Abstract
Phthalates are a diverse group of chemicals, including five with production volumes of over 1 million pounds per year in the United States (U.S.). They are used as plasticizers in a variety of plastics including polyvinyl chloride (PVC), medical devices (e.g., intravenous bags and tubing), food contact materials (FCMs), toys, and household goods, and as solvents in fragranced personal care and household products. Although not all phthalates have been evaluated for their toxic effects, many that are in widespread use have displayed endocrine disrupting properties on the developing reproductive system, especially in males, in laboratory, animal, and human studies. Widespread exposure to phthalates has been documented in the U.S. and in European countries, with some examples of unusually high exposures in certain populations, such as neonates with intravenous interventions in hospital settings. For the majority of the population, the primary route of exposure to the endocrine disrupting phthalates produced in the highest volume, bis(2-ethylhexyl) phthalate (DEHP) and diisononyl phthalate (DINP), is through diet. DEHP is used in food packaging, and also has been found to contaminate food sources directly. Some newer phthalates that have been introduced as alternatives to phthalates with known health concerns are also endocrine disruptors, while others have not been evaluated. Regulatory agencies are considering ways to define phthalates and assess their risk as a group based on chemical structure.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Phthalates
- Testosterone
- Male reproduction
- Female reproduction
- Reproductive toxicity
- Sperm count
- Plastics
- Vinyl
- Polyvinyl chloride
- PVC
- Food
- Food packaging
- Food contact material
- Consumer products
- Children’s toys
- Medical equipment
- Neonatal intensive care unit
- Intravenous bags
- DEP
- DBP
- DnBP
- DiBP
- BBzP
- DEHP
- DINP
2.1 Key Take Home Points
-
Phthalates are ubiquitous in the modern indoor environment, and they have a variety of uses and diverse exposures.
-
Food packaging and processing, vinyl household products, and vinyl flooring are the major contributors of exposure to bis(2-ethylhexyl) phthalate (DEHP) and several other phthalates that are used as plasticizers, while fragranced products are an important source of diethyl phthalate (DEP) exposure.
-
Population-level variations in exposures are influenced by factors such as geography, dietary habits, gender, and personal care product use.
-
Some phthalates, such as DEHP, are endocrine disruptors that affect the developing male reproductive system by interrupting the production of fetal testosterone and the protein involved in testicular descent, insulin-like 3 (insl3), while other phthalates, such as DEP, are not endocrine disruptors.
-
Because phthalates vary in their potency, and some do not show endocrine disruption, all phthalates cannot be grouped together or be grouped based on molecular weight.
-
Since mixtures of phthalates act in a dose-additive manner, risks should be considered cumulatively.
2.2 Introduction
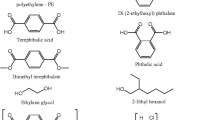
Phthalates are a group of compounds commonly used in plastics and personal care products. Although they are often referred to collectively by the single term “phthalates,” there are many types of phthalates in widespread commercial use with different predominant uses, physical and chemical characteristics, and toxicologic profiles. Examples of phthalates, U.S. production volumes, endocrine disrupting properties, and major uses are given in Table 2.1. DEHP and butylbenzyl phthalate (BBzP) are often used as plasticizers in flexible vinyl materials, such as items ranging from food containers to vinyl flooring to medical devices. Lower molecular weight phthalates such as dibutyl phthalate (DBP) and diethyl phthalate (DEP) are used in personal care products, pesticides, glues, and paints as solvents, and in time-released pharmaceuticals (Meeker and Ferguson 2012).
Phthalates are weakly bound to their incorporating substrate, and are thus easily leached from products, enabling them to enter the environment. They are high-production volume chemicals present in food packaging, foods, and a variety of consumer products, and they are some of the most abundant chemicals measured in indoor air and house dust (Schecter et al. 2013; Dodson et al. 2012; Rudel et al. 2003). Not surprisingly, greater than 98 % of the U.S. population is considered exposed to some widely used phthalates, although urinary concentrations of different phthalate metabolites typically span orders of magnitude within a population (Silva et al. 2004; Aylward et al. 2011). The variability in concentrations of phthalates in environmental media and biologic samples arises primarily from variation in the sources and concentrations, individuals’ behaviors, and the chemicals’ short biological half-lives.
Some phthalates are considered to be endocrine disruptors, thereby disrupting normal hormonal signaling and functioning in the body. Consistent laboratory evidence shows that DEHP, DBP, BBzP, and DINP are anti-androgenic; they adversely affect the developing male reproductive system through inhibition of testosterone and insl3 synthesis during fetal development, and there are supporting human associations for these observations (Wilson et al. 2004; Swan et al. 2005). Adverse effects on male and female reproduction have also been demonstrated in animal studies (Gray et al. 2006a). In humans there is also some evidence of effects on metabolism, neurological development, asthma, and allergy (Meeker and Ferguson 2012). While DEP is commonly found in personal care products, particularly as a solvent for fragrance compounds, it has not been demonstrated to show anti-androgenic endocrine disrupting activity.
2.3 Human Exposure and Biomonitoring
Widespread human exposure to phthalates has been documented by independent studies and representative samples in the U.S., as well as European countries, Mexico, Taiwan, and other populations (Meeker and Ferguson 2012). Phthalates have been measured in indoor and outdoor air and house dust, foods and food packaging, consumer products, and other media (Rudel et al. 2003; Dodson et al. 2012; Schecter et al. 2013; Rudel et al. 2010, 2011).
2.3.1 Exposure Biomarkers
Human exposure to phthalates is most commonly measured by chemical analysis of urine to detect monoester and oxidative metabolites (Silva et al. 2007). Table 2.2 lists the common metabolites for biomonitoring major phthalates. Phthalates are ubiquitous in the environment and also in sample collection and laboratory equipment, so great care is required to produce valid measurements. The most reliable exposure biomarkers are monoesters and oxidative metabolites in urine. Measurements of the parent compound are vulnerable to contamination from laboratory or sampling equipment. Additionally, hydrolytic enzymes found in blood, breast milk, and other biologic matrices like amniotic fluid and meconium, but not urine, can convert these contaminants into the monoesters after sample collection (Calafat et al. 2006). Measurements of oxidative metabolites in blood are thought to be reliable because they are not formed as a result of sample contamination.
Urinary levels of phthalate metabolites are considered the best measure of exposure as phthalates are rapidly excreted within hours, primarily in the urine (Janjua et al. 2008). The measurement of phthalates in urine is a valuable biomarker for epidemiologic studies of health effects because it integrates exposure across multiple routes: ingestion, inhalation, and dermal absorption. The contribution of each of the routes of exposure appears to vary between phthalates, with some phthalates primarily coming from dietary sources, while others come from personal care products (Wormuth et al. 2006; Fromme et al. 2007). Because of the short half-lives of phthalates and because urine levels vary throughout the day, average exposure levels are best estimated using 24-hour (h) urine samples, possibly from multiple days.
Urinary concentrations of phthalate metabolites are available for many populations, geographies, and age groups, and these provide useful information on relative exposure levels among these groups. Pharmacokinetic models and other techniques have been used to back-calculate exposure intake levels that correspond to the urine levels (Lorber and Calafat 2012; Aylward et al. 2009; Koch et al. 2011). Based on these models, researchers have estimated that the typical intake for DEHP in the U.S. 2001 general population was in the range of 0.6–2.2 microgram/kg per day (μg/kg per day), corresponding to median urine concentrations of MEHP, 5OH-MEHP, and 5oxo-MEHP of 4.1, 20.1, and 14.0 μg per liter (L), respectively (Lorber and Calafat 2012). Others have used these relationships and a cumulative assessment of exposure to three common phthalates (DEHP, DnBP and DiBP) and showed that 24 % of German children exceeded the health-based guidance value, the Tolerable Daily Intake (TDI) (Koch et al. 2011).
Because phthalates are not listed on ingredient labels in most product categories in the U.S., there is no way to know which phthalates may be commonly found in everyday products (Dodson et al. 2012). Since formulations change regularly, substitutes may enter the market without the public knowing. As a result, scientists may not always know which phthalates to measure in biomonitoring studies.
2.3.2 Diet as a Primary Route of Exposure
Several studies provide evidence that exposure to DEHP and its substitutes is primarily from diet. In two similar studies, volunteers participated in a 48-h fast. DEHP, DINP, DnBP, DiBP, and BBzP metabolites were measured in urine before and during the fasting period (Koch et al. 2013; Wittassek et al. 2011). Within 24 h, DEHP and DINP metabolites were reduced to levels five to ten times lower than pre-fasting measurements. DINP is a widely used substitute for DEHP. However, no significant changes were seen for the urinary excretion of DnBP, DiBP, or BBzP metabolites during the fasting period (Fig. 2.1), suggesting their exposures were from non-food sources. In a similar attempt to reduce dietary exposure sources, another intervention study removed food packaging from five families’ diets (Rudel et al. 2011). Switching from a conventional diet to a diet of whole foods and beverages that had limited contact with plastic, aluminum, or canned packaging resulted in an average decrease of urinary DEHP metabolites levels by over 50 % during the 3-day intervention period (Fig. 2.2). Reductions were even more pronounced for people with the highest pre-intervention exposures, which resulted in more than a 90 % reduction of urinary DEHP. Levels of the urinary metabolites of DMP, DEP, DBP, and BBzP did not significantly change during the intervention period, again suggesting that exposure to these phthalates is largely from non-food related sources.
Excretion of several phthalate metabolites during 48-h fasting. Credit figure reproduced with permission from John Wiley and Sons, Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim; original figure appeared in: Wittassek et al. (2011) as Fig. 1
Change in phthalate excretion during a dietary intervention to reduce food packaging. Credit figure reproduced with permission from Environmental Health Perspectives; original figure appeared in: Rudel et al. (2011) as Fig. 2
2.3.3 Foods and Food Packaging
Phthalates, especially DEHP, have been reported at high concentrations in some foods. In a dietary intervention study similar to that discussed in Sect. 2.3.2 (Rudel et al. 2011), researchers substituted food with limited contact with plastic packaging into ten families’ diets, and an unexpected spike in DEHP measurements occurred during the dietary intervention period (Sathyanarayana et al. 2013). This was an unexpected finding, and the authors concluded that the prepared meals that were served to the participants were contaminated with DEHP. Milk, cream and coriander used for meal preparation in the study had higher DEHP levels than normally found in those foods, demonstrating food contamination may occur even in the most careful of circumstances. Another study revealed phthalate contamination in Taiwanese sport drinks, which contained high levels of DEHP added illegally as a clouding agent (Yang et al. 2013).
Additional evidence that food and food packaging are important sources of DEHP exposure come from observational human epidemiologic and panel studies. For example, in analyses adjusted for body mass index (BMI), each ounce of poultry consumed in the previous day was associated with an approximately 6 % higher level of urinary DEHP metabolites in the 2003–2004 National Health and Nutrition Examination Survey (NHANES), which is representative of the U.S. non-institutionalized population above 6 years of age (Colacino et al. 2010). In a biomonitoring study collecting all urine specimens over a week from eight adults, the highest excretion of DEHP metabolites among 427 specimens was subsequent to a meal of packaged food and coffee purchased at a gas station (Lorber and Calafat 2012).
Phthalate levels in foods, based on limited testing, are highly variable and suggest sporadic contamination events which may occur in the source or original collection of the food, in the packaging or processing prior to market, or in the preparation leading to consumption. Phthalate levels are generally higher in fatty foods, including dairy products, meats, and vegetable oils. Phthalate esters are considered lipophilic with higher lipophilicity in phthalates with longer side chains, such as DEHP (Agency for Toxic Substances and Disease Registry 1995). A 1990 study demonstrated that collection tubing used in a commercial dairy resulted in DEHP-contaminated milk, and that the level of DEHP was at a higher level in cream (Castle et al. 1990). This suggests that the DEHP migrated from the collection tubing to the fat component of the milk and cream.
Recent testing of 72 foods purchased from a U.S. supermarket demonstrated that various phthalates were detectable in all classes of food with DEHP being the highest of the phthalates tested in most food categories, particularly in pork, dairy products, vegetable oils and grains, although the sample sizes were limited (Schecter et al. 2013). This is largely consistent with concentrations found in European foods, reviewed in a scenario-based model that found that diet had a major influence on DEHP exposure (Wormuth et al. 2006). The sporadic contamination of food was seen in the three U.S. cooking oil samples, which had concentrations of BBzP of 459, 2.20 and 0.35 nanograms per gram (ng/g), with the highest value detected in virgin olive oil from a glass container, although the source of phthalates found in that sample was unknown (Schecter et al. 2013). A study of FCMs by the European Food Safety Authority (EFSA) found that the use of gaskets for metal lids made from PVC on imported foods accounted for several of the highest observed concentrations of phthalates in foods, although conveyor belts, gloves, and tubes for liquids were also contributing sources (Petersen and Jensen 2010). Items used in the handing, processing, and packaging of foods and beverages are all considered to be FCMs. The packaging (polyethylene coated aluminum dishes sealed with polyethylene terephthalate-coated foil) of hot cooked foods led to further increases in both DEHP and DnBP in a study examining Italian school lunches (Cirillo et al. 2011).
It is unclear which food packaging materials contain phthalates and how different foods become contaminated. On the one hand, the American Plastics Council states that:
phthalates are not used in plastic beverage bottles, nor are they used in plastic food wrap, food containers, or any other type of plastic food packaging sold in the United States (Enneking 2006).
On the other hand, the studies described above show that diet remains an important source of phthalate exposure, and that food packaging is one established contributing source (Schecter et al. 2013; Rudel et al. 2011). Some possible explanations for this discordance include inconsistent compliance with industry claims, particularly in imported foods; introduction of phthalates from recycled content or during manufacturing; use of packaging on food that was not intended for food use; and use of PVC and other phthalate-containing plastics in food processing and handling (Petersen and Jensen 2010; Tsumura et al. 2001; Montuori et al. 2008). Identifying the specific sources of phthalate contamination in food has been difficult because of the variety and vast assortment of FCMs in use, and the large number of manufacturers of foods and food packaging materials. Surveys of phthalates in materials and foods are further limited by the expense of laboratory measurements and the technical challenge of directly measuring phthalate diesters that are prone to laboratory contamination.
2.3.4 Non-dietary Sources
Exposures to phthalates other than DEHP and its substitutes are primarily from non-dietary sources, possibly from consumer goods in the indoor environment and personal care products. A study that paired indoor and outdoor air measurements found that phthalates were the most abundant chemicals in indoor air and house dust, with maximum levels of DBP in air of 1.1 micrograms per cubic meter (μg/m3) and levels in house dust approaching 1 mg per gram (g) (Rudel et al. 2003, 2010). There was no correlation between indoor and outdoor air measurements of DEP, DBP, DiBP, or DEHA, an alternate plasticizer, demonstrating that phthalate exposures are primarily from indoor sources. In a cohort in New York City, homes with vinyl floors were correlated with indoor air and urine measurements of BBzP, but not DEHP, which is further support for the idea that DEHP exposure is primarily from food and food packaging (Adibi et al. 2008; Just 2012). In a Swedish study of infants, the urinary metabolites of BBzP, but not DEHP, were higher among those with PVC flooring in their bedrooms (Carlstedt et al. 2013). These studies demonstrate that BBzP used in vinyl migrates out of its substrate, entering indoor air and dust, leading to exposures through inhalation or ingestion of dust. Recent research has shown that exposure via dermal absorption in the indoor environment is a major contributor to the total intake for certain phthalates that predominantly partition to the gas-phase. A Danish study of children aged 3–6 estimated that dermal absorption of DEP, DnBP, and DiBP was the dominant exposure pathway of these phthalates in the indoor environment. More research in the dermal exposure pathway from ambient indoor air is forthcoming (Bekö et al. 2013).
DEHP and BBzP are found in consumer products at high concentrations. DEHP is especially high in vinyl products, up to 40 % by weight (Agency for Toxic Substances and Disease Registry 2002). DEP, also commonly found in the indoor environment, is used as a solvent for fragrance in perfumes, lotions, hair products, and other personal care products. A 2012 study tested 213 commercial products, including “conventional” products and some “alternative,” or advertised as healthier, products (Dodson et al. 2012). DEHP was detected at the highest concentration of the phthalates tested: 28 % by weight of a vinyl shower curtain, and 14 % by weight of a vinyl pillow protector; BBzP was also detected in the pillow protector at lower levels. DEHP also was detected in conventional diapers, hand sanitizer, shaving cream, deodorant, lipstick, car air freshener, dryer sheet, nail polish, and sunscreen. DEHP also was found in alternative products, including hand sanitizer, shaving cream, lipstick, and an alternative pillow protector (at very low levels). DEP was one of the most commonly detected chemicals in both conventional products and alternative products. Of the ten phthalates tested for, four were found in both conventional products and alternative products, including DEP, di-n-butyl phthalate (DnBP), BBzP, and DEHP. Other types of conventional products with additional phthalates, such as di-n-octyl phthalate (DnOP), di-n-hexyl phthalate (DnHP), and diisobutyl phthalate (DiBP) included foundation makeup, lipstick, and perfume. Other alternative products that contained DnOP, DINP, and di-cyclohexyl phthalate were laundry detergent, shaving cream, bar soap, baking soda, and borax. Exposure to these phthalates may result from direct contact with the product, such as shaving cream applied directly to skin, and also from inhalation or ingestion of phthalates that migrate out of the product, as in the case of DEHP in vinyl shower curtains. The European Union (EU) also identified DEHP, DnBP, and DiBP in PVC-containing sandals and DEHP in PVC-containing sex toys as potentially significant sources of exposure (European Chemicals Agency 2012). Although there are now some restrictions on phthalates in children’s toys in the EU and U.S., high levels have been reported in PVC-based toys, some containing up to 40 % DINP or DEHP (Schettler 2006; Stringer et al. 2000).
Phthalates have also been detected in art supplies, including modeling clay. High concentrations of DnOP, DnHP, BBzP, and DEHP have been reported in Sculpey™ and Fimo® modeling clay, with levels up to 14 % by weight (Schettler 2006). Contact with these concentrated sources can likely be associated with much higher exposure levels than reported in the general population, especially for children who may exhibit greater hand-to-mouth-activity resulting in incidental ingestion of DEHP. Inhalation exposure may also be important when the clay products are baked dry.
A survey of phthalates in personal care products purchased in the U.S. in 2002, with a follow-up published in 2010, found that eight of 25 products that were re-tested no longer contained phthalates, possibly indicating reformulation following market pressures (Hubinger and Havery 2006; Hubinger 2010). In the 2010 follow-up study, while DEP was still widely found and in high concentrations, the only other phthalate that was found in adult-use cosmetic products was DnBP in 11 of 24 nail products with widely ranging concentrations (<10–62,607 μg/g) (Hubinger 2010). Of the phthalates tested, only DEP was detected in a survey of 24 baby-care products in the U.S. (baby shampoo, body wash, cream, lotion, and oil products) (Hubinger 2010). Similar results, also showing DEP detection, were reported in a survey of personal care products purchased in Canada (Koniecki et al. 2011). These changes may reflect restrictions in the EU on DEHP, DBP, and BBzP in personal care products that were enacted in 2004 (European Union 2004).
Several studies have demonstrated that adult use of personal care products, particularly the use of fragrance (cologne or perfume), the use of multiple products, and use reported in the house before urine collection, is associated with higher levels of urinary DEP metabolites in Israel and the U.S. (Berman et al. 2009; Duty et al. 2005a; Just et al. 2010; Parlett et al. 2013; Braun et al. 2013). In one study, a model adjusting for urinary dilution, age, education, and use of perfume or cologne in the previous 24 h explained 41 % of the population variance in urinary DEP metabolites, with fragrance users having almost three times the concentration of non-users on average (Parlett et al. 2013). In two separate studies, personal care product use in Mexico was associated with higher urinary levels of DEHP and DEP metabolites among adult women, and among both boys and girls 8–13 years of age (Romero-Franco et al. 2011; Lewis et al. 2013). A study measuring phthalate metabolites in infant urine found that reported use of baby care products (lotion, powder, and shampoo) was associated with higher levels of infant exposure to and urinary excretion of DMP, DEP, and DiBP (Sathyanarayana et al. 2008).
Contact with certain phthalate-containing products may lead to unusually high exposure levels. For example, Hauser et al. reported that study participants using phthalate-containing medications had urinary DBP levels 100 times above the NHANES 95th percentile (Hauser et al. 2004). Use of phthalate-containing medical devices can also affect potential exposures. For instance, infants in a neonatal intensive care unit showed elevated urinary levels of DEHP, DBP, and BBzP metabolites, with highest levels observed in conjunction with the most intensive use of medical devices that contained or contacted PVC (Weuve et al. 2006).
2.3.5 Exposure Trends: Demographic and Geographic Patterns
Results from a biobank of samples from German university students show that the daily intake of both DEHP and DnBP, as estimated from urinary excretion patterns of metabolites, decreased substantially over 15 years, with the median DnBP intake falling to less than one-third of 1988 levels by 2003. Over the same period, there was some evidence of increasing exposure to the replacement phthalates DiNP (a DEHP replacement) and DiBP (a DnBP replacement) (Wittassek et al. 2007). It also was noted that the annual median intake of DEHP for German students, estimated from urine samples from this biobank, had an extremely close correlation (r = 0.97) with yearly German industrial DEHP production data over the same period (Helm 2007).
Gender and age may also affect use patterns of phthalate-containing products. DEP exposures were higher in females than in males, and higher in adults and adolescents compared to children in one U.S. study, likely reflecting patterns of personal care product use (Silva et al. 2004). DBP, BBzP, and DEHP metabolites were higher among children and among females than in males (Silva et al. 2004). In a study conducted in Mexico City, levels of metabolites of DEP, DnBP, DiBP, BBzP and DEHP in stored maternal urine collected during the third trimester of pregnancy were compared with paired urine samples from the delivered children when they were aged 8–13 years; children had higher levels than their mothers except for the DEP metabolite which is found in personal care products, particularly perfume (Just et al. 2010; Hubinger and Havery 2006).
Biomonitoring results from NHANES demonstrate that socioeconomic factors are associated with large differences in population exposure to different phthalates. This may be the result of economic and social differences in dietary preferences, housing materials, and consumer product use patterns. In a pooled analysis of female NHANES 2001–2008 participants 20–39 years of age, those in the lowest quartile of overall socioeconomic status (SES) had lower urinary DEHP metabolite levels but an average of 1.83 times the BBzP metabolite concentrations of those in the highest quartile of SES. Non-White ethnicities had higher urinary DBP and DEP metabolite levels compared with Non-Hispanic Whites but lower urine BBzP after accounting for SES (Kobrosly et al. 2012). Whether these phthalate exposure and urinary excretion patterns would apply to other populations and other demographic groups requires further research. Women in a Mennonite community that had a limited exposure to plastic food packaging, cosmetics, and automobiles had lower exposures to DEP, DiBP, and DEHP, measured from urinary excretion than the general population (Martina et al. 2012).
2.4 Health Effects
2.4.1 Reproductive Toxicity: Laboratory Animals
Animal studies provide consistent evidence that certain phthalates target the developing male reproductive system. These effects in animal studies have been termed Phthalate Syndrome (PS), and the effects mirror a set of reproductive symptoms seen in human males, termed Testicular Dysgenesis Syndrome (TDS). TDS and PS include symptoms that arise from insufficient testosterone production during in utero development, including undescended testes, malformations of the penis, reduced anogenital distance (AGD), decreased sperm motility and mobility, infertility, and testicular cancer (Meeker and Ferguson 2012). Phthalates that have exhibited these effects in animal studies are DiBP, DnBP, BBzP, DEHP, diisoheptyl phthalate (DiHP), and DiNP (Gray et al. 2000; Hannas et al. 2011). Testicular toxicity has also been observed following pubertal exposure, and, at higher doses, in adult males (Mahood et al. 2007; Noriega et al. 2009).
Although the effects of phthalates on the male reproductive system are the most fully described, DEHP has been shown to affect the female reproductive system as well. Female rats treated with DnBP from weaning through pregnancy aborted litters subsequent to decreases in progesterone levels, and these effects are observed at similar doses that induce testicular toxicity in adult male rats (Gray et al. 2006a). Female marmosets (primates) showed effects on ovarian and uterine weight and blood levels of estradiol following 65 weeks of pubertal exposure to DEHP (Tomonari et al. 2006). In a study that examined generational affects, female pups of mice orally exposed to high doses of MEHP during pregnancy had shorter life expectancy, prolonged estrus cycles, and hyperplasia of mammary tissue (Moyer and Hixon 2012). At extremely high doses, adult female rats orally exposed to DEHP have lowered circulating estradiol, prolonged estrus cycles, and experience anovulation (Lovekamp-Swan and Davis 2003).
Phthalates are thought to cause their toxic effects on male reproductive development by interrupting testosterone and insl3 production in the testes during the sensitive in utero masculinization programing window. In male mammals, testosterone and insl3 are necessary to change the default phenotype from female to male during fetal development. Therefore, the fetus is at the most sensitive developmental stage for the reproductive health effects of anti-androgenic chemicals. The specific developmental period of greatest sensitivity to these effects is from 15.5 to 17.5 days gestation in the rat and 8–20 weeks gestation in the human (Scott et al. 2009). Rodent studies with DEHP and DiBP show that the inhibition of testosterone production in Leydig cells, which produce testosterone in the testes, may occur through down-regulation of key genes, including StAR, HMC-CoA synthase, and SRB1 (involved in cholesterol uptake), and the enzymes CYP11A, 3B-Hsd, and CYP17 (involved in steroid biosynthesis). Because cholesterol is the first building block of testosterone synthesis, down-regulation of its uptake and transport may result in less testosterone production. CYP11A is the rate-limiting step of testosterone biosynthesis, and CYP17 is regarded as the qualitative regulator of steroidogenesis because it is necessary in more than half of the conversions from cholesterol to testosterone (Scott et al. 2009). During pubertal development, Sertoli cells are also a target of phthalate toxicity. Some phthalates, such as DEHP, impair the production of spermatozoa by interrupting the Sertoli cells’ ability to nourish the germ cells (Hauser et al. 2006). Insl3, which is a protein involved in controlling testicular descent, is adversely affected by DEHP’s monoester metabolite, MEHP (Gray et al. 2006b). One study found that DINP reduced immunostaining intensity in Leydig cells for StAR, P45scc, and CYP17 in similar manner as DEHP (Boberg 2007). Scientists expect harm to the male reproductive system observed in animal models to be relevant to humans because similar effects have been observed in many species of mammals, including rats, mice, hamsters, ferrets, and guinea pigs (Voss et al. 2005; Hannas et al. 2012; Hotchkiss et al. 2008).
Differences in phthalate structure determine how they are metabolized and their toxic effects. MBuP and MBzP, metabolites of DBP and BBzP, have been identified as active metabolites toxic to reproductive endpoints, including sperm concentration in adult males (Hauser et al. 2006). Direct measures of parent phthalates or phthalate monoester metabolites in blood are difficult to interpret because of the high likelihood of contamination from sampling or from lab materials given that esterase activity after sample collection can generate monoester metabolites in samples contaminated after collection (Calafat et al. 2013).
Phthalates differ in their ability to affect androgen pathways, with some showing anti-androgenic effects and others showing no effect (see Table 2.1). These effects may partially be due to the potency of the phthalate. Dr. Earl Gray, a reproductive biologist and toxicologist at U.S. Environmental Protection Agency (USEPA), has conducted experiments to assign relative potencies to different phthalates. DMP, DEP, BBzP, DEHP, dioctyl tere-phthalate (DOTP), and DINP were orally administered to pregnant rats perinatally, and their offspring were assessed for androgen-sensitive endpoints. While administration of DEP, DMP, and DOTP did not exhibit adverse effects on the male reproductive system, DEHP and BBzP produced male malformations, including reduced AGD and nipple retention, with similar potencies and frequency. At the same dose, DINP produced these malformations with approximately ten times less prevalence (Gray et al. 2000). DnBP and DiBP have also shown to exhibit similar potencies to DEHP and BBzP, while dipentyl phthalate (DPP) was three times more potent than DEHP (Howdeshell et al. 2008). Examples of human reproductive toxicity with exposure to various phthalates are provided in Sect. 2.4.4.
2.4.2 Epigenetic Changes
There is some evidence that phthalates can cause other types of toxicity, including epigenetic changes. Epigenetic effects are changes to genetic expression, which can occur through the way DNA is packaged, such as histone modification and methylation patterns. Changes to genetic expression may have adverse effects, and heritable epigenetic changes may alter DNA expression across several generations. A study with human breast cell line MCF10A and human breast cancer cell line MCF7 found that exposure to BBzP led to demethylation of a promoter region of estrogen receptor alpha (ERα) (Kang and Lee 2005). ERα is a cellular receptor for the hormone estrogen, and it is necessary for normal estrogen function in both men and women. In another study, DnBP was associated with hypomethylation in the c-myc proto-oncogene in liver cells (Ge et al. 2002). Prenatal exposure to DEHP in mice was found to induce PS, increase DNA methylation of testes, and increase methyltransferase expression (Wu et al. 2010).
2.4.3 Carcinogenicity and Genotoxicity
In addition to having adverse effects on the male reproductive system, DEHP has also been found to produce liver tumors in both male and female Fischer-344 rats and B6C3F1 mice. Animals fed DEHP in a 2-year cancer bioassay had significantly higher incidence of malignant liver tumors (hepatocellular carcinomas), compared to control groups (Integrated Risk Information System 1997). In a study with male Sprague-Dawley rats fed DEHP in their diet for 3 years, benign Leydig cell tumors in the testes occurred with nearly twice the incidence than the control animals (Voss et al. 2005). Testicular tumors are a symptom of TDS and the finding is consistent with the testicular damage associated with phthalates. A 2-year study with Fischer-344 rats fed DEHP in their diet resulted in significantly higher incidence of benign pancreatic tumors in the male rats in the highest dose group (David et al. 2000). DEHP has been rated as reasonably anticipated to be a human carcinogen by the National Toxicology Program (NTP) based on evidence from laboratory animal cancer bioassays (National Toxicology Program 2011). DINP has structural and mechanistic similarities to DEHP and was recently identified as a carcinogen based on several rodent studies (Office of Environmental Health Hazard Assessment California Environmental Protection Agency 2013). Like DEHP, DINP also has been found to cause increased hepatocellular carcinomas in male and female Fischer-344 rats and B6C3F1 mice. Male and female Fischer-344 rats also experienced significantly increased incidence of mononuclear cell leukemia following chronic DINP exposure. Increased incidence of renal tubular cell carcinoma was observed in male Fischer-344 rats, although a statistically significant association was observed in only one study. Incidence of pancreatic islet cell tumors, Leydig cell tumors, and uterine tumors all increased in Sprague-Dawley rats following DINP exposure, although findings did not reach statistical significance.
Additional genotoxic effects in vivo also have been observed, such as DNA damage, chromosomal aberrations, and tumor promotion (International Agency for Research on Cancer 2012). The mono-ester metabolite of DEHP, MEHP, activates peroxisome proliferation activated receptor alpha (PPARα) in the liver, which is thought to lead to liver tumors, and it has been hypothesized that rats are more sensitive to this liver effect than humans because of species differences in PPARα (Melnick 2001; Rusyn and Corton 2012). DINP also activates PPARα, as well as PPAR gamma (γ). However, a 2-year dietary DEHP study with PPARα-null mice found significantly increased incidence of malignant liver tumors in the PPARα-null mice compared to wild-type mice, demonstrating the liver effects are not entirely PPARα-dependent. The authors hypothesize DEHP exposure may lead to the formation of reactive oxygen species, causing DNA damage in liver tissue, contributing to tumor effects through other pathways (Ito et al. 2007).
2.4.4 Reproductive Toxicity: Human Epidemiology
Epidemiologic studies measuring phthalate exposure largely show consistency with animal models’ evidence of harm to the male reproductive system (Meeker and Ferguson 2012). One longitudinal cohort study in the U.S. described decreased AGD in male children whose mothers had higher levels of MEP, MBP, MBzP, and MiBP in their urine (Swan et al. 2005). AGD is a testosterone-dependent endpoint of genital development. Males have longer AGDs due to increased fetal testosterone levels as compared to females, who have shorter AGDs due to lower testosterone levels. This finding is consistent with evidence from animal models for exposures to the anti-androgenic phthalates BBzP and DBP, although DEP does not produce anti-androgenic effects in animal models. These results suggest that phthalates are interfering with fetal testosterone levels. A Danish-Finish cohort study examined the levels of phthalate monoester metabolites in breast milk of mothers of 3-month old male infants (Main et al. 2005). Higher breast milk levels of MBP, the active metabolite of DBP, were statistically significantly negatively correlated with free serum testosterone levels in male offspring. Breast milk levels of MiNP, the primary metabolite of DINP, were significantly positively correlated with serum levels of luteinizing hormone (LH) in the boys. Increased LH is an indirect indicator of anti-androgenic effects because its production is stimulated in the presence of low testosterone levels. MMP (a metabolite of DMP) and MBP (a metabolite of DBP and BBzP), measured in mother’s breast milk, were both significantly positively correlated with LH:free testosterone ratio in male offspring. This finding may indicate that impaired testosterone production has been compensated by increased levels of LH (Scott et al. 2009). Of these associations, DBP and DINP, but not DMP, are supported by anti-androgenic activity in animal models. The authors found no statistically significant difference between lactational phthalate exposure and the presence or absence of cryptorchidism (undescended testes). Another study found higher risk of pubertal gynecomastia (breast tissue development) in boys aged 11–15 years in Turkey, who had higher plasma concentrations of DEHP and MEHP (Durmaz et al. 2010). Additional studies with adult men have shown associations with phthalate exposures and reduction in serum free testosterone and increased DNA damage in sperm (Duty et al. 2005b; Hauser et al. 2006; Pan et al. 2006).
Although few epidemiological studies have focused on female reproductive endpoints, one study that examined MEHP levels in women near the time of conception found that increased urinary levels of MEHP were associated with an increased risk of miscarriage, compared to women with lower MEHP levels, consistent with a study in female rats (Toft et al. 2012; Gray et al. 2006a).
Gender-specific behavioral endpoints also have been assessed in relation to phthalate exposure. Sexually dimorphic play behavior was observed among children as measured by a validated questionnaire; elevations in mid-pregnancy urinary concentrations of MnBP, MiBP, MEOHP, and MEHP in the mothers were significantly positively correlated with decreased masculine play in their male children (Swan et al. 2010). All of these phthalates show anti-androgenic activity in animals, and this study supports the idea that androgen production may also alter sexual differentiation in the brain during fetal development. Confounding is an important consideration and limitation of epidemiological studies, and it is discussed in Sect. 2.4.8.
2.4.5 Epidemiological Associations with Evidence of Neurotoxicity
Neurological effects associated with phthalate exposure also have been reported in human studies. Although a mechanism of action has not been demonstrated, it is hypothesized that disruption of maternal thyroxine levels may contribute to the downstream clinical effects of thyroid dysfunction (Meeker et al. 2007). Measurements of maternal urinary phthalate measurements in the third trimester, specifically low molecular weight phthalates, including MBP, MEP, MiBP, and MMP, were associated with symptoms of attention deficit hyperactive disorder in follow up of their children at ages 4 through 9. Statistically significant poorer results in emotional control and Global Executive Composite scale, a measure of executive function or regulation of cognitive processes, were seen among boys with increasing third trimester measurements of MEP, MBP, and MMP (Engel et al. 2010).
2.4.6 Epidemiologic Associations with Metabolic Effects
Metabolic health effects as a result of phthalate exposure are thought to occur via the PPAR pathway and through endocrine disruption of the thyroid hormones. The sodium/iodide symporter, necessary for normal thyroid function, can be down-regulated by some phthalates in animal models (Breous et al. 2005). The PPARs are nuclear receptor proteins that are involved in metabolism, cellular differentiation, and development. Two of these receptors, PPARα and PPARγ, have been shown to be activated by MEHP, MBzP, and MBuP. PPARγ is primarily found in adipose tissue and controls adipogenesis, or fat cell creation (Hurst and Waxman 2003). Because of the phthalates’ interaction with fat cells and the endocrine system, there is concern that these chemicals may have health effects related to metabolism, such as obesity and diabetes. In a study based on NHANES data, children in the 2003–2008 surveys that had higher levels of urinary DEHP metabolites had elevated systolic blood pressure. This cross-sectional analysis adjusted for diet, BMI, and demographic characteristics (Trasande et al. 2013). Decreased levels of thyroid hormones in adult males and females has been observed in association with MEHP and MBP exposure (Meeker and Ferguson 2012). Decreased insulin resistance and increased waist circumference in adult males has been observed in association with BBzP, DEHP, and DEP exposures estimated from urinary excretion (Meeker and Ferguson 2012).
2.4.7 Epidemiologic Associations with Immune System and Respiratory Effects
Phthalate exposure has been associated with respiratory symptoms in both male and female children and adults. Individual studies have used a variety of endpoints and identified links with several different phthalates. BBzP in house dust was associated with childhood eczema, a persistent skin rash that may be related to the development of an allergic phenotype, in a cross-sectional Swedish case control study (Bornehag et al. 2004). Similarly, prenatal concentrations of the urinary metabolite of BBzP were positively associated with a report of early eczema in an urban cohort of children from New York City (Just et al. 2012b). Although lacking direct exposure measures, PVC materials in the home such as plastic wall materials or PVC flooring (potential sources of BBzP and/or DEHP) were associated with wheezing and asthma symptoms in several cross-sectional and prospective studies of children (Jaakkola et al. 1999, 2000; Larsson et al. 2010). Increased urinary metabolites of DEP and DBP were associated with decreased lung function but only among males in a cross-sectional sample of 240 U.S. adults (Hoppin et al. 2004). Metabolites of DEP and BBzP, both believed to have substantial contributions from inhalation, were associated with higher airway inflammation as measured by fractional exhaled nitric oxide among children 5–9 years old. There were stronger associations for BBzP among children with report of recent wheeze who may be more susceptible to inflammatory effects of pollutants (Just et al. 2012a). In a study of Norwegian children at age 10, the urinary metabolites mono(carboxyoctyl) phthalate and mono(carboxynonyl) phthalate, but not the metabolites of other common phthalates, were associated with current asthma in a cross-sectional design (Bertelsen et al. 2013). Given the etiologic period of allergy and asthma that develops over a period of months or years, more longitudinal studies are needed to assess whether exposure to different types of phthalates could contribute to incident allergy or asthma.
2.4.8 Limitations of Epidemiological Studies
Confounding is important to consider in epidemiological studies. Exposure studies have demonstrated that people with higher levels of DEP use many more fragranced and personal care products, and these products contain many different chemicals, including endocrine disruptors such as parabens and some fragrances (Duty et al. 2005a; Dodson et al. 2012). Thus, MEP in urine may be a proxy for a complex set of exposures that could be important for the health endpoints in studies. Similarly, DEHP in urine was reduced in people eating a fresh food diet with limited packaged food. DEHP metabolite levels may be a proxy for people who eat a less healthy diet of more processed and packaged foods. Therefore, health outcomes associated with the phthalate levels may be due to nutritional factors, or other chemicals that may migrate from packaging to foods or beverages (Rudel et al. 2011). Additionally, cross-sectional analyses, such as those using NHANES, must be interpreted with caution when used to draw associations between short-lived exposure and complex chronic diseases (LaKind et al. 2012).
2.5 Risk Assessment and Cumulative Effects
Phthalates act in a dose-additive manner; exposure to multiple anti-androgenic phthalates will result in additive risk to reproductive harm, so risks must be evaluated considering cumulative exposure (Hotchkiss et al. 2010; Koch et al. 2011). Phthalates also act additively with other chemicals that disrupt fetal testosterone synthesis by different mechanisms (Howdeshell et al. 2008). These findings prompted the National Research Council and others to report that phthalates, along with other compounds with similar actions, should be considered for their cumulative effects on the male reproductive system (National Research Council 2008; Koch et al. 2011).
A recent study in Germany with 111 school children aged 5–6 conducted a cumulative risk assessment, using an approach that combined the ratios of daily exposure and TDI for each phthalate measured in the study. Roughly a quarter of the children exceeded the cumulative TDI for DEHP, DnBP and DiBP, the three phthalates with the most evidence of male reproductive toxicity (Koch et al. 2011). This study’s findings indicate that although these phthalates are regulated in the EU in children’s toys, childcare goods, and cosmetics, exposures are currently above acceptable levels for male reproductive health.
2.6 Regulations and Policies
2.6.1 United States
In the U.S., the Food and Drug Administration (FDA) has issued guidance for restricting the use of DBP and DEHP in prescription and nonprescription products (Food and Drug Administration 2012). This does not address phthalate use in drug delivery systems, packaging, or medical equipment. The FDA states that there is no health risk posed by phthalates used in cosmetics, and labeling of phthalate ingredients is not required if they are used in fragrances or in professional salon products (Food and Drug Administration 2013). Three phthalates, DEHP, DnBP, and BBzP are banned in the U.S. in children’s toys and some child care articles intended to facilitate feeding, sucking, or teething under the Consumer Product Safety Improvement Act (United States Congress 2008). Three additional phthalates, DINP, DIDP, DnOP are temporarily banned from children’s toys that can be placed in a child’s mouth, or children’s toys smaller than 5 centimeters (cm). The ban does not apply to inaccessible parts of a toy (United States Congress 2008). The Consumer Product Safety Commission’s (CPSC) Chronic Hazard Advisory Panel is conducting a hazard assessment on these phthalates to determine if their ban will be lifted or remain in place (United States Environmental Protection Agency 2012b).
The USEPA developed an Action Plan for phthalates based upon their toxicity, widespread use, and human exposure. Eight phthalates are included in the Action Plan: DBP, DIBP, BBzP, di-n-pentyl phthalate (DnPP), DEHP, DnOP, DINP, and DIDP. The USEPA will coordinate with the CPSC and FDA on regulatory action to address the manufacturing, use, sale, and distribution of these compounds in the U.S. (United States Environmental Protection Agency 2012b). To date, a Significant New Use Rule has been proposed for DnPP, which requires manufacturers or processors of the chemical to obtain USEPA approval (United States Environmental Protection Agency 2013). Levels of DEHP in drinking water are regulated by the Clean Drinking Water Act, with a maximum contaminant level (MCL) of 0.006 mg/L for DEHP. DEHP and DBP are listed as hazardous pollutants under the Clean Air Act (United States Environmental Protection Agency 2012b).
California’s Safe Drinking Water and Toxic Enforcement Act of 1986, more commonly known as Proposition 65, requires products that contain chemicals identified as carcinogens or toxic to development or reproduction by the state of California’s Office of Environmental Health Hazard Assessment to be labeled as such in the state. DEHP is labeled as a carcinogen and male developmental toxicant. DINP is recognized as a carcinogen, and DBP, DIDP, BBzP, and DnHP are developmental toxicants under this act (State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment 1986). This rule can provide an incentive for companies to reformulate products with safer alternatives.
Several phthalates are listed among the risks to public health associated with PVC materials, and the American Public Health Association, which represents a broad array of public health professionals, urges federal and local governments to replace PVC when possible in medical care settings, schools, public housing, and building materials (American Public Health Association 2011).
Screening phthalates for anti-androgenic activity has been proposed by the USEPA’s Endocrine Disruptor Screening Program (EDSP). DnBP, BBzP, and DEHP are included in the first group of 67 chemicals to be screened as part of the EDSP. Because phthalates’ anti-androgenic activity is not a result of direct action of the chemical on the androgen receptor, receptor-based screening assays will not detect the anti-androgenic activity of phthalates or evidence of other indirect endocrine disruption (Ankley and Gray 2013).
2.6.2 European Union
Food, food packaging, and pharmaceuticals have been shown to increase human exposure to phthalates. To address this, the EFSA restricted the use of DBP, DIDP, BBzP, DEHP, and DINP in FCMs (Petersen and Jensen 2010). In the EU, DEHP is allowed in food production facilities, such as in conveyor belts, provided it does next exceed the substance migration limit of 1.5 mg/kg of food, although it is prohibited from single-use lips or caps (European Union 2007). DEHP, DBP, and BBzP are banned from children’s toys and child care articles intended to be placed in the mouth by children under age 3, and DINP, DIDP, and DnOP are banned from toys and childcare articles that can be placed in the mouth by children (European Parliament Council 2005). DEHP, DBP, and BBzP are prohibited from use in cosmetics (European Union 2004). Regulations in the EU require human health and environmental testing and data sharing for chemicals within its legislative chemical framework under Registration, Evaluation, Authorization and Restriction of Chemicals (REACH). Substances of Very High Concern are evaluated for restriction, or authorization for certain uses only. DnPP, bis(2-methoxyethyl) phthalate, DiBP, BBzP, DEHP, and DnBP are on the candidate list for REACH authorization as toxic for reproduction (European Chemicals Agency 2013).
The European Medicines Agency recently released a guidance document recommending a reduction in the content of DEP and DBP in medicines in order to protect the safety of all patient populations, in recognition that phthalate exposure through medicines may contribute to the overall burden of phthalate exposure (European Medicines Agency 2013).
2.7 Alternatives
A variety of chemicals are commonly used as alternatives to phthalate plasticizers. Di(isononyl) cyclohexane 1,2-dicarboxylate (DINCH), di(ethylhexyl) adipate (DEHA) and O-acetyl tributyl citrate (ATBC) have been reported as substitutes that are commonly used in DEHP-free products in medical settings, in children’s products, and in plastic cling wrap for food storage (Health and Consumer Protection 2007; United States Environmental Protection Agency 2012b; Lowell Center for Sustainable Production 2011). Some alternatives have been evaluated for health effects, and there is evidence of both reproductive and non-reproductive health endpoints associated with these chemicals. In a 2-year study, DINCH administration was associated with increased thyroid weight in both male and female rats, and a 90-day repeated dose study in rats resulted in increased liver and testes weight (Lowell Center for Sustainable Production 2011; Health and Consumer Protection 2007). Studies have shown that DEHA leaches from its plastic polymer to a greater degree than DEHP. There is limited evidence of DEHA’s carcinogenicity from two rodent feeding studies. There was no evidence of carcinogenicity in rats, while liver carcinomas and adenomas were observed in DEHA-treated male and female mice, leading to its Group 3 classification by IARC (Group 3: not classifiable as to its carcinogenicity in humans) (Van Vliet et al. 2011; World Health Organization 2000). ATBC is a citric acid-derived plasticizer with high potential to leach from plastic and health effects noted in T cells, increased liver weight, and reduced body weight rat pups following in utero exposure (Health and Consumer Protection 2007). Biomonitoring data on these alternatives are limited, although German urine samples show an increased in detectable levels of DINCH metabolites from 7 % in 2006 to 98 % of urine samples in 2012 (Schutze et al. 2014).
2.8 Summary
While phthalates are a diverse group of compounds that are used in many applications, they have toxicological differences, and they cannot all be considered as one group. Humans are primarily exposed to DEHP through their diet, although consumer products and building materials in the indoor environment are also significant contributors to exposure to DEP, DBP, BBzP, DEHP, and DINP. Since phthalates are so commonly used, most people experience constant exposure, although levels vary throughout the day due to the compounds’ short half-lives. Because of this rapid clearance from the body, studies have indicated it is possible to reduce exposure levels quickly by eating fresh whole foods and avoiding foods or beverages stored in plastic or cans. The best understood toxic effect of some phthalates is on the developing male reproductive system. Their toxicological action on this system is known to be additive, and thus phthalates and other compounds that exert similar effects should be considered cumulatively in a risk assessment. Other toxic endpoints that have been studied include fertility, cancer, epigenetic changes, neurotoxicity, metabolic changes, and immune and respiratory effects. While some phthalates have been restricted in FCMs and children’s products due to health concerns, suitable alternatives have not undergone rigorous evaluation for health effects.
2.9 Data Gaps
Risk assessments indicate that current exposure levels for hormonally active phthalates are above a level of concern even for the general population, and there are subpopulations with much higher exposure, for example from medical uses (Koch et al. 2011). Therefore, the most urgent data needs are to identify ways to reduce exposure to these active phthalates and to evaluate potential health effects of substitutes prior to putting them into use. Specific data gaps include:
-
Knowledge of major exposure pathways for hormonally active phthalates and identification of opportunities for intervention to reduce exposures. Currently there is limited disclosure of product ingredients and this makes it difficult to identify important pathways or intervene. It is also important to identify phthalate uses that lead to highly exposed subpopulations.
-
Sensitive toxicological screening tests that recognize the specific mechanisms of action of phthalates for use in high-throughput chemical testing programs. The initial priority is to be able to screen for the developmental inhibition of testosterone synthesis, but tests are also needed for other biological pathways that may be important for phthalates or their substitutes.
-
More comprehensive health effect studies of chemicals used in consumer products and food processing and packaging, especially chemicals being introduced as substitutes for phthalates. Systematic assessments of potential health effects of this family of compounds and better exposure models are needed as a basis for reformulation and regulation.
References
Adibi JJ, Whyatt RM, Williams PL et al (2008) Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect 116(4):467–473
Agency for Toxic Substances and Disease Registry (1995) Toxicological profile for diethyl phthalate. U.S. Department of Health and Human Services, Atlanta
Agency for Toxic Substances and Disease Registry (2002) Toxicological profile for di(2-ethylhexyl) phthalate. U.S. Department of Health and Human Services, Atlanta
American Public Health Association (2011) Reducing PVC in facilities with vulnerable populations. http://www.apha.org/advocacy/policy/policysearch/default.htm?id=1419. Accessed 15 Nov 2013
Ankley GT, Gray LE (2013) Cross-species conservation of endocrine pathways: a critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environ Toxicol Chem 32(5):1084–1087
Aylward LL, Hays SM, Gagne M et al (2009) Derivation of biomonitoring equivalents for di(2-ethylhexyl)phthalate (CAS No. 117-81-7). Regul Toxicol Pharmacol 55(3):249–258
Aylward LL, Lorber M, Hays SM (2011) Urinary DEHP metabolites and fasting time in NHANES. J Expo Sci Environ Epidemiol 21(6):615–624
Bekö G, Weschler CJ, Langer S et al (2013) Children’s phthalate intakes and resultant cumulative exposures estimated from urine compared with estimates from dust ingestion, inhalation and dermal absorption in their homes and daycare centers. PLoS ONE 8(4):e62442
Berman T, Hochner-Celnikier D, Calafat AM et al (2009) Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environ Int 35(2):353–357
Bertelsen RJ, Carlsen KC, Calafat AM et al (2013) Urinary biomarkers for phthalates associated with asthma in Norwegian children. Environ Health Perspect 121(2):251–256
Boberg J (2007) Endocrine disrupters affecting male rat reproductive development—focus on phthalates and the fetal testis. Technical University of Denmark. http://orbit.dtu.dk/fedora/objects/orbit:79926/datastreams/file_3195804/content. Accessed 15 Nov 2013
Bornehag CG, Sundell J, Weschler CJ et al (2004) The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect 112(14):1393–1397
Braun JM, Sathyanarayana S, Hauser R (2013) Phthalate exposure and children’s health. Curr Opin Pediatr 25(2):247–254
Breous E, Wenzel A, Loos U (2005) The promoter of the human sodium/iodide symporter responds to certain phthalate plasticisers. Mol Cell Endocrinol 244(1–2):75–78
Calafat AM, Ye X, Silva MJ et al (2006) Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl 29(1):166–171 (discussion 181-165)
Calafat AM, Koch HM, Swan SH et al (2013) Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res 15(5):403
Carlstedt F, Jonsson BA, Bornehag CG (2013) PVC flooring is related to human uptake of phthalates in infants. Indoor Air 23(1):32–39
Castle L, Gilbert J, Eklund T (1990) Migration of plasticizer from poly(vinyl chloride) milk tubing. Food Addit Contam 7(5):591–596
Centers for Disease Control and Prevention (2009) Fourth national report on human exposure to environmental chemicals. U.S. Department of Health and Human Services. http://www.cdc.gov/exposurereport/pdf/FourthReport.pdf. Accessed 15 Nov 2013
Cirillo T, Fasano E, Castaldi E et al (2011) Children’s exposure to di(2-ethylhexyl)phthalate and dibutylphthalate plasticizers from school meals. J Agric Food Chem 59(19):10532–10538
Colacino JA, Harris TR, Schecter A (2010) Dietary intake is associated with phthalate body burden in a nationally representative sample. Environ Health Perspect 118(7):998–1003
David RM, Moore MR, Finney DC et al (2000) Chronic toxicity of di(2-ethylhexyl)phthalate in mice. Toxicol Sci 58(2):377–385
Dodson RE, Nishioka M, Standley LJ et al (2012) Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect 120(7):935–943
Durmaz E, Ozmert EN, Erkekoglu P et al (2010) Plasma phthalate levels in pubertal gynecomastia. Pediatrics 125(1):e122–e129
Duty SM, Ackerman RM, Calafat AM et al (2005a) Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ Health Perspect 113(11):1530–1535
Duty SM, Calafat AM, Silva MJ et al (2005b) Phthalate exposure and reproductive hormones in adult men. Hum Reprod 20(3):604–610
Engel SM, Miodovnik A, Canfield RL et al (2010) Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118(4):565–571
Enneking PA (2006) Phthalates not in plastic food packaging. Environ Health Perspect 114(2):A89–A90
European Chemicals Agency (2012) Opinion on an Annex XV dossier proposing restrictions on four phthalates. http://echa.europa.eu/documents/10162/77cf7d29-ba63-4901-aded-59cf75536e06. Accessed 17 Dec 2013
European Chemicals Agency (2013) Candidate list of substances of very high concern for authorisation. http://echa.europa.eu/candidate-list-table. Accessed 15 Nov 2013
European Medicines Agency (2013) Guideline on the use of phthalates as excipients in human medicinal products http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/05/WC500143140.pdf. Accessed 6 Dec 2013
European Parliament Council (2005) Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the member states relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles). http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:344:0040:0043:en:PDF. Accessed 6 Dec 2013
European Union (2004) Commission Directive 2004/93/EC. Official J Eur Union. http://www.dehp-facts.com/upload/documents/webpage/Cosmetics.pdf. Accessed 12 Dec 2013
European Union (2007) Commission Directive 2007/19/EC. http://www.dehp-facts.com/upload/documents/webpage/foodcontact%20leg.pdf. Accessed 12 Dec 2013
Food and Drug Administration (2012) Guidance for industry limiting the use of certain phthalates as excipients in CDER-regulated products. U.S. Department of Health and Human Services. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM294086.pdf. Accessed 15 Nov 2013
Food and Drug Administration (2013) Phthalates and cosmetic products. U.S. Department of Health and Human Services. http://www.fda.gov/cosmetics/productandingredientsafety/selectedcosmeticingredients/ucm128250.htm. Accessed 6 Dec 2013
Fromme H, Gruber L, Schlummer M et al (2007) Intake of phthalates and di(2-ethylhexyl)adipate: results of the integrated exposure assessment survey based on duplicate diet samples and biomonitoring data. Environ Int 33(8):1012–1020
Ge R, Tao L, Kramer PM et al (2002) Effect of peroxisome proliferators on the methylation and protein level of the c-myc protooncogene in B6C3F1 mice liver. J Biochem Mol Toxicol 16(1):41–47
Gray LE Jr, Laskey J, Ostby J (2006a) Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female Long Evans hooded rats. Toxicol Sci 93(1):189–195
Gray LE Jr, Wilson VS, Stoker T et al (2006b) Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int J Androl 29 (1):96–104 (discussion 105-108)
Gray LE Jr, Ostby J, Furr J et al (2000) Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58(2):350–365
Hannas BR, Lambright CS, Furr J et al (2011) Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci 123(1):206–216
Hannas BR, Lambright CS, Furr J et al (2012) Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci 125(2):544–557
Hauser R, Meeker JD, Park S et al (2004) Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112(17):1734–1740
Hauser R, Meeker JD, Duty S et al (2006) Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology 17(6):682–691
Health and Consumer Protection (2007) Preliminary report on the safety of medical devices containing DEHP plasticized PVC or other plasticizers on neonates and other groups possibly at risk. European Commission. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_008.pdf. Accessed 15 Nov 2013
Helm D (2007) Correlation between production amounts of DEHP and daily intake. Sci Total Environ 388(1–3):389–391
Hoppin JA, Ulmer R, London SJ (2004) Phthalate exposure and pulmonary function. Environ Health Perspect 112(5):571–574
Hotchkiss A, Ankley GT, Wilson VS et al (2008) Of mice and men (and mosquitofish): antiandrogens and androgens in the environment. Bioscience 58(11):1037–1050
Hotchkiss AK, Rider CV, Furr J et al (2010) In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats. Reprod Toxicol 30(2):261–270
Howdeshell KL, Wilson VS, Furr J et al (2008) A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague-Dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105(1):153–165
Hubinger JC (2010) A survey of phthalate esters in consumer cosmetic products. J Cosmet Sci 61(6):457–465
Hubinger JC, Havery DC (2006) Analysis of consumer cosmetic products for phthalate esters. J Cosmet Sci 57(2):127–137
Hurst CH, Waxman DJ (2003) Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 74(2):297–308
Integrated Risk Information System (1997) Di(2-ethylhexyl)phthalate (DEHP) summary. United States Environmental Protection Agency. http://www.epa.gov/iris/subst/0014.htm. Accessed 18 Nov 2013
International Agency for Research on Cancer (2012) IARC monographs on the evaluation of carcinogenic risk to humans: di(2-ethylhexyl)phthalate, vol 101. International Agency for Research on Cancer, Lyon, France. http://monographs.iarc.fr/ENG/Monographs/vol101/mono101-006.pdf. Accessed 12 Dec 2013
Ito Y, Yamanoshita O, Kurata Y et al (2007) Induction of peroxisome proliferator-activated receptor alpha (PPARalpha)-related enzymes by di(2-ethylhexyl) phthalate (DEHP) treatment in mice and rats, but not marmosets. Arch Toxicol 81(3):219–226
Jaakkola JJ, Oie L, Nafstad P et al (1999) Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo. Norway. Am J Public Health 89(2):188–192
Jaakkola JJ, Verkasalo PK, Jaakkola N (2000) Plastic wall materials in the home and respiratory health in young children. Am J Public Health 90(5):797–799
Janjua NR, Frederiksen H, Skakkebaek NE et al (2008) Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl 31(2):118–130
Just AC (2012) Exposure to phthalate mixtures and inner-city pediatric allergic disease and airway inflammation. Ph.D. dissertation, Columbia University http://academiccommons.columbia.edu/catalog/ac%3A156937. Accessed 15 Nov 2013
Just AC, Adibi JJ, Rundle AG et al (2010) Urinary and air phthalate concentrations and self-reported use of personal care products among minority pregnant women in New York City. J Expo Sci Environ Epidemiol 20(7):625–633
Just AC, Whyatt RM, Miller RL et al (2012a) Children’s urinary phthalate metabolites and fractional exhaled nitric oxide in an urban cohort. Am J Respir Crit Care Med 86(9):830–837
Just AC, Whyatt RM, Perzanowski MS et al (2012b) Prenatal exposure to butylbenzyl phthalate and early eczema in an urban cohort. Environ Health Perspect 120(10):1475–1480
Kang SC, Lee BM (2005) DNA methylation of estrogen receptor alpha gene by phthalates. J Toxicol Environ Health A 68(23–24):1995–2003
Kobrosly RW, Parlett LE, Stahlhut RW et al (2012) Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environ Res 115:11–17
Koch HM, Wittassek M, Bruning T et al (2011) Exposure to phthalates in 5–6 years old primary school starters in Germany—a human biomonitoring study and a cumulative risk assessment. Int J Hyg Environ Health 214(3):188–195
Koch HM, Lorber M, Christensen KL et al (2013) Identifying sources of phthalate exposure with human biomonitoring: results of a 48 h fasting study with urine collection and personal activity patterns. Int J Hyg Environ Health 216(6):672–681
Koniecki D, Wang R, Moody RP et al (2011) Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res 111(3):329–336
LaKind JS, Goodman M, Naiman DQ (2012) Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS One 7(12):e51086
Larsson M, Hagerhed-Engman L, Kolarik B et al (2010) PVC—as flooring material—and its association with incident asthma in a Swedish child cohort study. Indoor Air 20(6):494–501
Lewis RC, Meeker JD, Peterson KE et al (2013) Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere 93(10):2390–2398
Lorber M, Calafat AM (2012) Dose reconstruction of di(2-ethylhexyl) phthalate using a simple pharmacokinetic model. Environ Health Perspect 120(12):1705–1710
Lovekamp-Swan T, Davis BJ (2003) Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 111(2):139–145
Lowell Center for Sustainable Production (2011) Phthalates and their alternatives. University of Massachusetts Lowell. http://www.sustainableproduction.org/downloads/PhthalateAlternatives-January2011.pdf. Accessed 15 Nov 2013
Mahood IK, Scott HM, Brown R (2007) In utero exposure to di(n-butyl) phthalate and testicular dysgenesis: comparison of fetal and adult end points and their dose sensitivity. Environ Health Perspect 115(Suppl 1):55–61
Main KM, Mortensen GK, Kaleva MM et al (2005) Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in 3 month old infants. Environ Health Perspect 114(2):270–276
Martina CA, Weiss B, Swan SH (2012) Lifestyle behaviors associated with exposures to endocrine disruptors. Neurotoxicology 33(6):1427–1433
Meeker JD, Ferguson KK (2012) Dioxins and health: including other persistent organic pollutants and endocrine disruptors. In: Phthalates: human exposure and related health effects. Hoboken, Wiley (Chapter 13)
Meeker JD, Calafat AM, Hauser R (2007) Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115(7):1029–1034
Melnick RL (2001) Is peroxisome proliferation an obligatory precursor step in the carcinogenicity of di(2-ethylhexyl)phthalate (DEHP)? Environ Health Perspect 109(5):437–442
Montuori P, Jover E, Morgantini M et al (2008) Assessing human exposure to phthalic acid and phthalate esters from mineral water stored in polyethylene terephthalate and glass bottles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25(4):511–518
Moyer B, Hixon ML (2012) Reproductive effects in F1 adult females exposed in utero to moderate to high doses of mono-2-ethylhexylphthalate (MEHP). Reprod Toxicol 34(1):43–50
National Research Council (2008) Phthalates and cumulative risk assessment the task ahead. National Academy of Sciences. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=202508#Download. Accessed 15 Nov 2013
National Toxicology Program (2011) Twelfth report on carcinogens. U.S. Department of Health and Human Services. http://ntp.niehs.nih.gov/ntp/roc/twelfth/roc12.pdf. Accessed 15 Nov 2013
Noriega NC, Howdeshell KL, Furr J et al (2009) Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci 111(1):163–178
Office of Environmental Health Hazard Assessment California Environmental Protection Agency (2013) Evidence on the carcinogenicity of diisononyl phthalate (DINP). http://oehha.ca.gov/prop65/hazard_ident/pdf_zip/DINP_HID100413.pdf. Accessed 31 Dec 2013
Pan G, Hanaoka T, Yoshimura M et al (2006) Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect 114(11):1643–1648
Parlett LE, Calafat AM, Swan SH (2013) Women’s exposure to phthalates in relation to use of personal care products. J Expo Sci Environ Epidemiol 23(2):197–206
Petersen JH, Jensen LK (2010) Phthalates and food-contact materials: enforcing the 2008 European Union plastics legislation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 27(11):1608–1616
Romero-Franco M, Hernandez-Ramirez RU, Calafat AM et al (2011) Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ Int 37(5):867–871
Rudel RA, Camann DE, Spengler JD et al (2003) Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ Sci Technol 37(20):4543–4553
Rudel RA, Dodson RE, Perovich LJ et al (2010) Semivolatile endocrine-disrupting compounds in paired indoor and outdoor air in two northern California communities. Environ Sci Technol 44(17):6583–6590
Rudel RA, Gray JM, Engel CL et al (2011) Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect 119(7):914–920
Rusyn I, Corton JC (2012) Mechanistic considerations for human relevance of cancer hazard of di(2-ethylhexyl) phthalate. Mutat Res 750(2):141–158
Sathyanarayana S, Karr CJ, Lozano P et al (2008) Baby care products: possible sources of infant phthalate exposure. Pediatrics 121(2):e260–e268
Sathyanarayana S, Alcedo G, Saelens BE et al (2013) Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J Expo Sci Environ Epidemiol 23(4):378–384
Schecter A, Lorber M, Guo Y et al (2013) Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121(4):473–479
Schettler T (2006) Human exposure to phthalates via consumer products. Int J Androl 29(1):134–139
Schutze A, Kolossa-Gehring M, Apel P et al (2014) Entering markets and bodies: increasing levels of the novel plasticizer Hexamoll DINCH in 24 h urine samples from the German Environmental Specimen Bank. Int J Hyg Environ Health 217(2–3):421–428
Scott HM, Mason JI, Sharpe RM (2009) Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr Rev 30(7):883–925
Silva MJ, Barr DB, Reidy JA et al (2004) Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112(3):331–338
Silva MJ, Samandar E, Preau JL Jr et al (2007) Quantification of 22 phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 860(1):106–112
State of California Environmental Protection Agency Office of Environmental Health Hazard Assessment (1986) Safe Drinking Water and Toxic Enforcement Act of 1986. California, United States
Stringer R, Labunska I, Santillo D et al (2000) Concentrations of phthalate esters and identification of other additives in PVC children’s toys. Environ Sci Pollut Res Int 7(1):27–36
Swan SH, Main KM, Liu F et al (2005) Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113(8):1056–1061
Swan SH, Liu F, Hines M et al (2010) Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl 33(2):259–269
Toft G, Jonsson BA, Lindh CH et al (2012) Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect 120(3):458–463
Tomonari Y, Kurata Y, David RM et al (2006) Effect of di(2-ethylhexyl) phthalate (DEHP) on genital organs from juvenile common marmosets: I. Morphological and biochemical investigation in 65-week toxicity study. J Toxicol Environ Health A 69(17):1651–1672
Trasande L, Sathyanarayana S, Spanier AJ et al (2013) Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr 163(3):747–753 (e741)
Tsumura Y, Ishimitsu S, Kaihara A et al (2001) Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit Contam 18(6):569–579
United States Congress (2008) Consumer product safety improvement act of 2008 http://www.cpsc.gov//PageFiles/113865/cpsia.pdf. Accessed 15 Nov 2013
United States Environmental Protection Agency (2012a) Chemical data access tool. http://java.epa.gov/oppt_chemical_search/. Accessed 15 Nov 2013
United States Environmental Protection Agency (2012b) Phthalates action plan. http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/phthalates_ap_2009_1230_final.pdf. Accessed 15 Nov 2013
United States Environmental Protection Agency (2013) Benzidine-based chemical substances; di-n-pentyl phthalate (DnPP); and alkanes, C12–13, chloro; proposed Significant New Use Rules. Federal Register. http://www.gpo.gov/fdsys/pkg/FR-2012-03-28/pdf/2012-7208.pdf. Accessed 15 Nov 2013
Van Vliet ED, Reitano EM, Chhabra JS et al (2011) A review of alternatives to di (2-ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. J Perinatol 31(8):551–560
Voss C, Zerban H, Bannasch P et al (2005) Lifelong exposure to di-(2-ethylhexyl)-phthalate induces tumors in liver and testes of Sprague-Dawley rats. Toxicology 206(3):359–371
Weuve J, Sanchez BN, Calafat AM et al (2006) Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect 114(9):1424–1431
Wilson VS, Lambright C, Furr J et al (2004) Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett 146(3):207–215
Wittassek M, Wiesmuller GA, Koch HM et al (2007) Internal phthalate exposure over the last two decades—a retrospective human biomonitoring study. Int J Hyg Environ Health 210(3–4):319–333
Wittassek M, Koch HM, Angerer J et al (2011) Assessing exposure to phthalates—the human biomonitoring approach. Mol Nutr Food Res 55(1):7–31. doi:10.1002/mnfr.201000121
World Health Organization (2000) IARC monographs on the evaluation of carcinogenic risks to humans, vol 77. International Agency for Research on Cancer, Lyon, France
Wormuth M, Scheringer M, Vollenweider M et al (2006) What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal 26(3):803–824
Wu S, Zhu J, Li Y et al (2010) Dynamic effect of di-2-(ethylhexyl) phthalate on testicular toxicity: epigenetic changes and their impact on gene expression. Int J Toxicol 29(2):193–200
Yang J, Hauser R, Goldman RH (2013) Taiwan food scandal: the illegal use of phthalates as a clouding agent and their contribution to maternal exposure. Food Chem Toxicol 58:362–368
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Cite this chapter
Rodgers, K.M., Rudel, R.A., Just, A.C. (2014). Phthalates in Food Packaging, Consumer Products, and Indoor Environments. In: Snedeker, S. (eds) Toxicants in Food Packaging and Household Plastics. Molecular and Integrative Toxicology. Springer, London. https://doi.org/10.1007/978-1-4471-6500-2_2
Download citation
DOI: https://doi.org/10.1007/978-1-4471-6500-2_2
Published:
Publisher Name: Springer, London
Print ISBN: 978-1-4471-6499-9
Online ISBN: 978-1-4471-6500-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)