Abstract
Aneurysmal sinus Valsalva (congenital or iatrogenic) is usually asymptomatic but when it ruptured, this catastrophic event lead to sudden onset chest chain, dyspnea, syncope or even sudden death. In this situation, right coronary cusp is the most one which frequently ruptured to the RVOT or RA with significant left to right shunt and eventually biventricular failure. It is obligatory to repair the ruptured cusp. Recently, trans-catheter device closure seems to be safe and effective strategy to close this shunt with minimal side effects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
History
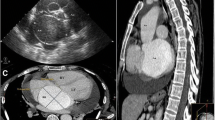
A 35 years old man was referred to ER with complaints of sudden onset chest pain and dyspnea since 6 h ago who admitted with the impression of ACS. In TTE ruptured aneurysmal sinus Valsalva (NCC) was diagnosed.
Sinus of Valsalva aneurysm (SOVA) is an abnormal dilatation of the aortic root between the aortic annulus and ST junction. The estimated rate is about 0.09% of the general population. SOVA can be either congenital (connective tissue disease like Marfan syndrome) or acquired (bacterial endocarditis, Takayasu’s arteritis, or trauma), the consequence of elastic lamina weakening and medial cystic necrosis. It can be an isolated anomaly or be associated with other CHD like: Bicuspid AV, Aortic regurgitation, VSD, PDA, and Coarctation of aorta. The most affected valve is RCC, followed by NCC (5–15%) and finally LCC. Non-ruptured ones are usually asymptomatic but can lead to sinus thrombosis or coronary artery compression and acute coronary syndrome. Ruptured sinus Valsalva aneurysm is a fatal event. In majority of cases, RCC or NCC ruptured results in communication between the aorta and RA or RVOT (significant left-to-right shunt with RV and LV overload and failure) who present with sudden onset chest pain, dyspnea, orthopnea, syncope, infective endocarditis, tamponade, hemodynamic instability or sudden cardiac death but ruptured LCC with communication to LVOT, is less clinically significant [1].
Diagnostic Work-Up
SOVA rupture diagnosis requires a low index of suspicion. In physical exam, a typical continues murmur with trill is sound in right and left LSB with bounding pulses and sometimes the murmur of AI is audible. In long-standing cases, the ECG shows LVH and RVH criteria. TTE is the first modality which shows LV and RV size and functions, aortic valve morphology and any aneurysmal or ruptured cusp with continues flow in systole and diastole into the communicated chambers, any vegetation and other anomalies and finally for more information, TEE is confirming tool for SOVA rupture and would differentiate it from a combination of VSD and AI. Cardiac CT is the test of choice for quantifying the size and morphology of SOVA.
Management
The ACC-AHA 2010 guideline-recommended surgical repair for those with non-ruptured sinus of Valsalva greater than 5.5 cm, greater than 5 cm in those with BAV and greater than 4.5 cm in those with connective tissue disease. But in SOVA rupture, the surgical or tans-catheter repair is urgent and mandatory as patients may quickly deteriorate. Medical treatment is just for stabilizing the patients before invasive procedures. Surgical intervention is recommended for a ruptured SOVA and/or a SOVA with associated intracardiac abnormalities such as VSD or significant AI. If ruptured SOVAs remain untreated, the prognosis is poor with a 1-year life expectancy [2].
Procedural Technique
The technique is similar to the TCC of perimembranous VSD, although the defect is located just above the aortic valve instead of below. Periprocedural TEE and color Doppler interrogation help us in sizing the defect, device selection (2–4 mm larger than the aortic end), delineating the SOVA anatomy in regards to its neighboring structures namely the aortic valve, tricuspid valve, and RVOT, ensuring proper seating of the aortic disk on the aortic side without slipping into the body of the aneurysm, and most importantly, monitoring AR and TR occurrence and residual shunting on color Doppler.
After loading ASA 325 mg and Plavix 600 mg and insertion of right femoral artery and vein sheaths (6F) and completed IV Heparin to achieved ACT > 200 s, Under general anesthesia, TEE guidance and re-evaluation of the defect (assessment of the maximum diameter of the aortic end of the RSOV, the minimum diameter and the length of the windsock, and the distance of the aortic end of the RSOV from the coronary ostium), aortic root and LV angiography was done for more evaluation (Fig. 71.1a, b). Then via retrograde approach, from the aortic side, after crossing the defect (Noncoronary cusp rupture to RA) via 0.035 inch, 260 cm long straight tipped Terumo wire and passing the JR catheter to the RA and manipulated to reach the IVC, the wire was snared from Femoral vein to create an arterio-venous loop (Fig. 71.2). After that, the delivery sheath (Occlutech 12F) was passed from the venous side over the wire across the RSOV. According to the defect size and length, Muscular VSD 8 mm (Occlutech) was chosen. After loading the device and connecting to the delivery cable and fully de-aired, the device was passed through the delivery sheath, under TEE guidance and fluoroscopy. Aortic disk was opened in the ascending aorta and the whole system was pulled back till it anchored at the aortic end of the RSOV and it was ensured that aortic valve leaflets were free as seen on TEE, then proximal disk was deployed by retracting the sheath (Fig. 71.3a). After confirming the correct position of the device by aortic root injection (Fig. 71.3b) and TEE (no interference with AV or occlusion of coronary ostia and the residual shunt), the device was released. Final angiography showed mild residual shunt, stable device position, and no AI (Fig. 71.4a, b).
(a, b) According to the defect size and length, Muscular VSD 8 mm (Occlutech) was chosen. Aortic disk was opened in the ascending aorta and the whole system was pulled back till it anchored at the aortic end of the RSOV and it was ensured that aortic valve leaflets were free as seen on TEE, then the proximal disk was deployed by retracting the sheath. After confirming the correct position of the device by aortic root injection and TEE (no interference with AV or occlusion of coronary ostia and the residual shunt), the device was released
Conclusion
Since the first report of device closure of RSOVA in 1994, it is increasingly being reported as either case reports or case series and is gradually replacing surgical correction with cardiopulmonary bypass, conventionally the mainstay of treatment. Transcatheter closure (TCC) had been performed using Amplatzer duct occluder (ADO), ventricular septal defect (VSD) occluder, atrial septal defect (ASD) occluder, Gianturco coils, and Rashkind umbrella. To conclude, transcatheter closure of RSOV is an effective and safe treatment modality for isolated RSOV. In patients where on-pump surgery is a high risk, due to poor general condition and comorbidities, transcatheter device closure can be lifesaving. An extended follow-up is required to assess the long-term outcome of these patients [3].
References
Serban AM, Batrana N, Cocoi M, et al. The role of echocardiography in the diagnosis and management of a giant unruptured sinus of Valsalva aneurysm. Med Ultrson. 2019;21(2):194–6.
Sakar M, Wehman B, Mukherjee R, et al. Left sinus of Valsalva aneurysm presenting as myocardial ischemia. J Thorac Cardiovasc Surg. 2018.
Supratim S, Amitabha C, Mily R, et al. Transcatheter device closure of ruptured sinus of Valsalva: immediate results and short term follow up. Ann Pediatr Cardiol. 2009;2(1):79–82.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer-Verlag London Ltd., part of Springer Nature
About this chapter
Cite this chapter
Firouzi, A., Hosseini, Z. (2021). Transcatheter Device Closure of Ruptured Sinus of Valsalva. In: Maleki, M., Alizadehasl, A. (eds) Case-Based Clinical Cardiology. Springer, London. https://doi.org/10.1007/978-1-4471-7496-7_71
Download citation
DOI: https://doi.org/10.1007/978-1-4471-7496-7_71
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-7495-0
Online ISBN: 978-1-4471-7496-7
eBook Packages: MedicineMedicine (R0)