Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is a transient disturbance in cerebral vascular tone with subsequent multifocal segmental intracerebral arterial constriction and dilation. It typically presents with recurrent thunderclap headaches, and with focal neurological deficits if cerebral infarction has occurred. Cerebral angiography demonstrates multifocal segmental narrowing and dilatation which normalises within 3 months. Differentiation from primary CNS angiitis can be made clinically, and from aneurysmal subarachnoid haemorrhage radiologically. Management includes enquiring about and discontinuing any potential precipitant. commencement of calcium channel blockers may be beneficial. Prognosis is favourable, but dependent on the extent of cerebral infarction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vasoconstriction
- Vasopasm
- Headache
- Thunderclap headache
- Angiography
- Cerebral haemorrhage
- Cerebral infarction
- Subarachnoid haemorrhage
Clinical History
A 22-year-old female right-handed investment banker presented with a 3-week history of recurrent thunderclap occipital headaches. The headaches were initially frontal and left sided, then right sided, and then became occipital. She described the headaches as “being hit from behind by a bat” and subsequently “pounding” with associated photophobia and hyperacusis. Each headache was precipitated by physical exertion at the gym. The headaches initially lasted approximately 30 min but in the week before presentation were lasting 9–10 hours. There were no relieving factors.
She had no past medical history and was not on any regular medications. There was no family history of note. She did not smoke or drink alcohol, and denied recreational drug use. She had recently started taking diphenhydramine sleeping tablets and unnamed Chinese herbal supplements.
Examination
She was alert and oriented. Blood pressure was 110/68 mmHg. Cardiac, respiratory, and abdominal examination was unremarkable. Neurological examination including fundoscopy was normal. There were no visible rashes.
Investigations
Routine blood tests including CRP and ESR were normal. ECG displayed sinus rhythm.
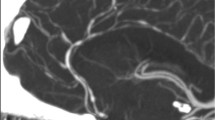
MRI Brain revealed normal brain parenchyma, however MR Angiogram of the intracranial arteries (Fig. 34.1) demonstrated a widespread irregular calibre with beading of multiple arterial segments of the circle of Willis bilaterally consistent with a vasculopathic process. These findings were confirmed on a four-vessel catheter angiogram (Fig. 34.1). CT Abdomen with contrast revealed normal extra-cranial blood vessels.
Vasculitis and thrombophilia screens were negative. Cerebrospinal fluid opening pressure at lumbar puncture was 14 cm. It was acellular with normal protein and glucose, culture and viral serology was negative.
She was diagnosed with cerebral vasoconstriction syndrome and commenced on oral nimodipine. She was discharged home and her headaches gradually subsided. Repeat MR Angiogram (Fig. 34.2) of the intracranial vessels 5 months post presentation revealed normal intracranial vessels, confirming the diagnosis of reversible cerebral vasoconstriction syndrome (RCVS). Nimodipine was gradually withdrawn, and she remains headache free.
Discussion
Reversible cerebral vasoconstriction syndrome (RCVS) is characterised by transient dysregulation of cerebral vascular tone leading to prolonged but reversible constriction of intracranial arteries, with resolution within 3 months. Peak incidence is between 20 and 50 years of age, with a female predominance (2–10:1) [1, 2]. Accurate incidence is difficult to define as it is underdiagnosed, particularly in pure cephalgic cases.
The most common clinical presentation is with severe acute headache (typically thunderclap), with or without additional neurological symptoms including focal neurology (9–64%) and seizures (0–21%) [3–5]. The acute headache is typically bilateral, with posterior onset and associated nausea, vomiting, and photophobia. Most patients (>90%) will present with recurrent thunderclap headaches recurring 7 days apart on average, with a milder background headache between attacks [2, 5].
The pathophysiology of RCVS is not fully understood. Brain biopsies have not revealed abnormalities, and have not demonstrated vascular inflammation [6]. The prevailing hypothesis is that it is secondary to transient and reversible disturbance in the control of cerebral vascular tone, with subsequent multifocal and segmental arterial constriction and dilatation [4, 7]. Since intracranial vascular tone and calibre is dependent on both vascular receptor activity and sensitivity, a central vascular discharge (either spontaneous or evoked) with a multifactorial trigger may determine the relapsing and reversible nature of RCVS [3]. The temporal clinical progression suggests that the underlying disturbance first involves small distal arteries, potentially causing haemorrhage and a clinical syndrome identical to the posterior reversible encephalopathy syndrome, and then progresses to involve medium and large calibre vessels, with subsequent ischaemia and potential infarction [2].
The syndrome may be spontaneous (idiopathic), or may occur secondary to a precipitant (25–60%) [4]. Precipitants have been implicated from a temporal relationship with RCVS which may occur several months after precipitant use [3, 4]. Reported precipitants include vasoactive substances, pregnancy and the puerperium, catecholamine-secreting tumours, and immunosuppressants or blood products (Table 34.1). The most commonly reported precipitants are serotonin selective re-uptake inhibitors, cannabis, and over-the-counter nasal decongestants [2]. A trigger immediately prior to the onset of headache is recounted by 80% of patients, including sexual intercourse, straining, physical exertion, urination, high emotional state, showering, and head movements [4].
Complications of RCVS include seizures, PRES, subarachnoid haemorrhage, and intracerebral haemorrhage during the first 7 days after headache-onset when small calibre vessels are affected, followed by ischaemic infarcts during the second week when medium and larger calibre arteries are affected [4]. Subarachnoid haemorrhage is of small volume and tends to be localised, overlying the cortical surface with limited spread over 1–3 sulci [1, 4, 5]. Infarction is due to ischaemia distal to affected vessels, typically within arterial borderzone regions, and occurs in 7–50% of cases [1, 3–5].
CT/MR Brain imaging at presentation is frequently normal. Angiography, either transfemoral or indirect (MRA/CTA) shows multifocal segmental narrowing and dilatation, in a “string of beads” pattern. Typically the anterior and posterior cerebral circulations are bilaterally and diffusely affected, with potential involvement of the carotid artery and basilar siphon [4]. If performed in the first few days after presentation, angiography may appear normal, due to the poor sensitivity of angiography to small vessel involvement. Follow-up cerebral imaging is abnormal in three quarters of cases, including infarcts (39%), subarachnoid haemorrhage (34%), intracerebral haemorrhage (20%), and oedema (20%) [5]. Serial angiography has found peak involvement of arterial segments 16 days after headache onset, with unsynchronised progression and regression of vasoconstricted arterial segments [1]. Complete reversal of angiographic abnormalities is apparent between 1 and 3 months after presentation.
Cerebrospinal fluid at presentation is frequently abnormal, and may show mild elevation in protein and white cell count [4]. Serum inflammatory markers may show a modest elevation [4].
The differential diagnosis for a single thunderclap headache includes primary headache syndromes, aneurysmal subarachnoid haemorrhage, intracranial haemorrhage, cervical or intracranial dissection, hypertensive encephalopathy, post-partum vasculopathy, venous thrombosis, giant cell arteritis, and pituitary apoplexy. Recurrent thunderclap headaches are much more likely to be RCVS or primary headache syndromes. Appropriate imaging and serum inflammatory markers should differentiate the above presentations. RCVS-SAH with vasospasm is distinguished from aneurysmal-SAH on imaging with typical appearances of a small volume bleed overlying the superior or lateral cortical surface, with multifocal vessel abnormalities remote from the site of bleeding, and no evidence of aneurysm rupture [1, 4, 5]. The differential diagnosis for the imaging appearances of RCVS is primary CNS angiitis but the clinical presentation of the two syndromes is very different. CNS angiitis rarely presents with thunderclap headache, progresses over more than 4 weeks, and has cerebrospinal fluid and serum abnormalities [6].
The following diagnostic criteria for RCVS have been proposed [3]:
-
1.
Acute severe headache with or without neurological signs or symptoms
-
2.
Multifocal segmental vasoconstriction of cerebral arteries demonstrated by angiography
-
3.
No evidence for aneurysmal subarachnoid haemorrhage
-
4.
Normal or near-normal cerebrospinal fluid analysis (protein <1 g/l, white cells <15 mm [4], glucose normal)
-
5.
Reversibility of angiographic abnormalities within 12 weeks after onset
Initial management includes cessation of potential precipitants, blood pressure control, and medical management of seizures. There have been no clinical trials evaluating pharmacotherapy in RCVS and open studies have not found any significant clinical benefit with calcium channel blockers or glucocorticoids. Calcium channel blockers (particularly nimodipine) are frequently used as vasodilators, but care must be taken to avoid hypotension and subsequent borderzone infarction in areas perfused by the constricted artery [3, 5]. The optimum treatment duration is unclear. Patients with RCVS are sometimes given glucocorticoids at presentation due to diagnostic uncertainty of primary CNS angiitis and evidence of benefit in aneurysmal subarachnoid haemorrhage in animal studies [6]. However, there is no clear clinical indication for glucocorticoids in RCVS, and their use has been associated with a trend towards poor outcomes [5].
The prognosis in RCVS in favourable, with excellent recovery in uncomplicated cases. One series reported modified Rankin of 0–1 in 78% of patients, 2–3 in 11%, and 4–5 in 9% [5]. Cerebral infarction (but not haemorrhage) is associated with a poor outcome [5]. Deaths have rarely been reported. Relapse is very uncommon.
Key Clinical Learning Points
-
RCVS is a transient disturbance in cerebral vascular tone with subsequent multifocal segmental intracerebral arterial constriction and dilatation
-
RCVS should be considered in all presentations with cryptogenic strokes or thunderclap headache, particularly if recurrent
-
Differentiation from primary CNS angiitis can be made clinically, and from aneurysmal subarachnoid haemorrhage radiologically
-
Management includes enquiring about and discontinuing potential precipitants. Calcium channel blocker initiation may be beneficial
-
Prognosis is favourable, but dependent on the extent of cerebral infarction
Key Radiological Learning Points
-
All patients with recurrent thunderclap headaches with or without cerebral haemorrhage should have intracranial angiography
-
Multifocal segmental narrowing and dilatation with a “string of beads” pattern on angiography (transfemoral or indirect), with normalisation within 3 months is diagnostic of RCVS
-
Angiography at presentation may be normal, and repeat angiography may be required to demonstrate vascular abnormalities
References
Chen SP, Fuh JL, Wang SJ, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010;67:648–56.
Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:133–9.
Calabrese LH, Dodick DW, Schwedt TJ, et al. Narrative review: reversible cerebral vasoconstriction syndrome. Ann Intern Med. 2007;146:34–44.
Ducros A, Bousser MG. Reversible cerebral vasoconstriction syndrome. Pract Neurol. 2009;9:256–67.
Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes. Analysis of 139 cases. Arch Neurol. 2011;68(8):1005–12.
Sattar A, Manousakis G, Jensen MB. Systematic review of reversible cerebral vasoconstriction syndrome. Expert Rev Cardiovasc Ther. 2010;8(10):1417–21.
Schwedt TJ, Matharu MS, Dodick DW. Thunderclap headache. Lancet Neurol. 2006;5:621–31.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Brown, N.F., Brown, M.M. (2015). Recurrent Thunderclap Headaches. In: Gill, S., Brown, M., Robertson, F., Losseff, N. (eds) Stroke Medicine. Springer, London. https://doi.org/10.1007/978-1-4471-6705-1_34
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6705-1_34
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6704-4
Online ISBN: 978-1-4471-6705-1
eBook Packages: MedicineMedicine (R0)