Abstract
Optical coherence tomography (OCT) is an evolving technology, developed in the 1990s to provide noninvasive imaging of tissues. Using low-coherence light and ultra-short laser pulses, it generates cross-sectional images of biologic structures with great detail. OCT has become valuable in medicine, especially in ophthalmology. This technology allows the detection of early or subtle pathology and provides a critical tool in the diagnosis and management of ocular diseases. OCT is routinely used in the fields of retina and glaucoma, and now has become more popular in neuro-ophthalmology. In this chapter the role of OCT technology, its limitations, and use in the differentiation of optic nerve disorders from retinal disorders will be discussed. Its potential role in the diagnosis and monitoring of multiple sclerosis, Alzheimer’s disease, and Parkinson’s disease will also be highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optical Coherence Tomography
- Retinal Nerve Fiber Layer
- Optic Neuritis
- Retinal Nerve Fiber Layer Thickness
- Spectral Domain Optical Coherence Tomography
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Basic Principles of Optical Coherence Tomography (OCT)
OCT technology is based on the principle of “indirect inferometry” which superimposes light waves for imaging [1]. The summation of waves results in either constructive or destructive interference of light, and this information is then translated into an A-scan. The horizontal summation of a series of A-scans is a B-scan, which is represented as the OCT cross-sectional image. A summation of B-scans results in C-scans or three-dimensional cross-sections.
Early OCT employed time domain technology. In time domain OCT, the reference arm contains a light source and mobile mirror, which changes the path length of the light with time [2]. When the time delay from both arms is congruent, constructive interference occurs, and when they are not the same, varying levels of constructive and destructive interference patterns are formed. An example of an OCT using time domain technology is Zeiss Stratus OCT.
More recently, spectral, or Fourier domain OCT, has been developed. In this technology, the reference arm is fixed, resulting in a spectrum of interference pattern detected by the camera at any time point [3]. A mathematical algorithm known as the Fourier transformation is then carried out to generate the OCT image [3]. With this technology, the imaging speed is dramatically improved, allowing for acquisition of a dense level of data and in effect decreasing motion artifacts and generation of three-dimensional data. In addition, the use of a broad wavelength light source results in high-resolution imaging. Examples of OCT devices using spectral domain are Spectralis (Heidelberg Engineering, Carlsbad, CA), Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA), 3D-OCT (Topcon Medical Systems, Oakland, NJ), and RTVue (Optovue, Fremont, CA) [4, 5].
Although both technologies follow the same basic principle, studies comparing the two types of machines have found differences in the results generated. In addition to variability between time domain and spectral domain OCT, there is also variability in the results between the different spectral domain OCTs. This variability limits the longitudinal assessment of data when a patient’s results are obtained on different machines over time. The differences between the reproducibility of data among the different machines will also be highlighted.
OCT in the Analysis of Neuro-Ophthalmic Disorders
OCT does not replace the role of a careful neuro-ophthalmic evaluation. It supplements the clinical examination and can provide quantitative and longitudinal information. In this section, examples of neuro-ophthalmic disorders are shown in which OCT demonstrates areas of pathology not always clearly visualized on fundus examination.
Non-arteritic Ischemic Optic Neuropathy with Subretinal Fluid
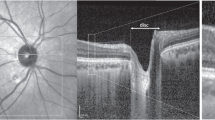
Patients with a non-arteritic ischemic optic neuropathy (NAION) typically present with altitudinal field loss. Central vision may be mildly to moderately affected. Hedges et al. first reported several patients with central vision loss due to subretinal fluid (SRF) associated with NAION [6]. These patients, unlike those with classic NAION, have more central visual loss due to SRF extending to the fovea. SRF can be difficult to identify on fundus exam (Fig. 11.1) but can be clearly seen on macular OCT (Fig. 11.2). As treatment of NAION is developed, exactly how therapy affects the nerve and/or the retina must be considered.
Fast macular scan in above patient with non-arteritic ischemic optic neuropathy (NAION) in the left eye revealing increased macular thickness in the affected eye (a). Line scan through the optic nerve and macula clearly revealing severe disc edema with intraretinal and subretinal fluid in the macula (b)
Papilledema with Secondary Choroidal Neovascularization and Subretinal Fluid
Patients with papilledema typically do not present with acute central visual loss. An exception is when there are associated macular changes from SRF or a choroidal neovascular membrane (CNV) (see Fig. 11.3). Line scans through the macula help in locating the heme as subretinal and identifying the CNV (see Fig. 11.4). Avastin injections have been beneficial in treating CNV associated with papilledema. SRF involving the macula, as described by Hoye et al. in 1998, usually resolves, and visual acuity improves spontaneously as the cause of the papilledema is treated [7, 8].
Toxic Optic Neuropathy
Patients with visual loss from toxic, hereditary, or nutritional optic neuropathies maybe mistaken for functional visual loss due to minimal abnormalities on fundus examination. The temporal pallor and dull foveal reflex on fundus exam is subtle (see Fig. 11.5). Retinal nerve fiber layer (RNFL) and macular OCT studies clearly demonstrate abnormalities in RNFL thickness temporally and in the distribution of the papillomacular bundle fibers (see Fig. 11.6) [9].
Optic Nerve Drusen
Optic nerve drusen can give the appearance of blurred disc margins, similar to the appearance of true disc edema, or papilledema (see Fig. 11.7). RNFL scans show atrophy in optic nerve head drusen, whereas it shows increased RNFL thickness in optic nerve edema (see Fig. 11.8). In addition, line scans through the optic nerve reveal differences in the contour of the optic nerve. Various studies have shown a characteristic “bumpy” appearance of the internal contour of the optic nerve in optic nerve drusen, compared to the more smooth borders of elevation seen with true disc edema (see Fig. 11.9) [10, 11].
Optic Nerve Pit
Patients with optic nerve pits have an optic nerve appearance that may be mistaken for an optic neuropathy or glaucoma. In characteristic cases, there may be a grayish appearance to the inferior temporal rim (see Fig. 11.10). In more subtle cases, this may not be present. OCT can help clarify the diagnosis. Optic nerve pits often have inferior-temporal RNFL thinning on fast RNFL scan (see Fig. 11.11). When patients with an optic nerve pit present with central visual loss from neurosensory retinal fluid, this may be further mistaken for an acquired optic neuropathy (see Fig. 11.12). The correct diagnosis can be made by doing a line scan from the optic nerve to the macula, which reveals the “pit” as a schisis contiguous with fluid in the neurosensory retina (see Fig. 11.13).
Macular Edema from Fingolimod
A rare complication in the use of fingolimod in multiple sclerosis (MS) patients is visual loss from macular edema [12]. Macular edema may sometimes be difficult to diagnose on fundus examination (see Fig. 11.14). When MS patients present with central visual loss, it may be inaccurately diagnosed as retrobulbar optic neuritis. Macular OCT (see Fig. 11.15) helps distinguish visual loss from macular edema versus that from retrobulbar optic neuritis by clearly demonstrating the intraretinal fluid in the macula.
Central Serous Retinopathy
Central serous retinopathy and optic neuritis can present with similar symptoms which can be especially confusing in MS patients. The findings of central visual loss, color defect, and a relative afferent pupillary defect can be present in both central serous retinopathy and optic neuritis. Similar to macular edema, central serous retinopathy can be difficult to identify on fundus exam. Macular OCT (see Fig. 11.16) is especially helpful in these cases by demonstrating the SRF (Fig. 11.17).
Central Retinal Artery Occlusion
Patients with central retinal artery occlusion (CRAO) may also have clinical features suggestive of an optic neuropathy because of the presence of central visual loss, color defect, and relative afferent pupillary defect, with occasional optic nerve edema (see Fig. 11.18). In the early stages of CRAO, the fundus examination often shows mild changes. Macular OCT more clearly demonstrates acute retinal thickening (see Fig. 11.19) [13] followed by more chronic inner retinal thinning in the chronic stages of CRAO.
Demyelinative and Neurodegenerative Disorders
The use of OCT extends beyond the evaluation of the eye and retrobulbar optic nerve to provide insight into intracranial pathology. MS, Parkinson’s disease (PD), and Alzheimer’s disease (AD) all show changes on OCT specific to central nervous system disease, which may help in the diagnosis and management of these conditions.
A current area of intensive investigation is the use of OCT in demyelinating disease. When a patient presents with new onset optic neuritis, it is important to distinguish if he or she has MS, neuromyelitis optica (NMO), or a clinically isolated syndrome (CIS). Unlike CIS patients, those with MS may show subclinical RNFL loss in the unaffected eye. Over time, MS patients also develop further RNFL loss in the affected eye, presumably from additional optic neuritis events compared to those patients with only CIS [14–16].
OCT can help distinguish patients with NMO from MS. Studies have shown that more severe RNFL loss occurs after a single episode of optic neuritis in NMO compared to that in MS [17]. It is important to distinguish among patients with CIS, MS, and NMO, as the overall prognosis and management of these diseases are different.
OCT can also provide information regarding the type of MS. In a study by Costello, lower RNFL values were found with more severe forms of MS such as primary and secondary progressive MS compared to patients with relapsing, remitting MS [18].
In addition to the RNFL, recent OCT studies evaluating macular thickness have provided further insight into the pathologic course of MS. Macular thinning was found to be at the level of the inner and outer nuclear layer, and the ganglion cell layer (GCL) [19]. These patients with macular thinning did not have RNFL thinning, as one would expect, but did have more severe forms of MS. Multiple sclerosis not only causes demyelination, but also neuronal loss. Microcystic changes in the retina have also been seen in optic neuritis, but this pathology is now being interpreted as a nonspecific secondary change that can be seen in a variety of other optic nerve disorders [20–23].
In other neurodegenerative diseases, such as PD and AD, the distinctive patterns of RNFL thinning on OCT may play a role in the diagnosis and monitoring of these disorders and may give further insight into the understanding of associated visual symptoms. Degenerative changes occurring in the optic nerve in AD patients are seen as RNFL thinning. Marziani et al. [24] demonstrated that the RNFL and RNFL and GCL thickness measurements on spectral domain OCT were reduced in all RNFL quadrants in AD patients compared with healthy subjects. In another study by Moreno-Ramos et al. [25], RNFL thinning was statistically correlated with both the Mini Mental State Examination and the Mattis Dementia Rating Scale scores in not only AD, but also in PD and dementia of Lewy bodies. Greater cognitive deterioration correlated with more severe RNFL thinning. These studies support RNFL measurement as a possible biomarker for the diagnosis and monitoring of various dementias.
Patients with PD have decreased dopamine levels that play an important role for various visual functions, including color vision and spatial sensitivity. Dopamine is an important neurotransmitter in the visual pathways, and pathology specimens have revealed its widespread location in the retina [26, 27]. Previous studies have shown ERG and VEP changes in patients with PD [28–30]. Time domain OCT can show macular and RNFL layer changes in patients with early stages of PD [31]. Moschos et al. [32] found that patients with PD with no clinical optic nerve pathology had statistically significant RNFL thinning in the inferior and temporal quadrants. Hajee et al. [33] also found paramacular thinning in PD patients without associated macular pathology. In both of these studies, the OCT findings support that subclinical visual dysfunction can be detected in the early stages of PD.
Limitations of OCT in Neurologic Disorders
Limitations in OCT Software
Accurate quantitative analysis of the macular and RNFL thickness in neuro-ophthalmic disorders requires proper segmentation of the RNFL anatomy. In cases where the anatomy is altered from pathology or media opacities, “software breakdown” may occur and limit the accuracy of the data. For example, with optic nerve pathologies such as severe disc edema or papilledema, placement of the 3.4 mm circular scan in the center of the optic nerve can be challenging because of the uneven elevation of the optic nerve along its circumference. This contour distortion can result in inaccurate measurements and difficulty in assessing progression or improvement of optic nerve edema. In media opacities like cataract or corneal scarring, degradation of the signal strength can limit the quality of the scan and give inaccurate results, especially with signal strengths under 7 [34]. Therefore, clinical decisions cannot be based solely on numeric RNFL thickness measurements, and the quality and reliability of each OCT scan must be individually evaluated for various possible artifacts. Research in improving the retinal segmentation software to reduce the incidence of “software breakdown” is currently underway.
With spectral domain OCT, limitations from motion and spatial resolution have been reduced. Scanning speed has tremendously increased with spectral domain OCT so that blink artifact, an inherent limitation in time domain technology, is decreased. Newer generation spectral domain technology also uses a broader imaging wavelength, which improves axial resolution from approximately 10 μm to about 4 μm for most commercial devices. This improved resolution has allowed greater accuracy in detecting subtle pathology with spectral domain OCT.
Limitations of OCT Based on Patient Cooperation, Operator Skills, and Ocular Anatomy
Patient cooperation is an important factor that may influence the quality of a scan. Patients with neurologic disorders often have cognitive difficulties that impair attention and concentration during the OCT procedure. For example, if a patient cannot maintain fixation on the scan target or if he/ she blinks during the scan, this may result in motion or blink artifacts. These errors are seen as distorted, or “cut- off” OCT images. Spectral domain OCT technology decreases the incidence of these errors by operating at a faster scan speed. Some spectral domain OCTs, such as the Heidelberg Spectralis, also have a retinal tracker feature which decreases the incidence of motion artifact.
In addition to patient cooperation, signal strength is also affected by the operator and it can vary depending on the operator’s skill. Scans with a signal strength of less than 7 are associated with lower reproducibility [34]. Different results in the peripapillary RNFL measurements in time domain and spectral domain OCT can also be seen because of the variability in the manual placement of the 3.4 mm circle around the optic nerve among operators. Even in situations where the patient is cooperative and the operator is skilled, OCT values can have reduced reliability based on the patient’s ocular anatomy. For example, a longer axial length in high myopia, or a markedly temporally tilted optic disc [35] can give higher variability in RNFL thickness measurements. (For further details regarding how ocular anatomy and age-related changes can affect RNFL measurement, see “Limitations of OCT in Retinal Nerve Fiber Layer Measurement” in the chapter entitled, “Approach to the Diagnosis and Differentiation of Glaucomatous and Nonglaucomatous Optic Neuropathies.”)
Measurement Variability in OCT Technology in Multiple Sclerosis
Although the mean RNFL thickness in patients with MS can be reproducibly measured by skilled technicians with excellent interrater (ICC, 0.89), intrarater (ICC, 0.98), and intervisit (ICC, 0.91) results [36], the differences in technology and software can result in different RNFL measurements between the time and spectral domain OCT devices. Although the RNFL thickness measurements in optic neuritis scanned from Stratus OCT have been shown to correlate well with those taken from Cirrus OCT, these measurements are not the same [37]. In a study of 18 patients with monocular acute optic neuritis within 6 months of presentation, Rebolleda et al. [37] showed that the RNFL in the nasal and temporal quadrants was thicker with Stratus OCT than with Cirrus OCT, except when the RNFL was very thin. When the average RNFL thickness was ≤56 μm, Cirrus OCT gave a higher value than Stratus. These measurement differences may be due to differences in registration, processing, and analysis. Differences in software segmentation algorithms may also account for this variation. The Cirrus spectral domain OCT aims to measure the bottom of the RNFL layer, whereas the Stratus time domain OCT focuses more at the top of the GCL [38].
Furthermore, the Cirrus spectral domain OCT, compared with the Stratus time domain OCT, identified a higher proportion of eyes with optic neuritis based on the internal normative database (44.4 % vs. 38 %). This greater sensitivity in measurement could be attributed to higher scan resolution and more accurate data registration of the instrument. In another study by Lange et al. [39] comparing MS eyes without optic neuritis to those with optic neuritis and to normal controls using Spectralis spectral domain OCT and Stratus time domain OCT, measurements between time domain and spectral domain OCT were highly correlated, but the absolute measurements were not interchangeable. These results were also similar to those in a study done by Bock et al. [40] in which they compared differences in RNFL thicknesses in RRMS patients compared to normal controls, using time domain and Cirrus spectral domain OCT.
Studies have also shown differences in measurements generated among the different spectral domain OCT equipment themselves. In a study by Watson et al. [4] five different OCT devices (the Stratus time domain OCT, along with four spectral domain OCTs, including Topcon 3D OCT 1000, Optovue RTVue-100, Cirrus HD OCT, and Heidelberg Spectralis SD OCT) yielded statistically significant differences in RNFL and central macular thickness (CMT) measurements in 92 eyes of patients with a history of optic neuritis and/or MS. The Cirrus and Spectralis OCT yielded lower RNFL measurements, while the RTVue-100 and 3D OCT 1000 yielded higher measurements compared to those of the Stratus OCT. The differences in CMT could arise from the nonstandard placement of the outer macular segmentation boundary line. The boundary line of the Stratus OCT aligns at the inner segment/outer segment junction, while it aligns at the outer RPE of the Cirrus/ RTVue-100, at the inner RPE in the 3D OCT-1000, and at the Bruchs membrane in the Spectralis. Differences in sampling density, in the placement of the outer circle (Stratus/Cirrus at 3.46 mm, RTVue-100/Spectralis at 3.45 mm, and 3D OCT-1000 at 3.40 mm), and in the signal strength scale are all likely to contribute to RNFL and CMT measurement differences.
Because of this variability between spectral domain and time domain OCT and among various spectral domain OCTs, it is important to use the same instrument when following patients longitudinally.
References
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81.
Fujimoto JG, Brezinski ME, Tearney GJ, et al. Optical biopsy and imaging using optical coherence tomography. Nat Med. 1995;9:970–2.
Yaqoob Z, Wu J, Yang C. Spectral domain optical coherence tomography: a better OCT imaging strategy. Biotechniques. 2005;9(6 Suppl):S6–13.
Watson GM, Keltner JL, Chin EK, Harvey D, Nguyen A, Park SS. Comparison of retinal nerve fiber layer and central macular thickness measurements among five different optical coherence tomography instruments in patients with multiple sclerosis and optic neuritis. J Neuroophthalmol. 2011;31:110–6.
Buchser NM, Wollstein G, Ishikawa H, Bilonick RA, Ling Y, Folio LS, et al. Comparison of retinal nerve fiber layerthickness measurement bias and imprecision across three spectral-domain opticalcoherence tomography devices. Investig Ophthalmol Vis Sci. 2012;53(7):3742–7.
Hedges 3rd TR, Vuong LN, Gonzalez-Garcia AO, Mendoza-Santiesteban CE, Amaro-Quierza ML. Subretinal fluid from anterior ischemic optic neuropathy demonstrated by optical coherence tomography. Arch Ophthalmol. 2008;126(6):812–5.
Hoye 3rd VJ, Berrocal AM, Hedges 3rd TR, Amaro-Quireza ML. Optical coherence tomography demonstrates subretinal macular edema from papilledema. Arch Ophthalmol. 2001;119(9):1287–90.
Savini G, Barboni P, Carbonelli M, Carelli V, Sadun AA. Optical coherencetomography for optic disc edema. Arch Ophthalmol. 2011;129(9):1245–6.
Moura FC, Monteiro ML. Evaluation of retinal nerve fiber layer thickness measurements using optical coherence tomography in patients with tobacco-alcohol-induced toxic optic neuropathy. Indian J Ophthalmol. 2010;58(2):143–6.
Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127:45–9.
Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillaryretinal pigment epithelium layer in papilledema. Investig Ophthalmol Vis Sci. 2011;52(9):6558–64.
Afshar AR, Fernandes JK, Patel RD, Ksiazek SM, Sheth VS, Reder AT, et al. Cystoid macular edema associated with fingolimod use for multiple sclerosis. JAMA Ophthalmol. 2013;131(1):103–7.
Rodrigues IA. Acute and chronic spectral domain optical coherence tomography features of branch retinal artery occlusion. BMJ Case Rep. 2013;pii:bcr2013009007.
Costello F, Hodge W, Pan YI, Eggenberger E, Coupland S, Kardon RH. Tracking retinal nerve fiber layer loss after optic neuritis: a prospective study using optical coherence tomography. Mult Scler. 2008;14(7):893–905.
Costello FE, Klistorner A, Kardon R. Optical coherence tomography in the diagnosis and management of optic neuritis and multiple sclerosis. Ophthalmic Surg Lasers Imaging. 2011;42(Suppl):S28–40.
Talman LS, Bisker ER, Sackel DJ, Long Jr DA, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiberlayer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–60.
Fernandes DB, Raza AS, Nogueira RG, Wang D, Callegaro D, Hood DC, et al. Evaluation of inner retinal layers in patients with multiple sclerosis orneuromyelitisoptica using optical coherence tomography. Ophthalmology. 2013;120(2):387–94.
Costello F, Hodge W, Pan YI, Eggenberger E, Freedman MS. Using retinal architecture to help characterize multiple sclerosis patients. Can J Ophthalmol. 2010;45(5):520–6.
Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, et al. Primary retinal pathology in multiple sclerosis asdetected by optical coherence tomography. Brain. 2011;134(Pt 2):518–33.
Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786–93.
Gelfand JM, Cree BA, Nolan R, Arnow S, Green AJ. Microcystic inner nuclear layer abnormalities and neuromyelitisoptica. JAMA Neurol. 2013;70(5):629–33.
Abegg M, Zinkernagel M, Wolf S. Microcystic macular degeneration from optic neuropathy. Brain. 2012;135(Pt 12):e225.
Wolff B, Basdekidou C, Vasseur V, Mauget-Faÿsse M, Sahel JA, Vignal C. Retinal inner nuclear layer microcystic changes in optic nerve atrophy: a novel spectral-domain OCT finding. Retina. 2013;33:2133–8.
Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, et al. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2013;54(9):5953–8.
Moreno-Ramos T, Benito-León J, Villarejo A, Bermejo-Pareja F. Retinal nerve fiber layer thinning in dementia associated with Parkinson’s disease, dementia with lewy bodies, and Alzheimer’s disease. J Alzheimers Dis. 2013;34(3):659–64.
Spund B, Ding Y, Liu T, Selesnick I, Glazman S, Shrier EM, et al. Remodeling of the fovea in Parkinson disease. J Neural Transm. 2013;120(5):745–53.
Bodis-Wollner I. Foveal vision is impaired in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19(1):1–14.
Jaffe MJ, Bruno G, Campbell G, Lavine RA, Karson CN, Weinberger DR. Ganzfeldelectroretinographic findings in parkinsonism: untreated patients and the effect of levodopa intravenous infusion. J Neurol Neurosurg Psychiatry. 1987;50(7):847–52.
Bodis-Wollner I, Yahr MD. Measurements of visual evoked potentials in Parkinson’s disease. Brain. 1978;101(4):661–71.
Gawel MJ, Vincent S, Clifford RF. Visual evoked potentials in patients with Parkinson disease. J Neurol Neurosurg Psychiatry. 1981;44:227–32.
Kirbas S, Turkyilmaz K, Tufekci A, Durmus M. Retinal nerve fiber layer thickness in Parkinson disease. J Neuroophthalmol. 2013;33(1):62–5.
Moschos MM, Tagaris G, Markopoulos I, Margetis I, Tsapakis S, Kanakis M, et al. Morphologic changes and functional retinal impairment in patients with Parkinson disease without visual loss. Eur J Ophthalmol. 2011;21(1):24–9.
Hajee ME et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127:737–41.
Wu Z, Huang J, Dustin L, Sadda SR. Signal strength is an important determinant of accuracy of nerve fiber layer thickness measurement by optical coherencetomography. J Glaucoma. 2009;18(3):213–6.
Hwang YH, Yoo C, Kim YY. Myopic optic disc tilt and the characteristics ofperipapillary retinal nerve fiber layer thickness measured by spectral-domainoptical coherence tomography. J Glaucoma. 2012;21(4):260–5.
Cettomai D, Pulicken M, Gordon-Lipkin E, Salter A, Frohman TC, Conger A, et al. Reproducibility of optical coherence tomography in multiple sclerosis. Arch Neurol. 2008;65(9):1218–22.
Rebolleda G, García-García A, Won Kim HR, Muñoz-Negrete FJ. Comparison ofretinal nerve fiber layer measured by time domain and spectral domain opticalcoherence tomography in optic neuritis. Eye. 2011;25(2):233–8.
Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherenttomography. Ophthalmology. 2009;116(7):1271–7.
Lange AP, Sadjadi R, Saeedi J, Lindley J, Costello F, Traboulsee AL. Time-domain and spectral-domain optical coherence tomography of retinal nervefiber layer in MS patients and healthy controls. J Ophthalmol. 2012;2012:564627.
Bock M, Brandt AU, Dörr J, Pfueller CF, Ohlraun S, Zipp F, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: acomparative cross-sectional study. Mult Scler. 2010;16(7):893–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media New York
About this chapter
Cite this chapter
Ho, J., Athappilly, G., Tivnan, T.B., Hedges, T.R. (2014). Application of Optical Coherence Tomography in Neuro-Ophthalmic Disorders. In: Chan, J. (eds) Optic Nerve Disorders. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-0691-4_11
Download citation
DOI: https://doi.org/10.1007/978-1-4614-0691-4_11
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-0690-7
Online ISBN: 978-1-4614-0691-4
eBook Packages: MedicineMedicine (R0)