory responses are reported to be 540 nm (Uchida and Morita 1994), which is not in accordance with the absorption maximum of lamprey rhodopsin (Hisatomi et al. 1997) bearing the native chromophore 11-cis 3,4-dehydroretinal (retinal A2) (λ max = 525 nm) (Fig. 1.3a). Therefore, it is possible that photoreceptor cells containing an opsin other than lamprey rhodopsin could be involved in the excitatory response.
1.1.2.6 Opsin-Based Pigment Involved in the Electrophysiological Response in the Other Pineal-Related Organs
Parapineal organs, frontal organs, and parietal eyes are known as pineal-related organs. The lamprey has a well-developed parapineal organ, where parapinopsin and rhodopsin are present (Koyanagi et al. 2004); however, an electrophysiological response has not yet been reported. On the other hand, the lizard parietal eye, which evolved from the parapineal, can detect the ratio of UV/blue to longer wavelength light. Interestingly, in the parietal eye, chromatic antagonism resides in the photoreceptor cells themselves; that is, the photoreceptor cells hyperpolarize and depolarize to light in a wavelength-dependent manner (Solessio and Engbretson 1993; Su et al. 2006). This chromatic antagonism mechanism in a single photoreceptor cell is unique to the parietal eye photoreceptor cells. The photoreceptor cells in the side-blotched lizard (Uta stansburiana) have two antagonistic light signaling pathways, which lead to hyperpolarizing and depolarizing responses with maximal sensitivity to blue and green lights, respectively. Recently, the molecular basis of these two pathways was revealed: the blue-sensitive pinopsin and the green-sensitive parietopsin are colocalized in a single photoreceptor cell and underlie antagonistic light responses (Su et al. 2006). The hyperpolarizing response to light is mediated by pinopsin, which activates a cGMP-phosphodiesterase through gustducin to lower cGMP concentrations, consequently closing cyclic nucleotide-gated (CNG) channels. On the other hand, the depolarizing light response is mediated by parietopsin (named after a parietal eye opsin). Light absorption by parietopsin causes elevation of cGMP levels through activation of Go, which is suggested to inhibit the cGMP phosphodiesterase, consequently opening CNG channels. Interestingly, the recent study by Wada et al. (2012) indicated that parietopsin colocalizes with the UV-sensitive pigment parapinopsin instead of pinopsin in the parietal eye of iguana (Iguana iguana), which can discriminate UV and visible light. This finding strongly suggests that parapinopsin may serve as a UV-sensitive pigment in the wavelength discrimination pathway in a single photoreceptor cell. It is very interesting that the photopigment used in combination with parietopsin varies between two lizard species.
The electrophysiological responses have also been recorded from chromatic- and achromatic-type ganglion cells in the frog frontal organ, which differentiated from a frontal portion of the pineal organ (Dodt and Heerd 1962). It was reported that the wavelengths of maximum sensitivity for inhibitory and excitatory responses were 355 nm and 515 nm, respectively. Because parapinopsin was isolated from the clawed frog (Xenopus tropicalis) tissue containing the frontal organ (Koyanagi et al. 2004), it is suggested that parapinopsin may control UV reception for the inhibitory response.
Overall, parapinopsin may be a common UV-sensitive pigment for wavelength discrimination of UV and visible light in various pineal-related organs.
1.2 The Evolution of Pineal and Parapineal Photopigments
More than 2,000 types of opsins have been identified thus far and are classified into at least eight groups on the basis of amino acid sequence similarity: vertebrate visual and non-visual opsin, Opn3 (encephalopsin)/TMT-opsin, invertebrate Go-coupled opsin, cnidarian Gs-coupled opsin, Opn5 (neuropsin), Gq-coupled visual opsin and Opn4 (melanopsin), peropsin and retinal photoisomerase (Fig. 1.4a) (Terakita 2005; Terakita et al. 2012). Since the discovery of pinopsin (Okano et al. 1994), multiple types of non-visual opsins have been identified. Several lines of evidence suggest that these different types of opsins are expressed in pineal and related organs in a wide variety of non-mammalian vertebrates. Immunohistochemical and molecular biological investigations revealed that non-visual opsins, pinopsin (Okano et al. 1994), parapinopsin (Blackshaw and Snyder 1997), exo-rhodopsin (Mano et al. 1999), VA opsin (Philp et al. 2000), melanopsin (Bailey and Cassone 2005), and parietopsin (Su et al. 2006) are present in the non-mammalian pineal and related organs in addition to the rod and cone visual pigments (Vigh et al. 2002). As seen in the phylogenetic tree, opsins specific to pineal and related organs, exo-rhodopsin, pinopsin, parapinopsin, and parietopsin, are closely related to vertebrate visual opsins, suggesting an important evolutionary connection to vertebrate visual opsins (Fig. 1.4b).
The molecular phylogenetic tree including the members of Opn3 (encephalopsin)/TMT opsin as an out-group suggests that visual opsins arose from non-visual opsins. Previous studies revealed that upon light absorption, non-visual opsin-based pigments, such as Opn3 (encephalopsin)/TMT-opsin, Opn4 (melanopsin), and Opn5 (neuropsin)-based pigments, convert to a stable photoproduct, which activates G proteins and then reverts back to the original dark state by subsequent light absorption, as found for invertebrate visual pigments (Gq-coupled visual opsin) (Koyanagi et al. 2005, 2013; Koyanagi and Terakita 2008; Yamashita et al. 2010; Kojima et al. 2011; Matsuyama et al. 2012). The photoreversible or photointerconvertible property of the two stable states (dark state and photoproduct) is called a bistable nature, which is quite different from the molecular properties of the vertebrate rod and cone visual pigments (Fig. 1.5) (Terakita 2005; Terakita et al. 2012). The photoproduct of such visual pigments releases the chromophore retinal and bleaches (becomes colorless) (Fig. 1.5). Detailed spectroscopic investigations indicated that pinopsin and exo-rhodopsin also have the bleaching property but their photoproduct (Meta II) decay rates are between those of rod and cone visual pigments (Nakamura et al. 2001; Tarttelin et al. 2011). In addition, parietopsin and VA-opsin also have the bleaching property (Sato et al. 2011; Sakai et al. 2012). However, parapinopsin is bistable, similar to Opn3 (encephalopsin)/TMT-opsin homologs and Gq-coupled visual opsin, as suggested by reversible photoisomerization of chromophore retinal in the lamprey pineal organ (Tamotsu and Morita 1990).
Schematic drawings of molecular and spectroscopic properties of opsin-based pigments. (a) Opsin-based pigments are divided into two groups based on the molecular properties of their photoproducts, bleaching pigments and bistable pigments. (b) In the physiological conditions, the photoproduct of bleaching pigments become “colorless” with its absorption maximum at ~380 nm (UV region), as a result of chromophore release (a, upper diagram), whereas the photoproducts of bistable pigments have absorption maxima in the visible region, as a result of retaining the chromophore (a, lower diagram)
1.2.1 The Molecular and Biochemical Properties of Parapinopsin as an Evolutionary Intermediate
Parapinopsin is a member of the Gt-coupled opsin group composed of vertebrate visual and non-visual pigments but has a bistable nature, unlike other members of this group (Koyanagi et al. 2004). Our previous studies suggested that the amino acid residue that serves as the counterion is related to the molecular properties of the photoproduct. For opsin-based pigments, the chromophore retinal binds to the highly conserved lysine residue at position 296 (Lys 296, bovine rod opsin (rhodopsin) numbering system) through a Schiff base linkage (Pitt et al. 1955; Hargrave et al. 1983; Findlay and Pappin 1986). Various types of opsin-based pigments with absorption maxima in the visible light region possess a “protonated” Schiff base linkage. In the protein moiety, the positive charge on the protonated Schiff base is unstable, and therefore a counterion, a negatively charged amino acid residue is needed to stabilize the positive charge. In vertebrate visual pigment, glutamic acid at position 113 serves as the counterion (Sakmar et al. 1989; Zhukovsky and Oprian 1989; Nathans 1990). In contrast, Glu181 acts as the counterion in invertebrate pigments as well as in retinochrome, a retinal photoisomerase in squid photoreceptor cells, suggesting that Glu181 was the counterion in the ancestral vertebrate visual pigments (Terakita et al. 2000, 2004). The counterion position is therefore a key site in defining the diversity of opsins and their pigments.
Although parapinopsin has an amino acid sequence similar to those of vertebrate visual pigments, it has the molecular properties of a bistable pigment, similar to invertebrate visual pigments (Gq-coupled visual opsin) and Opn3 (encephalopsin)/TMT-opsin-based pigments. These observations indicate that parapinopsin is one of the key pigments for understanding the molecular evolution of vertebrate visual pigments. Parapinopsin has glutamic acid residues at both positions 113 and 181, similar therefore to vertebrate visual pigments. However, mutational analyses have revealed that Glu181 is the functional counterion residue, as found for invertebrate rhodopsins (Terakita et al. 2004). Therefore, this suggests that the molecular properties of photoproducts, namely photoregeneration (bistability) and bleaching, may relate to counterion position and that vertebrate visual pigments having bleaching property might have evolved from an ancestral vertebrate bistable pigment similar to parapinopsin.
Interestingly, the G protein activation efficiency of both parapinopsin and invertebrate-type pigment, which have Glu181 as the counterion, is much lower (1/20–1/50) than that of vertebrate visual pigments (Terakita et al. 2004). However, previous studies revealed that the rhodopsin–G protein interaction sites are located far from amino acid residues at positions 113 and 181, indicating that the different positions of counterion residues do not account by themselves for the difference in G protein activation efficiency. Thus, it is of interest to establish how the different G protein activation efficiencies are generated. Upon photoreception, some conformational changes take place in opsin-based pigments to activate the G protein. Site-directed spin labeling studies using bovine rod opsin-based pigment (rhodopsin) have revealed that movement of the cytoplasmic end of the sixth transmembrane helix is essential for pigment activation (Farrens et al. 1996; Hubbell et al. 2003; Altenbach et al. 2008). Using a site-directed fluorescence labeling technique, the difference in G protein activation efficiency between parapinopsin and bovine rod opsin-based pigment was investigated in relation to differences in the movement of helix VI (Tsukamoto et al. 2009). The movement of helix VI was similar in the two pigments, but the movement was greater in bovine rod opsin-based pigment than in parapinopsin. Amplitude differences of conformational changes likely led to the different G protein activation efficiencies between these pigments (see Chap. 7).
1.2.2 The Evolutionary Interaction of the Phototransduction Molecules with Opsins
The light response of vertebrate visual cells is achieved by light-absorbing visual pigments coupled to signal transduction proteins such as visual G protein transducin and visual arrestin, the latter of which binds to the light-stimulated visual pigment to shut off G protein-mediated signaling (Yau and Hardie 2009). As described above, the molecular properties of the photoproduct, which activates the G protein, are different between parapinopsin and vertebrate visual pigments. Therefore, we speculated that the signal transduction mechanism driven by parapinopsin was different from that of vertebrate retinal photoreceptors. Our immunohistochemical study suggested that transducin is the G protein coupled to the pigment in the parapinopsin-containing photoreceptor cells (Kawano-Yamashita et al. 2011). Arrestin binding to the parapinopsin photoproduct in the lamprey pineal organ however was found to involve β-arrestin, which is generally not bound to opsin-based G protein-coupled receptors (GPCRs); it is present in the parapinopsin-containing photoreceptor cells and translocates to the outer segments in a light-dependent manner (Kawano-Yamashita et al. 2011). These findings suggest that β-arrestin binds to light-stimulated parapinopsin to shut off signaling of the G proteins in the pineal photoreceptor cells, which is similar to the function of visual arrestin binding to light-activated rod opsin-based pigment (Philp et al. 1987).
In various mammalian GPCR systems, β-arrestin generally has two major functions that are carried out through binding to stimulated GPCRs (Lohse et al. 1990; Ferguson et al. 1996a, b; Goodman et al. 1996; Krupnick and Benovic 1998): termination of GPCR signaling to G proteins, and an involvement in the clathrin-mediated internalization process that removes receptors from the membrane to desensitize the cell. With respect to the latter function, β-arrestin has a clathrin binding domain, which visual arrestin lacks (Fig. 1.6). Interestingly, β-arrestin colocalizes with parapinopsin and not with the G protein in the granules observed in the parapinopsin-expressing cells under light illumination (Kawano-Yamashita et al. 2011). Our analysis of the interaction of parapinopsin with β-arrestin in HEK 293S cells revealed that lamprey β-arrestin modulated the internalization of parapinopsin in a clathrin- and light-dependent manner, similar to mammalian β-arrestin. It was therefore suggested that the granules in the cell body were generated in a light-dependent manner by β-arrestin-mediated internalization of parapinopsins from the outer segment, photoreceptive portions.
A schematic presentation of the correlation between the molecular evolution of photopigments and arrestins. The right and left trees show the phylogenetic relationships of photopigments and arrestins, respectively. Three types of arrestins are shown but only two have a clathrin-binding domain (the filled circle). The arrows that connect the trees indicate the biochemical interactions between photopigments and arrestins
Internalization mediated by β-arrestin, namely, the removal of the light-activated parapinopsin, may be responsible for cell recovery after activation. The photoproduct of the visual pigment rhodopsin is unstable, and therefore, visual cells recover to the original dark state in the time taken to release of the chromophore, all-trans retinal, from the photoproduct. However, parapinopsin converts to a photoproduct that is stable and does not bleach. Therefore, the parapinopsin photoproduct does not release the chromophore retinal or is not degraded, even under strong light (Koyanagi et al. 2004). In this context, parapinopsin internalization mediated by β-arrestin may play an important role in photoproduct removal in the course of recovery to the original dark state. In Drosophila visual cells, where the visual pigment is converted to a stable photoproduct, the visual pigment interacts with invertebrate-type arrestins to terminate signal transduction (Dolph et al. 1993) and trigger light-induced internalization of visual pigments (Alloway et al. 2000; Kiselev et al. 2000; Satoh and Ready 2005). Interestingly, although invertebrate-type arrestins do not contain a clathrin-binding domain, they are implicated in light-induced clathrin-mediated internalization of visual pigments through interaction with another adaptor protein, AP-2 (Orem et al. 2006). As a result, this internalization leads to photoreceptor cell degeneration. Therefore, it can be speculated that the arrestin-mediated internalization of bistable pigments is a general strategy for completely eliminating the light-activated pigment from the signaling cascades to restore photoreceptor cell conditions to the original dark state. In addition, the removal of photoproduct from the outer segments results in the down-regulation of parapinopsin function. This down-regulation may partially contribute to light adaptation and desensitization of photoreceptor cells to light, similar to the down-regulation of ligand-binding GPCRs through internalization (Lohse et al. 1990; Ferguson et al. 1996a, b; Goodman et al. 1996; Krupnick and Benovic 1998; Lefkowitz 1998).
Vertebrate visual arrestins are found in a wide variety of vertebrates, including the lamprey (Kawano-Yamashita et al. 2011). In most of these animals, visual arrestin is localized not only to the visual cells of the retinal but also to the pineal photoreceptor cells, which contain a pigment that bleaches (Collin et al. 1986). In other words, most visual arrestins function with bleaching pigments, regardless of their localization. This observation strongly supports the functional relationship between visual arrestin and bleaching visual pigments. Interestingly, in ascidians, which are the invertebrates that are most closely related to vertebrates, opsin-based pigments bind to arrestin, which has a function similar to the vertebrate β-arrestin (Nakagawa et al. 2002). Therefore, vertebrate visual arrestins appear to have diversified from their ancestral vertebrate “β-like” arrestin, for function in visual cells. It is possible therefore that vertebrate visual arrestins lack a clathrin-binding domain and are hence unable to function as a mediator of internalization because of the newly acquired bleaching property of the associated visual pigments that no longer require internalization for the inactivation of photoproducts. This is a strong argument in support of the notion that the evolution of visual pigments promoted the diversification of other signal transduction proteins and the acquisition of a phototransduction cascade that is unique to the vertebrate visual cell. That is, we can speculate that opsin evolution is correlated with the evolution of visual arrestin based on the findings of a pineal opsin, parapinopsin (Fig. 1.6).
1.3 Conclusion
Various types of opsin-based pigments are expressed in the pineals and related organs. Parapinopsin, which functions as the UV-sensitive photopigment in wavelength discrimination, is an important opsin-based pigment for understanding the molecular evolution of vertebrate visual opsins. Although parapinopsin has an amino acid sequence similar to those of vertebrate visual opsins, it shows photoreversibility and is therefore bistable in nature, similar to invertebrate visual pigments and unlike vertebrate visual pigments, which have a bleaching property. Based on the spectroscopic and biochemical properties of parapinopsin, vertebrate visual pigments that undergo bleaching may have evolved from the ancestral vertebrate opsin-based pigment with a bistable nature, similar to parapinopsin. Moreover, acquisition of the bleaching property during molecular evolution of vertebrate visual pigment may have promoted the emergence of visual arrestin. Thus, we can predict that the bleaching property of opsin-based pigment may have facilitated the molecular evolution of other signaling proteins that specifically couple to bleaching visual pigments.
References
Alloway PG, Howard L, Dolph PJ. The formation of stable rhodopsin-arrestin complexes induces apoptosis and photoreceptor cell degeneration. Neuron. 2000;28(1):129–38.
Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci U S A. 2008;105(21):7439–44.
Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280(1759):20122987.
Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res. 2005;134(2):345–8.
Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6(7): 544–56.
Blackshaw S, Snyder SH. Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family. J Neurosci. 1997;17(21):8083–92.
Collin JP, Mirshahi M, Brisson P, Falcon J, Guerlotte J, Faure JP. Pineal-retinal molecular relationships: distribution of “S-antigen” in the pineal complex. Neuroscience. 1986;19(2):657–66.
Collin JP, Voisin P, Falcon J, Faure JP, Brisson P, Defaye JR. Pineal transducers in the course of evolution: molecular organization, rhythmic metabolic activity and role. Arch Histol Cytol. 1989;52(Suppl):441–9.
Deguchi T. Rhodopsin-Like Photosensitivity of Isolated Chicken Pineal-Gland. Nature. 1981; 290(5808):706–7.
Dodt E. The parietal eye (pineal and parietal organs) of lower vertebrates. In: Jung R, editor. Handbook of sensory physiology. Berlin: Springer; 1973. p. 113–40.
Dodt E, Heerd E. Mode of action of pineal nerve fibers in frogs. J Neurophysiol. 1962;25: 405–29.
Dodt E, Meissl H. The Pineal and Parietal Organs of Lower-Vertebrates. Experientia. 1982;38(9):996–1000.
Dolph PJ, Ranganathan R, Colley NJ, Hardy RW, Socolich M, Zuker CS. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science. 1993;260(5116): 1910–6.
Falcon J. Cellular circadian clocks in the pineal. Prog Neurobiol. 1999;58(2):121–62.
Falcon J, Marmillon JB, Claustrat B, Collin JP. Regulation of melatonin secretion in a photoreceptive pineal organ: an in vitro study in the pike. J Neurosci. 1989;9(6):1943–50.
Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274(5288):768–70.
Ferguson SS, Barak LS, Zhang J, Caron MG. G-protein-coupled receptor regulation: role of G-protein-coupled receptor kinases and arrestins. Can J Physiol Pharmacol. 1996a;74(10): 1095–110.
Ferguson SS, Downey 3rd WE, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996b;271(5247):363–6.
Findlay JB, Pappin DJ. The opsin family of proteins. Biochem J. 1986;238(3):625–42.
Frigato E, Vallone D, Bertolucci C, Foulkes NS. Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles. Naturwissenschaften. 2006;93(8): 379–85.
Gern WA, Greenhouse SS. Examination of in vitro melatonin secretion from superfused trout (Salmo gairdneri) pineal organs maintained under diel illumination or continuous darkness. Gen Comp Endocrinol. 1988;71(1):163–74.
Goodman Jr OB, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383(6599):447–50.
Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, Fong SL, et al. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9(4):235–44.
Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295(5557):1065–70.
Hisatomi O, Ishikawa M, Tonosaki A, Tokunaga F. Characterization of lamprey rhodopsin by isolation from lamprey retina and expression in mammalian cells. Photochem Photobiol. 1997;66(6):792–5.
Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. Rhodopsin structure, dynamics, and activation: a perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking. Adv Protein Chem. 2003;63:243–90.
Kappers JA. Survey of the innervation of the epiphysis cerebri and the accessory pineal organs of vertebrates. Prog Brain Res. 1965;10:87–153.
Kawamura S, Yokoyama S. Expression of visual and nonvisual opsins in American chameleon. Vision Res. 1997;37(14):1867–71.
Kawano-Yamashita E, Terakita A, Koyanagi M, Shichida Y, Oishi T, Tamotsu S. Immunohistochemical characterization of a parapinopsin-containing photoreceptor cell involved in the ultraviolet/green discrimination in the pineal organ of the river lamprey Lethenteron japonicum. J Exp Biol. 2007;210(Pt 21):3821–9.
Kawano-Yamashita E, Koyanagi M, Shichida Y, Oishi T, Tamotsu S, Terakita A. beta-arrestin functionally regulates the non-bleaching pigment parapinopsin in lamprey pineal. PLoS One. 2011;6(1):e16402.
Kiselev A, Socolich M, Vinos J, Hardy RW, Zuker CS, Ranganathan R. A molecular pathway for light-dependent photoreceptor apoptosis in Drosophila. Neuron. 2000;28(1):139–52.
Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6(10):e26388.
Korf HW, Liesner R, Meissl H, Kirk A. Pineal complex of the clawed toad, Xenopus laevis Daud.: structure and function. Cell Tissue Res. 1981;216(1):113–30.
Korf HW, Foster RG, Ekstrom P, Schalken JJ. Opsin-like immunoreaction in the retinae and pineal organs of four mammalian species. Cell Tissue Res. 1985a;242(3):645–8.
Korf HW, Moller M, Gery I, Zigler JS, Klein DC. Immunocytochemical demonstration of retinal S-antigen in the pineal organ of four mammalian species. Cell Tissue Res. 1985b;239(1):81–5.
Koyanagi M, Terakita A. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 2008;84(4):1024–30.
Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, et al. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci U S A. 2004;101(17):6687–91.
Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15(11):1065–9.
Koyanagi M, Takada E, Nagata T, Tsukamoto H, Terakita A. Homologs of vertebrate Opn3 potentially serve as a light sensor in nonphotoreceptive tissue. Proc Natl Acad Sci U S A. 2013;110(13):4998–5003.
Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319.
Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273(30):18677–80.
Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248(4962):1547–50.
Mano H, Kojima D, Fukada Y. Exo-rhodopsin: a novel rhodopsin expressed in the zebrafish pineal gland. Brain Res Mol Brain Res. 1999;73(1–2):110–8.
Masuda H, Oishi T, Ohtani M, Michinomae M, Fukada Y, Shichida Y, et al. Visual pigments in the pineal complex of the Japanese quail, Japanese grass lizard and bullfrog: immunocytochemistry and HPLC analysis. Tissue Cell. 1994;26(1):101–13.
Matsushita A, Yoshikawa T, Okano T, Kasahara T, Fukada Y. Colocalization of pinopsin with two types of G-protein alpha-subunits in the chicken pineal gland. Cell Tissue Res. 2000;299(2): 245–51.
Matsuyama T, Yamashita T, Imamoto Y, Shichida Y. Photochemical properties of mammalian melanopsin. Biochemistry. 2012;51(27):5454–62.
Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, et al. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267(5203):1502–6.
Meiniel A. Detection and localization of biogenic amines in the pineal complex of Lampetra planeri (Petromizontidae). Prog Brain Res. 1979;52:303–7.
Meiniel A. Ultrastructure of serotonin-containing cells in the pineal organ of Lampetra planeri (Petromyzontidae). Cell Tissue Res. 1980;207(3):407–27.
Morita Y. Lead pattern of the pineal neuron of the rainbow trout (Salmo irideus) by illumination of the diencephalon. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;289(3):155–67.
Morita Y. Wave length discriminators in the intracranial pineal organ of Rana catesbyana. Experientia. 1969;25(12):1277.
Morita Y, Dodt E. Slow photic responses of the isolated pineal organ of lamprey. Nova Acta Leopold. 1973;38:331–9.
Morita Y, Samejima M, Tamotsu S. Response patterns and neuronal networks of photosensory pineal organs. Arch Histol Cytol. 1989;52(Suppl):469–75.
Nakagawa M, Orii H, Yoshida N, Jojima E, Horie T, Yoshida R, et al. Ascidian arrestin (Ci-arr), the origin of the visual and nonvisual arrestins of vertebrate. Eur J Biochem. 2002;269(21): 5112–8.
Nakamura A, Kojima D, Imai H, Terakita A, Okano T, Shichida Y, et al. Chimeric nature of pinopsin between rod and cone visual pigments. Biochemistry. 1999;38(45):14738–45.
Nakamura A, Kojima D, Okano T, Imai H, Terakita A, Shichida Y, et al. Regulatory mechanism for the stability of the meta II intermediate of pinopsin. J Biochem. 2001;129(2):329–34.
Nathans J. Determinants of visual pigment absorbance: role of charged amino acids in the putative transmembrane segments. Biochemistry. 1990;29(4):937–42.
Okano T, Fukada Y. Phototransduction cascade and circadian oscillator in chicken pineal gland. J Pineal Res. 1997;22(3):145–51.
Okano T, Fukada Y. Photoreception and circadian clock system of the chicken pineal gland. Microsc Res Tech. 2001;53(1):72–80.
Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372(6501):94–7.
Okano T, Takanaka Y, Nakamura A, Hirunagi K, Adachi A, Ebihara S, et al. Immunocytochemical identification of pinopsin in pineal glands of chicken and pigeon. Mol Brain Res. 1997;50(1–2):190–6.
Oksche A. Survey of the Development and Comparative Morphology of the Pineal Organ. Prog Brain Res. 1965;10:3–29.
Oksche A. Sensory and glandular elements of the pineal organ. In: Wolstenholme GEWKJ, editor. The pineal gland (A Ciba Foundation Symposium). London: Churchill; 1971. p. 127–46.
Orem NR, Xia L, Dolph PJ. An essential role for endocytosis of rhodopsin through interaction of visual arrestin with the AP-2 adaptor. J Cell Sci. 2006;119(Pt 15):3141–8.
Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002; 298(5601):2213–6.
Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225(1–2):127–32.
Philp AR, Garcia-Fernandez JM, Soni BG, Lucas RJ, Bellingham J, Foster RG. Vertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar). J Exp Biol. 2000;203(Pt 12):1925–36.
Pitt GA, Collins FD, Morton RA, Stok P. Studies on rhodopsin. VIII. Retinylidenemethylamine, an indicator yellow analogue. Biochem J. 1955;59(1):122–8.
Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95(1):340–5.
Sakai K, Imamoto Y, Su CY, Tsukamoto H, Yamashita T, Terakita A, et al. Photochemical nature of parietopsin. Biochemistry. 2012;51(9):1933–41.
Sakmar TP, Franke RR, Khorana HG. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci U S A. 1989;86(21):8309–13.
Samejima M, Tamotsu S, Uchida K, Moriguchi Y, Morita Y. Melatonin excretion rhythms in the cultured pineal organ of the lamprey, Lampetra japonica. Biol Signals. 1997;6(4–6):241–6.
Samejima M, Shavali S, Tamotsu S, Uchida K, Morita Y, Fukuda A. Light- and temperature-dependence of the melatonin secretion rhythm in the pineal organ of the lamprey, Lampetra japonica. Jpn J Physiol. 2000;50(4):437–42.
Sato K, Yamashita T, Ohuchi H, Shichida Y. Vertebrate ancient-long opsin has molecular properties intermediate between those of vertebrate and invertebrate visual pigments. Biochemistry. 2011;50(48):10484–90.
Satoh AK, Ready DF. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr Biol. 2005;15(19):1722–33.
Solessio E, Engbretson GA. Antagonistic Chromatic Mechanisms in Photoreceptors of the Parietal Eye of Lizards. Nature. 1993;364(6436):442–5.
Su CY, Luo DG, Terakita A, Shichida Y, Liao HW, Kazmi MA, et al. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311(5767):1617–21.
Tamotsu S, Morita Y. Blue sensitive visual pigment and photoregeneration in pineal photoreceptors measured by high performance liquid chromatography. Comp Biochem Physiol. 1990;96B:487–90.
Tamotsu S, Korf H-W, Morita Y, Oksche A. Immunocytochemical localization of serotonin and photoreceptor-specific proteins (rod-opsin, S-antigen) in the pineal complex of the river lamprey, Lampetra japonica, with special reference to photoneuroendocrine cells. Cell Tissue Res. 1990;262(2):205–16.
Tamotsu S, Oishi T, Nakao K, Fukada Y, Shichida Y, Yoshizawa T, et al. Localization of iodopsin and rod-opsin immunoreactivity in the retina and pineal complex of the river lamprey, Lampetra japonica. Cell Tissue Res. 1994;278:1–10.
Taniguchi Y, Hisatomi O, Yoshida M, Tokunaga F. Pinopsin expressed in the retinal photoreceptors of a diurnal gecko. FEBS Lett. 2001;496(2–3):69–74.
Tarttelin EE, Fransen MP, Edwards PC, Hankins MW, Schertler GF, Vogel R, et al. Adaptation of pineal expressed teleost exo-rod opsin to non-image forming photoreception through enhanced Meta II decay. Cell Mol Life Sci. 2011;68(22):3713–23.
Terakita A. The opsins. Genome Biol. 2005;6(3):213.
Terakita A, Yamashita T, Shichida Y. Highly conserved glutamic acid in the extracellular IV-V loop in rhodopsins acts as the counterion in retinochrome, a member of the rhodopsin family. Proc Natl Acad Sci U S A. 2000;97(26):14263–7.
Terakita A, Koyanagi M, Tsukamoto H, Yamashita T, Miyata T, Shichida Y. Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol. 2004;11(3): 284–9.
Terakita A, Kawano-Yamashita E, Koyanagi M. Evolution and diversity of opsins. WIREs Membr Transp Signal. 2012;1:104–11.
Tsukamoto H, Farrens DL, Koyanagi M, Terakita A. The magnitude of the light-induced conformational change in different rhodopsins correlates with their ability to activate G proteins. J Biol Chem. 2009;284(31):20676–83.
Uchida K, Morita Y. Intracellular responses from UV-sensitive cells in the photosensory pineal organ. Brain Res. 1990;534(1–2):237–42.
Uchida K, Morita Y. Spectral sensitivity and mechanism of interaction between inhibitory and excitatory responses of photosensory pineal neurons. Pflugers Arch. 1994;427(3–4):373–7.
Uchida K, Nakamura T, Morita Y. Signal transmission from pineal photoreceptors to luminosity-type ganglion cells in the lamprey, Lampetra japonica. Neuroscience. 1992;47(1):241–7.
Underwood H. Pineal melatonin rhythms in the lizard Anolis carolinensis: effects of light and temperature cycles. J Comp Physiol A. 1985;157(1):57–65.
Vigh B, Manzano MJ, Zadori A, Frank CL, Lukats A, Rohlich P, et al. Nonvisual photoreceptors of the deep brain, pineal organs and retina. Histol Histopathol. 2002;17(2):555–90.
Wada S, Kawano-Yamashita E, Koyanagi M, Terakita A. Expression of UV-sensitive parapinopsin in the iguana parietal eyes and its implication in UV-sensitivity in vertebrate pineal-related organs. PLoS One. 2012;7(6):e39003.
Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci U S A. 2010;107(51): 22084–9.
Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139(2):246–64.
Yoshikawa T, Okano T, Oishi T, Fukada Y. A deep brain photoreceptive molecule in the toad hypothalamus. FEBS Lett. 1998;424(1–2):69–72.
Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246(4932):928–30.
Acknowledgements
This work was supported in part by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (to A.T., E.K.-Y. and M.K.). E.K.-Y. is supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kawano-Yamashita, E., Koyanagi, M., Terakita, A. (2014). The Evolution and Diversity of Pineal and Parapineal Photopigments. In: Hunt, D., Hankins, M., Collin, S., Marshall, N. (eds) Evolution of Visual and Non-visual Pigments. Springer Series in Vision Research, vol 4. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-4355-1_1
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4355-1_1
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-4354-4
Online ISBN: 978-1-4614-4355-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)