Abstract
Every student of chemistry, material science, and chemical engineering should be schooled in catalysis and catalytic reactions. The reason is quite simple; most products produced in the chemical and petroleum industry utilize catalysts to enhance the rate of reaction and selectivity to desired products. Catalysts are also extensively used to minimize harmful byproduct pollutants in environmental applications. Enhanced reaction rates translate to higher production volumes at lower temperatures with smaller and less exotic materials of construction necessary. When a highly selective catalyst is used, large volumes of desired products are produced with virtually no undesirable byproducts. Gasoline, diesel, home heating oil, and aviation fuels owe their performance quality to catalytic processing used to upgrade crude oil.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The Importance of Catalysis
Every student of chemistry, material science, and chemical engineering should be schooled in catalysis and catalytic reactions. The reason is quite simple; most products produced in the chemical and petroleum industry utilize catalysts to enhance the rate of reaction and selectivity to desired products. Catalysts are also extensively used to minimize harmful byproduct pollutants in environmental applications. Enhanced reaction rates translate to higher production volumes at lower temperatures with smaller and less exotic materials of construction necessary. When a highly selective catalyst is used, large volumes of desired products are produced with virtually no undesirable byproducts. Gasoline, diesel, home heating oil, and aviation fuels owe their performance quality to catalytic processing used to upgrade crude oil.
Margarine, cakes, chocolate, salad oils, and other everyday edible products are produced from natural oils via catalytic hydrogenation. Polyethylene and polypropylene plastics, commonly used in packaging of foods, films, fibers, liquid containers, etc., require catalysts for cost-effective high volume production. Because of highly active and selective catalysts, polyester fibers used in clothing can be produced at reasonable prices for the mass market. Catalysts enhance the production of ammonia-based fertilizers that enrich the earth’s nutrient deficient soils for efficient agriculture. Catalytically produced ethylene oxide is a precursor to antifreeze. Formaldehyde is produced catalytically and used as a preservative and as a component in some polymer resins.
It is good to keep in mind the importance of catalysts in protecting the environment. They are frequently installed in the exhaust ducts from chemical operations to convert volatile organic compounds (VOC) generated during manufacturing operations, into harmless products. Catalysts also provide the environmental benefit of clean air by abating pollutants generated from stationary and mobile combustion sources. In many locations around the industrialized world, coal- and gas-fired power plants have special catalysts installed in the ducts to eliminate pollutants dangerous to our health. Many gas-fired compressors that pump natural gas through millions of miles of pipelines are also equipped with exhaust catalysts to clean emissions at moderate conditions. Even fastfood restaurants are being equipped with catalysts to eliminate odors from the cooking process. The most widely used treatment of exhaust pollutants is that of the catalytic converter present in the exhaust manifold that cleans emissions from the internal combustion engines of gasoline- and diesel-fueled automobiles and trucks. As modern commercial passenger jets fly above 30,000 feet, there is a need to destroy the few ppm ozone that enters the airplane with make-up air to ensure passenger and crew comfort and safety. Radiators on select vehicles have a catalytic coating deposited on their surface that decomposes harmful groundlevel ozone as the vehicle is driven.
All of this gives the consumer the benefits of readily available high-quality products at reasonable prices. From food to clothing to medicines to clean energy, catalysts play a major role in products people use in everyday life.
The forthcoming description of catalysts and catalytic processes should only serve as a primer towards understanding the basic principles with some examples of applications in the field of petroleum processing, alternative fuels, chemical production, and environmental air purification. Table 6.1 gives a list of some of the many commercial catalytic applications.
How Does a Catalyst Work?
A catalyst increases the reaction rate or activity relative to an uncatalyzed process by providing a less energetic pathway for conversion of reactants to products. In this regard the catalyst provides a chemical and energetic shortcut by lowering the energy barrier (i.e., activation energy) of reactants going to products. If no catalyst were present, higher temperature would be required to initiate the reaction. Higher temperatures often lead to undesirable byproducts and sometimes decomposition of one of the reactants. Therefore, by initiating the reaction at a lower temperature, the process is more controlled and the desired product can be produced. This is the most important advantage for catalytic processes that is exploited in many product applications.
The catalyst is not consumed in the process; it accelerates but does undergo various chemical changes during the process by interacting with the reactants and products. Mechanistically some or all of the reactants adsorb onto active sites of the catalyst where bonds are rapidly made or broken. For a heterogeneous solid catalyst processing a liquid and/or gas, the adsorption of reactants is called chemisorption that has the kinetics and reaction energies equivalent to a chemical reaction. Frequently chemisorbed species decompose to an intermediate that is rapidly converted to other intermediates or the final product. After the reaction is complete, the catalyst returns to its original state. In this regard there is no net change of the catalyst. Therefore, a very small amount of catalyst can process many molecules.
What Are the Catalytic Metals and Metal Oxides?
Most catalytic metals and metal oxides are derived from Group VIII of the periodic table. Of special importance are Fe, Co, Ni, Rh, Pd, and Pt, but also of importance are Cu and Ag in Group 1b, V in Group Vb, and Cr and Mo in Group V1b. Three of the precious metals Rh, Pd, and Pt are extensively used in many industries due to their extremely high activity and selectivity. They are rare in nature and very expensive, and thus spent catalysts are routinely recycled, purified, and reused. However, the so-called base metals Fe, Co, Ni, Cu, V, Cr, and Mn but especially Ni and Cu are used for specialty chemical applications. Base metal catalysts usually have modest activities, but are much less expensive and in certain cases more selective than the precious metals. Therefore, it is always desirable to search for less expensive base-metal catalysts whenever possible. This has been especially the case for replacing precious metal-containing automotive emission control catalysts, but because of lower activity and stability in the severe environment of an automobile exhaust they are only used as promoters.
More examples of the efficient use of catalytic metals and metal oxides will be given in the applications section of this brief review.
The Structure of Heterogeneous Catalysts
The process of chemisorption of reactants requires adsorption on the surface of the catalyst. Therefore to maximize the rate, the catalytic surface area should also be maximized. This is achieved by dispersing the catalytic species onto a high surface area inorganic carrier. An ideal dispersion of Ni on Al2O3 is shown in Fig. 6.1.
Ideally every Ni atom should be accessible to the reactants for maximum efficiency in the conversion process. Although this is possible when the catalyst is first prepared, the dynamics of the catalytic reactions lead to some agglomeration. Catalyst scientists, however, have developed procedures and stabilizers to minimize the extent of agglomeration and therefore dispersed catalysts can be classified as nanomaterials with sizes only slightly greater than 1 nm or 10 Å.
The carrier can be thought of as a sponge with pores from 1 to 100 nm (10–1,000 Å) in diameter. If one were to measure the internal surface area of just 20 g with an internal surface area of 200–300 m2/g, it would be equivalent to about 1 football field. Carriers such as Al2O3, SiO2, TiO2, CeO2, ZrO2, C, and combinations of these materials are commonly used. All have different surface properties and are used in applications dependent on the requirement for acidity, inertness to solubility, interactions with reactants, affinity for catalytically active components, and resistance to components in the gas phase. High surface area Al2O3 is not well suited for combustion reactions in which SO2/SO3 are present due to the formation of Al2(SO4)3. In such cases, high area TiO2 and/or ZrO2 are used because of their inertness. Carbons are mostly used for supporting precious metals in hydrogenation reactions. In addition to their chemical role, precious metal recovery is achieved simply by burning the carbon.
The most common carrier is gamma alumina (γ-Al2O3). It has an internal area of >200–300 m2/g. Its surface is highly hydroxylated (i.e., Al-O−H+). The H+ sites provide acidity required for many reactions and exchange sites for catalytic metal cations.
Zeolites are combinations of Al2O3 and SiO2 that are crystalline structures with precisely defined pore structures in the molecular size range (0.4–1.5 nm or 4–15 Å). A related group of materials known as mesoporous silica–alumina has extended the range of pore sizes attainable in ordered SiO2–Al2O3 supports to 4 nm (40 Å). They are commonly used in the chemical and petroleum industry due to their surface acidity and ability to exclude molecules larger than the pore diameter. For this reason, they are often referred to as molecular sieves. Their surfaces contain Al–OH groups with acidic and exchangeable H+. In the application section some of these materials will be more thoroughly discussed.
Rate-Limiting Steps for a Supported Catalyst
Supporting a catalytic component introduces a physical size constraint dictated by the pore size of the carrier. Thus, a key consideration is the accessibility of the reactants to the active catalytic sites within the high surface area carrier. Consider a hydrogenation reaction in which Ni is located in extremely small pores (i.e., 1 nm or 10 Å). The H2 molecule has easy access, but a large molecule, having a size comparable to the diameter of the pore, would experience great resistance moving towards the active sites. If large amounts of Ni are present in pores and are not accessible to the molecules to be hydrogenated, the reaction rate will not be enhanced to its fullest potential. Thus, the carrier with its geometric sizes and its pore size distribution must be carefully designed to permit the reagents and products to move in the pores with minimum resistance.
Following are the seven fundamental steps in converting a reagent molecule(s) to its product(s) using a supported heterogeneous catalyst.
-
1.
Bulk diffusion of all reactants to the outer surface of the catalyzed carrier from the external reaction media
-
2.
Diffusion through the porous network to the active sites
-
3.
Chemisorption onto the catalytic sites (or adjacent sites) by one or more of the reactants
-
4.
Conversion and formation of the chemisorbed product
-
5.
Desorption of the product from the active site
-
6.
Diffusion of the products through the porous network to the outer surface of the catalyzed carrier
-
7.
Bulk diffusion of the products to the external fluid
Steps 1, 2, 6, and 7 depend on the physical properties of the catalyzed carrier and are not activated processes (no intermediate chemical complex is formed). For this reason, we use the term apparent activation energy which is a term useful for comparing temperature dependence as will be described later. Steps 3–5 are chemically activated (with intermediate complexes formed during conversion to products) and depend on the chemical nature of the interaction of the reactants and products with the active sites [1–3]. Step 1 is referred to as bulk mass transfer. It describes the transfer of reactants from the bulk fluid to the surface of the catalyzed carrier. When this step is rate limiting, reactant molecules arriving at the external surface of the catalyst are converted instantaneously resulting in zero concentration of reactants at the surface. Thus, the internal surface of the catalyst is not used. Such as mass transfer controlled process is nonactivated and we assign an apparent activation energy of less than 2 kcal/mol. Rates vary only slightly with temperature (T 3/2) which, as will be shown below, allows it to be distinguished from other rate-limiting steps. Step 7 is similar to Step 1 except that the products diffuse from the external surface of the catalyst particle into the bulk fluid. The temperature dependence of this phenomena is relatively weak and has an apparent activation energy similar to that observed in Step 1 when it is rate limiting. When only the external surface of the catalyst particle is participating in the catalysis, it is said to have a low effectiveness factor. The effectiveness factor is defined as the actual rate divided by the maximum rate achievable when all catalytically active sites participate in the reaction. In the case of bulk mass transfer, the effectiveness factor approaches zero.
Steps 2 and 6 are both pore diffusion processes with apparent activation energies between 2 and 10 kcal/mol. This apparent activation energy is stated to be about 1/2 that of the chemical rate activation energy. The concentration of reactants decreases from the outer perimeter towards the center of the catalyst particle for Step 2. In this case some of the interior of the catalyst is being utilized but not fully. Therefore, the effectiveness factor is greater than zero but considerably less than one. These reactions are moderately influenced by temperature, but to a greater extent than bulk mass transfer.
Steps 3, chemisorption of the reactant(s), 4, chemical reaction forming the adsorbed product, and 5, desorption of the product(s) from the active site(s) are dependent on the chemical nature of the molecule(s) and the nature of their interaction with the active site(s). Activation energies are typically greater than 10 kcal/mol for kinetically or chemically controlled reactions. Chemical kinetic phenomena are controlling when all transport processes are fast relative to the reactions occurring at the surface of the active species so the effectiveness factor is one. All available sites are being utilized and the concentration of reactants and products is uniform throughout the particle. These reaction processes are affected by temperature more than either transport mechanisms. Figure 6.2 shows the conversion vs. temperature and concentration profiles of a reagent (R) for the three regimes of rate control.
Conversion of a reactant vs. temperature. The concentration of reactants [R] within the porous catalyst structure. Concentration of R is (a) uniform for kinetic control, (b) decreasing within the catalyst for pore diffusion control, and (c) zero immediately at the surface of the catalyst for bulk mass transfer
Because of the significant differences in temperature dependence the kinetically limited reactions can be distinguished from pore diffusion that in turn can be differentiated from bulk mass transfer. This is shown in Fig. 6.2 in which conversion of reactants is measured against temperature. The first evidence of conversion is the sharply increasing slope that indicates kinetic control, whereas pore diffusion shows a lower change in slope as the temperature increases. The bulk mass transfer process shows little change in conversion with increasing temperature. Thus, at low temperature the reaction is controlled by chemical reactions (3, 4, or 5) and pore diffusion limited reactions exist when the supply of reactants to the active sites within the particle is limiting the rate (2 or 6). Finally, at the highest temperature, chemical reactions at the external surface are faster than bulk mass transfer (1 or 7) and the reaction is considered limited by bulk mass transfer.
The corresponding concentration of reactant R is also shown for each regime. The concentration of R is constant within the catalyst for kinetically limited reactions. The concentration of reactant gradually decreases within the catalyst particle for the pore diffusion limited case because the rate is limited by transport through the porous network. For bulk mass transfer limited cases, the concentration of R is zero at the gas/solid interface.
Activation Energies and Rate-Limiting Steps. The heterogeneous catalyzed NH3 synthesis from N2 and H2 will be used to illustrate the relationship between rate and activation energy. There are a series of steps in the Fe catalyzed process.
The process steps within the pore structure of the catalyst are
-
1.
Diffusion of N2 and H2 to the active Fe site within the catalyst pore structure
-
2.
Chemisorption of H2 on the active Fe surface
-
3.
Dissociation of chemisorbed H2 to H atoms on the Fe site
-
4.
Chemisorption of N2 on the Fe site
-
5.
Dissociation of N2 to N atoms on the Fe surface
-
6.
Surface reaction between adsorbed N and H atoms forming chemisorbed NH3
-
7.
Desorption of NH3 from the surface
-
8.
Diffusion of the NH3 into the bulk gas
Dissociation of chemisorbed N2 (Step 5) is the slowest and thus is rate limiting.
The overall rate of reaction is determined by the slowest of these steps. In other words, the overall reaction cannot be faster than the slowest step. The slow step and hence the overall reaction rate is characterized by the apparent activation energy.
An important detail is that an individual rate-limiting step may be endothermic, whereas the overall reaction is exothermic as in this case. This is illustrated in Fig. 6.3. The chemisorption of N2 is exothermic and its dissociation is endothermic (1A). However, the overall reaction of N2 + H2 to NH3 is exothermic (1B). The overall activation energy and kinetics are dictated by the slow step. The reaction heat liberated (ΔH 25°C) = −11 kcal/mole is the thermodynamic value associated with the overall reaction.
It is very important to understand that the catalyst only promotes the rate of a reaction and cannot change the equilibrium concentrations of reactants and products. It cannot make a thermodynamically unfavorable reaction occur. It increases the rate at which equilibrium is achieved while always respecting the thermodynamics of the equilibrium constant and the enthalpy (ΔH) and free energy (ΔG) of the overall reaction. Process conditions (T&P) are changed to give more favorable thermodynamics and rates.
Consider an everyday example of how we are all influenced by rate-limiting steps. If you are driving on a one-lane road behind a slow-moving truck, your speed and that of those behind you is no greater than that of the truck although you certainly have the potential to increase your rate. Thus, the time required to arrive at your destination is controlled by the speed of the truck. Taking an analogy, we can liken the truck to a slow chemical reaction step where the overall reaction rate, and the time required to achieve products, is no faster than the speed of the conversion at the surface of the catalyst. Returning to our highway story if you take a bypass road you can increase your rate of speed and decrease the time needed for you to arrive at your destination. The new road is analogous to a catalyst that provides a different pathway to the final product. It is likely, however, that you will again be limited by another obstacle (narrowing of the new road due to construction) that will slow you and the others behind you, as you maneuver through it. This may be likened to pore diffusion where you are limited by the width of the passage. Mass transfer control can be thought of as reaching the maximum speed your vehicle can safely achieve within the local speed limits. The activation energy reflects the slow step and the kinetics of the overall reaction rate.
Selectivity
In many processes, multiple reaction pathways are possible and it is the role of the catalyst and reaction conditions to maximize the rate along the desired path. Selectivity is defined as the amount of desired product divided by reactant converted. A practical example of the catalyst directing reactants to a selective product is shown by the oxidation of ammonia to nitric oxide, which is used in the production of fertilizers.
The operating temperature of the process is 900°C and both the standard free energy of Δ G25 = −57.2 kcal/mol of NH3 and the equilibrium constant of K NO = 1010 are very favorable.
However, the decomposition pathway to N2 is even more thermodynamically favorable with ΔG 25 = −77.9 kcal/mol of NH3 and an equilibrium constant of K N2 = 1015 at 900°C.
The presence of a PtRh gauze catalyst catalyzes the reactants along the NO pathway with a selectivity of 98%. Therefore, although the free energy is more favorable and the equilibrium constant for the N2 reaction is 105 times greater, the highly selective PtRh catalyst promotes the NH3 oxidation reaction to NO. In contrast, the presence of Pd favors the N2 product. In each case the catalyst respects the equilibrium constant, but directs the reactants to specific products.
A second reaction that is currently receiving a great amount of attention because of low-temperature fuel cells is the purification of traces of CO present in a H2 stream. The fuel cell directly converts chemical energy (H2 and O2) to electricity bypassing the mechanical (pistons, turbines, etc.) and combustion steps associated with conventional power generation. The mechanical step limits efficiency and combustion generates pollutants (CO, HC, and NOx). The heat and power generated from the fuel cell hold promise for powering vehicles and for providing heat and electricity to residential and commercial buildings with the only product being H2O. H2 and CO are produced by catalytic steam reforming of a hydrocarbon (e.g., natural gas). The subsequent water gas shift reaction generates more H2 from the CO + H2O reaction. Traces of CO exiting the shift reactor must be removed from the H2 because it poisons the anode of the low-temperature fuel cell. The H2 content of the gas is about 75%, and the CO is about 0.1% (balance is H2O and CO2). Although both standard state free energies are similar, a highly selective Pt containing catalyst promotes the oxidation of the CO with minimum oxidation of the H2 purifying the latter for a low-temperature fuel cell.
A small amount of air is injected into the reactor. The inlet H2/CO ratio is 750, whereas the exit ratio must be 75,000. Thus, the free energy for CO oxidation is becoming less favorable (more positive) as CO is reduced below 10 ppm. An effective catalyst [4] currently in use commercially is Pt on Al2O3 promoted with a small amount of Fe. It operates at an inlet of 90°C and reduces the CO to less than 0.001% with a selectivity of well over 50% depending on management of the exothermic heat of reaction. This is quite remarkable given the increasingly large excess of H2 as the reaction approaches completion. The same catalyst, but without the Fe, requires 170°C to achieve the same conversion of CO but with a selectivity less than 25%.
Catalyst Preparation
In the example given above, a small amount of Fe is added to a Pt on Al2O3 catalyst. The catalyst is prepared by a very unique procedure that must be strictly adhered to in order to achieve the desired results. The Pt and Fe must be in such close proximity that the CO chemisorbs on the Pt and the O2 on the Fe after which they react to form CO2 [4]. Simply reproducing the composition will not give acceptable performance. The specific details of catalyst preparation may be confidential and are most often covered by patents and trade secrets.
Some general guidelines for supported catalyst preparations are presented below; however, the reader should consult the many references and patents available on the subject [5]. Even by doing so the precise details used by industry to optimize the catalyst will often not be found.
Known amounts of salt(s) of catalytic metals are dissolved in aqueous solutions and impregnated into carrier materials. The wet mass is dried at 110°C and calcined in air at 300–500°C, releasing the decomposable salt components and depositing the metal oxide on the surface within the depths of the porous carrier. For many oxidation reactions, the catalyst is now ready for use, but for hydrogenation it is necessary to reduce the impregnated metal oxide or salt chemically. Usually this is accomplished by flowing H2, under conditions consistent with the maximum temperature of use for the reaction of interest.
The carrier can be in the form of a powder used for slurry reactions or a particulate such as a sphere, cylinder, or tablet (typically a few mm in diameter) used in fixed bed reactors. The size and shape depend very much on what is anticipated to be the rate-limiting step. For example, for a reaction limited by pore diffusion, it is customary to use a smaller particle in the shape of a star, trilobe, or wagon wheel to decrease the diffusion path while increasing the external geometric surface area.
Mechanical strength and solubility under reaction conditions must be considered in the selection. Although it is often stated that the carrier is inert, there are many cases where this is not the case. Some carriers provide acid or basic sites that act as cocatalysts with the metal or metal oxides performing other functions. Petroleum reforming (discussed later) requires a hydrogenation function, provided by the metal, to dissociate H2 and the carrier provides the acid site to enhance isomerization reactions.
Multi-channel ceramic monoliths (Fig. 6.4) are now the primary choice as support structures to carry the active catalytic species for cleaning emissions from various sources of pollution [6]. Figure 6.4 shows the shapes used for both automotive and stationary pollution abatement applications.
The largest application is the automotive catalytic converter that converts carbon monoxide (CO), hydrocarbons (HC), and nitric oxides (NOx) to nontoxic compounds. The monolith structure offers high cell densities and thus geometric surface area upon which the catalyst is deposited permitting smaller reactor sizes, high mechanical strength, excellent thermal shock resistance, and low-pressure drop [6]. A powdered carrier, usually stabilized Al2O3, is impregnated with catalyst precursor salts. A slurry of the impregnated carrier is prepared and milled to some desirable particle size. The monolith is dipped into the slurry and “washcoated” onto the walls of all of the channel surfaces (see Fig. 6.4). Air is blown through the channels to remove excess slurry. It is then dried and calcined in air at about 500°C. The finished structure now contains the proper amount of catalyst uniformly deposited throughout the channel length. The washcoat thickness is greatest at the corners or fillets of the cell due to its sharp angle. The reactants flow through the channels and catalysis occurs on the washcoated walls. There are many other variations of preparing monolith catalysts with different carriers and compositions. There are monoliths made of metal, some of which have parallel channels and others with nonparallel channels designed for tortuous flow to enhance mass transfer.
A Heterogeneous Catalytic Reaction: An Example
There is a great desire to use naturally occurring and renewable biomass for producing useful products. Furfural is extracted from cornhusks and contains an aldehyde functional group. If this group is selectively hydrogenated to the corresponding alcohol, the product furfuryl alcohol can be used to make corrosion-resistant resins for preparing polymers to make molds for shaping products. This reaction is selectively carried out with a Cu, Cr2O3 catalyst (copper chromite powder) in a slurry phase stirred batch reactor (see “Reactor Types” below) at 3,000 psig and 150°C.

Hydrogen gas is dissociatively chemisorbed onto the surface of the Cu-containing catalyst producing highly active hydrogen atoms. The high pressure is needed to ensure adequate solubility of the H2 in the furfural liquid. The aldehyde functional group forms a weak bond with these active adsorbed atoms and is hydrogenated to the finished product. In the absence of the catalyst, the diatomic hydrogen molecule would have to be dissociated in the gas phase at a much higher temperature leading to the decomposition of the aldehyde group.
Active Catalytic Sites
Not all catalytic sites are equal. Ideally each catalytic site is an atom having equal activity. This is never the case for a supported heterogeneous catalyst. One of the great mysteries in catalysis is the exact nature of the active site. Some catalytic species may be so well dispersed that they have no defined structure or are amorphous (no long-range structural order), whereas others may be highly crystalline. Amorphous catalytic components have greater catalytic surface area because fewer atoms are buried within a large crystal. However, the nature of the carrier and the catalytic species and the method used to deposit it on the carrier gives rise to a very heterogeneous surface with different sites having different surface energies and different activities. For example, it is believed that defects in the crystal structure produce highly energetic and active sites for catalytic reactions. This may be true but the more crystalline the catalytic site, the lower is the number of surface atoms and the lower is its catalytic surface area. All this being said, there are reactions that favor certain catalyst crystalline sizes and are said to be structure sensitive. The above discussion points to the mystery of catalysis. The goal of finding a universal model describing the nature of the active catalytic site still eludes us today and will undoubtedly be the subject of fundamental research for years to come.
Reactor Types
There are many different reactor designs, but the two most commonly used are fixed bed and batch slurry phase. For a fixed bed reactor, a given volume of solid particulate or monolith supported catalyst is fixed in a heated tube located within a furnace and liquid and/or gaseous reactants flow through the bed. This type of process is commonly used for large continuous-volume production where the reactor is dedicated to making only one product such as a bulk chemical or petroleum product.
Monolithic supports are commonly used for environmental applications and will be discussed in more detail later [6]. Batch reactors are used mostly for small-scale production such as the hydrogenation of intermediates in the production of medicines in the pharmaceutical industry. The catalyst powder is mixed in a precise amount of reactant in a pressurized-stirred autoclave. A gaseous reactant, usually H2, is introduced at elevated pressures and the reaction proceeds with continuous monitoring of the H2 consumed. The catalyst is separated from the product via filtration and is often used again depending on its retained activity and selectivity.
For the production of gasoline and other fuels by catalytic cracking of oils, a fluid bed reactor is used. This is a hybrid of a fixed bed and slurry phase reactor. The catalyst is fluidized as it interacts with the feed to be processed. This application is so important that it will be highlighted in the application section of this review.
Kinetics
The overall kinetics of a heterogeneous catalytic reaction can be controlled by any of the seven steps listed above [7–10]. We can distinguish which is rate controlling by determining the temperature dependence of the reaction. Once we know this we can design the catalyst to enhance the rate of the slowest step.
For example, bulk mass transfer (Steps 1 and 7) can be enhanced by increasing the turbulence of the reactants by increased stirring for a batch process or by increasing the linear velocity (see below) in the case of a fixed bed reactor. Increasing the geometric surface area of the catalyst also favors a reaction limited by bulk mass transfer. This is accomplished by decreasing the particle size of a particulate or by increasing the number of channels in a monolithic structure. Turbulence can be introduced in a monolith channel by modifying the surface from smooth to rough. Because kinetically controlled reactions have a stronger temperature dependence than transport controlled reactions, they are affected the most by increasing temperature. Pore diffusion resistance is decreased by increasing the pore size of the carrier or by using a smaller diameter carrier. One may also deposit the active catalytic species nearer the surface of the carrier to decrease the diffusion path length. The rate of a reaction limited by pore diffusion is moderately enhanced with temperature.
For chemically controlled reactions, one must modify the catalyst itself by increasing the number of active sites (increasing the catalytic surface area) or finding a crystal size that is more active for a given reaction. Often the activity is increased by the addition of promoters to the catalyst (i.e., Fe addition to Pt described under “Selectivity”) that enhance the activity. Having the highest activation energy, it is affected more than the transport regimes by increasing the temperature. Many examples of this will be given in the example section of this chapter.
General Kinetic Rate Equations. The rate of a bi-molecular reaction is given by
Rate is the disappearance of reactants with time expressed as the derivative—d[A]/d(t). [A] and [B] are the concentrations of reactants and [C] and [D] are the concentrations of the products. The exponents a, b, c, and d are the reaction orders for each compound. The rate constants are k F for the forward and k REV for the reverse reaction. For those cases where the reaction is far away from equilibrium, the reverse rate is negligible and this term is dropped from the rate expression.
To determine the rate constant and the reaction order at a specific temperature, it is often convenient to increase the concentration of one reactant at least 20 times that of the other to maintain it relatively constant during the reaction. Thus, with a high concentration of reactant B one my write k F [B]b = k *F
If the reaction order is to be determined, one may take the natural log of the rate equation and obtain
A plot of the –ln(d[A]/dt) vs. ln[A] will produce a straight line with a slope equal to a and intercept ln k *F
If one assumes a = 1 and integrates the rate expression
Integration from the initial concentration A o to A at anytime and from t = 0 to t
Plotting A o ln[1/(1−x)] vs. t will give a straight line with a slope equal to k *F Kinetics for Fixed Bed Continuous Reactions. For continuous flow reactors, we use the term space velocity (SV) defined as the volumetric flow rate at STP divided by the volume of catalyst. That ratio yields the reciprocal of the residence or space-time (t)
Thus, the rate equation for continuous flow reactions is
The linear velocity (LV) or superficial velocity is an important engineering term because it relates to pressure drop and turbulence. This parameter is often increased in fixed bed reactors to enhance bulk mass transfer and heat transfer.
Kinetics of a Slurry Phase Reaction in a Batch Process. This example is for the liquid phase hydrogenation of nitrobenzene to aniline with a powdered catalyst. These reactions typically are controlled by the supply of H2 to the active sites.
H2 must be
-
1.
Transported from the bulk gas phase and dissolved in the liquid nitrobenzene.
-
2.
Diffuse to the outside of the catalyst particle and into the pore structure.
-
3.
H2 and nitrobenzene react at the catalytic site.
-
4.
Products diffuse through the pores and into the bulk liquid.
Steps 1, 2, and 4 are mass transfer phenomena while step 3 is kinetic.
At steady state the rate of mass transfer of reactants (Rate)M is equal to the kinetic rate (Rate)R. This assumes Step 4 is fast and not rate limiting.
where H2g = H2 concentration in the gas
H2s = H2 concentration at catalyst surface
k m = Mass transfer rate constant
where k R = kinetic rate constant
Q = the amount of catalyst
Equating (Rate)MT and (Rate)R and rearranging one obtains
Taking the inverse for the general rate equation and dividing both sides by k R k m Q, one obtains
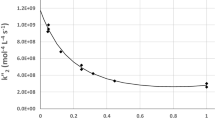
A plot of inverse (Rate)net vs. the inverse of Q yields a straight line with the slope equal to the inverse of k R and the intercept the inverse of k m. This is shown in Fig. 6.5.
When the amount of catalyst Q is large k R Q ≫ k m
The rate is limited by mass transfer because the reactants are consumed immediately at the outer surface of the catalyst. For small amounts of catalysts k m ≫ k R Q
The reaction is kinetically controlled limited by the amount of catalyst.
Arrhenius Equation. The general rate constant (k) is an exponential function of temperature as described by the Arrhenius equation
where
E = Activation energy for chemical control (“apparent” for diffusion limited processes)
R = Universal gas constant
T = Absolute temperature
k o = Absolute rate constant
Taking the natural log of the equation gives
The plot of ln (k) vs. T −1 gives a straight line with a slope equal to −E/R and intercept the absolute rate constant k o as shown in Fig. 6.6. The lowest slope represents reactions controlled by bulk mass transfer, and the largest is for chemical or kinetic control. This method allows for the comparison of different rate-limiting steps, but it must be clearly understood that diffusion processes are not activated and thus we use the term apparent activation energy for them only to allow comparisons to activated processes such as chemically controlled processes.
Rate Models
The Langmuir-Hinshelwood kinetic model describes a reaction in which the rate-limiting step is reaction between two adsorbed species such as chemisorbed CO and O reacting to form CO2 over a Pt catalyst. The Mars-van Krevelen model describes a mechanism in which the catalytic metal oxide is reduced by one of the reactants and rapidly reoxidizd by another reactant. The dehydrogenation of ethyl benzene to styrene over Fe2O3 is another example of this model. Ethyl benzene reduces the Fe+3 to Fe+2, whereas the steam present reoxidizes it, completing the oxidation-reduction (redox) cycle. This mechanism is prevalent for many reducible base metal oxide catalysts. There are also mechanisms where the chemisorbed species reacts with a gas phase molecule and the combination rapidly converts to the final product. There are many kinetic models that describe different mechanisms and the reader is directed to some outside references [7–10].
Catalyst Deactivation
The first indication of catalyst deactivation is a significant change in the activity/selectivity of the process. Catalyst deactivation occurs in all processes, but it often can be controlled if its causes are understood. This subject is very extensive and the reader is encouraged to seek additional information in references given here [11, 12]. In the following we will present some of the most common deactivation modes especially for heterogeneous catalysts. These are pictorially shown in cartoon form in Fig. 6.7.
Sintering of the Active Components. Catalytic scientists go to great lengths to disperse the active catalytic species over the surface of a carrier to maximize the number of sites available to the reactants. Small particles or crystallites have a high surface-to-volume ratio that is a highly unstable thermodynamic condition. The simple principle of Ostwald ripening indicates that small crystallites tend to grow to larger ones to bring the surface to volume condition to a favorable low free energy state. Thermal sintering occurs when small particles of active catalyst migrate over the surface of the carrier and agglomerate to form larger particles. There are other mechanisms of sintering, but conceptually this is the easiest to understand. The net effect is the loss of catalytic surface area that leads to loss of activity. The most frequently encountered cause is high temperature. This condition is encountered in Pt-, Pd-, and Rh-containing catalytic converters present in automobile exhausts where temperatures close to 1,000°C are commonly experienced. An oxidizing environment promotes the sintering of Pt by the formation of highly mobile or volatile Pt oxides. PdO, on the other hand, tends to form a stronger bond with the Al2O3 surface and thus sintering is not significant at modest temperatures. In contrast, it does sinter more readily in reducing environments. A catalytic species strongly bound to the surface is less likely to sinter. For this reason, a carrier such as SiO2, which contains few OH groups on the surface relative to Al2O3, leads to sintering of the supported metal or metal oxide more readily. Catalyst companies have incorporated “rare earth stabilizers” into the formulations to minimize the rate of growth of the metal and metal oxide components. Stabilizers slow the rate of sintering, but do not completely prevent it due to the thermodynamic nature of the phenomenon. The goal is to minimize the rate to acceptable levels to ensure acceptable life of the catalyst.
Carrier Sintering. The purpose of the carrier is to provide a high surface area upon which the catalytic components can be dispersed. The high surface area leads to sintering by collapse of the pore structure that subsequently blocks (or occludes) the active sites by preventing access of the reactant. For some carriers such as Al2O3, there are changes to the crystal structure that occur as the temperature is increased. The most common is the conversion of high surface area (gamma) γ-Al2O3 (200 m2/g) to low area (alpha) α-Al2O3 (1–5 m2/g) at temperatures greater than about 800°C. This process occludes the catalytic components within the carrier and prevents the reactants from having access. The easiest analogy to understand is the truck that breaks down at the tunnel entrance; it prevents other vehicles from entering. High temperatures and steam are two of the most significant contributors to carrier sintering. Catalyst companies have incorporated metal oxides, such as Ba and La in precise percentages, into the carrier to minimize the sintering rate.
Poisoning. Specific components present in the reactant feed can adsorb selectively onto active catalytic sites rendering them inactive, in much the same way as CO can react with Fe-hemoglobin in the blood. For heterogeneous catalysts, sulfur compounds are the most universal poisons for both base metal catalysts and to a lesser extent precious metals. Sulfur compounds present in petroleum, chemical, and environmental streams adsorb on the surface of Ni, Cu, Co, etc. forming metal sulfides that have little or no activity. In general, poisoning by sulfur compounds is irreversible. For this reason, upstream processes are used to reduce the sulfur to acceptable levels.
Sulfur oxides (SO2 and SO3) present in flue gases from upstream combustion operations adsorb onto the catalyst surface and in many cases form inactive metal sulfates. It is the presence of sulfur compounds in petroleum-based fuels that prevent the super-sensitive base metal catalysts (i.e., Cu, Ni, Co, etc.) from being used as the primary catalytic components for many environmental applications. Precious metals are inhibited by sulfur and lose some activity, but usually reach a lower but steady state activity. Furthermore, the precious metals are reversibly poisoned by sulfur compounds and can be regenerated simply by removing the poison from the gas stream. Heavy metals such as Pb, Hg, As, etc. alloy with precious metals and permanently deactivate them. Basic compounds such as NH3 can deactivate an acidic catalyst such as a zeolite by adsorbing and neutralizing the acid sites.
Water is a reversible poison in that it will weakly adsorb (physically adsorb) on sites at low temperature, but readily desorbs as the temperature is increased.
One interesting example of different selective poisoning mechanisms is that of SO3 deactivation of Pt on Al2O3 used for abating emissions from combustion reactions. The Pt oxidizes the SO2 to SO3 and the latter adsorbs onto the Al2O3 forming a sulfate. Slowly the carrier surface becomes so sulfated that it occludes the Pt within the pores and the catalyst slowly deactivates. By using a nonsulfating carrier such as TiO2 or ZrO2, deactivation can be prevented. In contrast, SO3 directly adsorbs on Pd sites and deactivation occurs rapidly.
Poisoning is not always bad. There are situations where a catalyst is intentionally poisoned to decrease activity towards an undesirable reaction. In the hydrodesulfurization and -demetallization of a petroleum feedstock, the catalyst is presulfided prior to introducing the feed to decrease its activity and minimize cracking reactions that will produce unwanted gases. Another is the use of ammonia to slightly poison a Pt catalyst used in the hydrogenation of fats and oils to decrease undesirable oversaturation.
Nonselective poisoning or masking is caused by debris depositing on the surface of the catalyst physically blocking sites. Corrosion products from the reactor walls and contaminants such as dust, oil, etc. can be eliminated by careful filtration upstream, but this mechanism of deactivation is a constant problem in many applications. Regeneration is possible for precious metal oxidation catalysts designed to abate VOC from flue gases. The reactor is bypassed when the activity begins to decline to unacceptable levels. High-velocity air is passed through the catalyst bed and loosely held debris is dislodged. In some cases chelating solutions are used to solubilize the metal contaminants such as Fe without destroying the catalyst. Coking is a common phenomenon when petroleum and/or high molecular weight chemical compounds are processed. Hydrogen-deficient-hydrocarbons are formed from undesirable side reactions and block access to the catalytic sites deep within the pores of the catalyst. This deactivation mode has been positively integrated into the fluid catalytic cracking process for converting heavy oils to useful products. The coked catalyst is regenerated with air in a separate reactor and the heat liberated used to preheat the feed as it enters the cracker.
Catalyst Characterization
The goal of catalyst characterization is to relate the physical and/or chemical properties of the catalyst to performance. Some of the most important catalytic properties are physical surface area, pore size distribution, active catalytic surface area, the morphology or crystal structure of the carrier and active components, the location of the active components within the carrier, and the presence of surface contaminants or poisons on the surface. Fortunately, there are many instrumental tools readily available in modern laboratories to measure these properties for fresh and spent catalysts. There are many reference books and monographs available that describe the strengths and limitations of the instrumental methods used in characterizing catalysts [13, 14].
The chemical composition can be measured by traditional wet and instrumental methods of analysis. Physical surface area is measured using the N2 adsorption method at liquid nitrogen temperature (BET method). Pore size is measured by Hg porosimetry for pores with diameters larger than about 3.0 nm (30 Å) or for smaller pores by N2 adsorption/desorption. Active catalytic surface area is measured by selective chemisorption techniques or by X-ray diffraction (XRD) line broadening. The morphology of the carrier is viewed by electron microscopy or its crystal structure by XRD. The active component can also be measured by XRD, but there are certain limitations once its particle size is smaller than about 3.5 nm (35 Å). For small crystallites, transmission electron microscopy (TEM) is most often used. The location of active components or poisons within the catalyst is determined by electron microprobe. Surface contamination is observed directly by X-ray photoelectron spectroscopy (XPS).
Making the characterization measurements is of critical importance in the diagnostics of the catalysts, but interpreting those most responsible for changes in activity or selectivity requires experience and good comparative kinetics for fresh and aged materials. It should be standard practice to compare fresh and aged catalytic performance with the changes observed in your characterization diagnostics. Measuring rate-limiting steps and activation energies will provide invaluable insight into the major causes of deactivation.
Homogeneous Catalytic Reactions
In a homogenous catalytic reaction, the reactants and catalysts are in the same phase. The catalyst is a metal (Rh, Co, Ni, etc.) chelated with organic ligands (often phosphine-containing) soluble in the reaction media, and because no support is used, pore diffusion does not exist. However, bulk mass transfer is a concern especially when the reaction is a hydrogenation because H2 must be dissolved in the liquid and make contact with the catalyst. This is accomplished by using high pressure and vigorous stirring. Homogeneous catalysis is most often used in the pharmaceutical industry where the desired selectivity can only be achieved with active complexes. A significant issue is separation of the catalyst from the final product to achieve the required purity. Furthermore, recovery of the catalyst is most often necessary especially for expensive precious metal containing complexes such as Rh. Distillation is sometimes used, provided there is a significant difference in vapor pressure of the product from the catalyst. The catalyst is also recovered by ion exchange with a suitable sequestering agent such as an amine compound. The efficiency of the separation allows for catalyst reuse and is essential for an economic process.
An example will be given in “Commercial Applications.”
Commercial Applications
There are literally hundreds of commercial catalytic processes carried out for high and low volume premium products. Only a few have been selected below as examples of everyday products essential for a high-quality life. Table 6.1 also presents listings of some of the major catalytic processes, but the reader is directed to references given in this review for a more complete listing [10].
Petroleum Processing
Hydro-Demetallization (HDM) and -Desulfurization (HDS) of Heavy Oils. The hydrocarbon petroleum fractions separated by distillation contain varying amounts of inorganic impurities such as nickel, vanadium, and sulfur-containing compounds, all of which must be removed to make high-quality products both functionally and environmentally. The high-boiling fractions contain the highest concentration of metals and sulfur [15, 16]. Metals, if present in gasoline or diesel, will create significant engine wear and the sulfur would produce sulfur oxides during combustion and ultimately sulfuric acid in the atmosphere. Furthermore, they will deactivate the catalysts used in the petroleum upgrading processes and in their ultimate application as a fuel will damage the performance of the abatement catalyst.
Crude oil contains about 0.01% metals and up to 5% sulfur present in large aromatic structures. These levels are highly dependent on the origin of the crude. For example, California crude is relatively low in sulfur but higher in metals than crude from Kuwait. Any process to remove them must be economical with little destruction of the hydrocarbons and minimum consumption of H2. The catalyst is Co, Mo/Al2O3 with particles a few mm in diameter. Although sulfur is usually a poison for catalytic reactions, it is used here in a positive function to control selectivity. It is presulfided to decrease activity towards excessive consumption of H2 that leads to unwanted saturation of aromatic molecules.
R and R′ = organic host of metals and sulfur M = metal (Ni or V)
The hydrogenation process is carried out at 500°C and pressures in excess of 30 atm in fixed bed reactors containing catalysts with varying physical properties to accommodate the metal deposition that occurs during the reaction. In some cases moving bed reactors are used where spent catalyst is continuously removed and fresh catalyst added.
The first reactor contains the Co, Mo deposited on a low surface area Al2O3 with large pores to allow deep penetration of the metals into the particle. The second bed will treat a feed with less metal so its pore size is smaller and surface area slightly larger. The metal penetration here is less deep than in the first bed and allows for some hydrodesulfurization. The final bed contains the highest surface area and smallest pores and is designed to perform most of the desulfurization.
The catalyst is regenerated frequently during its useful life, but once spent it is leached and the metals recovered.
Catalytic Cracking for the Production of Useful Fuels. Gasoline and diesel fuel, home and commercial heating oil, kerosene, jet fuel, etc. are all produced by catalytically cracking fractions of distilled crude oil. Crude oil is distilled in large vertical towers where the various fractions present are separated according to their boiling ranges. The light gases (C3 and C4 propane and butane, respectively) are distilled first and the light/heavy naphtha fraction (C5 to C10 pentanes to branched cyclopentanes), the precursors to gasoline, distill between roughly 70 and 200°C. Diesel fuel and heating oils (No. 1 and 2) are collected between 200 and 340°C. The remaining heavy hydrocarbons (called vacuum distillates) are used for lubricants and road construction.
The composition and molecular weight distribution of the crude oil depends on its origin, but generally less than 50% is within the molecular and boiling range for transportation and heating fuels. Thus, the role of the cracking process is to break or crack the higher molecular weight fractions into lower molecular weight compounds to be used for more useful products. Therefore, the catalyst is at the heart of the refining industry.
Cracking Catalysts. The catalysts used for cracking are called zeolites [17, 18]. They are SiO2–Al2O3 materials in which Si, in its tetrahedral SiO2 structure, is replaced with Al cations. They are produced by reacting sodium silicate with a water-soluble salt of Al followed by hydrothermal treatment in an autoclave. The zeolite is unique in that it has a well-defined crystal structure with a precise pore size (or aperture) ranging typically from 0.3–4 nm or 3–40 Å. This unique pore structure is responsible for separating molecules in accordance with their cross-sectional area. A molecule smaller than the aperture can enter the interior although a larger one cannot. Hence, the term molecular sieve is often used to describe zeolites. The composition and pore size can be varied giving rise to a large number of different zeolites with different pore sizes and crystal structures. They are usually identified by the Si/Al ratio, the crystal structure, and the size and shape of the pore. The Si+4 is bonded to 4 O−2 and each is bonded to another Si+4 establishing charge neutrality. Substituting Al+3 for Si cation upsets charge neutrality and requires another positive charge to satisfy the oxygen ions.

Neutrality is satisfied by a cation (e.g., M+), which is usually Na+ derived from the salts used in the synthesis. When the cation is exchanged with a proton, an acid site is created. This is the key active site for catalytic cracking reactions. The first exchange is with NH +4 which when heat-treated decomposes to NH3 and the H+ is retained on the zeolite. The acid zeolite is designated HZ.

The active zeolite for cracking reactions is called Faujasite and is classified as an X zeolite (HX). It has a Si/Al ratio of 1.0–1.5 with a pore or aperture size of 0.74 nm or 7.4 Å forming an aperture composed of 12 oxygen anions as shown in Fig. 6.8. The midpoint of each line represents an O−2 bonded to either Si+4 or Al+3. It is the AlO− site that requires a metal cation for charge balance. For cracking catalysts these sites are H+. The higher the Al content (lower Si/Al), the greater the number of acid sites, but the lower the thermal stability. The zeolite is embedded within an amorphous SiO2–Al2O3 structure that initiates the cracking of the large molecules, but also captures impurities such as organic compounds containing Ni and V that will severely deactivate the zeolite. Having its own acid sites, it also functions to break large molecules into smaller sizes where the zeolite can polish them to desired products. Catalyst particle sizes vary between 50 and 100 μm depending on the fluidization dynamics of the process.
During the fluidized catalytic cracking (FCC) process, a C–C bond is broken and a proton transferred from the catalyst to the molecule forming a positively charged carbocation. This ion can react with other hydrocarbons transferring its proton generating new carbo cations. Ultimately the large molecule is cracked to a smaller alkane and alkene with the regeneration of the protonated zeolite completing the catalytic cycle.
Excessive extraction of H leads to the formation of hydrogen-deficient, high-boiling hydrocarbons called coke. Coking reactions are catalyzed by acid. The coke masks the surface and blocks the pore of the catalyst preventing access of the feed molecules leading to a loss in activity.
Cracking is carried out in a fluid bed process as shown in Fig. 6.9. Catalyst particles are mixed with feed and fluidized with steam upflow in a riser reactor where the reactions occur at around 500°C. The active life of the catalyst is only a few seconds because of deactivation caused by coke formation. The deactivated catalyst particles are separated from the product in a cyclone separator and injected into a separate reactor where they are regenerated with a limited amount of injected air. The regenerated catalyst is mixed with the incoming feed which is preheated by the heat of combustion of the coke.
Zeolites play a major role as catalysts and/or adsorbents in the petroleum, chemical, and lately in a growing number of environmental applications. The reader should consult references available [18].
Naphtha Reforming for High-Octane Gasoline. Gasoline is volatilized and injected into the cylinders of the internal combustion engine where it is ignited under compression by a spark plug in the power stroke. Maximum power occurs when the cylinder reaches top dead center (maximum compression) and the mixture ignited by the spark plug. A high-octane gasoline is formulated not to preignite before reaching top dead center during compression to avoid the pinging or “knocking” sound that detracts from power. Before the mid-1970s, tetraethyl lead was added to quench preignition reactions, but because lead is no longer permitted the gasoline must be formulated to resist combustion until initiated by the spark. High-octane compounds such as aromatics and branched-paraffins are used in place of lead compounds. Today oxygenates are added to boost octane allowing decreases in carcinogenic aromatics.
Fuel-quality gasoline is made by a process called catalytic reforming [19, 20] in which molecules in the gasoline boiling range (called naphtha) are isomerized, dehydrogenated, and aromatized to make high-octane products. The most widely used reforming catalyst is Pt, Re on chlorinated Al2O3 particles (3–5 mm diameter). The Pt is the active component primarily for dehydrogenation and aromatization reactions and the Cl adds to the acidity of the carrier and is the active site for isomerization. The Re is believed to minimize coke formation. Dehydroisomerization requires both metal and acid functions. Some reactions are endothermic (dehydrogenation and dehydroisomerization) and others are exothermic (isomerization and dehydroaromatization). One can see below that the reactions lead to an increase in octane number
The dehydrogenation of cyclohexane to benzene and H2 increases the octane number from 75 to 106.
Isomerization of n-butane to i-butane increases octane from 94 to 101.
Heptane has a defined octane number of 0 and when dehydroaromatization occurs toluene is formed with an octane number of 116.
The formation of benzene by the dehydroaromatization coupled with isomerization of methyl cyclopentane also increases octane from 76 to 106.
The reforming process operates with three or four reactors in series. The feed is delivered to the first reactor at 500°C that is charged with the smallest amount of catalyst (5% of the total and the highest space velocity) to perform the easy but highly endothermic dehydrogenation reactions. To minimize coke formation, a small amount of H2 is recycled from the product. The products and unreacted feed are then reheated to 500°C and fed to a second bed containing about 15% of the total catalyst charge where isomerization reactions occur. The unreacted feed and product are then reheated to 500°C where the more difficult dehydroisomerization reactions take place with 20% of the total catalyst charge. The final reactor contains 60% of the total catalyst charge and performs dehydrocyclization. Swing reactors are in place to allow the process to continue as each bed is being regenerated by coke burn-off. After regeneration, the catalyst must be rejuvenated by the addition of chloride. The final step is reduction of the metal to its active state.
Alternative Fuels
A secure energy supply and the need for reduction of greenhouse gas emissions will continue to be a technological goal for the world throughout the next few decades. It has become apparent that we need a fossil free energy supply. Solar, wind geothermal, tidal, etc. are natural sources of energy that are beginning to be utilized for stationary power generation. The promise of a hydrogen economy and fuel cell vehicles is also on the horizon. These technologies are slowly being introduced; however, we need a transitional approach for liquid fuels for vehicular applications. Biofuels, derived from plants, are already making an impact partially replacing fossil fuels in both gasoline and diesel applications [21]. In the US, ethanol is commonly added (10%) to gasoline, while bio diesel additions to fossil derived diesel can range from 2 to 100% depending on the locations and country of use.
Ethanol is derived primarily for corn, and other starchy plants, by the well known fermentation process where enzymes (nature’s catalysts) accelerate the conversion of starch to ethanol. Bio diesel is synthesized by the homogeneous alkali catalyzed transesterification of triglyceride oils derived from plants such as soy and canola. Both alternative fuels are derived from edible plants which conflict with the food chain.
For this reason, there is a strong interest in utilizing non-edible portions of the plant, called lignin-cellulose as a source of fuel, especially ethanol. One of the main issues is the efficient penetration of the lignin (un-reactive aromatic polymer) portion of the plant which exists as a protective fiber surrounding the hemi cellulose and cellulose (sugar polymers of glucose), both of which are somewhat reactive towards enzymatic catalyzed fermentation to ethanol. This problem has stimulated the chemical and biochemical approach of developing new pretreatment techniques, including new enzymes, which can breakdown the lignin without destroying the ability to ultimately ferment the cellulose to ethanol. Alternatively, the thermal-chemical approach of gasification or pyrolysis of lignin-cellulose to produce gases and bio oils is being explored as a source of fuel. The gaseous and liquid products will require more traditional heterogeneous catalysts for upgrading to useable fuels.
Biodiesel can be derived also from non-edible plants such as jathropa and pennycress, both of which are rich in triglyceride oils relative to edible plants. These plants grow on arid lands during summer and winter seasons with little need for fertilizers. Research in harvesting and utilizing these plants is in progress with catalysts being used to upgrade the products to useful fuels.
Catalysts for Controlling Automotive Emissions
Oxidation Catalysts to Abate Unburned Hydrocarbon and CO Emissions
Catalytic converters were first installed in U.S. cars in 1976 [22, 23]. They were passive devices in that they were simply placed in the exhaust with no communication with the engine or its control strategy. It catalyzed the oxidation of the unburned hydrocarbons (CyHn) and carbon monoxide (CO) emitted during the incomplete combustion of the fuel. In some vehicles, excess air was pumped into the exhaust to ensure sufficient oxygen to complete the catalytic oxidation. This resulted in about a 90% reduction of these two pollutants relative to the uncontrolled uncatalyzed vehicle.
The presence of the catalyst provides a lower-energy chemical path than that offered by a thermal reaction. A catalyst accelerates oxidation of hydrocarbon/carbon monoxide/air mixtures that lie outside the flammability range required for thermal reactions. In the exhaust of the automobile, the composition of the pollutants is far below the flammability range, yet the oxidation reactions occur by the catalyst providing a lower-energy chemical path to that offered by the thermal reaction. An excellent example is the oxidation of CO with and without a catalyst. Without a catalyst, the rate-limiting step is O2 dissociation at 700°C followed by reaction with gas phase CO. In the presence of the Pt catalyst, O2 dissociation is rapid and the rate-limiting step becomes the surface reaction between adsorbed O atoms and CO that occurs below 100°C.
Two approaches were used in the design of the converters, both of which were positioned in the exhaust physically under the driver’s seat. Both used precious metals (Pt and Pd) as the active catalytic components dispersed on Al2O3 (stabilized against carrier sintering with 1–2% CeO2 and sometimes alkaline earth metal oxides).
One major automobile company used catalyzed Al2O3 beads (4 mm in diameter) and a spring-loaded pancakelike vessel to decrease the linear velocity and thus pressure drop. This design decreases back pressure that detracts from the power by offering less resistance to flow. Another used a new ceramic monolithic structure with hundreds of parallel channels (see Fig. 6.4). Upon the walls was deposited a coating of stabilized Al2O3 containing the active precious metals. The cordierite structure (2MgO–5SiO2–2Al2O3) has a melting point over 1,300°C sufficiently high to withstand the expected temperatures in the exhaust. It was extruded and had excellent resistance to breakage due to thermal shock experienced during the transient operation of the vehicle. The cellular structure had between 200 and 400 cells per square inch (cpsi) parallel to the flow. With channel diameters of 0.059 in. (200 cpsi) and 0.044 in. (400 cpsi), they had open frontal areas of about 70%, offering little resistance to flow and thus low back pressure. It was incorporated into the exhaust system with retainer rings and surrounded by layers of insulation to minimize breakage due to vibration and heat. The regulations required that the converter have a life of 50,000 miles. To ensure this life, it was necessary to remove the tetraethyl lead, used to boost octane, in gasoline because the Pb poisoned the Pt and Pd by alloy formation.
Oxidation catalysts were used until 1979 in both the particulate (bead) form and monolith structure. Road experience demonstrated that the particulate beds were not mechanically stable and were breaking apart. In contrast, the washcoated monoliths were found to be highly reliable so they became the structure of choice.
Three-Way Catalytic Conversion
In 1980, additional regulations imposed by the U.S. Environmental Protection Agency (EPA) required control of NOx (NO, NO2, N2O) emissions. Its removal coupled with the continuing need to remove CO and CyHn proved to be quite challenging because the latter had to be oxidized and the former reduced. Thus, it appeared two separate environments were needed. This problem was solved by the development of the three-way catalyst or TWC capable of catalyzing the conversion of all three pollutants simultaneously, provided the exhaust environment could be held within a narrow air-to-fuel range. This is shown in Fig. 6.10.
This range was defined between the fuellean and fuel-rich sides of the stoichiometric point, where the amount of O2 is precisely sufficient for oxidizing both the CO and CyHn. This control required an O2 sensor that is discussed below.
In the TWC, the Pt functions primarily as the catalyst for the oxidation reactions and the Rh catalyzes the NOx reduction.
The second reaction requires H2 that is produced catalytically by the steam reforming reaction that occurs when excess CyHn is present.
O 2 Sensor. The control of the exhaust composition was essential to maintain the air-to-fuel ratio close to stoichiometric for simultaneous conversion of all three pollutants. This control came about with the invention of the O2 sensor [23, 24]. The sensor head of this device was installed in the exhaust immediately at the inlet to the catalyst and was able to measure the O2 content instantly and precisely. It generates a voltage consistent with the Nernst equation in which the partial pressure of O2 (PO2)exhaust in the exhaust develops a voltage (E) relative to a reference. The exhaust electrode was Pt deposited on a solid oxygen ion conductor of yttrium-stabilized zirconia (ZrO2). The reference electrode, also Pt, was deposited on the opposite side of the electrolyte, but was physically mounted outside the exhaust and sensed the partial pressure (PO2)ref in the atmosphere. E o is the standard state or thermodynamic voltage. R is the universal gas constant, T the absolute temperature, n the number of electrons transferred in the process, and F the Faraday constant.
The CO and CyHn catalytically react with the O2 at the surface of the Pt electrocatalyst. When the O2 content is below stoichiometric, the electrode surface is depleted of O2 causing an increase in the (PO2)ref/(PO2)exhaust generating a large voltage. When the O2 is higher than stoichiometric, the voltage is decreased. Thus, the electrodes must also function as catalysts. The voltage signal generated continuously fluctuates as the O2 content is adjusted from sub to excess stoichiometric. Naturally, the exhaust electrode had to be resistant to exhaust poisons and temperature variations, so it was engineered with great care.
The voltage signal is fed back to the air/fuel intake system of the engine through an electronic control unit that controls the ratio necessary to maintain the proper window in the exhaust. Given the finite time necessary for the feedback system to function, it creates a perturbation of the O2 content in the exhaust. The TWC catalyst had to be engineered to respond to these changes. The catalyst was composed of Pt, Rh on stabilized Al2O3 on a ceramic monolith, but an oxygen storage component (OSC) capable of storing and releasing O2 was added. When the engine momentarily delivers less O2 than the stoichiometric amount, the hydrocarbons present reduce the OSC. During higher O2 spikes, the excess is stored on the OSC according to the fuel lean reaction below.
The current OSC material is CeO2–ZrO2 (proprietary promoters are added to stabilize it against sintering) where the oxidation state of the cerium is sufficiently labile to respond to the requirements for the OSC. The ZrO2 is added to enhance thermal stability of the OSC.
U.S. federal regulations require that the driver be alerted to a malfunctioning catalyst through a signal on the dashboard. Currently, there is no instrumentation commercially available to sense the effectiveness of the catalyst to meet the onboard diagnostic requirement. An indirect solution is to place a second O2 sensor at the exit of the catalytic converter. If the OSC in the catalyst is working properly, its voltage signal would have virtually no fluctuations because the O2 content would be always zero. If the OSC is not functioning properly, O2 will break through at the exit and the sensor would undergo similar fluctuations as the inlet sensor. Comparing these two signals generates the diagnostic that informs the operator of a malfunction.
A modern converter with dual O2 sensors (one at the inlet and one at the outlet) is shown as Fig. 6.11.
Modern Catalytic Converter Systems
Modern TWC-equipped vehicles are required to meet minimum emission standards for 150,000 miles [23, 24]. It should be understood that after this time period the catalyst is still extremely active, but has lost sufficient activity that it no longer meets the stringent EPA standards. The source of deactivation is sintering of the catalytic metals, especially the OSC and the carrier due to the extremely high temperatures (900–1,000°C) experienced in the exhaust. The steam produced from combustion enhances the degree of sintering. There are proprietary stabilizers added to the formulations that minimize the extent of sintering. Poisoning effects by sulfur and oil components (Zn, P, Ca, S, etc.) have been minimized by reductions in fuel sulfur and careful design of the washcoat to prevent contact of the poisons with the catalytic components. These catalyst improvements, coupled with enhanced engine control, have resulted in lifetimes of at least 150,000 miles.
At start-up, the catalyst is cold and there is a substantial emission of hydrocarbons. It is during the first 1 or 2 min of cold-start operation that the vehicle can fail the federal test procedure. Here the reaction is kinetically controlled. Once it gets sufficiently warm, the reaction exotherm quickly raises the temperature and the reaction becomes limited by bulk mass transfer. The space velocity varies between 5,000 (idle) and 75,000 h−1 at high speed. So manufacturers had to design the catalyst for kinetic control and bulk mass transfer conditions. The cold-start issue was addressed by positioning a small oxidation catalyst (close coupled) up against the exhaust ports of the engine to ensure rapid heat up and light-off. This is shown in Fig. 6.11. The newest cordierite monoliths have lower weights for faster light-off and high geometric areas (600–900 cpsi) to ensure adequate bulk mass transfer area and lower pressure drop to meet modern driving demands and ever-increasing regulations.
It is truly remarkable that catalysts can function so well in the exhaust of the modern high-speed vehicle. This fact has raised confidence in industry to use different monolithic (ceramic and metal) structures as supports for catalysts for other environmental applications such as diesel exhausts, power and chemical plants, restaurants, and even on widebody aircraft.
Controlling Emissions from Diesel Engines
The diesel engine was invented by Rudolph Diesel in the latter part of the nineteenth century. Due to significant benefits in fuel economy, they have enjoyed a surge in popularity in recent years, particularly in Europe where they represent approximately 50% of new cars sold. Although improved fuel efficiency is the key driver, improvements in the drivability of diesel vehicles as well as a reduction in their tailpipe emissions have also helped improve their image and spurred further growth. Unlike gasoline emissions that are mainly gaseous in nature, diesel emissions from passenger cars, buses, and trucks contain solid, liquid, and gaseous components. Both diesel fuel and operation of the engine differ significantly from gasoline spark ignited engines and therefore their emission profiles are much more complicated. Diesel fuel has a boiling range from 200 to 350°C. The four-stroke engine compresses air and at maximum compression (top dead center) injects liquid fuel into the mixture where combustion occurs spontaneously driving the piston downward in the work stroke. Diesel engines operate with a large excess of air (λ ≫ 1) and therefore three-way catalysts, which operates at (λ ~ 1), will not catalyze the reduction of NOx. Furthermore, the reduction of total particulate matter (TPM) must be addressed with new technology [23].
Based on the combustion characteristics of lean burn compression ignition diesel engines, the focus of emission regulations is on two key tailpipe pollutants—TPM and NOx. Both of these are produced in high quantity during combustion. Since diesel fuel is less volatile than gasoline and is injected directly into the cylinder as a liquid, combustion initiates before the fuel has had sufficient time to vaporize and mix completely with the air. As a result, combustion begins at the gas–liquid interface of the fuel spray and then progresses towards the center. This “diffusion combustion” phenomenon results in the production of significant amounts of NOx at the fuel liquid–gas interface where the local temperature and oxygen concentrations are high. In contrast, high amounts of soot (dry carbon) are generated in the interior of the spray where the temperature and oxygen concentrations are lower. As a result, both soot and NOx are produced simultaneously in large amounts from diesel engines. This relationship between combustion temperature-particulates and NOx is called the NOx-particulate trade-off. When NOx emissions are high (e.g., at high combustion temperatures), particulate emissions are low. In contrast, NOx emissions are low (e.g., at lower combustion temperature), but the particulate emissions are high.
Also shown on this profile are the emission standards to be met for different countries and years. Clearly, regulations are requiring that both particulate and NOx emissions be reduced to close to zero by 2010 and beyond in the US with other countries following closely.
Due to the adverse health effects associated with diesel soot and the ozone forming potential of NOx, both are the major focus for emissions regulations. Although CO and HC are also regulated, the amounts produced by diesel engines are generally low enough not to be a major obstacle for meeting emission regulations. This is particularly true for heavy duty diesel applications.
The solids emitted from diesel engines are essentially dry soot (carbon rich particles). The liquids are primarily unburned diesel fuel and lubricating oils (commonly referred to as soluble organic fraction or SOF) and to some extent sulfates originating from the combustion of the sulfur compounds present in the diesel fuel. The combination of solid and liquid pollutants is referred to as particulates or TPM. Note that H2SO4 derived from the combustion of sulfur compounds in diesel fuel is included since it is a liquid at the collection conditions for TPM. The gaseous pollutants are CO, HC, and NOx.
Total Particulate Matter
-
Dry soot
-
Liquids (oil, fuel) called SOF
-
H2SO4
Gases
-
CO, HC, NOx
Diesel Oxidation Catalysts
The diesel oxidation catalyst (DOC) has two primary functions (1) to oxidize the CO and HC’s emitted from the engine and (2) oxidize injected diesel fuel to generate heat for regenerating the particulate filter (to be discussed next). This catalyst is primarily a mixture of Pt and Pd on stabilized Al2O3 deposited on a ceramic monolith.
Regulations for dry soot reduction required an additional solution. This led to the introduction of the wall flow or diesel particulate filter (DPF). A DPF is primarily a cordierite honeycomb structure with alternating adjacent channels plugged at opposite ends. Exhaust enters the open channels, but only the gaseous components can pass through the porous wall exiting via the adjacent channel. Soot that is entrained in the exhaust stream is trapped on the wall while the gaseous components pass unrestricted. Periodically (e.g., every 1,000 km of driving), the filter is heated to a temperature high enough (ca. 550°C) to combust the soot and regenerate the filter. This heat is generally provided by oxidizing the diesel fuel injected into the DOC generating at least 550°C to initiate combustion of the soot. Alternatively, for vehicles without a DOC, fuel is injected into the cylinders during the exhaust stroke to promote combustion in the exhaust manifold, thereby raising the exhaust and DPF temperature. For the light duty market, DPF are usually silicon carbide or aluminum titanate.
A DPF may also contain a Pt containing catalyst (i.e., a catalyzed soot filter or CSF) to assist with the combustion of soot and to oxidize CO generated during the soot regeneration process.
Controlling NOx in Diesel Engines
The US and European standards require the reduction of all three phases of diesel emissions (i.e., solids, liquids, and gases). In particular, reduction of NOx will offer considerable challenges due to the lean nature (large excess air) of the exhaust. Two of the most promising technologies for controlling NOx at the tailpipe are Selective Catalytic Reduction (SCR) and lean NOx Traps (LNT). Both utilize catalytic processes to eliminate NOx by chemically reducing it to N2.
SCR relies on the reduction of NOx by ammonia (NH3) over either a vanadia (V2O5) supported on titania or metal-exchanged zeolite-based catalyst. The major desired reactions are below
Although commonly used in stationary source applications, SCR is relatively new for vehicle applications. Since handling gaseous ammonia is not practical in automobiles or trucks, an ammonia surrogate such as urea is utilized to generate ammonia in-situ in the vehicle exhaust. Typically, a solution of urea and water is injected into the exhaust stream before the SCR catalyst, and in the presence of water vapor, is hydrolyzed to ammonia which can participate in the SCR/NOx reduction reactions. In addition, an ammonia “cleanup” catalyst (often referred to as an AMOX catalyst) may be required to remove any ammonia “slip” that may pass through the SCR catalyst unconverted. Clearly, integration of the SCR catalyst within the exhaust system is a challenge. Depending on the specific application, an oxidation catalyst (DOC), a CSF, an SCR catalyst, and an ammonia destruction catalyst (AMOX) may all be required to meet the combined CO, HC, and NOx regulations especially for heavy duty trucks. Sophisticated engine controls are required to ensure proper operation of all components within the system. The schematic in Fig. 6.12 shows the catalytic unit operations for meeting 2010 diesel emission standards using an SCR system.
The second promising technology for controlling NOx at the tailpipe is LNT. The technology utilizes a Pt and Rh-based TWC catalyst in combination with an NO2 trapping agent (e.g., an alkaline earth compound such as BaO). During the normal lean operation mode of the diesel engine, NO is oxidized to NO2 over the Pt catalyst and the NO2 is adsorbed by the BaO within the catalyst washcoat. Periodically (e.g., every 60–120 s), the trap is regenerated by introducing a “rich pulse” of reductant (e.g., diesel fuel) into the exhaust stream or by switching the engine operating mode to stoichiometric or rich for 1–2 s. This rich pulse provides the necessary chemical reductant to convert the adsorbed nitrate to nitrogen over the Rh catalyst.
Pt
Rh
LNT technology has been successfully demonstrated in light duty vehicle applications; however, its primary disadvantages are high Pt levels are required to maintain sufficient catalyst durability and a fuel penalty (ca. 3–5%) results from the periodic trap regeneration. In addition, the SOx derived from the fuel-borne sulfur forms BaSO4 that is much more stable than the corresponding nitrates and are not removed during the stoichiometric or rich operation mode. Therefore, the trap becomes progressively poisoned by sulfates. Complicated engine control strategies are being developed to desulfate the poisoned trap by operating the engine at a high temperature (>550°C) and rich of the stoichiometric air/fuel ratio for a short period of time. In addition, the air-to-fuel ratio must be carefully controlled to avoid the formation of H2S during excessive rich conditions. LNT technology has the capability of removing up to 90% of the NOx in the exhaust. Having lower sulfur fuels available will favor high NOx conversion levels and also reduce the requirements for desulfation. Improvements in catalyst and engine control technologies hold promise for the future.
Catalytic Hydrogenation of Vegetable Oils for Edible Food Products
Triglycerides
Plant-derived oils such as soy, cottonseed, peanut, canola, corn, etc. are natural sources of edible products such as baking dough for cakes, cooking oils, salad dressing, chocolates, margarine, etc. Non-edible products such as lubricants, creams, lotions, etc. can also be produced depending on the processing of the oils. Natural oils are composed of long chains of fatty acid esters called triglycerides as shown in Fig. 6.13. The triglyceride chains are polyunsaturated, the degree of which influences their stability against oxidation in air.
Catalytic hydrogenation of the double bonds improves the stability against air and raises the melting point such that solids can be produced. Thus, the precursor to chocolate candy, margarine, or a cake mix is liquid oil that upon hydrogenation becomes an edible solid at room temperature. The more double bonds hydrogenated (the more saturated) the higher istowards air but often more injurious to our health by deposition of cholesterol in our blood the melting point, the lower is the reactivity vessels. The goal of a good catalyst coupled with the proper process conditions is to produce a reasonably healthy product with the desired melting point range with sufficient air stability to permit good shelf life.
Oils are classified by the length of the glyceride chain and degree of polyunsaturation. Typically nature produces oils with each chain length between 12 and 22 carbons with up to three unsaturated bonds usually all in the cis-form. Triglycerides with 18 carbons per length and three double bonds at positions 9, 12, and 15 counting from the first carbon in the ester group are called linolenic and designated C18:3. This structure is shown in Fig. 6.13. The outermost double bond is so reactive towards air that oils with three double bonds in the alkyl chain are rare. Therefore, the most prevalent in nature have double bonds at positions 9 and 12 and are referred to as linoleic (C18:2). Its reactivity is about half that of linolenic. The least reactive is oleic (1/20 that of linolenic) with only one double bond per length at position 9 (C18:1). Stearic is the term used for glycerides with all bonds saturated (C18:0). Not surprisingly, this form has virtually no reactivity towards air, has a high melting point, and is unhealthy.
The source of the oils plays a major role in producing a product with the desired melting point, stability, and health consequences. Cotton, sunflower, corn, and soy bean oils are a mixture of the four basic triglycerides with 50–70% C18:2 being the most dominant followed by 20–30% C18:1 with less than 1% C18:3. Less than 10% are other saturated oils such as C16:0. Palm kernel and coconut oils have almost 80% saturated triglycerides (C12:0, C14:0, and C16:0), have high melting points, and are stable against air but not healthy. They are used for protecting the skin against excessive sun exposure. Olive oil has up to 80% C18:1 and is therefore relatively healthy.
The most common oil hydrogenation catalysts are 20–25% Ni on Al2O3 and SiO2. The nickel salts are either impregnated or co-gelled with a carrier precursor such as a soluble Al or Si salt. The catalytically active state of Ni is the reduced (metallic) form. The activation step is performed during manufacture at which time the catalyst is coated with a fatty gel to protect it from air oxidation during shipment.
Less than 1% by weight of catalyst is added to the batch reactor where the fatty protective gel slowly dissolves and the hydrogenation reaction commences. Temperatures of 100°C and H2 pressures of 3–5 atmospheres are used to ensure adequate dissolution of the H2 into the feed stream. Reactions are carried out between 100 and 160°C. Stirring is vigorous to maximize bulk gas diffusion to the catalyst surface. The catalyst particles are small to minimize pore diffusion resistance and increase liquid–solid mass transfer area.
Cu supported on Al2O3 is less active than Ni and this property is used to “brush” hydrogenate. Only minimum hydrogenation occurs maintaining the melting point but sufficient to improve stability against air. This is sometimes used for producing salad oils.
The reaction profile is generally sequential with hydrogenation first occurring on the most active double bonds followed by those less active [24–26]. Time distribution shows the linoleic form decreasing as the oleic form increases. After extended reaction time, the stearic begins to form as the oleic is slowly hydrogenated. Thus, one can design the process to control the product distribution in a predictable manner.
Hydrogenation of the linoleic form with a melting point of −13°C will produce a oleic product with a melting point of 5.5°C very suitable for consumption. During the partial hydrogenation process, it is most desirable to minimize isomerization to the trans isomer (the hydrocarbon groups are trans to each other across the remaining unsaturated bonds) because this structure raises the “bad” cholesterol or LDL (low-density lipids). A partially hydrogenated cis-structure may have a melting point of 6°C, whereas its trans isomer melts at 40°C. The trans isomer is more readily formed at high reaction temperatures, high Ni catalyst loadings, and at low hydrogen pressures (low concentration of H2 at the catalyst surface). Pt containing oil hydrogenation catalysts produce considerably less trans than Ni; however, the high activity causes excessive hydrogenation of the double bonds. To minimize this effect, NH3 is intentionally added to the feed or catalyst to poison its activity towards hydrogenation. By so doing, a low trans oil is produced without excessive saturation of the double bonds. In 2006, the U.S. Food and Drug Administration required labels that report the amount of trans components present in edible products.
Catalyst deactivation is mainly caused by mechanical attrition due to the rigorous stirring. In most cases, adsorption guard beds are used upstream to remove most of the impurities such as sulfur and phosphorous often found in the feed. Recognizing that some poisons may break through, the catalyst has an average pore size sufficiently large to admit the triglycerides but smaller than the average size of the organic compounds containing P and S. The spent catalyst is separated from the product by filtration. Given the increasing cost of Ni, it is recovered, refined, and used to make fresh catalyst.
Fertilizers and Hydrogen Generation
General Reactions
Ammonium nitrate (NH4NO3) and urea (CO(NH2)2) are two major sources of the world’s fertilizers. The nitrate is produced by reaction of ammonia and nitric acid.
Urea is produced by the reaction of NH3 with CO2 and its subsequent decomposition.
Heart
Ammonia is produced by the catalytic hydrogenation of N2 with H2
Hydrogen is produced by a series of catalytic reactions, the first of which is hydrocarbon reforming, and the second is water gas shift. Considering natural gas (CH4) as the starting hydrocarbon
Nitric acid is produced by the selective catalytic oxidation of NH3 and its subsequent hydration
Each catalytic step will be discussed in this section.
Hydrogen Generation for the Production of NH3
Producing H2 from hydrocarbons such as natural gas is currently practiced in the chemical industry [27–30] under steady state conditions with carefully controlled catalytic unit operations. The overall process is as shown in Fig. 6.14.
Traces of organic sulfur compounds such as mercaptans, thiosulfides, and alkyl sulfides are added to natural gas to impart odor for safety detection of leaks. Because sulfur compounds are poisons to the downstream catalysts, they must be removed. The technology of choice is hydrodesulfurization or HDS.
The reaction is carried out at about 300–400 psig and 300–400°C, but these conditions vary with the hydrocarbon feed. The catalyst is 3% Co, 15% Mo deposited on spheres (25–75 m2/g) Al2O3 with diameters of 2–3 mm. The catalyst is presulfided to decrease its activity towards undesirable side reactions such as coking.
The H2S produced is removed downstream from the HDS reactor by adsorption on ZnO particles at about 400–500°C.
Primary reforming of the sulfur-free natural gas (i.e., CH4) is the first step to produce a H2-rich gas.
The reaction is highly endothermic and thus is favored at high temperatures. The maximum temperature achievable is limited by metallurgy of the reactor. Given the increase in gas volume, the reaction is favored by low pressures.
The catalyst is approximately 30% Ni with about 14% CaO on highly densified alpha alumina (α-Al2O3) with a surface area about 2–5 m2/g. The CaO reacts with the Al2O3 forming CaAl2O4 for added mechanical strength under the severe operating conditions of 800°C and 300–400 psig and a steam environment up to 75%. The reaction rate is limited by a combination of heat transfer and pore diffusion, the latter due to the inadequate accessibility of the reactants to the catalyst interior. To counter pore diffusion limitations, the catalyst is manufactured as a donut with two to three holes to increase the external contact area and decrease the diffusion path. The space velocity is between 1,000 and 2,000 h−1. The active catalyst is Ni metal, so it must be reduced carefully with H2 prior to introducing the feed. The reaction is carried out in a series of tubular parallel reactors located in a large fired box furnace to provide the necessary heat.
Deactivation is due mostly to the slow accumulation of sulfur that breaks through the upstream HDS/ZnO guard beds. Sulfur irreversibly decreases the activity of the Ni that allows the methane decomposition rate to become significant leading to the accumulation of hydrogen-deficient carbon or “coke.” This builds up within and between catalyst particles, leading to its fracture and an increase in pressure drop. During process shut down, the catalyst must be “passivated” to protect against air oxidation of the Ni and a subsequent fire due to its strong exotherm creating a safety hazard at the plant site. This is accomplished by periodically injecting small amounts of air and carefully oxidizing the surface of the Ni while monitoring the exotherm.
Equilibrium limits conversion of the CH4, so partial oxidation or secondary reforming of the unreacted CH4 is used to generate more heat and H2 in a secondary reforming step. The addition of air also serves the purpose of providing the required N2 for the subsequent ammonia synthesis reaction. Secondary steam reforming also uses a high-temperature resistant Ni containing catalyst that must retain its strength after prolonged exposure to close to 1,200°C due to the oxidation in the front end of the bed. The catalyst used is α-Al2O3 impregnated with about 18–20% Ni and 15% CaO.
The exit from the secondary reformer contains about 10–12% CO, is cooled to about 350°C, and fed to a high-temperature water gas shift (HTS) reactor.
The particulate catalyst is composed of 90% Fe and 10% Cr. The Cr minimizes sintering of the active Fe phase. The catalytic reaction is limited by pore diffusion, so small particles are used. The exit process gas contains about 2% CO as governed by the thermodynamics and kinetics of the reaction. This reaction is slightly exothermic and thermodynamics favor low temperatures that decrease the reaction rate. It is therefore necessary to further cool the mix to about 200°C where it is fed to a low-temperature shift reactor (LTS) containing another particulate catalyst composed of 30–35% Cu, 45% ZnO, and 13–20% Al2O3. The catalyst is active in the reduced form, so it must be carefully activated with H2 avoiding excessive overheating which will cause sintering. The Zn and Al2O3 are added to stabilize the Cu because it is sensitive to sintering. The CO is decreased to its thermodynamic limit as imposed by the temperature and gas compositions. Typically, the CO is reduced to less than about 0.5%. The catalyst deactivates by traces of sulfur and sintering of the active Cu phase. Because of the necessity to operate at low temperatures, the reaction rate is slow and large volumes and low space velocities (1,500–2,500 h−1) are used.
The active Cu-containing catalyst is also very air sensitive (like the Ni reforming catalyst) and will spontaneously oxidize generating uncontrolled reaction heats. Thus, it must be passivated before discharged and exposed to air. A small amount of air is added to the reactor and the temperature monitored. This process is continued until the exotherm is small enough that the catalyst can be safely removed from the reactor.
The remaining CO, which poisons the downstream ammonia synthesis catalyst, is removed by methanation using either a Ni or Ru on Al2O3 catalyst at 300°C
The CO2 is scrubbed in an amine solution.
Ammonia Synthesis
The Haber process for the synthesis of ammonia from H2 and N2 has been practiced since the beginning of the twentieth century always with a massive Fe catalyst [31].
It is mildly exothermic, so the reaction is thermodynamically favored at lower temperatures but at high pressures due to the contraction of gaseous product volume. To obtain reasonable rates, the process is operated at about 450°C and pressures approaching 5,000 psig at a space velocity of 10,000–15,000 h−1. The process is operated in a recycle mode, so ammonia is continuously removed aiding the equilibrium.
The active catalyst is 75–80% Fe metal, 10% Fe2O3, 4% Al2O3, less than 5% alkali and alkaline earth (Li, Ca, and Mg), with 1% SiO2 added to minimize sintering of the Fe. The promoters are added to a melt of magnetite (Fe2O3). The solid mass is then ground to 1 mm particles, charged to the fixed bed reactor, and slowly reduced with H2 at 500°C. The reduction generates active Fe metal with some porosity due to liberation of oxygen forming H2O. The surface area is increased from about 1 m2/g to about 20 m2/g. The small particle size of the finished catalyst is necessary to minimize pore diffusion limitations. Special precaution is necessary during discharge from the reactor because air exposure will spontaneously oxidize the Fe surface generating large quantities of heat.
The catalyst is poisoned by CO, CO2, and H2O, so they must be rigorously removed upstream in the hydrogen synthesis process. Oxygen-containing molecules are permanent poisons. Other poisons such as sulfur, arsenic, halides, and phosphorous must be carefully removed upstream in as much as they too are permanent poisons.
Nitric Acid Synthesis
Nitric acid is produced by the selective oxidation of NH3 over a gauze catalyst composed of 90%Pt, 10%Rh (some gauze is 90% Pt, 5% Rh, and 5% Pd) [32]. This reaction was used in the “Selectivity” section to demonstrate the high efficiency with which PtRh leads to NO production as opposed to more thermodynamically favored N2.
The low-pressure process (15–30 psig) produces NO with a selectivity of 98% and the high-pressure process (150 psig) has a selectivity of 94%. The high-pressure plant allows for a smaller reactor (and gauze) diameter (3 ft) compared to 12 ft for the low-pressure process.
The feed is composed of 10–12% NH3 in air and is fed to the reactor at an inlet temperature of about 250°C and a space velocity approaching 50,000 h−1. For a high-pressure plant, the exotherm generates an outlet temperature of 900°C. The NO product is cooled and noncatalytically converted to NO2, its thermodynamically favored state at low temperatures, which reacts with H2O forming HNO3.
The finished alloy catalyst is manufactured by a knitting process to form a wire gauze that looks like a door screen. The Rh is added to impart mechanical strength during the wire drawing operations. During reaction, the catalyst undergoes an unusual morphology change. The Pt forms an oxy–nitrogen species and volatilizes from the gauze. The smooth wires become roughened and sprout resembling cauliflower. The surface area of the gauze increases by 20 times. The loss of Pt enriches the surface with Rh and the catalyst slowly loses activity. After approximately 90 days of operating a high-pressure plant, the Pt content of the gauze is reduced to 50%. The volatile Pt is captured downstream on a “getter gauze” made of Pd. The Pd surface catalytically decomposes the gaseous oxy–nitro Pt species and a Pt–Pd alloy forms that allows for easy recovery of the precious metals. The spent catalyst is returned to the supplier where the precious metal is recovered for future use.
Another source of deactivation is Fe contamination originating from the corrosion of upstream equipment depositing on the gauze resulting in decomposition of the NH3 to N2. Another source is from the Fe-containing ammonia synthesis catalyst.
Although the largest use for nitric acid is NH4NO3 fertilizers, it is also used for explosives and nylon polymers.
Pure Hydrogen Generation with Pressure Swing Adsorption Purification
For applications in which N2 is not needed, such as H2 or alcohol production, pressure swing adsorption (PSA) is used. The process flow diagram is shown in Fig. 6.15.
There is a renewed interest in hydrogen generation for the developing hydrogen economy with the anticipated use of fuel cells as a power source for vehicles [33]. The fuel cell generates electricity by electrochemically oxidizing H2 and reducing O2. Because it directly converts chemical to electrical energywithout using the traditional mechanical steps of piston-driven engines and turbines, it promises to be more efficient, cleaner, decrease our dependence on oil, and generate less greenhouse gas. The small-scale generation of H2 for cost-effective refueling stations is a major issue that is aggressively being studied. Ultimately, it will be derived from water by electrolyzers using natural sources such as solar, wind, and geothermal energy. Until these technologies are available, natural gas reforming is a likely source because infrastructures exist in many cities in the world. The fuel cell powered vehicle will require an infrastructure similar to gasoline and diesel service stations. High-pressure H2 will have to be available to refuel the vehicles. Such demonstration stations are now being built in various parts of the world. PSA is used for final H2 purification. The partial oxidation and the LTS are eliminated. There is therefore a loss of some hydrogen production, but the final H2 is not diluted with N2. The PSA purification unit replaces the methanator and CO2 scrubber and produces pure H2. Some of the H2 is recycled for HDS and some combusted to provide the heat for steam reforming.
Given the need for smaller size reformers to be operated in local communities, safety will be an elevated concern. These reformers must contain nontoxic and air-insensitive catalysts, thus eliminating Ni, Cu, and Cr from consideration. A major research effort is underway to redesign the entire H2 generation process system using modern materials such as monoliths and precious metal catalysts [33].
For fuel processors directly integrated to a residential fuel cell, H2 must be maximized and no pressure is available for PSA. In these cases, a high- and LTS catalyst will be required. Hydrogen purification to reduce the CO to less than 10 ppm will be managed by preferential oxidation [34].
Production of Butyraldehyde: A Homogeneous Catalytic Reaction
Butyraldehyde
The incorporation of an inner layer of poly(vinyl butyral) or (PVB) in the glass of an automobile windshield protects against serious head injuries when a passenger strikes it during an accident. The strongly adherent coating is optically transparent and maintains the glass intact (antishattering agent) when a foreign object hits the surface. Thus, the glass does not shatter when a stone strikes its surface. PVB is produced by reaction of polyvinyl alcohol (PVA) with linear butylraldehyde (CH3CH2CH2CHO).

Another important application of butyraldehyde is in the production of oxo-alcohols for use as plasticizers used to improve mixing of solid compounds that must be molded or extruded into specific shapes. The hydrogenation catalyst is Ni/Al2O3.

Butylraldehyde is produced by a homogeneous catalytic process called hydroformulation in which CO and H2 are added to liquid propylene using a soluble cobalt-containing complex catalyst Co(CO)6. The reaction is carried out with a butanol solvent
The conditions needed to catalyze the reaction are very severe; pressure = 3,000–4,500 psig and 150°C. The high pressure maintains the propylene in solution, ensures sufficient solubility of the H2 and CO, and maintains the Co-carbonyl complex stable against decomposition. The product distribution is 4:1 linear to branch.
Much less severe conditions can be used with the Wilkinson homogeneous catalyst rhodium tricarbonyl triphenyl phosphate, HRh(CO)3(PC6H5)3. Pressures equal to 225 psig and temperatures of 100°C selectively produce the more useful linear form [35]. The milder conditions more than compensate for the more expensive Rh (1,000 times that of Co). The aldehyde product is distilled leaving the catalyst in the solvent ready for reuse.
Homogeneous catalysts are structurally well- defined complexes and, because they are soluble in the reaction mix, are not subject to pore diffusion limitations as are heterogeneous catalytic materials. They are almost always highly selective towards desired products. The main consideration is that the complex be stable and reactor conditions chosen such that all the gaseous reactants are adequately dissolved and mixed in the liquid phase. Homogeneous catalysts are easily characterized by standard instrumental methods for compound identification such as XRD or spectroscopy. Deactivation is associated with attack by traces of carboxylic acidic byproducts and impurities in the feed such as O2 and chlorides that attack the ligand groups.
Polyethylene and Polypropylene for the Production of Plastics
Polyethylene
Specially prepared plastics are rapidly replacing traditional metal components because of their strength, transparency, resilence, lighter weight, and greater corrosion resistance. The largest volume products are polyethylene and polypropylene. Each has its own contributions to the marketplace where the former is primarily used for low-strength applications such as milk and food containers. Polypropylene is used when enhanced strength, higher melting temperatures, and greater resistance to chemicals such as chemical holding tanks and automobile bumpers are required.
There are two prevalent methods of producing polyethylene, both of which involve heterogeneous catalysts. A slurry phase process utilizes chromium oxide deposited on SiO2 dispersed in a solvent such as cyclohexane at 80–150°C and a pressure between 300 and 500 psig. The process operates in a recycle mode with a residence time of 2–3 h. The product containing the solvent and polymer is flashed leaving the polymer. The catalyst is usually left in the polymer because its concentration is extremely low. The operating conditions are adjusted to produce both high- and low-density polyethylene. The active site is Cr+2 produced by the reduction of Cr+6 by ethylene. The reaction mechanism proposed is that the polymer coordinates with one of the Cr+2 sites and the incoming ethylene coordinates with another site. Insertion of the ethylene into the double bond of polymer propagates its growth.
A second method of production utilizes the Ziegler–Natta TiCl4 catalyst with liquid cocatalysts such as an alkyl aluminum halide. This is a reactive catalyst that must be prepared at the exclusion of air and water. The alkyl group of the cocatalyst coordinates with the Ti+3 site. The polymer grows by insertion of the ethylene into the double bond of the adsorbed polymer on another site.
Polypropylene
The most modern production route for polypropylene (PP) is also the Zeigler–Natta catalyst [36–38]. The catalyst is TiCl4 supported on MgCl2 along with aluminum alkyl halide cocatalyst such as diethyl aluminum fluoride (CH3CH2)2AlF. The MgCl2 is milled to a very disordered but active structure and the TiCl4 is added. Production of the PP can be carried out in a fluidized gas phase reactor between 50 and 100°C and 100–600 psig. Ethyl benzoate is also used as part of the catalyst preparation and functions to reduce the TiCl4 to active TiCl3. The role of the alkyl component of the cocatalyst is to coordinate with the Ti+3 site where it inserts into the adsorbed polypropylene continuing the chain growth. The amount of catalyst used is so small it is retained in the final polymer product with no negative consequences. Unreacted gases are removed and recycled at the completion of the process. The most desirable product for the largest market is the isotactic form in which all CH3 groups are on the same side of the polymer chain. Typically, it has a density of 0.9 g/cm3, a melting point of 170°C, and an average molecular weight of 500,000. The polypropylene product is mixed in a separate reactor with ethylene to make a block polymer with enhanced mechanical properties.
Water, CO, and O2 are the most significant poisons and are carefully removed upstream of the process.
The catalyst preparation and the process are far more complicated than presented here, so the reader is encouraged to refer to more detailed references [36–38].
Catalyst Challenges
Catalysts will have additional challenges as we move forward in the twenty-first century. In this author’s mind, one of the most critical is the need to balance our rapidly expanding energy needs with the environment. Catalysts are already playing a dominant role in pollution abatement and in the production of specialty petroleum and chemical products. The main challenge will be to use bio-renewable energy sources (Chap. 33) as well as solar, wind, geothermal, etc. with the specific goal of freeing us from the use of fossil fuels. The hydrogen economy coupled with the fuel cell holds great promise as one road to meet this challenge [39]. Catalysts will play a key role in this pursuit, although the road map is not yet complete. This will be an exhilarating ride as we find our way to clean energy.
Another area of great importance is the application of bio-catalysis using enzymes to produce a growing number of pharmaceutical agricultural products and fuels such as ethanol. This subject is outside the scope of this review so other sections of this Handbook (see Chap. 31) should be consulted.
References
Smith J (1981) Chemical reaction engineering. McGraw-Hill, New York
Schweich D (2003) Transport effects. In: Horvath I (ed) Encyclopedia of catalysis, vol 6. Wiley, New York, p 507
Fogler H (1992) Elements of chemical reaction engineering, 2nd edn. Prentice Hall, Englewood Cliffs, NJ
Liu X, Korotkikh O, Farrauto R (2002) Selective catalytic oxidation of Co in H2: structural study of Fe-oxide promoted Pt/alumina catalyst. Appl Catal Gen 226:293
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 2
Heck R, Farrauto R, Gulati S (2009) Catalytic air pollution control: commercial technology, 3rd edn. Wiley, New York, Chapters 2, 4, 7, and 9
Missen R, Mims C, Saville B (1999) Introduction to chemical reaction engineering and kinetics. Wiley, New York, Chapter 4
Broadbelt L (2003) Kinetics of catalyzed reactions. In: Horvath I (ed) Encyclopedia of catalysis, vol 4. Wiley, New York, p 472
Chorkendorff I, Niemantsverdrietm J (2003) Concepts of modern catalysis and kinetics. Wiley-VCH, Weinheim, Germany, Chapters 2 and 9
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 1
Bartholomew C (2003) Catalyst deactivation/regeneration. In: Horvath I (ed) Encyclopedia of catalysis, vol 2. Wiley, New York, p 82
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 5
Knozinger H, Maximlians L (2003) Catalyst characterization. In: Horvath I (ed) Encyclopedia of catalysis, vol 2. Wiley, New York
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 3
Van Rysaselberghe V, Froment G (2003) Hydrodesulfurization. In: Horvath I (ed) Encyclopedia of catalysis, vol 3. Wiley, New York, p 667
Brunet S, Mey D, Perot G, Bauchy C, Diehl F (2005) On the hydrodesulfurization of FCC gasoline: A Rev Appl Catal Gen 278(2):143
Gates B (2003) Catalysis by solid acid. In: Horvath I (ed) Encyclopedia of catalysis, vol 2. Wiley, New York, p 104
Csicsery S, Kiricsi I (2003) Shape selective catalysis. In: Horvath I (ed) Encyclopedia of catalysis, vol 6. Wiley, New York, p 307
Moser M (2003) Reforming. In: Horvath I (ed) Encyclopedia of catalysis, vol 6. Wiley, New York, p 1
Antos G, Aitani A, Parera J (eds) (1995) Catalytic naphtha reforming, science and technology. Marcel Dekker, New York
Soetaert W, Vandamme E (2008) Biofuels. Wiley, UK
Heck R, Farrauto R (2003) Automotive catalysis. In: Horvath I (ed) Encyclopedia of catalysis, vol 1. Wiley, New York, p 517
Heck R, Farrauto R, Gulati S (2009) Catalytic air pollution control: commercial technology, 3rd edn. Wiley, New York, Chapter 6
Albright L (1978) Hydrogenation (partial) of triglycerides. In: Peterson S, Johnson H (eds) Encyclopedia of food science. Avi Publishing, Westport, CT, p 398
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 7
Albright L, Wisniak J (1962) Selectivity and isomerization during partial hydrogenation of cottonsead oil and methyl oleate: effect of operating variables. J Am Oil Chem Soc 39:14
Rostrup-Nielsen J, Nielsen TR (2002) Steam methane reforming. CATTECH 6(4):150
Osterkamp P (2003) Synthesis gas. In: Horvath I (ed) Encyclopedia of catalysis, vol 6. Wiley, New York, p 456
Nielsen J (2003) Hydrogen generation by catalysis. In: Horvath I (ed) Encyclopedia of catalysis, vol 4. Wiley, New York, p 1
Kondrantenko E, Baerns M (2003) Synthesis gas generation. In: Horvath I (ed) Encyclopedia of catalysis, vol 6. Wiley, New York, p 424
Pesce L, Jenks W (2006) Synthetic nitrogen products. In: Kent J (ed) Riegels handbook of industrial chemistry, 11th edn. Kluwer, New York
Bartholomew C, Farrauto R (2006) Fundamentals of industrial catalytic processes, 2nd edn. Wiley, New York, Chapter 8
Farrauto RJ, Hwang S, Shore L, Ruettinger W, Lampert J, Giroux T, Liu Y, Ilinich O (2003) New material needs for hydrocarbon reforming; Generating hydrogen for the prom fuel cell. Ann Rev Mater Res Soc 33:1
Shore L, Farrauto R (2003) Preferential oxidation of CO in H2 streams. In: Vielstich W, Lamm A, Gasteiger H (eds) Handbook of fuel cells, 3, Part 2. Wiley, West Sussex, England, p 211
Kohlpaintner C (2003) Hydroformulation. In: Horvath I (ed) Encyclopedia of catalysis, vol 3. Wiley, New York, p 787
Vlad G (2003) Polymerization. In: Horvath I (ed) Encyclopedia of catalysis, vol 5. Wiley, New York, p 611
Rodriguez F (1996) Principles of polymer systems, 4th edn. Taylor & Francis, Washington, DC
Knapczyk J, Simon R (2006) Synthetic resins and plastics. In: Kent J (ed) Riegels handbook of industrial chemistry, 11th edn. Kluwer, New York
McNicol B, Williams K (2003) Catalysts for fuel cells. In: Horvath I (ed) Encyclopedia of catalysis, vol 2. Wiley, New York, p 387 and 42
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media New York
About this chapter
Cite this chapter
Farrauto, R.J. (2012). Industrial Catalysis: A Practical Guide. In: Kent, J. (eds) Handbook of Industrial Chemistry and Biotechnology. Springer, Boston, MA. https://doi.org/10.1007/978-1-4614-4259-2_6
Download citation
DOI: https://doi.org/10.1007/978-1-4614-4259-2_6
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4614-4258-5
Online ISBN: 978-1-4614-4259-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)