Abstract
The endocannabinoid system modulates the release of excitatory and inhibitory neurotransmitters in several brain areas implicated in motor control. Cannabinoid and dopamine receptors are highly abundant and often co-expressed in the basal ganglia circuitry, and the cross talk between these two systems regulates short- and long-term synaptic plasticity in the striatum. Dysregulation of the endocannabinoid system has been reported in animal models of Parkinson’s disease and parkinsonian patients and is exacerbated in dyskinetic states, following chronic levodopa administration.
This chapter reviews recent investigations on the relationships between endocannabinoids and other neurotransmitter/neuromodulator systems in the basal ganglia, with the intent to underline their relevance for the pathophysiology of levodopa-induced dyskinesia and discuss new pharmacological approaches for their treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Although levodopa remains the gold standard for the treatment of motor symptoms in Parkinson’s disease (PD), its long-term use leads to the development of abnormal involuntary movement, collectively termed dyskinesia, in as many as 90–95 % of PD patients receiving treatment [1–3].
The molecular mechanisms associated with LID development are not fully understood, but several factors, including neurotransmitter abnormalities, pulsatile stimulation of dopamine receptors, and maladaptive plasticity within the striatum, are known to play a role [4].
To date, the only FDA-approved drug for the treatment of dyskinesia is the NMDA antagonist amantadine [5, 6]. This drug, however, has a short therapeutic time window, is poorly tolerated, and can worsen dyskinesia upon discontinuation or induce psychiatric complications [5, 7]. Thus, there is an urgent need to develop alternative antidyskinetic therapies targeting non-dopaminergic systems to avoid possible interferences with the antiparkinsonian effects of L-DOPA.
In the last decade, several studies have pointed to the endocannabinoid system as an important modulator of synaptic transmission and plasticity in the basal ganglia circuitry. As this system regulates dopamine-induced motor activation and is required for the coordination and fine-tuning of movement [8, 9], it represents a potential pharmacological target for the treatment of motor disorders. Indeed, both exogenous and endogenous cannabinoids show antiparkinsonian and antidyskinetic activity in animal models of PD and patients.
In this chapter, we will review the most relevant studies on the role played by the endocannabinoid system in LID, discuss the complex interactions between endocannabinoids and several neurotransmitters regulating basal ganglia function [10, 11], and provide a conceptual frame to address some conflicting findings reported in the literature.
The Endocannabinoid System
The endocannabinoid system consists of a family of lipid signaling molecules (endocannabinoids) released on demand from membrane lipid precursors, the enzymes responsible for their synthesis and degradation and distinct metabotropic (cannabinoid), ionotropic, and nuclear receptors activated by these ligands [12, 13].
Among the multiple endocannabinoids identified so far [14], arachidonoyl ethanolamine (anandamide) [15, 16] and 2-arachidonoyl glycerol (2-AG) [17] represent the two most studied examples.
Anandamide is synthesized in a Ca++-dependent manner from N-arachidonoyl phosphatidylethanolamine by phospholipase D (PLD) [18, 19] or via alternative pathways, such as those initiated by alpha-beta-hydrolase 4 [20]. 2-AG is produced by diacylglycerol lipases (DAGLα and β) acting on membrane acyl arachidonoyl glycerols [21, 22]. As in the case of anandamide, multiple biosynthetic pathways have been reported for 2-AG, which can also derive from the hydrolysis of phosphatidic acid or lysophospholipids [23, 24].
The biological actions of anandamide are terminated by facilitated diffusion into cells via a carrier-mediated transport [25], followed by enzymatic hydrolysis via a fatty acid amide hydrolase (FAAH) [26–28]. To date, there is no consensus on the existence of an endocannabinoid transporter [29], and its molecular identity has not been yet identified. Anandamide can be also metabolized by lipoxygenases [30] and cyclooxygenases (such as COX-2) [31–33]. In particular, the COX-2 metabolic pathway may become physiologically relevant under conditions promoting endocannabinoid or COX-2 upregulation, as in the course of neurodegenerative processes [34].
Concerning 2-AG, although this lipid can be metabolized by FAAH and cyclooxygenases [35, 36], in the brain it is mainly hydrolyzed by a monoacylglycerolipase (MAGL), which is localized in presynaptic elements [37]. Interestingly, pharmacological blockade of FAAH by URB597 may decrease brain 2-AG in vitro via a mechanism involving TRPV1 activation and DAGL inhibition [38, 39]. This decrease, however, has not been confirmed in vivo by other groups [40–43], suggesting that it might be limited to specific brain areas.
The endocannabinoids can activate Gi/o protein-coupled cannabinoid receptors (CB1 and CB2), some members of the transient receptor potential (TRP) family, as well as nuclear peroxisome proliferator-activated receptors (PPAR) [44]. Endocannabinoids can also serve as allosteric modulators or bind to other metabotropic receptors, including GPR55 [45–47] – a cloned orphan receptor activated by the CB1 antagonists rimonabant and AM251 [48] – and GPR18 [49]. However, the physiological roles of these receptors remain unknown, and neither anandamide nor 2-AG has shown consistent pharmacological effects following GPR55 stimulation [50].
In rodents and humans, CB1 receptors are highly expressed in the peripheral and central nervous system (CNS) [51, 52], whereas CB2 receptors are mainly restricted to immunocompetent cells, lymphoid organs, and microglia [44, 53, 54]. The “segregation” of CB2 to the immune system has been challenged by recent studies showing their presence in neurons and glial cells throughout the brain, including the substantia nigra pars reticulata (SNpr) and the striatum [55–58]. Also, CB2 receptors are upregulated in activated microglia and astrocytes in response to neurotoxic insults and neuroinflammatory events [59–62].
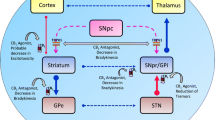
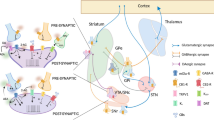
Within the basal ganglia, CB1 receptors are generally expressed on presynaptic elements, including GABAergic striatofugal neurons [63, 64], striatal parvalbumin-positive interneurons [65, 66], glutamatergic terminals from the cortex [67] and the subthalamic nucleus [68], and serotonergic afferents [69, 70] (see Fig. 14.1). It is now well established that activation of presynaptic CB1 receptors by retrogradely mobilized endocannabinoids inhibits the release of several neurotransmitters involved in basal ganglia function [71, 72].
Schematic illustration of the basal ganglia motor circuit showing striatofugal “direct” and “indirect” projections to the output nuclei and afferent projections from the cortex and raphe nuclei to the striatum. GPi globus pallidus pars interna, GPe globus pallidus pars externa, SNpc substantia nigra pars compacta, SNpr substantia nigra pars reticulata, STN subthalamic nucleus. Glutamatergic (green), GABAergic (red), and serotonergic projections and expression of different receptor subtypes are also indicated
Endocannabinoids and CB1 receptors have been implicated in three main forms of plasticity at striatal synapses: (1) short-term depolarization-induced suppression of excitation (DSE) or inhibition (DSI), (2) short-term depression dependent by activation of postsynaptic Gq-coupled receptors, and (3) long-term depression (LTD) (for review, see [73]). Also, concomitant activation of CB1 and other metabotropic receptors can promote the coupling of CB1 to G isoforms other than Gi/o [74, 75] or the formation of heterodimers with D2 and mu-opioid receptors [76, 77], leading to different downstream signaling pathways than those traditionally activated by cannabinoids.
Studies on cannabinoid agonists administered to CB1 knockout mice support the existence of non-CB1/CB2 receptors regulating synaptic transmission throughout the body (for review, see [52]).
As previously mentioned, some exogenous and endogenous cannabinoids can target at least five distinct TRP channels [78]. In particular, anandamide can bind to TRPV1 receptors [79], which are expressed in the striatum, globus pallidus, and the substantia nigra pars compacta (SNpc) [80–82]. As anandamide affinity for TRPV1 is quite low, it is not clear whether this lipid might serve as an endovanilloid ligand under physiological conditions [83, 84]. Nevertheless, blockade of FAAH activity has been shown to enhance anandamide potency at TRPV1 receptors in vitro [85]. In addition, there is evidence for a cross talk between CB1 and TRPV1 receptors, as CB1 stimulation can alter the phosphorylation state of TRPV1 and consequently its function [86].
Some cannabinoid compounds, including anandamide, noladin ether, virodhamine, and WIN55,212-2, can also bind different subtypes of PPAR receptors and enhance the expression of their target genes [87]. In particular, anandamide has been shown to activate PPARα [88] and PPARγ [89]. These receptors, which are known to increase insulin sensitivity and modulate glucose and lipid metabolism, are also expressed in neuronal and glial cells of the basal ganglia [90, 91]. Although their role in the CNS is still largely unexplored, recent studies indicate that PPARα and PPARγ agonists have antioxidant and neuroprotective activity in animal models of PD [92–95], Alzheimer’s disease [96, 97], cerebral ischemia [98], and traumatic brain injury [99, 100], and they can reverse haloperidol-induced oral dyskinesia in rats [101].
Pharmacological Effects
Effects on PD Motor Symptoms
In general, systemic administration of exogenous cannabinoids, or enhancement of endocannabinoid tone via pharmacological blockade of their catabolic enzymes or reuptake, decreases locomotor activity in a CB1-dependent manner [28, 102–104]. In line with these observations, CB1 knockout mice exhibit motor abnormalities [105, 106] and suppression of cocaine-induced hyperlocomotion [107]. However, some of the cannabinoid-induced motor effects are not elicited via activation of CB1 receptors. For instance, anandamide produces catalepsy in both CB1 knockout mice and wild-type controls [108], and elevation of endocannabinoid tone in these animals produces hypokinesia via a TRPV1-mediated mechanism [109]. Also, pharmacological blockade of TRPV1 receptors in 6-OHDA rats has been shown to unmask the antidyskinetic effects of the FAAH inhibitor URB597 [103] (see below). These observations suggest that, under conditions in which anandamide reaches supraphysiological concentrations and consequently activates TRPV1, these channels can influence motor behaviors presumably by affecting the firing rate of nigrostriatal neurons and dopamine transmission [110].
In the context of PD, several research groups have found increased CB1 mRNA and receptor binding in the striatum of animal models [111, 112] and PD patients [113]. Numerous studies have also shown abnormal endocannabinoid levels, although there is no consensus on the direction of endocannabinoid fluctuations. While some reports indicate an increase of endocannabinoid levels in the basal ganglia of dopamine-depleted rodents [114–116], other studies showed decreased or unaltered endocannabinoid tone [103, 117, 118]. These discrepancies may be attributable to species-specific differences among PD models or to the physiological state of the animals at the time of the experiments, which is known to affect endocannabinoid release. Interestingly, the administration of levodopa to 6-OHDA rats failed to elevate anandamide levels [103, 117] and further increased CB1 expression in the striatum [119], suggesting that levodopa is not able to correct the endocannabinoid dysfunction associated with dopamine denervation. This dysfunction likely causes the disruption of the plasticity observed at corticostriatal synapses in PD models [118–121]. In this regard, elevation of endocannabinoid tone has been shown to rescue striatal LTD and to alleviate motor deficits associated with the nigrostriatal lesion [118]. Although these data point to a deficit (rather than an enhancement) of endocannabinoid mobilization in PD, improvement of motor symptoms has been achieved not only with administration of cannabinoid agonists but also with CB1 receptor antagonists in either rodents [122–124] or nonhuman primates [125] (Table 14.1). Explaining these paradoxical findings is challenging, although the answer may lie in the multiple site of actions engaged by cannabinoid drugs when administered systemically. Indeed, while increased endocannabinoid transmission may alleviate PD symptoms by reducing striatal glutamate release [71, 115], on the other hand, activation of CB1 on striatofugal terminals of the “indirect” pathway may lead to increased GABAergic drive to the external globus pallidus (GPe), which may amplify the inhibitory output of the basal ganglia and consequently contribute to PD symptoms. Therefore, in this case, CB1 antagonism may produce antiparkinsonian effects by limiting GABA release from striatopallidal projections. Finally, other studies have hypothesized that CB1 antagonists elicit antiparkinsonian effects only in animals with severe nigrostriatal lesions [123, 126], which may differentially affect endocannabinoid production and CB1 expression in the striatum and GPe of these animals versus those with less severe lesions.
Effects on LID
As endocannabinoids counteract dopamine-mediated hyperactivity [103, 136, 137] and given the fact that increased corticostriatal glutamate transmission contributes to dyskinesias [138, 139], stimulation of CB1 receptors should alleviate dyskinetic symptoms by (1) reducing levodopa-induced sensitization of dopamine receptors, (2) normalizing aberrant glutamate release, and (3) rebalancing maladaptive plasticity in the denervated striatum. In support of this hypothesis, several groups have shown cannabinoid-mediated improvement of levodopa-induced abnormal involuntary movements (AIMs) in rodent models and nonhuman primates [103, 118, 128, 131, 132] and PD patients [129] (Table 14.1).
The antidyskinetic effects of cannabinoid agonists do not seem to result from a generalized motor suppression, as they were obtained using doses that did not produce hypomotility or catalepsy [103, 126]. Nevertheless, as in the case of PD motor deficits, significant antidyskinetic effects [125, 130], or no effects [134], were also observed with CB1 antagonists (Table 14.1). The rationale for blocking CB1 receptors as a pharmacological approach to treat dyskinesia is based on the observations that endocannabinoid transmission is elevated in dyskinetic animals [140] and PD patients [113] and that genetic deletion of CB1 receptors prevents the development of severe abnormal movements in mice [140]. However, neither striatal endocannabinoid levels nor CB1 upregulation has been correlated to LID expression or severity [125, 141].
Overall, these discrepancies reveal some limitations in generalizing cannabinoid effects across different animal models and may be ascribed to the multiple sites of action of cannabinoid agents (see above), which complicate the translation of these findings into new pharmacotherapies.
So far, studies carried out in PD patients have been inconclusive. While a randomized, double-blind, placebo-controlled pilot study by Sieradzan et al. [129] has shown an antidyskinetic action of the cannabinoid agonist nabilone in PD patients [129], other reports have not confirmed any beneficial effects of either cannabinoid agonists [127] or antagonists [133] on LID. However, the study of Carroll et al. [127] evaluated the effects of oral cannabis, which has a highly variable pharmacokinetics and a more complex pharmacological profile than synthetic cannabinoid agonists. In addition, the assessment of dyskinesia was based on patient self-reported questionnaires, which are often inaccurate in identifying symptoms [142]. On the other hand, the dose of the CB1 antagonist rimonabant used in the study of Mesnage et al. [133] was significantly lower than that used by van der Stelt and coworkers [125]. Thus, new and larger-scale clinical studies are necessary to confirm the antidyskinetic properties of cannabinoid agents in humans.
Pharmacological blockade of FAAH, which elevates anandamide and other acylethanolamides in those brain areas where they are actively synthesized, did not reduce levodopa-induced AIMs in 6-OHDA rats [103]. These findings suggest that increasing anandamide tone is not sufficient to alleviate dyskinesia, possibly because of the concomitant stimulation of CB1 and TRPV1 receptors, which exert opposite effects within the basal ganglia circuitry. In support of this hypothesis, coadministration of the FAAH inhibitor URB597 and the TRPV1 antagonist capsazepine produced a significant antidyskinetic effect in 6-OHDA rats [103, 131]. In addition, a recent study by Gonzalez-Aparicio [143] has shown that oleylethanolamide (OEA), a structural analog of anandamide that does not bind to CB1 but has antagonistic activity at TRPV1 receptors, can reduce levodopa-induced AIM via a TRPV1-mediated mechanism [135]. These observations differ from those reported by Lee et al. [143], showing that the administration of either URB597 or the TRPV1 agonist capsaicin alone reduced levodopa-induced hyperactivity in reserpine-treated rats [143]. However, it is important to note that hyperactivity in reserpine-treated rodents has not been validated as an appropriate measure of dyskinesia [144].
Although TRPV1 blockade seems necessary to unmask the antidyskinetic effect of URB597, the beneficial action of this drug is only partially mediated by CB1 receptors, since pretreatment with the CB1 antagonist AM251 did not fully reverse the combined effect of URB597 and capsazepine (CPZ) [103]. Interestingly, administration of the nonselective PPAR antagonist BADGE completely blocked the URB597 + CPZ antidyskinetic effect (unpublished observations), suggesting a PPAR-dependent mechanism. Whether the involvement of PPAR in this response reflects a direct action of anandamide, or of other lipid signaling molecules elevated by FAAH blockade, on these nuclear receptors is still unclear. Nevertheless, a recent study has shown that PPARα and PPARγ agonists administered individually or in combination with antipsychotics can alleviate haloperidol-induced oral dyskinesias [101].
Endocannabinoid Modulation of Basal Ganglia Circuitry: Pathophysiology and Implications for LID
According to the classical model of basal ganglia organization (see Fig. 14.1), striatal MSN receive excitatory glutamatergic projections from the cerebral cortex. MSN are in turn modulated by nigrostriatal dopaminergic afferents that exert excitatory or inhibitory effects on “direct” and “indirect” striatofugal pathways via dopamine D1 and D2 receptors, respectively.
Although CB1 are not present on dopaminergic neurons [145], they co-localize with D1/D2-like receptors in the dorsal striatum and indirectly affect dopamine output by modulating neurotransmitter release from projecting inhibitory and excitatory terminals via stimulation of CB1 receptors [64, 67, 68, 72, 146, 147]. The overall effect of cannabinoids on dopamine release in the caudate-putamen remains controversial, as some studies have shown a decrease [72], an increase [148], or no effect at all [149, 150]. Anandamide- and endocannabinoid-enhancing drugs, such as FAAH inhibitors, can also modulate nigrostriatal dopamine transmission by acting at TRPV1 [109, 110, 151, 152] or PPAR receptors [153].
Stimulation of dopamine D1- and D2-like receptors has been shown to affect striatal endocannabinoids in opposite ways: for example, while D1 agonists tend to decrease anandamide [154], D2-like agonists increase it [103, 117, 136, 155]. These effects may depend on the ability of D1 and D2 agonists to enhance or diminish excitatory postsynaptic currents in striatal MSN, respectively, and suggest a dopamine-mediated control of endocannabinoid mobilization [156]. Indeed, studies have shown that LTD at corticostriatal synapses is regulated by D2 receptors [118, 157]. Although the precise site of this modulation is still the subject of debate, it appears to be restricted to glutamatergic projections onto MSN of the indirect pathway [118, 158] and to be mediated by anandamide or 2-AG, depending on the frequency of stimulation applied to the glutamatergic afferents [159–161].
Endocannabinoids, in particular anandamide, also mediate synaptic depression at GABAergic afferents onto striatal MSN [155, 162–164] to produce disinhibition of MSN activation.
Interestingly, endocannabinoid-mediated LTD at corticostriatal synapses is profoundly compromised after striatal dopamine denervation [118] or blockade of D2 receptors [157, 165, 166] and completely lost in dyskinetic – but not in non-dyskinetic – parkinsonian rats treated with levodopa [167].
In line with these observations, behavioral studies indicate that the anandamide elevation observed after administration of dopaminergic agonists may serve as an inhibitory feedback signal to offset dopamine-induced hyperactivity [136, 137, 168]. Thus, abnormalities in dopamine and endocannabinoid-mediated plasticity may disrupt this feedback mechanism and lead to motor disturbances, particularly upon long-term activation of dopamine receptors.
Recent studies have added a further level of complexity, showing a competitive interaction between dopamine D2 and adenosine A2A receptors in the induction of endocannabinoid-mediated plasticity, such that D2 receptor activation promotes LTD, whereas A2A activation promotes LTP [158, 169]. Also, coadministration of A2A and CB1 agonists has been shown to partially inhibit the CB1-dependent decrease of glutamate transmission [170]. The presence of A2A receptors on glutamatergic terminals projecting onto MNS spines [171] suggests that these might be the anatomical substrate for these complex interactions.
CB1 receptors are also expressed on serotonergic raphe-striatal fibers [69] (Fig. 14.1), which are able to (1) convert levodopa into dopamine and release it as a “false neurotransmitter,” thus contributing to LID development [172]; (2) influence nigrostriatal dopamine release [173]; and consequently (3) affect the dopamine-mediated and CB1-dependent control of glutamate release [174]. Therefore, we could speculate that cannabinoid agents may exert their antidyskinetic effects by dampening the ectopic dopamine release from serotonergic terminals and/or by controlling dopamine transmission indirectly via inhibition of 5-HT release [175, 176].
Molecular Mechanisms
Overactivity of D1-positive striatofugal neurons of the direct pathway has been long known to be involved in LID [177–179]. Dopamine denervation leads to a high-affinity D1 receptor state in 6-OHDA rats [180, 181], and D1 agonist-induced GTPγS binding has been correlated with LID severity in MPTP-treated primates [182]. D1 overactivity is also accompanied by dysregulation of the cAMP/protein kinase A (PKA) signaling cascade and increased signaling of the dopamine- and cAMP-regulated phosphoprotein-32 kDa (DARPP-32), a key integrator of dopaminergic and glutamatergic inputs in the striatum [131, 183, 184].
Administration of the cannabinoid agonist WIN55,212-2 has been shown to alleviate levodopa-induced AIM in 6-OHDA rats and to reverse the concomitant overactivity of striatal PKA [131]. In keeping with these observations, blockade of PKA signaling has been proven as an effective strategy to reduce AIM expression [185, 186], possibly by preventing PKA-mediated cytoskeleton modifications, which may contribute to the long-term aberrant plasticity underlying striatal dysfunction in dyskinesia [185, 187]. The reduction of PKA activity elicited by cannabinoids may result from the direct activation of CB1 receptors, which are negatively linked to adenylyl cyclase and co-localized on D1-positive striatal neurons [69].
PKA-induced phosphorylation at the threonine (Thr)-34 site converts DARPP-32 into an inhibitor of protein phosphatase-1 (PP1) [188]. Although DARPP-32 phosphorylation appears to be required for the expression of CB1-mediated motor effects, such as catalepsy [189], WIN55,212-2 administration to dyskinetic rats produced a dephosphorylation of DARPP-32 at Thr-34 that was only partially reversed by the CB1 antagonist AM251 even at doses that fully blocked WIN55,212-2 antidyskinetic effect [131]. This discrepancy may depend on different biochemical or functional aspects underlying the behaviors measured in these studies (catalepsy versus AIM) and/or, as previously mentioned, on species-specific differences among animal models. Interestingly, Polissidis et al. [190] have shown that WIN55,212-2 can produce opposite effects on striatal Thr-34 phosphorylation across different rat strains [190].
Concluding Remarks
Experimental evidence indicates that systemic administration of cannabinoids reverses the aberrant levodopa-induced overactivity of downstream signaling that may lead to long-term maladaptive changes in striatal plasticity. However, both direct (or indirect) cannabinoid agonists and antagonists have shown antidyskinetic actions in preclinical models, and experimental evidence for their efficacy in clinical settings is still limited. Given the modulatory action played by the endocannabinoid system in the basal ganglia, understanding its dysfunction in PD and reconciling conflicting data may have important implications for the pathophysiology and treatment of levodopa-associated motor complications.
In addition, the therapeutic potentials of modulating endocannabinoid levels or targeting non-CB receptors activated by endocannabinoids, such as TRP channels and PPAR receptors, have not been fully explored. These approaches may offer more effective and specific pharmacological actions than those observed with traditional cannabinoid agents. Furthermore, as some of these drugs have shown anti-inflammatory and neuroprotective properties in the CNS [191], their application in PD therapy appears particularly appealing, as they may delay/halt the progressive neurodegenerative process occurring in this pathology.
References
Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–58.
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22:1379–89.
Hely MA, Morris JG, Reid WG, Trafficante R. Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord. 2005;20:190–9.
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222.
Verhagen Metman L, et al. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998;50:1323–6.
Del Dotto P, et al. Intravenous amantadine improves levodopa-induced dyskinesias: an acute double-blind placebo-controlled study. Mov Disord. 2001;16:515–20.
Sawada H, et al. Amantadine for dyskinesias in Parkinson’s disease: a randomized controlled trial. PLoS One. 2010;5:e15298.
Benarroch E. Endocannabinoids in basal ganglia circuits: implications for Parkinson disease. Neurology. 2007;69:306–9.
Morera-Herreras T, Miguelez C, Aristieta A, Ruiz-Ortega JA, Ugedo L. Endocannabinoid modulation of dopaminergic motor circuits. Front Pharmacol. 2012;3:110.
Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462.
Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–12.
Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80.
O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–82.
Lopez-Moreno JA, Gonzalez-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol. 2008;13:160–87.
Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9.
Di Marzo V, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–91.
Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90.
Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–42.
Sugiura T, et al. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: involvement of Ca(2+)-dependent transacylase and phosphodiesterase activities. Biochem Biophys Res Commun. 1996;218:113–7.
Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–72.
Bisogno T, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8.
Basavarajappa BS. Neuropharmacology of the endocannabinoid signaling system-molecular mechanisms, biological actions and synaptic plasticity. Curr Neuropharmacol. 2007;5:81–97.
Carrier EJ, et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007.
Sugiura T, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97.
Beltramo M, et al. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–7.
Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7.
Wei BQ, Mikkelsen TS, McKinney MK, Lander ES, Cravatt BF. A second fatty acid amide hydrolase with variable distribution among placental mammals. J Biol Chem. 2006;281:36569–78.
Giuffrida A, McMahon LR. In vivo pharmacology of endocannabinoids and their metabolic inhibitors: therapeutic implications in Parkinson’s disease and abuse liability. Prostaglandins Other Lipid Mediat. 2010;91:90–103.
Hillard CJ, Jarrahian A. Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol. 2003;140:802–8.
Hampson AJ, et al. Anandamide hydroxylation by brain lipoxygenase:metabolite structures and potencies at the cannabinoid receptor. Biochim Biophys Acta. 1995;1259:173–9.
Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–6.
Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–8.
Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007;152:594–601.
Vila M, et al. The role of glial cells in Parkinson’s disease. Curr Opin Neurol. 2001;14:483–9.
Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73.
Kozak KR, et al. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–85.
Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–24.
Justinova Z, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–7.
Maccarrone M, et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–9.
Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81.
Gobbi G, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–5.
Maione S, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–82.
Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2010;13:373–86.
Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:4–15.
Begg M, et al. Evidence for novel cannabinoid receptors. Pharmacol Ther. 2005;106:133–45.
Sharir H, et al. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J Neuroimmune Pharmacol. 2012;7:856–65.
Henstridge CM, et al. Minireview: recent developments in the physiology and pathology of the lysophosphatidylinositol-sensitive receptor GPR55. Mol Endocrinol. 2011;25:1835–48.
Johns DG, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–31.
McHugh D, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:44.
Yin H, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–38.
Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318.
Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8:E298–306.
Walter L, Stella N. Cannabinoids and neuroinflammation. Br J Pharmacol. 2004;141:775–85.
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci. 2005;25:1904–13.
Van Sickle MD, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32.
Gong JP, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23.
Suarez J, et al. Early maternal deprivation induces gender-dependent changes on the expression of hippocampal CB(1) and CB(2) cannabinoid receptors of neonatal rats. Hippocampus. 2009;19:623–32.
Onaivi ES, Ishiguro H, Gu S, Liu QR. CNS effects of CB2 cannabinoid receptors: beyond neuro-immuno-cannabinoid activity. J Psychopharmacol. 2012;26:92–103.
Benito C, et al. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci. 2003;23:11136–41.
Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem. 2005;95:437–45.
Price DA, et al. WIN55,212-2, a cannabinoid receptor agonist, protects against nigrostriatal cell loss in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:2177–86.
Palazuelos J, et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132:3152–64.
Wallmichrath I, Szabo B. Cannabinoids inhibit striatonigral GABAergic neurotransmission in the mouse. Neuroscience. 2002;113:671–82.
Martin AB, et al. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology. 2008;33:1667–79.
Fusco FR, et al. Immunolocalization of CB1 receptor in rat striatal neurons: a confocal microscopy study. Synapse. 2004;53:159–67.
Uchigashima M, et al. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–76.
Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–71.
Morera-Herreras T, Ruiz-Ortega JA, Gomez-Urquijo S, Ugedo L. Involvement of subthalamic nucleus in the stimulatory effect of Delta(9)-tetrahydrocannabinol on dopaminergic neurons. Neuroscience. 2008;151:817–23.
Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–60.
Haring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–9.
Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–51.
Sidlo Z, Reggio PH, Rice ME. Inhibition of striatal dopamine release by CB1 receptor activation requires nonsynaptic communication involving GABA, H2O2, and KATP channels. Neurochem Int. 2008;52:80–8.
Mathur BN, Lovinger DM. Endocannabinoid-dopamine interactions in striatal synaptic plasticity. Front Pharmacol. 2012;3:66.
Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–33.
Mukhopadhyay S, Howlett AC. Chemically distinct ligands promote differential CB1 cannabinoid receptor-Gi protein interactions. Mol Pharmacol. 2005;67:2016–24.
Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–704.
Hojo M, et al. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci. 2008;108:308–19.
Akopian AN, Ruparel NB, Jeske NA, Patwardhan A, Hargreaves KM. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol Sci. 2008;30:79–84.
Caterina MJ, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24.
Mezey E, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–60.
Micale V, et al. Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology. 2009;34:593–606.
Cristino L, et al. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–15.
Szolcsanyi J. Are cannabinoids endogenous ligands for the VR1 capsaicin receptor? Trends Pharmacol Sci. 2000;21:41–2.
Zygmunt PM, Julius I, Di Marzo I, Hogestatt ED. Anandamide – the other side of the coin. Trends Pharmacol Sci. 2000;21:43–4.
De Petrocellis L, et al. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–63.
Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33.
Sun Y, Bennett A. Cannabinoids: a new group of agonists of PPARs. PPAR Res. 2007;2007:23513.
Sun Y, et al. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–43.
Bouaboula M, et al. Anandamide induced PPARgamma transcriptional activation and 3 T3-L1 preadipocyte differentiation. Eur J Pharmacol. 2005;517:174–81.
Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–45.
Cimini A, et al. Expression of peroxisome proliferator-activated receptors (PPARs) and retinoic acid receptors (RXRs) in rat cortical neurons. Neuroscience. 2005;130:325–37.
Carta AR, et al. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience. 2011;194:250–61.
Carroll CB, Zeissler ML, Hanemann CO, Zajicek JP. Delta(9)-tetrahydrocannabinol (Delta(9)-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol Appl Neurobiol. 2012;38:535–47.
Swanson CR, et al. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91.
Galan-Rodriguez B, et al. Oleoylethanolamide exerts partial and dose-dependent neuroprotection of substantia nigra dopamine neurons. Neuropharmacology. 2009;56:653–64.
Xiang GQ, et al. PPARgamma agonist pioglitazone improves scopolamine-induced memory impairment in mice. J Pharm Pharmacol. 2012;64:589–96.
Dhikav V, Anand K. Potential predictors of hippocampal atrophy in Alzheimer’s disease. Drugs Aging. 2011;28:1–11.
Medhi B, Aggarwal R, Chakrabarti A. Neuroprotective effect of pioglitazone on acute phase changes induced by partial global cerebral ischemia in mice. Indian J Exp Biol. 2010;48:793–9.
Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388:7–12.
Thal SC, et al. Pioglitazone reduces secondary brain damage after experimental brain trauma by PPAR-gamma-independent mechanisms. J Neurotrauma. 2011;28:983–93.
Grover S, Kumar P, Singh K, Vikram V, Budhiraja RD. Possible beneficial effect of peroxisome proliferator-activated receptor (PPAR)–alpha and gamma agonist against a rat model of oral dyskinesia. Pharmacol Biochem Behav. 2013;111:17–23.
Compton DR, Martin BR. The effect of the enzyme inhibitor phenylmethylsulfonyl fluoride on the pharmacological effect of anandamide in the mouse model of cannabimimetic activity. J Pharmacol Exp Ther. 1997;283:1138–43.
Morgese MG, Cassano T, Cuomo V, Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson’s disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–9.
Giuffrida A, Seillier A. New insights on endocannabinoid transmission in psychomotor disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38:51–8.
Ledent C, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–4.
Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5.
Li X, et al. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacology (Berl). 2009;204:1–11.
McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6 J mice. Eur J Pharmacol. 2007;569:70–6.
Tzavara ET, et al. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: therapeutic implications. Biol Psychiatry. 2006;59:508–15.
Marinelli S, et al. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–44.
Romero J, et al. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–94.
Lastres-Becker I, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–32.
Van Laere K, et al. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol Aging. 2012;33(620):e621–8.
Di Marzo V, Hill MP, Bisogno T, Crossman AR, Brotchie JM. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–8.
Gubellini P, et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–7.
Maccarrone M, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem. 2003;85:1018–25.
Ferrer B, Asbrock N, Kathuria S, Piomelli D, Giuffrida A. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–14.
Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–7.
Zeng BY, et al. Chronic L-DOPA treatment increases striatal cannabinoid CB1 receptor mRNA expression in 6-hydroxydopamine-lesioned rats. Neurosci Lett. 1999;276:71–4.
Calabresi P, Giacomini P, Centonze D, Bernardi G. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol. 2001;47:60–8.
Picconi B, et al. Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology. 2005;26:779–83.
Meschler JP, Howlett AC, Madras BK. Cannabinoid receptor agonist and antagonist effects on motor function in normal and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP)-treated non-human primates. Psychopharmacology (Berl). 2001;156:79–85.
Fernandez-Espejo E, et al. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with severe nigral lesion in experimental parkinsonism. Neurobiol Dis. 2005;18:591–601.
Kelsey JE, Harris O, Cassin J. The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson’s disease. Behav Brain Res. 2009;203:304–7.
van der Stelt M, et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson’s disease. FASEB J. 2005;19:1140–2.
van Vliet SA, Vanwersch RA, Jongsma MJ, Olivier B, Philippens IH. Therapeutic effects of Delta9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol. 2008;18:383–9.
Carroll CB, et al. Cannabis for dyskinesia in Parkinson disease. Neurology. 2004;63:1245–50.
Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord. 2002;17:1180–7.
Sieradzan KA, et al. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology. 2001;57:2108–11.
Segovia G, Mora F, Crossman AR, Brotchie JM. Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesia induced by high-dose levodopa in the reserpine-treated rat model of Parkinson’s disease. Mov Disord. 2003;18:138–49.
Martinez A, Macheda T, Morgese MG, Trabace L, Giuffrida A. The cannabinoid agonist WIN55212-2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res. 2012;72:236–42.
Walsh S, Gorman AM, Finn DP, Dowd E. The effects of cannabinoid drugs on abnormal involuntary movements in dyskinetic and non-dyskinetic 6-hydroxydopamine lesioned rats. Brain Res. 2010;1363:40–8.
Mesnage V, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004;27:108–10.
Cao X, et al. Blockade of cannabinoid type 1 receptors augments the antiparkinsonian action of levodopa without affecting dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys. J Pharmacol Exp Ther. 2007;323:318–26.
Gonzalez-Aparicio R, Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson s disease. Neurobiol Dis. 2013;62C:416–25.
Giuffrida A, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–63.
Masserano JM, Karoum F, Wyatt RJ. SR 141716A, a CB1 cannabinoid receptor antagonist, potentiates the locomotor stimulant effects of amphetamine and apomorphine. Behav Pharmacol. 1999;10:429–32.
Bido S, Marti M, Morari M. Amantadine attenuates levodopa-induced dyskinesia in mice and rats preventing the accompanying rise in nigral GABA levels. J Neurochem. 2011;118:1043–55.
Dupre KB, et al. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–99.
Perez-Rial S, et al. Increased vulnerability to 6-hydroxydopamine lesion and reduced development of dyskinesias in mice lacking CB1 cannabinoid receptors. Neurobiol Aging. 2011;32:631–45.
Van Laere K. In vivo imaging of the endocannabinoid system: a novel window to a central modulatory mechanism in humans. Eur J Nucl Med Mol Imaging. 2007;34:1719–26.
Vitale C, et al. Unawareness of dyskinesias in Parkinson’s and Huntington’s diseases. Neurol Sci. 2001;22:105–6.
Lee J, Di Marzo V, Brotchie JM. A role for vanilloid receptor 1 (TRPV1) and endocannabinnoid signalling in the regulation of spontaneous and L-DOPA induced locomotion in normal and reserpine-treated rats. Neuropharmacology. 2006;51:557–65.
Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–9.
Matyas F, et al. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–61.
Szabo B, Wallmichrath I, Mathonia P, Pfreundtner C. Cannabinoids inhibit excitatory neurotransmission in the substantia nigra pars reticulata. Neuroscience. 2000;97:89–97.
Julian MD, et al. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–18.
Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128:21–6.
Szabo B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73:1084–9.
Kofalvi A, et al. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–84.
de Lago E, de Miguel R, Lastres-Becker I, Ramos JA, Fernandez-Ruiz J. Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res. 2004;1007:152–9.
Marinelli S, et al. N-arachidonoyl-dopamine tunes synaptic transmission onto dopaminergic neurons by activating both cannabinoid and vanilloid receptors. Neuropsychopharmacology. 2007;32:298–308.
Melis M, et al. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008;28:13985–94.
Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;306:880–8.
Centonze D, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal gabaergic Transmission. Neuropsychopharmacology. 2004;29:1488–97.
Andre VM, et al. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28.
Tang K, Low MJ, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci U S A. 2001;98:1255–60.
Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–51.
Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. J Neurosci. 2007;27:2403–9.
Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J Neurosci. 2009;29:1375–80.
Lerner TN, Kreitzer AC. RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits. Neuron. 2012;73:347–59.
Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403.
Narushima M, Uchigashima M, Hashimoto K, Watanabe M, Kano M. Depolarization-induced suppression of inhibition mediated by endocannabinoids at synapses from fast-spiking interneurons to medium spiny neurons in the striatum. Eur J Neurosci. 2006;24:2246–52.
Adermark L, Talani G, Lovinger DM. Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci. 2009;29:32–41.
Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–45.
Wang Z, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–52.
Picconi B, et al. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134:375–87.
Beltramo M, et al. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–7.
Tozzi A, et al. The distinct role of medium spiny neurons and cholinergic interneurons in the D/AA receptor interaction in the striatum: implications for Parkinson’s disease. J Neurosci. 2011;31:1850–62.
Martire A, et al. Pre-synaptic adenosine A2A receptors control cannabinoid CB1 receptor-mediated inhibition of striatal glutamatergic neurotransmission. J Neurochem. 2011;116:273–80.
Quiroz C, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. ScientificWorldJournal. 2009;9:1321–44.
Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–33.
Abdallah L, et al. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci. 2009;29:8156–65.
Mathur BN, Capik NA, Alvarez VA, Lovinger DM. Serotonin induces long-term depression at corticostriatal synapses. J Neurosci. 2011;31:7402–11.
Nakazi M, Bauer U, Nickel T, Kathmann M, Schlicker E. Inhibition of serotonin release in the mouse brain via presynaptic cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:19–24.
Haring M, Grieb M, Monory K, Lutz B, Moreira FA. Cannabinoid CB(1) receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology. 2013;65:83–9.
Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–88.
DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4.
Koprich JB, Johnston TH, Huot P, Fox SH, Brotchie JM. New insights into the organization of the basal ganglia. Curr Neurol Neurosci Rep. 2009;9:298–304.
Pifl C, Reither H, Hornykiewicz O. Functional sensitization of striatal dopamine D1 receptors in the 6-hydroxydopamine-lesioned rat. Brain Res. 1992;572:87–93. doi:0006-8993(92)90455-I [pii].
Corvol JC, et al. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24:7007–14.
Aubert I, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57:17–26.
Oh JD, Del Dotto P, Chase TN. Protein kinase A inhibitor attenuates levodopa-induced motor response alterations in the hemi-parkinsonian rat. Neurosci Lett. 1997;228:5–8.
Santini E, Valjent E, Fisone G. Parkinson’s disease: levodopa-induced dyskinesia and signal transduction. FEBS J. 2008;275:1392–9.
Lebel M, Chagniel L, Bureau G, Cyr M. Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis. 2010;38:59–67.
Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005.
Cyr M, et al. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc Natl Acad Sci U S A. 2003;100:11035–40.
Hemmings Jr HC, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–5.
Andersson M, et al. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25:8432–8.
Polissidis A, et al. Individual differences in the effects of cannabinoids on motor activity, dopaminergic activity and DARPP-32 phosphorylation in distinct regions of the brain. Int J Neuropsychopharmacol. 2010;13:1175–91.
Carta AR. PPAR-gamma: therapeutic prospects in Parkinson’s disease. Curr Drug Targets. 2013;14:743–51.
Funding Sources
NS 050401–07 and M.J. Fox Foundation (to A.G.); 1F31NS073411-03 (to A.M.)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Giuffrida, A., Martinez, A. (2014). Cannabinoids and Levodopa-Induced Dyskinesia. In: Fox, S., Brotchie, J. (eds) Levodopa-Induced Dyskinesia in Parkinson's Disease. Springer, London. https://doi.org/10.1007/978-1-4471-6503-3_14
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6503-3_14
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6502-6
Online ISBN: 978-1-4471-6503-3
eBook Packages: MedicineMedicine (R0)