Abstract
This is a case of recurrent TIA in a young child; the diagnosis of moyamoya disease was made on radiological features. Moyamoya disease is typically associated with recurrent ischaemic symptoms in young people; haemorrhage is more common in adults. The diagnosis is based on radiological criteria. Surgical revascularisation should be considered to prevent recurrent ischaemic stroke or TIA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Clinical History
An 11-year-old caucasian girl was assessed for recurrent episodes of transient hemiparesis affecting right and left sides on separate occasions that had begun whilst she was on holiday in the Canary Islands. Each lasted up to 30 min and she was having 2–3 attacks each week, with complete resolution of symptoms in between. Previous health and development were normal.
Examination
Clinical examination was unremarkable; in particular, cutaneous and cardiovascular examination (including blood pressure) were normal.
Investigations
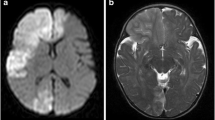
Brain MRI was initially thought unremarkable but, on review, numerous flow voids were observed in the basal ganglia (Fig. 37.1); MRA of the circle of Willis confirmed occlusive disease of both terminal ICAs. These findings were suggestive of moyamoya disease. Catheter cerebral angiography, undertaken to characterise the cerebrovascular disease and evaluate the collateral circulation, confirmed this diagnosis, and in addition showed occlusive disease of the left posterior cerebral artery; in the absence of an associated condition, a diagnosis of moyamoya disease was made.
She was treated with low-dose aspirin but continued to have attacks. As the angiogram showed minimal external to internal carotid artery collaterals (Fig. 37.2a), she underwent bilateral surgical revascularisation with pial synangiosis (superficial temporal artery laid onto brain surface). Post-operative angiography showed intracranial supply from the external carotid artery (Fig. 37.2b). Aspirin was discontinued at 18 years of age.
Catheter cerebral angiogram (a) pre- and (b) post-operatively in patient described above. The external carotid injection in (a) does not demonstrate intracranial filling. In (b), undertaken after right pial synangiosis, common carotid injection demonstrates both occlusion of the right ICA and intracranial filling via the donor right ECA (arrow)
Discussion
Moyamoya disease is an occlusive intracranial arteriopathy of unknown aetiology that involves the terminal internal carotid arteries (ICA) and the proximal MCA and ACA. The posterior circulation (especially posterior cerebral artery) may also be affected. The other characteristic of this pattern of arteriopathy is development of an abnormal network of collateral vessels in the basal ganglia that manifests on catheter angiography as the pathognomonic “puff of smoke” [1].
“Moyamoya disease” is by definition idiopathic and bilateral; the term “moyamoya syndrome” is used for cases with an associated diagnosis known to be associated with intracranial arteriopathy [1, 2]. These include:
-
genetic conditions (sickle cell disease, neurofibromatosis type 1, Trisomy 21, Alagille syndrome),
-
vascular disorders (congenital heart disease, renal artery stenosis, giant cervicofacial haemangiomas, PHACE syndrome),
-
other recognised causes (CNS infection, head trauma, previous cranial radiotherapy, SLE, dissection)
Moyamoya disease is endemic in East Asia, with a prevalence of 3.16/100,000 in Japan [1]; linkage studies in these populations have identified several genetic loci [3]. The natural history of moyamoya disease in East Asia is for a high rate of recurrent TIA/ischaemic stroke in childhood. Moyamoya can also present in adult life with TIA or ischaemic stroke. Presentations with cerebral haemorrhage are more common in adulthood than in childhood. It is unclear whether a similarly progressive and aggressive course occurs in people of other ethnicities or in secondary disease born in Asia. Moyamoya disease is less common in Western populations and appears to run a more benign course in many patients, especially in those presenting in adult life. Other presentations include severe focal migraine, seizures, acute chorea and progressive cognitive decline.
The diagnosis of moyamoya disease should be considered in young people with recurrent symptoms of cerebral ischaemia (TIA or stroke) especially if precipitated by hyperventilation (or in hot weather, as in the case above) or if there is an associated condition. Adults with perfusion failure secondary to intracranial occlusive disease often present with “haemodynamic TIAs”. These are high frequency attacks often occurring when perfusion falls a critical level, often after taking antihypertensives, or after eating or on exercise. Very often these start with jerking of a limb, mimicking partial epilepsy. Patients with moyamoya disease should be evaluated for the presence of an associated diagnosis. Vascular disease in other systems, especially in the renal arteries, is common [4].
The diagnosis of moyamoya disease is based on radiological criteria (see below), either MRI/MRA or cerebral angiography. Cerebral perfusion studies (MR, Xe-CT, PET, SPECT) can be used to characterise regional cerebral perfusion or cerebrovascular reactivity; however, interpretation of these studies in relation to indications for surgery are not established.
Antiplatelet treatment (aspirin) is usually offered for patients with ischaemic presentations; anticoagulation is generally avoided due to the risk of intracranial haemorrhage from friable collaterals. As the primary mechanism for the ischaemic events is the haemodynamic insufficiency, revascularisation surgery may be considered to provide an alternative collateral circulation. There is a large body of observational evidence to suggest that revascularisation is effective in reducing stroke and TIA [5] but the specific clinical and radiological indications for surgery in individual patients remain controversial. Patients should therefore be referred to a specialised service for moyamoya disease. The AHA childhood stroke guidelines suggest that children with ongoing ischaemic symptoms and evidence of compromised cerebral blood flow or cerebral perfusion reserve should be considered as surgical candidates [2]. Adequate intravenous hydration, maintenance of blood pressure at normal or slightly elevated levels, good pain management (to avert crying) and maintenance of oxygenation are recommended in the perioperative period [2]. If ischaemic symptoms manifest in childhood they commonly improve or resolve during adolescence and into adulthood. Revascularisation is not necessarily indicated in adult patients. Longer-term important management considerations include minimising vascular risk factors (e.g., avoid smoking, manage cholesterol and blood pressure). Blood pressure management is especially important around pregnancy and this may require input from a specialist centre. Revascularisation surgery is not indicated to prevent haemorrhage. The complex nature and location of disease makes endovascular treatments inappropriate.
Key Clinical Learning Points
-
1.
Moyamoya disease should be considered in young patients with recurrent or bilateral stroke or TIA, especially if they have one of the associated conditions
-
2.
Ischaemic symptoms predominate in childhood; haemorrhagic presentations are more common in adults
-
3.
The natural history of the disease is for a high rate of recurrent stroke or TIA
-
4.
Surgical revascularisation is safe and appears effective in preventing recurrent stroke in childhood, although indications are not clearly established
-
5.
Patients commonly have vascular disease in other organs, especially renal arteries
Key Radiological Learning Points
-
1.
Moyamoya is a radiological, rather than clinical, diagnosis
-
2.
Diagnostic criteria on catheter angiography (see Fig. 37.3) are:
Fig. 37.3 Catheter cerebral angiogram, left CCA injection, lateral view. There is occlusion of the terminal ICA and profuse basal collaterals, as well as collateral supply via the ophthalmic artery. The posterior communicating artery is patent and the CCA injection fills the posterior cerebral artery territory by this route
-
(a)
Occlusive disease affecting terminal ICA and/or proximal ACA and/or MCA
-
(b)
Abnormal vascular networks in the vicinity of the occlusive or stenotic lesions in the arterial phase
-
(c)
Bilateral involvement in “Moyamoya disease”
-
(a)
-
3.
In addition to the occlusive disease, the presence of more than two flow voids in at least one side of the basal ganglia are sufficient to make the diagnosis on MRI (see Figs. 37.1 and 37.4)
Fig. 37.4 (a) Axial T2-weighted (b) FLAIR and (c) 2D time of flight magnetic resonance angiography of the circle of Willis (frontal view) from a 3-year-old child with an acute left hemiparesis. There is an acute right MCA territory infarct with high signal and swelling on T2 and FLAIR; in addition there are cavitated gliotic scars in the white matter of the left hemisphere, representing areas of previous ischaemic injury. The mesh of basal collaterals is apparent in (a; see arrow). The MRA shows evidence of an arteriopathy affecting terminal ICA and proximal MCA and ACA bilaterally, with profuse proximal “moyamoya” collaterals
-
4.
Additional radiological features include:
-
(a)
Multiple infarcts of different age in different arterial territories (see Fig. 37.4)
-
(b)
diffuse leptomeningeal enhancement on post-contrast MR images or on FLAIR images (“ivy” sign)
-
(a)
-
5.
Catheter angiography is useful to characterise disease morphology, to assess the pattern of collateralisation pre-operatively and to evaluate results of revascularisation surgery (see Fig. 37.2)
References
Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (“moyamoya” disease). Clin Neurol Neurosurg. 1997;99 Suppl 2:S238–40.
Smith ER, Scott RM. Spontaneous occlusion of the circle of Willis in children: pediatric moyamoya summary with proposed evidence-based practice guidelines. A review. J Neurosurg Pediatr. 2012;9:353–60.
Achrol AS, Guzman R, Lee M, et al. Pathophysiology and genetic factors in moyamoya disease. Neurosurg Focus. 2009;26(4):E4.
Wilsher A, Roebuck D, Ng J, et al. How commonly do children with complex cerebral arteriopathy have renovascular disease? Dev Med Child Neurol. 2013;55(4):335–40.
Ng J, Thompson D, Lumley JP, et al. Surgical revascularisation for childhood moyamoya. Childs Nerv Syst. 2012;28:1041–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Niotakis, G., Ganesan, V. (2015). Cerebrovascular Disease in Childhood. In: Gill, S., Brown, M., Robertson, F., Losseff, N. (eds) Stroke Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-6705-1_37
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6705-1_37
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6704-4
Online ISBN: 978-1-4471-6705-1
eBook Packages: MedicineMedicine (R0)