Abstract
The rapid economic development occurring in developing countries in recent decades has been accompanied with increasingly worsened air quality. In recent years, severe air pollution episodes have occurred frequently in the cities of some developing countries. The goal of this chapter is to describe outdoor air pollution issues with a focus on urban areas in developing countries. Topics include global air pollution trends, differences in emissions and concentrations of key air pollutants in different regions, emission sources disproportionately affecting developing countries, estimating disease burden in developing countries, and recommendations based on historical successes and lessons from developed countries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
A number of countries have rapidly industrialized in the past few decades, leading to severe decreases in air quality associated with industrial processes, urbanization, and population growth. Meanwhile, indoor air pollution resulting from household combustion of solid fuels remains severe, affecting more than half of the population. Each of these two problems contributes greatly to the global burden of disease, as outdoor air pollution and household air pollution from solid fuels were responsible for 3.4 and 3.5 million premature deaths, respectively, worldwide in 2010 (Lim et al. 2012). The majority of this disease burden due to air pollution, however, occurs in developing countries. Since indoor air pollution from household biomass combustion is covered in Chap. 14, the goal of this chapter is to describe outdoor air pollution problems in developing countries with a focus on urban areas.
Wealthier nations underwent similar processes decades earlier in a much slower fashion, and most have since at least partially addressed the severe air pollution that used to afflict their urban areas. In cities such as Beijing and many northern Chinese cities, severe winter smog episodes have frequently occurred in recent years, replaying the historical air pollution episodes of the twentieth century (e.g., Meuse River Valley, Belgium in 1930; Donora, Pennsylvania in 1948; and London in 1952). For example, daily PM2.5 concentrations measured on the grounds of the U.S. Embassy in Beijing in the month of January from 2010 to 2014 frequently exceeded 100 μg/m3 and reached as high as 552 μg/m3, about 16 times the US EPA’s 24-h standard for PM2.5 of 35 μg/m3 (see Fig. 13.1). Given that the highest air pollutant concentrations are found in rapidly industrializing developing nations, it is important to understand what types of exposures are occurring there and why. The following specific questions will be addressed:
January PM 2.5 concentrations measured at the U.S. Embassy in Beijing 2010–2014. Winter PM2.5 concentrations in Beijing far exceed international standards. In this boxplot, the top whisker line corresponds with the maximum value, the top line of the box corresponds with the 75th percentile, the blue diamond is the mean, the middle box line is the median, the bottom box line is the 25th percentile, and the bottom whisker line is the minimum (Data were retrieved from the Beijing U.S. Embassy air monitoring Twitter feed at https://twitter.com/BeijingAir)
-
1.
What are the levels and composition of the urban air pollution mixture in developing countries in comparison to those in developed countries? What are the trends in emissions?
-
2.
What are the major sources that contribute to urban air pollution in developing countries?
-
3.
What are the disease burdens attributable to outdoor air pollution in developing countries? Is it appropriate to use concentration-response relationships derived from studies at lower concentrations in health risk assessment for developing countries?
-
4.
What are the recommendations for reducing health risks associated with urban outdoor pollution in developing countries?
13.2 Pollution Levels and Emission Trends
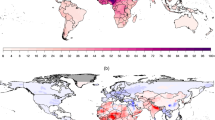
Urbanization has been a dominant trend in the developing world that has expanded at a much higher rate than what occurred previously in the developed world. For example, it took the United States 90 years to go from 40 % urbanized in 1900 to over 75 % urbanized in 1990, but the same process took only 20 years in South Korea and only 30 years in Brazil (Henderson 2002). This urbanization can lead to worsening air pollution as increased vehicle traffic, household emissions, and power plant and factory emissions resulting from the tightly packed population centers create areas of high emissions and high exposure. Currently, 19 of the top 25 most populous cities in the world are in developing countries, and many of these megacities have annual average concentrations of air pollutants in excess of health-based standards and guidelines, as can be seen in Fig. 13.2. In particular, megacities in developing countries tend to have higher air pollution concentrations than those in developed countries, consistent with the PM10 emission trends described below.
Annual mean urban PM 10 levels versus international standards in the 25 most populous cities . Many of the top 25 most populous cities in the world have annual PM10 levels above national and international standards. The cities are ordered in terms of increasing annual mean PM10 concentrations, which are based on WHO data from 2003 to 2010. The top 25 most populous cities were determined based on the 2011 population in the “urban agglomeration” as defined by the UN (UNDESA 2011). Shenzhen was in the top 25, but PM10 data was not available for that city. Therefore we used Jakarta (#26) instead. The US EPA annual standard for PM10 was obtained from their website, but this standard was revoked in 2006 in favor of only having a 24-h average standard of 150 μg/m3 for PM10 that is not to be exceeded more than once a year on average. The EU annual standard of 40 μg/m3 was established in 2005 and obtained from the European Commission website. The WHO annual guideline of 20 μg/m3 was obtained from their website and is up to date as of 2014
Over the past four decades, the developed countries in North America, Europe, and the Pacific have implemented policies to curtail the severe air pollution events that had plagued them during the early and mid-twentieth century. One of the consequences of the increased stringency of pollution control regulations in the developed, industrialized world is the outsourcing of more polluting manufacturing jobs into the developing world by multi-national corporations. This process was coupled with domestically driven industrial development by a number of countries that saw industry as a path to national prosperity. Increased national wealth is coupled with a desire for increased living standards among the population. For example, the use of cars as a means of personal transportation rose rapidly with per capita income. Many of these developing countries have among the highest population density in the world, and so the rise of personal vehicles has become a major source of air pollution and transportation congestion in the developing world. Furthermore, these higher living standards led to increased energy consumption. These trends coupled with higher rates of garbage and agricultural combustion, low quality fuel use, coal combustion and other major polluting energy sources, and construction-related dust have led to much higher air pollutant concentrations in developing countries in comparison with the developed world. Figure 13.3 shows how drastic the air pollution burden shifts of the past four decades have been, using PM10 emissions as a surrogate marker for other major air pollutants in terms of global trends (PM2.5 data are largely unavailable historically).
Global annual PM 10 emissions in 1970 and 2008. The annual emissions scale ranges from green, marking 0 to 10 tons, to blue, marking greater than 100 tons. The lines over bodies of water reflect the PM10 contribution of major international shipping and aviation routes (These maps were obtained from the European Commission Joint Research Centre Emission Database for Global Atmospheric Research (EDGAR) http://edgar.jrc.ec.europa.eu/overview.php?v=42)
There are many noticeable differences between these two time periods. PM10 distributed across the eastern area of the United States and Canada diminished over the decades. Southern Mexico, Central America, and the Caribbean have increased their air pollution emissions, though Cuba produces much less PM now. PM10 emissions in South America attributable to wide-scale forest burning have abated, though the population centers of the continent in Venezuela, Brazil, and Argentina have much worse air quality currently. Europe has generally seen remarkable improvements in air quality, though Asia Minor and parts of Northern Africa have gotten worse. Most of the line of PM10 across the former Soviet Union and Kazakhstan has disappeared with the collapse of the USSR. A thick belt of heavy PM pollution has developed between the Saharan Desert and the equator in Africa, though Southern Africa has improved. The already thick pockets of air pollution over South, East, and South-East Asia have intensified in the past four decades. Finally, it is apparent that the developed countries of the Pacific, namely Australia, New Zealand, Japan, and South Korea, have experienced air quality improvements. These global trends only account for PM10, but there are similar patterns of other major air pollutants on the global scale.
To get a clearer picture of just how the contribution of different countries to global air pollution burdens has changed, Fig. 13.4 maps out the regional trends in annual PM10 emissions from 1970 to 2008.
Global PM 10 emission trends by region from 1970 to 2008. The regions with more developing nations have grown to comprise a greater share of the global PM10 emissions. The portion of total PM10 emissions contributed by each world region is shown by the thickness of the space between lines that represents each region. The regions are organized from highest total PM10 emissions from 1970 to 2008 on top to the lowest total emission regions on bottom. Each line represents the trend in total PM10 annual emissions for that region and all the ones below it. All data were obtained from the European Commission Joint Research Centre Emission Database for Global Atmospheric Research (EDGAR) (http://edgar.jrc.ec.europa.eu/overview.php?v=42). Asia-Stan: Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan; China +: China, Hong Kong, Macao, Mongolia, Taiwan; India +: Afghanistan, Bangladesh, Bhutan, India, British Indian Ocean Territory, Sri Lanka, Maldives, Nepal, Pakistan; Indonesia +: Indonesia, Papua New Guinea; Russia +: Armenia, Azerbaijan, Georgia, Russian Federation; Ukraine +: Belarus, Republic of Moldova, Ukraine (For more information on the regional categories, please visit http://edgar.jrc.ec.europa.eu/methodology.php)
The starkest trend in this figure is that of the Northern and Western African regions, which have dramatically increased particulate emissions in the latter half of the 2000s. This has been driven by rapid industrialization in the coastal West African countries and the belt of Central and Eastern African nations just south of the Sahel Desert, where some of the fastest urbanization rates in the world are occurring. On the other end of the emissions scale, the Middle Eastern countries have gone from being barely visible on the figure in the 1970s to just becoming apparent in the mid-1990s and 2000s. The regions that are steadily increasing include Northern and Western Africa, China and surrounding countries, India and surrounding countries, and the Middle East. There have been increases that seem to oscillate in a less discernible pattern for Eastern and Southern Africa, Southeast Asia and Indonesia +, and South America. Asia-Stan and Russia + and Central America peaked in the late 1990s and are on the decline now. The regions that have experienced a steady decline in air pollutant emissions are North America, OECD Europe, Central Europe, and Japan and Korea. Finally, Oceania, Turkey and Ukraine +, and international shipping and aviation have been relatively steady emitters over these past four decades. These trends show that the greatest burden of air pollution exposure falls on the developing countries, in particular those in the process of massive urbanization and industrialization.
13.3 Criteria Air Pollutants in Developing Countries
The urban air pollution mixture is very complex in terms of its chemical composition. In the United States, common pollutants in this mixture that affect the general population are regulated as criteria air pollutants, including particulate matter (PM10 and PM2.5), lead, ozone, nitrogen dioxide, carbon monoxide, and sulfur dioxide. Similarly, some or all of these pollutants have national standards in many other countries and have guidelines by WHO. In the previous section, we have described emission trends and typical ambient concentrations of PM10, because PM (especially PM2.5) has been most commonly linked to various adverse health effects. Although PM2.5 is a more health-relevant measure of PM, historical data are less available for PM2.5 than for PM10. Given the focus on PM in the previous section, the following section will not emphasize that criteria pollutant. Below we describe how developing countries differ in other common pollutants of the urban air pollution mixture from developed countries.
13.3.1 Lead
Lead can enter the air through a variety of natural processes, such as volcanic eruptions, forest fires, and soil erosion. The natural background concentration of lead in the air can vary due to local natural sources. The background lead concentration was 5–15 ng/m3 in Central Europe in 2003 (UNEP 2010), but it was approaching 100 ng/m3 in 2005 in Australia due to a higher prevalence of brush fires (Heritage 2005). The corresponding background child blood lead levels worldwide are generally in the range of 10–30 μg/l (WHO 2000). Anthropogenic sources include lead smelters, waste incineration, battery recycling, mining operations, lead paint dust, and leaded gasoline. The latter two sources have been reduced significantly through legislation either banning or significantly reducing lead content in certain countries. Today only some of the developed countries have significant regulations on paint lead content, whereas all but six countries in the world have bans on leaded gasoline (see the Sect. 13.4.3 for more information on leaded gasoline). Nevertheless, some illegal production of leaded gasoline still occurs. The widespread banning of leaded gasoline has reduced the impact of this air pollutant, particularly in developed countries. Finland began phasing out leaded gasoline in the early 1980s, and from 1980 to 1991 the mean ambient air lead concentrations dropped from 0.335 to 0.041 μg/m3 in Helsinki (Ponka et al. 1993). Though lead air concentrations have dropped in developed countries, they are still an issue due to mining and industrial processes. The United States banned lead paint in 1977 and leaded gasoline in 1995, but 21 cities or counties exceeded the 3 month rolling average standard of 0.15 μg/m3 as of 2014. Lead exposure through ambient air may still be an issue in some developed countries, but the problem is more severe in developing countries.
Although the US EPA monitors ambient air concentrations of lead, many countries do not, and so it is difficult to assess lead exposure via air in developing countries. More countries monitor blood lead levels (BLLs) in children, and so this can be used as a marker of the total exposure that a child receives in a given country. The WHO based the establishment of its 2000 air lead concentration guideline for Europe, 0.5 μg/m3, on the assumption that 1 μg/m3 lead in the air corresponds to 50 μg/l lead in the blood through inhalation exposure and other indirect routes (WHO 2000). The U.S. phasing out and eventual ban of leaded gasoline contributed to an 84 % reduction (from 8.6 to 1.4 %) in the percentage of children with elevated blood lead levels (≥100 μg/l) from 1988 to 2004 (Jones et al. 2009). The banning of leaded gasoline in 2000 in China was a large factor in the 29 % reduction (from 33.8 to 23.9 %) in the mean percentage of children with elevated blood lead levels from the 1995–2003 sampling period to the 2001–2007 period (He et al. 2009), but the fraction of children with elevated blood lead levels still remain much higher in China than in the U.S.
Similarly, blood lead levels measured in Kinshasa, the capital of the Democratic Republic of the Congo, are much higher than those measured in the U.S., even after the banning of leaded gasoline. In 2008, the ambient lead concentration in the urban air ranged from 0.57 to 5.22 μg/m3. Sixty three percent of children measured in 2004 and 71 % of children measured in 2008 had elevated BLLs (Tuakuila et al. 2013a). Leaded gasoline was banned in 2009, and by 2011 only 41 % of children measured had elevated BLLs, a 42 % reduction. Nevertheless, this is still a very high incidence of hazardous lead exposure, indicating that the children of Kinshasa are still being exposed to significant amounts of lead through other routes (e.g., inhalation and ingestion of lead-contaminated soil, the use of fired clay as a traditional treatment for gastritis during pregnancy, household paint chips, and household Portland cement) (Tuakuila et al. 2013b). The post-leaded gasoline phase-out incidence of elevated BLLs is higher in Kinshasa in comparison to other areas of Africa. In 1991, over 90 % of the children attending inner city schools in the Cape Peninsula of South Africa had elevated BLLs, but by 2002, when leaded gasoline represented only 30 % of the market, only 10 % of children in those same schools had elevated BLLs (Mathee et al. 2006). Leaded gasoline was phased out of Uganda in 2005, and a 2009 study of schoolchildren in Kampala found that 20.5 % had elevated BLLs, less than the percentage found in Kinshasa and China but still far greater than that found in the USA (Graber et al. 2010). These examples indicate that the phasing out of leaded gasoline has significantly reduced ambient air lead concentrations and children’s blood lead levels, but lead exposure through airborne and other routes still remains much more of a problem in the developing world.
13.3.2 Sulfur Dioxide
SO2 is used as an indicator for the sulfur oxides (SOX), as it is the sulfur oxide with the highest concentration in the atmosphere. SO2 is also a precursor of particulate sulfate formed photochemically in the atmosphere. SO2 has been dramatically reduced in developed countries due to regulations concerning industrial emission controls and other important sources. The US EPA Clean Air Act resulted in a 78 % reduction in national SO2 levels (CAI-Asia 2010). On the whole, global SO2 emissions have declined from 1990 to 2010 (from 121 to 103 Tg SO2), led mostly by declines in the developed world (Klimont et al. 2013). The greatest sources for 2011 in order of contribution are the energy sector (40 Tg; primarily coal with petroleum combustion as the next largest source), industry (38.7 Tg), shipping (13.6 Tg), residential sources (6.4 Tg), transportation (1.6 Tg), and waste (0.3 Tg).
China contributed 29.1 % of global SO2 emissions in 2011, showing a peak in 2005, after which the control measures in the 11th Five Year Plan (2006–2010) resulted in a 14 % reduction in SO2 emissions and a concomitant 13–15 % and 8–10 % reduction in ambient SO2 and SO4 2−concentrations, respectively, over eastern China (Wang et al. 2014). Nevertheless, China is still the largest contributor to global SO2 emissions of any country. India is the next largest contributor, just behind total international shipping, and it has been steadily increasing SO2 emissions from 1990 to 2011, rising from 2.8 to 10 % of the global total (Klimont et al. 2013). Much higher coal usage in China and India is the main factor leading to their relatively high SO2 levels. Coal use in U.S. is relatively high for an industrialized nation, making it the next greatest contributor to emissions behind India (6.2 % of the global total in 2011). Over 70 % of total SO2 emissions in the U.S. came from coal-fired power plants in 2008 (USEPA 2012). However, cleaner coal processing and increased removal of gaseous byproducts from flue gas in the U.S. have made it so that SO2 ambient levels in 2012 are nearly all below the US EPA 1 h maximum standard (99th percentile of 1 h daily maximum concentrations, averaged over 3 years) of 75 ppb (196 μg/m3) (USEPA 2013a). For most other countries in the world, regardless of economic status, SO2 is a relatively minor pollutant, though it may still have a significant health impact below national standards. A recent study in Seoul on ambient pollution levels and tuberculosis incidence found that SO2 was the only pollutant correlated with a significantly increased risk of TB in males, even though SO2 levels (annual average of 6.1 ppb) were below Korean standards (annual average of 20 ppb) (Hwang et al. 2014). It is not clear if the health effects of SO2 are mainly attributable to the pollutant itself or to the secondary pollutants it creates through atmospheric chemical reactions.
13.3.3 Nitrogen Dioxide
NO2 is used as an indicator of the nitrogen oxides (NOX) due to its more common prevalence than NO and N2O, much like SO2 and SOX. NO2 is formed by high temperature combustion, in which nitrogen reacts with oxygen to form NO and then NO2. NO2 and other NOX contribute to the formation of secondary pollutants such as particulate nitrate (He et al. 2014). For example, due to increasing emissions of NOx, the nitrate/sulfate ratio in PM2.5 increased from 0.43 to 0.75 from 2000 to 2009 in Shanghai, China (Huang et al. 2012a).
In many areas the greatest source of NOX is traffic-related pollution. Overall, mobile sources (57.5 %), fuel combustion from stationary sources (24.2 %), and industrial processes (8.4 %) account for the majority of the 14.1 Tg of NOX emissions in the U.S. (USEPA 2014a). As a result, the cities with the greatest concentrations of NO2 in the U.S. often have the worst traffic problems, such as downtown Los Angeles, which had an annual 98th percentile of daily 1-h maximum average of 67 μg/m3 in 2011 (USEPA 2013b). In the U.S., over 90 % of NO2 concentrations have been below the national annual 98th percentile of daily maximum 1-h average standard of 100 ppb (188 μg/m3) at least since the early 2000s, and they continue to show a decline. The Japanese government enacted the Automobile NOX Law in 1992 to ban vehicles in certain areas not conforming to emission standards and strengthened it to include PM considerations and stricter standards in 2001. Areas in which the law was enforced had half the average annual NO2 concentration that unenforced areas did (21.8 versus 39.3 μg/m3 for 2006–2009), and this was correlated with a reduction in asthma and atopic dermatitis prevalence even after controlling for PM (Hasunuma et al. 2014).
China is the largest producer of NOX, emitting 23.4 Tg in 2012. However, according to the Chinese Ministry of Environmental Protection, 70.9 % of this was from industrial sources, with motor vehicles only accounting for 27.4 % (MEP 2013a). Beijing has a high concentration of vehicle traffic, but it also receives about 50 % of its NOX from regional and not local sources (Ma et al. 2014). The high industrial contribution to Beijing NOX levels is likely due to sources in the greater Beijing/Tianjin/Hebei area, which accounts for 43 % of national coal consumption, 30 % of national thermal power consumption, and 50 % of national steel and coke production. Beijing had a monthly mean concentration of 100 ppb NOX in January 2013 (He et al. 2014). During this time levels spiked to around 350 ppb a few times and over 200 ppb several times, and so the 98th percentile of daily maximum 1-h average would likely be much higher than the monthly mean. Nationwide average concentrations of NO2 show a slightly increasing trend.
In India, NO2 pollution shows increasing trends in many areas. In the National Capital Region and surrounding states, increasing trends from 2007 to 2011 have been seen in the annual mean NO2 concentrations of the National Capital Territory of Delhi (49.67–57 μg/m3, 15 % increase), Uttar Pradesh (36.17–44 μg/m3, 22 % increase), and Haryana (25–54 μg/m3, 116 % increase) (Board, N.C.R.P 2013). By 2011, each of these regions exceeded the national annual average NO2 air quality standard of 40 μg/m3.
13.3.4 Carbon Monoxide
As a principal product of incomplete combustion, CO has global background concentrations ranging from 0.06 to 0.14 mg/m3 depending on natural sources such as photochemical synthesis, volcanic eruptions, and forest fires (WHO 2000). Mobile sources account for the bulk of CO emissions (50.8 % of total U.S. emissions in 2011 (USEPA 2014b)), and so concentrations of this pollutant tend to be higher in urban environments with dense traffic. Vehicle emission controls greatly influence CO emissions, as average Pakistani vehicles emitted 25 times more CO than the average U.S. vehicle in 2000 (Barletta et al. 2002). The EU has made great strides to reduce CO emissions from 1990 to 2010, with all but one member country showing reductions ranging from 92 % in Luxembourg to 18 % in Romania, in large part through vehicle emission regulation (EEA 2010).
CO pollution has been very effectively curbed in the U.S., so much so that no counties exceeded the 8-h average standard of 9 ppm (10.3 mg/m3) more than once per year in 2012 (USEPA 2014c). The last nonattainment area for CO in the U.S. was redesignated as being in attainment in September 2010. CO concentrations dropped 83 % from 1980 to 2012 in the U.S., from a mean annual second maximum 8-h average of 8.9 ppm (10.2 mg/m3) to 1.5 ppm (1.7 mg/m3). CO levels have also been dropping in Chinese cities. In Shanghai, CO annual averages have dropped from about 1.4 mg/m3 in 2008 to 0.88 mg/m3 (37 % decrease) in 2012 (EPB-Shanghai 2013), and Beijing annual averages have dropped from about 2.5 mg/m3 in 2006 to 1.4 mg/m3 (44 % decrease) in 2012 (EPB-Beijing 2013). However, increasing vehicle traffic in East Asia, South Asia, Africa, and other rapidly urbanizing environments may lead to increased CO exposure to urban populations. In Lagos, short-term air monitoring along a busy road found CO values exceeding the US EPA 8-h standard (Olajire et al. 2011). The average roadside concentration seen in Lagos, 19.27 ppm, is much higher than that seen roadside in central London, 0.53 ppm (von Schneidemesser et al. 2010). Furthermore, a global comparison found higher CO concentrations in Latin America and Asia when compared to the United States and U.K. (von Schneidemesser et al. 2010).
13.3.5 Ozone
Ozone is a major component of photochemical smog. Concentrations of ozone typically have large daily variations depending on variations in heat, sunlight, and precursor gas concentrations. Monitoring in Agra, India found a diurnal ozone cycle with an average maximum concentration of 117 μg/m3 in the peak noontime and an average minimum concentration of 23.2 μg/m3 at sunrise (Saini et al. 2005). Though ozone concentrations can be high in urban environments, the presence of NO in the local urban air can “quench” ozone, causing there to be lower concentrations of O3 in urban areas and possibly higher O3 in non-urban areas where NO concentrations are low.
Ozone is still a major problem in both the developing and developed world. In July 2006, afternoon mean surface ozone concentrations show the highest levels between the 30° N and 60° N parallels, especially the Eastern and Western U.S., Europe, the Middle East, Central Asia, and the Yellow Sea area (WHO 2007). Other areas of high ozone include the Arabian Peninsula and southern Africa. In Delhi, average hourly concentrations measured in 2008 ranged from about 20 μg/m3 at 9:00 pm to over 70 μg/m3 at 3:00 pm (Guttikunda 2009). Ozone concentrations are much higher in the summer, and so there is considerable seasonal variation. 2010 peak pre-monsoon summer average ozone concentrations in the twin Pakistani cities of Islamabad and Rawalpindi were about 42.7 μg/m3, as compared with about 31.8 μg/m3 in the deep winter (Ahmad and Aziz 2013). In the United States, ozone levels decreased the least of all the criteria air pollutants following the Clean Air Act and other relevant air pollution legislation. There was only a 9 % reduction in O3 from 2000 to 2012, compared to the next smallest reduction, 27 % for PM10, and the largest reduction, 57 % for CO (USEPA 2014c). Furthermore, 94 % of people in the United States estimated to live in counties with at least one air pollutant concentration exceeding its relevant standard in 2012 live in counties with ozone exceedances, far greater than for any other criteria pollutant. The high burden of ozone in the U.S. is apparent in that the US EPA 1-h and 8-h ozone standards are higher than those for most Asian nations or territories with the exception of the Hong Kong SAR (1-h), Indonesia (1-h), Singapore (8-h), and Bangladesh (1 and 8-h) (CAI-Asia 2010). Interestingly, unlike other directly emitted air pollutants, ozone is not necessarily higher in developing countries.
13.4 Air Pollution Sources More Common in Developing Countries
Common anthropogenic sources of air pollution in urban atmospheres in both developed and developing countries include fuel combustion for energy production (e.g., power plants, steam generation, household and commercial boilers/heaters), industrial processes (e.g., oil refinery), solvent utilization, gasoline or diesel powered vehicles, and fugitive dust (for PM only). In addition, the pollutants generated outside a city can be transported, along with secondary pollutants that are formed via photochemical reactions, contributing to the complex mixture of urban air pollution. Here we describe some unique air pollution sources in developing countries.
13.4.1 Industrial Emissions Especially from Coal Combustion
Industries emit air pollutants in every country, but more developed countries have much more stringent standards in regards to point source emission control. For instance, the EU has experienced significant reductions in industrial air pollutants as a result of its Large Combustion Plant (LCP) Directive, implemented in 2001, which required that new plants follow strict standards and that old plants exhibit significant reductions in criteria pollutants by 2008 (Commission 2014). From 2007 to 2009, LCPs reduced emissions of SO2 by 44 %, NOX by 27 %, and dust by 44 % (Grebot et al. 2012). Nearly all member nations showed negative trends for these pollutants, with the exceptions of Romania, Sweden, and Slovakia for SO2; Greece, Hungary, and Sweden for NOX; and Cyprus, Hungary, Lithuania, and Latvia for dust. In terms of meeting emissions ceilings, compliance was exceedingly good, with only Estonia’s and Romania’s SO2 emissions as well as Bulgaria’s SO2, NOX, and dust levels exceeding their limits. Even with a number of developing countries in its member nations, the EU was able to successfully curb industrial emissions with good compliance rates using well-enforced legislation. Many developing countries suffer much higher air pollution due to a lack of enforcement of emission standard laws.
In more developed industrialized nations, policies requiring flue gas scrubbers and other technologies have been instrumental in reducing industrial sources of air pollution. The common technologies used to clean flue gases include electrostatic precipitators and fabric filters for reducing PM; flue-gas desulfurization (FGD) for reducing SO2; flue-gas denitrification for reducing NOX through selective catalytic reduction (SCR) and selective non-catalytic reduction (SNCR); and wet and dry scrubbing, absorbers, flue gas recirculation, etc. for a variety of air pollutants. China has adopted most of these technologies to varying extents, including circulating fluidized beds (CFB), in which a gas or fluid is passed through a high kinetic energy solid-fluid mixture to increase gas/fluid-solid contact, to increase coal combustion efficiency and reduce emissions (Chen and Xu 2010). In addition, they have extensive FGD system use in coal-fired plants due to the 11th Five Year Plan, which has helped to reduce SO2 emissions from the 2005 peak though enforcement and implementation across provinces is still an issue. However, the relatively high operating costs of NOX removal systems such as SNCR/SCR, which inject urea or ammonia into the flue gas to reduce NOX into N2, have impeded its large scale installation in China until the 12th Five Year Plan (2011–2015) required that all new thermal power plants be fitted and old, large plants be retrofitted with SCR/SNCR (Chen and Xu 2010; Zhao et al. 2013). Though China has issued laws requiring the use of these technologies, enforcement appears to be too relaxed given the number of plants and factories above emission standards.
The severe, prolonged smog episodes in Northern China have major inputs from industrial plants in Hebei and Shandong Province as well as other northern and central provinces. Unprecedented transparency in air pollutant monitoring results ordered by the Ministry of Environmental Protection (MEP) in July 2013 revealed that several large scale steel factories and thermal power plants were consistently breaching discharge standards. From October to December 2013, a comparison of eight major pollution sources each in Hebei and Shandong showed that NOX emissions were 30 and 37 times greater, respectively, than eight major sources in Beijing (Ma et al. 2014). This contributed to Hebei and Shandong being the No. 1 and No. 2 greatest emitters of NOX of any Chinese province in 2012 (MEP 2013b). Industrial emissions accounted for 84–91 % of total national SO2 emissions for China in 2011, and the industries monitored in these provinces also showed a high frequency of exceeding SO2 standards. During a month and a half period in fall 2013, 13 cities in Shandong had a 24-h moving average air quality index over 200, and many industries in those cities were exceeding NOX and SO2 emission standards every hour during the worst days (Ma et al. 2014). Shandong has already implemented more stringent emission standards, but most companies will require significant measures to bring their emissions down. Thermal power and cement production facility emission standards are lacking in Hebei, and so are municipal standards in other major polluting provinces, such as Jiangsu, Zhejiang, and Liaoning. The use of coal to power industrial and power plants is a major source of many air pollutants. Coal use is extremely high in the Yangtze River Delta region (Jiangsu/Zhejiang/Shanghai, 1.2 billion metric tons in 2011), with the industrial sector being the greatest consumer (39 %). This region consumes more coal than the entire U.S. (807 million metric tons in 2012 (USEIA 2014)), which contributed to it being the area of China with the greatest discharge intensity (tons per km2 land area) for SO2, NOX, and VOCs (Ma et al. 2014). Though China has adopted some technologies to clean flue gas on a case by case basis, it lacks widespread legislation and enforcement measures to ensure these technologies are used across the country. Issues with power plant and factory emissions is not a problem unique to China, but it provides an extreme example of how a lack of regulation enforcement can contribute significantly to ambient pollution. Other less industrialized developing countries also lack legislation concerning industrial emissions.
13.4.2 Open Burning
The widespread burning of trash and other waste can be a major contributor to ambient air pollution, particularly on a local scale. Garbage burning releases particulate matter, carbon monoxide, toxic organics from plastics, toxic metals, PAHs, and to a lesser extent SOX and NOX. Many developing countries lack regulations or sufficient enforcement concerning garbage disposal or other forms of open burning. Open burning is defined as combustion that releases emissions directly into the open air without passing through an adequate chimney or duct. This is an issue that affects both rural and urban settings in a local sense, but the health burden of this practice is especially severe and wide reaching in crowded urban settings where more people are exposed to these emissions. However, no data are available on the quantitative assessment of the health impact from such unique sources.
Open burning exists even where it is illegal when laws are not sufficiently enforced. This practice is illegal in Mumbai, but about 2 % of the solid waste generated in each of the city’s wards is disposed of in this fashion (NEERI 2010). Open burning in Mumbai accounts for 8.7 % of all annual combustion-related emissions of PM, CO, NOX, SO2, and hydrocarbons (HC). More specifically, it accounts for 23.9 %, 15.7 %, and 26.1 % of all annual combustion-related PM, CO, and HC emissions, respectively. This open burning includes the combustion of municipal waste, auto parts, wood refuse, small-scale industrial waste, and leaves. One of the more distinctive components of garbage is plastic materials, which give off some unique compounds when combusted. Plastic combustion mainly produces non-specific even-carbon-chain alkanes, terephthalic acid, phthalates, and 4-hydroxybenzoic acid. Minor products include PAHs (in particular triphenylbenzenes) and tris(2,4-di-tert-butylphenyl)-phosphate, and air sampling in Chile suggested that 1,3,5-triphenylbenzene and tris(2,4-di-tert-butylphenyl)-phosphate could be used as unique markers of plastic burning (Simoneit et al. 2005).
These trash-burning operations can be a significant source of fine particulate matter in certain areas. For example, a study in Accra, Ghana found that an increase of 5 open trash burning spots per hectare was associated with a 27 % increase in local PM2.5 concentrations (Rooney et al. 2012). Garbage burning in the home in Accra was also associated with a 195 % increased risk of low birth weight (Amegah et al. 2012). There is not only an increase in fine particulate matter surrounding the burning sites, but also an increase for the entire urban environment. An analysis of monitoring stations around Hyderabad, India found that waste burning accounted for 12 % of PM2.5 in the summer and winter (7 % during rain), and 4–6 % of PM10 depending on weather and season conditions (Guttikunda et al. 2013). A study of the Mexico City Metropolitan Area found that there were about 25 tons of primary organic aerosols (POAs) emitted in the area each day as a result of trash burning, which is a similar amount to fossil fuel POA emissions for that city (Hodzic et al. 2012). The authors concluded that reducing or eliminating trash burning could reduce ambient POA concentrations by 2–40 % and PM2.5 concentrations by 1–15 % depending on the location within the city.
In contrast, developed countries do use burning as a means of eliminating waste, but this is usually done in incineration plants with much higher temperatures and scrubbers to remove pollutants from the emissions. These strategies lead to a much lower exposure burden for the local population. In terms of particulate matter pollution, sampling and modeling of British incineration plants found that the levels of PM10 immediately at the mouth of the flue, or waste gas duct, varied between 0 and 10 μg/m3 depending on use, and ground concentrations were modelled to not exceed 0.01 μg/m3 even under the most intense use conditions (Ashworth et al. 2013). Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) are carcinogenic byproducts of combustion that can be associated with waste incineration, especially if it is performed at lower temperatures or if the flue contains electrostatic precipitators that can provide a surface that catalyzes these compounds’ formation. However, commonly employed wet scrubbers in the flue are efficient at removing these pollutants (Karademir and Korucu 2013). Even workers inside of the incineration plants are exposed to metal, VOC, PM, and airborne microorganism levels well below international occupational exposure standards (Sabatini et al. 2013). The contribution of open waste burning to air pollution in developing countries is largely absent from developed countries, and this leads not only to higher levels of general combustion products such as CO, PM, PAHs, but also some unique pollutants such as chlorinated compounds.
13.4.3 Low Quality Gasoline and Diesel
Mobile sources are significant contributors to urban air pollution in megacities. Emission rates of pollutants depend on engine combustion efficiency, whether there is a catalytic converter or other pollution control device, and fuel quality. The composition of gasoline and diesel can vary significantly based on refining processes, and these variations affect the emissions produced through gasoline combustion. Various regulations in developed and developing countries have phased out harmful additives and components. However, much of the gasoline in developing countries still has not caught up with the standards of developing countries.
One of the first initiatives to improve gasoline quality was the international effort to phase out tetraethyl lead (TEL) as an anti-knocking agent in gasoline. Knocking is a process by which small pockets of the air/fuel mixture in gasoline combust outside of the normal combustion front caused by spark plug ignition, and the effects of this phenomenon were potentially destructive to early engines. TEL was not only able to reduce knocking, but also to boost octane and subsequently improve engine efficiency. The use of TEL as a gasoline additive began in the 1920s, but its contribution to neurotoxic lead exposures and coating and inactivating of catalytic converters led to it being phased out in the U.S. beginning in the mid-1970s. Catalytic converters have been used starting in 1975 in the U.S. to catalyze redox reactions that decrease hydrocarbon, CO, and NOX emissions, but they can become less useful if gasoline components coat the converter and prevent harmful chemicals from interacting with the catalytic surface. Japan enacted the first ban on leaded gasoline in 1986, and most countries had officially phased out TEL use by the early 2000s. Only six countries continue to use leaded gasoline today (2014): Afghanistan, Algeria, Iraq, Myanmar, North Korea, and Yemen. However, there is evidence that illegal leaded gasoline production in some developing nations such as China is being performed despite national regulations (Chung 2013). Though there has been dramatic international improvement in TEL regulation in the past decade, other potentially hazardous gasoline additives are not as controlled.
High sulfur content is another gasoline component that can increase harmful emissions through disruption of the catalytic converter and increasing SOX emissions. Sulfur is a naturally occurring component of crude oil, and its levels vary by region. It is present in the fuel in the form of thiols, thiophenes, and disulfides. When combusted in a vehicle, SO2 is formed, and this can adsorb onto palladium, platinum, or rhodium catalytic converters, in order of most to least sensitivity to sulfur adsorption (Truex 1999). This adsorbed sulfur both physically and electrically blocks the binding of other emission chemicals to the catalytic surface. The “dose-response” curve for fuel sulfur content and catalytic inhibition is supralinear, meaning that lower levels of SO2 have a disproportionately high effect on inhibition. Refining processes are capable of reducing sulfur content in both gasoline and diesel fuels, resulting in a greater efficiency of the catalytic converter and a subsequent reduction in the emissions of several pollutants. The reduction of sulfur content in fuels from 450 to 50 ppm has led to reductions of 35 % for acetaldehyde, 21 % for benzene, 19 % for CO, 17 % for hydrocarbons, and 8 % for NOX (Schuetzle et al. 1994). Though high sulfur content in fuel can lead to direct increases in SO2 tailpipe emissions, traffic is generally a much lower source of SO2 than energy sector or industrial emissions. SO2 reacts with hydroxyl radicals in the air to form sulfuric acid (H2SO4) particles that can combine with each other in a rare process known as homogeneous nucleation. The EPA estimates that over 12 % of SO2 emitted in the urban U.S. is converted into sulfate fine and ultrafine particulate matter, which indicates that gasoline and diesel vehicles may be responsible for eight times more than what is accounted for in direct diesel emission inventories of PM (Blumberg et al. 2003).
The most stringent sulfur standards for gasoline and diesel (below 15 ppm for diesel) tend to be in the wealthier nations, particularly those in Europe, North America, and Australia (Programme., P.f.C.F.a.V.U.N.E 2014). Moderate to high diesel sulfur standards (between 50 and 500 ppm) exist in Mexico, southern and parts of central Africa, Russia, South Asia, East Asia, and Southeast Asia. The least stringent standards (greater than 500 ppm) predominate in South America, Central Asia, the Middle East, and most of Africa. Hence, the relative contribution of fuel sulfur in gasoline and diesel to SO2 and fine particles emissions would be higher in developing countries where fuel sulfur content is higher.
13.5 Disease Burdens Attributable to Air Pollution in Developing Countries
The disease burden attributable to air pollution is the product of population, exposure concentration, and the concentration-response relationship. Hence, large disease burdens are expected to occur in heavily polluted, populous urban areas of developing countries.
Figure 13.5 shows the relationship between regional PM10 concentration, gross national income per capita, and the PM10-attributable disease burden in disability-adjusted life years (DALYs). DALYs can be defined as healthy years lost due to a disease-causing factor, and they are calculated as the sum of years of life lost (YLL) from premature mortality and years lost due to disability (YLD) from a disease or set of diseases (DALY = YLL + YLD). The WHO-defined world regions are organized by increasing annual mean PM10 levels, and in general all the high-income regions have the lowest PM10 concentrations. (The Eastern Mediterranean regions are an exception due to the contribution of sandstorms and desert dust to ambient PM10 levels.) The relationship between particulate matter concentrations and disease burden is less clear on the regional scale, as it is a function of air pollution levels, the size of the affected population, and the population of the WHO region. The low-middle income European region has a high disease burden despite having only moderate annual PM10 levels because those areas over which the PM10 is distributed are consistently highly populated across the region, and the relatively small population of the lower income European countries inflates the per capita DALY value. For the Western Pacific region, countries like China have high air pollution over densely populated areas, but the region also contains countries with sparse populations, such as Mongolia, and has a large total population, which dampens the effect of high air pollution there on DALYs. Generally, the high-income WHO regions tend to have smaller total populations than the low to middle income regions because there are fewer countries that meet the high income criteria, thus inflating their DALYs per capita values. Nevertheless, the global data shows that regions with lower average GNIs tend to have higher exposures to PM10 and higher overall burdens of disease attributable to PM10. Two issues, however, arise when comparing DALYs between developing and developed countries, namely the nature of the concentration-response relationship and the influence of regional differences on air pollution composition.
Trends between annual mean urban PM10, GNI, and PM 10 -attributable DALYs by region. Wealthier nations in more developed regions tend to have lower annual PM10 and DALYs attributable to air pollution. All data were obtained from the WHO Global Health Observatory Data Repository. The annual mean PM10 data was averaged over the period of 2003–2010, the regional mean GNI data is averaged over the period of 1980–2012, and the regional mean PM10 burden of disease data was collected in 2004. Amr Americas (includes North, Central, and South America and the Caribbean), Eur Europe (includes all of Europe, Israel, and some of Central Asia), Wpr Western Pacific (includes Pacific nations, East Asia, and eastern South-east Asia), Sear South-east Asia (includes South Asia, western South-east Asia, and some Pacific nations), Emr Eastern Mediterranean (includes the Middle East and some northern African nations), Afr Africa (includes most African nations), HI High income, LMI low-middle income (For more information on the WHO regions, please visit http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_AnnexC.pdf?ua=1)
13.5.1 Concentration-Response Relationship
A linear dose-response relationship (slope factor) has been applied to estimate the global burden of disease attributable to air pollution. This slope factor, however, has been largely derived from observations made in developed countries where the pollution level was substantially lower. Hence, questions arise as to whether this would have resulted in an overestimation or underestimation of the actual burden. Such questions are related to fundamentals on the shape of dose-response curves, as depicted in Fig. 13.6. The prevailing evidence, particularly in terms of PM effects on mortality, suggests that the response is linear or approximately linear across a wide range of concentrations measured in both developed and developing countries. Figure 13.7 shows that meta-analyses of multi-city studies of air pollution and mortality have found similar effect sizes of PM10 on mortality in all regions of the world, even though the concentration ranges differ greatly between these regions.
Excess risk of mortality and 95 % confidence intervals associated with a 10 μg/m 3 increase in PM 10 concentration. There appear to be small variations between the increased all-cause mortality risk associated with unit increases in PM10 between countries and regions, suggesting a linear dose-response relationship between air pollutants and mortality. Dots represent mean increases in percent mortality risk per 10 μg/m3 increase in PM10 from multi-city or multi-study models. The bars represent 95 % confidence intervals. Any mean change in risk with confidence interval bars not overlapping the dashed zero line represents a significant change. This figure is based on a similar one in (Anderson et al. 2010) (Sources: Levy et al. 2000; Stieb et al. 2002; Schwartz 2004; PAHO 2005; Anderson et al. 2010; Chen et al. 2012; Romieu et al. 2012)
The studies summarized in Fig. 13.7 show an excess risk of all-cause mortality per 10 μg/m3 increase in PM10 ranging from 0.27 % (95 % CI, 0.12–0.42 %) in the 8 Asian studies reviewed by Anderson et al. to 0.77 % (95 % CI, 0.6–1 %) in the 2012 Latin American nine-city study conducted by Romieu et al. The variation in these estimates is not apparently correlated with PM10 levels, as similar estimates were reported in two studies that were conducted in areas with several-fold differences in PM10 (Schwartz 2004; Chen et al. 2012). Considering likely contributions of inter-population variability and differences in the statistical methods to the variations in the concentration-response relationship between different studies, the estimates are remarkably similar, suggesting that the dose-response relationship between PM10 exposure and mortality is fairly constant across air pollution levels. However, from a toxicological standpoint, differences in PM10 chemical composition are expected to cause differences in PM toxicity. This is supported by limited epidemiological evidence. For example, PM2.5 rich in secondary species, such as sulfate and nitrate, and certain organic compounds has been associated with greater effects on mortality. Specifically, it was found that transmural myocardial infarctions (MIs), blockages of major coronary arteries, were more associated with PM2.5 that was composed mostly of nitrate, sulfate, and ammonium and had the least elemental carbon (Rich et al. 2013). The fact that similar PM10-mortality relationships (effect size) were observed as shown in Fig. 13.7 may not reflect PM10 toxicity or effects. In these studies, PM10 concentrations may have simply served as a surrogate for exposure to the whole pollution mixture.
13.5.2 Composition of Pollution Mixture
Another issue that needs to be considered in understanding the health effects of air pollution in developing countries is the difference in the pollution mixture composition compared to that in developed countries. In most areas of developed countries, concentrations of sulfur dioxide and carbon monoxide, for example, are low and potentially below a threshold level for health effects. This is perhaps a major reason that PM has often been used in epidemiological studies and has been regarded as the outdoor air pollutant most relevant to health in estimating risk (as done in the global burden of disease assessment). In contrast, the gaseous pollutants in areas of developing countries are still high enough to cause significant health problems, or they may be a more accurate surrogate for certain air pollution sources (e.g., SO2 for coal and high-sulfur oil combustion) than PM10 or PM2.5. For example, ambient SO2 concentrations in Chinese cities have been associated with various adverse health effects, such as pathophysiological biomarkers of adverse cardiopulmonary events (Huang et al. 2012b; Rich et al. 2012), respiratory symptoms and lung function (Roy et al. 2012; Zhang et al. 2002), and low birth weight (Wang et al. 1997). The associations were stronger with SO2 than PM2.5 or PM10 in some of these studies.
13.6 Historical Lessons and Recommendations
Since the Industrial Revolution, urban air pollution has been an incessant problem. Local air quality became so severe in the early and mid-twentieth century that industrialized countries such as the U.K. and the U.S. had to establish the Clean Air Act or similar laws to protect the public from the harmful impact of air pollution. Pollution control technologies have been developed and advanced consistently to meet the increasingly stringent air quality standards. For example, although gross domestic product (GDP) and vehicle miles traveled increased by 133 % and 92 %, respectively, from 1980 (10 years after the US Clean Air Act was passed) to 2012 in the U.S., the aggregate emissions of the six criteria pollutants decreased by 67 % in the same period (see Fig. 13.8). A recent analysis of the relationship between reductions in ambient PM2.5 concentrations and increases in life expectancy in U.S. cities suggests that the 1970 Clean Air Act alone may have extended life expectancy by a half year to a year (Pope et al. 2009). The additional measures of the 1990 Clean Air Act Amendment have and will continue to reduce a number of disease burdens, as is shown in Table 13.1. The formula for this kind of success may be simply described as:
The 1970 Clean Air Act lowered emissions of key air pollutants even as energy consumption, GDP, vehicle miles, and population increased (This graph was adapted directly from the US EPA website USEPA 2014c)
Legislation + Technology + Enforcement = Clean Air
On the contrary, air quality in some developing countries such as China has actually become worse long after the establishment of national air quality standards (China’s first clean air law was passed in 1989). The key issue, hence, is not the lack of laws or regulations, but the effectiveness in enforcing the laws, pointing to the importance of “Enforcement” in the clean air success formula. Today in places like China, air pollution problems are so severe and widespread that they consistently become major topics in the press. During severe smog episodes schools are closed, people are advised to stay indoors, and people use air filters or purifiers and wear dust masks. There is strong public support to combat the air pollution. The timing for the above success formula is ideal, as developing countries do not have to go through the long history during which developed countries learned about various aspects of air pollution (e.g., sources, fate and transport, and health effects). Most importantly, developing countries have opportunities to utilize the most advanced emission control technologies that were nonexistent when developed countries experienced their worst air pollution problems.
References
Ahmad SS, Aziz N (2013) Spatial and temporal analysis of ground level ozone and nitrogen dioxide concentration across the twin cities of Pakistan. Environ Monit Assess 185(4):3133–3147
Amegah AK, Jaakkola JJ, Quansah R, Norgbe GK, Dzodzomenyo M (2012) Cooking fuel choices and garbage burning practices as determinants of birth weight: a cross-sectional study in Accra Ghana. Environ Health 11:78
Anderson HR, Atkinson R, Balbus J, Brauer M, Chapman R, Chowdhury Z, Cohen A, Demerjian KL, Ebi K, Favarato G, Greenbaum DS, Mehta S, North T, O’Keefe RM, Pandey KD, Pope CA III, Smith KR, Speizer F, Walsh M, Zhang J (2010) Outdoor air pollution and health in the developing countries of Asia: a comprehensive review. Health Effects Institute, Boston
Ashworth DC, Fuller GW, Toledano MB, Font A, Elliott P, Hansell AL, de Hoogh K (2013) Comparative assessment of particulate air pollution exposure from municipal solid waste incinerator emissions. J Environ Public Health 2013:560342
Barletta B, Meinardi S, Simpson IJ, Khwaja HA, Blake DR, Rowland FS (2002) Mixing ratios of volatile organic compounds (VOCs) in the atmosphere of Karachi Pakistan. Atmos Environ 36(21):3429–3443
Blumberg KO, Walsh MP, Pera C (2003) Low-sulfur gasoline & diesel: the key to lower vehicle emissions. Available online [April 24] at: http://www.theicct.org/documents/Low-Sulfur_Exec_Summ_ICCT_2003.pdf
Board, N.C.R.P (2013) Draft revised regional plan 2021: national capital region. Ministry of Urban Development, Government of India, New Delhi
CAI-Asia (2010) Air quality in Asia: status and trends 2010 edition. Clean Air Initiative for Asian Cities Center, Pasig City
Chen WY, Xu RN (2010) Clean coal technology development in China. Energy Policy 38(5):2123–2130
Chen R, Kan H, Chen B, Huang W, Bai Z, Song G, Pan G, Group CC (2012) Association of particulate air pollution with daily mortality: the China Air Pollution and Health Effects Study. Am J Epidemiol 175(11):1173–1181
Chung K (2013) TEL for MOGAS manufacture in China – LEAD project final report. The Lead Education and Abatement Design Group, Inc
Commission E (2014) Large combustion plants directive. Available from: http://ec.europa.eu/environment/air/pollutants/stationary/lcp/legislation.htm. 4 Nov 2014
EEA (2010) Change in CO emissions 1990–2010 (EEA member countries). Available from: http://www.eea.europa.eu/data-and-maps/figures/change-in-co-emissions-1
EPB-Beijing (2013) 2012北京市环境状况剬报 2012 Beijingshi Huanjing Zhuangkuang Gongbao [Beijing environmental statement 2012]. Beijing Environmental Protection Bureau
EPB-Shanghai (2013) 2012上海市环境状况剬报 2012 Shanghaishi Huanjing Zhuangkuang Gongbao [Shanghai environmental bulletin 2012]. Shanghai Environmental Protection Bureau
Graber LK, Asher D, Anandaraja N, Bopp RF, Merrill K, Cullen MR, Luboga S, Trasande L (2010) Childhood lead exposure after the phaseout of leaded gasoline: an ecological study of school-age children in Kampala, Uganda. Environ Health Perspect 118(6):884–889
Grebot B, Hill J, Lee J, Scarborough T (2012) Analysis and summary of the Member States’ emission inventories 2007–2009 and related information under the LCP Directive (2001/80/EC): final report. European Commission
Guttikunda SK (2009) Photochemistry of air pollution in Delhi, India: a monitoring based analysis, in SIM-air working paper series: 25–2009. Simple Interactive Models for Better Air Quality
Guttikunda SK, Kopakka RV, Dasari P, Gertler AW (2013) Receptor model-based source apportionment of particulate pollution in Hyderabad, India. Environ Monit Assess 185(7):5585–5593
Hasunuma H, Ishimaru Y, Yoda Y, Shima M (2014) Decline of ambient air pollution levels due to measures to control automobile emissions and effects on the prevalence of respiratory and allergic disorders among children in Japan. Environ Res 131:111–118
He K, Wang S, Zhang J (2009) Blood lead levels of children and its trend in China. Sci Total Environ 407(13):3986–3993
He H, Wang Y, Ma Q, Ma J, Chu B, Ji D, Tang G, Liu C, Zhang H, Hao J (2014) Mineral dust and NOx promote the conversion of SO2 to sulfate in heavy pollution days. Sci Rep 4:4172
Henderson V (2002) Urbanization in developing countries. World Bank Res Obs 17:89–112
Heritage, D.o.t.E.a. (2005) Lead (Pb): air quality fact sheet
Hodzic A, Wiedinmyer C, Salcedo D, Jimenez JL (2012) Impact of trash burning on air quality in Mexico City. Environ Sci Technol 46(9):4950–4957
Huang K, Zhuang G, Lin Y, Fu JS, Wang Q, Liu T, Zhang R, Jiang Y, Deng C, Fu Q, Hsu NC, Cao B (2012a) Typical types and formation mechanisms of haze in an Eastern Asia megacity Shanghai. Atmos Chem Phys 12(1):105–124
Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman-Strickland P, Diehl SR, Zhu P, Tong J, Gong J, Zhu T, Zhang J (2012b) Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the Beijing Olympics. Am J Respir Crit Care Med 186(11):1150–1159
Hwang SS, Kang S, Lee JY, Lee JS, Kim HJ, Han SK, Yim JJ (2014) Impact of outdoor air pollution on the incidence of tuberculosis in the Seoul metropolitan area South Korea. Korean J Intern Med 29(2):183–190
Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ (2009) Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988–2004. Pediatrics 123(3):e376–e385
Karademir A, Korucu MK (2013) Assessment of emissions and removal of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) at start-up periods in a hazardous waste incinerator. J Air Waste Manag Assoc 63(7):788–795
Klimont Z, Smith SJ, Cofala J (2013) The last decade of global anthropogenic sulfur dioxide: 2000–2011 emissions. Environ Res Lett 8(1):1–6
Levy JI, Hammitt JK, Spengler JD (2000) Estimating the mortality impacts of particulate matter: what can be learned from between-study variability? Environ Health Perspect 108(2):109–117
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260
Ma J, Gu B, He J, Shen S, Liu J, Sun Z, Yan Y, Yi Z, Lu X, Guo Y, Chang Z, Wang J, Song G, He W, Chen J, Xi T (2014) Blue sky roadmap report II: real-time disclosure begins. The Institute of Public and Environmental Affairs, Renmin University of China Institute of Environmental Policy and Planning, Society of Entrepreneurs and Ecology, Friends of Nature, Envirofriends, and Nature University Beijing, PRC
Mathee A, Rollin H, von Schirnding Y, Levin J, Naik I (2006) Reductions in blood lead levels among school children following the introduction of unleaded petrol in South Africa. Environ Res 100(3):319–322
MEP (2013a) 2012年中国环境状况剬报:大气环境 2012 Nian Zhongguo Huanjing Zhuangkuang Gongbao: Daqi Huanjing [2012 Chinese environmental condition report: air environment]. Available from: http://jcs.mep.gov.cn/hjzl/zkgb/2012zkgb/201306/t20130606_253402.htm. 6 June 2013
MEP (2013b) 2012年环境统计年报:废气 2012 Nian Huanjing Tongji Nianbao: Feiqi [2012 annual environment statistical report: air emissions]. Ministry of Environmental Protection, People’s Republic of China
NEERI (2010) Air quality assessment, emissions inventory and source apportionment studies: Mumbai. National Environmental Engineering Research Institute, New Delhi
Olajire AA, Azeez L, Oluyemi EA (2011) Exposure to hazardous air pollutants along Oba Akran road, Lagos-Nigeria. Chemosphere 84(8):1044–1051
PAHO (2005) An assessment of health effects of ambient air pollution in Latin America and the Caribbean. Pan American Health Organization (PAHO), Santiago
Ponka A, Salminen E, Ahonen S (1993) Lead in the ambient air and blood specimens of children in Helsinki. Sci Total Environ 138(1–3):301–308
Pope CA, Ezzati M, Dockery DW (2009) Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 360(4):376–386
Programme., P.f.C.F.a.V.U.N.E. (2014) Diesel fuel sulfur levels: global status April 2014. Global Clean Fuels and Vehicles Database
Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang JJ (2012) Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 307(19):2068–2078
Rich DQ, Ozkaynak H, Crooks J, Baxter L, Burke J, Ohman-Strickland P, Thevenet-Morrison K, Kipen HM, Zhang J, Kostis JB, Lunden M, Hodas N, Turpin BJ (2013) The triggering of myocardial infarction by fine particles is enhanced when particles are enriched in secondary species. Environ Sci Technol 47(16):9414–9423
Romieu I, Gouveia N, Cifuentes LA, de Leon AP, Junger W, Vera J, Strappa V, Hurtado-Diaz M, Miranda-Soberanis V, Rojas-Bracho L, Carbajal-Arroyo L, Tzintzun-Cervantes G, H.E.I.H.R. Committee (2012) Multicity study of air pollution and mortality in Latin America (the ESCALA study). Res Rep Health Eff Inst 171:5–86
Rooney MS, Arku RE, Dionisio KL, Paciorek C, Friedman AB, Carmichael H, Zhou Z, Hughes AF, Vallarino J, Agyei-Mensah S, Spengler JD, Ezzati M (2012) Spatial and temporal patterns of particulate matter sources and pollution in four communities in Accra, Ghana. Sci Total Environ 435–436:107–114
Roy A, Hu W, Wei F, Korn L, Chapman RS, Zhang JJ (2012) Ambient particulate matter and lung function growth in Chinese children. Epidemiology 23(3):464–472
Sabatini L, Pianetti A, Cecchetti G, Bruner A, Citterio B, Barbieri F, Bruscolini F (2013) Chemical and microbiological monitoring of air in two waste incineration plants. Ig Sanita Pubbl 69(1):13–37
Saini R, Masih A, Satsangi GS, Taneja A (2005) Surface levels of ozone and its precursor NO2 during winter season at urban and rural sites of a semi arid region in India. In: The atmospheric sciences and air quality conference. American Meteorological Society, San Francisco
Schuetzle D, Siegl WO, Jensen TE, Dearth MA, Kaiser EW, Gorse R, Kreucher W, Kulik E (1994) The relationship between gasoline composition and vehicle hydrocarbon emissions: a review of current studies and future research needs. Environ Health Perspect 102(Suppl 4):3
Schwartz J (2004) The effects of particulate air pollution on daily deaths: a multi-city case crossover analysis. Occup Environ Med 61(12):956–961
Simoneit BRT, Medeiros PM, Didyk BM (2005) Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ Sci Technol 39(18):6961–6970
Stieb DM, Judek S, Burnett RT (2002) Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc 52(4):470–484
Truex TJ (1999) Interaction of sulfur with automotive catalysts and the impact on vehicle emissions – a review. Proceedings of the society of automotive engineers, SAE Paper 1999-01-1543, Detroit
Tuakuila J, Kabamba M, Mata H, Mata G (2013a) Blood lead levels in children after phase-out of leaded gasoline in Kinshasa, the capital of Democratic Republic of Congo (DRC). Arch Public Health 71(1):5
Tuakuila J, Lison D, Mbuyi F, Haufroid V, Hoet P (2013b) Elevated blood lead levels and sources of exposure in the population of Kinshasa, the capital of the Democratic Republic of Congo. J Expo Sci Environ Epidemiol 23(1):81–87
UNDESA (2011) On-line data: urban agglomerations, population of urban agglomerations with 750 000 or more in 2011. Available from: http://esa.un.org/unpd/wup/unup/index_panel2.html
UNEP (2010) Final review of scientific information on lead. United Nations Environment Programme. Chemicals Branch, DTIE, Geneva, Switzerland
USEIA (2014) Short-term energy and summer fuels outlook. US Energy Information Administration, Washington, DC
USEPA (2011) The benefits and costs of the Clean Air Act from 1990–2020. US Environmental Protection Agency, Washington, DC
USEPA (2012) 2008 national emissions inventory, version 2. Technical support document. U.S. Environmental protection agency. Office of air quality planning and standards. Air quality assessment division. Emissions Inventory and Analysis Group, Research Triangle Park
USEPA (2013a) Air trends: sulfur dioxide. Available from: http://www.epa.gov/air/airtrends/sulfur.html. 3 Sept 2013
USEPA (2013b) Air trends: nitrogen dioxide. Available from: http://www.epa.gov/airtrends/nitrogen.html. 3 Sept 2013
USEPA (2014a) Air emission sources: nitrogen oxides. Available from: http://www.epa.gov/cgi-bin/broker?_service=data&_debug=0&_program=dataprog.national_1.sas&polchoice=NOX 22 Apr 2014
USEPA (2014b) Air emission sources: carbon monoxide. Available from: http://www.epa.gov/cgi-bin/broker?_service=data&_debug=0&_program=dataprog.national_1.sas&polchoice=CO. 24 Apr 2014
USEPA (2014c) Air trends: air quality trends. Available from: http://www.epa.gov/airtrends/aqtrends.html. 21 Apr 2014
von Schneidemesser E, Monks PS, Plass-Duelmer C (2010) Global comparison of VOC and CO observations in urban areas. Atmos Environ 44(39):5053–5064
Wang X, Ding H, Ryan L, Xu X (1997) Association between air pollution and low birth weight: a community-based study. Environ Health Perspect 105(5):514
Wang S, Xing J, Zhao B, Jang C, Hao J (2014) Effectiveness of national air pollution control policies on the air quality in metropolitan areas of China. J Environ Sci (China) 26(1):13–22
WHO (2000) Air quality guidelines for Europe. In: WHO regional publications, European series, no. 91. World Health Organization Regional Office for Europe, Copenhagen
WHO (2006) Air quality guidelines: global update 2005. Particulate matter, ozone, nitrogen dioxide, and sulfur dioxide. World Health Organization
Zhang JJ, Hu W, Wei F, Wu G, Korn LR, Chapman RS (2002) Children’s respiratory morbidity prevalence in relation to air pollution in four Chinese cities. Environ Health Perspect 110(9):961–967
Zhao B, Wang SX, Liu H, Xu JY, Fu K, Klimont Z, Hao JM, He KB, Cofala J, Amann M (2013) NOx emissions in China: historical trends and future perspectives. Atmos Chem Phys 13(19):9869–9897
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag London
About this chapter
Cite this chapter
Zhang, J.(., Day, D. (2015). Urban Air Pollution and Health in Developing Countries. In: Nadadur, S., Hollingsworth, J. (eds) Air Pollution and Health Effects. Molecular and Integrative Toxicology. Springer, London. https://doi.org/10.1007/978-1-4471-6669-6_13
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6669-6_13
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6668-9
Online ISBN: 978-1-4471-6669-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)