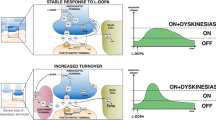
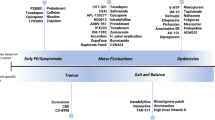
Abstract
Levodopa-induced dyskinesia represents an on-going challenge in the management of PD. Clinicians and scientists need to continue to work together in developing strategies to reduce and prevent LID. Here, we review and summarize the field to date as presented in the book and give an overview of future perspectives.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
We would like to take the opportunity to conclude this book by thanking all the authors who have contributed their time and expertise to make it a comprehensive survey of LID as it exists in 2014. As editors, it has been our pleasure to work with such esteemed, and valued, colleagues and friends in the field. We are also extremely grateful for the support and enabling role of the team at Springer who made the book possible. We would like to conclude by sharing our personal views on how we see the field moving forward. The views are solely our own and based upon experience, and perhaps prejudice, developed in our work over the last decades where we have been involved in the assessment of more than 40 potential antidyskinetic therapies in nonhuman primates and more than a dozen in clinical trials.
Throughout this book, authors have provided reviews of the state of the art with respect to clinical management (Chaps. 1, 3, 5, and 6) and understanding of the pathophysiology of LID (Chaps. 4, 7, and 8). Levodopa is still the most effective antiparkinsonian drug with least propensity for side effects and most cost-effective at improving PD patients’ quality of life. The development of LID becomes part of the “cost” of this potential improvement in PD symptoms. Despite decades of study, the pharmacological properties of levodopa preclude long-term administration of the drug in a way that does not result in LID (Chaps. 9 and 10).
There is increasing understanding that for many PD subjects, putting up with a small degree of LID is better than the opposite clinical state of being off, slow, and stiff. We know that PD patients often do not appreciate that they even have LID, and so the clinical necessity to treat such movements may be driven by family and the physician, rather than the patient themselves. However, there still exists a significant number of PD patients, for whom LID is a bothersome symptom, including young-onset patients who will need a lifetime of levodopa therapy and an increasing proportion of patients who do not tolerate levodopa-sparing agents in particular dopamine agonists. The early use of surgical options (e.g., bilateral STN DBS) is still only for a select group. There thus remain a significant number of PD patients requiring management of LID. Targeting certain individuals based on genetic propensity to develop LID (Chap. 4) may be a future option, to rationalize and optimize such therapies.
We have come to understand that there exists a panoply of neurotransmitter systems, including mu-opioid, alpha-adrenergic, 5-HT-1A, −1B and 2A serotonergic, nicotinic cholinergic, CB1 cannabinoid, and both inotropic and metabotropic glutamate receptors, that have been validated as potential antidyskinetic therapeutic targets (Chaps. 11, 12, 13, 14, 15, and 16). Such validation has been delivered in rodent and nonhuman primate models (Chap. 18) and in many cases, in proof-of-concept Phase II clinical trials. Potential therapeutics acting at these targets have in common that their anticipated mode of utilization would most likely be as adjunctive therapy to levodopa. That is, they show potential to reduce the expression of dyskinesia once it has been established, without reducing the antiparkinsonian benefits of levodopa. In this scenario, they are analogous to, and potentially an extension of, the current use of amantadine. However, these new targets offer hope to provide benefit to those patients who currently do not benefit, or receive nonoptimal benefit, from combination of dopamine replacement therapy and amantadine. A major challenge in delivering this promise appears to be successful translation from demonstration of efficacy in nonhuman primates and Phase II clinical studies into success at Phase III trials and ultimately regulatory approval for clinical use. Thus, the nonhuman primate models of LID, based upon MPTP administration and chronic levodopa therapy, have proved extremely reliable in defining compounds and target drug exposure levels that show efficacy in Phase II. Moreover, the availability of the intravenous levodopa Phase IIa trial paradigm, pioneered by Dr Chase in the 1990s and early twenty-first century, allows rapidly demonstration of clinical proof of concept of approaches with efficacy in nonhuman primate, though admittedly in a non-real-world situation, in a small number of patients. Beyond Phase II, success seems more difficult to attain; in Phase III, we only have trials showing no significant efficacy, compared to placebo, to report.
The reasons behind the lack of success beyond Phase II are likely multitude, including inappropriate dosing, trial design, and the impact of a strong placebo effect. However, these issues are now becoming the focus of investigation (for instance, the work lead by Dr Goetz, see Chap. 2). With this emerging understanding, and new validated clinical rating scales, we see clear hope that the success of preclinical science and early-stage clinical development can be capitalized upon in the coming decade (Chap. 17).
Two features of LID research described above, strong predictive value of animal models, and increasing understanding of clinical translation should de-risk the process of investing in developing therapies for LID and are beginning to make LID an attractive indication for pharmaceutical companies. This is particularly the case with respect to indication switching of compounds, where the mechanism of action of such agents overlaps with one of the validated targets for LID. In such instances, LID can represent an attractive opportunistic route for a rapid transition to demon-stration of efficacy, firstly in a nonhuman primate model and subsequently clinical proof of concept, for a compound for which the pharmacokinetic, metabolism, and safety properties are already well understood.
It is clear from the above discussion, and indeed the spectrum of transmitter systems covered in individual chapters herein, that LID is not a simple problem of enhanced dopaminergic signalling. Complex cascades of compensation, for loss of dopaminergic transmission, and plasticity, driven by pulsatile dopaminergic stimulation, impact on multiple transmitter systems and contribute to the development and expression of LID. It is clear, in both nonhuman primates and in Phase II clinical studies, where available, that the actions of agents acting on any single pharmacological target are likely to have a range of efficacy across a patient population. Thus, in any individual, the contribution of different transmitter systems to the mechanisms underlying their LID is likely idiosyncratic. A corollary of this is that no single antidyskinetic agent is likely to be able to completely suppress the expression of LID once established. It is thus, perhaps, surprising that, hitherto, therapeutic approaches modulating multiple targets have been little studied. Indeed, for most of the potential therapeutic agents/targets discussed in the preceding chapters, combination with standard clinical care, amantadine has not been rigorously investigated. As we move forward, an understanding of such interactions could prove extremely valuable. Firstly, on a purely logistical level, it could define whether clinical trials to assess efficacy of an approach should/could include patients already receiving benefit from amantadine therapy and indeed should exclude those who have not received such amantadine benefit. Such an understanding could dramatically empower our ability to demonstrate efficacy in clinical studies, for instance, optimizing power calculations of study sample size and recruitment. Secondly, and more importantly, by overlooking potential interactions between different targets, we may be missing significant opportunities for synergy and improved efficacy. The idea of developing therapies that combine actions at multiple targets is beginning to gain traction in the serotonergic space. Thus, there is some perception that combination of 5-HT1A and 5-HT1B agonists might, by allowing the use of lower doses of both, be able to deliver benefits of both targets while minimizing any adverse effects of one or the other. One approach to this is to develop compounds that are multifunctional, acting on more than one receptor. The problem we envisage with this approach is that it seems unlikely that a single molecule can capture the relative combination of multiple receptor blockade/stimulation that would provide optimal efficacy. This is compounded by our impression that there is likely no single optimal dose for any compound across a population. Certainly, in nonhuman primates, we find that the lowest effective dose of drug, in terms of antidyskinetic action, varies by a factor of tenfold or more even within a study. For a combination of two more targets, such variability would be compounded. A more attractive approach to modulating multiple targets is, to our minds, polypharmacy where the dose of each agent can be tailored/titrated to an individual’s response. This might be achieved with a therapy combining multiple active molecules, though this is associated with multiple development challenges and, as with the single multifunctional molecule, a combination therapy may be limited by being only available in one, or at least invariable, combinations. We therefore propose that the therapeutic landscape for LID will/should evolve in a way in which multiple drugs/targets are developed in parallel and that the armamentarium should be built organically. As compounds are developed, studies in nonhuman primates should be expanded to focus on synergy, additivity, or lack of, between targets and also on defining whether certain populations of patients might befit more than others from drugs for a particular target. With respect to the latter, it is already clear that some classes of drug act preferentially to reduce LID of a choreic, rather than dystonic, phenotype, and vice versa, while others reduce dyskinesia elicited by levodopa but not dopamine receptor agonists. At present, such considerations are rarely taken into account when transitioning a development project from nonhuman primate to clinical development but could become even more important in defining how to employ therapies once they reach the market. Moreover, as agents other than amantadine become available, neurologists will learn in an empirical manner which patients respond best to which combinations, and best practice for combining the multiple pharmacologies will become defined.
The discussion above, and indeed the vast majority of the chapters preceding, has focussed primarily upon the issue of understanding and managing LID once it has become established. A major opportunity to reduce the impact of LID on patients with PD exists if we can prevent, de novo, the development of LID once dopamine replacement therapy is initiated. Over the last decade, and more, the issue of continuous dopaminergic stimulation to prevent the development of dyskinesia has gained much attention. However, to date, it has proved impossible to deliver antiparkinsonian benefits equivalent to levodopa while avoiding the development of LID. Alternative strategies should be investigated, and we have been attracted to the potential of adjunctive therapies that leverage the antiparkinsonian benefit of levodopa but combine levodopa with an agent that reduces its propensity to lead to the development of LID. Compounds with potential to achieve this goal, as indicated by nonhuman primate studies, include those acting as A2A adenosine receptor, D3 dopamine receptor, and NR2B NMDA receptor antagonists. Indeed, we have long espoused that such an indication represents the biggest opportunity for A2A adenosine antagonists in PD. It should be noted that of these three potential therapeutic targets for reducing the development of LID, none would be considered, by us at least, as having significant potential in reducing LID once it has been established. Thus, we note that the pharmacology of the development of LID is very different than that of its expression. Moreover, this leads us to believe that separate paths of drug development are needed to prevent rather than diminish previously established dyskinesia. One attraction of developing agents to prevent the development of LID is that, unlike approaches to suppress established LID, which as discussed above, have yet to succeed at Phase III, a path through Phase III to market has already been demonstrated for de novo therapy that leads to reduced development of LID, for dopamine agonists. However, none of the three targets for preventing development of LID proposed above has been investigated for such potential in clinical studies. The reasons for this are likely not solely scientific. The magnitude of a clinical proof-of-concept study, likely several hundred patients over 3–5 years, is perceived as too large to justify the potential investment for anticipated reward. We feel this undervalues the impact of LID, both from a clinical perspective and also from a commercial market perspective. In the absence of a true disease-modifying agent in PD, an agent that was able to prevent development of LID while allowing the antiparkinsonian benefit of levodopa would form the basis of product with potential for annual sales in excess of $1bn. A major challenge that faces us today is to convince our partners and colleagues in the pharmaceutical sector that a de novo therapy to slow or prevent LID development to represent an unmet need with potential impact equivalent to a disease-modifying therapy or a symptomatic therapy in a disorder with greater incidence than PD. The translatability of our animal models and their value in de-risking investment should help in this respect.
In conclusion, the reviews presented through this book illustrate the significant advances that have been made in LID over recent years. They highlight the rapidly changing face of this important disease area. The discussion presented in this last chapter is given to encourage thought and debate and highlight the opportunities that remain ahead of us.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Brotchie, J.M., Fox, S.H. (2014). Final Thoughts: Summary and Future Therapeutic Strategies in Levodopa-Induced Dyskinesia. In: Fox, S., Brotchie, J. (eds) Levodopa-Induced Dyskinesia in Parkinson's Disease. Springer, London. https://doi.org/10.1007/978-1-4471-6503-3_19
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6503-3_19
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6502-6
Online ISBN: 978-1-4471-6503-3
eBook Packages: MedicineMedicine (R0)